Abstract
Actinobacillus pleuropneumoniae, which was formerly classified in the genus Haemophilus, is a pathogen causing swine pleuropneumonia. We found that aspoxicillin showed strong activity and that meropenem had better lytic activity against this pathogen. In the present study, we for the first time identified penicillin-binding proteins (PBPs) of A. pleuropneumoniae in order to elucidate the relationship between the antibacterial and lytic activities of β-lactam antibiotics and affinities of the PBPs. The competitive assay using 3H-labeled benzylpenicillin revealed seven PBPs in A. pleuropneumoniae; they were determined to be PBPs 1a, 1b, 2, 3, 4, 5, and 6, and the molecular masses of these PBPs were estimated to be 92, 80, 76, 72, 50, 44, and 30 kDa, respectively, by comparison with those of Haemophilus influenzae. Our detailed analysis of the affinities of the PBPs of A. pleuropneumoniae and of the bacterial lysis kinetics for several β-lactam antibiotics revealed that the strong antibacterial activity of aspoxicillin against this strain could be related to the higher affinity of PBP 3 and that preferential inactivation of PBP 1b could cause rapid lysis.
Actinobacillus pleuropneumoniae and Haemophilus influenzae are the most prevalent pathogens of respiratory infections in swine and humans, respectively. A. pleuropneumoniae, a small gram-negative capsulated rod, had been classified as belonging to the genus Haemophilus (26). But in 1983, this bacterium was transferred to the genus Actinobacillus based on a study comparing the biological phenotypes and DNA base compositions between Actinobacillus lignieresii and H. influenzae (21). The biotype 1 strain (NAD dependent) can cause swine pleuropneumonia, which is a major bacterial disease of swine, resulting in great economic losses (24, 27). Several β-lactam antibiotics have been frequently used for its treatment (22). This pathogen has distributed around the world, and β-lactam-resistant strains that can produce ROB-1 β-lactamase have been isolated in some countries (9, 17). But in Japan, the resistant strains have been observed only in a small percentage of total isolates (1, 25, 29).
Aspoxicillin, an injectable penicillin derivative (33; T. Nishino, N. Ishii, T. Tanino, S. Ohshima, and T. Yamaguchi, Program Abstr. 19th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 241, 1979), has been used for treating human infections, especially for the treatment of respiratory and abdominal infections and otitis, because of its strong activity against Streptococcus pneumoniae, H. influenzae, Escherichia coli, Staphylococcus aureus, and Streptococcus pyogenes (4, 16) and because of its high level of distribution to the infected tissues (19). The antibacterial activity of β-lactam antibiotics is dependent on the affinity of the target molecules, the penicillin-binding proteins (PBPs). It has been reported that, in E. coli, aspoxicillin showed bactericidal activity with lysis due to high affinities of PBPs 1a, 1b, 2, and 3 (20). But the antibacterial properties of aspoxicillin for A. pleuropneumoniae and H. influenzae have not yet been well investigated.
In the present study, we found that aspoxicillin was potent against a susceptible strain of A. pleuropneumoniae and that the antibacterial activity of aspoxicillin against this strain was stronger than that against H. influenzae. The increase in antibacterial activity was observed in several other β-lactams also; therefore, we thought that these results could be correlated with their affinities of the PBPs of A. pleuropneumoniae and H. influenzae. But the PBP profile of A. pleuropneumoniae has not been completely characterized (11), and the affinities of PBPs of H. influenzae for aspoxicillin have not yet been investigated. In the present study, therefore, we characterized for the first time the PBP profile of A. pleuropneumoniae and conducted a detailed analysis of the antibacterial properties and the affinities of PBPs of A. pleuropneumoniae and H. influenzae for aspoxicillin and seven β-lactam antibiotics.
MATERIALS AND METHODS
Bacterial strain.
Beta-lactamase nonproducing strains of A. pleuropneumoniae NB001 and H. influenzae IID983 were provided by the Japanese Association of Veterinary Biologics (Tokyo, Japan) and the Institute of Medical Science, University of Tokyo, respectively.
Culture media.
Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 25 μg of β-NAD (Nacalai tesque, Kyoto, Japan) per ml and the same broth supplemented with 15 μg each of hemin (Sigma, St. Louis, Mo.) and of β-NAD per ml were used to culture A. pleuropneumoniae and H. influenzae, respectively. Cultivation of each strain was performed under natural aerobic condition.
Antibiotics.
Aspoxicillin was synthesized by Tanabe Seiyaku Co., Ltd. (Osaka, Japan). The following antibiotics were purchased from the respective manufacturers: piperacillin, cefotaxime, and cefsulodin (Sigma); mecillinam (Takeda Pharmaceutical Co., Ltd., Osaka, Japan); aztreonam (Eisai Co., Ltd., Tokyo, Japan); cefdinir (Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan); and meropenem (Sankyo Co., Ltd., Tokyo, Japan).
Susceptibility testing.
The MICs of antibiotics were determined by the agar dilution method, which has been authorized by the Japan Society of Chemotherapy (3). The cells of each strain were grown at 37°C for 15 h in the above-mentioned broth. The cultures were diluted with Mueller-Hinton broth to 5 × 106 CFU/ml. The cell suspension was inoculated onto chocolate agar plates prepared from Mueller-Hinton agar (Difco) containing twofold serial dilutions of each antibiotic with a multipoint inoculator (Sakuma, Tokyo, Japan). The MICs were determined after incubation for 18 h at 37°C.
Time-kill and lytic studies and microscopic examination.
Time-kill and lytic studies on A. pleuropneumoniae were performed at the MIC, 4× the MIC, and 16× the MIC of aspoxicillin, and the lytic studies on the strain were also performed at the same concentrations of meropenem, cefsulodin, cefdinir, and piperacillin. Time-kill and lytic studies on H. influenzae were performed at the MIC and at 4× the MIC of aspoxicillin and piperacillin. Cell suspensions of the two strains were prepared to give 5-ml cultures of 106 CFU/ml with each supplemented broth. The cultures were incubated under continuous shaking in a water bath at 37°C. After 1 h of incubation, a test antibiotic was added to a culture. At selected times, 30 μl each of the culture was serially diluted with saline and plated on a chocolate agar plate to determine the viable cell count (CFU/milliliters). The culture turbidity was monitored to examine bacteriolytic activity by recording the optical density at 620 nm (OD620) with a spectrophotometer (Spectronic 301; Milton Roy Company, Rochester, N.Y.). At 2 h after the addition of each antibiotic, the cells were microscopically examined with a differential interference contrast microscope (NTF2; Nikon, Tokyo, Japan).
Preparation of cell membrane fractions.
The cells of each strain were grown in the above-mentioned broth in a shaking incubator at 37°C for 6 h. The following process was implemented at 4°C or in an ice bath. The cells of each strain in the exponential phase were collected by centrifugation at 5,000 × g, and disrupted by sonication. The cell debris was removed by centrifugation at 10,000 × g, and the membrane fraction was collected by centrifugation at 100,000 × g for 1 h. The pellet was resuspended in phosphate buffer (pH 7.4) and stored at −80°C until use for the following examination.
PBP affinities in cell membrane fractions.
PBP affinities in the cell membrane fractions of the two strains were determined by the competitive assay using 3H-labeled benzylpenicillin (3H-PCG, [phenyl-4(n)-3H]benzylpenicillin; 37 MBq/ml; 20 μg of PCG/ml; Amersham, Buckinghamshire, United Kingdom) according to the methods of Spratt (28) and Tuomanen et al. (32). PBPs labeled with 3H-PCG were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a running gel consisting of 12.5% acrylamide (GIBCO-BRL/Life Technologies, Inc., Gaithersburg, Md.) and 0.083% N,N-methylenebisacrylamide (GIBCO-BRL). After electrophoresis, the fractionated PBPs were transferred electrically to a nitrocellulose membrane (Immobirone PSQ; Millipore Corp., Bedford, Mass.) under a constant current of 150 mA for 50 min with a semidry blotting apparatus (Nihon Eido, Tokyo, Japan) using a buffer consisting of 50 mM Tris base, 200 mM glycine, and 20% methanol. The transferred proteins on the membrane were fixed by methanol-acetic acid (6/1) and then rinsed with methanol. The membrane was dried and attached to a phosphorimaging plate of the BAS-2000 image-analyzing system (Fujifilm, Tokyo, Japan) at room temperature for 3 days. The radioactivity of the PBPs on the plate was scanned and analyzed by the image-analyzing system. The affinity of PBP for a β-lactam was expressed as a 50% inhibitory concentration (IC50) value, which represents the concentration of a β-lactam needed to cause a 50% inhibition of 3H-PCG binding.
RESULTS AND DISCUSSION
PBPs, which are essential enzymes involved in the last step of peptidoglycan synthesis (15), have been identified in various species of bacteria (14), since they are target molecules for β-lactam antibiotics. These physiological functions have been well investigated in E. coli: the high-molecular-weight PBPs (PBPs 1s, 2, and 3) are key roles for cell growth and division, whereas the physiological roles of low-molecular-weight PBPs (PBPs 4, 5, 6, and 7) remain to be elucidated. PBP 3 and two unknown PBPs of A. pleuropneumoniae have been revealed by binding 35S-PCG to the whole cells (11); however, the complete profile and molecular masses of the PBPs had been hitherto unknown.
In this study, the method using 3H-PCG, which was developed by a slight modification of the method of Spratt (28) and Tuomanen et al. (32), for the first time revealed seven PBPs of A. pleuropneumoniae (Fig. 1) numbered PBPs 1a, 1b, 2, 3, 4, 5, and 6 on the basis of the PBP numbering of H. influenzae (12) and the affinities of mecillinam and aztreonam (Table 1), which have been known to be bound selectively by PBPs 2 and 3 of E. coli (31), respectively. Our method could more clearly and easily visualize PBPs on nitrocellulose membrane by an image analyzing system than did previously reported methods (5, 10). The subtype of PBP 3, as shown in H. influenzae, was not observed. The molecular masses of PBPs 1a, 1b, 2, 3a, 3b, 4, 5, and 6 of H. influenzae have been reported to be 85, 80, 72, 65, 63, 50, 45, 42, and 30 kDa, respectively (12). On the basis of these observations, the molecular masses of PBPs 1a, 1b, 2, 3, 4, 5, and 6 of A. pleuropneumoniae were estimated to be 92, 80, 76, 72, 50, 44, and 30 kDa, respectively.
FIG. 1.
Comparative profiles of PBPs of A. pleuropneumoniae NB001 and H. influenzae IID983. PBPs in the cell membrane preparations labeled with 3H-PCG were fractionated by 12.5% polyacrylamide gel electrophoresis and visualized with the BAS-2000 image-analyzing system. Lanes A and B show PBPs untreated and PBPs treated with PCG (100 μg/ml), respectively. The molecular mass (in kilodaltons) of each PBP, indicated in parentheses, of A. pleuropneumoniae NB001 was estimated from that of H. influenzae (12).
TABLE 1.
Affinities of PBPs of A. pleuropneumoniae NB001 for aspoxicillin and other β-lactams
| PBP | IC50 (μg/ml) ofa:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Aspoxicillin (0.016) | Piperacillin (0.016) | Mecillinam (0.125) | Aztreonam (0.008) | Cefdinir (0.016) | Cefotaxime (0.002) | Cefsulodin (2) | Meropenem (0.031) | |
| 1a | 1.51 | 0.676 | >32 | 0.669 | 0.699 | 0.372 | 4.54 | 0.208 |
| 1b | 0.444 | 0.070 | 25.2 | 0.025 | 0.007 | 0.013 | 0.156 | 0.011 |
| 2 | 0.383 | 0.112 | 0.009 | >2 | 5.82 | 39.8 | >256 | 0.021 |
| 3 | 0.022 | 0.002 | >32 | 0.003 | 0.020 | 0.003 | 2.06 | 0.034 |
| 4 | 1.25 | 0.454 | >32 | >2 | 0.094 | 0.300 | 181 | 0.128 |
| 5 | 9.33 | 1.28 | >32 | >2 | >32 | >32 | >256 | 0.365 |
| 6 | 0.363 | >8 | 27.1 | 1.25 | 0.109 | 0.067 | 1.79 | 0.012 |
Values in parentheses are MICs, in micrograms per milliliter, as determined by the agar dilution method (3).
The MICs of aspoxicillin and seven β-lactams against A. pleuropneumoniae NB001 and the affinities of PBPs for those β-lactams are shown in Table 1. Aspoxicillin had better activity than mecillinam, cefsulodin, and meropenem, and the MIC of aspoxicillin was equivalent to those of piperacillin and cefdinir. Aztreonam was more active than aspoxicillin, and cefotaxime was most active in all of the tested β-lactams. Aspoxicillin was bound preferentially to PBP 3, and the IC50 values of aspoxicillin for PBPs 1a, 1b, and 2 were 69-, 20-, and 17-fold higher than that for PBP 3, respectively. Piperacillin, aztreonam, and cefotaxime, like aspoxicillin, were bound preferentially to PBP 3. These results suggest that the antibacterial activity against A. pleuropneumoniae may correlate with the affinity of PBP 3.
Table 2 shows the MICs of aspoxicillin, piperacillin, and mecillinam and the affinities of PBPs of H. influenzae for those β-lactams. We found that aspoxicillin and piperacillin had better activities against A. pleuropneumoniae than against H. influenzae, and the affinities of PBP 3 of A. pleuropneumoniae for the two β-lactams were higher than those of PBP 3a of H. influenzae. In addition to these β-lactams, aztreonam, cefdinir, cefotaxime, and cefsulodin were more active against A. pleuropneumoniae than against H. influenzae and were bound to PBP 3 of A. pleuropneumoniae with higher affinities than to PBP 3a, but not PBP 3b, of H. influenzae (7).
TABLE 2.
Affinities of PBPs of H. influenzae IID983 for aspoxicillin, piperacillin, and mecillinam
| PBP | IC50 (μg/ml) ofa:
|
||
|---|---|---|---|
| Aspoxicillin (0.063) | Piperacillin (0.031) | Mecillinam (256) | |
| 1a | 7.95 | 9.64 | 252 |
| 1b | 0.082 | 0.080 | 33.9 |
| 2 | 0.041 | 0.029 | 0.008 |
| 3a | 0.026 | 0.006 | >256 |
| 3b | 0.010 | 0.003 | >256 |
| 4 | 0.073 | 0.050 | 51.8 |
| 5 | 5.43 | 2.28 | >256 |
| 6 | 3.77 | 12.5 | 103 |
Values in parentheses are MICs, in micrograms per milliliter, as determined by the agar dilution method (3).
Since bacterial lysis induced by β-lactam antibiotics can lead to rapid and irreversible death in bacterial cells, detailed analysis of relationship between lysis and inactivation of PBPs was performed. It has been reported in E. coli that inhibition of PBP 1a and/or PBP 1b is associated with rapid cell lysis (30, 34); however, there are some evidences that PBPs 2 and 3 have important roles in the process of cell lysis (6, 11), suggesting that the mechanism of bacterial lysis induced by β-lactam antibiotics has been uncertain.
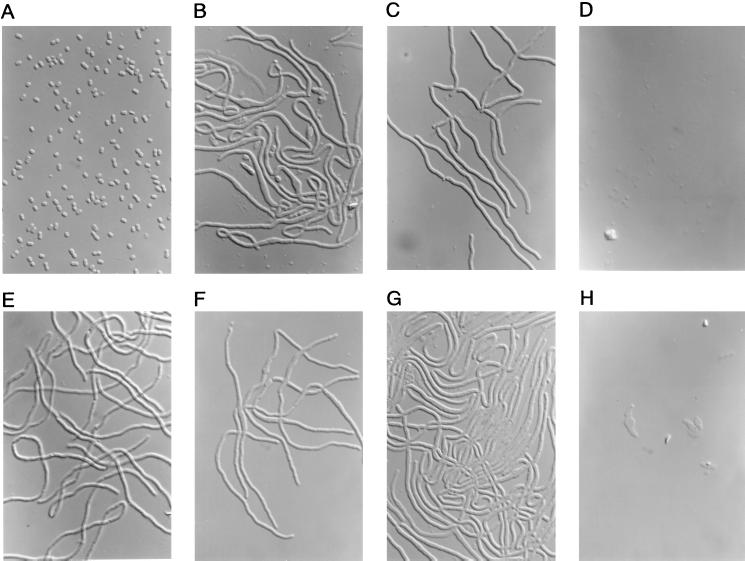
To investigate the relationship between lysis and the inactivation of PBPs of A. pleuropneumoniae, we performed time-kill and lytic studies and microscopic examination for aspoxicillin and some characteristic β-lactams. Figure 2 shows the time-kill and lysis kinetics of aspoxicillin against A. pleuropneumoniae, and Fig. 3 shows the lysis kinetics of meropenem, cefsulodin, cefdinir, and piperacillin against the strain. Photomicrographs of A. pleuropneumoniae cells treated with 4× the MICs of aspoxicillin, piperacillin, mecillinam, aztreonam, cefdinir, cefsulodin, and meropenem for 2 h are shown in Fig. 4.
FIG. 2.
Time-kill (A) and lytic (B) studies on aspoxicillin against A. pleuropneumoniae NB001. Viable cell counts were determined by the colony-counting method, and the turbidity of the culture was represented as an OD620 value. Aspoxicillin was added to the culture at time zero at concentrations of its MIC (circles), 4× its MIC (triangles), and 16× its MIC (squares). The viable cell counts and the OD of a control culture at times of −1, 0, 1, 2, 4, and 8 h are represented by dotted lines without symbols.
FIG. 3.
Bacteriolytic kinetics of meropenem (A), cefsulodin (B), cefdinir (C), and piperacillin (D) against A. pleuropneumoniae NB001. Each drug was added to the culture at time zero in the exponential phase at concentrations of its MIC (circles), at 4× its MIC (squares), and at 16× the MIC (squares). The OD of a control culture at times of −1, 0, 1, 2, 4, and 8 h are represented by dotted lines without symbols.
FIG. 4.
Photomicrographs of A. pleuropneumoniae NB001 treated with each antibiotic at 4× the MIC for 2 h and are depicted as follows: none (A), aspoxicillin (B), piperacillin (C), mecillinam (D), aztreonam (E), cefdinir (F), cefsulodin (G), and meropenem (H). Magnification, ×1,000.
The number of viable cells treated with aspoxicillin decreased rapidly in a concentration-dependent manner (Fig. 2A). Evident decreases in the OD of cultures were not observed at its MIC and at 4× the MIC; however, the OD of culture in presence of 16× the MIC decreased gradually after 2 h and reached 0.016 at 8 h (Fig. 2B). The cells treated with aspoxicillin at 4× its MIC showed a filamentous shape with a bulge in the middle of the cell body (Fig. 4B).
The OD of culture treated with meropenem at its MIC was 0.025 at 2 h, which was much lower than those of cefsulodin, cefdinir, piperacillin, and aspoxicillin (Fig. 3). Meropenem rapidly lysed the cells after 2 h in a concentration-dependent manner. The OD of culture treated with meropenem at its MIC, at 4× its MIC, and at 16× its MIC reached 0.010, 0.002, and 0.001, respectively, at 8 h. Meropenem caused formation of the spindle and short filamentous cells with a bulge at 4× its MIC, and lysed cells were observed (Fig. 4H). The OD of culture treated with cefsulodin at its MIC and at 4× the MIC gradually decreased to 0.053 and 0.027, respectively, at 8 h, which were higher than those of meropenem; however, a rapid decrease in turbidity was observed at 16× the MIC after 1 h. Treatment with cefsulodin at 4× its MIC resulted in formation of filamentous cells without any obvious lysis (Fig. 4G), but at 16× the MIC lysed cells were predominantly observed (not shown). These antibiotics were preferentially bound to PBP 1b, suggesting that the preferential inactivation of PBP 1b could be essential for inducing the lysis of A. pleuropneumoniae.
Cefdinir, as well as meropenem and cefsulodin, was preferentially bound to PBP 1b and less strongly to PBP 3 (Table 1); however, the OD of culture treated with cefdinir at its MIC, at 4× its MIC, and at 16× its MIC increased for up to 4 h and decreased slowly to 0.076, 0.066, and 0.030, respectively, at 8 h (Fig. 3C). The cells treated with cefdinir at 4× its MIC showed filamentous shape without any lysed cells (Fig. 4F) and even at 16× the MIC (not shown). Meropenem was bound to PBP 2 with a higher affinity than cefdinir. The affinity of PBP 2 for cefsulodin, which had less lytic activity than meropenem, was poor. Because it has been reported that inactivation of PBP 2 could result in lysis of E. coli (6), it is possible that inactivation of PBP 2 might also be involved in rapid lysis in A. pleuropneumoniae.
There was a slight decrease in turbidity of a culture treated with piperacillin at 16× the MIC, and the OD reached 0.048 at 8 h; however, rapid lysis was not observed at its MIC and at 4× the MIC as well as with aspoxicillin (Fig. 3D). Filamentous cells were predominantly observed in cultures treated with piperacillin and aztreonam at 4× the MIC without any obvious bulge and lysed cells, as shown in Fig. 4C and E, respectively. Aspoxicillin and these two β-lactams were preferentially bound to PBP 3, suggesting that inactivation of PBP 3 may interfere with lysis, as was previously reported for E. coli (23).
The time-kill and lytic kinetics of aspoxicillin against H. influenzae were compared to those of piperacillin (Fig. 5), and photomicrographs of H. influenzae treated with those antibiotics at 4× the MIC for 2 h are shown in Fig. 6. Viable cell counting of H. influenzae treated with each antibiotic decreased at its MIC and at 4× its MIC. A rapid decrease in turbidity of cultures treated with aspoxicillin at 4× the MIC was observed, and the OD of the culture reached 0.012 at 8 h. Slow lysis was observed in cultures treated with aspoxicillin at its MIC, and the OD of the culture was 0.052 at 8 h. The spindle-shaped cells with a bulge were predominantly observed by treatment of aspoxicillin, and lysed cells were partially observed (Fig. 6B). The OD of cultures treated with piperacillin at its MIC and at 4× its MIC decreased slowly to 0.075 and 0.047, respectively, at 8 h. The cells treated with piperacillin showed a filamentous shape without bulge and lysis (Fig. 6C) even at 16× its MIC (not shown). Aspoxicillin and piperacillin were preferentially bound to PBP 3b of H. influenzae and less strongly to PBP 3a (Table 2). The affinity of PBP 1b for piperacillin was equivalent to that for aspoxicillin; however, the affinity of PBP 3a for piperacillin was much higher than that for aspoxicillin. It has been reported that inactivation of PBPs 3a and 3b of H. influenzae could interfere with lysis (7). These evidences suggested that the higher affinities of PBPs 3a and 3b of H. influenzae for piperacillin than for aspoxicillin could result in the lower lytic activity of piperacillin. Mecillinam, which was selectively bound to PBP 2, had much less antibacterial activity than aspoxicillin and piperacillin (Table 2).
FIG. 5.
Time-kill (A) and lytic (B) studies with aspoxicillin and piperacillin against H. influenzae IID983. Viable cell counts were determined by the colony-counting method, and the turbidity of culture was represented as the OD620. Closed and opened symbols represent aspoxicillin and piperacillin, respectively. Each antibiotic was added to the culture at time zero at concentrations of its MIC (circles) and at 4× its MIC (triangles). The viable cell counts and the OD values of a control culture at times of −1, 0, 1, 2, 4, and 8 h are represented by dotted lines without symbols.
FIG. 6.
Photomicrographs of H. influenzae IID983 treated with aspoxicillin and piperacillin at 4× the MIC for 2 h are depicted as follows: none (A), aspoxicillin (B), and piperacillin (C). Magnification, ×1,000.
The most important results of this work include the complete PBP profile of A. pleuropneumoniae and the correlation between the antimicrobial activity of β-lactams and binding to PBP 3. Recently, the numbers of β-lactamase-nonproducing, ampicillin-resistant strains of H. influenzae have been increasing in some countries (8, 13, 18). The resistant strains showed decreased affinities of many β-lactams for PBPs 3a and 3b (2). Although this type of resistant strain of A. pleuropneumoniae has not yet been reported, we should continue to survey the affinities of the PBPs of this pathogen for β-lactams in the field of veterinary medicine.
ACKNOWLEDGMENT
We thank Shigeyuki Takeyama for critical reading of the manuscript.
REFERENCES
- 1.Asawa T, Kobayashi H, Mitani K, Ito N, Morozumi T. Serotypes and antimicrobial susceptibility of Actinobacillus pleuropneumoniae isolated from piglets with pleuropneumonia. J Vet Med Sci. 1995;57:757–759. doi: 10.1292/jvms.57.757. [DOI] [PubMed] [Google Scholar]
- 2.Clairoux N, Picard M, Brochu A, Rousseau N, Gourde P, Beauchamp D, Parr T R, Jr, Bergeron M G, Malouin F. Molecular basis of the non-β-lactamase-mediated resistance to β-lactam antibiotics in strains of Haemophilus influenzae isolated in Canada. Antimicrob Agents Chemother. 1992;36:1504–1513. doi: 10.1128/aac.36.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee for Revision of MIC Determination Method. Revision of minimal inhibitory concentration (MIC) determination method. Chemotherapy (Tokyo) 1981;29:76–79. [Google Scholar]
- 4.Deguchi K, Yokota N, Koguchi M, Suzuki Y, Suzuki K, Fukayama S, Ishihara R, Oda S. Antimicrobial activities of aspoxicillin of fresh clinical isolates. Jpn J Antibiot. 1993;46:295–309. [PubMed] [Google Scholar]
- 5.Denome S A, Elf P K, Henderson T A, Nelson D E, Young K D. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol. 1999;181:3981–3993. doi: 10.1128/jb.181.13.3981-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutmann L, Vincent S, Billot-Klein D, Acer J F, Mrèna E, Williamson R. Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis of Escherichia coli by some β-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam) Antimicrob Agents Chemother. 1986;30:906–912. doi: 10.1128/aac.30.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inui T, Oshida T, Endo T, Matsushita T. Potent bacteriolytic activity of ritipenem associated with a characteristic profile of affinities for penicillin-binding proteins of Haemophilus influenzae. Antimicrob Agents Chemother. 1999;43:2534–2537. doi: 10.1128/aac.43.10.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen J H, Maher L A, Howell A W. Activity of a new carbapenem antibiotic, meropenem, against Haemophilus influenzae strains with β-lactamase- and non-enzyme-mediated resistance to ampicillin. Antimicrob Agents Chemother. 1991;35:600–602. doi: 10.1128/aac.35.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juteau J M, Sirois M, Medeiros A A, Levesque R C. Molecular distribution of ROB-1 β-lactamase in Actinobacillus pleuropneumoniae. Antimicrob Agents Chemother. 1991;35:1397–1402. doi: 10.1128/aac.35.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao X, Hancock R E W. Cloning and characterization of the Pseudomonas aeruginosa pbpB gene encoding penicillin-binding protein 3. Antimicrob Agents Chemother. 1995;39:1871–1874. doi: 10.1128/aac.39.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malouin F, Bryan L E. Haemophilus influenzae penicillin-binding proteins 1a and 3 possess distinct and opposite temperature-modulated penicillin-binding activities. Antimicrob Agents Chemother. 1988;32:498–502. doi: 10.1128/aac.32.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malouin F, Parr T R, Jr, Bryan L E. Identification of a group of Haemophilus influenzae penicillin-binding proteins that may have complementary physiological roles. Antimicrob Agents Chemother. 1990;34:363–365. doi: 10.1128/aac.34.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manninen R, Huovinen P, Nissinen A the Finnish study group for antimicrobial resistance. Increasing antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Finland. J Antimicrob Chemother. 1997;40:387–392. doi: 10.1093/jac/40.3.387. [DOI] [PubMed] [Google Scholar]
- 14.Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob Agents Chemother. 1998;42:1–17. doi: 10.1128/aac.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuhashi M. Utilization of lipid-linked precursors and the formation of peptidoglycan in the process of cell growth and division: membrane enzyme involved in the final step of peptidoglycan synthesis and the mechanism of their integration. In: Ghuysen J M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Biomedical Press; 1994. pp. 55–71. [Google Scholar]
- 16.Matsumoto K. Review: new antimicrobial agent series XXII: aspoxicillin. Jpn J Antibiot. 1987;40:1221–1242. [PubMed] [Google Scholar]
- 17.Medeiros A A, Levesque R, Jacoby G A. An animal source for ROB-1 β-lactamase of Haemophilus influenzae type b. Antimicrob Agents Chemother. 1986;29:212–215. doi: 10.1128/aac.29.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelman P M, Chaffin D O, Stull T L, Rubens C E, Mack K D, Smith A L. Characterization of non-β-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984;26:235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai K, Tokushima T, Hirota Y, Nagamatsu M, Takeda K, Mizobe M, Takai C. Pharmacokinetic study on aspoxicillin transfer into pulmonary and tracheal tissues. Jpn J Antibiot. 1993;46:367–373. [PubMed] [Google Scholar]
- 20.Nakanishi N, Shibata K, Matsushita T, Tani K, Yamaguchi T. A study on the bactericidal action of aspoxicillin against Escherichia coli. Jpn J Antibiot. 1988;41:427–436. [PubMed] [Google Scholar]
- 21.Pohl S, Bertschinger H U, Frederiksen W, Mannheim W. Transfer of Haemophilus pleuropneumoniae and the Pasteurella haemolytica-like organism causing porcine necrotic pleuropneumonia to the genus Actinobacillus (Actinobacillus pleuropneumoniae comb. nov.) on the basis of phenotypic and deoxyribonucleic acid relatedness. Int J Syst Bacteriol. 1983;33:510–514. [Google Scholar]
- 22.Raemdonck D L, Tanner A C, Tolling S T, Michener S L. Antimicrobial susceptibility of Actinobacillus pleuropneumoniae, Pasteurella multocida and Salmonella choleraesuis isolates from pigs. Vet Rec. 1994;134:5–7. doi: 10.1136/vr.134.1.5. [DOI] [PubMed] [Google Scholar]
- 23.Satta G, Cornaglia G, Mazzariol A, Golini G, Valisena S, Fontana R. Target for bacteriostatic and bactericidal activities of β-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob Agents Chemother. 1995;39:812–818. doi: 10.1128/aac.39.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebunya T N K, Saunders J R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983;182:1331–1337. [PubMed] [Google Scholar]
- 25.Shimizu M, Kuninori K, Sakano T, Terashima T. Antibiotic susceptibility of Haemophilus pleuropneumoniae and Pasteurella multocida isolates from swine. Jpn J Vet Sci. 1982;44:359–363. doi: 10.1292/jvms1939.44.359. [DOI] [PubMed] [Google Scholar]
- 26.Shope R E. Porcine contagious pleuropneumonia. I. Experimental transmission, etiology, and pathology. J Exp Med. 1964;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shope R E, White D C, Leidy G. Porcine contagious pleuropneumonia. II. Studies of the pathogenicity of the etiological agent, Haemophilus pleuropneumoniae. J Exp Med. 1964;119:369–375. doi: 10.1084/jem.119.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spratt B G. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem. 1977;72:341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Ohmae K, Ohishi K, Muramatsu M, Takahashi T. Antimicrobial susceptibility of Actinobacillus (Haemophilus) pleuropneumoniae isolated from pigs with pleuropneumonia. Jpn J Vet Sci. 1989;51:450–452. doi: 10.1292/jvms1939.51.450. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki S, Nakajima S, Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci USA. 1977;74:5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of β-lactam antibiotics. Rev Infect Dis. 1986;8(Suppl. 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- 32.Tuomanen E, Gilbert K, Tomasz A. Modulation of bacteriolysis by cooperative effects of penicillin-binding proteins 1a and 3 in Escherichia coli. Antimicrob Agents Chemother. 1986;30:659–663. doi: 10.1128/aac.30.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagatsuma M, Seto M, Miyagishima T, Kawazu M, Yamaguchi T, Ohshima S. Synthesis and antibacterial activity of asparagine derivatives of aminobenzylpenicillin. J Antibiot. 1983;36:147–154. doi: 10.7164/antibiotics.36.147. [DOI] [PubMed] [Google Scholar]
- 34.Yousif S Y, Broome-Smith J K, Spratt B G. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985;131:2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]