Abstract
Implanted medical devices such as central venous catheters are highly susceptible to microbial colonization and biofilm formation, and are a major risk factor for nosocomial infections. The opportunistic pathogen Pseudomonas aeruginosa uses exopolysaccharides, such as Psl, for both initial surface attachment and biofilm formation. We have previously shown that chemically immobilizing the Psl-specific glycoside hydrolase, PslGh, to a material surface can inhibit P. aeruginosa biofilm formation. Herein we show that PslGh can be uniformly immobilized on the lumen surface of medical-grade, commercial polyethylene, polyurethane, and polydimethylsiloxane (silicone) catheter tubing. We confirmed the surface-bound PslGh was uniformly distributed along the catheter length and remained active even after storage for 30 days at 4 °C. P. aeruginosa colonization and biofilm formation under dynamic flow culture conditions in vitro showed a 3-log reduction in the number of bacteria during the first 11 days, and a 2-log reduction by day 14 for PslGh modified PE-100 catheters, compared to untreated catheter controls. In an in vivo rat infection model, PslGh-modified PE-100 catheters showed ~1.5-log reduction in the colonization of the clinical P. aeruginosa ATCC 27853 strain after 24 hours. These results demonstrate the robust ability of surface-bound glycoside hydrolase enzymes to inhibit biofilm formation, and their potential to reduce rates of device-associated infections.
Keywords: catheters, biomaterials, bacterial biofilms, medical device infection, Pseudomonas aeruginosa, enzyme immobilization, glycoside hydrolases, PslGh
Graphical Abstract
1. Introduction
Biofilms formed on implanted medical devices are responsible for 50–70% of all hospital acquired infections, and can be tremendously difficult to treat 1–4. Biofilms are surface-attached microbial colonies embedded within a self-produced extracellular matrix, composed of exopolysaccharide, extracellular DNA and proteins 5. Annually, there are an estimated 14 million biofilm infections in the US, causing at least 350,000 deaths 4. The vast majority of these infections (86–95%) are associated with microbial colonization of catheters e.g., central-line associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), and ventilator-associated pneumonia (VAP) 6–7. The annual cost of the top five hospital-acquired infections is ~$9.8 billion in the US alone 8. Indwelling central venous catheters (CVC) are routinely used to administer medications, blood products, and nutritional fluids to patients, for both temporary and long-term use9. The clean surfaces of newly-implanted catheters are highly susceptible to microbial colonization 10–13. The exopolysaccharide component of the matrix impairs antibiotic penetration 14–15 and provides a barrier against phagocytosis by host immune cells 16. Within biofilms, microbes are up to 1000 times more tolerant to antimicrobials than planktonic cells 1, 10, 17–18. As a result, device-related biofilm infections are difficult to treat using systemic antibiotics 19, and often require device extraction and replacement.
Medical biofilms can involve a wide variety of organisms, but the bacterium Pseudomonas aeruginosa accounts for ~28% of device-related infections 20–23. P. aeruginosa is an ubiquitous, Gram-negative, opportunistic pathogen associated with a wide array of life-threatening acute and chronic nosocomial infections, particularly in patients with compromised host-defense mechanisms 24. P. aeruginosa infections are becoming more challenging to treat due to the emergence of multidrug resistant strains 14–15, 25–28. P. aeruginosa produces three distinct biofilm exopolysaccharides: Psl, Pel, and alginate 29–31. The cationic Pel and neutral Psl polysaccharides are produced in varying amounts in different strains 32, while the anionic alginate is synthesized exclusively by mucoid strains in the lungs of cystic fibrosis patients 29–30. Both Pel and Psl play a significant role in the formation and maintenance of biofilm structure 14, 33, and recent work has shown the role that polysaccharides (such as Psl) play to enable the cell-cell and cell-surface adhesion necessary for biofilm formation 14, 16, 32, 34–35.
Antimicrobial coatings for medical devices often incorporate silver, chlorhexidine, or triclosan for diffusive release 13, 36–38. These coatings are often ineffective over time as they do not directly prevent microbial attachment, and new cells eventually accumulate on dead cells 39. Diffusional release is also limited by a number of factors, including: (i) slow diffusion rates to the material surface; (ii) decreased activity over time as the antimicrobial concentration decreases and bacterial biomass accumulates 40; and (iii) antimicrobial tolerance 41–42. These limitations have led to an alternative trend to develop non-adhesive, non-fouling surfaces that target the early stages of biofilm development. These materials are designed to keep bacteria in a planktonic (swimming) state 43–45 and thus more susceptible to antibiotics and the host immune system 10. Non-fouling, ‘bio-passive’ polymer brush surfaces, such as hydrophilic polyethylene glycol (PEG) are effective, but tend to fail after a period of days 46–47. Slippery liquid infused porous surfaces (SLIPS) show highly-effective, long term anti-fouling properties but clinical performance is still unclear 48.
Recently, we developed novel ‘bioactive’ biomaterial surfaces by covalently immobilizing the glycoside hydrolase, PslGh. In solution, this enzyme has been shown to disrupt P. aeruginosa biofilms in vitro 49–50. We immobilized PslGh to flat squares of glass, polydimethylsiloxane (PDMS) and polystyrene (PS), and demonstrated that the surfaces prevented adsorption by hydrolyzing Psl 51. The PslGh-bound surfaces reduced P. aeruginosa biofilms by 3–4 log units (8 d in static culture), compared to controls. However, to be suitable for clinical application, it is important to uniformly immobilize PslGh along the length of the (inner) lumen surface of medical-grade catheters, and demonstrate long term activity to prevent P. aeruginosa biofilm growth. Herein, we show that PslGh surface-modified catheters of polyethylene (PE), PDMS and polyurethane (PU) not only exhibit significantly reduced rates of biofilm formation under flow culture in vitro but also in vivo using a rat catheter infection model.
2. Materials and Methods
2.1. Materials
Aminopropyl trimethoxysilane (APTMS), glutaraldehyde (GDA), ethanol (HPLC grade), Fetal bovine serum (FBS) and all other reagents were of high analytical grade and were purchased from Sigma Aldrich (Mississauga, ON). Commercial polyethylene (PE), polyurethane (PUR) and polydimethylsiloxane silicone (PDMS) tubing with identical dimensions (0.3 cm ID and 0.6 cm OD) from McMaster-Carr (Ohio, USA), and medical grade polyethylene (PE-100) catheters (0.8 mm, ID) (Intramedic, Becton Dickinson and Company, NJ) were used as biomaterial surfaces.
2.2. Strains, media and growth conditions
Strains used in this study are P. aeruginosa PAO1 (wild-type strain; serotype O5) and P. aeruginosa ATCC 27853 (clinical strain). Lysogeny broth (LB) and agar were used for standard culture conditions. To prepare a pre-culture of tested strains, a single colony of freshly grown bacterial cells grown on LB agar overnight at 37 °C was inoculated in 5 mL LB and incubated at 37 °C overnight with agitation. Bacterial biofilms were grown in LB without salt (LBNS). Serum treatment was performed by coating the catheter segment with 100 μL of FBS then incubating without shaking at room temperature for 30 min.
2.3. Enzyme purification
The hydrolase domain of PslGh was cloned into an expression plasmid and purified from E. coli as previously described 50, 52. Briefly, the enzyme was recombinantly expressed in E. coli cells and grown in autoinduction media overnight at 37 °C with 50 μg/mL kanamycin. The protein was purified by Ni-NTA affinity chromatography using an N-terminal His6-tag present on the enzyme followed by size exclusion chromatography (HiLoad 16/600 Superdex 200 pg, GE Healthcare). The protein was eluted in 20 mM Tris (pH 7.5), 150 mM NaCl, and 2% (vol/vol) glycerol and stored at −80 °C until required. The His6-tag was left intact for all experiments.
2.4. Surface modification and enzyme immobilization
Enzyme immobilization by covalent binding for the activated surfaces was performed as previously described 51. We took advantage of surface hydroxyl or carboxylic groups as reactive sites for the APTMS, creating primary amines to be covalently linked to those of the PslGh by GDA (Figure 1A). All polymer sheets and tubing (PE, PU and PDMS) and medical grade PE catheters (PE-100) were sonicated in absolute ethanol for 15 min then oven dried. Dried samples were exposed to atmospheric plasma (Harrick Plasma PDC001) at high power for up to 10 min. To ensure an even hydroxylation of the lumen surface, sections were flipped end to end after 5 min then exposed to plasma for additional 5 min. Samples were then functionalized with amine (NH2) groups by immersion in APTMS (Sigma-Aldrich, 97%) solution (50 mg/mL in 80% (v/v) ethanol) for 2 h with shaking at 25 °C according to a published protocol 53. Tubing segments were checked for any air bubbles that may prevent the APTMS contact with the lumen. After silanization, the surfaces were washed three times with 80% (v/v) ethanol to remove unreacted APTMS. The amino-functionalized surfaces were then immersed in 2% (v/v) GDA in 1×PBS buffer solution (pH 7.2) for 2 h under gentle stirring at 25 °C. The surfaces were rinsed 3 times with sterile 1× PBS to remove unreacted GDA. Functionalized (NH2-GDA) surfaces were immersed in PslGh solution (80 μg/mL in 1x PBS buffer) and incubated overnight at 4 °C then washed several times with 1× PBS buffer to remove the unbound enzyme. The concentration of immobilized enzyme was determined from the difference between the concentration of PslGh in solution prior to immobilization minus the amount of protein that remained in the solution after the immobilization and washings steps. The enzyme concentration before and after immobilization and in washing buffers was measured using the Bradford protein assay (B6916, Sigma) with bovine serum albumin as a standard.
Figure 1.
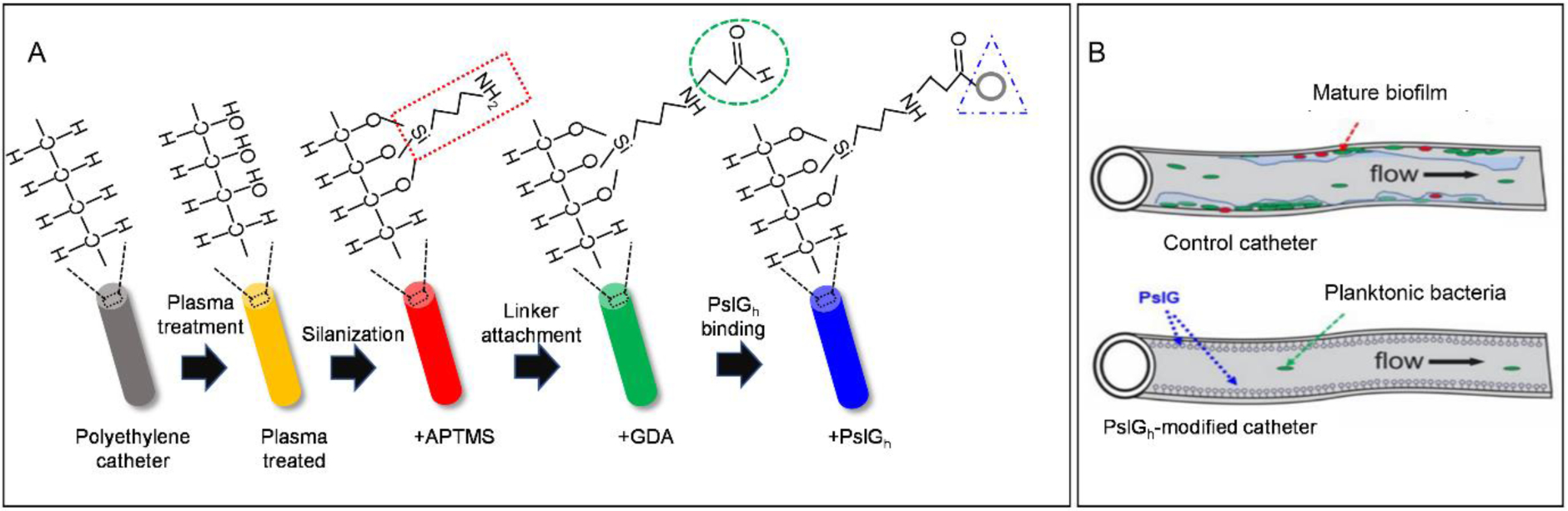
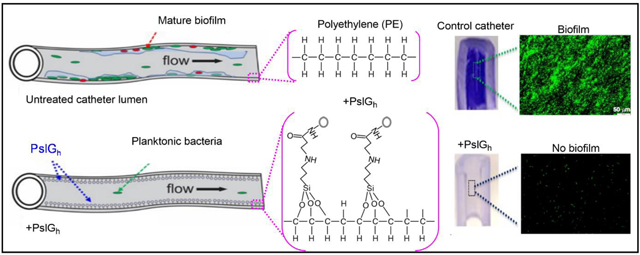
(A) Schematic illustration of the sequence of steps involved in enzyme immobilization: surface activation by plasma treatment; amine (NH2) functionalization with 3-aminopropyltrimethoxysilane (APTMS) via silanization; activation of the catheter lumen surface using the bifunctional agent (GDA) under the formation of Schiff base and finally coupling of the enzyme. (B) Chemical immobilization of PslGh prevents P. aeruginosa biofilm formation on the luminal surface of catheter tubing. Schematic representation demonstrating cell attachment and biofilm formation on untreated catheters (top) and inhibition of biofilm formation when the catheter is treated with PslGh (bottom).
2.5. Physical and chemical surface characterization
Surface wettability measurements and attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer Spectrum 100) were used to monitor each step of the process including the surface activation (hydroxylation), silanization (APTMS), cross linking (GDA), and enzyme covalent binding (correlated with the spectra of native components). After a background scan, spectra were collected from 4000 to 625 cm−1 using 64 scans at 4 cm−1 resolution. The reflection intensities were corrected to compensate for their frequency (wavenumber) dependence (i.e., neglect the effect of frequency). Surface wettability of ‘wide’ tubing segments was assessed by measuring the static water contact angle using the sessile drop method. Tubing segments were cut longitudinally into two halves and a 1 μL drop of dH2O was placed on the lumen surface and imaged. The angle between the horizontal plane and the tangent to the drop at the point of contact with the surface was analyzed by ImageJ 54 using the contact angle plugin 55. For thin PE-100 catheters, the lumen surface wettability was evaluated by lowering the catheters into identical vials half filled with distilled water and measuring the water level change inside the lumen. To evaluate the surface chemistry of the lumen, tubing was cut longitudinally into small, low curvature segments and the interior surface (lumen) was pressed firmly against the face of the ATR crystal to improve surface contact and obtain high quality absorbance/transmission signals.
2.6. Antibiofilm activity under static culture conditions
PAO1 cells were grown in LB medium at 37 °C overnight with shaking at 200 rpm. Cell cultures were normalized to an OD600 nm of 0.5 and diluted in LBNS (1:100). 5 mL of the diluted culture was added to sterile 6-well polystyrene microplates (Thermo Scientific cat. no. 243656) containing the tested catheters segments (2.5 cm). The culture plates were incubated for 24 h at 25 °C. After incubation, catheters were removed, drained, and rinsed 3x with 10 ml of sterile 1× PBS buffer (pH 7.2) to remove non-adherent cells and media. Under strict sterile conditions, the catheters were cut into 1 cm segments and transferred to separate sterile vials for Sytox Green, crystal violet (CV) staining and colony forming units (CFU) counts, respectively.
2.7. Antibiofilm activity in vitro under dynamic flow conditions
P. aeruginosa PA01 biofilms were grown in a lab-made ‘two catheters’ device, in which the sterile untreated (control) and PslGh bound PE-100 (10 cm) were installed together with one inlet and one outlet device (Figure S1). P. aeruginosa PAO1 was grown in LB Broth overnight, with shaking at 37 °C. The culture was then diluted 1:100 into 150 mL of fresh LBNS Broth in a 250 mL Erlenmeyer flask and stirred. The device inlet was connected to the inoculated media, and the outlet was connected to a tube mounted into a peristaltic pump (Cole Parmer) head and into a waste media bottle. The experiment began once the inoculated medium started flowing from the end of PE-100 catheters, and the flow was stopped to allow for cell attachment and biofilm growth under static conditions. After 24 h, under aseptic conditions the Erlenmeyer flask was disconnected and 20 L of diluted (1/10) and sterilized LBNS media in a container was connected to the device and media flow (0.4 mL/min) was started. LBNS media was pumped through the reactor from the top, and the waste was dispensed from the side to ensure removal of all free, unattached cells and allow the attached cells to form biofilms under flow conditions for 14 d. A filtered port was used to provide air to the bacteria. A 1 cm section of each tubing was taken daily under sterile conditions and used in a crystal violet (CV) assay, and for SYTOX Green and CFU measurements. For PE catheters, multiple 10 cm sections of the control and PslGh-bound were connected between two multichannel devices. In this case, after long-term continuous flow (~14 d), a whole catheter was disassembled and cut into sections under sterile conditions for biofilm analysis (CV, fluorescence microcopy and CFU).
2.8. Biofilm inhibition in vitro assay
To examine biofilm inhibition using a microtiter plate assay, P. aeruginosa strain ATCC27853 was grown at 37 °C overnight with shaking at 200 rpm. The cultures were normalized to an OD600 of 0.5 and then diluted 1:100 in LBNS. Diluted culture was added to sterile 96-well polystyrene microtiter plates (Thermo Scientific cat. no. 243656), and varying concentrations of PslGh (0.1 to 10 μM) were added. The cultures were incubated statically for 24 h at 37 °C to allow for biofilm formation. To eliminate edge effects, ~200 μL of sterile water was placed in all outside wells, and the plate was sealed with parafilm. After incubation, nonadherent cells and media were removed by thoroughly washing the plate with deionized water. The wells were stained with 150 μL of 0.1% (w/v) crystal violet for 10 min and then rinsed with water. The remaining dye was solubilized by addition of 150 μL of 95% (v/v) ethanol and left for 10 min, after which the absorbance was measured at 595 nm using a SpectraMax M2 spectrophotometer (Molecular Devices). The amount of biofilm was proportional to the absorbance from staining with crystal violet. Experiments were performed in triplicate and all statistical analysis were performed using GraphPad Prism.
2.9. Antibiofilm activity in vivo
P. aeruginosa ATTC 27853 biofilm formation on coated catheters in vivo was evaluated using a rat venous catheter model previously developed to study Candida albicans biofilm formation 63. Pathogen-free female Sprague-Dawley rats weighing 400 g (Harlan Sprague-Dawley, Indianapolis, Ind.) were used. Animals were maintained in accordance with the American Association for Accreditation of Laboratory Care criteria, and all studies were approved by the institutional animal care committee. Briefly, 24 h after implantation, the jugular venous catheters (PE-100) were inoculated (107 cells/mL) and allowed to dwell for 6 h before washing excess inoculum. Rats were sacrificed after 24 h and the catheters collected for bacterial enumeration or imaging by scanning election microscopy (SEM)56.
2.10. Cell viability assay
To quantify viable bacteria as colony forming per units (CFU), catheters segments were immersed in 10 mL of 1× PBS, sonicated at low power for 5 min and vortexed for 15 s to release viable cells. Aliquots were diluted 102-to108-fold, and 100 μL of each dilution was plated in duplicate on LB plates. The number of colonies was counted after incubation at 37 °C for 24 h.
2.11. Fluorescence imaging of attached bacteria and biofilm
After catheter segments were rinsed with 1×PBS at pH 7.2, the adherent bacteria were fixed by 2.5% (v/v) glutaraldehyde (GDA) in 1×PBS solution for at least 1 h, then rinsed 3 times with 1×PBS to remove unreacted GDA. After fixation, samples were treated with Tween-20 (0.05% (v/v) in PBS buffer) for 10 min, rinsed with sterile PBS, and stained in the dark with SYTOX green (Life Technologies, CA, USA) for 30 min 37, 56, 62. Samples were imaged by fluorescence microscopy (Olympus BX63, Tokyo, Japan) using a 20X dry objective. The green fluorescence of the biofilm was observed through a GFP filter (λex/λem 395/470 nm). The fluorescence densities of biofilms were quantified using computerized image analysis with Olympus CellSens micro imaging software. Each data point represents the average of six images. Error bars represent standard deviation from the mean (n = 6).
2.12. Crystal violet staining of biofilms
Crystal Violet (CV) staining was used to visualize the biofilm biomass on catheters. Briefly, after the catheters were rinsed with 1× PBS buffer and air dried for 1 h at 25 °C, they were stained with 300 μL of 0.5% (w/v) CV for 30 min, then rinsed with dH2O. CV-stained catheters segments were air dried and photographed.
2.13. Scanning electron microscopy
Catheters segments were rinsed with 1× PBS buffer and placed in fixative (1% (v/v) GDA and 4% (v/v) formaldehyde) overnight. The samples were rinsed in 1× PBS buffer two times and then placed in 1% (v/v) osmium tetroxide for 30 min, followed by immersion in hexamethyldislilazane (Polysciences, Inc., Warrington, Pa.). The samples were subsequently dehydrated in a series of ethanol washes (30% (v/v) for 10 min, 50% for 10 min, 70% for 10 min, 95% for 10 min, and 100% for 10 min). Final desiccation was accomplished by critical-point drying (Tousimis, Rockville, MD). Specimens were mounted on aluminum stubs and sputter coated with gold. Samples were observed in a scanning electron microscope (Hitachi S-5700) in the high-vacuum mode at 10 kV. The images were processed for display by using Adobe Photoshop version 5.0.
3. Results
3.1. Immobilization of PslGh on polymer tubing by covalent binding
In our previous study, we covalently immobilized PslGh to flat glass, PDMS and PS squares, to demonstrate that a continuous enzymatic degradation of Psl disrupted P. aeruginosa attachment and biofilm formation51. Extending this approach to medically relevant devices, we first tested conditions for uniform immobilization of PslGh to the lumen surface of catheters of reasonable length (>10–20cm), where physical access is difficult. The materials of commercial catheters, PE, PDMS and PU, are useful for their chemical inertness (in addition to low friction coefficient, strength, and sterilizability), but also make them difficult to chemically functionalize.
For PslGh immobilization, we used a 4-step process: surface hydroxylation by plasma oxidation; amine-functionalization by APTMS; GDA cross-linking and PslGh covalent binding (Figure 1A)51. Surface hydroxylation is the key step in PslGh immobilization to generate active sites (e.g. OH and COOH) for APTMS57. The plasma treatment time to hydroxylate PE surface was tested first for simple PE sheets (1cm2), and their surface wettability was evaluated by water contact angle. Treating PE sheets with 5 min plasma exposure significantly reduced the contact angle from 92° ±2 (hydrophobic) to 21° ±1 (hydrophilic) (Figure S2A), indicating an increase in surface polar groups. ATR-FTIR confirmed a weak O-H broad band at ~3400 cm−1 (Figure S2B). The low intensity of the O-H signal is due the thin surface layer and the limited sensitivity of ATR-FTIR 57. For PDMS and PU sheets, atmospheric plasma treatment for 5 min also caused a reduction in contact angle from 108° ±4 and 69° ±2 to 21° ±3 and 20° ±1, respectively.
Tubing segments (0.5 cm, ID) of PE, PDMS and PU were then treated with plasma for 5 min, silanized with APTMS and the PslGh cross-linked with GDA. The success of chemical immobilization along tubing length was confirmed by ATR-FTIR after each step (Figure 2A–C). ATR-FTIR spectrum of the lumen surface of untreated (control) PE tubing (Figure 2A) showed three ethylene bands at 3000–2800 cm−1 (CH2 asymmetric and symmetric stretching), 1500–1400 cm1 (1473 and 1463 cm−1 bending and wagging deformation at 1366) and 730–720 cm−1 (rocking). After plasma treatment, we observed a small O-H broad band centered at ~3400 cm−1 of the hydroxyl (OH) groups56. Addition of APTMS shows FTIR spectra with peaks belonging to C-H (~2900 cm−1) and N-H (~3300 cm−1) stretching vibrations, and peaks corresponding to hydroxyl groups (~3400 cm−1) and C=O (~1635 cm−1) stretching vibrations after the addition of GDA. After incubating with PslGh, the spectrum showed a signal at 1635 cm−1 associated with the carbonyl (C=O) vibrations of an amide I band, and a broad band of O-H and N-H stretching vibrations centered at 3343 cm−1. These signals were similar to those obtained from the IR analysis of pure PslGh in buffer solution 51. For PDMS and PU tubing, similar modifications in the surface chemistry were detected by ATR-FTIR (Figure 2B and C) confirming the attachment of PslGh to these two surfaces. Collectively, these surface characterizations confirm successful covalent immobilization of PslGh to the lumen surface of PE, PDMS and PU tubing.
Figure 2.
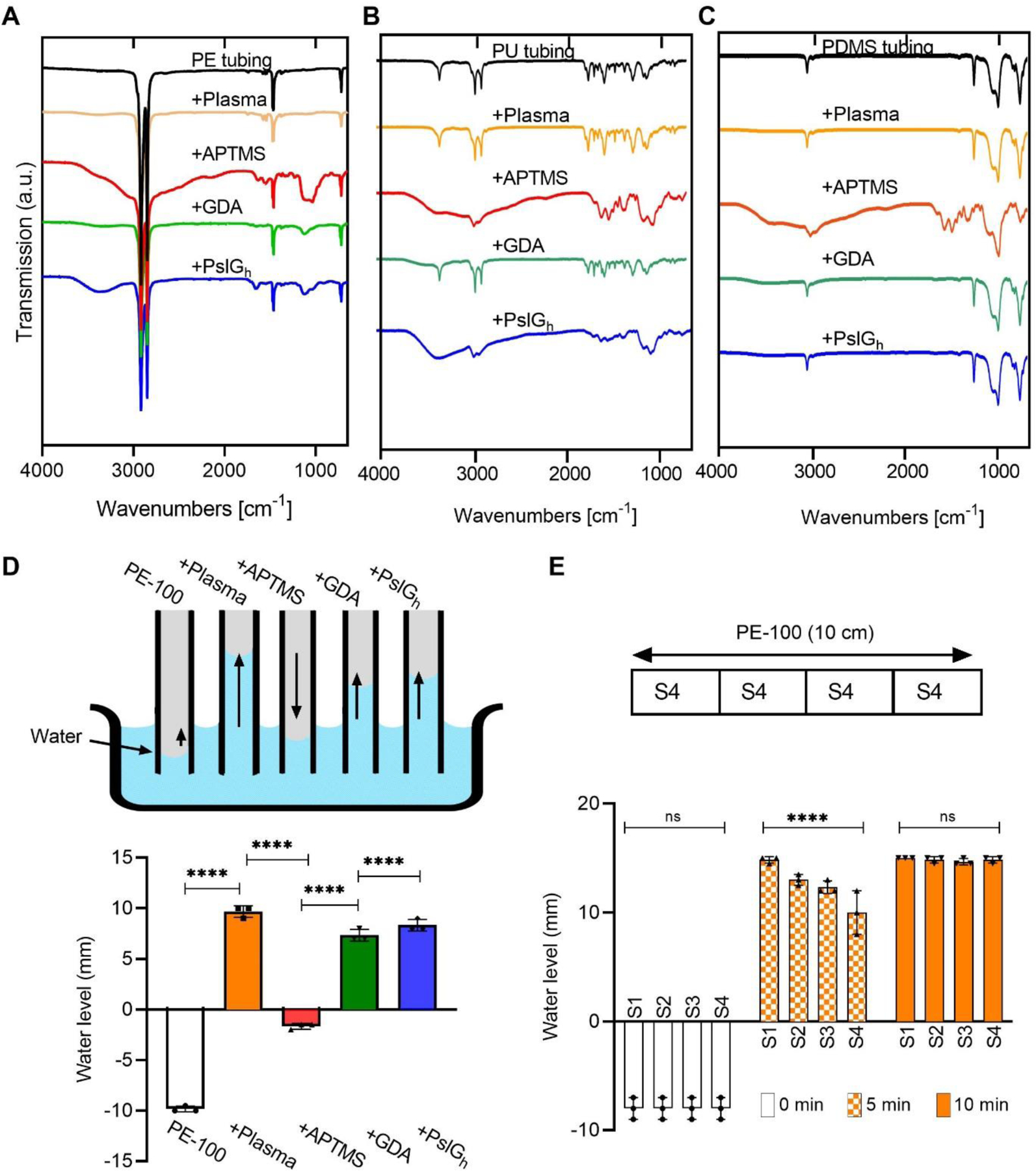
Chemical and physical characterization of the lumen of commercial polymer tubing and a medical grade catheter. (A-C) ATR-FTIR spectra showing the surface chemical groups lining the PE, PU and PDMS tubing lumen during immobilization; (D) Variation in the relative water level inside PE tubing after each immobilization step. A rise in the water level within catheter lumen is indicative of hydrophilic character; and (E) Relative displacement of water inside 4 lumen segments (S1-S4) of a 10cm long PE-100 catheter after treatment with atmospheric plasma for 10 min. ****P ≤ 0.0001. NS, no significant difference.
3.2. Immobilization of PslGh on PE-100 microcatheters
After PslGh immobilization on wide tubing (0.5 cm, ID), we next tested short segments (2.5 cm) of medical grade PE-100 microcatheter (0.8 mm, ID). As shown in Figure 2D, we could measure changes in lumen hydrophobicity by the relative capillary rise of water due to capillary action 58. The capillary height for the control PE-100 catheters was ~ 1 cm below the water level (Figure 2D) due to its hydrophobic surface. Plasma treatment increased the water level by ~ 2 cm, showing a hydrophilic character. Adding APTMS, the water level decreased below the surface level due to the increased number of hydrophobic C-H and N-H groups on the surface (Figure 2D), while GDA increased the capillary rise again due to the hydrophilic hydroxyl (O-H) and carbonyl (C=O) groups. After PslGh immobilization, the lumen surface maintained its hydrophilic character.
The average length of a CVC catheter insertion is 11–14 cm 9, so for the in vitro and in vivo-testing we prepared 14 cm lengths of PE-100 medical grade tubing with PslGh. As the lumen of a longer catheter is not directly exposed inside the plasma treatment chamber, the efficiency of hydroxylation may be affected. To test the uniformity along the tubing length, the catheters were cut into 4 equal segments after each immobilization step, and their hydrophobicity and surface chemistry measured by capillary rise and ATR-FTIR, respectively. We found that plasma treatments of < 10 min resulted in variations in the level of water reached within each segment (S1-S4) of the lumen, but exposures of 10 min or more resulted in uniform hydroxylation with even capillary rise for all 4 segments (Figure 2E). Importantly, identical water level (indicative of hydrophilicity) (Figure S3A) and ATR-FTIR spectra (indicative of surface chemistry) (Figure S3B) were also observed for all 4 sections after PslGh immobilization, suggesting that PslGh was also evenly immobilized along the entire length of these PE-100 catheters.
3.3. PslGh modified polymer tubing inhibit biofilm formation
We first tested the resistance of covalently-bound PslGh on PE, PDMS, PU tubing to P. aeruginosa PAO1 biofilm formation under static culture conditions. Fluorescence microscopy using SYTOX green was used to visualize attached bacterial cells and biofilm formation after 24 h growth. While dense biofilms were observed on the three untreated controls, those with bound PslGh showed significantly lower cell attachment and colony formation (Figure 3A). Image analysis (cell count/cm2) revealed that the bound PslGh significantly reduced the number of attached cells by at least 2-log (Figure 3B). These results confirm the uniformity of bound PslGh along the lumen length of PU and PDMS and that the surfaces can effectively inhibit the adhesion, colonization and subsequent biofilm formation of P. aeruginosa PAO1.
Figure 3.
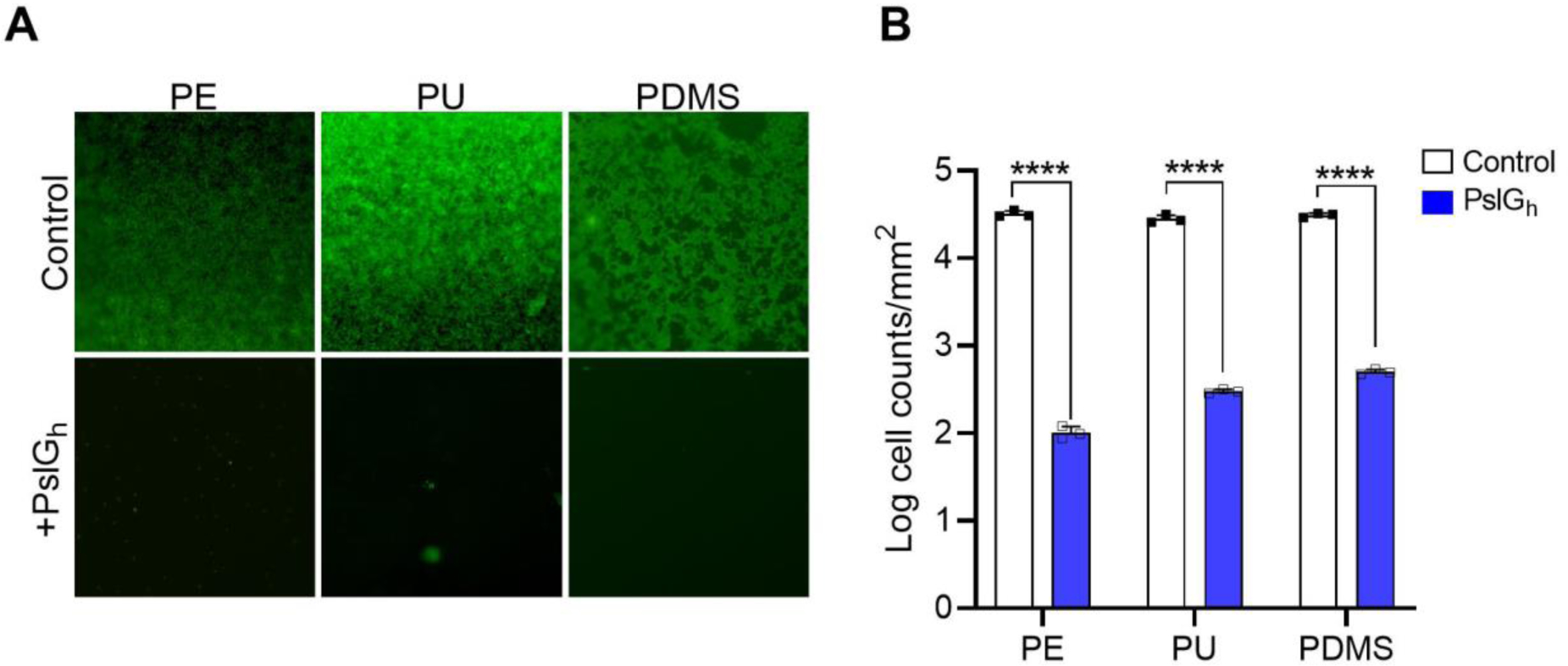
Bound PslGh prevents P. aeruginosa biofilm formation on the luminal surface of polymer tubing. (A) Florescence images showing the inhibition of biofilm formation by bound PslGh on the luminal surface of PE, PU and PDMS tubing, but not for the untreated control. (B) Corresponding cell counts (per cm2) calculated from the image analysis of surfaces in (A). ****P ≤ 0.0001.
3.4. PslGh modified PE-100 microcatheters inhibit biofilm formation
Previously, we found that the enzymatic activity of covalently-bound PslGh on glass surfaces was dependent on the enzyme surface density51. To maximize the immobilized PslGh surface density on the lumen surface of PE-100 microcatheters, we varied the PslGh concentration (0, 20, 40, 80 and 160 μg/ml) in the immobilization buffer. The surface density of bound PslGh was determined from the difference between its concentration before and after immobilization using the Bradford protein assay 51. The amount of surface-bound PslGh increased exponentially with increasing PslGh concentration in the buffer up to a PslGh concentration of 80 μg/ml, at which point the maximum immobilization capacity is likely reached (Figure 4A). The maximum PslGh surface density was estimated to be about 1.86 μg/cm2. Fluorescence microscopy images showed a thick biofilm developed on the lumen surface of short (2.5 cm) untreated PE-100 microcatheter after exposure to P. aeruginosa PAO1 under static culture condition for 24 h (Figure 4A). However, a moderate reduction in the biofilm growth was observed even at a low density (1.18 μg/cm2) of surface-bound PslGh, and complete inhibition of bacterial attachment and biofilm formation was observed at a PslGh surface density of 1.78 μg/cm2 and above (Figure 4A).
Figure 4.
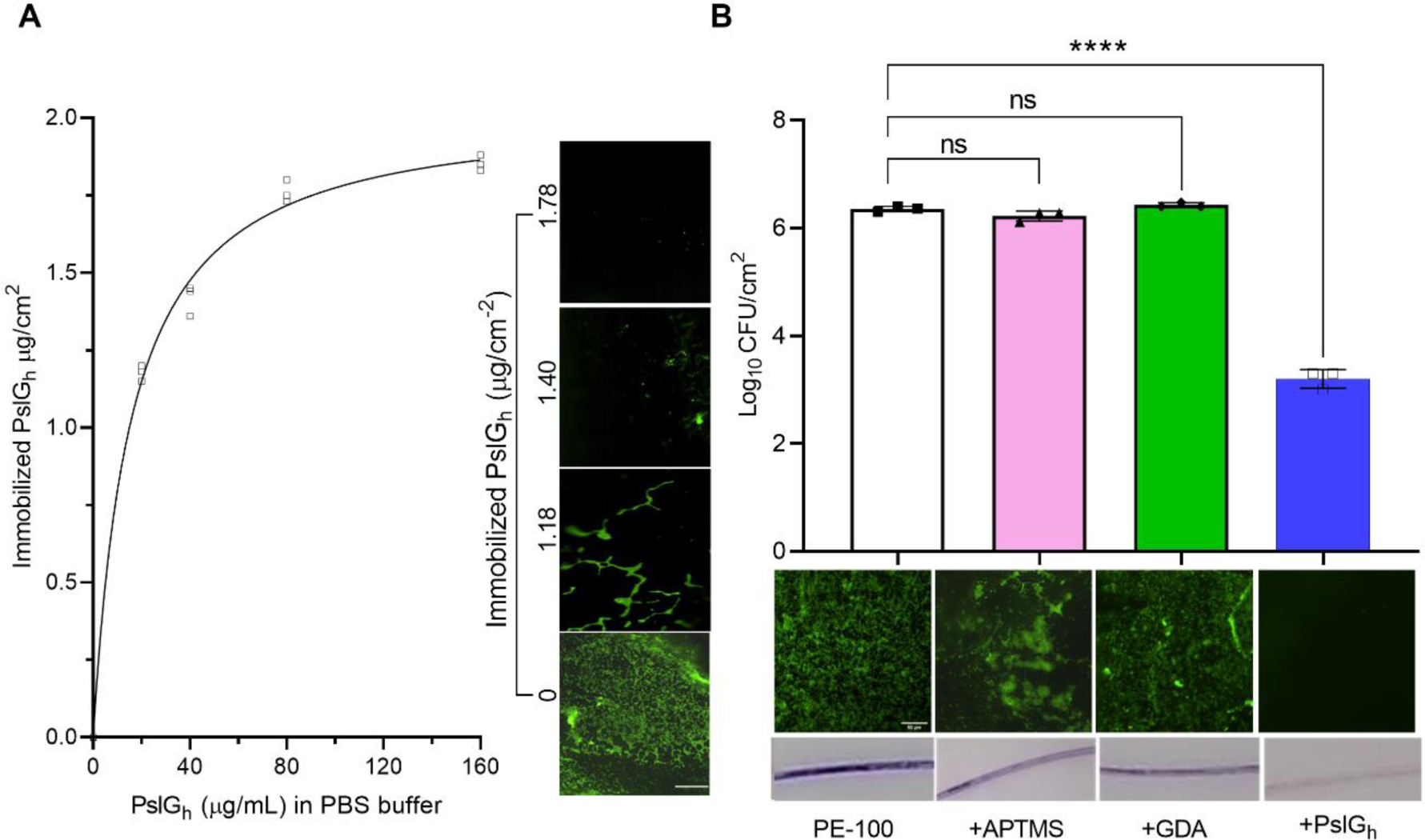
PslGh-modified PE-100 catheter inhibits biofilm formation (A) Effective concentration of immobilized PslGh required for biofilm inhibition. Effect of PslGh concentration (μg/mL) in immobilization solution on its binding capacity to catheter luminal surface (μg/cm) (left). Effect of immobilized PslGh surface density on biofilm formation of Pseudomonas aeruginosa PAO1 (right). (B) Anti-biofilm activity of the PE catheter lumen with bound PslGh, relative to the untreated control, or APTMS and GDA functionalized surfaces. (A) Colony forming units per cm2 (CFU) (top), corresponding florescence images (middle), and crystal violet (CV) stained images (bottom). All measurements were acquired after incubation for 24 h in bacterial culture. Biofilms were stained with SYTOX Green (middle) and CV (bottom) (scale bar, 50 μm). ****P ≤ 0.001. NS, no significant difference.
To ensure that the anti-biofilm activity was due to the bound PslGh specifically, we tested the effects of surface functionalization with APTMS and GDA on P. aeruginosa biofilm formation. The number of bacterial cells attached to APTMS (1.7 ×106 CFU/cm2) and GDA (2.7 ×106 CFU/cm2)-treated lumen surfaces after 24 h were not significantly different from that of the untreated microcatheter lumen (2.3 ×106 CFU/cm2). However, a ~3-log reduction in attached cell numbers was observed with PslGh-immobilized vs untreated microcatheters (1.7 ×103 vs 2.3 ×106 respectively, Figure 4B). In addition, SYTOX green (SG) and crystal violet (CV) staining demonstrated the formation of thick biofilm on the lumen surface of untreated microcatheters and those treated with APTMS or GDA, while biofilms were absent from the lumen of PslGh-treated PE-100 microcatheters (Figure 4B). Combined, these results demonstrate that the anti-biofilm activity of the PE-100 microcatheters against P. aeruginosa is due to the immobilized PslGh rather than other effects of the immobilization process on the microcatheter lumen surface.
To test the activity of the modified PE-100 microcatheters against clinical strains, we used P. aeruginosa strain ATCC 27853, a clinical strain with great ability to produce a more robust biofilm compared to the lab PAO1 strain59. We first tested the ability of PslGh in solution to inhibit its biofilm formation. Our results demonstrate that the PslGh in solution is effective at inhibiting P. aeruginosa ATCC 27853 biofilms with an EC50 of ~ 1 nM (Figure S4A). Fluorescence microscopy images of the lumen surface of untreated PE-100 microcatheter showed a thick biofilm after exposure to P. aeruginosa ATCC 27853 under static culture condition for 24 h (Figure S4B). However, a significant reduction in the number of attached cells (~3-log) was observed compared to the control (Figure S4C).To ensure the activity of immobilized PslGh along the entire microcatheter length (10 cm), we tested the anti-biofilm activity against P. aeruginosa PAO1 biofilm under 24 h static culture. After incubation, CV staining of biofilm mass along the 10 cm microcatheter showed almost no biofilm formation on the PslGh-bound lumen surface, whereas a heavily CV-stained biofilm appeared within the untreated PE-100 control catheter after 24 h (Figure 5A). To test the uniformity of the anti-biofilm activity along the entire 10 cm catheter lumen, catheters were cut into ten 1-cm long segments after incubation in the same conditions as above. Then, 5 alternating segments (Figure 5B) were tested for resistance to P. aeruginosa PAO1 biofilm formation using fluorescence microscopy (S2, S4, S6, S8, S10) and CFU (S1, S3, S5, S7, S9). Microscopic images showed thick biofilms within all 5 segments of the untreated catheter lumen (Figure 5C). In comparison, the covalently-bound PslGh significantly eliminated biofilm formation of P. aeruginosa for all PE-100 segments (Figure 5C). Quantitative culture confirmed that the PslGh-treatment of catheters significantly reduced attached cell density from 6.5–6.8 ×106 CFU/cm2 (untreated control) to 1–8.3 ×103 CFU/cm2 (PslGh-bound) after 24 h, representing a ~3-log reduction in CFU (Figure 5D). These results confirm we succeeded in generating a uniform, high density PslGh coating of the lumen surface, with strong anti-biofilm activity against P. aeruginosa along the length of these medical grade PE-100 catheters.
Figure 5.
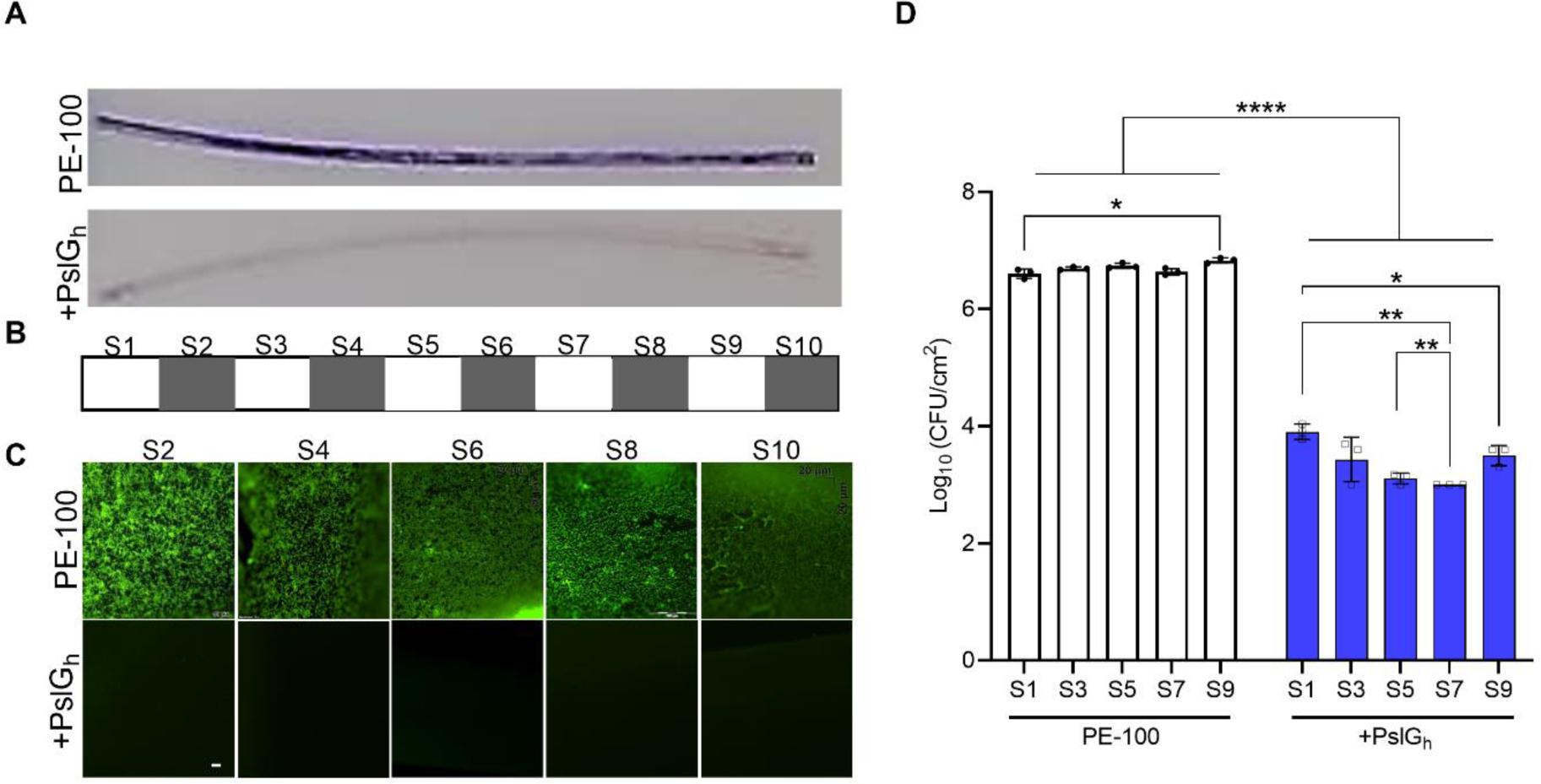
Antibiofilm activity of the lumen of high density PslGh-bound 10 cm PE-100 catheters against P. aeruginosa. (A) Photos of CV stained 10 cm PE-100 catheters; (B) Fluorescence images of 5 alternating segments (i.e., S2, 4, 6, 8 and 10); and (C) Colony forming units per cm2 (CFU) of the other 5 alternating segments (i.e., S1, 3, 5, 7 and 9). All measurements were acquired after incubation for 24 h in bacterial culture. Biofilms were stained with CV in (A) and with SYTOX Green in (B) (scale bar, 50 μm). *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001.
3.4. In vitro flow culture
We next tested the PslGh-modified catheters in an in vitro flow culture model (Figure S1). P. aeruginosa cells were allowed to adhere under static culture conditions for 1 day after which a constant flow (0.6 mL/min) of fresh LBNS media was passed through the lumen of both untreated control and PslGh-bound PE-100 catheters in parallel. Fluorescence microscopy (Figure 6A) demonstrated that the PslGh-bound catheters were resistant to biofilm formation on the lumen surface for the 14 d test period under dynamic culture flow conditions, as compared to the control PE-100 catheters. The rate of cell attachment and biofilm formation was also measured under these flow culture conditions. Quantitative culture confirmed that PslGh-bound catheters exhibited an ~ 3 log reduction in attached cell numbers during the first 11 d of flow, as compared to untreated catheters. Bacterial counts for the untreated catheters decreased between days 11 and 14, which resulted in a reduction of only 2 log between the treated and control catheters at day 14 (Figure 6B). Taken together, these results indicated that PslGh immobilized on the catheter lumen surface inhibits biofilm growth for at least 14 d under dynamic flow culture conditions.
Figure 6.
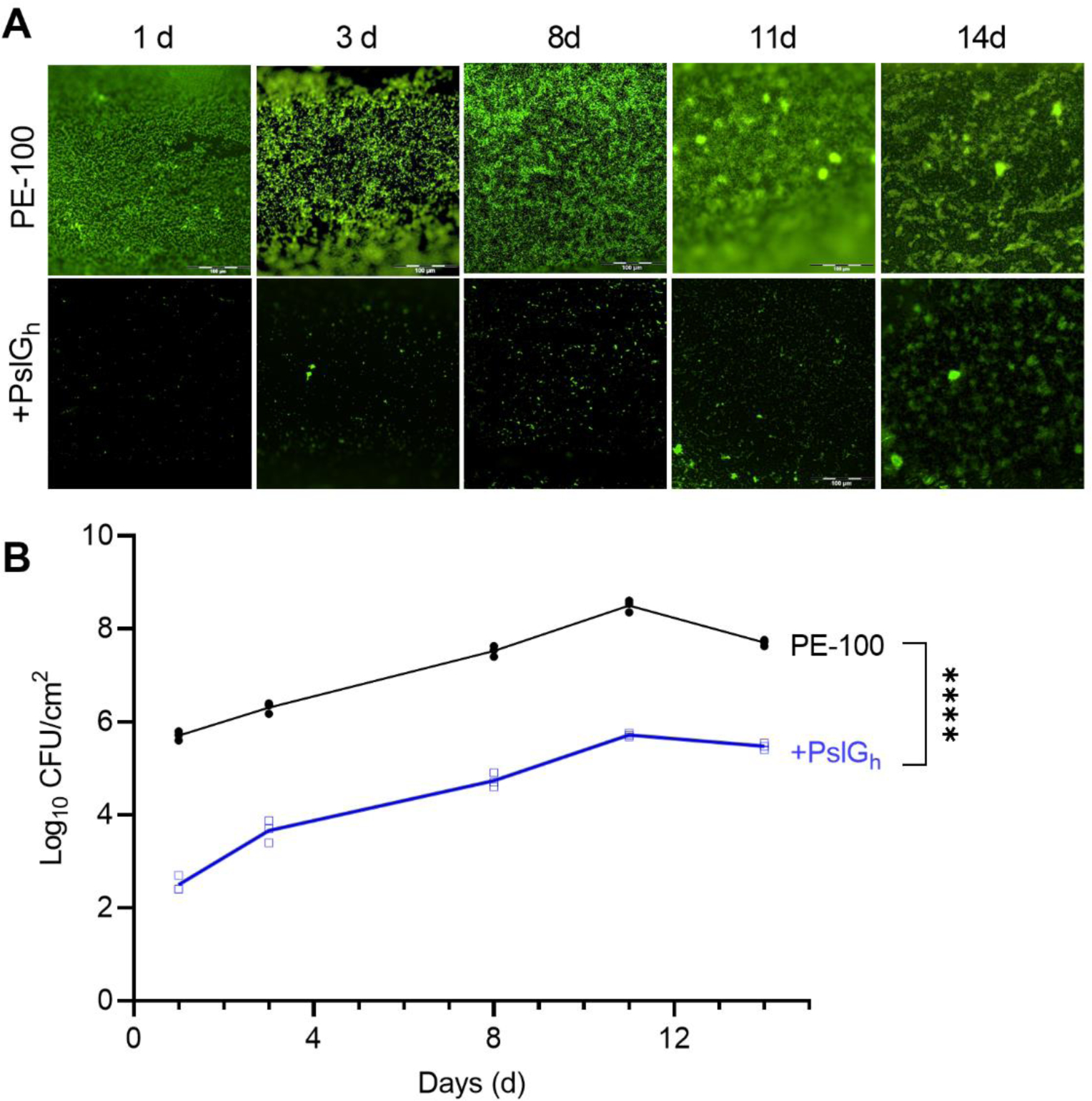
Inhibition of P. aeruginosa biofilm formation by PslGh bound to the lumen surface of PE-100 catheter under dynamic flow culture conditions. (A) Fluorescence images of biofilm growth for up to 14 days on the lumen surface of untreated (top) and PslGh-bound PE-100 catheter (bottom). (B) Colony forming units (CFU/cm2) of P. aeruginosa PAO1 on the lumen surface of untreated and PslGh-bound PE-100 catheter after 14 d incubation. Biofilms were stained with SYTOX Green in (A) (scale bar, 100 μm). ****P ≤ 0.0001.
3.5. Antibiofilm activity of PslGh modified catheter in presence of serum
As a prelude to an in vivo study, we investigated the effect of blood proteins, storage stability and catheter sterility on the antibiofilm activity of the PslGh-bound catheters. Immediately after catheterization, the surface of indwelling catheters becomes covered with plasma proteins that could potentially interfere with the function of the immobilized enzyme. Therefore, the activity of the lumen-bound PslGh was tested after being treated with serum for 24 h. In general, there were significant increases in the number of cells that adhered on both untreated and treated catheters after serum treatment (Figure S4). Nevertheless, CV staining (Figure S4A) and SYTOX green (Figure S4B) both confirmed a reduction in bacterial biofilm biomass on the lumen surface of PslGh-bound catheters relative to the untreated control catheter, despite treatment with serum. A few cell clusters were observed by fluorescence microscopy of the PslGh-bound catheter lumen (Figure S4B). Quantitative culture showed that, relative to the control catheters, immobilized PslGh reduced attached cell density within the catheter lumen before and after serum treatment by 3-log and ~2.5-log, respectively, (Figure S4C). Our results show that while serum proteins increase the adherence of cells to both the control and enzyme-treated surfaces, the PslGh-bound PE catheters remain more resistant to cell attachment even in the presence of serum.
3.6. Sterility and shelf-life storage of the catheter and PslGh modified catheter
Immobilization procedure (Figure 1) was carried out under aseptic conditions. Prior to immobilzations, caatheters were either pre-sterilized using70% ethanol or ethylene oxide. The immobilzation steps involve the use of atmospheric plasma, 80% ethanol, 2% GDA and filter sterilized PslGh in PBS buffer which are sufficient to maintain the sterility of catheters during immobilization. To confirm, we tested the sterility of PslGh-bound catheters with a standard plate counting method. Results confirmed the absence of contamination.
In addition, we tested the storage stability of PslGh-bound catheters after storage for 30 d at 4 °C under both dry and wet conditions. PslGh-bound catheters were found to remain resistant to biofilm formation as shown from fluorescence microscopy (Figure S6A) and their corresponding cell counts, which exhibited a 2.5–3 log reduction (Figure S6B).
3.7. PslGh modified catheter inhibits biofilm formation in vivo
Finally, we tested the anti-biofilm activity of these PslGh-bound PE-100 catheters in the complex environment found in vivo, using a central venous catheter infection (biofilm) rat model 65. In this model, the clinical strain P. aeruginosa ATCC 27853 was chosen as it was isolated from a hospital blood specimen and has been shown to produce a more robust biofilm compared to the lab PAO1 strain 59. Twenty-four hours after intravascular placement, SEM imaging of untreated catheter segments (Figure 7A) showed a dense biofilm network that consists of a relatively large number of cells enclosed in an extracellular matrix. In comparison, segments from the PslGh-bound catheter contained scattered and weakly attached cells and a lack of biofilm matrix. Quantitative culture of the PslGh-bound catheter showed a significant inhibition of biofilm formation 24 h after infection, as compared to the untreated catheters (~2 log CFU reduction) (Figure 7B).
Figure 7.
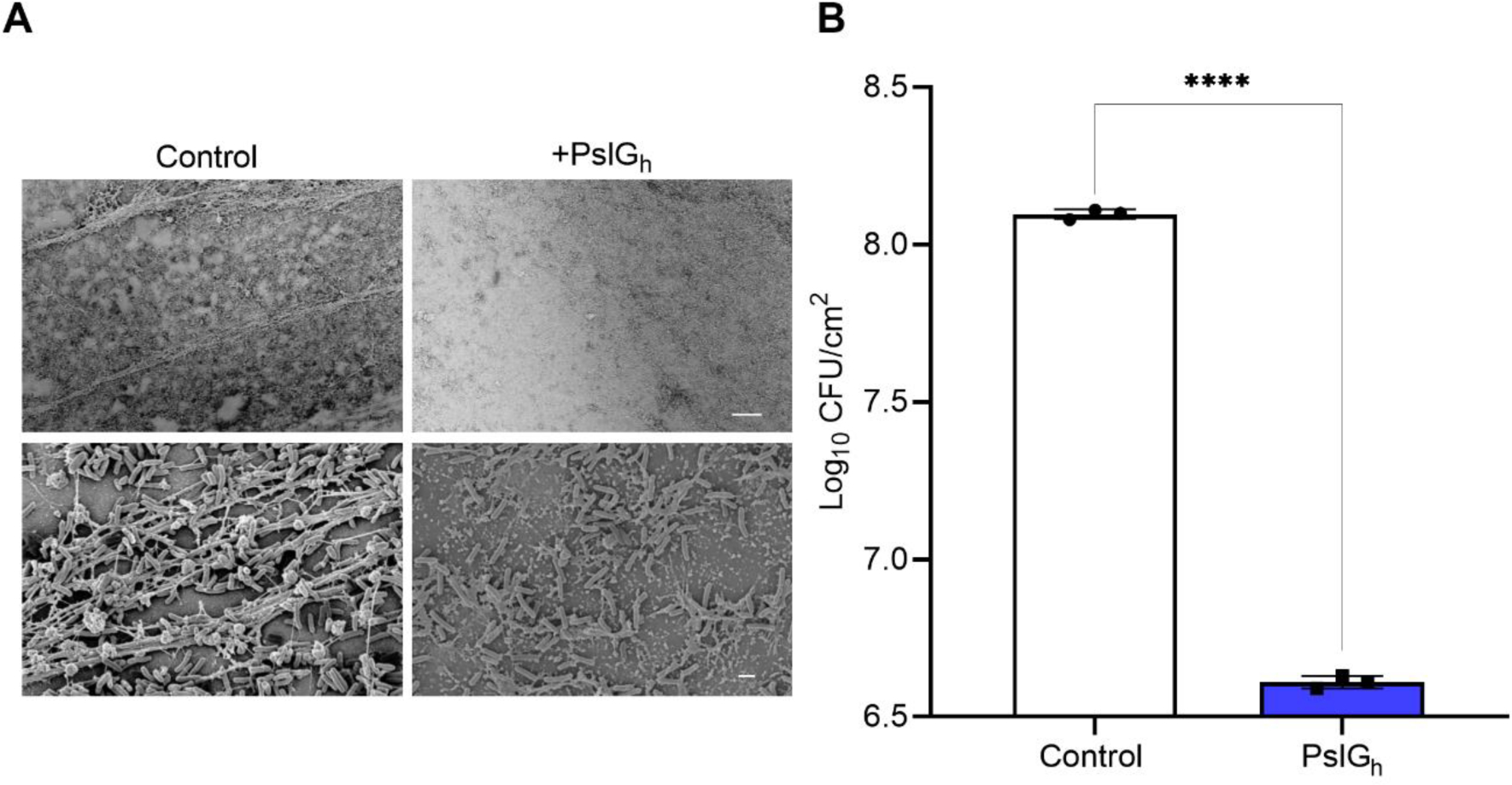
In vivo inhibition of biofilm formation of P. aeruginosa ATCC 27853 on the PslGh-treated lumen surface of PE catheter. (A) SEM images of biofilm growth on untreated and treated PE-100 catheters at low magnification (1,000X), and (B) corresponding bacterial burden showing ~ 2 log CFU /cm2 reduction in P. aeruginosa ATCC 27853 cells after 1d of placement in vivo. The scale bars represent 20 μm, respectively. ****P ≤ 0.0001.
4. Discussion
Developing robust approaches to limit bacterial biofilm formation on biomaterials through non-fouling (non-biocidal) mechanisms remains relatively new for antimicrobial biomedical devices. Antimicrobial strategies that do rely on biocidal drug release 60 are generally ineffective over time as they cannot prevent surface colonization (new cells adhering to attached dead cells), as evidenced by the high rates of biofilm-related disease that persist globally 4, 39. Since Psl adsorption plays a key role in the mechanism of P. aeruginosa cell attachment and biofilm formation, our approach specifically targets and disrupts this microbial adhesion mechanism. This approach differs from non-specific mechanisms to non-fouling such as SLIPS (slippery liquid infused porous surfaces) 61–62, and other non-wetting surfaces. For well-known, common pathogens it seems advantageous to target cell attachment, at the early stages of biofilm development. It has also been reported that, in mixed species biofilms, Psl can also contribute to the protection of other pathogens such as Escherichia coli and Staphylococcus aureus from antimicrobials15. Therefore, specific, selective prevention of P. aeruginosa biofilm formation should not only help enable antibiotic therapies to eradicate infections and reduce the development of acquired resistance, but also help the host immune system to eliminate infections associated with medical devices that are caused by both biofilm and non-biofilm producers.
The immobilization of the PslGh glycoside hydrolase uniformly along the full length of these medical grade, small-diameter PE catheters, has been a significant technical achievement due to the inert nature of PE, and the physically confinement of access to the lumen surface (inside a 1mm tube, 14 cm long) for the plasma and solution penetration. The lumen surface of implanted catheters can become exposed to blood and body fluids, and has a high risk of biofilm infection. Previous efforts to functionalize catheter lumen surfaces, such as uniform activation with polar groups, was shown to be a challenge 63. The narrow PE-100 catheters used herein thus represent a kind of ‘worst case’ application for medical devices, and our results suggest that a wide range of device sizes, material types and morphologies should be possible for enzyme immobilization.
A key finding in our work here is stability. The results show a consistent, stable activity of these enzymes to disrupt biofilm formation (through continuous Psl polysaccharide degradation) under in vitro flow conditions for extended periods (14 d), after extensive sterilization and storage (30 d, 4°C), and in the complexity of an in vivo environment. These results suggest that PslGh remains active after surface immobilization, which may make them suitable for a wide range of medical devices. Importantly, the immobilization procedure helped to maintain sterility of the catheters as conventional methods such as ethylene oxide did not work as the sterilized PslGh surfaces lost their anti-biofilm activity (Figure S5). The difference between the CFU log reduction observed in vivo (Figure 7B) and that observed in the in vitro assay (Figure 6B) likely reflects the enhanced biofilm formation capacity of strain ATCC 27853 59 as well as the effect body fluids and blood serum on promoting the biofilm growth 64. Overall, our work demonstrates that covalent immobilization of enzymes on biomedical tubing surfaces can represent an effective and feasible design strategy for non-fouling antimicrobial material design. These represent a ‘bio-active’ interface to specifically prevent bacterial adhesion mechanisms through continuous hydrolytic activity of a biofilm matrix component.
5. Conclusions
In summary, we have generated a sterile, uniform, and high-density surface of immobilized PslGh enzyme throughout the lumen of long, small diameter, medical grade catheters, in an effort to improve the performance of indwelling catheters against microbial biofilm growth. The catheters were highly effective at preventing P. aeruginosa biofilm growth under both static and dynamic flow conditions in vitro, and even for long periods of time (11 days). Finally, preliminary in vivo results show the PslGh-bound catheters dramatic resistance to biofilm growth in the complex environment of an infection model. These results demonstrate for the first time that ‘bio-active’ enzyme-modification of surfaces can realistically be applied to a wide range of biomaterials, uniformly over large areas, and with long-term activity. There is a wide range of biomedical devices that may benefit from these effects on P. aeruginosa biofilm growth, to significantly impact the problem of hospital acquired infections, complications and costs 54.
Supplementary Material
References
- 1.Costerton J; Stewart PS; Greenberg E, Bacterial biofilms: a common cause of persistent infections. Science 1999, 284 (5418), 1318–1322. [DOI] [PubMed] [Google Scholar]
- 2.Del Pozo J; Patel R, The challenge of treating biofilm-associated bacterial infections. Clinical Pharmacology & Therapeutics 2007, 82 (2), 204–209. [DOI] [PubMed] [Google Scholar]
- 3.Percival SL; Suleman L; Vuotto C; Donelli G, Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. Journal of medical microbiology 2015, 64 (4), 323–334. [DOI] [PubMed] [Google Scholar]
- 4.Wolcott R; Rhoads D; Bennett M; Wolcott B; Gogokhia L; Costerton J; Dowd S, Chronic wounds and the medical biofilm paradigm. Journal of wound care 2010, 19 (2), 45–53. [DOI] [PubMed] [Google Scholar]
- 5.Flemming H-C; Wingender J, The biofilm matrix. Nature Reviews Microbiology 2010, 8 (9), 623. [DOI] [PubMed] [Google Scholar]
- 6.Richards MJ; Edwards JR; Culver DH; Gaynes RP, Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Critical care medicine 1999, 27 (5), 887–892. [DOI] [PubMed] [Google Scholar]
- 7.Ryder MA, Catheter-related infections: it’s all about biofilm. Topics in Advanced Practice Nursing eJournal 2005, 5 (3), 2005. [Google Scholar]
- 8.Zimlichman E; Henderson D; Tamir O; Franz C; Song P; Yamin CK; Keohane C; Denham CR; Bates DW, Health care–associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine 2013, 173 (22), 2039–2046. [DOI] [PubMed] [Google Scholar]
- 9.Mermel LA; Allon M; Bouza E; Craven DE; Flynn P; O’Grady NP; Raad II; Rijnders BJ; Sherertz RJ; Warren DK, Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clinical infectious diseases 2009, 49 (1), 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L; Costerton JW; Stoodley P, Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology 2004, 2 (2), 95–108. [DOI] [PubMed] [Google Scholar]
- 11.Reid G, Biofilms in infectious disease and on medical devices. International journal of antimicrobial agents 1999, 11 (3), 223–226. [DOI] [PubMed] [Google Scholar]
- 12.Loo C-Y; Lee W-H; Young PM; Cavaliere R; Whitchurch CB; Rohanizadeh R, Implications and emerging control strategies for ventilator-associated infections. Expert Review of Anti-infective Therapy 2015, 13, 379–393. [DOI] [PubMed] [Google Scholar]
- 13.Campoccia D; Montanaro L; Arciola CR, A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34 (34), 8533–8554. [DOI] [PubMed] [Google Scholar]
- 14.Colvin KM; Gordon VD; Murakami K; Borlee BR; Wozniak DJ; Wong GC; Parsek MR, The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS pathogens 2011, 7 (1), e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billings N; Millan MR; Caldara M; Rusconi R; Tarasova Y; Stocker R; Ribbeck K, The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS pathogens 2013, 9 (8), e1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra M; Byrd MS; Sergeant S; Azad AK; Parsek MR; McPhail L; Schlesinger LS; Wozniak DJ, Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cellular microbiology 2012, 14 (1), 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhede M; Bjarnsholt T; Givskov M, Pseudomonas aeruginosa biofilms: mechanisms of immune evasion. Adv Appl Microbiol 2014, 86, 1–40. [DOI] [PubMed] [Google Scholar]
- 18.Davies D, Understanding biofilm resistance to antibacterial agents. Nature reviews Drug discovery 2003, 2 (2), 114–122. [DOI] [PubMed] [Google Scholar]
- 19.Høiby N; Ciofu O; Johansen HK; Song Z.-j.; Moser C; Jensen PØ; Molin S; Givskov M; Tolker-Nielsen T; Bjarnsholt T, The clinical impact of bacterial biofilms. International journal of oral science 2011, 3 (2), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes L; Cooper I, Biomaterials and Medical Device-Associated Infections. Elsevier: 2014. [Google Scholar]
- 21.Phillips KS; Patwardhan D; Jayan G, Biofilms, medical devices, and antibiofilm technology: Key messages from a recent public workshop. American journal of infection control 2015, 43 (1), 2. [DOI] [PubMed] [Google Scholar]
- 22.Treter J; Macedo A, Catheters: a suitable surface for biofilm formation. Handbook: science against microbial pathogens: communicating current research and technological advances. Spain: Formatex Research Center 2011, 835–842. [Google Scholar]
- 23.Giesecke MT; Schwabe P; Wichlas F; Trampuz A; Kleber C, Impact of high prevalence of pseudomonas and polymicrobial gram-negative infections in major sub-/total traumatic amputations on empiric antimicrobial therapy: a retrospective study. World J Emerg Surg 2014, 9 (1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciofu O; Tolker-Nielsen T, Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents—How P. aeruginosa Can Escape Antibiotics. Frontiers in Microbiology 2019, 10 (913). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livermore DM, Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clinical infectious diseases 2002, 34 (5), 634–640. [DOI] [PubMed] [Google Scholar]
- 26.Kang C-I; Kim S-H; Kim H-B; Park S-W; Choe Y-J; Oh M.-d.; Kim E-C; Choe K-W, Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clinical infectious diseases 2003, 37 (6), 745–751. [DOI] [PubMed] [Google Scholar]
- 27.Khan W; Bernier SP; Kuchma SL; Hammond JH; Hasan F; O’Toole GA, Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. International microbiology: the official journal of the Spanish Society for Microbiology 2010, 13 (4), 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zegans ME; Wozniak D; Griffin E; Toutain-Kidd CM; Hammond JH; Garfoot A; Lam JS, Pseudomonas aeruginosa exopolysaccharide Psl promotes resistance to the biofilm inhibitor polysorbate 80. Antimicrobial agents and chemotherapy 2012, 56 (8), 4112–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjarnsholt T; Jensen PØ; Fiandaca MJ; Pedersen J; Hansen CR; Andersen CB; Pressler T; Givskov M; Høiby N, Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatric pulmonology 2009, 44 (6), 547–558. [DOI] [PubMed] [Google Scholar]
- 30.Deschaght P; De Baere T; Van Simaey L; De Baets F; De Vos D; Pirnay J-P; Vaneechoutte M, Comparison of the sensitivity of culture, PCR and quantitative real-time PCR for the detection of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. BMC microbiology 2009, 9 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt KD; Tümmler B; Römling U, Comparative genome mapping of Pseudomonas aeruginosa PAO with P. aeruginosa C, which belongs to a major clone in cystic fibrosis patients and aquatic habitats. Journal of bacteriology 1996, 178 (1), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colvin KM; Irie Y; Tart CS; Urbano R; Whitney JC; Ryder C; Howell PL; Wozniak DJ; Parsek MR, The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental microbiology 2012, 14 (8), 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L; Wang J; Wang S; Anderson EM; Lam JS; Parsek MR; Wozniak DJ, Synthesis of multiple Pseudomonas aeruginosa biofilm matrix exopolysaccharides is post-transcriptionally regulated. Environmental microbiology 2012, 14 (8), 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L; Conover M; Lu H; Parsek MR; Bayles K; Wozniak DJ, Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS pathogens 2009, 5 (3), e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao K; Tseng BS; Beckerman B; Jin F; Gibiansky ML; Harrison JJ; Luijten E; Parsek MR; Wong GC, Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 2013, 497 (7449), 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zilberman M; Elsner JJ, Antibiotic-eluting medical devices for various applications. J Control Release 2008, 130 (3), 202–15. [DOI] [PubMed] [Google Scholar]
- 37.Donelli G; Francolini I, Efficacy of antiadhesive, antibiotic and antiseptic coatings in preventing catheter-related infections: review. J Chemother 2001, 13 (6), 595–606. [DOI] [PubMed] [Google Scholar]
- 38.Kluin OS; van der Mei HC; Busscher HJ; Neut D, A surface-eroding antibiotic delivery system based on poly-(trimethylene carbonate). Biomaterials 2009, 30 (27), 4738–4742. [DOI] [PubMed] [Google Scholar]
- 39.Koo H; Allan RN; Howlin RP; Stoodley P; Hall-Stoodley L, Targeting microbial biofilms: current and prospective therapeutic strategies. Nature Reviews Microbiology 2017, 15 (12), 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostendorf T; Meinhold A; Harter C; Salwender H; Egerer G; Geiss HK; Ho AD; Goldschmidt H, Chlorhexidine and silver-sulfadiazine coated central venous catheters in haematological patients—a double-blind, randomised, prospective, controlled trial. Supportive care in cancer 2005, 13 (12), 993–1000. [DOI] [PubMed] [Google Scholar]
- 41.Bridges K; Kidson A; Lowbury E; Wilkins M, Gentamicin-and silver-resistant pseudomonas in a burns unit. BMJ 1979, 1 (6161), 446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Percival S; Bowler P; Russell D, Bacterial resistance to silver in wound care. Journal of hospital infection 2005, 60 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- 43.Chen M; Yu Q; Sun H, Novel strategies for the prevention and treatment of biofilm related infections. International journal of molecular sciences 2013, 14 (9), 18488–18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bazaka K; Jacob MV; Crawford RJ; Ivanova EP, Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Applied microbiology and biotechnology 2012, 95 (2), 299–311. [DOI] [PubMed] [Google Scholar]
- 45.Salwiczek M; Qu Y; Gardiner J; Strugnell RA; Lithgow T; McLean KM; Thissen H, Emerging rules for effective antimicrobial coatings. Trends in biotechnology 2014, 32 (2), 82–90. [DOI] [PubMed] [Google Scholar]
- 46.Harris JM, Poly (ethylene glycol) chemistry: biotechnical and biomedical applications. Springer Science & Business Media: 2013. [Google Scholar]
- 47.Michel R; Pasche S; Textor M; Castner DG, Influence of PEG architecture on protein adsorption and conformation. Langmuir 2005, 21 (26), 12327–12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong T-S; Kang SH; Tang SK; Smythe EJ; Hatton BD; Grinthal A; Aizenberg J, Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477 (7365), 443–447. [DOI] [PubMed] [Google Scholar]
- 49.Baker P; HIll PJ; Snarr BD; Alnabelseya N; Pestrak MJ; Lee MJ; Jennings LK; Tam J; Melnyk RA; Parsek MR, Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Science Advances 2016, 2 (5), e1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker P; Whitfield GB; Hill PJ; Little DJ; Pestrak MJ; Robinson H; Wozniak DJ; Howell PL, Characterization of the Pseudomonas aeruginosa Glycoside Hydrolase PslG Reveals That Its Levels Are Critical for Psl Polysaccharide Biosynthesis and Biofilm Formation. J Biol Chem 2015, 290 (47), 28374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asker D; Awad TS; Baker P; Howell PL; Hatton BD, Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [DOI] [PubMed] [Google Scholar]
- 52.Pestrak MJ; Baker P; Dellos-Nolan S; Hill PJ; Passos da Silva D; Silver H; Lacdao I; Raju D; Parsek MR; Wozniak DJ; Howell PL, Treatment with the <span class=“named-content genus-species” id=“named-content-1”>Pseudomonas aeruginosa Glycoside Hydrolase PslG Combats Wound Infection by Improving Antibiotic Efficacy and Host Innate Immune Activity. Antimicrobial Agents and Chemotherapy 2019, 63 (6), e00234–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buthe A; Wu S; Wang P, Nanoporous silica glass for the immobilization of interactive enzyme systems. Enzyme Stabilization and Immobilization: Methods and Protocols 2011, 37–48. [DOI] [PubMed] [Google Scholar]
- 54.Rasband WS, ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2008. http://rsb.info.nih.gov/ij/. [Google Scholar]
- 55.Brugnara M, Contact Angle Plugin. University of Trento, Trento, Italy: 2010. [Google Scholar]
- 56.Andes D; Nett J; Oschel P; Albrecht R; Marchillo K; Pitula A, Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infection and immunity 2004, 72 (10), 6023–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutowski W; Wu DY; Li S, Surface silanization of polyethylene for enhanced adhesion. The Journal of Adhesion 1993, 43 (1–2), 139–155. [Google Scholar]
- 58.Onyshchenko I; De Geyter N; Nikiforov AY; Morent R, Atmospheric pressure plasma penetration inside flexible polymeric tubes. Plasma Processes and Polymers 2015, 12 (3), 271–284. [Google Scholar]
- 59.Cao H; Lai Y; Bougouffa S; Xu Z; Yan A, Comparative genome and transcriptome analysis reveals distinctive surface characteristics and unique physiological potentials of Pseudomonas aeruginosa ATCC 27853. BMC Genomics 2017, 18 (1), 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatton BD, 13 - Antimicrobial coatings for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials, Wen C, Ed. Woodhead Publishing: 2015; pp 379–391. [Google Scholar]
- 61.Lavielle N; Asker D; Hatton BD, Lubrication dynamics of swollen silicones to limit long term fouling and microbial biofilms. Soft Matter 2021, 17 (4), 936–946. [DOI] [PubMed] [Google Scholar]
- 62.Awad TS; Asker D; Hatton BD, Food-Safe Modification of Stainless Steel Food-Processing Surfaces to Reduce Bacterial Biofilms. ACS Applied Materials & Interfaces 2018, 10 (27), 22902–22912. [DOI] [PubMed] [Google Scholar]
- 63.Zhou C; Wu Y; Thappeta KRV; Subramanian JTL; Pranantyo D; Kang E-T; Duan H; Kline K; Chan-Park MB, In vivo anti-biofilm and anti-bacterial non-leachable coating thermally polymerized on cylindrical catheter. ACS applied materials & interfaces 2017, 9 (41), 36269–36280. [DOI] [PubMed] [Google Scholar]
- 64.Batoni G; Maisetta G; Esin S, Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochimica et Biophysica Acta (BBA)-Biomembranes 2016, 1858 (5), 1044–1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


