Abstract
Study design
A retrospective population-based study.
Objectives
Describe the incidence of traumatic spinal cord injury (TSCI) and mortality risks, based on the characteristics of the patient, anatomical level of the lesion, setting/cause of the injury, and type of healthcare support received within the regional trauma network (highly specialized trauma center or spoke hospital).
Setting
Between 2011 and 2020, 1303 patients with incident TSCI were identified in a population of 4.9 million inhabitants.
Methods
Hospital discharge records and mortality records were used to identify patients and outcomes. Cox regression models were fitted to estimate mortality risks across several subgroups.
Results
Over the past decade, age-sex-standardized TSCI incidence rates remained stable with 26.5 cases (95% CI, 25.0–27.9) per 1,000,000 inhabitants (mean age 59.2 years) and most cases were males (68.3%). Incidence was directly associated with age while the male to female ratio was inversely related. Most TSCIs were cervical lesions (52.1%), and the most common cause of injury were traffic crashes (29.9%) followed by occupational accidents (29.8%). Sex, cause of the trauma, or inpatient hospital management were not associated with an increased risk of death. Mortality rates were greater for cervical lesions, and increased with age, remaining stably high among older individuals even 12 months after the accident. One-month mortality risk was significantly higher at ≥75 years compared to <55 years (adjusted HR 9.14 (95% CI, 4.17–20.03)).
Conclusion
Public health policies should aim at reducing preventable TSCIs, and special attention should be drawn to long-term management of elderly patients in the attempt to decrease mortality rates.
Subject terms: Epidemiology, Risk factors
Introduction
Traumatic spinal cord injuries (TSCI) are increasingly recognized as a global health priority [1, 2]. Worldwide, TSCIs have a considerable impact in terms of mortality and morbidity, and represent a relevant burden for healthcare systems due to the expensive and complex medical support required by patients with TSCI, in addition to economic consequences deriving from loss in productivity [1, 3, 4]. This condition is a leading cause of disability especially among younger people, with a high impact on years lived with disability [5, 6]. Traffic crashes, falls, and self-inflicted violence are among the most common causes of injury-related TSCIs worldwide [1]. Marked variations in incidence and prevalence across countries are present, with differences by sex, mechanism of injury, level and severity of the lesion [1, 7–10]. This variability can be partly explained by geographical and cultural conditions, as well as relevant infrastructural inequalities, but it also reflects the presence of diverse criteria used to identify and classify patients with spinal cord injuries [9, 11–14]. Reported incidence ranges between 12.1 and 57.8 cases per million inhabitants in high-income countries, and between 12.7 and 29.7 in low-income countries [15, 16]. Large differences are also present within Europe, where the highest incidence rate is reported in Portugal (57.8 cases per million) [17] and Russia (44.0 cases per million) [18], while the lowest in Italy (14.7 cases per million) [10].
Given the preventable nature of occupational accidents, traffic crashes, and falls, that are leading causes of TSCIs, public health experts should monitor TSCI trends and identify groups at increased risk, to implement targeted prevention policies [19]. Furthermore, given the burden of mortality related to TSCIs, both the characteristics of the trauma and the type of healthcare support provided to patients should be examined to determine the characteristics that are associated with better outcomes in terms of short- and long-term mortality.
The aim of the present study is to perform grouped analyses on incidence rates and trends of TSCI and examine the factors that are most strongly associated with short- and long-term mortality.
Methods
Study population
This is a retrospective population-based study conducted in Veneto Region, northeastern Italy, with a population of about 4.9 million residents. In Veneto Region health services are provided by 9 local social and healthcare units, 2 hospital enterprises, 2 hospitals for scientific research and private certified providers, based on a hub and spoke hospital model. Given the universalistic model of the Italian National Health Service, data from hospital episodes fully reflect the incidence of TSCI in the study population. Healthcare support for traumatic injuries is provided by a regional trauma network composed of 5 highly specialized trauma centers (one per million inhabitants) with spinal cord units in neurosurgical departments, operating at all times and located in the major cities of the Veneto Region [20] and 22 spoke hospitals [21], that are distributed in the entire Region, providing an integrated healthcare support tailored on individual patient’s needs.
Data sources
All residents in Veneto Region, assisted by the regional health service, were identified by means of a population registry. All emergency department records and hospital discharge records from January 1, 2011, to December 31, 2020, were collected. The regional mortality database includes information relating to any death that occurred in the Region. Linkage with this database was used to define overall mortality at 1, 6 and 12 months from the date of the injury until December 31, 2021.
Hospital discharge records database contains socio-demographic data (sex, age, address, city of birth, educational level), and clinical and hospital information (hospitalization ward, admission and discharge date, primary and secondary diagnoses, any surgical or medical procedure performed on the patient, and the mode of discharge (intra-hospital transfer, discharge at home or death)). Diagnoses and procedures are coded using the International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) coding system, currently used in Italy.
Emergency department records database provides information on all emergency room visits and includes patient demographic data, reason for the visit, and a description of the type of trauma for visits due to traumatic injuries.
Regional health records are routinely submitted to a standardized anonymization process that assigns a unique anonymous code to each subject, allowing linkage between electronic health records without any possibility of back-retrieving the subject’s identity. Since all analyses were carried out on routinely collected anonymized records, the study was deemed exempt from approval by the Local Ethics Committee
Incident traumatic spinal cord injury cohort
All patients with TSCI admitted to any hospital in the Region during the study period, alive at the time of hospital admission, resident in Veneto Region and insured by the regional health service, were included in the cohort. Only patients at the first hospital admission for TSCI, with a primary or secondary diagnosis of spinal cord injury with vertebral fracture (ICD-9-CM 806.x) or without evidence of vertebral fracture (ICD-9-CM 952.x) were included in the analyses. To remove possible inpatient episodes of prevalent TSCI cases, the following exclusion criteria were applied: hospitalized patients with TSCI that were not present in the population registry, cases with a diagnosis of spinal column fracture without spinal cord injury, and patients with conditions indicative of a non-traumatic spinal cord injury (pathologic fracture of vertebrae (ICD-9-CM 733.13) or secondary malignant neoplasm of the bone and bone marrow (ICD-9-CM 198.5). To further exclude possible prevalent cases, only TSCI hospitalizations that followed an emergency department visit or an urgent admission to a neurosurgery department were included. Diagnoses retrieved from planned hospitalizations or admissions to rehabilitation facilities have been excluded from the analyses.
Patient characteristics
Patients were grouped by sex, age, clinical characteristics of the injury, such as the anatomical level of lesion, the cause of the lesion (traffic crashes or other), the setting in which the injury occurred (occupational accident or other), and the characteristics of the healthcare support received by the patient (accessed a highly specialized trauma center, transferred to a highly specialized trauma center, or assisted exclusively in a spoke hospital).
Age at the time of the injury was categorized as <15 years, in 10-year intervals from 15 to 84 years, and ≥85 years. When analyzed as a covariate, age was categorized as ≤54, 55–74, and ≥75 years. Based on specific ICD-9-CM diagnostic codes reported in the discharge record, the anatomical level of the injury was defined as high cervical (C1–C4), low cervical (C5–C7), thoracic (T1–T12), and lumbar or sacral. In case of lesions at multiple levels, the patient was included in the group that corresponded to the most severe level of the lesion, from top to bottom.
The cause of the trauma was classified as traffic crashes and other causes (i.e., falls, cuts, firearm injury). Due to missing data and a lower frequency of other causes, this category was considered as a whole. The setting of the injury was classified as occupational accidents (workplace or while commuting) or other (i.e., domestic accidents, sports accidents). When estimating incidence rates by sex and age, domestic accidents were analyzed separately.
Given the presence of an efficient trauma network in the Region, patients were also classified based on the type of hospital support they received. Three categories were defined: (1) patients initially assisted by a highly specialized trauma center, (2) patients transferred from a spoke hospital to a highly specialized trauma center, (3) patients that received support exclusively from a spoke hospital.
Inter-hospital patient transfers were defined as any new admission from a hospital to another within one day of the patient’s prior discharge date. Hospital length of stay was defined as the number of days spent in the hospital from the admission date to the discharge date, considering the last date among all inter-hospital transfers, if any were present, but excluding days spent in rehabilitation departments.
Statistical analysis
Univariate and bivariate analyses were performed to summarize data with respect to the patient demographic and TSCI characteristics. Continuous variables were presented with descriptive statistics (mean, standard deviation, median and interquartile range). For categorical variables, frequencies and percentages were calculated. Student’s t test or Mann–Whitney test, as appropriate, were used to calculate the differences between patient groups. The difference between groups were examined by Pearson’s Chi-square or Fisher’s exact test, as appropriate. A p < 0.05 was considered statistically significant.
Annual TSCI incidence rate, both crude and age-sex-standardized, along with the 95% confidence interval (95% CI) were estimated per million inhabitants. Based on the population registry, annual incidence rates were calculated using the resident population insured by the regional health service in Veneto Region during the study period, as denominator. Standardization was performed by direct method using as reference the 2015 resident population of the Veneto Region. Cochran-Armitage test of trend was used to describe changes in yearly incidence rates over time. Age-specific rates were also estimated for the overall population and stratified by sex, along with a male to female ratio. Annual trends in age-standardized incidence rates for traffic crashes and occupational accidents were also estimated grouped by sex. Sex-stratified age-specific incidence rates were estimated for all TSCIs, for those that occurred in a domestic setting, in an occupational setting, and those that occurred due to traffic crashes, and plotted with the 95% CI.
Mortality rates were stratified by sex, age group, anatomical level of the injury, cause of injury, setting where the injury occurred, and hospital support received by the patient.
Cox regression models were fitted to estimate mortality risks and 95% CI at 1, 6, and 12 months (to ensure greater comparability with previous studies), and across several subgroups [22, 23]. Follow-up started at the moment of incident TSCI up to death, specific time point (1, 6, and 12 months) or December 31, 2021, whichever came first. Proportional hazards assumption was verified by plotting Schoenfeld residuals. Adjustment was performed by including all available covariates.
In a sensitivity analysis, to examine possible differences in trends of incident TSCI among younger individuals, yearly incidence rates were estimated only among people aged ≤30 years.
In a further sensitivity analysis, the proportion of TSCIs due to falls (ICD-9-CM codes E880-E888) was also calculated, although this analysis was restricted to 2016-2020 due to missing data for this specific cause.
All statistical analyses were conducted using SAS (Statistical Analysis System) software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
During the 10-year study period, from January 1, 2011 to December 31, 2020, 1303 incident cases of TSCI were identified. The mean age at the time of the injury was 59.2 years (SD 21.4), spanning from 0 to 99 years (median age 62, IQR 44–77 years), with a higher prevalence of male patients (68.3%). TSCIs occurred most frequently due to traffic crashes (29.9%) and occupational accidents (29.8%). On average, hospital length of stay was just above 20 days, although among patients that died within the first 12 months of the injury, the length of stay was significantly greater, corresponding to an average of 25 days (p = 0.0023). The majority of patients (60.7%) accessed the trauma network in a highly specialized trauma center, but 22% were transferred within the regional trauma network at some point. Due to hospital transfers, 1303 patients led to 1717 hospital discharges. Overall, 99 (7.6%) patients died in the hospital, while 85 (6.5%) died after discharge, but within the first 6 months since the injury, and 40 (3.1%) between 6 and 12 months. Despite a significant mean age difference among men and women, corresponding to 56 years (SD 21.0) and 64 years (SD 21.3) respectively (p < 0.0001), when comparing those who died in the first year after the injury and those who did not, no significant sex-based differences were found (p = 0.7522). Mean age was significantly higher among patients who died in the first year of the injury (76.6 years (SD 15.0)), compared to survivors (55.5 years (SD 20.7)). Most TSCI patients had a high cervical lesion (C1–C4), with or without lower lesions (29.9%). High cervical lesions were also the most common among patients that died in the first year (42.9%), followed by low cervical lesion (C5–C7) (26.8%). There were no significant differences in the distribution of decedents and survivors at one year based on the type of healthcare support provided, as reported in Table 1.
Table 1.
Descriptive characteristics of the cohort of patients with incident traumatic spinal cord injury according to mortality at one year.
| Total (n = 1303) | Died in the first 12 months (n = 224) | Alive at 12 months (n = 1079) | p value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Sex | ||||
| Male | 890 (68.3%) | 151 (67.4%) | 739 (68.5%) | |
| Female | 413 (31.7%) | 73 (32.6%) | 340 (31.5%) | 0.7522 |
| Age, mean (SD) | 59.2 (21.4) | 76.6 (15.0) | 55.5 (20.7) | <0.0001 |
| Number of hospitalizations, mean (SD) | 1.3 (0.7) | 1.4 (0.9) | 1.3 (0.7) | 0.6795 |
| Length of hospital stay*, mean (SD) | 20.4 (20.4) | 25.9 (25.0) | 19.2 (19.0) | 0.0023 |
| Inter-hospital transfers | 287 (22.0%) | 51 (22.8%) | 236 (21.9%) | 0.7685 |
| Level of the injury | ||||
| High cervical (C1–C4) | 389 (29.9%) | 96 (42.9%) | 293 (27.2%) | |
| Low cervical (C5–C7) | 290 (22.3%) | 60 (26.8%) | 230 (21.3%) | |
| Thoracic (T1–T12) | 283 (21.7%) | 31 (13.8%) | 252 (23.4%) | |
| Lumbar or sacral | 341 (26.2%) | 37 (16.5%) | 304 (28.2%) | <0.0001 |
| Cause | ||||
| Traffic crashes | 389 (29.9%) | 46 (20.5%) | 343 (31.8%) | |
| Other | 914 (70.1%) | 178 (79.5%) | 736 (68.2%) | 0.0008 |
| Setting | ||||
| Occupational accident | 388 (29.8%) | 40 (17.9%) | 348 (32.3%) | |
| Other | 915 (70.2%) | 184 (82.1%) | 731 (67.8%) | <0.0001 |
| Trauma network | ||||
| Primary access point | ||||
| Highly specialized trauma center | 791 (60.7%) | 133 (59.4%) | 658 (61.0%) | |
| Spoke | 512 (39.3%) | 91 (40.6%) | 421 (39.0%) | 0.6540 |
| Healthcare support provider | ||||
| Partly or exclusively highly specialized trauma center | 906 (69.5%) | 152 (67.9%) | 754 (69.9%) | |
| Only Spoke | 397 (30.5%) | 72 (32.1%) | 325 (30.1%) | 0.5496 |
*Comprising of all inpatients episodes, but excluding days spent in rehabilitation departments.
Overall, age-sex-standardized TSCI incidence rate was 26.5 (95% CI, 25.0–27.9) per 1,000,000 inhabitants. TSCIs were more common among males, with an overall male to female ratio of 2.3:1, as reported in Table 2. TSCI incidence rates were directly associated with age in both sexes, exceeding among older individuals (≥65 years), 59.2 and 23.3 per 1,000,000 for men and women, respectively. The male to female ratio decreased with age, despite TSCIs remaining at least twice as common among men compared to women.
Table 2.
Age- and sex-specific incidence rates of traumatic spinal cord injuries (cases per 1,000,000), and male to female ratio.
| Age group | Sex | M/F ratio | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Total | |||||
| N | Incidence (95% CI) | N | Incidence (95% CI) | N | Incidence (95% CI) | (95% CI) | |
| <15 | 21 | 6.0 (4.0–9.2) | 5 | 1.5 (0.7–3.6) | 26 | 3.9 (2.6–5.6) | 4.0 (1.5–10.5) |
| 15–24 | 59 | 24.9 (19.3–32.1) | 20 | 9.0 (5.8–13.8) | 79 | 17.2 (13.8–21.4) | 2.8 (1.7–4.6) |
| 25–34 | 65 | 24.6 (19.3–31.4) | 22 | 8.4 (5.5–12.7) | 87 | 16.5 (13.4–20.4) | 2.9 (1.8–4.8) |
| 35–44 | 109 | 29.6 (24.6–35.7) | 37 | 10.3 (7.4–14.1) | 146 | 20.0 (17.0–23.6) | 2.9 (2.0–4.2) |
| 45–54 | 122 | 30.3 (25.4–36.2) | 41 | 10.3 (7.6–14.0) | 163 | 20.4 (17.5–23.7) | 2.9 (2.1–4.2) |
| 55–64 | 153 | 48.8 (41.7–57.2) | 45 | 13.9 (10.4–18.6) | 198 | 31.0 (27.0–35.7) | 3.5 (2.5–4.9) |
| 65–74 | 149 | 59.2 (50.4–69.4) | 65 | 23.3 (18.3–29.7) | 214 | 40.3 (35.2–46.1) | 2.5 (1.9–3.4) |
| 75–84 | 149 | 94.0 (80.1–110.4) | 104 | 47.6 (39.3–57.7) | 253 | 67.1 (59.4–75.9) | 2.0 (1.5–2.5) |
| 85–100 | 63 | 136.9 (107.0–175.1) | 74 | 66.4 (52.9–83.3) | 137 | 87.0 (73.6–102.8) | 2.1 (1.5–2.9) |
| Total | 890 | 37.3 (34.9–39.8) | 413 | 16.5 (15.0–18.1) | 1303 | 26.5 (25.0–27.9)* | 2.3 (2.0–2.5) |
| p for trend | <0.0001 | <0.0001 | <0.0001 | ||||
*The estimate refers to the age-sex-standardized TSCI incidence rate.
Over the 10-year study period, TSCI incidence remained relatively stable. Trends related to the specific cause and setting that led to the injury are reported in Table 3. Traffic crashes represent 29.9% of all injuries, with a standardized incidence rate that is more than three times higher in males than females (12.3 and 3.7 cases per 1,000,000, respectively). Compared to traffic crashes, injury rates for other causes (such as violence, self-harm etc.), were twice as frequent in males and four times more frequent in females (data not shown). In the past decade, incidence rates remained substantially stable for both traffic crashes (p for trend among men p = 0.519 and women p = 0.404) and occupational accidents (p for trend among men p = 0.080 and women p = 0.777), except for 2020, where markedly lower rates have been observed. Estimates in 2020 are hardly comparable with previous years due to the COVID-19 pandemic and the strong restrictions on individual mobility with a reduced number of traffic crashes and occupational accidents in all age groups. Trends in the incidence rate of TSCI were also restricted to individuals aged ≤30 years, without observing any significant changes over time (p = 0.1084) (data not shown).
Table 3.
Trend of age-standardized incidence rate (IR) of traumatic spinal cord injury per 1,000,000 inhabitants/year* caused by traffic crashes and occupational accidents, grouped by sex (Veneto Region, 2011–2020).
| Traffic crashes | Occupational accidents | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | Male | Female | Male | Female | ||||
| N | Incidence rate (95% CI) | N | Incidence rate (95% CI) | N | Incidence rate (95% CI) | N | Incidence rate (95% CI) | |
| 2011 | 32 | 13.0 (8.8–18.7) | 12 | 4.8 (2.5–8.5) | 30 | 12.5 (8.4–17.9) | 6 | 2.4 (0.9–5.2) |
| 2012 | 25 | 10.6 (6.8–15.7) | 11 | 4.5 (2.2–8.1) | 20 | 8.3 (5.1–13.0) | 9 | 3.7 (1.7–7.0) |
| 2013 | 19 | 12.3 (8.2–17.7) | 4 | 1.6 (0.4–4.1) | 15 | 6.2 (3.5–10.4) | 5 | 1.9 (0.6–4.6) |
| 2014 | 21 | 8.7 (5.4–13.4) | 9 | 3.6 (1.6–6.8) | 22 | 9.2 (5.7–13.9) | 5 | 2.0 (0.6–4.7) |
| 2015 | 24 | 10.0 (6.4–14.9) | 11 | 4.4 (2.2–7.8) | 34 | 14.2 (9.8–19.8) | 11 | 4.4 (2.2–7.8) |
| 2016 | 40 | 16.6 (11.9–22.7) | 13 | 5.2 (2.8–8.9) | 43 | 17.9 (13.0–24.1) | 12 | 4.8 (2.5–8.3) |
| 2017 | 27 | 11.2 (7.4–16.3) | 10 | 4.0 (1.9–7.4) | 34 | 14.1 (9.8–19.8) | 10 | 4.0 (1.9–7.4) |
| 2018 | 34 | 14.0 (9.7–19.7) | 11 | 4.4 (2.2–7.9) | 39 | 16.1 (11.4–22.1) | 9 | 3.6 (1.6–6.9) |
| 2019 | 42 | 17.3 (12.5–23.6) | 10 | 4.0 (1.9–7.4) | 43 | 17.7 (12.8–23.9) | 10 | 4.1 (2.0–7.6) |
| 2020 | 28 | 9.4 (6.3–14.3) | 2 | 0.9 (0.1–3.3) | 28 | 11.6 (7.7–16.9) | 3 | 1.2 (0.2–3.7) |
| Overall | 296 | 12.3 (11.0–13.8) | 93 | 3.7 (3.0–4.5) | 308 | 12.9 (11.5–14.4) | 80 | 3.2 (2.5–4.0) |
| p trend | 0.519 | 0.404 | 0.080 | 0.777 | ||||
*Incidence were rates standardized using as reference the resident population of Veneto Region in 2015.
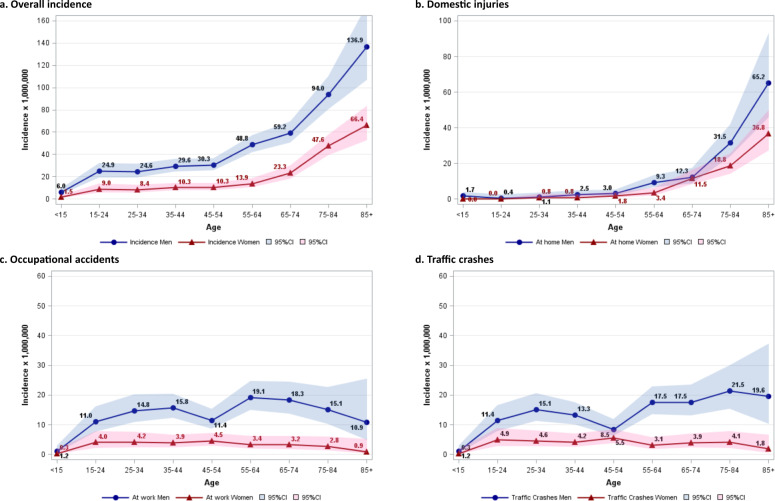
Age-specific sex-stratified incidence rates of TSCIs grouped by cause and setting that led to the onset of the injury are reported in Fig. 1. Overall, TSCIs increase exponentially with age, mainly due to domestic accidents, often related to falls. Incidence rates are always higher for men regardless of age, cause or setting. TSCIs related to traffic crashes do not show any clear association with age, with a similar pattern to that observed for occupational accidents that peak at working ages. In an analysis restricted to 2016–2020, TSCIs due to falls, stably represented 33% to 35% of all TSCIs, with this proportion increasing to 48.5% in 2020 (data not shown).
Fig. 1. Incidence rate of traumatic spinal cord injury (per 1,000,000 inhabitants) by age and sex.
Incidence rate in the overall cohort (a), and separately for patients with an injury that occurred in a domestic setting (b), in an occupational setting (c), and those caused by traffic crashes (d) (Veneto Region, 2011–2020).
As shown in Table 4, mortality rates were highest one month after the injury and progressively decreased with time, respectively from 2.1 to 0.5 deaths per 1000 person-days among males, and from 2.0 to 0.6 deaths per 1000 person-days among females. Risk of death among males was not significantly different compared to females at any time-point (12-month adjusted mortality HR 1.23 (95% CI, 0.91–1.66)). Mortality was strongly associated with age, with a significantly increased risk of 12-month mortality among individuals aged 75 years or more, compared to those aged 55 or less (adjusted HR 16.09 (95% CI, 9.38–27.60)). Patients with high cervical lesions had the highest 1-month mortality rate (4.1 deaths per 1000 person-days), and rates remained more than twice as high as those observed for patients with thoracic, and lumbar or sacral lesions, even 12 months after the injury (0.8, 0.3 and 0.3 deaths per 1000 person-days respectively). Traffic crashes and occupational accidents were not associated with any significantly increased risk of death. The type of healthcare support provided to the patient (highly specialized trauma center or spoke hospital) also did not modify the patient’s likelihood of dying. No changes in mortality risks could be observed based on the study period (12-months adjusted mortality HR 1.02 (95% CI, 0.98–1.07)).
Table 4.
Mortality hazard ratios and 95% confidence intervals (CI) of patients with traumatic spinal cord injury, at 1, 6, and 12 months since the date of the injury, based on characteristics of the patient, of the lesion, and of the hospital management.
| Parameter | N | 1-month mortality | 6-month mortality | 12-month mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Crude mortality rate x 1000 person-days | Hazard | Deaths | Crude mortality rate x 1000 person-days | Hazard | Deaths | Crude mortality rate x 1000 person-days | Hazard | ||
| Ratio (95% CI) | Ratio (95% CI) | Ratio (95% CI) | ||||||||
| Sex | ||||||||||
| Male | 890 | 55 | 2.1 | 1.08 (0.65–1.78) | 123 | 0.8 | 1.14 (0.82–1.58) | 151 | 0.5 | 1.23 (0.91–1.66) |
| Female | 413 | 24 | 2 | Ref. | 61 | 0.9 | Ref. | 73 | 0.6 | Ref. |
| Age | ||||||||||
| ≤54 years | 501 | 8 | 0.5 | Ref. | 14 | 0.2 | Ref. | 16 | 0.1 | Ref. |
| 55–74 years | 412 | 17 | 1.4 | 2.35 (1.01–5.49) | 40 | 0.6 | 3.27 (1.77–6.05) | 52 | 0.4 | 3.82 (2.17–6.72) |
| ≥75–100 years | 390 | 54 | 5.1 | 9.14 (4.17–20.03) | 130 | 2.4 | 14.27 (8–25.43) | 156 | 1.6 | 16.09 (9.38–27.6) |
| Cause | ||||||||||
| Traffic crashes | 389 | 20 | 1.8 | 1.18 (0.57–2.44) | 39 | 0.6 | 0.99 (0.6–1.64) | 46 | 0.4 | 0.89 (0.56–1.43) |
| Other | 914 | 59 | 2.2 | Ref. | 145 | 1 | Ref. | 178 | 0.6 | Ref. |
| Setting | ||||||||||
| Occupational accident | 388 | 16 | 1.4 | 0.96 (0.42–2.18) | 32 | 0.5 | 0.99 (0.56–1.75) | 40 | 0.3 | 1.10 (0.66–1.84) |
| Other | 915 | 63 | 2.4 | Ref. | 152 | 1 | Ref. | 184 | 0.6 | Ref. |
| Hospital management | ||||||||||
| Access from a highly specialized trauma center | 791 | 53 | 2.3 | Ref. | 111 | 0.9 | Ref. | 133 | 0.5 | Ref. |
| Transferred to highly specialized trauma center | 115 | 5 | 1.5 | 0.50 (0.20–1.27) | 16 | 0.9 | 0.80 (0.47–1.35) | 19 | 0.5 | 0.79 (0.49–1.29) |
| Only spoke | 397 | 21 | 1.8 | 0.66 (0.39–1.11) | 57 | 0.9 | 0.80 (0.57–1.11) | 72 | 0.6 | 0.83 (0.62–1.12) |
| Level of the injury | ||||||||||
| High cervical (C1–C4) | 389 | 44 | 4.1 | 1.69 (0.99–2.90) | 82 | 1.4 | 1.12 (0.79–1.59) | 96 | 0.8 | 1.13 (0.82–1.56) |
| Low cervical (C5–C7) | 290 | 19 | 2.3 | Ref. | 52 | 1.1 | Ref. | 60 | 0.7 | Ref. |
| Thoracic (T1–T12) | 283 | 9 | 1.1 | 0.51 (0.23–1.15) | 22 | 0.5 | 0.41 (0.25–0.68) | 31 | 0.3 | 0.49 (0.32–0.76) |
| Lumbar or sacral | 341 | 7 | 0.7 | 0.39 (0.16–0.94) | 28 | 0.5 | 0.51 (0.32–0.82) | 37 | 0.3 | 0.58 (0.38–0.89) |
| Year of event | 1.01 (0.93–1.09) | 1.01 (0.96–1.07) | 1.02 (0.98–1.07) | |||||||
Discussion
Incidence
Over the ten-year study period, in a large population of 4.9 million inhabitants in Veneto Region, the incidence of TSCIs was overall stable regardless of sex, setting, or cause that led to the onset of the injury. Most literature suggests there have been no significant changes in age-standardized incidence rates of TSCIs in the past decade [9, 24, 25]. Nevertheless, given the devastating effect of TSCIs on individual quality of life and the burden on healthcare systems, public health efforts should be aimed at reducing its incidence [19]. In the final year of our study, it was possible to observe how a significantly lower number of TSCIs was recorded as an effect of the COVID-19 pandemic and the governmental decisions to reduce the spread of the disease by restricting individual mobility, thereby impacting on a relevant number of traumatic injuries, primarily due to road and occupational accidents [26].
Age was strongly associated with incident TSCI, with especially high rates above 65 years, as previously reported in literature [1]. Strong sex-based differences have been observed, with stably higher incidence rates among men, that may be explained by behaviors at greater risk of trauma, with more relevant exposures to situations that increased risk of injury (i.e., work in more hazardous professions, higher propensity to driving), that are anyway not limited to any specific setting or cause, as previously reported [1, 27]. The male to female ratio decreased with age, from 4 to just above 2, suggesting TSCIs are more frequent among younger men compared to younger women, while they become progressively more relevant among women in late-life, despite remaining more than twice as common among men. The very high male to female ratio is comparable to that observed in previous studies [3, 10, 28]. A paper published on Lancet Neurology in 2019, on the global burden of TSCI showed that males have divergent incidence patterns compared to women below 60 years, while these trends overlap in older age [1]. These results are comparable with the ones reported in the present study, with the exception of older individuals, where sex-based differences persist. This could be partly explained by social and cultural differences in the present study population that maintain a significant differential risk between men and women. Anyway, the stably higher incidence of TSCIs among men at all ages warrants further studies to examine whether sex may actually be a risk factor per se, in order to promote targeted public health policies addressed to men.
The incidence of cervical, thoracic, and lumbar or sacral injuries, varies a lot in previous literature [3, 29]. In China, cervical lesions account for less than <5% of patients hospitalized with traumatic spinal cord injuries, while in Turkey this percentage rises to 92% [13]. This variability may be partly explained by different causal patterns, although the lack of adequate treatments both geographically and financially determined, is likely to contribute to a significant underreporting in several countries. Socio-economic disparities could play a role in the low rates of patients presenting with cervical injuries given their higher risk of death prior to reaching the hospital and being therefore identified as incident TSCI cases.
When examining the causes of the injury, it was possible to observe that traffic crashes accounted for about half of all TSCI cases among younger men and women. In most countries, in fact, traffic crashes represent the most common cause of TSCIs [3]. A previous article has reported an increase over time in TSCIs related to traffic crashes, especially among men aged ≤30 years [13]. Although no age-stratified cause-specific trend was examined in the present study, the stable incidence of TSCIs among individuals aged 30 years or less, and the stable trend of TSCIs due to traffic crashes in the overall population, are not suggestive of any similar change. Previous works have also observed stable if not decreasing trends in TSCIs caused by road accidents [13, 29, 30]. A possible underestimation of traffic crashes cannot be ruled out, but it is unlikely that this has changed over time, so the solidity of the trends should not have been affected. Variability in road accident rates across countries likely reflect different public health measures targeted at promoting safer driving conditions [31]. TSCIs due to traffic crashes in the present study showed a similar association with age as that observed for occupational accidents, and a similar pattern to that previously reported in literature, with the exception of stable or just slightly decreasing trends among older individuals [32].
Falls are reportedly one of the main mechanisms of TSCIs especially in late-life, with evidence suggesting trends are increasing over time [1, 29, 30]. Falls are a heterogeneous category that comprise a broad range of accidents. When restricting the analyses to the final study period, due to a greater completeness of the codes related to falls, it was possible to confirm how this cause represents more than one third of all TSCIs. Falls were especially relevant in 2020, likely due to lockdowns and restrictions on individual mobility for the COVID-19 pandemic, and a consequent reduction of other relevant causes of TSCIs, such as traffic crashes and occupational accidents. Violence accounts for a relevant number of TSCIs in specific countries. For example, 61–62% of all TSCIs in South Africa are related to violence, while this accounts for <5% of TSCIs in Europe, and it unlikely represents a relevant cause of injuries in Italy, and in the Veneto Region [13].
Mortality
Regardless of the setting and anatomical level of the spinal cord injury, patients with TSCI are at increased risk of premature death [13, 33, 34]. There are wide geographical variations in the reported incidence, prevalence, as well as mortality related to TSCIs. The variability of estimates across countries can be partly explained by differences in terms of mechanism of injury, demographic characteristics of patients, as well as cultural and life-style differences [13]. Different estimates are also likely to be affected by discrepancies in the algorithms used to identify patients from electronic health records, but also to treatment options. Progress in pre-hospital and hospital treatment have led to an improved survival and a consequent increase in prevalent cases with TSCIs. Nevertheless, mortality rates in the first year since the event are still generally elevated, and significantly higher than those observed at greater distance from the accident, but rates vary largely also within the first 12 months, and are especially elevated in the first 30 days [13].
Despite TSCIs being more common among men, no significant differences in adjusted mortality risks could be observed by sex, similarly to what has been previously reported [29]. This could be related to the presence of lesions of similar severity among men and women that did not differentially affect the risk of death.
Age was strongly associated with an increased mortality at all time-points. The present results add evidence in support of mortality rates remaining elevated among older patients even one year after the injury [13, 29, 35]. This finding is suggestive of a portion of deaths that could be related to long-term complications of TSCIs, rather than being primarily or exclusively associated with the most acute phase of the trauma. Older patients with TSCIs are more likely to develop complications and have an overall poorer prognosis with lower potential for rehabilitation than younger patients [13]. Comorbidity and increased frailty are likely to play a role in this finding. The especially high mortality risks observed in advanced age, and the number of TSCIs that is expected to increase due to ageing populations, suggests special attention should be drawn to short- and long-term management of older patients with TSCI [1, 25].
High cervical lesions (C1–C4) were associated with the highest mortality rates at all time-points, and especially one month after the lesion, as confirmed by previous studies [29, 36]. In fact, one month after the injury, mortality rates for patients with high cervical lesions were almost twice those observed for patients with low cervical lesions (C5-C7), and about four times greater than those observed for patients without cervical spinal cord lesions. A difference in mortality rates persisted across the study period, but was less marked, suggesting efforts in reducing mortality related to high cervical lesions should focus especially on the initial phases after the injury.
Occupational accidents and traffic crashes were not associated with higher adjusted mortality risks compared to injuries due to other causes or settings, similarly to what has been observed by Chamberlain et al. [29]. Nevertheless, given these types of accidents are frequently preventable and often affect younger individuals, they warrant public health efforts in promoting road and workplace safety policies.
The two main aspects of TSCI patient management within the regional trauma network are the primary access point (spoke or highly specialized trauma center), and patient transfers within the network. Regional guidelines define clinical criteria to centralize critical patients in highly specialized trauma centers [37]. Nevertheless, the severity of the clinical picture in TSCI patients could worsen, especially in the initial phases following the injury, and might end up requiring a transfer to a highly specialized trauma center, if the initial support has been provided by a spoke hospital. Transfers occurred frequently within the trauma network, concerning almost one fourth of all patients. The absence of differences in short- and long-term mortality risks based on the type of healthcare support provided within the regional trauma network is suggestive of an effective system, with a correct management of healthcare resources based on the stratification of patient needs. In fact, a clinical selection of patients based on an accurate assessment of the complexity of the overall clinical picture, the severity of the injury, and the patient’s need for surgery is performed to determine optimal treatment options. The importance of trauma centers in reducing mortality risks has been previously assessed [38]. Promoting trauma networks could allow healthcare systems to provide a dynamic and effective clinical support. The support of highly specialized spinal cord units within neurosurgical departments can be provided to critical patients that require surgical intervention, while other patients can be safely assisted in spoke hospitals. This allows to optimize expenditure by centralizing highly specialized personnel and equipment for more complex and critical TSCI patients, without hindering the overall quality of care.
Strengths and limitations
The main strength of this paper is a relatively long observation period and a large study population that allowed to perform numerous subgroup analyses. The retrospective study design by means of electronic health records allowed to rapidly identify the cohort of incident TSCI cases, and determine follow-up and outcomes. Although it was not possible to examine the specific setting or cause of the injury other than identifying occupational accidents and traffic crashes, separately analyzing the latter allowed us to obtain largely comparable results with those reported in literature. In fact, despite different classifications of settings and mechanisms of injury have been previously used, numerous studies have focused on traffic accidents [1, 8, 10]. Furthermore, by linkage with mortality records it was possible to monitor not only in-hospital mortality, but also longer term mortality, at 6 and 12 months. Finally, evaluating differences in hospital trajectories within the regional trauma network in relation to mortality risks, has provided evidence in support of the importance of a trauma network that allows to manage patients dynamically based on the individual complexity, adapting to possible changes in the clinical picture.
The main limitation of this paper is the absence of a clinical assessment of the severity of the spinal cord injury. Another limitation concerns the follow-up for mortality that was set at 12 months, but assessment of longer-term mortality could be warranted. Finally, stratification for the cause of death was not performed due to low numbers, but overall mortality was an effective indicator of what characteristics of the patient and of the injury were more strongly associated with an increased risk of death.
Future studies with an accurate assessment of the severity of TSCIs are warranted to better understand the importance of the type of healthcare support received within the trauma network. Studies with a complete and long-term assessment of the burden of TSCIs comprising outcomes and costs related to rehabilitation and disability are also needed to have a complete picture of the impact of TSCIs on public health systems.
Author contributions
CBA contributed conceiving the study and study design, interpreting the results, and writing the manuscript. LS performed the analyses and contributed conceiving the study and study design, interpreting the results, and writing the manuscript. SB contributed conceiving the study and study design, interpreting the results, and writing the manuscript. MS contributed conceiving the study and study design, interpreting the results, writing the manuscript and provided the data.
Data availability
The data used for the present study cannot be shared publicly. Data are available at Azienda Zero, Veneto Region, Clinical Governance of Health Systems Unit upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18:24–5. doi: 10.1016/S1474-4422(18)30444-7. [DOI] [PubMed] [Google Scholar]
- 3.Fehlings M, Singh A, Tetreault L, Kalsi-Ryan S, Nouri A. Global prevalence and incidence of traumatic spinal cord injury. CLEP. 2014;309. [DOI] [PMC free article] [PubMed]
- 4.van der Vlegel M, Haagsma JA, Havermans RJM, de Munter L, de Jongh MAC, Polinder S, et al. Long-term medical and productivity costs of severe trauma: Results from a prospective cohort study. Simmen H-P, editor. PLoS ONE. 2021;16:e0252673. doi: 10.1371/journal.pone.0252673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Burden of Traumatic Spinal Cord Injury in the United States: Disability-Adjusted Life Years - ScienceDirect [Internet]. [cited 2022 Feb 9]. Available from: https://www.sciencedirect.com/science/article/abs/pii/S000399931831298X?via%3Dihub. [DOI] [PubMed]
- 6.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S, et al. Life expectancy after spinal cord injury: a 50-year study. Spinal Cord. 2012;50:803–11. doi: 10.1038/sc.2012.55. [DOI] [PubMed] [Google Scholar]
- 7.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;2:110–6. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 8.Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D, et al. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord. 2011;49:493–501. doi: 10.1038/sc.2010.146. [DOI] [PubMed] [Google Scholar]
- 9.Wyndaele M, Wyndaele J-J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–9. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 10.for the Italian SCI Study Group. Ferro S, Cecconi L, Bonavita J, Pagliacci MC, Biggeri A, et al. Incidence of traumatic spinal cord injury in Italy during 2013–2014: a population-based study. Spinal Cord. 2017;55:1103–7. doi: 10.1038/sc.2017.88. [DOI] [PubMed] [Google Scholar]
- 11.Hagen EM, Rekand T, Gilhus NE, Gronning M. Diagnostic coding accuracy for traumatic spinal cord injuries. Spinal Cord. 2009;47:367–71. doi: 10.1038/sc.2008.118. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RL, Gabella BA, Gerhart KA, McCray J, Menconi JC, Whiteneck GG, et al. Evaluating sources of traumatic spinal cord injury surveillance data in Colorado. Am J Epidemiol. 1997;146:266–72. doi: 10.1093/oxfordjournals.aje.a009262. [DOI] [PubMed] [Google Scholar]
- 13.Hagen EM, Rekand T, Gilhus NE, Grønning M. Traumatic spinal cord injuries-incidence, mechanisms and course. Tidsskr Nor Laegeforen. 2012;132:831–7. doi: 10.4045/tidsskr.10.0859. [DOI] [PubMed] [Google Scholar]
- 14.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–18. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 15.Chiu W-T, Lin H-C, Lam C, Chu S-F, Chiang Y-H, Tsai S-H, et al. Review Paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health. 2010;22:9–18. doi: 10.1177/1010539509355470. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg MEL, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184–92. doi: 10.1159/000279335. [DOI] [PubMed] [Google Scholar]
- 17.Martins F, Freitas F, Martins L, Dartigues JF, Barat M. Spinal cord injuries – Epidemiology in Portugal’s central region. Spinal Cord. 1998;36:574–8. doi: 10.1038/sj.sc.3100657. [DOI] [PubMed] [Google Scholar]
- 18.Kondakov EN, Simonova IA. Poliakov IV [The epidemiology of injuries to the spine and spinal cord in Saint Petersburg]. Zh Vopr Neirokhir Im N N Burdenko. 2002 Jun:50–2; discussion 52–53. [PubMed]
- 19.WHO - Spinal cord injury. Available from: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury.
- 20.Deliberazione della Giunta Regionale del Veneto n. 1239 del 01 agosto 2016: Istituzione della Rete regionale per il Trauma. Piano Socio Sanitario Regionale (PSSR) 2012–2016. [Internet]. Available from: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=328301.
- 21.Deliberazione della Giunta Regionale del Veneto n. 614 del 14/05/2019, Allegato E [Internet]. Available from: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=394700
- 22.Whitney DG, Whibley D, Jepsen KJ. The effect of low-trauma fracture on one-year mortality rate among privately insured adults with and without neurodevelopmental disabilities. Bone. 2019;129:115060. doi: 10.1016/j.bone.2019.115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark DE, Qian J, Winchell RJ, Betensky RA. Hazard regression models of early mortality in trauma centers. J Am Coll Surg. 2012;215:841–9. doi: 10.1016/j.jamcollsurg.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck B, Cameron PA, Braaf S, Nunn A, Fitzgerald MC, Judson RT, et al. Traumatic spinal cord injury in Victoria, 2007–2016. 2019;7. [DOI] [PubMed]
- 25.Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, et al. Traumatic Spinal Cord Injury in the United States, 1993-2012. JAMA. 2015;313:2236. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paganini M, Barbiellini Amidei C, Valastro MF, Favaro A, Saia M, Buja A Adult emergency department visits during the COVID-19 pandemic in Veneto region, Italy: a time-trend Analysis. Intern Emerg Med [Internet]. 2021 Jul 30 [cited 2021 Dec 13]; Available from: https://link.springer.com/10.1007/s11739-021-02815-8. [DOI] [PMC free article] [PubMed]
- 27.for the Italian SCI Study Group. Franceschini M, Bonavita J, Cecconi L, Ferro S, Pagliacci MC. Traumatic spinal cord injury in Italy 20 years later: current epidemiological trend and early predictors of rehabilitation outcome. Spinal Cord. 2020;58:768–77. doi: 10.1038/s41393-020-0421-y. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AB, Dijkers M, DeVivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: Change and stability over 30 years. Arch Phys Med Rehabilitation. 2004;85:1740–8. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MWG. Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Neuroepidemiology. 2015;44:182–98. doi: 10.1159/000382079. [DOI] [PubMed] [Google Scholar]
- 30.Bárbara-Bataller E, Méndez-Suárez JL, Alemán-Sánchez C, Sánchez-Enríquez J, Sosa-Henríquez M. Change in the profile of traumatic spinal cord injury over 15 years in Spain. Scand J Trauma Resusc Emerg Med. 2018;26:27. doi: 10.1186/s13049-018-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor PJ. Trends in spinal cord injury. Accid Anal Prev. 2006;38:71–7. doi: 10.1016/j.aap.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Pérez K, Novoa AM, Santamariña-Rubio E, Narvaez Y, Arrufat V, Borrell C, et al. Incidence trends of traumatic spinal cord injury and traumatic brain injury in Spain, 2000–2009. Accid Anal Prev. 2012;46:37–44. doi: 10.1016/j.aap.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Hagen EM, Lie SA, Rekand T, Gilhus NE, Gronning M. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2010;81:368–73. doi: 10.1136/jnnp.2009.178798. [DOI] [PubMed] [Google Scholar]
- 34.Ahoniemi E, Pohjolainen T, Kautiainen H. Survival after spinal cord injury in Finland. J Rehabil Med. 2011;43:481–5. doi: 10.2340/16501977-0812. [DOI] [PubMed] [Google Scholar]
- 35.Sabre L, Rekand T, Asser T, Kõrv J. Mortality and causes of death after traumatic spinal cord injury in Estonia. J Spinal Cord Med. 2013;36:687–94. doi: 10.1179/2045772313Y.0000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S, et al. Life expectancy after spinal cord injury: a 50-year study. Spinal Cord. 2012;50:803–11. doi: 10.1038/sc.2012.55. [DOI] [PubMed] [Google Scholar]
- 37.Deliberazione della Giunta Regionale del Veneto n. 2127 del 23/10/2012, Allegato A [Internet]. Available from: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=243433.
- 38.MacKenzie EJ, Jurkovich GJ, Frey KP, Scharfstein DO A National Evaluation of the Effect of Trauma-Center Care on Mortality. n engl j med. 2006;13. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the present study cannot be shared publicly. Data are available at Azienda Zero, Veneto Region, Clinical Governance of Health Systems Unit upon reasonable request.