The emergence and spread of 2019 coronavirus disease (COVID-19) are causing a growing global public health crisis. Despite advances in treatment, vaccination remains the best way to contain the pandemic [1]. Vaccines are available by means of conditional marketing approval, full approval and emergency use authorisation pathways [2]. Evidence suggest that immunocompromised individuals, including solid-organ transplant recipients and patients under immunosuppressive treatment, may have increased mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection despite double-dose mRNA vaccine regimens [3]. This is partially attributed to blunted immune responses to vaccination, since only 38–54% of kidney and liver transplant recipients developed detectable SARS-CoV-2 antibodies following the second dose of mRNA vaccines [3, 4].
Short abstract
Patients with #IPF do not mount appreciable anti-spike antibody responses to two doses of #SARSCoV2 mRNA vaccine compared to the general population. National authorities should prioritise patients with IPF for booster doses. https://bit.ly/3K2KXQ0
To the Editor:
The emergence and spread of 2019 coronavirus disease (COVID-19) are causing a growing global public health crisis. Despite advances in treatment, vaccination remains the best way to contain the pandemic [1]. Vaccines are available by means of conditional marketing approval, full approval and emergency use authorisation pathways [2]. Evidence suggest that immunocompromised individuals, including solid-organ transplant recipients and patients under immunosuppressive treatment, may have increased mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection despite double-dose mRNA vaccine regimens [3]. This is partially attributed to blunted immune responses to vaccination, since only 38–54% of kidney and liver transplant recipients developed detectable SARS-CoV-2 antibodies following the second dose of mRNA vaccines [3, 4].
Patients with idiopathic pulmonary fibrosis (IPF) present with disrupted cellular and humoral immune responses [5]. In view of previous data during the first waves of the pandemic indicating increased risk of death from COVID-19 in unvaccinated patients with fibrotic interstitial lung diseases (ILDs) [6], we aimed to determine humoral responses to two doses of SARS-CoV-2 BNT162b2 mRNA vaccine in patients with IPF and compare them to those seen in the general population.
Therefore, we conducted a multicentre, prospective study between 22 July 2021 and 31 October 2021 including patients who received a multidisciplinary diagnosis of IPF in five ILD centres in Greece, and age-matched controls. Prospective data collection and analysis was approved by the Institutional Review Board and the Local Ethics Committee (protocol number 18482/21–7-21). IPF was diagnosed based on American Thoracic Society/European Respiratory Society 2018 guidelines [7].
We compared anti-SARS-CoV-2 antibodies 3 months after the second dose of the mRNA vaccine BNT162b2 in three subgroups: 1) patients with IPF receiving antifibrotics, 2) patients with IPF under no treatment and 3) age-matched controls. Patients receiving corticosteroids or steroid-sparing agents and patients with malignancies were excluded from the analysis. Subjects with history of prior SARS-CoV-2 infection were also excluded. The recruitment period was between 22 July 2021 and 31 October 2021. All measurements were performed using the authorised Abbott SARS-CoV-2 IgG assay. Positivity cut-off threshold for this assay was 50 AU·mL−1.
With regards to summary statistics, categorical data are presented as n (%). Continuous data are presented as mean±sd or median (95% CI) based on the Kolmogorov–Smirnov test for normality. The Kruskal–Wallis test was used to detect differences in the aforementioned three subgroups. A p-value <0.05 was considered statistically significant.
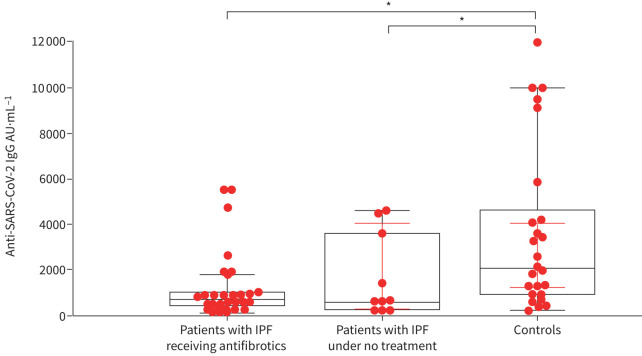
67 subjects were included in the analysis (patients with IPF receiving antifibrotics: n=32; patients with IPF under no treatment: n=10; controls: n=25). Groups were age- and sex-balanced (median age (95% CI): IPF/treatment 72.5 (69.9–75.0) years versus IPF/no treatment 73.0 (67.9–80.5) years versus controls 69.0 (65.5–74.2) years (p=0.15); sex: IPF/treatment 25 (78.1%) males out of 32 subjects, IPF/no treatment eight (80%) out of 10 subjects, controls 19 (76%) out of 25). Median (95% CI) forced vital capacity and diffusing capacity of the lung for carbon monoxide were 81.05% (68.8–89.4)% pred and 47.0% (40.9–57.4)% pred, 106.9% (66.1–121.1)% pred and 60.7% (50.7–85.1)% pred, and 91.5% (87.0–97.8)% pred and 87.0% (80.0–97.0)% pred, respectively, in the IPF/treatment, IPF/no treatment and control groups, respectively. The majority of subjects were current or ex-smokers in all groups (26 (81.3%) out of 32, eight (80%) out of 10 and 19 (76%) out of 25, respectively). Arterial hypertension was the most common comorbidity among all groups (14 (43.8%) out of 32, four (40%) out of 10 and 10 (40%) out of 25, respectively), while proportions of all other comorbidities, including obesity, were balanced among groups. Both groups of patients with IPF, whether receiving antifibrotic compounds or not, exhibited similarly reduced levels of anti-SARS-CoV-2 antibodies after two doses of the mRNA vaccine BNT162b2 compared to the general population (666.1 (540.1–900.0) AU·mL−1 versus 579.5 (232.4–4054.2) AU·mL−1 versus 2118.6 (1248.3–4035.5) AU·mL−1; p=0.002) (figure 1). The prevalence of anti-SARS-CoV-2 antibodies above the suggested threshold of 1000 AU·mL−1 was 21.9%, 40% and 72% in the IPF/treatment, IPF/no treatment and control groups, respectively.
FIGURE 1.
Both patients with idiopathic pulmonary fibrosis (IPF) receiving antifibrotics and patients with IPF under no treatment had reduced levels of anti-SARS-CoV-2 antibodies after two doses of SARS-CoV-2 mRNA vaccine compared to the general population (Kruskal–Wallis test, p=0.002). *: p<0.05.
This is the first study showing that patients with IPF did not mount appreciable anti-spike antibody responses to two doses of SARS-CoV-2 mRNA vaccine compared to the general population. Importantly, impaired immunogenicity was observed irrespective of antifibrotic treatment, further adding to the knowledge that current antifibrotics do not cause further immunosuppression but rather confer protection to acute disease exacerbations including those that are infection mediated [8, 9]. This report is of interest given that impaired immune response following vaccination in most diseases has not been attributed to the disease per se but to the compounds used to treat the disease. Our findings are in line with mechanistic data showing blunted cellular and humoral immune responses in patients with IPF potentially contributing to fibrosis development and progression. In particular, patients with IPF and poor disease outcomes present with T-cell exhaustion, as indicated by down-regulation of T-cell co-stimulatory markers. Importantly, the same high-risk genomic profile was also associated with increased mortality in patients with COVID-19 [10], indicating that similar aberrant immune responses predispose to severe SARS-CoV-2 pneumonitis and fibrotic ILD. The relationship between B-cells and IPF remains largely speculative, given the conflicting results of previous studies. On one hand, increased detection of CD20+ B-cells has been reported in lungs of patients with IPF [11]. Detection of the B-lymphocyte stimulator B-cell-activating factor (BAFF) is enriched in the peripheral blood and pulmonary parenchyma of patients with IPF, while neutralisation or genetic ablation of BAFF attenuated pulmonary fibrosis [12]. On the other hand, data derived from other animal models demonstrated that B-cells may suppress fibrotic response [13].
Limitations of our study include a moderate sample size that may lack generalisability, lack of serial measurements and an underpowered arm of patients with IPF under no treatment. However, treatment-naïve patients with IPF represent only a small minority in the era of antifibrotics and thus our sample size of untreated IPF patients is representative of everyday clinical practice. Moreover, we did not measure anti-SARS-CoV-2 antibody levels prior to vaccination, but we excluded subjects with history of SARS-CoV-2 infection. Another limitation is that we used the Abbott test, which quantifies IgG directed against an epitope of the spike protein, but we did not investigate specific immune responses, such as specific T-cell responses. We did not also record the exact time of the day that vaccination was performed to adjust for the effect of circadian rhythm. Finally, there was a small age difference between patients with IPF and controls, but this was not statistically significant.
Our findings are important, and should prompt national authorities to prioritise those subgroups of patients for booster doses and stringent precautions at the level of other groups of immunosuppressed individuals, considering that reduced levels of anti-SARS-CoV-2 protective antibodies as well as fibrotic lung diseases per se are independent risk factors for increased mortality following SARS-CoV-2 infection [6, 14]. Additional precautionary measures should include personal protective approaches such as protective facial masks and awareness of physical distancing, which played a beneficial role in the early phases of the pandemic [15]. However, the emergence of highly contagious variants of concern still mandates vaccination approaches to boost effectiveness and encourage fully vaccinated household contacts to offer optimal protection for at risk individuals.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: Z. Daniil, E. Manali, S. Papiris, D. Bouros and A. Tzouvelekis report receiving grants or contracts from Roche and Boehringer Ingelheim outside the submitted work, and honoraria received from Roche and Boehringer Ingelheim outside the submitted work. The remaining authors have nothing to disclose.
References
- 1.Karampitsakos T, Malakounidou E, Papaioannou O, et al. Tocilizumab improves 28-day survival in hospitalized patients with severe COVID-19: an open label, prospective study. Respir Res 2021; 22: 317. doi: 10.1186/s12931-021-01914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385: 661–662. doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325: 2204–2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Idiopathic Pulmonary Fibrosis Clinical Research Network . Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012; 366: 1968–1977. doi: 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med 2020; 202: 1656–1665. doi: 10.1164/rccm.202007-2794OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, Richeldi L, Kim DS, et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur Respir J 2017; 49: 1601339. doi: 10.1183/13993003.01339-2016 [DOI] [PubMed] [Google Scholar]
- 9.Ley B, Swigris J, Day BM, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196: 756–761. doi: 10.1164/rccm.201701-0091OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guardela BM J, Sun J, Zhang T, et al. 50-gene risk profiles in peripheral blood predict COVID-19 outcomes: a retrospective, multicenter cohort study. EBioMedicine 2021; 69: 103439. doi: 10.1016/j.ebiom.2021.103439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd NW, Scheraga RG, Galvin JR, et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res 2013; 6: 63–70. doi: 10.2147/JIR.S40673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.François A, Gombault A, Villeret B, et al. B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun 2015; 56: 1–11. doi: 10.1016/j.jaut.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Arras M, Louahed J, Simoen V, et al. B lymphocytes are critical for lung fibrosis control and prostaglandin E2 regulation in IL-9 transgenic mice. Am J Respir Cell Mol Biol 2006; 34: 573–580. doi: 10.1165/rcmb.2004-0383OC [DOI] [PubMed] [Google Scholar]
- 14.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv 2021; pre-print [ 10.1101/2021.08.09.21261290]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papiris SA, Bouros D, Markopoulou K, et al. Early COVID-19 lockdown in Greece and idiopathic pulmonary fibrosis: a beneficial “impact” beyond any expectation. Eur Respir J 2021; 57: 2003111. doi: 10.1183/13993003.03111-2020 [DOI] [PubMed] [Google Scholar]