Abstract
Background and Purpose
Cerebral venous sinus thrombosis (CVST) has been reported as a rare adverse event in association with thrombosis-thrombocytopenia syndrome (TTS) following COVID-19 vaccination.
Methods
We performed a systematic review and meta-analysis of investigator-initiated registries including confirmed CVST cases, with the aim to calculate (1) the odds ratio of TTS–CVST versus non-TTS–CVST after vector-based vaccines and (2) after non-vector-based vaccines, (3) the in-hospital mortality ratio of TTS–CVST compared to non-TTS–CVST; and (4) the dependency or death at discharge among TTS–CVST compared to non-TTS–CVST cases.
Results
Two eligible studies were included in the meta-analysis, comprising a total of 211 patients with CVST associated with COVID-19 vaccination. Vector-based COVID-19 vaccination was associated with a higher likelihood of TTS-associated CVST than with non-TTS–CVST (OR: 52.34, 95% CI 9.58–285.98). TTS–CVST was also associated with higher likelihood of in-hospital mortality (OR: 13.29; 95% CI 3.96–44.60) and death or dependency at discharge compared to non-TTS–CVST (OR: 6.70; 95% CI 3.15–14.26). TTS–CVST was recorded with a shorter interval between vaccination and symptom onset [Mean Difference (MD):-6.54 days; 95% CI − 12.64 to − 0.45], affecting younger patients (MD:-9.00 years; 95% CI − 14.02 to − 3.99) without risk factors for thromboses (OR:2.34; 95% CI 1.26–4.33), and was complicated more frequently with intracerebral hemorrhage (OR:3.60; 95% CI 1.31–9.87) and concomitant thromboses in other sites (OR:11.85; 95% CI 3.51–39.98) compared to non-TTS–CVST cases.
Conclusions
TTS–CVST following COVID-19 vaccination has distinct risk factor profile, clinical phenotype and prognosis compared to non-TTS–CVST. Further epidemiological data are required to evaluate the impact of different treatment strategies on outcome of TTS–CVST cases following COVID-19 vaccination.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-022-11101-2.
Keywords: COVID-19, Vector-based vaccine, Cerebral venous sinus thrombosis, Thrombotic-thrombocytopenia syndrome, Mortality
Introduction
Cerebral venous sinus thrombosis (CVST) has been reported as a rare, albeit very serious, adverse event associated with coronavirus disease-2019 (COVID-19) vaccination. Since the massive implementation of COVID-19 vaccination worldwide in late January 2021, various case reports and case series have been published presenting the clinical and laboratory characteristics and the outcomes of patients complicated with CVST, especially after vaccination with vector-based vaccines [1]. CVST has also been found to be four-times more common in the setting of thrombosis-thrombocytopenia syndrome (TTS) compared to other thrombotic complications [1]. Interestingly, prior to the COVID-19 pandemic, thrombocytopenia has been reported in only 8.4% of CVST patients [2]. Nevertheless, CVST cases following COVID-19 vaccination outside the spectrum of TTS have also been reported.
The largest volume of CVST cases in the setting of COVID-19 vaccination is derived by pharmacovigilance registries, such as the EudraVigilance [3, 4] or the VigiBase [5] databases. However, these data are limited due to the passive inclusion of suspected CVST cases by various sources (physicians, patients or others) without neuroimaging confirmation, and with limited clinical details. Here, we sought to provide high-quality data by inclusion of consecutive, confirmed CVST cases only, reported by physicians, to better characterize TTS-associated CVST, along with the therapeutic and prognostic implications, compared to non-TTS–CVST cases.
Methods
A systematic literature search was conducted up to October 12th, 2021, to identify investigator-initiated registries including confirmed cases of CVST associated with COVID-19 vaccination. The complete search algorithm is presented in the Supplement. Case reports, case series, pharmacovigilance registries and studies with high risk of potential duplicates were excluded. Eligible studies were subjected to quality control and bias assessment employing the Newcastle–Ottawa Scale [6]. Data extraction was performed in structured reports, including author names, date of publication, country, vaccine type, patients’ characteristics, management and outcomes.
An aggregate data meta-analysis was performed with the following outcomes of interest: (1) odds ratio (OR) of TTS–CVST among the total cases of CVST after vector-based vaccines (i.e., ChAdOx1 and Ad26.COV2.S) and after non-vector-based vaccines (i.e., mRNA vaccines, such as BNT162b2 and mRNA-1273, or vaccines using inactivated forms of the COVID-19 virus, such as the Sinovac-CoronaVac vaccine), (2) in-hospital mortality ratio of TTS–CVST compared to non-TTS–CVST; (3) dependency or death at discharge [as defined by modified Rankin Scale (mRS) 3–6] among TTS–CVST compared to non-TTS–CVST cases. Additional patient characteristics (age, sex, known risk factors for thrombosis, time interval from vaccination to symptom onset), complications (intracerebral hemorrhage, concomitant thromboses) and therapeutic management, including the potential association of anticoagulants and intravenous immunoglobulin (IVIG) with in-hospital mortality among TTS–CVST patients, were evaluated as secondary outcomes.
For each dichotomous outcome of interest, we calculated the corresponding ORs and 95% confidence intervals (95%CI). Continuous outcomes were assessed by mean difference (MD). For single-arm analyses, we calculated the corresponding pooled proportion with 95%CI, after the implementation of the variance-stabilizing double arcsine transformation. The random-effects model of meta-analysis (DerSimonian and Laird) [7] was used to calculate the pooled estimates. Heterogeneity was assessed with the I2 and Cochran Q statistics and the equivalent z test for each pooled estimate with a two-tailed p value < 0.05 was considered statistically significant. All statistical analyses were conducted using the Cochrane Collaboration’s Review Manager (RevMan 5.3) Software Package (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and the OpenMetaAnalyst [8].
The meta-analysis is reported according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9] and was written according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal [10]. All data generated or analyzed during this study are included in this article and its supplementary information files. This study did not require an ethical board approval or written informed consent by the patients according to the study design (systematic review and meta-analysis).
Results
The systematic database search yielded a total of 113 and 135 records from the MEDLINE and SCOPUS databases, respectively (Supplementary Figure-S1). After excluding duplicates and initial screening, we retrieved the full text of 8 records that were considered potentially eligible for inclusion. Six studies were further excluded [3–5, 11–13] (Supplementary Table-S1). Finally, we identified two eligible studies for inclusion in the meta-analysis [14, 15], comprising a total of 211 patients with CVST associated with COVID-19 vaccination (Table 1). The quality control and risk of bias of the included studies, as assessed by the Newcastle–Ottawa scale, is presented in Supplementary Table-S2. The overall score was 18 of 18 (100%), indicating high quality and low risk of bias of the included studies.
Table 1.
Main characteristics of the studies included in the systematic review
| Study | Date of publication | Country | Study duration | Vaccine type | N of total CVST cases | N of TTS–CVST cases | Interval in days (SD) to TTS–CVST cases | Female sex among TTS–CVST cases | Mean age in years (SD) of TTS–CVST cases | N of non-TTS–CVST cases | Interval (days) to non-TTS–CVST cases | Female sex among non-TTS–CVST cases | Mean age in years (SD) of non-TTS–CVST cases |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van Kammen et al.[14] | 28 Sep 21 | International | 30 Jan –14 Jun 21 | Vector-based | 97 | 77 | 9 ± 2 | 63 | 45 ± 14 | 20 | 15 ± 28 | 30 | 55 ± 20 |
| Non-vector-based | 19 | 1 | 18 | ||||||||||
| Perry et al.[15] | 6 Aug 21 | UK | 1 Apr –20 May 21 | Vector-based | 91 | 70 | 10 ± 3 | 39 | 45 ± 17 | 21 | 17 ± 21 | 11 | 53 ± 15 |
| Non-vector-based | 4 | 0 | 4 |
CVST cerebral venous sinus thrombosis, TTS thrombotic-thrombocytopenia syndrome
TTS was diagnosed in included studies in accordance with the Brighton Collaboration criteria [16]. In particular, in the study of van Kammen et al., patients with CVST following COVID-19 vaccination were diagnosed with TTS if they fulfilled all of the following criteria [14]: (1) confirmed thrombosis, (2) new-onset thrombocytopenia, and (3) no known recent exposure to heparin. In the study of Perry et al., TTS–CVST was diagnosed in accordance with the previous criteria, and additionally, in patients in whom D-dimer was measured, the highest value recorded had to be greater than 2000 μg/L [15].
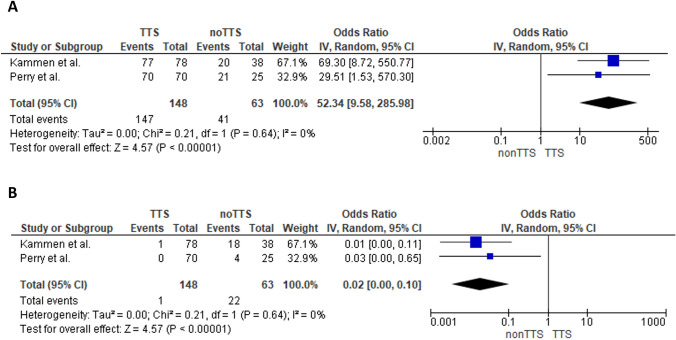
More than 70% of the CVST cases post COVID-19 vaccination were associated with TTS (95% CI 64–76%; I2 = 4%; p for Cochran Q = 0.31; Supplementary Figure-S2). Following vector-based vaccination, a higher likelihood of presenting TTS–CVST compared to non-TTS-CVST was noted (OR: 52.34; 95% CI 9.58–285.98; I2 = 0%; p for Cochran Q = 0. 0.64; Fig. 1A). On the contrary, non-vector-based vaccination was associated predominantly with non-TTS-CVST (OR:0.02; 95% CI0.00–0.1; I2 = 0%; p for Cochran Q = 0.64; Fig. 1B).
Fig. 1.
Forest plot presenting the association of vaccine-associated TTS–CVST with vector-based vaccines (Panel A). Forest plot presenting the association of vaccine-associated TTS–CVST with non-vector-based vaccines (Panel B)
TTS–CVST was recorded with a shorter interval between vaccination and symptom onset (MD:-6.54 days; 95% CI − 12.64 to − 0.45; I2 = 0%; p for Cochran Q = 0.87; Supplementary Figure-S3), affecting younger patients (MD:-9.00 years; 95% CI − 14.02 to − 3.99; I2 = 0%; p for Cochran Q = 0.70; Supplementary Figure-S4), without risk factors for thromboses (OR: 2.34; 95% CI 1.26–4.33; I2 = 0%; p for Cochran Q = 0.90; Supplementary Figure-S5), and was complicated more frequently with intracerebral hemorrhage (OR: 3.60; 95% CI 1.31–9.87; I2 = 60%; p for Cochran Q = 0.11; Supplementary Figure-S6) and concomitant thromboses in other sites (OR:11.85; 95% CI 3.51–39.98; I2 = 0%; p for Cochran Q = 0.57; Supplementary Figure-S7) compared to non-TTS–CVST cases. Female proportion was comparable between the two groups (OR:1.35; 95% CI 0.69–2.62; I2 = 0%; p for Cochran Q = 0.60; Supplementary Figure-S8).
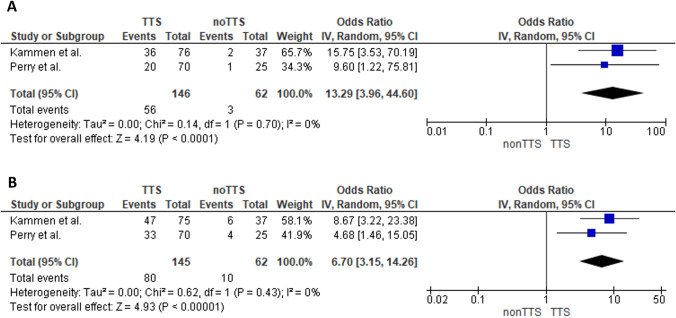
In-hospital mortality of TTS–CVST cases was significantly higher compared to non-TTS–CVST (OR: 13.29; 95% CI 3.96–44.60; I2 = 0%; p for Cochran Q = 0.70; Fig. 2A). Death or dependency at discharge was also found significantly higher in patients with TTS–CVST (OR: 6.70; 95% CI 3.15–14.26; I2 = 0%; p for Cochran Q = 0.43; Fig. 2B).
Fig. 2.
Forest plot presenting the association of TTS–CVST with in-hospital mortality (Panel A), and death or dependency at hospital discharge (Panel B)
With respect to TTS–CVST treatments, the pooled proportion of patients treated with non-heparin parenteral anticoagulants was 57% (95% CI 27–84%; 2 studies; I2 = 93%; p for Cochran Q < 0.01; Supplementary Table-S3), followed by heparin-anticoagulants in 31% (95% CI 17–47%; 2 studies; I2 = 77%; p for Cochran Q = 0.04; Supplementary Table-S3) and direct oral anticoagulants in 16% of cases (95% CI 0–49%; 2 studies; I2 = 95%; p for Cochran Q < 0.01; Supplementary Table-S3). Data regarding in-hospital mortality among patients treated with non-heparin-derived anticoagulants versus those administered heparin-derived anticoagulants could not be extracted in the study of van Kammen et al. and, thus, a comparative meta-analysis was not performed. As part of the single-arm meta-analysis, the pooled in-hospital mortality among patients with TTS–CVST treated with heparin-anticoagulants was calculated at 31% (95% CI 11–55%; 2 studies; I2 = 61%; p for Cochran Q = 0.11; Supplementary Figure-S9). It should be noted, however, that heparin-anticoagulants were mostly administered until March 2021, when TTS was first brought to wide attention and clinical practice guidelines were issued advising against heparin use in TTS.
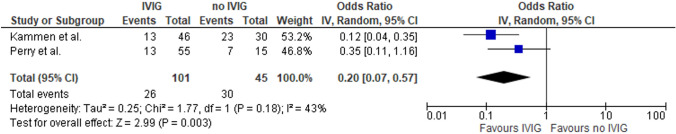
With respect to immunomodulation, the majority of TTS–CVST patients received IVIG (pooled rate: 70%; 95% CI 51–86%; 2 studies; I2 = 83%; p for Cochran Q = 0.02; Supplementary Table-S3), followed by corticosteroids (pooled rate: 53%; 95% CI 15–89%; 2 studies; I2 = 96%; p for Cochran Q < 0.01; Supplementary Table-S3) and plasmapheresis (pooled rate: 14%; 95% CI 0–32%; 2 studies; I2 = 86%; p for Cochran Q = 0.01; Supplementary Table-S3). Treatment of TTS–CVST patients with IVIG was associated with a significant reduction of in-hospital mortality compared to those not receiving IVIG (OR: 0.20; 95% CI 0.07–0.57; I2 = 43%; p for Cochran Q = 0.18; Fig. 3).
Fig. 3.
Forest plot presenting the association of treatment with intravenous immunoglobulin (IVIG) and in-hospital mortality among TTS–CVST patients
Discussion
In the present systematic review and meta-analysis, we found that vector-based COVID-19 vaccination was associated with an approximately 50-fold increase in the odds of TTS-related compared to non-TTS-related CVST. These findings add to the growing body of literature that ascertains a pathogenetic link between vector-based COVID-19 vaccines and TTS. In particular, antigenic complexes of vaccine components have been shown to bind to platelet factor 4 (PF4) on platelet surfaces, inducing proinflammatory reactions, anti-PF4 antibody formation and prothrombotic cascades [17]. Furthermore, our results show that non-vector-based vaccination was associated predominantly with non-TTS–CVST. These results, in conjunction with recent epidemiological evidence indicating that TTS- and CVST-incidence rates after non-vector-based COVID-19 vaccination are aligned to those in the general population [18, 19], refute direct causal associations between non-vector-based-vaccination, TTS and CVST.
With respect to clinical features, our findings show that TTS–CVST manifests earlier after vaccination, affects younger patients without known risk factors for thrombosis and manifests more frequently with intracerebral hemorrhage or concomitant thromboses in other sites, compared to non-TTS–CVST. Interestingly, no heterogeneity was uncovered for all previous associations. Additionally, in accordance with the findings of previous research [1], TTS–CVST was associated with higher likelihood of in-hospital mortality and death or dependency at discharge compared to non-TTS–CVST, highlighting the need for accurate diagnosis and prompt management of this syndrome.
With respect to therapeutic implications of TTS–CVST diagnosis, the majority of patients received non-heparin parenteral anticoagulants, followed by heparin-anticoagulants and direct oral anticoagulants. However, significant heterogeneity was uncovered concerning the choice of anticoagulation in the setting of TTS–CVST. These findings are most likely explained by the inclusion of patients that were diagnosed early in the course of the vaccination campaign, before awareness regarding TTS was raised and treatment guidelines that advise against heparin use in TTS were circulated and implemented [20, 21]. With respect to immunomodulation, the majority of TTS–CVST patients received IVIG treatment, followed by corticosteroids and plasma exchange. Crucially, our results corroborate significantly reduced in-hospital mortality in TTS–CVST patients that received IVIG compared to those that did not. According to current recommendations, treatment with IVIG and non-heparin-anticoagulants are recommended as first-line treatments in the setting of TTS–CVST, while heparin and platelet transfusion should be avoided. Since the establishment of awareness regarding TTS-syndrome among the scientific community, the proportion of patients under IVIG treatment increased with a simultaneous reduction in heparin-anticoagulation use, while in-hospital mortality of TTS–CVST cases decreased [4, 14].
Conclusions
The present systematic review and meta-analysis is the first to include confirmed CVST cases following COVID-19 vaccination as reported by physicians in dedicated registries, excluding passively collected pharmacovigilance data. Additionally, comparison between TTS–CVST and non-TTS–CVST cases was performed, underscoring the differences in clinical phenotypes and outcomes between those separate entities. Nevertheless, there are important methodological shortcomings related to our meta-analysis including the small number of included studies, the limited sample size and inability to perform subgroup analyses. Further high-quality epidemiological data are required to evaluate the impact of different treatment strategies on outcome of TTS–CVST cases following COVID-19 vaccination in prospective international clinical registries.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
LP, MIS and GT contributed to conception and study design. LP, MIS, DAS, JMC, MP and GT contributed to acquisition and analysis of data. LP, MIS, and GT contributed to drafting a significant portion of the manuscript or figures. DAS, JMC, MP, VP, TIV, ST and DKF contributed with critical comments during manuscript revision.
Funding
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
Dr. Palaiodimou—Nothing to report. Dr. Stefanou—Nothing to report. Dr. Aguiar de Sousa—Nothing to report. Dr. Coutinho—Nothing to report. Dr. Papadopoulou—Nothing to report. Dr. Papaevangelou—Nothing to report. Dr Vassilakopoulos -Nothing to report. Dr Tsiodras -Nothing to report. Dr Filippou -Nothing to report. Dr. Tsivgoulis—Nothing to report.
Ethical approval
This study did not require an ethical board approval or written informed consent by the patients according to the study design (systematic review and metaanalysis).
Footnotes
Lina Palaiodimou and Maria-Ioanna Stefanou have contributed equally to this work.
References
- 1.Palaiodimou L, Stefanou MI, Katsanos AH, et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based covid-19 vaccines: a systematic review and meta-analysis. Neurology. 2021;97(21):e2136–e2147. doi: 10.1212/WNL.0000000000012896. [DOI] [PubMed] [Google Scholar]
- 2.van Kammen MS, Heldner MR, Brodard J, et al. Frequency of thrombocytopenia and platelet factor 4/heparin antibodies in patients with cerebral venous sinus thrombosis prior to the covid-19 pandemic. Jama. 2021;326(4):332–338. doi: 10.1001/jama.2021.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krzywicka K, Heldner MR, van Kammen MS, et al. Post-Sars-Cov-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the european medicines agency. Eur J Neurol. 2021;28(11):3656–3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Munckhof A, Krzywicka K, de Sousa DA, et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after Sars-Cov-2 vaccination. Eur J Neurol. 2022;29(1):339–344. doi: 10.1111/ene.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination against covid-19: insight from arterial and venous thrombosis occurrence using data from vigibase. Eur Respir J. 2021 doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells G, Shea B, O'connell D, et al (2021) The Newcastle-Ottawa scale (Nos) for assessing the quality of nonrandomized studies in meta-analyses. Available From: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 16 Oct 2021
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the prisma 2020 statement. J Clin Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (Moose) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Pawlowski C, Rincón-Hekking J, Awasthi S, et al. Cerebral venous sinus thrombosis is not significantly linked to covid-19 vaccines or non-covid vaccines in a large multi-state health system. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2021;30(10):105923. doi: 10.1016/j.jstrokecerebrovasdis.2021.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and Sars-Cov-2 positive testing: self-controlled case series study. BMJ (Clinical research ed). 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz JB, Berlit P, Diener HC, et al. Covid-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):627–639. doi: 10.1002/ana.26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kammen MS, de Sousa DA, Poli S, et al. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in Sars-Cov-2 vaccine-induced immune thrombotic thrombocytopenia. JAMA Neurol. 2021;78(11):1314–1323. doi: 10.1001/jamaneurol.2021.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against covid-19 in the UK: a multicentre cohort study. Lancet. 2021;398(10306):1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brighton Collaboration (2022) Updated proposed brighton collaboration process for developing a standard case definition for study of new clinical syndrome X, as applied to thrombosis with thrombocytopenia syndrome (Tts). Available From: https://brightoncollaboration.us/Wp-Content/Uploads/2021/05/Tts-Interim-Case-Definition-V10.16.3-May-23-2021.Pdf. Accessed 19 Mar 2022
- 17.Greinacher A, Selleng K, Palankar R, et al. Insights in Chadox1 Ncov-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256–2268. doi: 10.1182/blood.2021013231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson CR, Shi T, Vasileiou E, et al. First-dose Chadox1 and Bnt162b2 covid-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr S, Joy M, Torabi F, et al. First dose Chadox1 and Bnt162b2 covid-19 vaccinations and cerebral venous sinus thrombosis: a pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med. 2022;19(2):e1003927. doi: 10.1371/journal.pmed.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Society of Hematology Thrombosis with Thrombocytopenia Syndrome (Also Termed Vaccine-Induced Thrombotic Thrombocytopenia) (Version 1.6; Last Updated August 12, 2021).Available At: https://www.hematology.org/Covid-19/Vaccine-Induced-Immune-Thrombotic-Thrombocytopenia. Accessed 16 Oct 2021
- 21.International Society on Thrombosis and Haemostasis Isth Interim Guidance for the Diagnosis and Treatment on Vaccine- Induced Immune Thrombotic Thrombocytopenia (Updated 20 April, 2021). Available At: https://cdn.ymaws.com/Www.Isth.Org/Resource/Resmgr/Isth_Vitt_Guidance_2.Pdf Accessed 16 Oct 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.