Dear Editor,
Molecular hydrogen (H2) has been recently put forward as a possible adjunct therapeutics in COVID-19 due to its anti-inflammatory and pulmo-protective effects.1,2 An open-label randomized trial in 44 patients with laboratory-confirmed COVID-19 from seven hospitals in China demonstrated that 3-day hydrogen inhalation resulted in significantly more patients with improved disease severity and reduced dyspnea comparing to patients who received standard-of-care treatment.3 The authors suggested that the clinical benefits were likely due to the ability of hydrogen to decrease the inspiratory efforts that consequently reduces chest distress and pain in COVID-19 patients. A recent report suggested that the administration of H2 dissolved in water to patient with COVID-19-like symptoms improves oxygen levels and exercise tolerance.4 However, whether the amelioration of respiratory symptoms after hydrogen administration was accompanied by other clinican- and patient-reported outcomes remained undetermined. In this case series, we evaluated the effects of drinking hydrogen-rich water (HRW) on various patient-reported outcomes, oxygen saturation, and biomarkers of inflammation and coagulation in COVID-19 patients.
A total of 24 COVID-19 patients (age 46.7 ± 10.6 years, 15 women) with mild to moderate disease severity (no hospital admission required at the enrolment) and no other co-morbidities were allocated to drink 1.5 L of HRW per day for 14 days in a double-blind randomized placebo-controlled design, with super-saturated HRW (hydrogen level 8 ppm) administered three times per day. Molecular hydrogen in HRW was produced by the following reaction Mg + H2O —> H2 + Mg(OH)2 and placebo drink was normalized for total magnesium amount and effervescent appearance. Both study participants and research personal were blinded to the treatment assignment. The primary outcomes (patient-reported symptoms and oxygen saturation) were assessed at baseline (pre-intervention) and at every 24-h interval during the duration of the trial; the secondary outcomes (circulatory biomarkers) were assessed at baseline and at 14-day follow-up. The study design was approved by the local IRB at the University of Novi Sad (# 2-CFHRW/2020 46–06-01/2020-1e1), with the study systematized following the Declaration of Helsinki and International Conference of Harmonization Efficacy Guidelines E6.
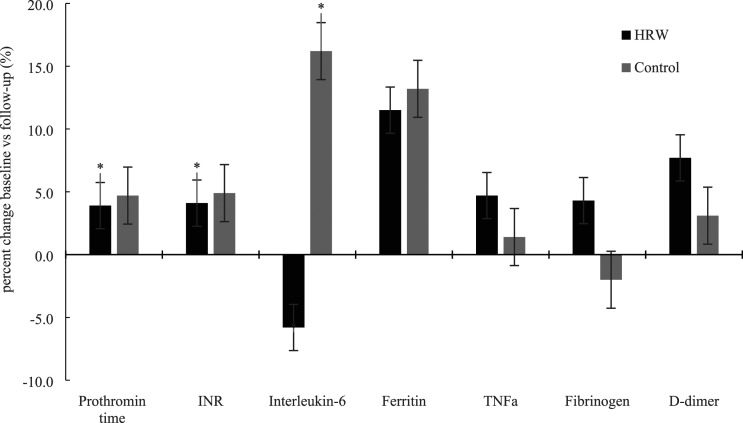
All patients were enrolled as soon as possible after a positive COVID-19 test, and the average delay for initiating the intervention was 3.1 ± 1.6 days (95% CI from 2.4 to 3.8 days), and the duration of the intervention was 13.4 ± 2.3 days (95% CI from 12.4 to 14.4 days). Regarding hospital admissions, bilateral severe pneumonia was developed in one patient (male, 62 years) from the control group who eventually was hospitalized; one patient from HRW group (woman, age 54) was hospitalized due to a COVID-induced relapse of thrombophlebitis. A two-way mixed ANOVA (treatment vs. time interaction) revealed no significant differences for patient-reported outcomes (e.g., cough, dyspnea, headache, chest pain) and oxygen saturation (p > 0.05), except for an attenuated fatigue after HRW intervention (p = 0.01). HRW significantly affected prothrombin time (12.9 ± 0.94 s at baseline vs. 13.4 ± 1.1 s at follow-up; p = 0.01) and INR (international normalized ratio) (0.97 ± 0.08 vs 1.01 ± 0.09; p = 0.01). Serum interleukin six dropped for 5.8% after HRW intervention and increased for 16.2% in the control group (p = 0.04) (Figure 1); the levels were reduced in all female patients after HRW intervention (10 out of 10 patients), and in 60.0% of women (3 out of 5 patients) in the control group (p = 0.02). No significant differences were found between interventions for other circulatory biomarkers (e.g., ferritin, tumor necrosis factor alpha, fibrinogen, D-dimer). No patients reported any side effects from any intervention or disturbances in liver and kidney function.
Figure 1.
Percent changes in biochemical markers during the study. Error bars indicate standard error. Asterisk (*) indicates significant differences at p < 0.05 between baseline and follow-up for each intervention.
This pilot case series with a convenient sample suggests possible beneficial effects and favorable safety of hydrogen-rich water in COVID-19 patients. Since placebo drink was normalized for total magnesium amount in this trial, the effects demonstrated in the HRW group (hydrogen plus magnesium) are likely due to biological effects of hydrogen owing to the fact that no significant effects were seen in the placebo group (magnesium only). It appears that HRW alleviates disease-related fatigue and modulates blood coagulation biomarkers, with possible anti-inflammatory effects being gender specific. Interleukin-6 dropped significantly after HRW intake which perhaps indicates an anti-inflammatory potential of the intervention. However, the changes in inflammatory response during COVID-19 are complex, and whether drop in interleukin-6 is beneficial by itself remains to be addressed. Our results are in line with previous studies showing anti-fatigue potential of HRW after sleep deprivation, heavy exertion, and other stress-related conditions.5 Still, we recruited here a rather small number of COVID-19 patients, with gender disbalance (e.g., women were 1.5 times as many as men), limited age range (e.g., no younger people or elderly were included), along with a short list of biochemical indicators related to inflammation and fatigue monitored in this pilot trial. Future research with magnesium-derived HRW should also address possible confounding effects of magnesium on viral RNA and protein synthesis, and perhaps assess the effects of magnesium-free HRW in this clinical population. Therefore, additional long-term well-sampled studies in COVID-19 cohorts are highly warranted to corroborate these initial findings.
Footnotes
Author contributions: All authors were involved in conception, data collection and analysis, and the writing of this manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Sergej M. Ostojic http://orcid.org/0000-0002-7270-2541
References
- 1.Ostojic SM. COVID-19 and molecular hydrogen inhalation. Ther Adv Respir Dis 2020; 14: 175346662095105. DOI: 10.1177/1753466620951051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen K-D, Lin W-C, Kuo H-C. Chemical and Biochemical Aspects of Molecular Hydrogen in Treating Kawasaki Disease and COVID-19. Chem Res Toxicol 2021; 34(4): 952–958. DOI: 10.1021/acs.chemrestox.0c00456. [DOI] [PubMed] [Google Scholar]
- 3.Guan W-J, Wei C-H, Chen A-L, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis 2020; 12(6): 3448–3452. DOI: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RB, Halabi G, Fatima G, et al. Molecular hydrogen as an adjuvant therapy may be associated with increased oxygen saturation and improved exercise tolerance in a COVID‐19 patient. Clin Case Rep 2021; 9(11): e05039. DOI: 10.1002/ccr3.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iida A, Nosaka N, Yumoto T, et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Medica Okayama 2016; 70(5): 331–337. DOI: 10.18926/AMO/54590. [DOI] [PubMed] [Google Scholar]