Abstract
Background
Hyponatremia is one of the most common electrolyte disturbances in advanced chronic kidney disease (CKD) and end-stage kidney disease (ESKD) patients, and has been shown to be associated with higher mortality risk. However, the relationship between hyponatremia during late-stage CKD and the risk of poor outcomes after ESKD transition is unknown.
Methods
We conducted a retrospective cohort study including 32 257 US veterans transitioning to ESKD from 1 October 2007 to 30 March 2015. We evaluated adjusted associations between the 3-month averaged pre-transition to ESKD serum sodium and all-cause mortality. Secondary outcomes included cardiovascular (CV) mortality, infection-related mortalities and hospitalization rate.
Results
Cohort mean ± standard deviation serum sodium was 139 ± 3 mEq/L, mean age was 67 ± 11 years, 98% were male and 28% were African American. Over a median (interquartile range) follow-up of 702 days (296, 1301) there were 17 162 deaths. Compared with the reference of 135 to <144 mEq/L, the lowest serum sodium group (<130 mEq/L) had a 54% higher all-cause mortality risk [hazard ratio 1.54 (95% confidence interval 1.34–1.76)] in the fully adjusted model. Associations were similar for CV and infection-related mortality, and hospitalization outcomes.
Conclusions
Hyponatremia prior to ESKD transition is associated with higher risk of all-cause, CV and infection-related mortalities, and hospitalization rates after ESKD transition. Future studies evaluating management of pre-ESKD hyponatremia may be indicated to improve patient outcomes for those transitioning to ESKD.
Keywords: end-stage kidney disease, ESKD transition, hospitalization, mortality, serum sodium
Key Learning Points
What is already known about this subject?
Hyponatremia is one of the most common electrolyte disturbances and is associated with increased morbidity and mortality in the general population, chronic kidney disease (CKD) and end-stage kidney disease (ESKD) population;
there is a U-shaped association between serum sodium levels and mortality in CKD and ESKD patients, with the lowest mortality being among patients with serum sodium in normonatremic range; however, it remains uncertain whether hyponatremia represents the underlying medical comorbidities or directly leads to poor clinical outcomes; and
while serum sodium of maintenance dialysis patients is affected by dialysis, the association between baseline serum sodium of advanced CKD patients and mortality after dialysis transition is unknown.
What this study adds?
Average serum sodium <130 mEq/L of advanced CKD patients during 3 months prior to dialysis transition was associated with greater risk of all-cause, cardiovascular (CV) and infection-related mortalities after dialysis initiation, while average serum sodium ≥144 mEq/L trended toward lower risk of all-cause and CV mortalities;
the rate of all-cause hospitalization after dialysis initiation was inversely associated with average serum sodium during 3 months prior to dialysis transition; and
serum sodium of advanced CKD patients with hyponatremia and hypernatremia increases and decreases toward the normal serum sodium level, respectively, after dialysis initiation and becomes plateaued after 3–6 months post-dialysis transition.
What impact this may have on practice or policy?
While the optimal serum sodium level during pre-dialysis initiation leading to favorable outcomes after dialysis transition remains unknown, hyponatremia in advanced CKD patients should prompt a search for an intervention of potential underlying comorbidities;
given the highest mortality during the early phase of dialysis initiation, providers may need to pay close attention to ESKD patients with a history of hyponatremia prior to dialysis transition; and
an appropriate time for dialysis initiation may be guided by the trend of serum sodium in advanced CKD patients and a rapidly decreased trend toward hyponatremia may require early dialysis initiation to minimize a period of hyponatremia after dialysis transition.
INTRODUCTION
Hyponatremia is one of the most common water and electrolyte disturbances in clinical practice [1] with approximately 8% of the general population aged 55 years and older being affected by hyponatremia [2]. Hyponatremia is a common sequelae of chronic kidney disease (CKD) [3–6], resulting from the nephron loss that leads to a diminished ability to appropriately handle salt and water [7, 8]. Despite these changes, the serum sodium concentration typically remains within a physiological range even in severe CKD [9].
Nonetheless, hyponatremia has been associated with higher mortality in acute kidney injury, CKD and end-stage kidney disease (ESKD), and this association remains consistent after adjustment for degree of CKD [4, 10–13]. Furthermore, even mild hyponatremia in a community-based population without underlying comorbidities has been shown to be an independent predictor of mortality [14]. This implies that abnormal serum sodium levels may be a risk factor for mortality distinct from the risk arising from the underlying cause(s) of the hyponatremia [5, 15]. Once patients transition to dialysis, serum sodium levels are affected by the dialysis procedure and it is unclear whether correcting underlying hyponatremia with hemodialysis can mitigate the poor outcomes associated with pre-existing hyponatremia.
In this study, we evaluate the association between hyponatremia in advanced CKD patients prior to ESKD transition and long-term major clinical outcomes after dialysis initiation including all-cause, cardiovascular (CV) and infection-related mortality, as well as hospitalization rate.
MATERIALS AND METHODS
Study population and data source
The analytical cohort was derived from the United States Renal Data System (USRDS) Special Study Center Transition of Care in CKD (TCCKD) database, a cohort of 102 447 US veterans who transitioned to ESKD between 1 October 2007 and 30 March 2015 [16–21].
Patients were excluded if they did not have follow-up data, were missing date of birth or if they did not have a serum sodium measurement within the 3 months prior to dialysis transition (hereby referred to as the ‘prelude’ period). Thus, our final analytical cohort consisted of 32 257 US veterans transitioning to ESKD (Supplementary data, Figure S1). Patients were followed from the initiation of ESKD until death, kidney transplantation, loss to follow-up or the date of final follow-up for all patients (1 September 2015 for all-cause mortality and hospitalization, and 31 July 2015 for CV and infection-related mortality).
Given the nonintrusive nature, patient anonymity and large sample size, the requirement for written informed consent was waived and the study was approved by the Memphis and Tibor Rubin Veterans Affairs (VA) Medical Centers Institutional Review Boards.
Demographic, clinical and laboratory measurements
Patient characteristics including demographic information and primary cause of ESKD collected during the prelude period were extracted from a composite of USRDS, Patient and Medical Evidence files, VA databases and Centers for Medicare and Medicaid Services (CMS) databases. VA and CMS data were used to determine pre-existing comorbidity status, according to the presence of International Classification of Disease-9 (ICD-9) codes, as well as the Charlson Comorbidity Index (CCI) at the time of ESKD transition. Prescribed medication information was extracted from CMS Medicare Part D and VA pharmacy dispensation records. Medication use in this study was defined as ever having a prescription filled during the prelude period.
Laboratory measurements, including serum sodium, were obtained from the VA Decision Support System National Data Extracts Laboratory Results file. All laboratory measurements during the 3-month period prior to ESKD transition were averaged into a single measurement used as prelude levels.
Exposure measurement
The main exposure of this study was average prelude (Q1) serum sodium. Prelude serum sodium levels were categorized into four exposure categories: (i) <130 (moderate–severe hyponatremia), (ii) 130 to <135 (mild hyponatremia), (iii) 135 to <144 (referent group) and (iv) ≥144 mEq/L (hypernatremia).
Outcome assessment
The primary outcome of interest was all-cause mortality after ESKD transition. Secondary outcomes of interest included CV mortality, infection-related mortality and hospitalization rates (Supplementary data, Table S1). Information on all outcomes and censoring events were obtained from VA, CMS and USRDS records using ICD-9 codes.
Statistical analysis
Patient demographic and clinical characteristics are presented as mean ± standard deviation (SD), median [interquartile range (IQR)] or percent as appropriate for the total cohort and stratified by prelude serum sodium groups.
To examine trajectories of quarterly (3-month) averaged sodium 1 year pre- and post-transition, we used a mixed-effects regression (random intercept and random slope) model to visualize trajectories stratified by prelude serum sodium groups.
Cox proportional hazards models were used to evaluate the association of the prelude serum sodium groups with all-cause, CV and infection-related mortalities. Proportional hazards assumptions were checked.
Hospitalization rates across the various sodium groups were analyzed using a Poisson model. Incidence rate ratios (IRRs) were obtained with serum sodium values between 135 and <144 as the referent range.
For each outcome, four hierarchical models of adjustment were used. Model 1: unadjusted. Model 2: Case Mix: age, sex, race, ethnicity, marital status, diabetes, cancer, CCI, cerebrovascular disease, congestive heart failure, anemia, depression, hyperlipidemia and liver disease. Model 3: Case Mix + malnutrition inflammation complex syndrome (MICS): Case Mix variables, body mass index (BMI), albumin, total serum bicarbonate (CO2), calcium, phosphorus, hemoglobin, white blood cells (WBCs) and glucose. Model 4: Case Mix + MICS + medication (Meds): Case Mix + MICS variables, use of antidepressants, lithium salts, thiazides, loop diuretics, potassium sparing diuretics, bicarbonate, insulin, oral hypoglycemic agents, intravenous (IV) solutions with electrolytes and IV solutions without electrolytes. Model 4 was our primary model of interest.
As a sensitivity analysis, restricted cubic spline functions were used to assess potential non-linear associations for prelude serum sodium modeled as continuous variables with all-cause mortality, CV mortality and infection-related mortality using the fully adjusted model (Model 4). Knots were placed at the 5th, 35th, 65th and 95th percentiles.
Missing categorical data on patient characteristics, including marital status, were <0.2% and were handled by creating missing categories. There were 13 patients (<0.05% of the total cohort) with missing comorbid data who were categorized as no presence of condition for each comorbidity. Missing values for laboratory measurements (including 36% of patients for phosphorus, 11% for albumin, 9% for hemoglobin, 8% for WBCs count, 7% for BMI and <5% for others) were imputed by multiple imputation using five imputed datasets.
All analyses were conducted using SAS Enterprise Guide, version 7.1 (Cary, NC, USA) and STATA version 14.2 (StataCorp, College Station, TX, USA).
RESULTS
Patient demographic, clinical and laboratory characteristics
The analytical cohort consisted of 32 257 patients who had at least one serum sodium measurement in the 3-month pre-ESKD initiation. Of these patients, the mean age was 67 ± 11 years, 98% were male, 75% were diabetic and 28% were African American (Table 1).
Table 1.
Prelude characteristics of 32 257 veterans transitioning to dialysis stratified by 3-month pre-ESKD sodium
| Variable | Total | Serum sodium (mEq/L) |
P for trend | |||
|---|---|---|---|---|---|---|
| <130 | 130 to <135 | 135 to <144 | ≥144 | |||
| N (%) | 32 257 | 341 (1) | 2896 (9) | 27 285 (85) | 1735 (5) | |
| Age (years) | 67 ± 11 | 64 ± 10 | 65 ± 11 | 67 ± 11 | 71 ± 11 | <0.0001 |
| Female (%) | 2 | 2 | 3 | 2 | 1 | 0.0042 |
| Race (%) | ||||||
| White | 68 | 75 | 70 | 64 | 65 | <0.0001 |
| African American | 28 | 21 | 26 | 33 | 33 | <0.0001 |
| Other races | 3 | 4 | 4 | 3 | 2 | <0.0001 |
| Hispanic | 8 | 11 | 8 | 8 | 7 | 0.0421 |
| Marital status (%) | ||||||
| Single | 10 | 11 | 12 | 9 | 8 | <0.0001 |
| Married | 51 | 48 | 47 | 52 | 57 | <0.0001 |
| Divorced | 29 | 33 | 33 | 29 | 22 | <0.0001 |
| Widowed | 10 | 8 | 9 | 10 | 14 | <0.0001 |
| Comorbidities (%) | ||||||
| CCI | 5 (3–7) | 4 (3–6) | 5 (3–7) | 5 (3–7) | 5 (3–7) | 0.8377 |
| Myocardial infarction | 28 | 24 | 30 | 27 | 30 | 0.6821 |
| Congestive heart failure | 59 | 52 | 61 | 58 | 63 | 0.1954 |
| Peripheral vascular disease | 46 | 38 | 47 | 47 | 51 | <0.0001 |
| Cerebrovascular disease | 37 | 29 | 36 | 39 | 44 | <0.0001 |
| Dementia | 4 | 3 | 3 | 4 | 6 | <0.0001 |
| Chronic pulmonary disease | 51 | 46 | 54 | 50 | 53 | 0.7108 |
| Connective tissue disease- rheumatic disease | 6 | 6 | 5 | 6 | 8 | 0.0189 |
| Peptic ulcer disease | 10 | 10 | 9 | 9 | 11 | 0.0296 |
| Liver disease | 18 | 31 | 23 | 18 | 16 | <0.0001 |
| Diabetes | 75 | 67 | 75 | 74 | 77 | 0.0070 |
| Paraplegia and hemiplegia | 5 | 3 | 5 | 5 | 7 | 0.0162 |
| Cancer | 28 | 26 | 26 | 28 | 32 | <0.0001 |
| AIDS/HIV | 2 | 2 | 2 | 2 | 1 | 0.0305 |
| Anemia | 79 | 72 | 78 | 81 | 85 | <0.0001 |
| Atrial fibrillation | 22 | 22 | 23 | 20 | 22 | 0.0636 |
| Depression | 35 | 36 | 39 | 36 | 30 | <0.0001 |
| Hyperlipidemia | 82 | 73 | 81 | 85 | 89 | <0.0001 |
| Hypertension | 96 | 91 | 96 | 98 | 99 | <0.0001 |
| Ischemic heart disease | 60 | 54 | 61 | 60 | 65 | 0.0033 |
| Laboratory tests | ||||||
| Sodium (mEq/L) | 138.86 ± 3.29 | 127.15 ± 2.69 | 133.25 ± 1.30 | 139.20 ± 2.18 | 145.07 ± 1.31 | <0.0001 |
| LDL (mg/dL) | 84.82 ± 40.28 | 74.61 ± 38.96 | 80.16 ± 42.46 | 85.36 ± 40.15 | 85.22 ± 38.56 | <0.0001 |
| HDL (mg/dL) | 39.14 ± 14.24 | 39.70 ± 19.10 | 38.48 ± 16.81 | 39.16 ± 13.97 | 39.80 ± 13.11 | 0.0664 |
| Triglycerides (mg/dL) | 145.22 ± 101.28 | 131.08 ± 79.79 | 149.93 ± 125.41 | 145.91 ± 99.97 | 130.17 ± 80.36 | 0.0024 |
| Cholesterol (mg/dL) | 152.16 ± 51.61 | 140.20 ± 50.70 | 147.28 ± 58.87 | 152.85 ± 51.13 | 151.00 ± 46.41 | 0.0003 |
| Hemoglobin (g/dL) | 10.23 ± 1.61 | 10.13 ± 1.71 | 10.14 ± 1.65 | 10.24 ± 1.60 | 10.33 ± 1.63 | 0.0001 |
| Potassium (mEq/L) | 4.49 ± 0.58 | 4.45 ± 0.67 | 4.44 ± 0.57 | 4.50 ± 0.58 | 4.53 ± 0.62 | <0.0001 |
| BUN (mg/dL) | 67.21 ± 24.96 | 69.59 ± 31.88 | 69.79 ± 28.50 | 66.95 ± 24.44 | 66.51 ± 24.84 | <0.0001 |
| WBCs (× 109/L) | 7.98 ± 4.01 | 9.82 ± 6.82 | 8.73 ± 4.03 | 7.89 ± 3.87 | 7.66 ± 5.10 | <0.0001 |
| Glucose (mg/dL) | 131.04 ± 48.98 | 160.94 ± 119.87 | 153.87 ± 70.00 | 129.38 ± 44.40 | 112.86 ± 34.35 | <0.0001 |
| HgbA1c (%) | 6.76 ± 1.47 | 7.18 ± 2.18 | 7.31 ± 1.94 | 6.71 ± 1.40 | 6.43 ± 1.09 | <0.0001 |
| Uric acid (mg/dL) | 8.13 ± 2.29 | 8.18 ± 2.70 | 8.53 ± 2.72 | 8.10 ± 2.24 | 7.95 ± 2.08 | <0.0001 |
| Platelets (× 109/L) | 205.79 ± 75.55 | 214.31 ± 98.41 | 212.59 ± 84.40 | 205.46 ± 74.63 | 196.63 ± 65.72 | <0.0001 |
| Thyroid stimulating hormone (mIU/L) | 3.46 ± 5.41 | 3.86 ± 8.37 | 4.17 ± 6.94 | 3.35 ± 5.02 | 3.71 ± 6.94 | 0.0012 |
| Alkaline phosphatase (IU/L) | 98.97 ± 75.28 | 117.91 ± 65.01 | 120.27 ± 122.90 | 96.80 ± 69.26 | 91.31 ± 43.91 | <0.0001 |
| Calcium (mg/dL) | 8.62 ± 0.80 | 8.41 ± 0.80 | 8.55 ± 0.74 | 8.62 ± 0.8 | 8.69 ± 0.87 | <0.0001 |
| Albumin (g/dL) | 3.30 ± 0.62 | 3.04 ± 0.69 | 3.07 ± 0.64 | 3.31 ± 0.61 | 3.50 ± 0.60 | <0.0001 |
| CO2 (mEq/L) | 22.63 ± 4.13 | 21.87 ± 4.54 | 22.63 ± 4.25 | 22.60 ± 4.07 | 23.25 ± 4.63 | <0.0001 |
| Phosphorus (mg/dL) | 5.34 ± 1.43 | 5.64 ± 1.91 | 5.39 ± 1.57 | 5.33 ± 1.40 | 5.39 ± 1.48 | 0.0212 |
| eGFR (mL/min/1.73 m2) | 11.8 (8.6–16.5) | 14.1 (9.4–25.9) | 13.4 (9.5–19.8) | 11.6 (8.5–16.2) | 11.5 (8.4–16.0) | <0.0001 |
| BMI (kg/m2) | 30.03 ± 6.81 | 28.97 ± 6.98 | 29.27 ± 6.95 | 30.08 ± 6.78 | 30.84 ± 6.89 | <0.0001 |
| Systolic blood pressure (mmHg) | 142.73 ± 19.07 | 132.93 ± 22.08 | 137.80 ± 20.11 | 143.26 ± 18.72 | 144.86 ± 20.30 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.16 ± 11.81 | 71.10 ± 12.53 | 73.50 ± 11.94 | 74.33 ± 311.74 | 73.23 ± 12.44 | 0.0065 |
| Weight (lbs) | 205.3 ± 50.1 | 199.6 ± 50.1 | 200.4 ± 51.4 | 205.6 ± 49.9 | 210 ± 50.6 | <0.0001 |
| Medications (%) | ||||||
| Thiazides | 20 | 30 | 30 | 20 | 14 | <0.0001 |
| Loop diuretics | 72 | 76 | 76 | 72 | 66 | <0.0001 |
| K-sparing diuretics | 6 | 19 | 12 | 6 | 4 | <0.0001 |
| Bicarbonate | 28 | 31 | 30 | 28 | 21 | <0.0001 |
| Oral hypoglycemic | 18 | 18 | 18 | 18 | 21 | 0.1175 |
| Insulin | 49 | 51 | 57 | 48 | 41 | <0.0001 |
| Antidepressants | 33 | 37 | 41 | 33 | 24 | <0.0001 |
| Lithium salts | 0.1 | 0 | 0.1 | 0.1 | 0.2 | 0.2574 |
| IV solutions w/ electrolytes | 35 | 50 | 49 | 35 | 19 | <0.0001 |
| IV solutions w/o electrolytes | 35 | 50 | 50 | 34 | 18 | <0.0001 |
Data presented as mean ± SD, median (IQR) or proportion where appropriate. Values might not add up to 100% due to rounding. BUN, blood urea nitrogen; CO2, bicarbonate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Hgb, hemoglobin; K, potassium; w/o, without; w/, with.
Patients with lower sodium level were more likely to be younger, not married, and have a lower prevalence of cerebrovascular disease, hyperlipidemia, cancer, anemia and dementia; however, they were more likely to have a higher prevalence of liver disease (mild and moderate–severe). Furthermore, patients with lower sodium level were more likely to have lower levels of serum albumin and lower BMI, yet higher levels of blood urea nitrogen, estimated glomerular filtration rate (eGFR), serum hemoglobin A1c, glucose, platelet count and WBCs.
Diuretics (loop and potassium-sparing) were prescribed more frequently in the lower serum sodium groups, as well as insulin, antidepressants and IV solutions with and without electrolytes.
Sodium trajectories before and after ESKD transition
In the 1-year prior to initiation of renal replacement therapy, patients with higher 3-month pre-ESKD serum sodium showed a gradual increasing trend toward higher serum sodium concentrations, whereas the lowest 3-month pre-ESKD serum sodium group showed a decline in serum sodium concentration as they approached ESKD (Figure 1). This alteration was most pronounced within the last 6 months prior to dialysis initiation while the change in sodium was most distinct in the highest (≥144 mEq/L) and lowest (<130 mEq/L) prelude serum sodium groups. After initiation of renal replacement therapy, there was a regression to the mean and all serum sodium groups plateaued after 3–6 months post-transition. The grading of the 3-month pre-ESKD serum sodium groups did not change during the 1 year pre- and post-transition observation period.
FIGURE 1.
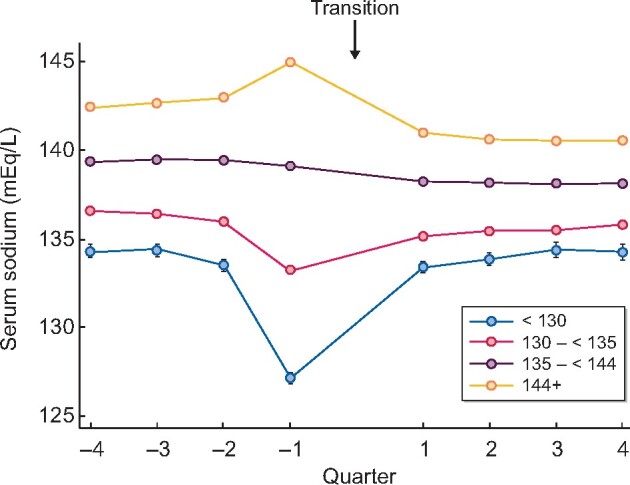
Trajectories of quarterly averaged sodium 1 year pre-transition (−4 quarters) and 1 year post-transition (4 quarters) to ESKD stratified by 3-month pre-ESKD serum sodium groups.
Pre-ESKD serum sodium and mortality
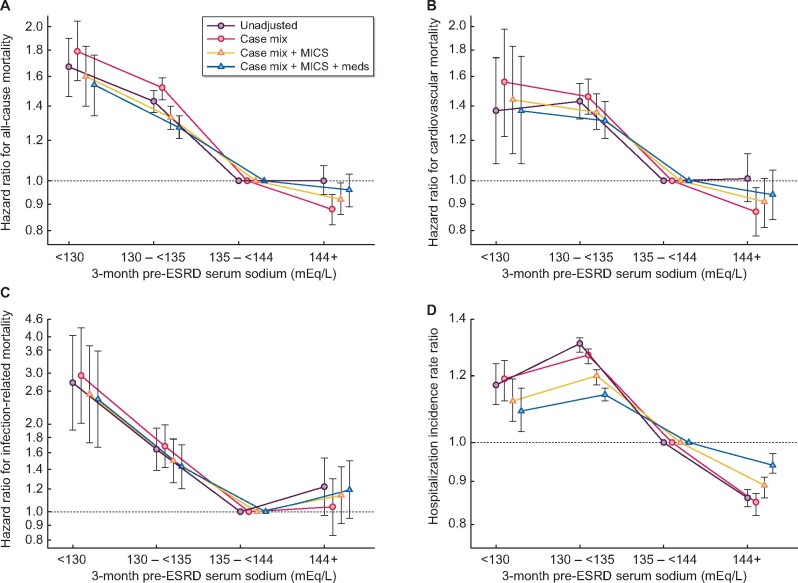
Median (IQR) follow-up time for the total cohort was 702 (296, 1301) days and 17 162 deaths occurred during the follow-up time, with an incidence rate of 22 (22–23) deaths per 100 patient-years. Lower serum sodium was associated with higher all-cause, CV and infection-related mortality risk across all models of adjustment (Figure 2A–C and Supplementary data, Table S2A–C). Patients with a prelude serum sodium <130 mEq/L had a 54%, 37% and 144% higher all-cause, CV and infection-related mortality risks, respectively, compared with those with serum sodium 135 to <144 mEq/L in fully adjusted models (Supplementary data, Table S2A–C and Figure 2A–C). Higher serum sodium (serum sodium ≥144 mEq/L) trended toward a lower all-cause and CV mortality risk in fully adjusted models.
FIGURE 2.
Association of 3-month pre-ESKD sodium with post-ESKD (A) all-cause mortality, (B) CV mortality, (C) infection-related mortality and (D) hospitalization incidence rate ratio in 32 257 veterans transitioning to ESKD.
Pre-ESKD serum sodium and hospitalizations
Finally, in our cohort, there were 136 302 hospitalizations over 76 464 person-years, resulting in a hospitalization rate of 1.78 [95% confidence interval (CI) 1.77–1.79] per patient year. There was an inverse linear association between serum sodium level and hospitalization rate. Compared with the reference group of 135 to <144 mEq/L, lower prelude serum sodium was associated with a 9% higher hospitalization IRR [1.09 (95% CI 1.03–1.16), for serum sodium group <130 mEq/L], while higher serum sodium was associated with a 6% lower hospitalization rate [IRR 0.94 (95% CI 0.92–0.97), for the highest serum sodium group ≥144 mEq/L] (Supplementary data, Table S2D and Figure 2D).
As a sensitivity analysis, we performed cubic spline analysis to assess the association between 3-month prelude serum sodium levels and all-cause, CV and infection-related mortality. We did not find any significant differences in the trends that we observed in prior analyses (Supplementary data, Figure S2).
DISCUSSION
In our cohort of 32 257 veteran patients transitioning to ESKD, we observed that moderate–severe hyponatremia (serum sodium <130 mEq/L) in the quarter prior to ESKD transition was associated with a higher risk of all-cause, CV and infection-related mortality, as well as hospitalization rate. Hypernatremia (≥144 mEq/L) was not associated with significantly different mortality and infection-related outcomes compared with our referent group but was associated with a lower risk of hospitalizations.
Our study is the first one to examines the association between serum sodium measured during the advanced CKD period (prelude) and outcomes after dialysis initiation (post-transition). Our analysis suggests that despite correction of hyponatremia with dialysis (as observed in the trajectories in Figure 1) as well as adjusting for comorbidities related to hyponatremia, hyponatremia remains associated with poor outcomes and increased rate of hospitalizations after dialysis transition.
Previously, Kovesdy et al. [4] demonstrated a U-shaped association between serum sodium and all-cause mortality in CKD patients, with the exception of patients with Stage 5 CKD who had lower mortality associated with hypernatremia. Our study results are similar showing higher mortality rates among patients with the lowest sodium values and suggests that even mild hyponatremia with serum sodium values between 130 and <135 are also associated with poor outcomes, even with attempting to correct hyponatremia after dialysis initiation.
Some studies have speculated that underlying causes of hyponatremia are associated with mortality, rather than the severity of hyponatremia itself [22–27]. The severity of underlying diseases may modulate serum sodium levels due to neurohormonal pathways that regulate antidiuretic hormone (ADH) secretion, which can lead to increased retention of electrolyte-free water and cause hyponatremia. Other studies have suggested that hyponatremia directly contributes to mortality [28–36]. While it remains unclear whether hyponatremia directly contributes to higher mortality and/or is a marker of underlying comorbidities, our study suggests that there remains an association between hyponatremia and mortality even after adjusting for comorbid conditions.
Moreover, a retrospective observational study of a large cohort of incident hemodialysis patients in the USA demonstrated a graded relationship between pre-dialysis serum sodium and all-cause mortality in ESKD patients. In this study, Rhee et al. [12] showed that incident hemodialysis patients with pre-dialysis serum sodium of <138 had the highest all-cause mortality, whereas patients with a serum sodium of ≥144 mEq/L had the lowest mortality. However, this association became a U-shape (the lowest mortality in patients with a serum sodium of 140 to <142 mEq/L) when modeled using time-varying analysis [12]. Our study adds to this previous study by specifically investigating both moderate–severe hyponatremia levels below 130 mEq/L as well as more mild hyponatremia levels between 131 and <144 mEq/L, and shows that even patients with mild hyponatremia continue to have a higher association with poor outcomes.
Hyponatremia was also associated with higher post-ESKD hospitalization rates. Our study is consistent with several previous studies showing that hyponatremia was associated with higher rates of infection [15], pulmonary diseases [37] and fracture [38, 39] related causes of hospitalizations. Interestingly, a higher serum sodium level (>144 mEq/L) was associated with a lower rate of hospitalizations even after adjusting for confounders. The pathophysiology behind this interesting association remains unclear, though it is possible these results are prone to bias in the positive direction from the competing risk of death not being considered. Further studies are likely needed to confirm our findings and elucidate any possible biological mechanisms behind this association.
The trajectory of serum sodium concentration from 1 year prior to and 1 year after dialysis initiation deserves discussion (Figure 1). Between 6 and 12 months prior to dialysis initiation, serum sodium likely reflects underlying comorbidities and progression of CKD. As expected, patients with hyponatremia were more likely to have known underlying conditions resulting in hyponatremia such as liver disease and use of medications such as antidepressants and diuretics compared with those for groups with a baseline serum sodium concentration ≥135 mmEq/L. Serum sodium appeared stable in each group until 3 months prior to dialysis initiation, when serum sodium decreased significantly in patients with the lowest serum sodium levels. The exacerbation of hyponatremia is likely due to a combination of factors including a further decrement in the ability of the native kidneys to excrete free water. In addition, acute illnesses are also highly prevalent around the time of dialysis initiation, which may exacerbate free water retention. After dialysis initiation, serum sodium levels appear to have normalized as a response to electrolyte control and appropriate fluid removal with dialysis treatments.
Several limitations of our study should be noted. First, as a retrospective study design, we cannot exclude the possibility of residual confounding by unmeasured variables such as inflammatory parameters (C-reactive protein), dietary patterns, other medication influencing serum sodium concentrations or the amount of excess fluid in patients prior to transition to ESKD. Second, causes of mortality and hospitalization were determined by physicians, collected in report forms and were missing in several patients. We believe these to be missing at random, which should potentially bias our results toward the null. In addition, the veteran population studied may limit external generalizability. Furthermore, our hospitalizations analysis may be prone to competing risk bias due to death, although our results are in line with other studies suggesting that there is an association between hyponatremia and increased risk of hospitalizations. We were also unable to assess individual reasons for hospitalization, especially incidence of falls and fractures, among our data. Given the known association between hyponatremia and these outcomes, further studies are necessary to see if these associations remain for patients after dialysis initiation and attempted correction of hyponatremia with dialysis.
In conclusion, we observed that hyponatremia, even mild degrees of hyponatremia (serum sodium between 130 and <135 mEq/L), in the 3-month pre-ESKD initiation period is associated with higher mortality and hospitalizations after dialysis transition despite correction of sodium levels after dialysis transition. Future studies targeting hyponatremia management in late stage CKD are needed and should help determine whether such management can improve health outcomes post-ESKD transition.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The work in this manuscript has been performed with the support of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) research grant U01-DK102163 to C.P.K. and K.K.-Z., and by resources from the US Department of Veterans Affairs. Support for Veterans Health Administration/CMS data are provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, Veterans Affairs Information Resource Center (Project Numbers SDR 02-237 and 98-004). K.K.-Z. has been supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) midcareer award K24-DK091419 as well as philanthropic grants from Mr Harold Simmons, Mr Louis Chang, Dr Joseph Lee and AVEO. C.M.R. has been supported by the NIH/NIDDK early career award K23-DK102903. E.S. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01). J.S., C.P.K., K.K.-Z. and E.S. are employees of the VA. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, AstraZeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor and ZS-Pharma.
DATA AVAILABILITY STATEMENT
Restrictions apply to the availability of data generated or analyzed during this study. The United States Department of Veterans Affairs (VA) places legal restrictions on access to veteran’s health care data, which includes both identifying data and sensitive patient information. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Contributor Information
Maria V Marroquin, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
John Sy, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Carola-Ellen Kleine, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA.
Justin Oveyssi, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA.
Jui-Ting Hsiung, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Christina Park, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Melissa Soohoo, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Csaba P Kovesdy, Division of Nephrology, University of Tennessee Health Science Center, Memphis, TN, USA; Nephrology Section, Memphis VA Medical Center, Memphis, TN, USA.
Connie M Rhee, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA.
Elani Streja, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Kamyar Kalantar-Zadeh, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA; Tibor Rubin VA Medical Center, Long Beach, CA, USA; Fielding School of Public Health at UCLA, Los Angeles, CA, USA.
Ekamol Tantisattamo, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine, School of Medicine, Orange, CA, USA.
REFERENCES
- 1. Lien YH. Are we ignoring dysnatremia? Am J Med 2012; 125: 1045–1046 [DOI] [PubMed] [Google Scholar]
- 2. Liamis G, Rodenburg EM, Hofman A. et al. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med 2013; 126: 256–263 [DOI] [PubMed] [Google Scholar]
- 3. Golestaneh L, Neugarten J, Southern W. et al. Improving the diagnostic workup of hyponatremia in the setting of kidney disease: a continuing medical education (CME) initiative. Int Urol Nephrol 2017; 49: 491–497 [DOI] [PubMed] [Google Scholar]
- 4. Kovesdy CP, Lott EH, Lu JL. et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012; 125: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoorn EJ, Zietse R.. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis 2013; 62: 139–149 [DOI] [PubMed] [Google Scholar]
- 6. Huang H, Jolly SE, Airy M. et al. Associations of dysnatremias with mortality in chronic kidney disease. Nephrol Dial Transplant 2017; 32: 1204–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayslett JP, Kashgarian M, Epstein FH.. Functional correlates of compensatory renal hypertrophy. J Clin Invest 1968; 47: 774–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bricker NS, Fine LG, Kaplan M. et al. “Magnification phenomenon” in chronic renal disease. N Engl J Med 1978; 299: 1287–1293 [DOI] [PubMed] [Google Scholar]
- 9. Wallia R, Greenberg A, Piraino B. et al. Serum electrolyte patterns in end-stage renal disease. Am J Kidney Dis 1986; 8: 98–104 [DOI] [PubMed] [Google Scholar]
- 10. Han SW, Tilea A, Gillespie BW. et al. Serum sodium levels and patient outcomes in an ambulatory clinic-based chronic kidney disease cohort. Am J Nephrol 2015; 41: 200–209 [DOI] [PubMed] [Google Scholar]
- 11. Mendes RS, Soares M, Valente C. et al. Predialysis hypernatremia is a prognostic marker in acute kidney injury in need of renal replacement therapy. J Crit Care 2015; 30: 982–987 [DOI] [PubMed] [Google Scholar]
- 12. Rhee CM, Ravel VA, Ayus JC. et al. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol Dial Transplant 2016; 31: 992–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravel VA, Streja E, Mehrotra R. et al. Serum sodium and mortality in a national peritoneal dialysis cohort. Nephrol Dial Transplant 2017; 32: 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sajadieh A, Binici Z, Mouridsen MR. et al. Mild hyponatremia carries a poor prognosis in community subjects. Am J Med 2009; 122: 679–686 [DOI] [PubMed] [Google Scholar]
- 15. Mandai S, Kuwahara M, Kasagi Y. et al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol 2013; 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalantar-Zadeh K, Kovesdy CP, Streja E. et al. Transition of care from pre-dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrol Dial Transplant 2017; 32: ii91–ii98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molnar MZ, Gosmanova EO, Sumida K. et al. Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis 2016; 68: 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gyamlani G, Molnar MZ, Lu JL. et al. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant 2017; 32: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sumida K, Molnar MZ, Potukuchi PK. et al. Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 2016; 91: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Renal Data System. USRDS 2017 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States (Chapter 8: Transition of Care in Chronic Kidney Disease). Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017
- 21. Obi Y, Park C, Soohoo M. et al. Association of pre-ESRD serum calcium with post-ESRD mortality among incident ESRD patients: A cohort study. J Bone Miner Res 2018; 33: 1027–1036 [DOI] [PubMed] [Google Scholar]
- 22. Baran D, Hutchinson TA.. The outcome of hyponatremia in a general hospital population. Clin Nephrol 1984; 22: 72–76 [PubMed] [Google Scholar]
- 23. Sterns RH. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med 1987; 107: 656–664 [DOI] [PubMed] [Google Scholar]
- 24. Ellis SJ. Severe hyponatraemia: complications and treatment. QJM 1995; 88: 905–909 [PubMed] [Google Scholar]
- 25. Clayton JA, Le Jeune IR, Hall IP.. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. QJM 2006; 99: 505–511 [DOI] [PubMed] [Google Scholar]
- 26. Gill G, Huda B, Boyd A. et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol 2006; 65: 246–249 [DOI] [PubMed] [Google Scholar]
- 27. Chawla A, Sterns RH, Nigwekar SU. et al. Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol 2011; 6: 960–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arieff AI, Guisado R.. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int 1976; 10: 104–116 [DOI] [PubMed] [Google Scholar]
- 29. Anderson RJ, Chung HM, Kluge R. et al. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med 1985; 102: 164–168 [DOI] [PubMed] [Google Scholar]
- 30. Tierney WM, Martin DK, Greenlee MC. et al. The prognosis of hyponatremia at hospital admission. J Gen Intern Med 1986; 1: 380–385 [DOI] [PubMed] [Google Scholar]
- 31. Terzian C, Frye EB, Piotrowski ZH.. Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med 1994; 9: 89–91 [DOI] [PubMed] [Google Scholar]
- 32. Nzerue CM, Baffoe-Bonnie H, You W. et al. Predictors of outcome in hospitalized patients with severe hyponatremia. J Natl Med Assoc 2003; 95: 335–343 [PMC free article] [PubMed] [Google Scholar]
- 33. Hoorn EJ, Lindemans J, Zietse R.. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant 2006; 21: 70–76 [DOI] [PubMed] [Google Scholar]
- 34. Zilberberg MD, Exuzides A, Spalding J. et al. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin 2008; 24: 1601–1608 [DOI] [PubMed] [Google Scholar]
- 35. Waikar SS, Mount DB, Curhan GC.. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009; 122: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wald R, Jaber BL, Price LL. et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010; 170: 294–302 [DOI] [PubMed] [Google Scholar]
- 37. Edmonds ZV. Hyponatremia in pneumonia. J Hosp Med 2012; 7 (Suppl 4): S11–S13 [DOI] [PubMed] [Google Scholar]
- 38. Tolouian R, Alhamad T, Farazmand M. et al. The correlation of hip fracture and hyponatremia in the elderly. J Nephrol 2012; 25: 789–793 [DOI] [PubMed] [Google Scholar]
- 39. Aicale R, Tarantino D, Maffulli N.. Prevalence of hyponatremia in elderly patients with hip fractures: A two-year study. Med Princ Pract 2017; 26: 451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study. The United States Department of Veterans Affairs (VA) places legal restrictions on access to veteran’s health care data, which includes both identifying data and sensitive patient information. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.