Abstract
The present quality control method of Chinese medicinal materials (CMM) has obvious deficiency, which cannot be compatible with the multi-target and multi-component characteristics and production process of CMM. Plant metabolomics with a huge impetus to comprehensively characterize the metabolites and clarify the complexity and integrity of CMM, has been widely used in the research of CMM. This article comprehensively reviewed the application of plant metabolomics in the quality control of CMM. It introduced the concept, technique, and application examples, discussed the prospects, limitations, improvements of plant metabolomics. MS and NMR, as important techniques for plant metabolomics, are mainly highlighted in the case references. The purpose of this article is to clarify the advantage of plants metabolomics for promoting the optimization of the CMM quality control system and proposing a system approach to realize the overall quality control of CMM based on plant metabolomics combined with multidisciplinary method.
Keywords: Chinese medicinal materials, Plant metabolomics, Quality control, Biomarkers, Multivariate statistical analysis
Introduction
Chinese medicinal materials (CMM) from plant sources are plants with preventive and therapeutic effects in diseases. China has more than 10,000 kinds of medicinal plants, about 87% of the total number of CMM resources. Different therapeutically active ingredients, from plant sources, have long been used in China. Some CMM with dual-purpose of drug and food, such as peach, jujube and plum, have been recorded as early as 2600 years ago. The Chinese pharmacopoeia (ChP) (2020 edition) has documented 499 categories of CMM from plant sources. In recent years, especially in fighting against COVID-19, CMM has played a significant role, such as forsythia, honeysuckle and ephedra in Lianhua Qingwen capsules (granules) [1, 2]. In so that, CMM attracted a growing amount of attention as the choice of clinical treatment and the source of new drug discovery. A controlled quality evaluation standard is a national strategy for the development of scientific industry, the modernization and internationalization of CMM, as well as the indispensable guarantee of safety and effectiveness in clinical treatment [3, 4].
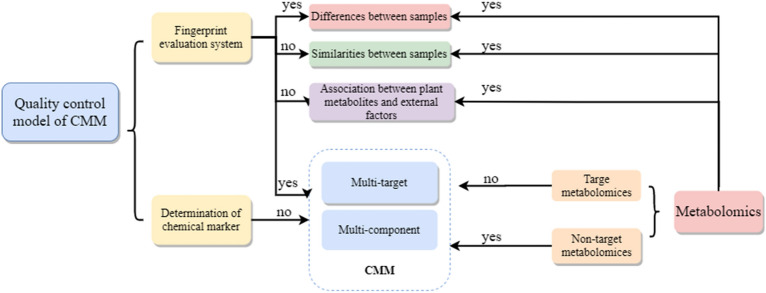
The quality of CMM is closely related to their metabolites. CMM biosynthetic secondary metabolites with changeable structure and diverse activities are usually the pharmacodynamic basis of CMM. During the production, these metabolites are prone to interference and produce changes, affecting the quality of CMM [5, 6]. It is difficult to achieve the scientific evaluation of the quality of CMM by current quality evaluation mode which is mainly consisted of determination of chemical markers and the fingerprint evaluation system. The former takes the single component as the evaluation mark, which cannot comprehensively represent the components of CMM [7], while the latter focuses on the similarity between samples and is therefore difficult to evaluate the differences [8, 9] (Fig. 1).
Fig. 1.
Comparison of existing CMM quality control system and metabolomics methods
Plant metabolomics is well suited for the quality control of CMM due to it can conduct a comprehensive detection of the metabolites of living organisms. It can be used to qualitatively and quantitatively analyze all small molecular metabolites of plants with different species, genotypes and ecological types, and find the difference between samples [10]. At present, plant metabolomic has been mainly applied to the source identification, authenticity identification, geoherbalism analysis, processing method evaluation and other quality control links of CMM [11, 12]. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) are important analytic techniques, widely used in metabolomics studies. We introduce the principles, the technical composition of plant metabolomics, and collect its application in the quality evaluation of CMM in recent years. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) are important detection techniques of plant metabolomics, their application were highlighted in the paper. Moreover, advantages and disadvantages of current analytical strategies in the innovations of CMM quality were discussed, and a systems strategy to substantially improve the performance of current strategies towards a new-horizon solution to systems-level quality evaluation of CMM was proposed.
The concept and major technology of plant metabolomics
The concept of plant metabolomics
Plant Metabolomics, a branch of metabolomics, is designed to study the overall changes in a large number of metabolites in plant samples and then conduct deep data mining and bioinformation analysis. Metabolites, components of defense systems developed by plants in response to pathogen attacks and other environmental stresses, are an important source of many natural pharmacological activity products which are often designated as specific biological activities associated with their biochemical structures [5, 6].
The main techniques of plant metabolomics
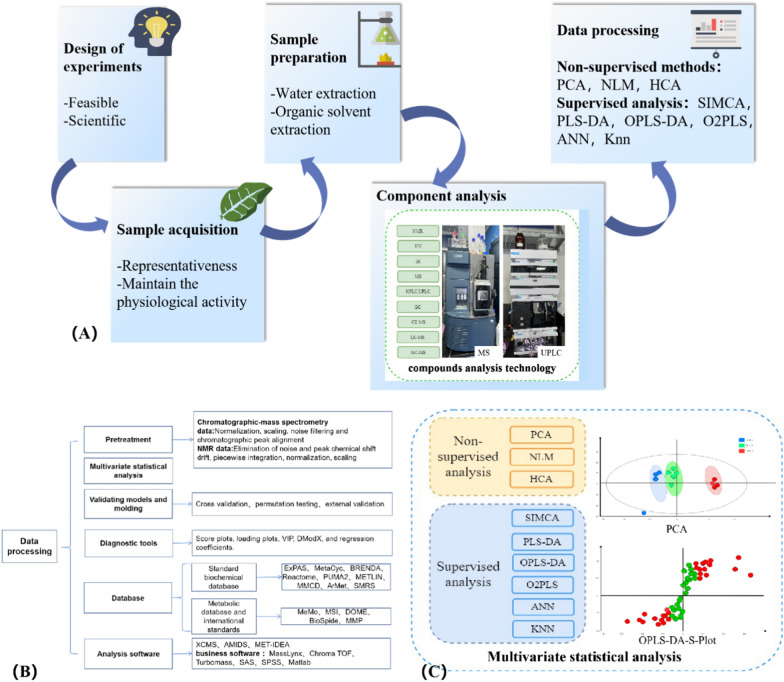
Complete plant metabolomics research process includes experimental design, sample collection, sample processing, sample preparation, detection and analysis, data processing, and metabolic pathway analysis/metabolic network analysis [13]. Analytical techniques and data processing techniques are the two important parts of metabolomics and metabolomics strategy is based on three main techniques: NMR, MS and chromatographic techniques [11, 12]. The common techniques applied to the studies of plant metabolomics are described below. Additionally, MS and NMR are the most advanced detection technology in recent years, with great application potential, and we mainly discuss their advantages and disadvantages.
Separation technology
Chromatography is mainly applied to the separation of metabolites GC is mostly used in the study of CMM rich in volatile oils, such as Cyperi Rhizoma, Myristica fragrans Houtt, and Rhizoma Chuanxiong [14–16]. High performance liquid chromatography (HPLC) and ultra performance liquid chromatography (UPLC) are appropriate for the separation and analysis of substances with high boiling points, macromolecules, strong polarity, and poor thermal stability [17–20]. CE achieves a breakthrough in analytical chemistry from microrise to nanorise, making a single cell or even single molecule analysis possible [21, 22]. CE is particularly suitable for the analysis of (highly) polarity and charged metabolites such as amino acids, nucleotides, small organic acids and phosphoric sugars [23–27].
Detection technology
The detection techniques of plant metabolomics mainly include UV, IR, MS, NMR. MS is characterised by extreme sensitivity and can provide qualitative and quantitative information according to the molecular weight [28–30]. Electrospray ionization (DESI) can achieve open environmental detection real-time analysis, which is more suitable for the analysis of drugs and endogenous small molecules. Orbitrap analyzer, time-of-flight (TOF) analyzer, and fourier transform ion cyclotron resonance (FTICR) analyzer for high resolution MS, enables the determination of fine structure [31]. The disadvantages of MS include inaccurate identification of unknown compounds, quantitative analysis depending on the reference substance, and the need of purity of the mixture and prior separation. MS is often coupled with a separation technique, such as liquid chromatography (LC), gas chromatography (GC) or capillary electrophoresis (CE) [32].
NMR, especially 1H-NMR, 1C-NMR and 31P-NMR, in plant metabolomics, commonly used in identification of species and growth years, metabolic pathways research, pharmacodynamics study, and methodology evaluation of CMM. NMR shows the absolute advantage in the identification for unknown compounds, which is of value in new compound discovery [33]. The sample preparation of NMR is simple, 1H high resolution magic angle rotation (HRMAS) can even be used for direct sample analysis [34]. In addition, sample detection of NMR is non-destructive and can be associated with downstream detection to achieve high-throughput determination [35]. However, even if new probes such as cryogenically cooled probes and microcoil probes appear, it is difficult to surpass the MS in sensitivity. In recent years, some studies have shown that the small high-temperature superconducting coils and ultra poltechnology can improve NMR sensitivity, but not widely used [36].
In addition, ultraviolet spectrum (UV) as a fundamental analysis technique has been widely used in the research of CMM [37, 38]. In recent years, infrared (IR) was applied to the study of enzyme activity, grease and fat, species difference, harvesting time, and source of CMM [39–43].
Data processing techniques
The pattern recognition technology is mainly for data processing, including unsupervised analysis and supervised analysis [44–47]. Principal components analysis (PCA) can clearly demonstrates repeatability within data group and differences between data groups [49], describing the data in a minimum dimensions but guarantee reproducibility. Partial least squares discriminative analysis (PLS-DA) and orthogonal projections to latent structures (OPLS-DA) reduce the data dimensionality and analyse them with a regression model [50]. Compared to PCA, PLS-DA/OPLS-DA establishes the model between metabolic expression and grouping relations, which better obtains intergroup difference information and predicts the grouping of samples [51]. In a practical application, several statistical methods are often combined according to the data characteristics and research requirement. Necessarily, these detection and analysis techniques depend on existing software and databases. (Fig. 2).
Fig. 2.
Process, technology and methods of plant metabolomics: A The process of plant metabolomics. B Contents included in the data processing [111–113]. C The classification of multivariate statistical methods and figure of PCA and OPLS-DA [114–118]
The application of plant metabolomics in quality evaluation of Chinese medicinal materials
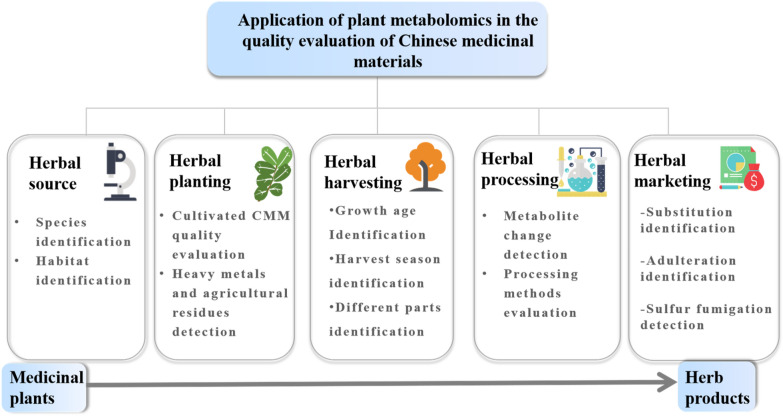
Unlike chemosynthetic drugs, CMM is a complex system composed of multiple chemical components. It is characterized by diverse chemical compositions, diverse structures and huge content differences [51, 52]. In addition, the species and contents of chemical components are susceptible to be influenced by factors such as germplasm resources, growth conditions, cultivation and processing methods, etc. [53–55]. MS-and NMR-based metabolomic techniques can be used to monitor key indicators of the production link of CMM by analyzing changes in the type and content of metabolites. Below we mainly discuss the application of MS-and MNR-based metabolomics techniques in five production links of CMM: sources, planting, harvesting, processing and market sales (Fig. 3).
Fig. 3.
The application of plant metabolomics in the quality control of CMM
Herbal source
The types and contents of the active metabolites in CMM are highly variable depending on the species, parts of the plant, cultivated geographic region, and planting period involved.
Species identification
Species genetically determine the difference in types and contents of plant metabolites, leading to differences in clinical effects. The current ChP stipulates that the original plant of licorice herbs is Glycyrrhiza Uralensis, Glycyrrhiza inflata or Glycyrrhiza glabra. The chemical composition and efficacy of the three kinds of licorice herbs differ significantly, but the mixing use of same generic plants often occurs frequently. Fukuda et al. used direct analysis in real time (DART)-MS to detect the metabolites of several licorice species, the peak at m/z 339 was mainly derived from [M + H]+ of licochalcone A, which is present in Glycyrrhiza inflata and is hardly detected in Glycyrrhiza glabra and Glycyrrhiza Uralensis. Therefore, licochalcone A can serve as a candidate biomarker to distinguish these three licorice species, which has great implications for ensuring the clinical efficacy of licorice [56]. In one study, in vitro activity experiments and NMR based metabolomic were combined to comprehensively measure the activity differences of Artemisia afra and Artemisia annua, two antimalarial drugs come from Africa and China. The antiplasmodial activity of Artemisia afra and Artemisia annua extracts were tested for activity against Plasmodiam falciparum 3D7, and the activity was only found in polar extracts of Artemisia annua. 1D- and 2D-NMR spectroscopy identified 24 semi-polar components including three novel phenylpropane for this species: caffeic acid, chlorogenic acid and 3,5-dicaffeoyl quinic acid, which can be seen as the biomarkers of this species. Combined with the in vitro experiments, metabolomics allows for a more comprehensive evaluation of the quality of CMM based on the compounds and activity [57]. The combination of metabolomics and molecular techniques shows the unique advantages of species identification. GC and TOF–MS combined with DNA molecular markers was applied to distinguish Astragalus mongolica and Astragalus membranaceus. In a result, three markers and eight metabolites were identified as candidate DNA and metabolic markers [58]. 1H-NMR combined with DNA barcoding technique ssuccessfully demonstrated that different genetic variation and chemical constituents existed among 3 different Seabuckthorns, Hippopahe gyantsensis., Hippopahe Neurocarpa and Hippopahe tibetana [59]. The combination of molecular techniques and MS and/or NMR based metabolomics reveals the nature of metabolite changes at the genetic level, which is conducive to the exploration of metabolic rules and functional compound synthesis using molecular technology.
Habitat identification
The variety, quantity and quality of CMM resources are restricted by the natural conditions of the cultivated geographic region. The habitat of ginseng has an important impact on the type and number of ginsenosides which are often used as biomarkers to evaluate the quality of Panax ginseng. Song et al. successfully identified the difference between Korean white ginseng (KWG) and Chinese white ginseng (CWG) using UPLC-Q-TOF–MS and OPLS-DA. Ginsenoside Rf and notoginsenoside R3 isomer in KWG can be used as biomarkers to make a distinction with the CWG which is rich in ginsenoside Ro and chikusetsusaponin IVa [60]. Dae et al. distinguished Panax ginseng grown in Korea, China and Japan by using rapid resolution liquid chromatography (RRLC)-QTOF/MS combined with PCA and PLS-DA. In the PCA score plot, ginseng samples from the three regions were significantly separated, revealing the obvious effect of the planting region on the metabolites [61]. The LC–MS based metabolomics technology has been widely used in the identification of Panax ginseng from different habitat, providing a powerful method to ensure the quality of valuable CMM. Astragalus membranaceus and Paeonia albiflora with different origins in Korea and China were analysed by 1H NMR and inductively coupled plasma atomic emission spectro/inductively coupled plasma-mass spectrometry (ICP-AES/ICP-MS). The results indicated that the complementary multielement and metabolomic data were more suitable for determining the geographical origin than the use of each individual data set alone [62]. HPLC coupled with Q-TOF–MS was used to analyze metabolites from dandelions (Taraxacum mongolicum) from four different geographical regions in China, namely Gansu, Henan, Shanxi, and Jiangsu. OPLS-DAD was applied to analysis metabolite and MBRoleto was used to identify potential metabolic pathways. This study found that the differential metabolites of dandelion in these four regions were mostly phenolic, and the biosynthesis of phenylpropanoids and flavonoids were main metabolic pathways involved. Environmental stress generates an enhancement of these metabolic pathways, causing elevated metabolite concentrations, so the samples from Gansu has higher quality relatly. This study not only analyze the metabolite differences between samples, but also conducted the exploration of metabolic pathways to speculate on the association between climate factors and metabolites, which can be used to guide production [63].
Herbal planting
The higher market demand and the sharp decrease of wild herbal resources encourage the development of herbal planting, which can ease the current contradiction between medicinal resources supply and demand. The problems such as evaluating and comparing the quality of the cultivated and wild, residues of pesticide and herbicides, and excessive use of chemical fertilizers have aroused widespread concern.
Cultivated CMM quality evaluation
Although artificially cultivated CMM are booming, cultivated products and wild products often differ in the content of active compounds, which may affect the clinical efficacy of CMM. Agarwood, an aromatic medicinal materials mainly formed from species of Aquilaria, is mainly rich in 2-(2-phenylethyl) chromones. Based on characterization of the 2-(2-phenylethyl) chromones via ultra-performance liquid chromatography coupled with electrospray ionization mass spectrometry (UPLC-ESI-QTOF-MS), 14 biomarkers were found from wild and cultivated agarwood. Unfortunately, some chromone isomers were difficult to distinguish clearly due to the most of the compounds have the same molecular formula and similar fragment ions, which requires more sensitive detection methods [64]. Cultivated Panax quinquefolius, a very common analogue of wild products, is hard to distinguish in the traditional way due to complex ingredients. 1H NMR-PCA was applied to study 37 batches of sample from 15 pharmacy stores over 5 provinces in China, which built a model which excises the most abundant metabolites of Panax quinquefolius extract from the NMR spectra before PCA, and correlated the differences between Panax quinquefolius types to the less abundant metabolites. It is the first time for the 1H NMR used in Panax quinquefolius and large sample size studies, which provided a new method for other CMM study [65]. According to the growth environment and the cultivation method, ginseng can be divided into three types: cultivated ginseng (CG), mountain-wild ginseng (MWG), and mountain-cultivated ginseng (MCG). The ChP also classified ginseng into CG and MCG groups, and there was no a viable way to distinguish them before. Xu et al. identified 40 ginsenosides in MCG and CG using a plant metabolomics method consisted of UPLC-QTOF-MS/MS and multivariate statistical methods. This is the first observed chemical composition differences between MCG and CG, providing theoretical guidance for the subsequent cultivation of high-quality ginseng [66]. The root of wild Salvia miltiorrhiza is red owing to the periderm is rich in tanshinones while the root in cultivated fields showed orange. In a study, metabolome and transcriptome analyses were integrated to analyze the differences of metabolites between two Danshen (Salvia miltiorrhiza) in different colors. UPLC/Q-TOf–MS based metabolomic detected 40 lipophilic components and 7 of them including tanshinone IIA and tanshinone I were obviously decreased in the orange one. The results of transcriptome analysis indicated that decreases in the content of dehydrogenated furanring tanshinones such as tanshinone IIA resulted in phenotypic change and quality degradation of Danshen. These changes may be related to environmental deterioration and biological stress. The combination of the metabolome and transcriptome can excavate the factors affecting the quality from the source while conducting the quality evaluation of CMM, which has the guiding significance of the actual production [67].
Heavy metals and agricultural residues detection
Heavy metals and agricultural residues are the safety control index of cultivation and the hotspot of quality control of CMM. The accumulation of secondary metabolites in many CMM is related to external environmental stress, such as heavy metals, drought, waterlogging. The research and analysis of stress resistance of CMM in extreme environments are the research trend of future CMM cultivation, which is the research basis for improving the quality of cultivated products. Luo et al. used GC–MS technique, combined with PCA and OPLS-DA, to explore metabolite differences in Sebum Alfredii root exudates in different environments. The result displayed that 12 compounds as biomarkers to distinguish different treatment conditions, indicating that Sedum Alfredii could tolerate super-rich heavy metal cadmium by regulating their secretion. With the deterioration of the ecological environment, the research on tolerating extreme environmental CMM will inevitably become a hotspot, and these studies will bring the cultivation of CMM to a more promising direction [68]. Moreover, the use of pesticides, including herbicides, fungicides, insecticides, and plant growth regulators, could also affect the secondary metabolism of CMM. UPLC/Q-Orbitrap-Full MS scan method combined with multivariate statistical analysis were employed to evaluate effect of two insecticides, imidacloprid and compound flonicamid and acetamiprid, on the overall composition of Lonicerae Japonicae Flos (LJF). The experiment obtained and characterized 29 metabolic markers including iridoids and carried out relative quantitative assay to monitor their changes at different times of flowering developments. These decreased iridoids should be considered as important markers in the holistic quality assessment of LJF. This is the first time to study the impact of pesticides on metabolites of LJF, which provides a basis of pesticide indicators for the quality control of bulk cultivated CMM [69]. The use of pesticides is a necessary link for the mass production of CMM. With the application of plant metabolomics technology, we can find pesticides friendly to plant growth or with less influence, which will promote quality improvement of CMM cultivation (Table 1).
Table 1.
Application of plant metabolomics in the quality evaluation of CMM
| Application | Species | Data processing technique | Analytical techniques | Number of metabolites | Biomarker | Effect | Refs. |
|---|---|---|---|---|---|---|---|
| Herbal source | |||||||
| Species identification | Glycyrrhiza Uralensis, Glycyrrhiza inflata or Glycyrrhiza glabra | / | DART (Direct Analysis in Real Time)-MS | 1 | Licochalcone A (LA) | Pain relief, antitussive, anti-inflammatory, anti-allergic, enhanced immunity | [56] |
| Artemisia afra and Artemisia annua | PCA | 1D- and 2D-NMR | 24 | 24 semi-polar components | Anti-malaria, anti-inflammatory, immunomodulatory | [57] | |
| Astragalus membranaceus and Paeonia albiflora | KNN, LDA, SVM, PLS-DA | 1H NMR and ICP-AES/ICP-MS | 8 | 4-aminobutyrate, acetate, alanine, arginine, asparagine, benzoate, choline, citrate, glucose, etc | Regulation of immunity, suppression of inflammatory response, anti-oxidation, anti-tumor, anti-infection | [58] | |
| Hippopahe gyantsensis., Hippopahe Neurocarpa and Hippopahe tibetana | PCA | 1H-NMR | 32 | Flavonoids, amino acids, organic acids, and fatty acids | Regulation of metabolism, anti-tumor | [59] | |
| Habitat identification | Panax ginseng | OPLS-DA | UPLC-Q-TOF–MS | 23 | Ginsenoside Rf, notoginsenoside R3 isomer, ginsenoside Ro and chikusetsusaponin Iva | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, enhanced immune function | [60] |
| Panax ginseng | PCA and PLS-DA | RRLC-QTOF/MS | 2 | Ginsenosides | [61] | ||
| Astragalus membranaceus and Paeonia albiflora | LDA, KNN, SVM, PLS-DA | 1H NMR and ICP-AES/ICP-MS | 7 | Calycosin, formononetin, linoleic acid, etc | Regulation of immunity, suppression of inflammatory response, anti-oxidation, anti-tumor, anti-infection | [62] | |
| Taraxacum mongolicum | PCA, OPLS-DA, | HPLC-Q-TOF–MS | 54 |
Guanosine 50 -monophosphate, phosphoric acid, gallic acid, catechol, citraconic acid, cis-aconitate,, etc |
diuresis, anti-inflammatory, and promote digestion | [63] | |
| Herbal planting | |||||||
| Cultivated CMM quality evaluation | Ggarwood | PCA, OPLS-DA | UPLC-ESI-QTOF-MS | 1 | 2-(2-phenylethyl) chromones | Antioxidant and bacteriostatic, analgesic and sedative, anti-inflammatory, anti-cancer | [64] |
| Panax quinquefolius | PCA | NMR | 6 | Sucrose, glucose, arginine, choline, and 2-oxoglutarate and malate | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, enhanced immune function,etc | [65] | |
| Panax ginseng | PCA, PLS | UPLC-ESI-QTOF-MS | 40 | Ginsenosides | [66] | ||
| Salvia miltiorrhiza | PCA, OPLS-DA | UPLC-Q-TOF/MS | 17 | Alkaloids | Anti-tumor, anti-atherosclerosis, anti-neuroinflammation, inhibiting myocardial hypertrophy | [67] | |
| Heavy metals and agricultural residues detection | Sedum Alfredii | PCA, OPLS-DA | GC–MS | 12 | / | / | [68] |
| Lonicerae Japonicae Flos | PCA.OPLS-DA | UPLC/Q-Orbitrap-Full MS scan | 29 | Chlorogenic acids, iridoids and organic acid-glucosides | Anti-virus, anti-lipids, anti-tumor factors, anti-bacterial, anti-allinflammatory | [69] | |
| Herbal harvesting | |||||||
| Growth age Identification | Panax ginseng | PCA | UPLC-QTOF/MS | 30 | / | Prevent aging, eliminate fatigue, improve memory, anti—aging, anti—tumor | [70] |
| Panax ginseng | / | HPLC-MRM/MSA | 301 | ginsenosides | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, enhanced immune function, etc | [32] | |
| Polygala tenuifolia Willd | PCA | UPLC-QTOF-MS | 26 | Tenufolia, fructose, sucrose, choline, glycine, raffinose, onjisaponin Fg, polygalasaponin XXVIII, etc | Enhance Intelligence, sedative-hypnotic, expelling phlegm and arresting coughing, anti—inflammatory, neuro protection, etc | [71] | |
| Harvest season identification | Panax ginseng | OPLS-DA | 1H- NMR | 24 | Ginsenosides, arginine, sterols, fatty acids, and uracil diphosphate glucose–sugars | Enhancing learning and memory, strong hear, antishock, anti-myocardial ischemia, enhanced immune function, etc | [72] |
| Piper Sarmentosum | PCA | FTIR | / | / | Anti-amoebic, antibacterial, anti-neoplastic, neuromuscular blocking, hypoglycemic, antioxidant,etc | [73] | |
| Pericarpium Citri Reticulatae (PCR) and Pericarpium Citri Reticulatae Viride (PCRV) | PCA | HPLC, HELP | 16 | Naringin, hesperidin, nobiletin, tangeretin | Anti-inflammatory, anti-oxidation, regulating metabolism, neuroprotection, anti-tumor | [74] | |
| Different parts identification | Cistanche deserticola | PLS | UPLC-PDA-Q/TOF–MS | 6 | Echinacoside, cistanoside A and 2 ' -acetylacteoside | Neuroprotection, immunomodulation, anti-aging, anti-osteoporosis, protecting liver and protecting liver | [75] |
| Ginseng | PCA, OPLS-DA | UHPLC-QTOF/MS | 81 | Rutinum, 20(R)-Notoginsenoside R2, Ginsenoside F3, etc | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, enhanced immune function, etc | [76] | |
| Gentiana crasicaulis | PCA, OPLS-DA | UPLC-ESI-HRMSn | 36 | Hydroxycinnamic acid amides, trans-N-caffeoyl phenethylamine | Anti-inflammatory, anti-allergy, acesodyne | [77] | |
| Mulberry | PCA, SIMCA | 1HNMR | 24 | / | Anti-inflammatory, anti-oxidation, anti-tumor, hypoglycemic, anti-hyperlipidemia, liver protection | [78] | |
| Clausena lansium (Lour.) Skeels | PCA, OPLS-DA | UPLC-Q-Orbitrap-MS | 364 | β-Alanine, 4-Pyridoxic acid, Phosphorylcholine, N-Acetylglutamic acid, N-Methyltyrosine, etc | Promote digestion, anti-influenza, relieve pain | [79] | |
| Herbal processing | |||||||
| Metabolite change detection | Astragali Radix | OPLS-DA, PCA | UPLC-QTOF-MS | 15 | Calycosin-7-O-β-Dglucopyranoside, Calycosin, formononetin, (6αR, 11αR)- 10- Hydroxy-3, etc | Anti-tumor, protection of cardio-cerebrovascular, improvement of immune function, etc | [80] |
| Radix Polygala | / | UPLC-QTOF-MS\NMR | 29 | 3,6′-disinapoylsucrose ester, amino acids,organic acid, and carbohydrates, total saponins | Enhance Intelligence, sedative-hypnotic, expelling phlegm and arresting coughing, anti—inflammatory, neuro protection, etc | [81] | |
| Panax notoginseng | / | 1H-NMR/ UPLC | 29 | Ginsenoside Rf, 20(S)‐pseudoginsenoside F11, malonyl gisenoside Rb1, etc | Anti-cancer, lipid-lowering, neuroprotection, endothelial cell protection, bone repair, anti-fibrosis | [82] | |
| Rheum palmatum | PCA, OPLS-DA | UPLC/Q-TOF–MS | 2 | Emodin-8-O-glucoside and gallic acid-3-O-glucoside | Improve the digestive system, regulate the blood system, promote metabolism, affect the nervous system, etc | [83] | |
| Aconitum carmichaelii Debx | PLS-DA, PCA | RPLC-Q-TOF/MS | 22 | Dehydrated Benzoylmesaconine, Dehydrated Benzoylaconine, 10-OH-Benzoylaconine, etc | Anti-inflammatory, relieve pain, anti-tumor, cardiovascular protection | [84] | |
| Euphorbia pekinensis Root | PCA | UPLC-MS | 7 | 3,3'-di-O-methylellagic acid-4'-O-β-D-xylopyranoside,3,3'-di-O-methyl ellagic acid, etc | Reversal of P-glycopprotein-mediated multidrug resistance, cytotoxicity, lipase inhibitory activity, etc | [85] | |
| Processing methods evaluation | Panax notoginseng | PLS-DA, PCA | UHPLC/TOF MS | 40 | Ginsenoside Ra3/isomer, gypenoside XVII, quinquenoside R1, ginsenoside Ra7, notoginsenoside Fe, etc | Anti-cancer, lipid-lowering, neuroprotection, endothelial cell protection, etc | [86] |
| Ginseng | ANOVA | HPLC | 10 | Ginsenosides | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, etc | [87] | |
| Notopterygium franchetii | PCA, HCA | UHPLC-QTOF-MS–MS | 30 | Nodakenin, psoralen, bergapten, notopterol, imperatorin, and isoimperatorin | Anti-inflammatory, anti-oxidation, anti-arrhythmic, anti-bacterial, etc | [88] | |
| Salvia miltiorrhiza | / | NMR and LC-DAD-MS | 81 | sugars, carboxylic acids and amino acids, polyphenolic acids and diterpenoids, etc | Anti-tumor, anti-neuroinflammation, inhibiting myocardial hypertrophy | [89] | |
| Astragalus root | PCA | H-1 NMR /UPLC-MS | 19 | acetate, alanine, arginine, caprate, fumarate, glutamate, valine, and some xylem-related compounds | Anti-tumor, protection of cardio-cerebrovascular, improvement of immune function, etc | [90] | |
| Herbal marketing | |||||||
| Hydrastis canadensis(goldenseal) canadensis | PCA | MS | 12 | Berberine, hydrastine, canadine, palmatine, coptisine, dihydrocoptisine | Antimicrobial, anti-inflammatory, hypolipidemic, hypoglycemic, antioxidant, neuroprotective, etc | [91] | |
| Panax ginseng | OPLS-DA | 1H NMR | 15 | / | Enhancing learning and memory, enhanced immune function, strong heart, antishock, etc | [92] | |
| Panax ginseng | OPLS-DA, PCA | UPLC-QTOF-MS/MS | 43 | ginsenoside Rg3, a nitrogen-containing component, ginsenoside 20(R)-Rh, etc | Enhancing learning and memory, strong heart, antishock, anti-myocardial ischemia, etc | [93] | |
| Trichosanthis Radix | OPLS-DA, PCA | UPLC-ESI-QTOF-MS/MS, UPLC-ESI-QTRAP-MS/MS | 25 | 5 sulfur-containing components, 20 non-sulfur marker metabolites | Anti virus, anti—tumor, odinopoeia | [94] | |
| Rhodiola | PCA | NMR, HPTLC | / | salidroside | Lowering blood lipids and blood sugar, improve physical, mental, memory, attention and immunity, anti-aging, etc | [95] | |
| Rhodiola | OPLS-DA | HPLC–UV, NMR | 5 | Coumarin, Crenulatin, rosavins | [96] | ||
| Angelica acutiloba | PCA | GC-TOF–MS | 22 | Sugars, fructose and glucose, phosphoric acid, proline, malic acid and citric acid | Improving anemia, protecting liver, immunomodulation, anti-tumor, anti-inflammatory | [97] | |
Herbal harvesting
The growth age and harvest season directly affect the quality, yield and harvest rate of CMM. Moreover, the types and content of active metabolites in different parts of CMM are different, which determines the clinical selection.
Growth age identification
Growth age has a significant impact on the contents of metabolites in plants. Ginseng is easily affected by differences in growth years, and 4, 5 and 6 years old Ginseng is in high demand in the market due to have higher levels of active substances. UPLC-Q-TOF–MS combined with PCA and HCA methods was developed to evaluate various ginsenosides in Panax ginseng roots between 1 and 6 years old. The results show that 30 ginsenosides profiling was carried out and 14 ginsenosides in P. ginseng roots cultivated for 4, 5, and 6 years can be clearly identified [70]. MALDI-MSI is a powerful tool to localize the components distribution and its cross sections showed the distribution of ginsenosides from P. ginseng roots cultivated for 4, 5, and 6 years, which provides the indicator of growth age for the quality control of ginseng [32]. The active compounds of Polygala tenufolia is apt to be affected by its growth age, and the market generally considers Polygala tenufolia aged 2–3 years of better quality. In Chp, Tenuifolin, Polygalaxanthone III, (3-sinapoyl) fructofuranosyl-(6-sinapoyl) glucopyranoside were measured as the detection index for quality of Polygala tenufolia. UPLC-QTOF-MS and NMR based metabolomic technology has found that 1 year-old Polygala tenufolia was significantly different from those of 2 and 3 years old due to differences between primary and secondary metabolites, and a portion of those index compounds will decrease accordingly as age grows. This result shows that those primary or secondary metabolites, with marked changes, are perhaps more appropriate quality markers [71]. Growth age is an important problem in CMM, which determines its quality. The UPLC-Q-TOF–MS and NMR methods were applied to the study of growth age of CMM, which effectively solves the problem of distinguishing different years.
Harvest season identification
Harvesting in different seasons affects the quality of CMM, because of the formation and enrichment of compounds are affected by seasonal factors. Lee et al. used 1H NMR metabolomics to explore the relationship between ginseng metabolites and climate. The results show that the growing season from March to October is of great importance for the synthesis of ginseng metabolites. Especially, it has been shown that March to May may be the optimal synthesis time for ginsenosides, arginine, steroids, fatty acids and uracil diphosphate glucose-sugar, a process accompanied by accelerating sucrose catabolism that may be associated with climate change, such as sunshine time and rainfall [72]. One study was designed to investigate the appropriate harvest time of Piper Sarmentosum fruits to maintain consistency of efficacy and batch reproducibility. Plant metabolomics was applied to detect, quantify and catalogue the time related metabolic processes of the samples. The Fourier transform infrared spectroscopy (FTIR) spectral data were analyzed by stoichiometry and PCA, and identification, classification and differentiation (ICD) evaluation were obtained using PerkinElmer software. These results indicated that the metabolites in the samples varied in all the batches. FTIR fingerprint profiles of the samples in combination with chemometrics is an effective tool. It is concluded from the results of this study that plant metabolomics in combination with chemometrics is an effective tool of fast and easy assessment of similarity of different samples and may be applied as an analytical tool in quality evaluation [73]. Pericarpium Citri Reticulatae (PCR) and Pericarpium Citri Reticulatae Viride (PCRV), two different maturation stages of the same plant source, have different clinical effects. Based the HPLC fingerprints, heuristic evolving latent projection (HELP) method was applied to obtain two-dimensional data and chemometrics. The growth footprint of Tangerine peels was given by PCA score chart, which show that July may be the best harvest time for PCRV, and November and December are better for PCR [74].
Different parts identification
Due to the differences in the distribution of the active compounds, different plant parts have been regarded as different drugs. The Chp also makes detailed requirements for the medicinal parts of medicinal plants. The succulent stem is the medicinal site of Cistanche deserticola, and few studies have focused on other parts. In one study, an UPLC-PA-QTOF-MS based metabolomic method was applied to identify the chemical constituents and screen the antioxidant activity profiles of 6 different parts from cultivated Cistanche deserticola. An obvious difference was observed between the chemical profiles and content distribution of phenylethanoid glycosides (PhGs). The results showed a significant decreasing trend from the bottom to the top of cultivated Cistanche deserticola and the highest content in the stems. This combination of metabolomics and antioxidant activity detection promotes the link between composition and activity, which will characterize the quality of CMM by a more comprehensive way and provide a guarantee for clinical efficacy [75]. Chang et al. utilized UHPLC-QTOF/MS combined with PCA and OPLS-DA analysis methods to identify different parts of ginseng, showing significant differences in chemical composition. A total of 81 major metabolites were detected from 4 different sites of the ginseng, and 70 metabolites was finally identified or preliminarily inferred. The plant metabolomics analysis of different parts of ginseng is beneficial to clarify the differences in pharmacodynamic substances of different parts of ginseng for treating different diseases and this method is fast, accurate, and reliable [76]. UPLC-ESI-HRMSn based metabolomic identified 36 biomarkers from different parts of Gentiana crasicaulis. The result shows that iridoids are mainly enriched in the root, while flavonoids and triterpenes were mainly concentrated in the aerial part. Through the detection of different compounds, different plant sites can be clearly distinguished [77]. Additionally, 1HNMR combined with PCA and SIMCA was successfully used to identify the differences on compounds from 6 sites of mulberry leaf [78]. In recent years, a chemical profiling of seven parts of Clausena lansium was built by a non‐targeted UPLC‐Q‐Orbitrap‐MS metabolomic method. 364 metabolites including 62 potential biomarkers were selected by the multivariate statistical analysis. Heat map and KEGG annotation showed a significant enrichment of the “Flavone and flavonol synthesis” and “Isoquinoline alkaloid biosynthesis” pathway. This investigation could provide a foundation for the isolation and identification of new constituents from Clausena lansium and further clarified the biosynthetic pathway of differential metabolites among various tissues of Clausena lansium, which can be used for reference by other studies of CMM [79].
Herbal processing
Processing of drugs necessarily leads to changes in metabolites that affect efficacy. The processing of CMM is called paozhi, an indispensable production process. The traditional processing methods are based primarily on the theory and the character of CMM which includes various methods, like parching, roasting, moistening, burning. The purpose of processing is eliminating or reducing the toxicity of drugs, strengthening the curative effect, facilitating the preparation or storage, and making the drugs pure.
Metabolite change detection
Changes in drug composition caused by processing will alter their clinical effects. UPLC-QTOF-MS based metabolomic paired with novel informatics UNIFI platform have been undertaken for evaluating the changes in chemical components of Astragali Radix after processing. PCA scores and OPLS-DAS maps indicate that there are some changes in compound composition of Astragali Radix processed by heating and auxiliary materials. For example, the content of Calycosin-7-O-β-Dglucopyranoside varies significantly after processing, while several unknown compounds are newly generated after processing, which can applied as the biomarkers. The present study provided a basis of chemical components for revealing connotation of different processing techniques on Astragali Radix [80]. Similarly, different processed and raw products of Radix Polygala were studied by NMR and UPLC. PCA analysis results showed that the metabolic composition of Radix Polygala was obviously different. Moreover, there are also differences in metabolomics between Radix Polygala honey products and licorice products [81]. Ginsenoside, a biomarker of raw and steamed Panax notoginseng, also was discoveried by UPLC/TOF–MS [82]. Through plant metabolomics, changes in metabolites of CMM before and after processing can be more intuitively found, and the relationship between processing and efficacy can be more directly clarified if combined with corresponding pharmacodynamic studies. The purpose of processing toxic drugs is often to reduce toxicity and increase efficiency, which can also be tested by plant metabolomics. Pre-use process is necessary due to long-term usage of Rheum palmatum may bring liver and kidney injury. In one study, UPLC/Q-TOF–MS based metabolomic was applied to study differential chemical composition of R. palmatum samples before and after processing. The results showed that Emodin-8-O-glucoside and gallic acid-3-O-glucoside can distinguish raw and processed products as biomarkers [83]. The detoxification effects of processing of Fuzi (the lateral root of Aconitum carmichaelii Debx) were evaluated via RPLC-Q-TOF/MS coupled with PCA, which found 19 key biomarkers associated with detoxification [84]. In addition, an UPLC-MS method was established to analyze the chemical composition before and after the processing of Euphorbia pekinensis root. The change of chemical composition caused by processing is obviously altered, which is supposed to be the material basis of the detoxification effect [85]. The reduction of toxic for CMM is an important target for processing, and mining attenuated related metabolites of toxic CMM with metabolomics provides criteria for clinical drug safety.
Processing methods evaluation
The evaluation of processing methods is advantageous to standardize the production process and excavate suitable and efficient processing methods of CMM. The processing method of ginseng is mainly steaming. Chan et al. utilized a UHPLC/TOF–MS based metabolomic strategy to discriminate the difference of steaming Panax notoginseng [86]. Moreover, Kim et al. compared the metabolite changes of steamed ginseng at 100 °C, 110 °C and 120 °C, and found that 120 °C is more conducive to its efficacy. At the same time, it was also noted that steaming caused the formation of new ginsenoside [87]. The quality of the drying method will affect the content of the active compounds in CMM. Chromatography-mass spectrometry coupled with targeted and untargeted analyses was applied to study on the relationship between drying method and chemical concentration of Notopterygium franchetii. 30 differentiated compounds are detected in seven drying methods. The results suggested that hot air drying is the best processing method, with minimal chemical changes at the lowest cost, and the largest chemical change caused by shadow drying [88]. Dai et al. used NMR and LC-DAD-MS method to study the effect of stress caused by water loss on metabolites in root of Salvia miltiorrhiza. Using freeze drying as reference, the effects of sun drying and air drying methods were compared. The results showed that water stress resulted in a significant change in the profile of metabolites in salvia miltiorrhiza, and content of tanshinone was significantly increased by air-drying [89]. Plant metabolomics has been widely used to evaluat the drying methods of CMM, but less research in other processing methods, which is also the need to be expanded later, such as stir-frying with bran, stir-frying with honey, steaming with wine and so on. Furthermore, evaluation of the postharvest peeling process of Astragalus root using 1HNMR and UPLC-MS has been demonstrated that there was a clear distinction between exfoliated and undelayed root of astragalus. Peeling can cause significant loss of several primary metabolites, while the content of xylem-related compounds were higher [90]. Postharvest processing often determines the quality of herbal medicines and rhizomes plants, but an full analysis using several complementary methods has not been developed. Plant metabolomics coupled with chemometric analysis can be applied to ensure proper postharvest processing by offering information of primary and secondary metabolites.
Herbal marketing
In drug sales, adulteration, staining, excessive sulfur fumigation will affect the changes of small molecular compounds in CMM, and finally reflect the difference of product quality. Plant metabolomics can be used to detect the indexes and evaluate the quality problems in the sales of CMM.
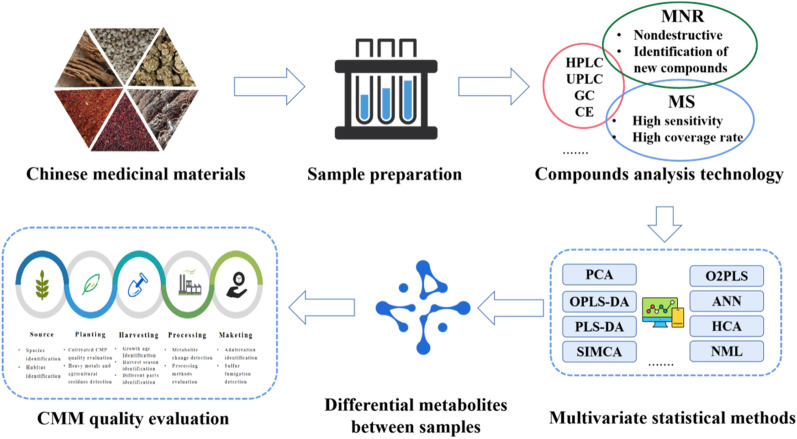
In recent years, UPLC-MS based metabolomics were applied to analysis 35 commercial products of Hydrastis canadensis. The obtained results demonstrate the potential for untargeted metabolomics to discriminate between multiple unknown products and predict possible adulteration [91]. While Panax ginseng adulteration is rampant, Nguyen et al. used 1H NMR combined with OPLS-DA to distinguish 60 Panax ginseng samples from Korea and China. 21 mixed samples of numerous Korea/China ratios were tested, showing satisfactory separation according to the proportion of mixing [92]. It is noteworthy that sulfur fumigation bleaching is also a common adulteration method of ginseng in the market which requires strict control. UPLC-QTOF-MS/MS-based metabolomics approach was developed to evaluate the holistic qualities of commercial white ginseng (WG) and red ginseng (RG) from herbal markets. 43 compounds including 3 sulfur-containing compounds were identified within 24 min suggested the inconsistencies in the quality of commercial WG and RG due to heat treatment and sulfur fumigation [93]. UPLC-ESI-QTOF-MS/MS-based non-targeted metabolomics and UPLC-ESI-QTRAP-MS/MS-based widely targeted metabolomics identified five characteristic sulfur fumigation markers of Trichosanthis Radix (TR) samples. At the same time, the different sulfur fumigation degree of TR samples was tested by chemical transformation analysis and sulfur dioxide residue test. Furthermore, 20 non-sulfur labeled metabolites were tested to evaluate the quality of samples of TR before and after sulfur fumigation. Sulfur fumigation of CMM may cause harm to human body or affect clinical effects and needs to be strictly controlled. The plant metabolome has provided technical support for the detection of sulfur-fumigated drugs by providing information about sulfur fumigation markers [94]. The scarcity of wild Rhodiola officinalis resources, coupled with strong market demand, led to the emergence of artificial cultivation of low quality varieties as adulterated drugs, seriously affected the CMM market. Booker et al. combined with NMR and high performance thin layer chromatography (HPTLC) technology to study the chemical composition differences of five varieties of Rhodiola officinalis in the market, proved that salidroside and tyrosine were the biomarkers, and established the method of content identification in mixed components to provide a methodological basis for distinguishing adulterated medicinal materials [95]. Metabolomics approaches have been applied to evaluate the quality and quantity of characteristic molecules in three different Rhodiola species, Rhodiola rosea, Rhodiola kirilowii and Rhodiola crenulata. Main molecules were identified by one and two-dimensional NMR metabolomics and quantified by HPLC–UV assay, and then OPLS-DA reveals specific patterns in the metabolite profiles. Therefore, plant metabolomics can be utilized to study the chemical diversity of different species, identify unique metabolites and identify adulterated products [96]. Degree of Angelica acutiloba root was evaluated by GC TOF–MS combined with PCA as the good, the moderate and the bad. Among the 22 metabolites identified, phosphoric acid, proline, malic acid, and citric acid showed an association with high-quality roots, while fructose and glucose are mostly found in bad roots. Moreover, PCA shows that malic acid is the most differentiated for medium quality roots [97]. The market price of different levels of CMM varies greatly. The adulteration of CMM occurs repeatedly due to the evaluation methods of different levels are not unified. Plant metabolomics can be applied as a evaluation methodology to provide the theoretical support for sales based on quality (Fig. 4).
Fig. 4.
Schematic diagram of experimental steps of plant metabolomic in quality evaluation of CMM
Concluding and future remarks
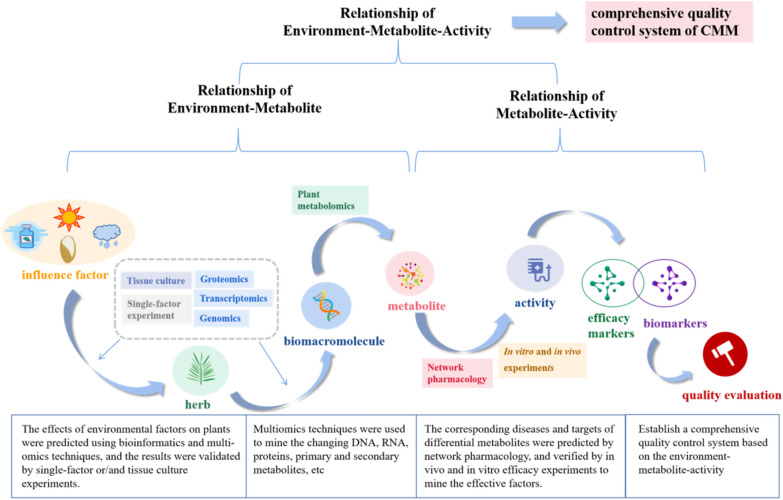
CMM is integrity due to complex composition, and various components interact to produce unique efficacy. Each link of CMM production may cause changes in metabolites and affect the efficacy. A quality control system to respectively evaluate production link from source, planting, harvesting, processing and marketing is urgently needed. Plant metabolomics technique can be used to analyze the composition and variation of metabolites from the view of the whole, which coincides with the “holistic” and “dynamic” views of CMM. In recent year, plant metabolomics have been applied for metabolites analysis to identify species, habitats, growth age, harvest season, parts and adulteration of CMM, which show an enormously potential for quality evaluation [98]. Although plant metabolomics develops rapidly, it is still in the early stage of development. There are still some limitations of plant metabolomics due to the diversity and complication of metabolites, and performance flaw of the instruments [99, 100]. First, the metabolites of CMM are complex and are vulnerable to impact by many factors, including heredity, temperature, humidity, light, chemical substances, etc., which poses challenges to metabolomic analysis [101]. Second, the application conditions of numerous technologies have limitations, where there is not an untargeted approach can profile all the metabolites of CMM. Third, the existing databases are not perfect because many studies on metabolism of medicinal plants remain blank, which limits the analysis and identification of chemical components. Therefore, breaking technical barriers and building more complete databases for comprehensively profiling metabolites are the primary problem of plant metabolomics. Systematically excavating and processing mass data is still the technical bottleneck of current plant metabolomics, which need a high coverage data acquisition strategy to min information of low abundance metabolites. Moreover, establishing a new approach for sample acquisition and preparation is beneficial to improve the experimental reproducibility and stability.
The application of plant metabolomics in the field of quality control will have more development space with the improvement of new systems and multidisciplinary integration applications. Establishing a comprehensive quality control system needs to improve the relationship of environment-metabolites and metabolites-activity. Combining multi-omics technology and bioinformatics technology, the influence of external factors on plant metabolism can be explored from the gene level and a comprehensive metabolic network can be established [102–105]. Based on the differential metabolites found by plant metabolomics, the network pharmacology can be used to predict the disease and target, and finally discover the therapeutic material basis and the predict the active mechanism of CMM. The relationship between different metabolites and their activity can be clarified by plant metabolomics combined with in vivo and in vitro efficacy experiments, then active markers of CMM can be discovered [106–110]. Ultimately, an associative network between environment-metabolite-efficacy will be established for a standardized cultivation-harvesting-processing system and a quality evaluation method of CMM. This system will substantially promote the production and application of CMM towards scientification and modernization. (Fig. 5).
Fig. 5.
Schematic diagram of multi-disciplinary application based on plant metabolomics to establish a comprehensive CMM quality control system
Acknowledgements
Not applicable.
Abbreviations
- CMM
Chinese Medicinal materials
- ChP
Chinese pharmacopoeia
- NMR
Nuclear magnetic resonance
- MS
Mass spectrometry
- LC
Liquid chromatography
- GC
Gas chromatography
- CE
Capillary electrophoresis
- HPLC
High performance liquid chromatography
- UPLC
Ultra performance liquid chromatography
- UV
Ultraviolet spectrum
- IR
Infrared
- EI
Electron impact ion source
- CI
Chemical ionization
- ESI
Electrospray ionization
- APCI
Atmospheric pressure chemical ionization
- MALDI
Matrix-assisted laser desorption ionization
- DESI
Desorption electrospray ionization
- Q
Quadrupole
- IT
Ion trap
- QQQ
Triple quadrupole
- TOF
Time-of-flight
- FTICR
Fourier transform ion cyclotron resonance
- HRMAS
1H high resolution magic angle rotation
- PCA
Principal components analysis
- PLS-DA
Partial least squares discriminative analysis
- OPLS-DA
Orthogonal projections to latent structures
- DART
Direct Analysis in Real Time
- KWG
Korean white ginseng
- CWG
Chinese white ginseng
- RRLC
Rapid resolution liquid chromatography
- ICP-AES
Plasma atomic emission spectro
- ICP-MS
Inductively coupled plasma-mass spectrometry
- FTIR
Fourier Transform infrared spectroscopy
- ICD
Identification, classification and differentiation
- PCR
Pericarpium Citri Reticulatae
- PCRV
Pericarpium Citri Reticulatae Viride
- HELP
Heuristic evolving latent projection
- WG
White ginseng
- RG
Red ginseng
- TR
T richosanthis Radix
- HPTLC
High performance thin layer chromatography
- Q-markers
Quality marker
Author contributions
QX: Investigation, Writing-original draft, Writing-review and editing; XLM: Visualization, Data Curation; JSL: Writing-review and editing, Funding acquisition; BL: Validation; HTL: Funding acquisition, Supervision, Conceptualization, Project administration; BGZ: Methodology; PGX: Writing-Review and Editing. All authors read and approved the final manuscript.
Funding
The ability establishment of sustainable use for valuable Chinese medicine resources (No. 2060302-2002-05), and the National Natural Science Foundation of China (No. 81872965).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Y, Fan X, Qu H, Gao X, Cheng Y. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr Top Med Chem. 2012;12(12):1356–1362. doi: 10.2174/156802612801319034. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Sun S, Ding X. The therapeutic effects of traditional chinese medicine on COVID-19: a narrative review. Int J Clin Pharm. 2020;89:56. doi: 10.1007/s11096-020-01153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu ZJ. Innovation and development of quality control and evaluation pattern of CMM. Clin J Chin Med. 2014;89:567. [Google Scholar]
- 4.Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines-A review. Int J Biodiver Conserv. 2012;4(3):101–112. doi: 10.5897/IJBC11.163. [DOI] [Google Scholar]
- 5.Guy C, Kopka J, Moritz T. Plant metabolomics coming of age. Physiol Plant. 2010;132(2):113–116. doi: 10.1111/j.1399-3054.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 6.Chunmei LU, Cui X. Research Progress in TCM Quality Control and Toxicity Evaluation Based on Metabolomics Technology. Chin J Inform Tradit Chin Med. 2019;8:78. [Google Scholar]
- 7.Heyman HM, Meyer JJM. NMR-based metabolomics as a quality control tool for herbal products. S Afr J Bot. 2012;82:21–32. doi: 10.1016/j.sajb.2012.04.001. [DOI] [Google Scholar]
- 8.Tu XM, Wu H, Wu KY. Research and Application on Traditional Chinese Medicine Fingerprint. Strait Pharma J. 2016;9:7. [Google Scholar]
- 9.Xie PS. A Feasible Strategy for Applying Chromatography Fingerprint to Assess Quality of Chinese Herbal Medicine. Tradit Chin Drug Res Clin Pharmacol. 2001;98:6799. [Google Scholar]
- 10.Hegeman AD. Plant metabolomics—meeting the analytical challenges of comprehensive metabolite analysis. Brief Funct Genomics. 2010;9(2):139–148. doi: 10.1093/bfgp/elp053. [DOI] [PubMed] [Google Scholar]
- 11.Barding GA, Salditos R, Larive CK. Quantitative NMR for bioanalysis and metabolomics. Anal Bioanal Chem. 2012;404(4):1165–1179. doi: 10.1007/s00216-012-6188-z. [DOI] [PubMed] [Google Scholar]
- 12.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26(1):51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HK, Verpoorte R. Sample preparation for plant metabolomics. Phytochem Anal. 2010;21(1):4–13. doi: 10.1002/pca.1188. [DOI] [PubMed] [Google Scholar]
- 14.Kanani H, Chrysanthopoulos PK, Klapa MI. Standardizing GC-MS metabolomics. J Chromatogr B. 2008;871(2):191–201. doi: 10.1016/j.jchromb.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 15.Covington BC, Mclean JA, Bachmann BO. Comparative mass spectrometry-based metabolomics strategies for the investigation of microbial secondary metabolites. Nat Prod Rep. 2016;6:78. doi: 10.1039/c6np00048g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren JL, Zhang AH, Ling K, Wang XJ. Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv. 2018;8(40):22335–22350. doi: 10.1039/C8RA01574K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Q, Wang P, Zhang A, Sun H, Wu X, Wang X. Ultra-performance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J Sep Sci. 2014;36(7):1238–1246. doi: 10.1002/jssc.201201198. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q, Zhang A, Zhang T, Hui S, Wang X. Dissect new mechanistic insights for geniposide efficacy on the hepatoprotection using multiomics approach. Oncotarget. 2017;8:65. doi: 10.18632/oncotarget.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimczak I, Gliszczyńska-Świgło A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015;2015(75):100–105. doi: 10.1016/j.foodchem.2014.11.104. [DOI] [PubMed] [Google Scholar]
- 20.Nováková L, Matysová L, Solich P. Advantages of application of UPLC in pharmaceutical analysis - ScienceDirect. Talanta. 2006;68(3):908–918. doi: 10.1016/j.talanta.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Ramautar R, Somsen GW, Jong G. CE-MS in metabolomics. Electrophoresis. 2010;30(1):276–291. doi: 10.1002/elps.200800512. [DOI] [PubMed] [Google Scholar]
- 22.Ramautar R, Somsen GW, Jong GD. CE-MS for metabolomics: developments and applications in the period 2012–2014. Electrophoresis. 2015;34(1):86–98. doi: 10.1002/elps.201200390. [DOI] [PubMed] [Google Scholar]
- 23.Ramautar R. CE-MS in metabolomics: Status quo and the way forward. Bioanalysis. 2016;8:5. doi: 10.4155/bio-2016-0001. [DOI] [PubMed] [Google Scholar]
- 24.Fu X, Liu Y, Li W, Pang N, Nie H, Liu H, Cai Z. Analysis of aristolochic acids by CE-MS with carboxymethyl chitosan-coated capillary. Electrophoresis. 2010;30(10):1783–1789. doi: 10.1002/elps.200800487. [DOI] [PubMed] [Google Scholar]
- 25.Liu JX, Zhang YW, Yuan F, Chen HX, Zhang XX. Differential detection of Rhizoma coptidisby capillary electrophoresis electrospray ionization mass spectrometry with a nanospray interface. Electrophoresis. 2014;35(21–22):3258–3263. doi: 10.1002/elps.201400334. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Yu B, Pang N, Liao Y, Liu H. Applications of LC-MS and CE-MS in the analysis of traditional Chinese medicine. Sciencepaper Online. 2009;89:6. [Google Scholar]
- 27.Sun Y, Sun G, Yu J. Quality control of traditional Chinese medicines by the capillary electrophoresis fingerprint and capillary electrophoresis-mass spectrometry. Chin J Chromatogr. 2008;26(2):160. doi: 10.1016/S1872-2059(08)60013-9. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Liu C, Zhang A. Rapid discovery and global characterization of multiple constituents from Kai-Xin-San using an integrated MS E data acquisition mode strategy based on ultra-performance liquid chromatography coupled to electrospray ionization/quadrupole-time-of-flight mass spectrometry. Anal Methods. 2015;7(1):279–286. doi: 10.1039/C4AY01954G. [DOI] [Google Scholar]
- 29.Sun H, Wang H, Zhang A. Berberine ameliorates nonbacterial prostatitis via multi-target metabolic network regulation. OMICS. 2015;19(3):186. doi: 10.1089/omi.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XJ, Ren JL, Zhang AH, Sun H, Yan GL, Han Y, Liu L. Novel applications of mass spectrometry-based metabolomics in herbal medicines and its active ingredients: current evidence. Mass Spectrom Rev. 2019;38:380–402. doi: 10.1002/mas.21589. [DOI] [PubMed] [Google Scholar]
- 31.Ren J, Zhang A, Kong LA, Han YA, Yan G, Sun HA, Wang XJ. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine. 2020;67:153165. doi: 10.1016/j.phymed.2019.153165. [DOI] [PubMed] [Google Scholar]
- 32.Hu C, Xu G. Metabolomics and traditional Chinese medicine. TrAC, Trends Anal Chem. 2014;61:207–214. doi: 10.1016/j.trac.2014.06.007. [DOI] [Google Scholar]
- 33.Markley JL, Brüschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS. The future of NMR-based metabolomics. Curr Opin Biotechnol. 2017;43:34–40. doi: 10.1016/j.copbio.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckonert O, Coen M, Keun HC, Wang Y, Ebbels TM, Holmes E, Lindon JC, Nicholson JK. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat Protoc. 2010;5(6):1019–1032. doi: 10.1038/nprot.2010.45. [DOI] [PubMed] [Google Scholar]
- 35.Alessia V, Veronica G, Gaia M, Cristina L, Takis PG, Leonardo T, Paola T, Claudio L. High-throughput metabolomics by 1D NMR. Angew Chem Int Ed. 2018;8:243. [Google Scholar]
- 36.Ramaswamy V, Hooker JW, Withers RS, Nast RE, Brey WW, Edison AS. Development of a 13C-optimized 15-mm high temperature superconducting NMR probe. J Magn Reson. 2013;235:58–65. doi: 10.1016/j.jmr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman A, Fayyadh MZ. Uv-Visible Metabolomics Approach For The Determination Of Selected Adulterants .In: Claimed Premixed Coffee, 2015.
- 38.Ke L, Yan Z, Yang J, Liang X. Simple quality assessment approach for herbal extracts using high performance liquid chromatography-UV based metabolomics platform. J Chromatogr A. 2010;1217(8):1414–1418. doi: 10.1016/j.chroma.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Saleh M, Siddiqui MJ, So'Ad SZM, Khatib A. FT-IR- based metabolomics approach to characterize the α-glucosidase inhibitory activity of Salak fruit; 2017.
- 40.Vlachos N, Skopelitis Y, Psaroudaki M, Konstantinidou V, Chatzilazarou A, Tegou E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal Chim Acta. 2006;573–574:459–465. doi: 10.1016/j.aca.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Kokalj M, Kolar J, Trafela T, Kreft S. Differences among Epilobium and Hypericum species revealed by four IR spectroscopy modes: transmission. KBr tablet, diffuse reflectance and ATR, Phytochem Anal. 2011;22:541–546. doi: 10.1002/pca.1315. [DOI] [PubMed] [Google Scholar]
- 42.Yusof NA, Isha A, Ismail IS. IR-metabolomics aprroach in detecting the possible changes of metabolites due to different harvesting ages and times. Open Conf Proc J. 2013;4(1):234–234. doi: 10.2174/2210289201304010234. [DOI] [Google Scholar]
- 43.Consonni R, Tsimidou M, Ordoudi S, Cagliani L. On the Traceability of Commercial Saffron Samples Using 1H-NMR and FT-IR Metabolomics. Molecules. 2016;21(3):286. doi: 10.3390/molecules21030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wishart DS. Metabolomics: applications to food science and nutrition research. Trends Food Sci Technol. 2008;19(9):482–493. doi: 10.1016/j.tifs.2008.03.003. [DOI] [Google Scholar]
- 45.Leenders J, Frédérich M, Tullio PD. Nuclear magnetic resonance: A key metabolomics platform in the drug discovery process. Drug Discovery Today Technol. 2015;13:39–46. doi: 10.1016/j.ddtec.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Ning Z, Lu C, Zhang Y, Zhao S, Liu B, Xu X, Liu Y. Application of Plant Metabonomics in Quality Assessment for Large-Scale Production of Traditional Chinese Medicine. Planta Med. 2013;79(11):897–908. doi: 10.1055/s-0032-1328656. [DOI] [PubMed] [Google Scholar]
- 47.Pan W, Wu M, Zheng Z, Guo L, Qiu B. Rapid authentication of Pseudostellaria heterophylla (Taizishen) from different regions by near infrared spectroscopy combined with chemometric methods. J Food Sci. 2020;85:11. doi: 10.1111/1750-3841.15171. [DOI] [PubMed] [Google Scholar]
- 48.Xia JM, Wu XJ, Yuan YJ. Integration of wavelet transform with PCA and ANN for metabolomics data-mining. Metabolomics. 2007;3(4):531–537. doi: 10.1007/s11306-007-0090-2. [DOI] [Google Scholar]
- 49.Kalivodová A, Hron K, Filzmoser P, Najdekr L, Janečková H, Adam T. PLS-DA for compositional data with application to metabolomics. J Chemom. 2015;29(1):21–28. doi: 10.1002/cem.2657. [DOI] [Google Scholar]
- 50.Triba MN, Moyec LL, Amathieu R. PLS/OPLS models in metabolomics: the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol BioSyst. 2014;11(1):13–19. doi: 10.1039/C4MB00414K. [DOI] [PubMed] [Google Scholar]
- 51.Chen YB, Tong XF, Ren J, Yu CQ, Cui YL. Current research trends in traditional Chinese medicine formula: a bibliometric review from 2000 to 2016. Evid Based Complement Alternat Med. 2019;2019:3961395. doi: 10.1155/2019/3961395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CX. Recognizing healthy development of Chinese medicine industry from resourcesquality-quality markers of Chinese medicine. Chin Tradition Herb Drugs. 2016;47:3149–3154. [Google Scholar]
- 53.Zhang T, Bai G, Han Y, Xu J, Gong S, Li Y, Zhang H, Liu C. The method of quality marker research and quality evaluation of traditional Chinese medicine based on drug properties and effffect characteristics. Phytomedicine. 2018;44:204–211. doi: 10.1016/j.phymed.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Zhang A, Zou D. Metabolomics coupled with pattern recognition and pathway analysis on potential biomarkers in liver injury and hepatoprotective effffects of yinchenhao. Appl Biochem Biotechnol. 2014;173(4):857–869. doi: 10.1007/s12010-014-0903-5. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Jia S, Zhan W. Study on Quality Influencing Factor and Preparation of CMM Hard Capsules. Western J Tradit Chin Med. 2013;98:5682. [Google Scholar]
- 56.Fukuda E, Baba M, Iwasaki N, Uesawa Y, Arifuku K, Kamoe O, Tsubono K, Okada Y. Identification of Glycyrrhiza species by direct analysis in real time mass spectrometry. Nat Prod Commun. 2010;5(11):1755–1758. [PubMed] [Google Scholar]
- 57.Ning QL, Cao M, Frédérich M, Choi YH, Verpoorte R. FVD Kooy, Metabolomic investigation of the ethnopharmacological use of Artemisia afra with NMR spectroscopy and multivariate data analysis. J Ethnopharmacol. 2010;128(1):230–235. doi: 10.1016/j.jep.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Qi X. Use of the Metabolomics Approach to Characterize Chinese Medicinal Material Huangqi. Mol Plant. 2012;5(2):376–386. doi: 10.1093/mp/ssr093. [DOI] [PubMed] [Google Scholar]
- 59.Yue L, Liu C, Tan E, Gang F, Yi Z. Genetic and chemical discrimination of traditional Tibetan medicine seabuckthorn based on DNA barcode and 1H-NMR metabolic method. China J Chin Materia Med. 2016;41(4):578–585. doi: 10.4268/cjcmm20160405. [DOI] [PubMed] [Google Scholar]
- 60.Song HH, Moon JY, Ryu HW, Noh BS, Kim JH, Lee HK, Oh SR. Discrimination of white ginseng origins using multivariate statistical analysis of data sets. J Ginseng Res. 2014;38:3. doi: 10.1016/j.jgr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dae-Young L, Jae K, Sabina S, Kyeong-Hwa S, Youn-Hyung L, Hyung-Jun N, Geum-Soog K, Yong-Bum K, Seung-Yu K, Nam-In B. Quality Evaluation of Panax ginseng Roots Using a Rapid Resolution LC-QTOF/MS-Based Metabolomics Approach. Molecules. 2013;18(12):14849–14861. doi: 10.3390/molecules181214849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shihao, H.U., Xinyue, Y.U., Ruiting, L.I., Xiaoxia, W.U., Yang, X., Han, Z., Huang, Y., Department, P. Analysis of the differential compounds in Astragalus membranaceus from different geographical origins by UFLC-IT-TOF/MS. J China Pharma Univer. 2019;89:78. [Google Scholar]
- 63.Zhang S, Li C, Gu W, Qiu R, Tian R. Metabolomics analysis of dandelions from different geographical regions in china. Phytochem Anayl. 2021;19:78. doi: 10.1002/pca.3033. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Sheng N, Wang L, Shijie L, Jiannan C, Xiaoping L. Analysis of 2-(2-phenylethyl) chromones by UPLC-ESI-QTOF-MS and multivariate statistical methods in wild and cultivated agarwood. Int J Mol Sci. 2016;17(5):771. doi: 10.3390/ijms17050771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao H, Xu J, Ghebrezadik H, Hylands PJ. Metabolomic quality control of commercial Asian ginseng, and cultivated and wild American ginseng using 1H NMR and multi-step PCA. J Pharm Biomed Anal. 2015;114:113–120. doi: 10.1016/j.jpba.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Xu XF, Cheng XL, Lin QH, Li SS, Jia Z, Han T. Identification of mountain-cultivated ginseng and cultivated ginsengusing UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J Ginseng Res. 2016;40(4):344–350. doi: 10.1016/j.jgr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhan Z, Fang W, Ma X, Chen T, Huang L. Metabolome and transcriptome analyses reveal quality change in the orange-rooted salvia miltiorrhiza (danshen) from cultivated field. Chin Med. 2019;14:1. doi: 10.1186/s13020-019-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Q, Sun LN, Hu XM. Metabonomics Study on Root Exudates of Cadmium Hyperaccumulator Sedum Alfredii. Chin J Anal Chem. 2015;43(1):7–12. doi: 10.1016/S1872-2040(15)60795-2. [DOI] [Google Scholar]
- 69.Pan HQ, Zhou H, Miao S, Guo DA, Shen JI. Plant metabolomics for studying the effect of two insecticides on comprehensive constituents of Lonicerae Japonicae Flos. Chin J Nat Med. 2021;19:1. doi: 10.1016/S1875-5364(21)60008-0. [DOI] [PubMed] [Google Scholar]
- 70.Kim N, Kim K, Choi BY, Lee DH, Shin YS, Bang KH, Cha SW, Lee JW, Choi HK, Jang DS. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-TOF-MS. J Agric Food Chem. 2011;59:10435–10441. doi: 10.1021/jf201718r. [DOI] [PubMed] [Google Scholar]
- 71.Xue Y, Li XW, Li ZY, Zeng ZP, Peng B. UPLC/Q-TOF MS and NMR plant metabolomics approach in studying the effect of growth year on the quality of Polygala tenuifolia. Acta pharmaceutica Sinica. 2015;50(3):340. [PubMed] [Google Scholar]
- 72.Lee HJ, Jeong J, Alves AC, Han ST, Hong YS. Metabolomic understanding of intrinsic physiology in Panax ginseng during whole growing seasons. J Ginseng Research. 2019;43:4. doi: 10.1016/j.jgr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Evaluation of Metabolic Changes in Fruit of Piper Sarmentosum in Various Seasons by Metabolomics Using Fourier Transform Infrared (FTIR) Spectroscopy. Int J Pharm Clin Res. 2009;1(2):68–71. [Google Scholar]
- 74.Yi L, Yuan D, Liang Y, Xie P, Zhao Y. Fingerprinting alterations of secondary metabolites of tangerine peels during growth by HPLC–DAD and chemometric methods. Anal Chim Acta. 2009;649:43–51. doi: 10.1016/j.aca.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Wang J, Guan H. Comparison of the Chemical Profiles and Antioxidant Activities of Different Parts of Cultivated Cistanche deserticola Using Ultra Performance Liquid Chromatography-Quadrupole Te-of-Flight Mass Spectrometry and a 1,1-Diphenyl-2-picrylhydrazyl-Based Assay. Molecules. 2017;22:11. doi: 10.3390/molecules22112011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang XW, Li D, Wang T, Wu YC, Ye ZL. Application of metabolomics approach to study of different parts of Mountain Cultivated Ginseng using UHPLC-QTOF/MS. Acta Pharmaceutica Sinica. 2016;51(10):1609–1615. [PubMed] [Google Scholar]
- 77.Chen, J.K., Zeng, R., University, S.M. Application of metabolomics approach to study on chemical constituents in different parts of Gentiana crasicaulis based on UPLC-ESI-HRMS~n. Chin Tradit Herbal Drugs. 2018;49(10):2328–2335. [Google Scholar]
- 78.Fukuda E, Yoshida M, Baba M, Uesawa Y, Ad AY. Application to classification of mulberry leaves using multivariate analysis of proton NMR metabolomic data. Nat Prod Commun. 2011;6(11):1621–1625. [PubMed] [Google Scholar]
- 79.Fan RY, Peng C, Zhang XX, Qiu DY, Mao GL, Lu YS, Zeng JW. A comparative UPLC-Q-Orbitrap-MS untargeted metabolomics investigation of different parts of Clausena lansium (Lour Skeels. Food Sci Nutr. 2020;8:5811–5822. doi: 10.1002/fsn3.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu PP, Shan GS, Zhang F, Zhang F, Chen JN, Jia TZ. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chin J Nat Med. 2018;16(09):82–88. doi: 10.1016/S1875-5364(18)30111-0. [DOI] [PubMed] [Google Scholar]
- 81.Meng Y, Peng WU, Zhang XL, Jiang HQ, Hui-Fen LI, Zhang QQ, Wang JX. Rapid Identification of Chemical Components in Raw and Processed Products of Polygalae Radix by HPLC-TOF/MS. Chin J Exp Tradit Med Formulae. 2015;34:5. [Google Scholar]
- 82.Sun BS, Xu MY, Zheng L, Wang YB, Sung CK. UPLC-Q-TOF-MS/MS Analysis for Steaming Times-dependent Profiling of Steamed Panax quinquefolius and Its Ginsenosides Transformations Induced by Repetitious Steaming. PubMed. 2012;36(3):277–290. doi: 10.5142/jgr.2012.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, Wang D, Zheng SH. Ultra-performance liquid chromatography-quadrupole\time-of- flight mass spectrometry with multivariate statistical analysis for exploring potential chemical markers to distinguish between raw and processed Rheum palmatum. BMC Complement Altern Med. 2014;14(1):302. doi: 10.1186/1472-6882-14-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun B, Li Y, Zhang Q, Ma K, Gao Y, Yan X. Metabonomic study on the toxicity of Hei-Shun-Pian, the processed lateral root of Aconitum carmichaelii Debx (Ranunculaceae) J Ethnopharmacol. 2008;116(3):561–568. doi: 10.1016/j.jep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Zeng Y, Hou PY, Chen XH. Investigation of Variations of Components in Euphorbia pekinensis Root and Its Processed Products Based on Plant Metabolomics. J Chin Med Mater. 2016;39(3):530–533. [PubMed] [Google Scholar]
- 86.Toh DF, New LS, Koh HL, Chan CY. Ultra-high performance liquid chromatography/time-of-flight mass spectrometry (UHPLC/TOFMS) for time-dependent profiling of raw and steamed Panax notoginseng. J Pharm Biomed Anal. 2010;52(1):43–50. doi: 10.1016/j.jpba.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Shin JH, Park YJ, Kim W, Kim DO, Kim BY, Lee H, Baik MY. Change of Ginsenoside Profiles in Processed Ginseng by Drying, Steaming, and Puffing. J Microbiol Biotechnol. 2019: 29(2):222–229. [DOI] [PubMed]
- 88.Su X, Wu Y, Li Y, Huang Y, Liu Y, Luo P, Zhang Z. Molecules. 2019;24:17. doi: 10.3390/molecules24173188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai H, Xiao C, Liu H, Tang H. Combined NMR and LC-MS analysis reveals the metabonomic changes in Salvia miltiorrhiza Bunge induced by water depletionJournal of Proteome Research. J Proteome Res. 2010;9(3):1460–1475. doi: 10.1021/pr900995m. [DOI] [PubMed] [Google Scholar]
- 90.Jung JY, Jung Y, Kim JS, Ryu DH, Hwang GS. Assessment of Peeling of Astragalus Roots Using 1H NMR- and UPLC-MS-Based Metabolite Profiling. J Agric Food Chem. 2013;61(43):10398–10407. doi: 10.1021/jf4026103. [DOI] [PubMed] [Google Scholar]
- 91.Wallace ED, Oberlies NH, Cech NB, Kellogg JJ. Detection of adulteration in Hydrastis canadensis (goldenseal) dietary supplements via untargeted mass spectrometry-based metabolomics. Food Chem Toxicol. 2018;120:439–447. doi: 10.1016/j.fct.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen HT, Lee DK, Choi YG, Choi YG, Min JE, Yoon SJ, Yu YH, Lim J, Lee J, Kwon SW, Park JH. A 1H NMR-based metabolomics approach to evaluate the geographical authenticity of herbal medicine and its application in building a model effectively assessing the mixing proportion of intentional admixtures: a case study of Panax ginseng: metabolomics for the authenticity of herbal medicine, Journal of pharmaceutical and biomedical analysis. J Pharm Biomed Anal. 2016;124:120–128. doi: 10.1016/j.jpba.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 93.Zhang HM, Li SL, Zhang H, Wang Y, Zhao ZL, Chen SL. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Kang C, Lv C, Yang J, Kang L, Guo L. A Practical Protocol for a Comprehensive Evaluation of Sulfur Fumigation of Trichosanthis Radix Based on Both Non-Targeted and Widely Targeted Metabolomics. Front Plant Sci. 2020;11:578086. doi: 10.3389/fpls.2020.578086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Booker A, Zhai LX, Gkouva C, Li S, Michael H. From traditional resource to global commodities:a comparison of Rhodiola species using NMR spectroscopy-metabolomics and HPTLC. Front Pharmacol. 2016;7:254. doi: 10.3389/fphar.2016.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marchev AS, Koycheva IK, Aneva IY, Georgiev MI. Authenticity and quality evaluation of different Rhodiola species and commercial products based on NMR-spectroscopy and HPLC. Phytochem Analy. 2020;31:6. doi: 10.1002/pca.2940. [DOI] [PubMed] [Google Scholar]
- 97.Tianniam S, Tarachiwin L, Bamba T, Kobayashi A, Fukusaki E. Metabolic profiling of Angelica acutiloba roots utilizing gas chromatography-time-of-flight-mass spectrometry for quality assessment based on cultivation area and cultivar via multivariate pattern recognition. J Biosci Bioeng. 2008;105(6):655–659. doi: 10.1263/jbb.105.655. [DOI] [PubMed] [Google Scholar]
- 98.Gonulalan EM, Nemutlu E, Demirezer LO. A new perspective on evaluation of medicinal plant biological activities: The correlation between phytomics and matrix metalloproteinases activities of some medicinal plant. Saudi Pharmac J. 2019;27(3):446–452. doi: 10.1016/j.jsps.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfender JL, Rudaz S, Hae Choi Y, Kim HK. Plant metabolomics: from holistic data to relevant biomarkers. Curr Med Chem. 2013;20(8):1056–1090. [PubMed] [Google Scholar]
- 100.Duan LX, Dai YT, Sun C, Chen SL. Metabolomics research of medicinal plants. China J Chin Materia Med. 2016;41(22):4090–4095. doi: 10.4268/cjcmm20162202. [DOI] [PubMed] [Google Scholar]
- 101.Ferry-Dumazet H, Gil L, Deborde C, et al. MeRy-B: a web knowledgebase for the storage, visualization, analysis and annotation of plant NMR metabolomic profiles. BMC Plant Biol. 2011;11:104. doi: 10.1186/1471-2229-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng TX, Sun X, Miao YJ, Gu SJ, Tian LX, Zheng Y, Jiang Y, Zhang XK, Feng Z, Pei J, Huang LF. Integrating bioclimatic factors and secondary metabolism to predict the suitable producing area of plants with high specific metabolite content in a real-world environment - a case of Carthamus tinctorius L. Ind Crops Products. 2022;177:114545. doi: 10.1016/j.indcrop.2022.114545. [DOI] [Google Scholar]
- 103.Tokimatsu T, Sakurai N, Suzuki H, Shibata D. KaPPA-View: A Tool for Integrating Transcriptomic and Metabolomic Data on Plant Metabolic Pathway Maps. Biotechnol Agric For. 2006;57:209. [Google Scholar]
- 104.Acharya S, Hedda GV, Kankariya AJ, et al. Hairy roots of ‘dashmula’ plant Uraria picta as a promising alternative to its medicinally valued true roots-functional and metabolomic analysis. Plant Cell Tiss Organ Cult. 2021;145:533–544. doi: 10.1007/s11240-021-02024-3. [DOI] [Google Scholar]
- 105.Villiers F, et al. Exploring the plant response to cadmium exposure by transcriptomic, proteomic and metabolomic approaches: potentiality of high-throughput methods. In: Promises of Integrative Biology, 2012.
- 106.Nkomo MM, Katerere DD, Vismer HH, et al. Fusarium inhibition by wild populations of the medicinal plant Salvia africana-lutea L linked to metabolomic profiling. BMC Complement Altern Med. 2014;14:99. doi: 10.1186/1472-6882-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Acharya S, Hedda GV, Kankariya AJ, et al. Hairy roots of ‘dashmula’ plant Uraria picta as a promising alternative to its medicinally valued true roots - functional and metabolomic analysis. Plant Cell Tiss Organ Cult. 2021;145:533–544. doi: 10.1007/s11240-021-02024-3. [DOI] [Google Scholar]
- 108.Pares RB, Alves DS, Alves LFA, et al. Acaricidal Activity of Annonaceae Plants for Dermanyssus gallinae (Acari: Dermanyssidae) and Metabolomic Profile by HPLC-MS/MS. Neotrop Entomol. 2021;50:662–672. doi: 10.1007/s13744-021-00885-z. [DOI] [PubMed] [Google Scholar]
- 109.Prinsloo G, Vervoort J. Identifying anti-HSV compounds from unrelated plants using NMR and LC–MS metabolomic analysis. Metabolomics. 2018;14:134. doi: 10.1007/s11306-018-1432-y. [DOI] [PubMed] [Google Scholar]
- 110.Niazian M, Sabbatini P. Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Planta. 2021;254:111. doi: 10.1007/s00425-021-03771-5. [DOI] [PubMed] [Google Scholar]
- 111.Andreev VP, Rejtar T, Chen HS, Moskovets EV, Ivanov AR, Karger BL. A universal denoising and peak picking algorithm for LC-MS based on matched filtration in the chromatographic time domain. Anal Chem. 2003;75(22):6314–6326. doi: 10.1021/ac0301806. [DOI] [PubMed] [Google Scholar]
- 112.Urban J, Štys D. Noise and Baseline Filtration in Mass Spectrometry. In: International Conference on Bioinformatics and Biomedical Engineering, 2015.
- 113.Andreev, V.P., Rejtar, T., Karger, B.L., Matched Filtration with Experimental Noise Determination for Denoising, Peak Picking and Quantitation in LC-MS (MEND).
- 114.Warmenhoven J, Coffey N, Liebl D, Harrison A, Hooker G. PCA of Waveforms and Functional PCA: A Primer for Biomechanics. J Biomech, 2020;89:12 [DOI] [PubMed]
- 115.Uarrota VG, Moresco R, Coelho B, Nunes E, Peruch L, Neubert E, Rocha M, Maraschin M. Metabolomics combined with chemometric tools (PCA, HCA, PLS-DA and SVM) for screening cassava (Manihot esculenta Crantz) roots during postharvest physiological deterioration. Food Chem. 2014;161(6):67–78. doi: 10.1016/j.foodchem.2014.03.110. [DOI] [PubMed] [Google Scholar]
- 116.Rukundo IR, Danao M. Identifying Turmeric Powder by Source and Metanil Yellow Adulteration Levels Using Near-Infrared Spectra and PCA-SIMCA Modeling. J Food Protection. 2020;83:6. doi: 10.4315/JFP-19-515. [DOI] [PubMed] [Google Scholar]
- 117.Boccard J, Rutledge DN. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal Chim Acta. 2013;769:30–39. doi: 10.1016/j.aca.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 118.Bylesj M, Eriksson D, Kusano M, Moritz T, Trygg J. Data integration in plant biology: the O2PLS method for combined modeling of transcript and metabolite data. Plant J. 2010;52(6):1181–1191. doi: 10.1111/j.1365-313X.2007.03293.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.