Abstract
Background
Disease recurrence and progression remain major challenges for the treatment of non‐muscle invasive bladder cancer. Narrow band imaging (NBI) is an optical enhancement technique that may improve resection of non‐muscle invasive bladder cancer and thereby lead to better outcomes for people undergoing the procedure.
Objectives
To assess the effects of NBI‐ and white light cystoscopy (WLC)‐guided transurethral resection of bladder tumor (TURBT) compared to WLC‐guided TURBT in the treatment of non‐muscle invasive bladder cancer.
Search methods
We performed a comprehensive literature search of 10 databases, including the Cochrane Library, the Cochrane Database of Systematic Reviews, MEDLINE, Embase, several clinical trial registries, and grey literature for published and unpublished studies, irrespective of language. The search was performed per an a priori protocol on 3 December 2021.
Selection criteria
We included randomized controlled trials of participants with suspected or confirmed non‐muscle invasive bladder cancer. Participants in the control group must have received WLC‐guided TURBT alone (hereinafter simply referred to as 'WLC TURBT'). Participants in the intervention group had to have received NBI‐ and WLC‐guided TURBT (hereinafter simply referred to as 'NBI + WLC TURBT').
Data collection and analysis
Two review authors independently selected studies for inclusion/exclusion, performed data extraction, and assessed risk of bias. We conducted meta‐analysis on time‐to‐event and dichotomous data using a random‐effects model in RevMan, according to Cochrane methods. We rated the certainty of evidence for each outcome according to the GRADE approach.
Primary outcomes were time to recurrence, time to progression, and the occurrence of a major adverse event, defined as a Clavien‐Dindo III, IV, or V complication. Secondary outcomes included time to death from bladder cancer and the occurrence of a minor adverse event, defined as a Clavien‐Dindo I or II complication.
Main results
We included eight studies with a total of 2152 participants randomized to the standard WLC TURBT or to NBI + WLC TURBT. A total of 1847 participants were included for analysis.
Based on limited confidence in the time‐to‐event data, we found that participants who underwent NBI + WLC TURBT had a lower risk of disease recurrence over time compared to participants who underwent WLC TURBT (hazard ratio 0.63, 95% CI 0.45 to 0.89; I2 = 53%; 6 studies, 1244 participants; low certainty of evidence). No studies examined disease progression as a time‐to‐event outcome or a dichotomous outcome. There was likely no difference in the risk of a major adverse event between participants who underwent NBI + WLC TURBT and those who underwent WLC TURBT (risk ratio 1.77, 95% CI 0.79 to 3.96; 4 studies, 1385 participants; low certainty of evidence).
No studies examined death from bladder cancer as a time‐to‐event outcome or a dichotomous outcome. There was likely no difference in the risk of a minor adverse event between participants who underwent NBI + WLC TURBT and those who underwent WLC TURBT (risk ratio 0.88, 95% CI 0.49 to 1.56; I2 = 61%; 4 studies, 1385 participants; low certainty of evidence).
Authors' conclusions
Compared to WLC TURBT alone, NBI + WLC TURBT may lower the risk of disease recurrence over time while having little or no effect on the risks of major or minor adverse events.
Plain language summary
Comparing narrow band imaging to regular cystoscopy for treatment of bladder cancer
Review question
How does a resection of bladder tissues guided by a special visualization method called narrow band imaging compare to a resection of bladder tissues guided by the standard visualization method (using white light) in people with tumors in their inner bladder wall?
Background
When people are suspected to have bladder cancer or have been diagnosed with bladder cancer, their doctors need to inspect the bladder closely and remove tissues for further examination. The removal of tumorous tissues also serves as treatment. The procedure to remove tumors in the bladder is called transurethral resection of bladder tumor, or TURBT. TURBT is done by passing a special instrument through the urethra and into the bladder. Sometimes, it is difficult to distinguish healthy normal bladder tissue from tumorous tissue. Some doctors use a special visualization method known as narrow band imaging to help visualize the tumorous tissues.
Study characteristics
We analyzed data from published studies called randomized controlled trials to understand if narrow band imaging reduces the risk of bladder cancer getting worse, and to see if there are any side effects. We only included randomized controlled trials because this study type is the most reliable.
Key results
We identified eight randomized controlled trials that addressed our review question. Participants included in these studies were suspected to have bladder cancer or were diagnosed with bladder cancer that was limited to the inner wall, meaning that the cancer did not invade the underlying muscle layers. Based on limited available data, the use of narrow band imaging may lower the risk of disease recurrence over time.
None of the randomized controlled trials examined whether the choice of visualization method made any difference to the risk of bladder cancer becoming worse or the risk of the person dying from bladder cancer, so we do not know if the use of narrow band imaging is effective in improving these two outcomes.
We found that the use of narrow band imaging may have little or no increased risk of complications, compared to the standard visualization method.
Quality of the evidence
Due to certain flaws in the design of these clinical trials and some contradictory findings between trials, our confidence in these findings was low. With more research in the future, more reliable data may likely change these findings. The evidence is up to date to December 2021.
Summary of findings
Summary of findings 1. NBI + WLC TURBT compared to WLC TURBT for transurethral resection of bladder tumors in people with suspected or diagnosed non‐muscle invasive bladder cancer.
| NBI + WLC TURBT compared to WLC TURBT for transurethral resection of bladder tumors in people with non‐muscle invasive bladder cancer | ||||||
| Patient or population: transurethral resection of bladder tumors in people with suspected or diagnosed non‐muscle invasive bladder cancer Setting: outpatient or inpatient setting Intervention: NBI + WLC TURBT Comparison: WLC TURBT | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens | |
| Risk with WLC TURBT | Risk difference with NBI + WLC TURBT | |||||
|
Time to disease recurrence Follow‐up: range 12 months to 35 months Assumed MCID: 5% (absolute effect size estimate based on events at 12 months) |
1244 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | HR 0.63 (0.45 to 0.89) | Lowc | In the low risk population, NBI + WLC TURBT may lower the risk of disease recurrence over time. We have limited confidence about this finding. | |
| 150 per 1000 | 53 fewer per 1000 (79 fewer to 15 fewer) | |||||
| ⊕⊕⊝⊝ LOWa,b | Highc | In the high risk population, NBI + WLC TURBT may lower the risk of disease recurrence over time. We have limited confidence about this finding. | ||||
| 610 per 1000 | 163 fewer per 1000 (265 fewer to 43 fewer) | |||||
|
Time to disease progression Follow‐up: not applicable Assumed MCID: 2% |
‐ | ‐ | ‐ | ‐ | ‐ | We found no data on time to disease progression. |
|
Major adverse event (Clavien‐Dindo grade III, IV, and V) Follow‐up: range 3 months to 24 months Assumed MCID: 2% |
1385 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | RR 1.77 (0.79 to 3.96) | Study population | NBI + WLC TURBT may have little to no effect on major adverse events. We have limited confidence about this finding. | |
| 13 per 1000 | 10 more per 1000 (3 fewer to 39 more) | |||||
|
Time to death from bladder cancer Follow‐up: not applicable Assumed MCID: 2% |
‐ | ‐ | ‐ | ‐ | ‐ | We found no data on time to death from bladder cancer. |
|
Minor adverse event (Clavien‐Dindo grade I and II) Follow‐up: range 3 months to 24 months Assumed MCID: 5% |
1385 (4 RCTs) | ⊕⊕⊝⊝ LOW a,b | RR 0.88 (0.49 to 1.56) | Study population | NBI + WLC TURBT may have little to no effect on the risk of a minor adverse event. We have limited confidence about this finding. | |
| 113 per 1000 | 14 fewer per 1000 (58 fewer to 63 more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based
on the assumed risk in the comparison group and the relative effect of the
intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MCID: minimal clinically important difference; NBI: narrow band imaging; RCT: randomized controlled trial; RR: risk ratio; TURBT: transurethral resection of bladder tumor; WLC: white light cystoscopy | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe rated down by one level for study limitations due to unclear or high risk of bias. bWe rated down by one level for imprecision given few events, wide confidence intervals, or both. cThe recurrence rate after TURBT for participants with non‐muscle invasive bladder cancer is highly variable in the literature. Among the pooled cohort of 2596 participants from seven European Organization for Research and Treatment of Cancer clinical trials, the probabilities of recurrence at one year after TURBT ranged from 15% to 61% (Sylvester 2006). Therefore, we considered 15% as low risk and 61% as high risk.
Background
Description of the condition
Bladder cancer is the tenth most common malignancy and the second most common urologic malignancy worldwide, with an estimated 550,000 new cases and 200,000 deaths per year (Bray 2018). Specifically, urothelial carcinoma accounts for 90% of all bladder cancers in the USA and Europe. Smoking contributes to 50% to 65% of all bladder cancer cases, increasing the risk of disease by up to four‐fold (Freedman 2011). Additional risk factors include environmental and occupational exposure to chemical carcinogens such as aromatic amines, and treatment of leukemia and lymphoma with cyclophosphamide (Chang 2016).
People with bladder cancer most often present with blood in their urine, but tumors can also be found during the evaluation for other symptoms, such as irritative voiding symptoms. The prevalence of bladder cancer ranges between 13% and 35% in people presenting with macroscopic hematuria and between 5% and 10% in those with microscopic hematuria (Sun 2015). People with bladder tumors then proceed to undergo transurethral resection of bladder tumor (TURBT), where tumorous tissues are removed for staging and treatment.
Pathologists classify tumors based on cell histology and the depth of invasion into the layers of the bladder wall (in order of depth: mucosa, lamina propria, and muscle layers). The majority of bladder cancers present as superficial tumors that do not invade the underlying bladder muscle at diagnosis. These superficial tumors are referred to as non‐muscle invasive bladder cancer, and have a 60% to 70% risk of recurrence (Aldousari 2010). Histologic characteristics can also predict the risk of progression. In general, papillary tumors on the mucosa of the bladder (Ta) are indolent, whereas the subset of superficial tumors called carcinoma in situ (CIS or Tis), and tumors that invade to the lamina propria layer (T1), are considered more aggressive and of concern for progression to muscle invasive disease (Humphrey 2016).
Once non‐muscle invasive bladder cancer invades the muscle layers, the disease is referred to as muscle invasive bladder cancer. Muscle invasive bladder cancer is associated with high rates of morbidity and mortality, and its treatment approach is highly invasive (Sun 2015). As bladder cancer progresses, the survival rate drops significantly. Within the USA, the five‐year survival rate for people with carcinoma in situ is 95.8%; for those with localized disease confined to primary site, it is 69.2%; for those with regional disease with spread of disease to regional lymph nodes, it is 36.5%; and for those with metastatic disease, it is 5.5% (SEER 2020).
Description of the intervention
Traditionally, TURBT is performed using white light cystoscopy (WLC). During this procedure, experienced urologists visually distinguish conspicuous lesions from normal mucosa. However, complete identification and removal of tumors during TURBT can be challenging, as the sensitivity of WLC ranges from 62% to 84% for identifying bladder tumors, and the specificity ranges from 43% to 98% (Sun 2015). The sensitivity of WLC is particularly low for small or flat lesions that are visually subtle or difficult to differentiate from areas of inflammation. Consequently, some recurrences can be attributable to, if not entirely consisting of, lesions left behind due to incomplete resection (Sylvester 2006). On second‐look TURBT using WLC, the residual tumor rate was reported to be as high as 43% to 62% (Goh 2009). Given these limitations, additional technologies are needed to augment the visual detection of tumors in the lower urinary tract. One such technology is narrow band imaging (NBI).
The NBI setting on the cystoscope changes the optical filters used to visualize the bladder, resulting in an image that may potentially improve visualization of tumors. At the time of the TURBT, urologists can toggle between white light and NBI using camera setting buttons, to help identify tumors.
How the intervention might work
The working principle of NBI relies on two phenomena. First, hemoglobin characteristically absorbs blue light at 415 nm and green light at 540 nm (Srivastava 2019). NBI uses optical filters to narrow the bandwidth of white light, such that only blue and green light pass through. NBI light is then preferentially absorbed by vessels, and reflected by mucosa. Second, the depth of light penetration into tissues depends on wavelength; the shorter the wavelength, the more superficial the penetration. The shorter NBI light wavelength only penetrates the superficial layers of the mucosa. Together, the differential absorption and penetration enhance the visibility of surface capillaries and blood vessels in the submucosa.
Under NBI mode, tumors with richer vasculature appear dark green or black in a background of normal urothelium, which appears mostly white (Naselli 2009). In contrast, lesions appear red under white light mode against the normal urothelium, which appears pink. Because tumors have more blood vessels than normal mucosa, NBI can potentially improve visualization of lesions that are difficult to see, and delineation of tumor margins, which together can enable more thorough tumor excision.
The impact of NBI on time‐to‐disease recurrence outcomes may not only be in the primary detection and resection of tumors, but also due to the fact that increased detection and resection of tumors may impact the individual's risk stratification and lead to more aggressive observation and intervention schedules (Chang 2016). Therefore, the benefit (or harm) of NBI + WLC TURBT may be compounded due to the impact of the intervention on subsequent patient care.
In contrast to blue light cystoscopy, another form of optical enhancement technology (Maisch 2021), NBI does not require any chemical to function. Moreover, systems integrating WLC and NBI are readily available (Naselli 2009). The NBI mode on a cystoscope can be activated with a control button, without adding significant risks or interruptions to the flow of the procedure.
Adverse effects of the intervention
NBI highlights areas of increased blood vessels, which is a surrogate for a potential tumor. The technology does not specifically identify tumors. The increased sensitivity compounded by decreased specificity may increase false positive rates. This can lead to more extensive resection and over‐treatment, increasing the risk of bleeding or complications, such as bladder perforation. Also, NBI cannot be used when there is active bleeding, since blood can absorb NBI light and obstruct visibility.
Why it is important to do this review
The impact of NBI during TURBT remains unclear. The detection and complete resection of non‐muscle invasive bladder cancer lesions are central to the treatment of the disease. Optical advances, such as NBI, offer the potential to improve clinicians’ ability to detect tumors. However, the effect of NBI on decreasing recurrence and progression is not well assessed.
Currently, the American Urological Association/Society of Urologic Oncology guideline suggests a conditional recommendation for the use of NBI + WLC TURBT (Chang 2016). While the European Association of Urology acknowledges that NBI may improve cancer detection, its guideline states that evidence for potential reductions in recurrence rate is limited (Babjuk 2019). Meanwhile, the National Institute for Health and Care Excellence guideline suggests that NBI may be offered to people with suspected bladder cancer in conjunction with WLC TURBT (NICE 2015). In this context, we conducted a stringent examination of current evidence to help inform clinicians and guideline developers on the use of NBI at time of TURBT.
Objectives
To assess the effects of NBI‐ and white light cystoscopy (WLC)‐guided transurethral resection of bladder tumor (TURBT) compared to WLC‐guided TURBT in the treatment of non‐muscle invasive bladder cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomized controlled trials, in which individual participants were randomized.
We excluded quasi‐experimental studies and cluster‐randomized trials due to their lack of random assignment at the individual level (Puffer 2005). We excluded cross‐over trials from our review, as both the comparator and the experimental interventions involve removal of bladder tumors. Inclusion of cross‐over trials would present a serious carry‐over effect (Higgins 2011).
Types of participants
We defined the eligible population as adults, aged 18 and over, with a suspected or established diagnosis of primary or recurrent urothelial carcinoma of the bladder, based on one of the following.
Bladder mass or abnormal bladder mucosa findings per clinic‐based WLC
Bladder mass on cross‐sectional imaging, such as bladder filling defects and hydronephrosis
Positive or atypical urinary cytology
Positive fluorescence in situ hybridization test
We excluded participants undergoing NBI‐guided surveillance, as we were only considering the use of NBI in the treatment setting. We also excluded participants with distant metastatic disease.
If studies included multiple participant groups or interventions, we only included the subset of participants of interest. If multiple articles were published by the same group with the same participant cohort, we merged and analyzed relevant data from each article as one study.
Types of interventions
Concomitant interventions had to be the same in the experimental and control groups to establish fair comparisons. Trials that described more than one use of NBI + WLC (for example: during subsequent cystoscopy or TURBT) were included, and this information was reported separately if available.
Experimental intervention
WLC‐ and NBI‐ guided TURBT (herein referred to as NBI + WLC TURBT)
Comparator intervention
WLC‐guided TURBT (herein referred to as WLC TURBT)
Comparison
NBI + WLC TURBT versus WLC TURBT in the treatment of non‐muscle invasive bladder cancer.
Types of outcome measures
We included studies assessing the impact of NBI + WLC TURBT on the recurrence or progression of non‐muscle invasive bladder cancer and excluded studies that reported data on NBI + WLC TURBT solely for the detection of bladder cancer.
Primary outcomes
Time to disease recurrence (time‐to‐event outcome)
Time to disease progression (time‐to‐event outcome)
Major adverse event: Clavien‐Dindo grade III, IV, or V (dichotomous outcome)
Secondary outcomes
Time to death from bladder cancer (time‐to‐event outcome)
Minor adverse event: Clavien‐Dindo grade I or II (dichotomous outcome)
Method and timing of outcome measurement
Primary outcomes
Time to disease recurrence: measured from the time of random sequence generation to time of any recurrence of bladder cancer, based on TURBT, regardless of tumor stage or grade.
-
Time to disease progression: measured from the time of random sequence generation to time of progression of bladder cancer as documented by histopathology. Consistent with the International Bladder Cancer Group (Lamm 2014), we defined progression as follows.
Increase in T stage from CIS or Ta to T1
Development of T2 or greater or lymph node disease or distant metastasis
Increase in grade from low to high, including CIS
Major adverse event: Clavien‐Dindo grade III, IV, or V (Clavien 2009), within 90 days of TURBT
Secondary outcomes
Time to death from bladder cancer: measured from the time of random sequence generation to the time of death from bladder cancer.
Minor adverse event: Clavien‐Dindo grade I or II (Clavien 2009), within 90 days of TURBT
When data on the time to disease recurrence, time to disease progression, or time to death from bladder cancer were incomplete and could not be analyzed as time‐to‐event outcomes, we had planned to analyze these outcomes as dichotomous outcomes (up to 12 months, or 13 to 24 months after randomization) in efforts to increase the comprehensiveness of our analysis. This was not necessary.
Thresholds for clinical relevance of outcomes
Primary outcomes
Time to disease recurrence: considered clinically relevant if the observed absolute difference is 5% or more at 12‐month follow‐up
Time to disease progression: considered clinically relevant if the observed absolute difference is 2% or more at 12‐month follow‐up
Major adverse event: considered clinically relevant if the observed absolute difference is 2% or more at initial TURBT or re‐resection
Secondary outcomes
Time to death from bladder cancer: considered clinically relevant if the observed absolute difference is 2% or more at 12‐month follow‐up
Minor adverse event: considered clinically relevant if the observed absolute difference is 5% or more at initial TURBT or re‐resection
We established these thresholds based on the expert opinions of the review authors, taking into consideration the relative importance of the given outcome, and the expected control event rate.
Search methods for identification of studies
We conducted a comprehensive search, inclusive of all languages and publication statuses. We reran the search within three months prior to anticipated publication of the review.
Electronic searches
We searched for relevant studies in the following ten databases from their respective dates of inception to 14 September 2020 and 3 December 2021, using the search strategies outlined in the Appendices.
Cochrane Library (via Wiley); Appendix 1
International Pharmaceutical Abstracts (via Ovid); Appendix 2
MEDLINE (via Ovid); Appendix 3
Embase (via embase.com); Appendix 4
Web of Science Core Collection (via Clarivate); Appendix 5
Scopus (via Scous.com); Appendix 6
Latin American and Caribbean Health Science Information database (LILACS; lilacs.bvsalud.org/en/)
Open Grey (www.opengrey.eu/); Appendix 7
ClinicalTrials.gov (www.clinicaltrials.gov/); Appendix 8
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialssearch/); Appendix 9
We incorporated additional relevant key words found during these searches into our search strategies, and documented changes accordingly.
Searching other resources
We attempted to identify other potentially eligible studies by searching the reference lists of included publications.
Data collection and analysis
Selection of studies
After removing duplicate records, two review authors (LL, ST) independently scanned the titles and abstracts of studies identified by the electronic search for eligibility. The two review authors (LL, ST) screened the full‐text reports for all potentially eligible studies, according to predefined criteria. They resolved any discrepancies through consensus or arbitration of a third review author (GL). For studies identified in trial registries, we contacted the authors or institutions recorded in the registry for trial reports. We translated papers that were published in languages other than English to assess eligibility. We presented a PRISMA flowchart, showing study selection, including reasons for exclusion of studies (Liberati 2009).
Data extraction and management
Two review authors (LL, ST or GL) conducted data extraction using an extraction form developed for this review, based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Li 2020). We resolved any disagreements by consensus, or by arbitration of a third review author (GL or LH) if needed.
We extracted the following data.
Study information: author, title, source, publication date, publication type, language, duplicate publications, source of funding, authors’ conflict of interest
Study characteristics: study design, randomization method, number of study center(s), country of study center(s), inclusion and exclusion criteria, subgroup analysis, statistical methods, period of enrollment, follow‐up period
Participants characteristics: number of participants, number of participants per study arm, age, gender, ethnicity, clinical stage of disease (presentation, focality, tumor size)
Intervention and comparator information: name, frequency, duration of treatment, adjuvant therapy, re‐intervention, follow‐up
Outcomes: according to the review's predefined primary and secondary outcomes (including tumor stage and grade), events of intervention and comparator groups, timing of outcome measurement, number of re‐resections
We extracted the relevant outcome data needed to calculate summary statistics and measures of variance. For dichotomous outcomes, we obtained numbers of events and totals to populate a 2 x 2 table, and calculated summary statistics with corresponding measures of variance. For continuous outcomes, we obtained means and standard deviations or data necessary to calculate this information. For time‐to‐event outcomes, we extracted hazard ratios with corresponding measures of variance or data necessary to calculate this information.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports associated with one primary study, we maximized the yield of information by mapping all publications to a unique study ID. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (LL, ST) independently assessed the risk of bias of each included study, using the Cochrane risk of bias assessment tool (Higgins 2011). They resolved disagreements by consensus, or through arbitration by a third review author (GL). We determined if risk of bias was low, high, or unclear, and presented our findings in a risk of bias summary figure. We assessed the trials for the following biases.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias.
We evaluated the risk of performance bias and detection bias separately for each outcome. We grouped outcomes according to whether they were measured subjectively or objectively in the risk of bias tables.
Performance bias ‐ susceptible
We considered that all outcomes were similarly susceptible to performance bias.
Detection bias ‐ susceptible
Time to disease recurrence (time‐to‐event outcome)
Time to disease progression (time‐to‐event outcome)
Time to death from bladder cancer (time‐to‐event outcome)
Minor adverse event: (Clavien‐Dindo grade I or II; dichotomous outcome)
Detection bias ‐ not susceptible
Major adverse event: (Clavien‐Dindo grade III, IV, or V; dichotomous outcome)
We assessed Incomplete outcome data, or attrition bias, on an outcome‐specific basis. We considered the rate of attrition as low (less than 10%), unclear (between 10% and 20%), and high (20% or more).
We assessed selective reporting bias on a study‐specific basis. We only considered the risk of selective reporting bias as low if we could identify an a priori protocol, and if the analyses and outcomes matched what the investigators preplanned.
We summarized the risk of bias within and across outcomes and studies in graphs. We used study‐specific risk of bias assessments to inform the preplanned sensitivity analyses.
Measures of treatment effect
We expressed outcomes with dichotomous data using risk ratios (RR), with 95% confidence intervals (CIs). We expressed outcomes with time‐to‐event data using hazard ratios (HR) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. For studies with repeated outcome measures, we performed time‐to‐event analysis. If this was not possible, we defined outcome measures as short term (< 12 months) versus long term (13 to 24 months) (Higgins 2020).
Dealing with missing data
We contacted study investigators for missing data. We only analyzed available data; we did not impute missing data. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward, if used by the study authors). We addressed the impact of missing data on the findings of the review in the Discussion section (Deeks 2020).
We conducted intention‐to‐treat analysis whenever possible. If intention‐to‐treat analysis was not possible, we conducted as‐treated and per‐protocol population analyses. We considered this a potential source of bias.
Assessment of heterogeneity
In the event of substantial clinical, methodological, or statistical heterogeneity, unexplained by subgroup analyses, we planned to exclude such results from the pooled effect estimate in the meta‐analysis and provide a narrative description of the results of each study instead.
We identified heterogeneity by assessing overlaps in CIs in forest plots. We also assessed the impact of heterogeneity on the meta‐analysis using the I² statistic (Higgins 2002; Higgins 2003). We interpreted the I² statistic as follows (Deeks 2020).
0% to 40%: may not be important
30% to 60%: may indicate moderate heterogeneity
50% to 90%: may indicate substantial heterogeneity
75% to 100%: considerable heterogeneity
The importance of the observed value of the I² statistic depended on the magnitude and direction of effects, and the strength of evidence for heterogeneity. When we identified heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting. Since there were not at least 10 studies available for meta‐analysis, we could not assess for publication bias by creating and visually inspecting funnel plots.
Data synthesis
We conducted a meta‐analysis with a random‐effects model for pooling data (Wood 2008). We conducted statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Li 2020). We analyzed dichotomous outcomes with the Mantel‐Haenszel method and continuous outcomes with the inverse variance method. We analyzed time‐to‐event outcomes using the generic inverse variance method. We used Review Manager 5 software to conduct all analyses (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
Certain tumor characteristics may impact outcomes. There was not sufficient data to conduct any of our preplanned subgroup analyses, listed as follows.
Setting: primary versus recurrent bladder cancer
Multifocality: solitary versus multiple lesions of bladder cancer
Tumor size: 3 cm or less versus larger than 3 cm
Stage: positive cytology, or history of carcinoma in situ (CIS; in the case of recurrent disease), or both, versus negative cytology, or the absence of history of CIS, or both
The rationale underlying these subgroup analyses was as follows.
Setting: per the European Organization for Research and Treatment of Cancer (EORTC) criteria, the setting of primary versus recurrent bladder cancer (primary with or without a recurrence, versus > 1 recurrence) can affect the risk of recurrence and progression (Sylvester 2006).
Multifocality: per the EORTC criteria, the number of tumors (1, 2 to 7, versus ≥ 8) can affect the risk of recurrence and progression (Sylvester 2006).
Tumor size: per the EORTC criteria, the size of tumors (< 3 cm versus ≥ 3 cm) can affect the risk of recurrence and progression (Sylvester 2006).
Stage: compared to other histological types, the detection of CIS is particularly difficult due to its flat growth within the cell level (Sylvester 2006).
Sensitivity analysis
There was an insufficient number of studies to conduct sensitivity analyses to evaluate differences in methodology that could impact the results of meta‐analyses. Therefore, we could not conduct preplanned sensitivity analyses by 1) excluding studies with high risk of bias (Deeks 2020), and 2) by excluding studies in which all participants underwent re‐resection on a routine basis, since routine re‐resection may mitigate potential benefits of NBI.
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity (directness of results) (Guyatt 2008). Two review authors (LL or GL) independently reviewed the quality of evidence for each outcome as high, moderate, low, or very low using GRADEpro GDT (GRADEpro GDT). We resolved any discrepancies by consensus, or if needed, by arbitration with a third review author (PD). We presented a summary of the evidence in a summary of findings table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2019). If meta‐analysis was not possible, we planned to present data in a narrative summary of findings table.
We presented a summary of findings table reporting the following outcomes, listed according to a priority rating established by the clinicians on our team with the input of external experts.
Time to disease recurrence
Time to disease progression
Major adverse event
Time to death from bladder cancer
Minor adverse event
Results
Description of studies
Results of the search
We identified 222 records during the first search in September 2020. We identified an additional 15 records during the repeated search in December 2021. After the exclusion of duplicates, we screened the title and abstract of 208 records. Of these, we deemed 160 to be irrelevant. We assessed 48 records (associated with 23 unique studies) for eligibility. Ultimately, eight randomized controlled trials, associated with 33 reports, met our inclusion criteria for the study question.
There were no ongoing studies nor studies awaiting classification. The details of study selection are presented as a PRISMA flow diagram in Figure 1.
1.
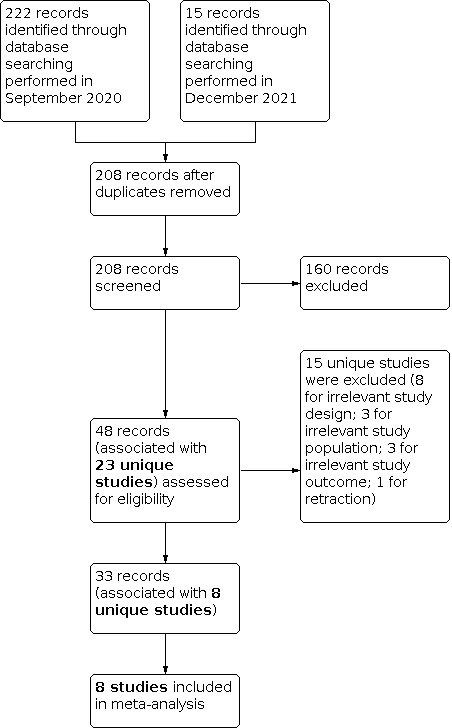
PRISMA flow diagram summarizing the study screening process
Included studies
We identified eight randomized controlled trials eligible for inclusion (Buaban 2018; Kim 2018; Lee 2014; Longo 2013; Ma 2015; Naito 2016; Naselli 2012; Stănescu 2014). For descriptions of the included studies, see Table 2 'Overview of included studies' and Characteristics of included studies.
1. Overview of included studies.
|
Reference identification |
Publication type | Trial period | Country |
Description of participants (number randomized) |
Comparator vs intervention | Outcome assessed (time point) | Mean age | Male, n (%) |
| Buaban 2018 | Full text | 2015 to 2016 | Thailand | Participants with histopathology‐visualized non‐muscle invasive bladder
cancer and complete resection of the visualized tumor (n = 158) |
WLC TURBT | Disease recurrence (3 months) Surgical complication |
Unclear. Characteristics were reported on a per‐procedure basis, and some participants had undergone more than one procedure. | Unclear. Characteristics were reported on a per‐procedure basis, and some participants had undergone more than one procedure. |
| NBI + WLC TURBT | See above | |||||||
| Kim 2018 | Full text | 2013 to 2017 | Korea | Participants who underwent TURBT for suspicion of a bladder tumor
discovered by use of cystoscopy or another imaging study (n = 198) |
WLC TURBT | Number of tumors identified Disease recurrence (12 months) |
66.96 ± 11.51 | 54 (81) |
| NBI + WLC TURBT | 64.54 ± 12.01 | 62 (73) | ||||||
| Lee 2014 | Abstract | 2010 to 2013 | Korea | Participants with overt or suspected non‐muscle invasive bladder cancer (n = 68 ) |
WLC TURBT | Disease recurrence (24 months) | 63.03 ± 12.43 | Not reported |
| NBI + WLC TURBT | 63.82 ± 12.31 | |||||||
| Longo 2013 | Abstract | Not reported | Italy | Not reported (n = 137) |
WLC TURBT | Surgical complication Mean time to catheter removal Absence of muscle tissue in specimen False positive rate |
Not reported |
|
| NBI + WLC TURBT | ||||||||
| Ma 2015 | Full text | 2013 to 2014 | China | Participants initially diagnosed with primary or recurrent non‐muscle
invasive bladder cancer (n = 178) |
WLC TURBT | Disease recurrence (3 months, 12 months) | 62 ± 8 | 79 (85) |
| NBI‐guided holmium laser resection of bladder tumor | 63 ± 9 | 69 (80) | ||||||
| Naito 2016 | Full text | 2010 to 2014 | 16 countries | Participants with primary non‐muscle invasive bladder cancer (n = 965) |
WLC TURBT | Surgical complication Disease recurrence (during re‐TURBT, 3 months, 12 months) |
65.8 ± 12.5 | 383 (80) |
| NBI + WLC TURBT | 66.7 ± 12.3 | 390 (81) | ||||||
| Naselli 2012 | Full text | 2009 to 2010 | Italy | Participants from two centers with overt or suspected bladder cancer (n = 188) |
WLC TURBT: white light cystoscopy guided TURBT exclusively | Detection rate Disease recurrence (12 months) |
71.6 ± 12.4 | 17 (24) |
| NBI TURBT: NBI guided TURBT exclusively | 70.8 ± 10.3 | 12 (16) | ||||||
| Stănescu 2014 | Full text | Not reported | Romania | Participants with non‐muscle invasive bladder cancer (n = 260) |
WLC TURBT | Tumor detection rate Surgical complication Disease recurrence (12 months, 24 months) |
64.8 (33 to 86) | Not reported |
| NBI‐guided bipolar plasma vaporization | 65.2 (32 to 87) | |||||||
NBI: narrow band imaging; TURBT: transurethral resection of bladder tumor; WLC: white light cystoscopy
Participants
In total, 2152 participants were randomized. Inclusion criteria varied widely between studies. All but the study by Longo 2013 specified their participant selection process, including participants with suspected or diagnosed non‐muscle invasive bladder cancer and excluding participants with muscle‐invasive bladder cancer. Methods used for the initial discovery of suspected bladder tumors varied from bladder lavage fluids and voided urine cytology to cystoscopies and other imaging studies. One study specified that only participants with primary bladder cancer were included (Naito 2016).
Interventions
Transurethral resection began in the white light mode with the introduction of the resectoscope. For the control arm, participants underwent cystoscopy, tumor resection, and coagulation in the white light mode only. For the intervention arm, the instruments were originally introduced into the bladder under the white light mode, then switched to the NBI mode for cystoscopy, tumor resection, and coagulation. One study specified that the intervention was carried out entirely in the white light or NBI mode, in which switching from white light to NBI mode during the procedure was prohibited (Naselli 2012). One trial used holmium laser for resection (Ma 2015), and another used NBI‐guided bipolar plasma vaporization (Stănescu 2014).
Outcomes
Outcomes were measured in ranges of 3 to 35 months. The studies reporting the primary outcomes for this review were as follows.
Time to disease recurrence, reported by six studies (Kim 2018; Lee 2014; Ma 2015; Naito 2016; Naselli 2012; Stănescu 2014)
-
Time to disease progression, reported by none
No studies reported the number of participants with disease progression at 12 months or 13 to 24 months, precluding the analysis of disease progression as a dichotomous outcome.
Major adverse events, reported by four studies (Buaban 2018; Longo 2013; Naito 2016; Stănescu 2014)
The studies reporting the secondary outcomes for this review were as follows.
-
Time to death from bladder cancer, reported by none.
No studies reported the number of participants with bladder cancer death at 12 months or 13 to 24 months, precluding the analysis of cancer death at 12 months as a dichotomous outcome.
Minor adverse events, reported by four studies (Buaban 2018; Longo 2013; Naito 2016; Stănescu 2014)
Excluded studies
We excluded 15 reports of 15 studies. Eight were excluded for irrelevant study design (Doehn 2014; Geavlete 2012; Herr 2019; Mukherjee 2019; Naya 2015; Shen 2010; Shen 2012; Ye 2015). Three were excluded for irrelevant study population (Hirner 2016; Mita 2018; Tschirdewahn 2020). Three were excluded for irrelevant study outcomes (Dogra 2016; Hah 2018; Lee 2017). One was excluded because the report was retracted (Herr 2015). The process of study exclusion is summarized in the PRISMA diagram. (Figure 1)
Risk of bias in included studies
Visual representations of the risk of bias of the included studies are presented in Figure 2 and Figure 3. Details are shown in Characteristics of included studies. Since there were less than 10 included studies available for meta‐analysis, we did not create a funnel plot to assess for publication bias.
2.

Risk of bias summary
3.

Risk of bias graph
Allocation
Six studies reported adequate efforts for random sequence generation (Buaban 2018; Kim 2018; Ma 2015; Naito 2016; Naselli 2012; Stănescu 2014). Two studies had no discussion of sequence generation; however, only the abstracts were available, so we deemed these to have an unclear risk of selection bias from random sequence generation (Lee 2014; Longo 2013).
Three out of eight studies reported adequate efforts to conceal allocation (Buaban 2018; Naito 2016; Stănescu 2014). Three full‐text studies did not describe methods used for allocation concealment, so we assessed these as having a high risk of selection bias (Kim 2018; Ma 2015; Naselli 2012). The remaining two studies, which only provided abstracts, did not describe allocation concealment (Lee 2014; Longo 2013); we rated these as having an unclear risk of selection bias.
Blinding
WLC mode and NBI mode were visually distinct. Given the nature of the intervention, no trial was able to blind the personnel involved. We considered all outcomes to be susceptible to performance bias. The risk of performance bias was high for all eight studies (Buaban 2018; Kim 2018; Lee 2014; Longo 2013; Ma 2015; Naito 2016; Naselli 2012; Stănescu 2014).
In judging the risk of detection bias, we categorized outcomes as subjective outcomes or objective outcomes. We considered time to disease recurrence and minor adverse events to be subjective outcomes, susceptible to detection bias, and grouped them together.
The detection of time to disease recurrence and minor adverse events involved clinical outcomes, so we considered studies that did not specify how, if any, blinding was conducted to be at high risk of detection bias for these outcomes (Buaban 2018; Kim 2018; Lee 2014; Longo 2013; Ma 2015; Naito 2016; Stănescu 2014). One study discussed the blinding of the pathologist, which reduced the risk of detection bias for time to disease recurrence (Naselli 2012); however, it did not state how, if any, blinding of outcome assessment for minor adverse events was conducted. As such, we judged the overall risk of detection bias to be unclear for that study.
We considered major adverse events to be objective outcomes, not susceptible to detection bias, because no clinical judgement was involved. The risk of detection bias for major adverse events was low for all five of the studies that assessed major adverse events (Buaban 2018; Longo 2013; Naito 2016; Naselli 2012; Stănescu 2014).
Incomplete outcome data
We rated the risk of attrition bias for each outcome based on the following predefined criteria: low (less than 10%), unclear (between 10% and 20%) and high (20% or higher).
Of the six studies that reported on time to disease recurrence or disease recurrence at 12 months or 13 to 24 months, one was at low risk of attrition bias (Ma 2015), one was at unclear risk of attrition bias (Lee 2014), and four were at high risk of attrition bias (Kim 2018; Naselli 2012; Naito 2016; Stănescu 2014).
Of the four studies that reported major and minor adverse events, one was at low risk of attrition bias (Longo 2013), and three were at high risk of attrition bias (Buaban 2018; Naito 2016; Stănescu 2014).
In total, 2152 participants were randomized but only 1847 participants were included in our analysis. This discrepancy was due to incomplete outcome data which precluded analysis.
Selective reporting
We assessed two studies to have a low risk of reporting bias as all outcomes were reported per trial registration (Naito 2016; Naselli 2012). None of the remaining studies had a trial registration or protocol available for comparison, so we assessed these to have an unclear risk of reporting bias.
Other potential sources of bias
We did not identify other potential sources of bias.
Effects of interventions
See: Table 1
NBI versus white light cystoscopy alone for transurethral resection
See: Table 1
Primary outcomes
Time to disease recurrence (time‐to‐event)
Based on six studies involving 1244 participants that reported time to disease recurrence (Kim 2018; Lee 2014; Ma 2015; Naito 2016; Naselli 2012; Stănescu 2014), participants who underwent NBI + WLC TURBT had lower risk of disease recurrence over time compared to participants who underwent WLC TURBT (hazard ratio 0.63 in favor of the NBI + WLC TURBT group, 95% CI 0.45 to 0.89; I2 = 53%; low certainty of evidence; Analysis 1.1). The anticipated absolute effects varied depending on the baseline risk of recurrence. For the low‐risk population, the use of NBI + WLC TURBT would lead to 53 fewer (range: 79 fewer to 15 fewer) recurrences per 1000 participants at 12 months. For the high‐risk population, the use of NBI + WLC TURBT would lead to 163 fewer (range: 265 fewer to 43 fewer) recurrences per 1000 participants at 12 months. The point estimates and confidence intervals for absolute effects did not meet the previously established thresholds of clinically relevant difference decrease (5% or more). All data were extrapolated from Kaplan‐Meier curves or outcomes data according to the Tierney method (Tierney 2007).
1.1. Analysis.

Comparison 1: NBI + WLC TURBT vs WLC TURBT alone, Outcome 1: Time to disease recurrence
Disease progression (time‐to‐event or dichotomous)
No studies reported data on disease progression.
Major adverse event (Clavien‐Dindo III, IV, V) (dichotomous)
Four studies involving 1385 participants reported on the occurrence of major adverse events. There were no major adverse events reported among either the NBI + WLC TURBT group or the WLC TURBT group in three of the four studies (Buaban 2018; Longo 2013; Stănescu 2014). One study found 16 major adverse events among 484 participants who underwent NBI + WLC TURBT and nine major adverse events among 481 participants who underwent WLC TURBT (Naito 2016) Considered together, there was likely no difference in the risk of major adverse events between the two groups (risk ratio 1.77, 95% CI 0.79 to 3.96; calculated from 1 study (Naito 2016) with 965 participants; low certainty of evidence; Analysis 1.2). The anticipated absolute effect was 10 more (range: 3 fewer to 39 more) major adverse events per 1000 participants. The point estimates and confidence intervals did not meet the previously established thresholds of clinically relevant difference for major adverse events (2% or more).
1.2. Analysis.

Comparison 1: NBI + WLC TURBT vs WLC TURBT alone, Outcome 2: Major adverse event (Clavien‐Dindo III, IV, V)
Secondary outcomes
Death from bladder cancer (time‐to‐event or dichotomous)
No studies reported data on death from bladder cancer.
Minor adverse event (Clavien‐Dindo I, II) (dichotomous)
Four studies involving 1385 participants reported on the occurrence of minor adverse events. All four studies had at least one minor adverse event in either the NBI + WLC TURBT group or the WLC TURBT group (Buaban 2018; Longo 2013; Naito 2016; Stănescu 2014). There were 78 minor adverse events among 704 participants who underwent NBI + WLC TURBT and 77 minor adverse events among 681 participants who underwent WLC TURBT. Considered together, there was likely no difference in risk of minor adverse events between the two groups (risk ratio 0.88, 95% CI 0.49 to 1.56; I2 = 61%; 4 studies, 1385 participants; low certainty of evidence; Analysis 1.3). The anticipated absolute effect was 14 fewer (range: 58 fewer to 63 more) minor adverse events per 1000 participants. The point estimates and confidence intervals did not meet the previously established thresholds of clinically relevant difference for minor adverse events (5% or more).
1.3. Analysis.

Comparison 1: NBI + WLC TURBT vs WLC TURBT alone, Outcome 3: Minor adverse event (Clavien‐Dindo grade I, II)
Subgroup Analysis
There were insufficient data available to conduct our planned subgroup analysis.
Sensitivity Analysis
There were no full‐text studies free of high risk of bias for all domains, precluding the ability to perform our prespecified sensitivity analyses.
Discussion
Summary of main results
In total, we identified eight randomized controlled trials, involving 2152 participants with suspected or diagnosed non‐muscle invasive bladder cancer, that compared NBI + WLC TURBT with WLC TURBT in the treatment of primary or recurrent non‐muscle invasive bladder cancer. Of the 2152 participants randomized, we included 1847 participants in the analysis. The discrepancy between participants randomized and those analyzed was most commonly due to incomplete outcome data.
Based on limited time‐to‐event data, the addition of NBI + WLC TURBT may lower the risk of disease recurrence over time. We were not able to examine death from bladder cancer as a time‐to‐event outcome or a dichotomous outcome. Our analysis suggests that there is no difference in risk of major or minor adverse events between the two groups. However, the certainty of evidence was low.
Given the limited data available, subgroup analysis and sensitivity analysis were not possible.
Overall completeness and applicability of evidence
This Cochrane Review is based on eight randomized controlled trials published between 2009 and 2017. The studies included participants undergoing transurethral resection of bladder tumor for suspected or pathologically confirmed (primary or recurrent), non‐muscle invasive bladder cancer (stage Ta, T1, and carcinoma in situ). This population is consistent with our inclusion criteria and represents a clinically relevant cohort for the use of NBI + WLC TURBT.
All but one study compared NBI + WLC TURBT to WLC TURBT alone. One study (Naselli 2012) compared NBI TURBT alone to WLC TURBT alone. We expect the results from this study to provide more conservative estimates for time to disease recurrence (favoring the null), since surgeons would be performing cystoscopy with only one light source (NBI) rather than with two light sources (NBI + WLC), which would theoretically lead to less opportunity for disease detection. We expect that the intervention of NBI TURBT alone vs WLC TURBT alone would underreport adverse events (favoring safety), since only one light source is used to examine the bladder. However, this particular study did not contribute data to adverse events, so our estimates are unchanged (Naselli 2012).
The majority of studies compared NBI + WLC TURBT with WLC TURBT alone using electrocautery to resect tumors, though one used laser resection of bladder tumor (Ma 2015), and another used bipolar plasma vaporization (Stănescu 2014). The influence on the type of resection of tumor performed on our outcomes is unknown and may introduce heterogeneity to our results. In addition to this, the type of WLC used (high definition versus standard definition) was not routinely described, and may influence the ability to detect tumors with WLC and the outcomes of the studies. The applicability of the intervention is improved by the fact that narrow band imaging is a standardized technology, though many differing manufacturers produce endoscopic cameras capable of NBI and not all studies described the NBI system they used.
Variation in the clinical management of people with non‐muscle invasive bladder cancer introduced heterogeneity and decreased the applicability of our results. For example, the use of immediate (within 24 hours of TURBT) postoperative chemotherapy instillations and intravesical therapy was not consistent between studies, so we could not include this in the analysis. Studies varied in their administration of mitomycin‐C, doxirubicin or epirubicin in the immediate (24‐hour) postoperative period and in the intravesical therapy regimens people with non‐muscle invasive bladder cancer may have received. In addition, the inclusion of people who underwent repeat transurethral resection and the timing of these resections were not well described and were likely to have been inconsistent between studies. These differences may impact the clinical applicability of our study results.
It is also important to discuss that the benefit of NBI + WLC TURBT on time to disease recurrence on clinical outcomes may be due to the fact that increased detection of tumors may lead to escalation of risk stratification, which then leads to more aggressive observation schedules and interventions. Therefore, the impact of NBI on disease recurrence may be indirect, through this cascade.
Quality of the evidence
We assessed the certainty of evidence using the GRADE approach, and considered it to be low for all outcomes. We consistently downgraded the certainty for risk of bias and imprecision.
Risk of bias: due to the nature of TURBT, personnel could not be blinded to treatment assignment. We considered the risk of performance bias for all outcomes, and the risk of detection bias for susceptible outcomes (i.e. time to disease recurrence, minor adverse events) to be high. However, we would not expect the lack of blinding to bias the detection of major adverse events because no clinical judgement would be involved. We downgraded the certainty of evidence by one level for issues of performance bias, detection bias, incomplete outcome data, or selective reporting.
Imprecision: we downgraded the certainty of evidence based on the confidence interval around the effect size, and whether it included both clinically relevant potential benefit and harm.
Potential biases in the review process
Few studies presented time to disease recurrence; among these, all data were presented in the form of Kaplan‐Meier curves or dichotomous outcome data only. We contacted all study authors to request additional data via email, but did not receive any additional data. Therefore, we derived hazard ratios for the outcome of time to disease recurrence based on dichotomous outcome data and Kaplan‐Meier curves, according to the Tierney method (Tierney 2007). This method can only reconstruct hazard ratios according to the information available and may have resulted in bias.
There were no data available on time to disease progression or time to death from bladder cancer, nor were dichotomous outcomes (disease progression, death from bladder cancer) reported.
Agreements and disagreements with other studies or reviews
Several systematic reviews have been performed on the topic of NBI + WLC TURBT for non‐muscle invasive bladder cancer. They are listed chronologically with a brief discussion on the conclusions of each review.
Lee 2015 performed a systematic review and network meta‐analysis on the use of blue light‐guided or NBI + WLC TURBT versus WLC TURBT alone. Of the 15 studies included, four were trials comparing NBI + WLC TURBT to WLC TURBT alone. Our review included three of the four trials included in their review (Geavlete 2012, which was an earlier publication for the Stănescu 2014 trial, Naselli 2012, Lee 2014). The remaining trial violated the randomization requirement (i.e. participants were consecutively enrolled) for eligibility of inclusion for our review. The authors used the Cochrane risk of bias assessment but did not grade certainty of evidence. Consistent with the directionality we found in our study, which favored NBI + WLC TURBT for decreasing disease recurrence at 12 months, the authors found that NBI + WLC TURBT was superior to that using WLC, with an odds ratio (OR) of 0.47 (95% CI 0.31 to 0.72; P < 0.001) for the outcome of recurrence rate, which was defined as the number of bladder cancer recurrences after initial TURBT.
Kang 2017 performed a systematic review of six studies on the use of NBI in reducing recurrence risk (dichotomous outcome at three months, one year, and two years). Of these, one was retrospective, and one prospective study has subsequently been retracted; we included all four eligible randomized controlled trials in this Cochrane Review. The authors used the Cochrane risk of bias tool and found that all RCTs were at high risk of performance bias and low or unclear risk of other forms of bias. The certainty of evidence was not evaluated. Kang and colleagues found that NBI + WLC TURBT was associated with improvements in the three‐month recurrence risk (relative risk [RR] 0.39, 95% CI 0.26 to 0.60; P < 0.0001), one‐year recurrence risk (RR 0.52, 95% CI 0.40 to 0.67; P < 0.00001) and two‐year recurrence risk (RR 0.60, 95% CI 0.42 to 0.85; P = 0.004) compared with WLC TURBT.
Xiong 2017 performed a systematic review of 25 studies, including 17 full texts and eight abstracts, to compare NBI + WLC TURBT with WLC TURBT alone in detection and recurrence risk. The meta‐analysis included both randomized and non‐randomized studies. In the risk of recurrence assessment, six studies were included, three of which were not randomized controlled trials. The study used the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) to assess the quality and risk of bias of the studies included in their review. The QUADAS‐2 only assesses four domains (participant selection, index test, reference standard, and flow and timing) versus the Cochrane risk of bias tool, which assesses six domains (selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias). Furthermore, Cochrane uses the GRADE approach to assess the quality of evidence. Consistent with our review, the authors found that NBI significantly reduced the recurrence rate of bladder cancer with a pooled risk ratio of 0.43 (95% CI 0.23 to 0.79) and 0.81 (95% CI 0.69 to 0.95) at three months and 12 months, respectively.
Chen 2017 performed a systematic review inclusive of 22 trials with a total of 7767 participants to assess the use of NBI and/or blue light TURBT relative to WLC TURBT alone. We were only able to locate an abstract and found no corresponding manuscript, so we were not able to discern the number of studies that were relevant to our outcomes of interest and compare methodology. Their outcomes of interest were one‐year, two‐year, and five‐year recurrence rates and progression rate, assessed with odds ratios. The authors found that the use of NBI + WLC TURBT was associated with lower odds of recurrence at one year (OR 0.56, 95% CI 0.31 to 0.91), compared to WLC TURBT. They reported that the use of NBI + WLC TURBT was associated with lower two‐year and five‐year recurrence rates, though odds ratios were not available. In addition, the authors concluded that NBI + WLC TURBT had "the higher probabilities to be the best intervention in lower 1‐year, 2‐year and 5‐ year recurrence rate" relative to WLC TURBT.
Motlagh 2021 performed a systematic review and Bayesian sensitivity network meta‐analysis of randomized trials, comparing the recurrence rates among the intervention group undergoing 1) blue light guided‐TURBT with or without single immediate intravesical chemotherapy, 2) NBI + WLC TURBT with or without single immediate intravesical chemotherapy, or 3) WLC TURBT with single immediate intravesical chemotherapy and the control group undergoing WLC TURBT alone. The authors concluded that NBI, with or without single immediate intravesical chemotherapy, was not associated with a significantly lower likelihood of 12‐month recurrence rate (OR 0.39, 95% CI 0.11 to 1.29; OR 0.65, 95% CI 0.343 to 1.15, respectively).
Gravestock 2021 performed a systematic review inclusive of three trials with a total of 921 participants to assess the effect of NBI + WLC TURBT compared with white light on recurrence rates in NMIBC. Our review included three of the three trials included in their review (Kim 2018, Naito 2016, Naselli 2012). Their outcomes of interest were recurrence at 12 and 24 months and adverse events. The authors did not find a statistically significant result. However, the analysis showed a trend in favor of NBI + WLC TURBT with a risk ratio of 0.75 (95% CI 0.50 to 1.14; P = 0.18; I2 = 61%). The authors also reported that only one of the included studies (Naito 2016) reported on adverse events and no significant difference was observed between the two cohorts.
In summary, several existing systematic reviews support a positive or neutral effect of NBI + WLC TURBT on reducing the risk of recurrence. Our rigorous inclusion of only randomized controlled trial data, extrapolation of time‐to‐event data, more recent systematic review, and assessment of the certainty of evidence rating on a per outcome basis distinguished our review from prior reviews.
Authors' conclusions
Implications for practice.
White light cystoscopy (WLC)‐ and narrow band imaging (NBI)‐guided transurethral resection of bladder tumor (TURBT) may lower the risk of disease recurrence over time compared to WLC TURBT in the treatment of non‐muscle invasive bladder cancer. Overall, our confidence in the effect estimates is limited. The true effects on disease recurrence may be substantially different from the effect estimates.
Unfortunately, no studies analyzed the effect of NBI + WLC TURBT on the risk of disease progression or bladder cancer death. In terms of patient safety, NBI + WLC TURBT may have little to no effect on major or minor adverse effects, though we have low confidence in the effect estimates.
However, unlike blue light cystoscopy, NBI + WLC TURBT does not require instillation of photosensitizing agents into the bladder. Further, given that many cystoscopes can be switched from the white light mode to the NBI mode with no significant interruption to the procedural workflow, NBI + WLC TURBT may be considered as an additional intervention to improve outcomes.
Implications for research.
The review identified only eight randomized controlled trials addressing certain aspects of the research question. The certainty of evidence was low. This underscores the importance of following higher methodological standards for designing trials, registering and peer reviewing trial protocols a priori, and using CONSORT guidelines when reporting trial results.
Further, few studies presented time‐to‐event data. While we were able to mitigate this limitation by using established methods to calculate time‐to‐event data, this additional calculation might have introduced bias in our results. When reporting time‐to‐event data, trialists may also consider including numbers at risk in their graphs or as supplementary data for improving data transparency. Standardizing data reporting and collection would facilitate additional systematic reviews and improve the quality of meta‐analyses. Future studies may also consider examining the effectiveness of WLC TURBT alone versus NBI + WLC TURBT for surveillance of people with non‐muscle invasive bladder cancer.
History
Protocol first published: Issue 3, 2021
Acknowledgements
We would like to acknowledge the support of all members of the Cochrane Urology Group, especially Robert Lane of the Cochrane Urology Editorial Teams in the USA and Yeeun Kim of the Korean Satellite of Cochrane Urology for their help and support. We also thank the Cancer Network for their support of this review.
We would like to thank several peer reviewers who provided invaluable feedback that has improved the review. These include: Rod Breau, Stefanie Schmidt, and Xiang Wang. Additional reviewers chose not to be acknowledged by name; we nevertheless appreciate their critical input.
Appendices
Appendix 1. Cochrane Library search strategy
#1 MeSH descriptor: [Urinary Bladder Neoplasms] explode all trees
#2 (bladder* near/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)):ti,ab,kw (Word variations have been searched)
#3 (NMIBC):ti,ab,kw (Word variations have been searched)
#4 (TURBT):ti,ab,kw (Word variations have been searched)
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Narrow band imaging] explode all trees
#7 (("narrow band" or narrowband or narrow‐band) near/3 imaging):ti,ab,kw
#8 nbi:ti,ab,kw
#9 #6 OR #7 OR #8
#10 #5 AND #9
Appendix 2. International Pharmaceutical Abstracts search strategy
1 (bladder$ adj3 (cancer$ or carcinoma$ or neoplas$ or tumo?r$)).tw.
2 NMIBC.tw.
3 TURBT.tw.
4 1 or 2 or 3
5 (("narrow band" or narrowband or narrow‐band) adj3 imaging).tw.
6 NBI.tw.
7 5 or 6
8 4 and 7
Appendix 3. MEDLINE Ovid search strategy
1 exp urinary bladder neoplasms/
2 (bladder$ adj3 (cancer$ or carcinoma$ or neoplas$ or tumo?r$)).tw.
3 NMIBC.tw.
4 TURBT.tw.
5 1 or 2 or 3 or 4
6 exp narrow band imaging/
7 (("narrow band" or narrowband or narrow‐band) adj3 imaging).tw.
8 NBI.tw.
9 6 or 7 or 8
10 5 and 9
11 randomized controlled trial.pt.
12 controlled clinical trial.pt.
13 randomized.ab.
14 placebo.ab.
15 drug therapy.fs.
16 randomly.ab.
17 trial.ab.
18 groups.ab.
19 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20 exp animals/ not humans.sh.
21 19 not 20
22 10 and 21
Appendix 4. Embase search strategy
#1 'bladder tumor'/exp
#2 (bladder* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)):ab,ti
#3 nmibc:ab,ti
#4 turbt:ab,ti
#5 #1 OR #2 OR #3 OR #4
#6 'narrow band imaging'/exp
#7 (("narrow band" or narrowband or narrow‐band) NEAR/3 imaging):ab,ti
#8 nbi:ab,ti
#9 #6 OR #7 OR #8
#10 #5 AND #9
#11 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti
#12 'animals'/exp NOT ('humans'/exp AND 'animals'/exp)
#13 #11 NOT#12
#14 #10 AND #13
Appendix 5. Web of Science search strategy
#1 TS=((bladder* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)) OR NMIBC OR TURBT)
#2 TS=((("narrow band" or narrowband or narrow‐band) NEAR/3 imaging) OR NBI)
#3 #1 AND #2
#4 TS=clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#5 #3 AND #4
Appendix 6. Scopus search strategy
#1 TITLE‐ABS‐KEY((bladder* W/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)) OR NMIBC OR TURBT)
#2 TITLE‐ABS‐KEY((("narrow band" or narrowband or narrow‐band) W/3 imaging) OR NBI)
#3 #1 AND #2
#4 ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomization" OR "placebo" ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐ Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical trial* OR trial* OR rct* OR random* OR blind* ) )
#5 #3 AND #4
Appendix 7. Open Grey literature search strategy
"Bladder Cancer" AND ("narrow band imaging" OR "narrow‐band imaging" OR "narrowband imaging" OR NBI)
Note: The Open Grey search was conducted during the initial search in September 2020. The service was discontinued in 2021, precluding its inclusion in the second search in December 2021.
Appendix 8. ClinicalTrials.gov search strategy
#1 Bladder Cancer
#2 Narrow band imaging OR narrow‐band imaging OR narrowband imaging OR NBI
#3 1 AND 2
Appendix 9. WHO search strategy
#1 bladder cancer AND narrow band imaging
#2 bladder cancer AND NBI
#3 bladder cancer AND narrow‐band imaging
#4 bladder cancer AND narrowband imaging
#5 1 OR 2 OR 3 OR 4
Appendix 10. LILACS search strategy
(mh:("Urinary Bladder Neoplasms") OR tw:(((bladder OR bexiga OR vejiga) AND (cancer$ OR carcinoma$ OR tumor$ OR tomour$ OR neoplasm$ OR neoplasia$ OR neoplasma$)) OR "NMIBC" OR "TURBT")) AND (mh:("Narrow band imaging") OR tw:("Narrow band imaging" OR "narrowband imaging" OR "narrow‐band imaging" OR "imagem de banda estreita" OR imágenes de banda estrecha" OR NBI)) AND (PT:"randomized controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomized controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter studies as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method" OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro")
Data and analyses
Comparison 1. NBI + WLC TURBT vs WLC TURBT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to disease recurrence | 6 | 1244 | Hazard Ratio (IV, Random, 95% CI) | 0.63 [0.45, 0.89] |
| 1.2 Major adverse event (Clavien‐Dindo III, IV, V) | 4 | 1385 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.79, 3.96] |
| 1.3 Minor adverse event (Clavien‐Dindo grade I, II) | 4 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.56] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buaban 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people with histopathology‐visualized non‐muscle
invasive bladder cancer and complete resection of the visualized tumor Exclusion criteria: people with muscle‐invasive bladder cancer, incomplete tumor resection, histopathology‐visualized non‐cancerous lesion, synchronous upper urinary tract cancer, and postoperative intravesical therapy before surveillance cystoscopy Number randomized: 79 participants to undergo WLC TURBT and 79 participants to undergo NBI + WLC TURBT Number analyzed by authors: 25 participants who underwent a total of 31 WLC TURBTs and 37 participants who underwent a total of 44 NBI + WLC TURBTs Baseline characteristics of participants analyzed. NOTE: The authors reported the characteristics using the total number of TURBTs as the denominator rather than the number of unique participants undergoing TURBT, which invariably double‐counted some participants. Comparator group: n = 25 participants who underwent a total of 31 WLC TURBTs.
Intervention group: n = 37 participants who underwent a total of 44 NBI + WLC TURBTs
Subsequent intravesical treatment among either group: no participant included had undergone subsequent intravesical treatment |
|
| Interventions |
Comparator arm: WLC TURBT alone Intervention arm: NBI + WLC TURBT using an EVIS EXERA II CLV‐180 (Olympus) |
|
| Outcomes | Disease recurrence at 3 months Complication rate |
|
| Funding sources | Not reported | |
| Declarations of interest | The authors reported having no conflict of interest to declare. | |
| Notes |
Contact with study author Date of contact attempt: 13 January 2021 Contact status: no additional data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The prospective, randomized, single‐blind control
study...After enrollment, participants were randomly allocated with a
computerized random permute block to TUR‐BT with NBI or TUR‐BT with
WLI." Comment: The allocation sequence was random using computers. |
| Allocation concealment (selection bias) | Low risk |
Quote: "After enrollment, participants were randomly allocated with
a computerized random permute block to TUR‐BT with NBI or TUR‐BT with WLI.
Both surgeons and participants were blinded from randomization before the
procedure." Comment: There was central randomization and the allocation sequence was concealed to both participants and clinicians until the participant was registered. |
| Blinding of participants and personnel (performance bias) | High risk |
Quote: "All TUR‐BTs were done by experienced urology staff or by
third‐year urology residents under supervision." Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence and minor adverse events would be affected by a lack of blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes: major adverse event | Low risk | Comment: The authors did not specify how, if any, blinding of outcome assessment was conducted. However, we do not expect the lack of blinding to bias the detection or reporting of major adverse events as no clinical judgement was involved. |
| Incomplete outcome data (attrition bias) Major adverse event | High risk | Comment: Of the 158 participants randomized (79 to each arm), only 25 and 37 were included in the analysis (32% and 47%, respectively). The attrition rate was greater than 20% in both arms. |
| Incomplete outcome data (attrition bias) Minor adverse event | High risk | Comment: Of the 158 participants randomized (79 to each arm), only 25 and 37 were included in the analysis (32% and 47%, respectively). The attrition rate was greater than 20% in both arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No associated trial registration was found. |
| Other bias | Low risk | Comment: No additional sources of potential bias were found. |
Kim 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people who underwent TURBT for suspicion of a
bladder tumor discovered by use of cystoscopy or another imaging study Exclusion criteria: people with muscle‐invasive tumors, those undergoing radical cystectomy, those receiving chemotherapy or radiotherapy, those with nonurothelial carcinoma, those who were not histologically diagnosed with cancer, those lost to follow‐up, and those who died for other reasons Number randomized: 198 (97 participants to undergo WLC TURBT and 101 participants to undergo NBI + WLC TURBT) Number analyzed by authors: 35 participants who underwent WLC TURBT and 39 participants who underwent NBI + WLC TURBT, and had follow‐up at 12 months Baseline characteristics of randomized participants who were not found to have muscle‐invasive tumor or nonurothelial carcinoma, those who did not undergo radical cystectomy during the study period, and those who did not receive chemotherapy (n = 67 comparison arm, n = 85 intervention arm) Comparator group, n = 67 participants
Intervention group, n = 85 participants
Subsequent intravesical treatment among either group: no documentation of subsequent intravesical treatment |
|
| Interventions |
Comparator group: WLC TURBT Intervention group: NBI + WLC TURBT |
|
| Outcomes | Number of tumors identified Disease recurrence at 12 months |
|
| Funding sources | Not reported | |
| Declarations of interest | The authors reported having no conflict of interest to declare. | |
| Notes |
Contact with study author Date of contact attempt: 13 January 2021 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The participants were randomly divided into the NBI
(experimental) and WLC (control) groups. Randomization was conducted by
means of a computer‐generated random sequence of numbers" Comment: Randomization was performed by a computer‐generated random sequence. |
| Allocation concealment (selection bias) | High risk |
Quote: "Before procedures, participants eligible for the study were
contacted by medical staff and provided with verbal and written
information." Comment: There was no mention of allocation concealment methods. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence would be affected by a lack of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | High risk | Comment: Of the 198 participants enrolled, 97 and 101 were randomized to the WLC TURBT arm and the NBI + WLC TURBT arm, respectively. Only 35 participants of the WLC TURBT arm and 39 participants of the NBI + WLC TURBT arm were included for analysis of disease recurrence at 12 months (36% and 39%, respectively). The attrition rate was greater than 20% in both arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: This study was registered in the Korea University Anam Hospital clinical trial center (approval number: MD13008). However, the registration protocol was not available for comparison. |
| Other bias | Low risk | Comment: No additional sources of potential bias were identified. |
Lee 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people with overt or suspected non‐muscle
invasive bladder cancer Exclusion criteria: people with muscle‐invasive bladder cancer, negative pathologic examination, or without follow‐up Number randomized: 68 (35 participants to undergo WLC TURBT alone and 33 participants to undergo NBI + WLC TURBT) Number analyzed by authors: 35 participants who underwent WLC TURBT alone and 33 participants who underwent NBI + WLC TURBT Baseline characteristics of participants analyzed Comparator group, n = 35
Intervention group, n = 33
Subsequent intravesical treatment among either group: no documentation of subsequent intravesical treatment |
|
| Interventions |
Comparator arm: WLC TURBT alone Intervention arm: NBI + WLC TURBT |
|
| Outcomes | Kaplan Meier curves of freedom from recurrence up to 35 months following treatment | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Contact with study author Date of contact attempt: 19 January 2021 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There were no discussions of sequence generation; however, only the abstract was available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The authors did not describe how, if any, concealment of allocation prior to assignment was conducted; however, only the abstract was available. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence would be affected by a lack of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | Unclear risk | Comment: While survival curves were available, it was unclear whether participants had an event occurrence or were censored. Only the abstract was available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No discussion of trial registration or associated registration was identified. |
| Other bias | Low risk | Comment: No additional sources of potential bias were identified. |
Longo 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people who underwent a TURBT for suspicious
bladder cancer diagnosed by WLC Exclusion criteria: not reported Number randomized: 66 participants to undergo WLC TURBT alone and 71 participants to undergo NBI + WLC TURBT Number analyzed by authors: 66 participants who underwent WLC TURBT alone and 71 participants who underwent NBI + WLC TURBT Baseline characteristics of participants analyzed Comparator group, n = 66
Intervention group, n = 71
Subsequent intravesical treatment among either group: no documentation of subsequent intravesical treatment |
|
| Interventions |
Comparator arm: WLC TURBT alone Intervention arm: NBI + WLC TURBT (Olympus) |
|
| Outcomes | Surgical complication (Clavien‐Dindo) Mean time to catheter removal Absence of muscle tissue in the specimen False‐positive rate |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Contact with study author Date of contact attempt: 5 October 2020 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Authors did not specify how random assignments were generated; however, only the abstract was available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The authors did not describe how, if any, concealment of allocations prior to assignment was conducted; however, only the abstract was available. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of minor adverse events would be affected by a lack of blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes: major adverse event | Low risk | Comment: The authors did not specify how, if any, blinding of outcome assessment was conducted. However, we do not expect the lack of blinding to bias the detection or reporting of major adverse events as no clinical judgement was involved. |
| Incomplete outcome data (attrition bias) Major adverse event | Low risk | Comment: Of the 137 participants randomized, 66 and 71 were randomized to the WLC TURBT arm and to the NBI + WLC TURBT arm, respectively. All participants were included in the analysis of adverse events. The attrition rate was less than 10% in both arms. |
| Incomplete outcome data (attrition bias) Minor adverse event | Low risk | Comment: Of the 137 participants randomized, 66 and 71 were randomized to the WLC TURBT arm and to the NBI + WLC TURBT arm, respectively. All participants were included in the analysis of adverse events. The attrition rate was less than 10% in both arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No associated trial registration was found. |
| Other bias | Low risk | Comment: No additional potential sources of bias were identified. |
Ma 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: between February 2013 and February 2014, 209
participants were initially diagnosed with original or recurrent bladder
cancer. They were randomized into WLC TURBT alone and NBI + WLC guided
Holmium laser resection of bladder tumor groups Exclusion criteria: presence of invasive bladder cancer; no immediate postoperative intravesical bladder instillation of therapeutic agent; underwent other treatment for cancer; presence of lymph node or distant metastasis Number randomized: 209 participants Number analyzed by authors: 92 participants who underwent WLC TURBT alone and 86 participants who underwent NBI‐guided holmium laser resection of bladder tumors Baseline characteristics of participants analyzed Comparator group, n = 92
Intervention group, n = 86
Subsequent intravesical treatment among either group: All participants underwent postoperative instillation. Intermediate‐ and high‐risk participants continued instillation for one year. |
|
| Interventions |
Comparator arm: WLC TURBT alone Intervention arm: NBI + WLC guided holmium laser resection of bladder tumor |
|
| Outcomes | Disease recurrence at 3 months Disease recurrence at 12 months In‐situ recurrence Ex‐situ recurrence (recurrence at other locations) |
|
| Funding sources | Two government sources | |
| Declarations of interest | Not reported | |
| Notes |
Contact with study author Date of contact attempt: 19 January 2021 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Authors generated two comparison groups using simple randomization. |
| Allocation concealment (selection bias) | High risk | Comment: The authors did not discuss the method used, if any, to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence would be affected by a lack of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | Low risk | Comment: Of the 209 participants enrolled and 178 randomized (92 of the WLC TURBT arm and 86 participants of the NBI + WLC TURBT arm), all participants were available for follow‐up at 12 months. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No associated trial registration was identified. |
| Other bias | Low risk | Comment: No additional sources of potential bias were found. |
Naito 2016.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study grouping: Parallel group |
|
| Participants |
Inclusion criteria: people scheduled for TURBT with papillary
bladder tumor(s) detected by imaging or cystoscopy or those scheduled for
random biopsies and/or TURBT because of bladder lavage fluid or voided urine
cytology with malignant (grade 3) cells Exclusion criteria: people with presence of tumors in the upper urinary tract, muscle‐invasive bladder tumour, previous irradiation of the pelvis, gross hematuria that might interfere with cystoscopy at the time of TURBT, participation in other clinical studies with investigational drugs either concurrently or within the previous 30 days, pregnancy, any condition associated with a risk of poor protocol compliance Number randomized: 481 participants to undergo WLC TURBT alone and 484 participants to undergo NBI + WLC TURBT Number analyzed by authors: 481 participants to undergo WLC TURBT alone and 484 participants to undergo NBI + WLC TURBT; of which 294 participants who underwent WLC TURBT alone and 303 participants who underwent NBI + WLC TURBT completed the 12‐month follow‐up. Baseline characteristics of intention‐to‐treat cohort Comparator group, n = 481
Intervention group, n = 484
Subsequent intravesical treatment among either group: no documentation of subsequent intravesical treatment |
|
| Interventions |
Comparator group: WLC TURBT Intervention group: NBI + WLC TURBT |
|
| Outcomes | Surgical complication (Clavien‐Dindo) Disease recurrence during re‐TURBT Disease recurrence at 3 months Disease recurrence at 12 months |
|
| Funding sources | Funded by Olympus through an unrestricted educational grant | |
| Declarations of interest | The authors reported having no conflict on interest to declare. | |
| Notes |
Contact with study author Date of contact attempt: 13 January 2021 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "After enrollment, participants were randomly allocated in a
1:1 ratio to parallel control (WL) and intervention (NBI) arms.
Randomization was conducted by means of a concealed computer‐generated
random sequence of numbers using permuted blocks..." Comment: Participants were randomized by means of a concealed computer‐generated random sequence of numbers. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The process was implemented through the online data
management system. Participants were blinded for the treatment arm to which
they were randomized." Comment: There was central randomization and participants were blinded, suggesting that adequate allocation sequence concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence and minor adverse events would be affected by a lack of blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes: major adverse event | Low risk | Comment: The authors did not specify how, if any, blinding of outcome assessment was conducted. However, we do not expect the lack of blinding to bias the detection or reporting of major adverse events as no clinical judgement was involved. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | High risk | Comment: Of the 481 and 484 participants randomized to the WLC TURBT arm and the NBI + WLC TURBT arm, respectively, 294 (61%) and 303 (63%) completed the study. The attrition rate was greater than 20% in both arms. |
| Incomplete outcome data (attrition bias) Major adverse event | High risk | Comment: Of the 481 and 484 participants randomized to the WLC TURBT arm and the NBI + WLC TURBT arm, respectively, 294 (61%) and 303 (63%) completed the study. The attrition rate was greater than 20% in both arms. |
| Incomplete outcome data (attrition bias) Minor adverse event | High risk | Comment: Of the 481 and 484 participants randomized to the WLC TURBT arm and the NBI + WLC TURBT arm, respectively, 294 (61%) and 303 (63%) completed the study. The attrition rate was greater than 20% in both arms. |
| Selective reporting (reporting bias) | Low risk | Comment: Trial was registered (nl3513) in the Netherlands Trial Register, and no discrepancies were identified between registration and the reported outcomes. |
| Other bias | Low risk | Comment: No additional sources of potential bias were identified. |
Naselli 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people from two centers with overt or suspected
bladder cancer. Exclusion criteria: invasive bladder cancer, absence of urothelial cancer after pathologic examination, or without follow‐up Number randomized: 188 (95 participants to undergo WLC TURBT alone and 93 participants to undergo NBI + WLC TURBT) Number analyzed by authors: 72 participants who underwent WLC TURBT and 76 participants who underwent NBI + WLC TURBT and had completed follow‐up at 12‐months. Baseline characteristics of participants analyzed Comparator group, n = 72
Intervention group, n = 76
Subsequent intravesical treatment among either group: no participant included had undergone subsequent intravesical treatment |
|
| Interventions |
Comparator group: WLC TURBT Intervention group: NBI + WLC TURBT |
|
| Outcomes | Detection rate Recurrence free survival rate at 12 months Logistic regression of 12 months and 3 months recurrence risks |
|
| Funding sources | None | |
| Declarations of interest | The authors reported having no conflicts of interest to declare | |
| Notes |
Contact with study author Date of contact attempt: 13 January 2021 Contact status: no reply to‐date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was centralized and performed by means of a
random table." Comment: Randomization was performed via a centralized random table. |
| Allocation concealment (selection bias) | High risk | Comment: The authors did not describe how, if any, concealment of allocation was conducted. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | Unclear risk |
Support: "The specimen of each lesion was analyzed individually by a
pathologist blinded to the mode of identification of the single lesion (WL
or NBI)." Comment: The pathologist was blinded. However, the authors did not specify if the personnel responsible for assessing minor adverse events were blinded. The detection of minor adverse events would be affected by a lack of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | High risk | Comment: Of the 93 participants who were randomized to the WLC TURBT arm, 72 (77%) had completed follow‐up. Of the 95 participants were randomized to the NBI + WLC TURBT arm, 76 (80%) had completed follow‐up. With attrition rates of 23% and 20%, the risk of attrition bias was deemed high. |
| Selective reporting (reporting bias) | Low risk | Comment: The trial was registered in the Netherlands Trial Register (NTR3645). Outcomes were reported as noted in registration. Primary and secondary outcomes reported as registered. |
| Other bias | Low risk | Comment: No additional sources of potential bias were identified. |
Stănescu 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Inclusion criteria: people with one or more suspected non‐muscle
invasive bladder cancer of over 3 cm in diameter. Exclusion criteria: people with muscle invasive bladder cancer Number randomized: 260 (130 participants to undergo WLC TURBT and 130 participants to undergo NBC+WLC guided bipolar plasma vaporization) Number analyzed by authors
Baseline characteristics (unclear if reported characteristics were of participants randomized or participants analyzed) Comparator group, n = unclear
Intervention group, n = unclear
Subsequent intravesical treatment: In all cases without bladder wall perforations, a single post‐operative mitomycin‐C instillation was performed. In all participants with NMIBT, standard monopolar repeat TUR was performed 4 weeks after the initial procedure followed by a 1‐year bacille Calmette‐Guérin intravesical instillation. |
|
| Interventions |
Comparator arm: WLC TURBT, followed by a single postoperative
instillation with doxorubicin or epirubicin within 24 hours after
surgery Intervention arm: NBI + WLC guided bipolar plasma vaporization (Visera video system), followed by a single postoperative instillation with doxorubicin or epirubicin within 24 hours after surgery |
|
| Outcomes | Adverse events Tumor detection rate by pathology Re‐TURBT rate Recurrence rate at 12 months Recurrence rate at 24 months |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Contact with study author Date of contact attempt: 13 January 2021 Contact status: no reply to‐date Time to disease recurrence data found in Geavlete 2012 (Urology) companion paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "All participants signed an approved written informed consent
form that properly explained the aims, methods, anticipated benefits,
potential hazards, and any other aspect of the study relevant to the
participant's decision to participate. They were subsequently randomized
using sealed envelopes and were unaware of which diagnostic and treatment
alternative was applied in each case." Comment: Sealed envelopes were used for sequence generation, which was deemed adequate. |
| Allocation concealment (selection bias) | Low risk |
Quote: "All participants signed an approved written informed consent
form that properly explained the aims, methods, anticipated benefits,
potential hazards, and any other aspect of the study relevant to the
participant's decision to participate. They were subsequently randomized
using sealed envelopes and were unaware of which diagnostic and treatment
alternative was applied in each case." Comment: There was central randomization with allocation sequence concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Personnel could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes: time to disease recurrence, minor adverse event | High risk | Comment: Authors did not specify how, if any, outcome assessment blinding was achieved. The detection of disease recurrence and minor adverse events would be affected by a lack of blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes: major adverse event | Low risk | Comment: The authors did not specify how, if any, blinding of outcome assessment was conducted. However, we do not expect the lack of blinding to bias the detection or reporting of major adverse events as no clinical judgement was involved. |
| Incomplete outcome data (attrition bias) Time to disease recurrence | High risk | Comment: Of the 260 participants randomized (130 to the WLC TURBT arm, and 130 to the NBI + WLC TURBT arm), 97 (75%) and 93 (72%) participants of the respective arms completed the follow‐up. The attrition rate was greater than 20% in both arms. |
| Incomplete outcome data (attrition bias) Major adverse event | High risk | Comment: Of the 260 participants randomized (130 to the WLC TURBT arm, and 130 to the NBI + WLC TURBT arm), 97 (75%) and 93 (72%) participants of the respective arms completed the follow‐up. The attrition rate was greater than 20% in both arms. |
| Incomplete outcome data (attrition bias) Minor adverse event | High risk | Comment: Of the 260 participants randomized (130 to the WLC TURBT arm, and 130 to the NBI + WLC TURBT arm), 97 (75%) and 93 (72%) participants of the respective arms completed the follow‐up. The attrition rate was greater than 20% in both arms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No associated trial registration found. |
| Other bias | Low risk | Comment: No additional sources of potential bias were identified. |
Abbreviations and descriptions:
CIS/Tis: urothelial carcinoma in situ, “flat tumor”; IQR: interquartile range; NBI: narrow band imaging; SD: standard deviation; pT0: no evidence of primary tumor (pathologically staged); pT1: tumor invades lamina propria (pathologically staged); pTa: noninvasive papillary carcinoma (pathologically staged); TURBT: transurethral resection of bladder tumor; TX: primary tumor cannot be assessed; WLC: white light cystoscopy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Doehn 2014 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT, blue light‐guided TURBT, and NBI + WLC TURBT in a randomized order. |
| Dogra 2016 | Irrelevant outcome: this study compared the detection of bladder cancer, rather than our outcomes of interest (disease recurrence, progression, death, adverse events). |
| Geavlete 2012 | Irrelevant study design: all participants underwent WLC TURBT, followed by NBI + WLC TURBT. |
| Hah 2018 | Irrelevant outcome: this study compared the detection of bladder cancer, rather than our outcomes of interest (disease recurrence, progression, death, adverse events). |
| Herr 2015 | Article was retracted. |
| Herr 2019 | Irrelevant design: editorial. |
| Hirner 2016 | Irrelevant study population: this study examined the use of NBI in participants on surveillance, a population which we have specified to exclude in our protocol since we are only considering the use of NBI in the treatment setting in this review. |
| Lee 2017 | Irrelevant outcome: this study compared the detection of bladder cancer, rather than our outcomes of interest (disease recurrence, progression, death, adverse events). |
| Mita 2018 | Irrelevant study population: this study examined the use of NBI in participants on surveillance, a population which we have specified to exclude in our protocol since we are only considering the use of NBI in the treatment setting in this review. |
| Mukherjee 2019 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT and NBI + WLC TURBT in a randomized order. |
| Naya 2015 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT and NBI + WLC TURBT in a randomized order. |
| Shen 2010 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT and NBI + WLC TURBT in a randomized order. |
| Shen 2012 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT and NBI + WLC TURBT in a randomized order. |
| Tschirdewahn 2020 | Irrelevant study population: this study examined the use of NBI in participants on surveillance, a population which we have specified to exclude in our protocol since we are only considering the use of NBI in the treatment setting in this review. |
| Ye 2015 | Irrelevant study design: this was a sequential intervention study where participants received WLC TURBT and NBI + WLC TURBT in a randomized order. |
NBI: narrow band imaging; TURBT: transurethral resection of bladder tumor; WLC: white light cystoscopy
Differences between protocol and review
We made the following changes between the protocol (Lai 2021) and the review.
We have revised the definition of disease progression to follow the standardized criteria published by the International Bladder Cancer Group.
The protocol stated that the detection of minor adverse events was not susceptible to bias. However, after further discussion, the author team recognized that significant clinical judgement was involved in assessing minor adverse events such that the detection of minor adverse was susceptible to bias.
Contributions of authors
Drafted the protocol: LL, ST, EG, LH, PD, PM, GL
Study selection: LL, ST, GL
Extracted data from studies: LL, ST, GL
Entered data into RevMan: LL, ST, EG
Carried out the analysis: LL, ST, PM, PD, GL
Interpreted the analysis: LL, ST, EG, LH, PM, PD, GL
Disagreement resolution: GL, PD
Sources of support
Internal sources
-
Minneapolis VA Healthcare, USA
Philipp Dahm received salary support from the Minneapolis VA Healthcare system.
External sources
-
Deutsche Forschungs Gemeinschaft, Germany
Philipp Maisch received salary support as a Cochrane Urology Fellow through the Deutsche Forschungs Gemeinschaft (DFG).
-
National Institutes of Health, USA
Lillian Y Lai is funded by the National Institutes of Health (NIH) National Cancer Institute (NCI) T32 T32CA180984 for separate projects not related to this review.
-
Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction and National Institutes of Health, USA
Giulia Ippolito Lane has been awarded grant funding, for separate projects, not related to this review, from the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) and from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) F32 DK126232.
Declarations of interest
LL, ST, EG, LH, PD, PM, and GL report no conflicts of interest.
New
References
References to studies included in this review
Buaban 2018 {published data only}
- Buaban K, Attawettayanon W, Sirisreetreerux P, Viseshsindh W, Kijvikai K, Kongcharoensombat W, et al.Comparison of 3-month recurrence rates after white-light versus narrow-band imaging transurethral resection for non-muscle invasive bladder cancer: a prospective, randomized control trial. Journal of the Medical Association of Thailand 2018;101(4):463-9. [Google Scholar]
Kim 2018 {published data only}
- Kim SB, Yoon SG, Tae J, Kim JY, Shim JS, Kang SG, et al.Detection and recurrence rate of transurethral resection of bladder tumors by narrow-band imaging: Prospective, randomized comparison with white light cystoscopy. Investigative and Clinical Urology 2018;59(2):98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee JY, Choi JH, Kim IK, Jung HD, Kang HW, Lee KS, et al.Recurrence rate of transurethral resection of bladder tumor using narrow band imaging: a randomized control trial, pilot study. Journal of Urology 2014;191(4):e240-1. [Google Scholar]
Longo 2013 {published data only}
- Longo F, Delor M, Mangiarotti B, Del Nero A, Montanari E.Feasibility of transurethral resection of the bladder (TURB) done by narrow-band imaging (NBI). European Urology 2013;12(1):e596. [Google Scholar]
Ma 2015 {published data only}
- Ma T, Wang W, Jiang Z, Shao G, Guo L, Li J, et al.Narrow band imaging-assisted holmium laser resection reduced the recurrence rate of non-muscle invasive bladder cancer: a prospective, randomized controlled study. National Medical Journal of China 2015;95(37):3032-5. [PubMed] [Google Scholar]
- Tianjia M, Junjia L, Zhaoqun J, Wenzhen W, Guangfeng S, Lei Z, et al.Narrow band imaging-assisted holmium laser resection reduces the residual tumor rate of primary non-muscle invasive bladder cancer; a comparison with the standard approach. National Medical Journal of China 2015;95(34):2775-8. [PubMed] [Google Scholar]
Naito 2016 {published data only}
- la Rosette J, Gravas S.A multi-center, randomized international study to compare the impact of narrow band imaging versus white light cystoscopy in the recurrence of bladder cancer. Journal of Endourology 2010;24(5):660-1. [DOI] [PubMed] [Google Scholar]
- Japan Primary Registries Network UMIN000004545.A multi-centre, international study to compare use of narrow band imaging (NBI) versus white light imaging (WLI) during TURBT to assess recurrence of bladder cancer in terms of safety and efficacy. trialsearch.who.int/?TrialID=JPRN-UMIN000004545 (first received 15 November 2010).
- Naito S, Algaba F, Babjuk M, Bryan RT, Sun YH, Valiquette L, et al.The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging-assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. European Urology 2016;70(3):506-15. [DOI] [PubMed] [Google Scholar]
- NCT01180478.Comparison study of narrow band imaging versus white light resection in patients with bladder tumors/cancer. clinicaltrials.gov/show/NCT01180478 (first received 12 August 2010).
- NTR3645.The Global Randomized NBI Bladder Cancer Study- a multi-centre, international study to compare use of narrow band imaging (NBI) versus white light (WL) during TURB to assess recurrence of bladder cancer in terms of safety and efficacy. trialregister.nl/trial/3513 (first received 10 February 2012).
Naselli 2012 {published data only}
- Introini C, Naselli A, Timossi L, Puppo P, Germinale F.A randomized prospective trial to assess impact of TUR performed in narrow band imaging modality on non muscle invasive bladder cancer recurrence. Anticancer Research 2012;32(5):1907. [DOI] [PubMed] [Google Scholar]
- Naselli A, Introini C, Bertolotto F, Spina B, Puppo P.Feasibility of transurethral resection of bladder lesion performed entirely by means of narrow-band imaging. Journal of Endourology 2010;24(7):1131-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Naselli A, Introini C, Germinale F, Bertolotto F.A randomized prospective trial to assess impact of TUR performed in narrow band imaging modality on non muscle invasive bladder cancer recurrence. European Urology Supplements 2012;11(1):e960. [DOI] [PubMed] [Google Scholar]
- Naselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, et al.A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. European Urology 2012;61(5):908-13. [DOI] [PubMed] [Google Scholar]
- NCT01004211.Prospective randomized comparison of transurethral resection by mean of white light and narrow band imaging. clinicaltrials.gov/ct2/show/NCT01004211 (first received 29 October 2009).
- Puppo P, Introini C, Germinale F, Naselli A.Is it possible to avoid second TUR in high grade bladder cancer after a first TUR performed in NBI modality? Journal of Endourology 2011;25:A130. [EMBASE: 70585038] [Google Scholar]
- Puppo P, Introini C, Germinale F, Naselli A.Randomized prospective trial to assess impact of TUR in narrow band imaging on non muscle invasive bladder cancer recurrence. Journal of Endourology 2011;25:A302. [DOI] [PubMed] [Google Scholar]
Stănescu 2014 {published data only}
- Geavlete B, Multescu R, Georgescu D, Moldoveanu C, Stanescu F, Jecu M, et al.The long term outcome of combined NBI-plasma vaporization approach in large NMIBT cases - a prospective, randomized controlled comparison to the standard management. European Urology Supplements 2014;13(5):105-6. [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Stanescu F, Jecu M, Geavlete P.Narrow band imaging cystoscopy and bipolar plasma vaporization for large nonmuscle-invasive bladder tumors-results of a prospective, randomized comparison to the standard approach. Urology 2012;79(4):846-51. [DOI] [PubMed] [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Stanescu F, Jecu M, Moldoveanu C, et al.Three-year impact of combined narrow band imaging cystoscopy and bipolar plasma vaporization in large non-muscle invasive bladder tumors' cases - a prospective, randomized comparison to the standard approach. European Urology Supplements 2013;12(4):e1229. [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Stanescu F, Jecu M, Moldoveanu C, et al.Three-year impact of combined narrow band imaging cystoscopy and bipolar plasma vaporization in large non-muscle invasive bladder tumors' cases - a prospective, randomized comparison to the standard approach. Journal of Endourology 2013;27:A151. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Geavlete P.Bipolar plasma vaporization and NBI for large non-muscle invasive bladder tumors: results of a prospective, randomized, long-term comparison to the standard approach. Urology 2011;78(3):S18-9. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Geavlete P.Bipolar plasma vaporization and NBI for large non-muscle invasive bladder tumors - results of a prospective, randomized, long term comparison to the standard approach. European Urology Supplements 2011;10(2):181. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Geavlete P.Bipolar plasma vaporization and NBI for large non-muscle invasive bladder tumors - results of a prospective, randomized, long-term comparison to the standard approach. Journal of Urology 2011;185(4):e663. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Moldoveanu C, et al.Narrow band imaging cystoscopy and bipolar plasma vaporization versus standard cystoscopy and monopolar resection in cases of large non-muscle invasive bladder tumors - a prospective, randomized, long term study. European Urology Supplements 2012;11(1):E959-U15. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Moldoveanu C, et al.Narrow band imaging cystoscopy and bipolar plasma vaporization versus standard cystoscopy and monopolar resection in cases of large non-muscle invasive bladder tumors - a prospective, randomized, long term study. Journal of Urology 2012;187(4):E673. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Moldoveanu C, et al.NBI cystoscopy and bipolar plasma vaporization versus standard cystoscopy and monopolar TURBT in cases of large non-muscle invasive bladder tumors. Journal of Endourology 2011;25:A140. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Jecu M, Georgescu D, Iacoboaie C, Geavlete P.Combined narrow band imaging cystoscopy in cases of large non-muscle invasive bladder tumors - better than the standard approach? Journal of Endourology 2012;26(Suppl 1):A92-3. [Google Scholar]
- Geavlete B, Stanescu F, Jecu M, Moldoveanu C, Multescu R, Georgescu D, et al.Combined narrow band imaging cystoscopy and bipolar plasma vaporization in cases of large non-muscle invasive bladder tumors - better than the standard approach? European Urology 2012;11(4):89. [DOI: 10.1016/S1569-9056(13)60032-9] [DOI] [PubMed] [Google Scholar]
- Geavlete B, Stanescu F, Multescu R, Georgescu D, Moldoveanu C, Jecu M, et al.Three-year impact of combined narrow band imaging cystoscopy and bipolar plasma vaporization in large non-muscle invasive bladder tumors' cases-a prospective, randomized comparison to the standard approach. Journal of Urology 2013;189(4):e523. [DOI] [PubMed] [Google Scholar]
- Geavlete BF, Multescu RD, Georgescu DA, Jecu M, Stanescu FA, Moldoveanu C, et al.Comparative therapeutic impact and long-term NMIBC recurrence rates specific for HAL blue light cystoscopy, combined NBI-bipolar plasma vaporization and the standard approach in randomized clinical settings. European Urology Supplements 2013;12(1):e570. [Google Scholar]
- Stănescu F, Geavlete B, Georgescu D, Jecu M, Moldoveanu C, Adou L, et al.NBI - plasma vaporization hybrid approach in bladder cancer endoscopic management. Journal of Medicine and Life 2014;7(2):155-9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Doehn 2014 {published data only}
- Doehn C, Krockenberger K, Zumbe J, Rassler J, Sommerauer M, Feller AC, et al.Comparison of white-light, photodynamic diagnosis and narrow-band imaging for the detection of non-muscle invasive bladder cancer: results from a randomized multicenter diagnostic phase-III study. European Urology 2014;13(1):e23. [DOI: 10.1016/S1569-9056(14)60025-7] [DOI] [Google Scholar]
Dogra 2016 {published data only}
- Dogra PN, Desai P, Singh P.Narrow band imaging cystoscopy in non-muscle invasive bladder cancer- a prospective randomized comparison to the standard white light cystoscopy. Journal of Urology 2016;195(4):e137. [Google Scholar]
Geavlete 2012 {published data only}
- Geavlete B, Jecu M, Multescu R, Geavlete P.Narrow-band imaging cystoscopy in non-muscle-invasive bladder cancer: a prospective comparison to the standard approach. Therapeutic Advances in Urology 2012;4(5):211-7. [DOI: 10.1177/1756287212454181] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hah 2018 {published data only}
- Hah YS, Lee KS, Koo KC, Chung BH.Identification of red/green/blue values from white-light imaging and narrow-band imaging for the discrimination of bladder cancer features. European Urology 2018;17(2):e1233. [DOI: 10.1016/S1569-9056(18)31704-4] [DOI] [Google Scholar]
Herr 2015 {published data only}
- Herr H.Randomized trial of narrow-band versus white-light cystoscopy for restaging (second-look) transurethral resection of bladder tumors. European Urology 2015;67(4):605-8. [DOI] [PubMed] [Google Scholar]
Herr 2019 {published data only}
- Herr H.The impact of narrow band imaging in the detection and resection of bladder tumor in transitional cell carcinoma of the bladder: a prospective, blinded, sequential intervention randomized controlled trial. Urology 2019;128:60. [DOI] [PubMed] [Google Scholar]
Hirner 2016 {published data only}
- Hirner L, Stagge E, Rübben H, Schenck M, Eisenhardt A.Narrow band imaging-assisted cystoscopy in bladder tumor follow-up: can more tumors be identified? Urologe A 2016;55(3):370-5. [DOI: 10.1007/s00120-015-3942-9] [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee KS, Koo KC, Kim DK, Lee J, Kim JW, Jeong JY, et al.Combination of RGB and narrow band imaging for discrimination of non-muscle invasive bladder cancer. Journal of Urology 2017;197(4):e92. [Google Scholar]
Mita 2018 {published data only}
- Mita K, Kobatake K, Ohara S, Kato M.Clinical benefits of transurethral resection under narrow band imaging for non-muscle invasive bladder cancer. Hinyokika Kiyo 2018;64(1):1-6. [DOI: 10.14989/ActaUrolJap_64_1_1] [DOI] [PubMed] [Google Scholar]
Mukherjee 2019 {published data only}
- Mukherjee P, George AJ, Yadav BK, Jeyaseelan L, Kumar RM, Mukha RP, et al.The impact of narrow band imaging in the detection and resection of bladder tumor in transitional cell carcinoma of the bladder: a prospective, blinded, sequential intervention randomized controlled trial. Urology 2019;128:55-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Naya 2015 {published data only}
- Naya Y, Oishi M, Yamada Y, Ueda T, Fujihara A, Nakanishi H, et al.Initial experience of combined use of photodynamic diagnosis and narrow band imaging for detection of flat urothelial lesion. International Journal of Clinical Oncology 2015;20(3):593-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shen 2010 {published data only}
- Shen Y, Zhu Y, Ye D, Yao X, Zhang S, Dai B, et al.Narrow-band imaging flexible cystoscopy in the detection of new non-muscle invasive bladder cancer: a randomized study. Journal of Urology 2010;183(4):e450. [Google Scholar]
Shen 2012 {published data only}
- Shen YJ, Zhu YP, Ye DW, Yao XD, Zhang SL, Dai B, et al.Narrow-band imaging flexible cystoscopy in the detection of primary non-muscle invasive bladder cancer: a "second look" matters? International Urology and Nephrology 2012;44(2):451-7. [DOI: 10.1007/s11255-011-0036-5] [DOI] [PubMed] [Google Scholar]
Tschirdewahn 2020 {published data only}
- Tschirdewahn S, Harke NN, Hirner L, Stagge E, Hadaschik B, Eisenhardt A.Narrow-band imaging assisted cystoscopy in the follow-up of patients with transitional cell carcinoma of the bladder: a randomized study in comparison with white light cystoscopy. World Journal of Urology 2020;38(6):1509-15. [DOI: 10.1007/s00345-019-02926-0] [DOI] [PubMed] [Google Scholar]
Ye 2015 {published data only}
- Ye Z, Hu J, Song X, Li F, Zhao X, Chen S, et al.A comparison of NBI and WLI cystoscopy in detecting non-muscle-invasive bladder cancer: a prospective, randomized and multi-center study. Scientific Reports 2015;5:10905. [DOI: 10.1038/srep10905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aldousari 2010
- Aldousari S, Kassouf W.Update on the management of non-muscle invasive bladder cancer. Canadian Urological Association Journal 2010;4(1):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babjuk 2019
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394‐424. [DOI: 10.3322/caac.21492] [DOI] [PubMed] [Google Scholar]
Chang 2016
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al.Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Joint Guideline.. Journal of Urology 2016;196:1021-9. [DOI] [PubMed] [Google Scholar]
Chen 2017
- Chen C, Feng S, Hao L, Thomas L, Huang J, Lin T.Comparative efficacy of new technology-assisted transurethral resection of patients with non-muscle invasive bladder cancer: systematic review and Bayesian network meta-analysis. International Journal of Urology 2017;24:33. [DOI: 10.1111/iju.13413] [DOI] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al .The Clavien-Dindo classification of surgical complications: five-year experience. Annals of Surgery 2009;250(2):187-96. [DOI: 10.1097/SLA.0b013e3181b13ca2] [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Freedman 2011
- Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC.Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306(7):737-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goh 2009
- Goh AC, Lerner SP.Application of new technology in bladder cancer diagnosis and treatment. World Journal of Urology 2009;27(3):301-7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University GRADEpro GDT.Version accessed 15 September 2020. Hamilton: McMaster University, 2020. Available from www.gradepro.org.
Gravestock 2021
- Gravestock P, Coulthard N, Veeratterapillay R, Heer R.Systematic review and meta-analysis of narrow band imaging for non-muscle-invasive bladder cancer. International Journal of Urology 2021;28:1212-7. [DOI: 10.1111/iju.14671] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al.GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Li T, Deeks JJ, editor(s).Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Humphrey 2016
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE.The 2016 WHO classification of tumours of the urinary system and male genital organs – Part B. Prostate and bladder tumours. European Urology 2016;70(1):106-19. [DOI: 10.1016/j.eururo.2016.02.028] [DOI] [PubMed] [Google Scholar]
Kang 2017
- Kang W, Cui Z, Chen Q, Zhang D, Zhang H, Jin X.Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: a systematic review and meta-analysis. Oncotarget 2017;8(14):23880-90. [DOI: 10.18632/oncotarget.13054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamm 2014
- Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al.Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. Journal of Urology 2014;191(1):20-7. [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee JY, Cho KS, Kang DH, Jung HD, Kwon JK, Oh CK, et al.A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection in patients with non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence versus hexylaminolevulinate fluorescence versus narrow band imaging. BMC Cancer 2015;15:1-10. [DOI: 10.1186/s12885-015-1571-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020
- Li T, Higgins JP, Deeks JJ, editor(s).Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maisch 2021
- Maisch P, Koziarz A, Vajgrt J, Narayan V, Kim MH, Damn P.Blue versus white light for transurethral resection of non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD013776. [DOI: 10.1002/14651858.CD013776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motlagh 2021
- Motlagh R, Mori K, Laukhtina E, Aydh A, Katayama S, Grossmann N, et al.Impact of enhanced optical techniques at time of transurethral resection of bladder tumour, with or without single immediate intravesical chemotherapy, on recurrence rate of non-muscle-invasive bladder cancer: a systematic review and network meta-analysis of randomized trials. British Journal of Urology International 2021;128(3):280-9. [DOI: 10.1111/bju.15383] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naselli 2009
- Naselli A, Introini C, Bertolotto F, Spina B, Puppo P.Narrow band imaging for detecting residual/recurrent cancerous tissue during second transurethral resection of newly diagnosed non-muscle-invasive high-grade bladder cancer. British Journal of Urology International 2009;105(2):208-11. [DOI] [PubMed] [Google Scholar]
NICE 2015
- NICE. Bladder cancer: diagnosis and management NICE guideline [NG2]. Published date: 25 February 2015; Updated April 2019. Available from www.nice.org.uk (accessed 15 September 2020).
Puffer 2005
- Puffer S, Torgerson DJ, Watson J.Cluster randomized controlled trials. Journal of Evaluation in Clinical Practice 2005;11(5):479-83. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.4. The Cochrane Collaboration, 2020.
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
SEER 2020
- Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute.Cancer Stat Facts: Bladder Cancer. seer.cancer.gov/statfacts/html/urinb.html (accessed 15 September 2020).
Srivastava 2019
- Srivastava R.History and physics of narrow band imaging. In: Atlas on Narrow Band Imaging in Upper Aerodigestive Tract Lesions. Singapore: Springer, 2019:7-10. [Google Scholar]
Sun 2015
- Sun M, Trinh QD.Diagnosis and staging of bladder cancer. Hematology/oncology Clinics of North America 2015;29(2):205-18. [DOI] [PubMed] [Google Scholar]
Sylvester 2006
- Sylvester RJ, Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al.Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49(3):466-77. [DOI: 10.1016/j.eururo.2005.12.031] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR .Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16):1-16. [DOI: 10.1186/1745-6215-8-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al.Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI: 10.1136/bmj.39465.451748.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiong 2017
- Xiong Y, Li J, Ma S, Ge J, Zhou L, Li D, et al.A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLoS ONE 2017;12(2):e0170819. [DOI: 10.1371/journal.pone.0170819] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lai 2021
- Lai LY, Tafuri SM, Ginier EC, Herrel LA, Dahm P, Maisch P, et al.Narrow band imaging versus white light cystoscopy alone for transurethral resection of non‐muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014887. [DOI: 10.1002/14651858.CD014887] [DOI] [PMC free article] [PubMed] [Google Scholar]


