Abstract
Organ fibrosis is a process in which cellular homeostasis is disrupted and extracellular matrix is excessively deposited. Fibrosis can lead to vital organ failure and there are no effective treatments yet. Although epithelial–mesenchymal transition (EMT) may be one of the key cellular mechanisms, the underlying mechanisms of fibrosis remain largely unknown. EMT is a cell phenotypic process in which epithelial cells lose their cell-to-cell adhesion and polarization, after which they acquire mesenchymal features such as infiltration and migration ability. Upon injurious stimulation in different organs, EMT can be triggered by multiple signaling pathways and is also regulated by epigenetic mechanisms. This narrative review summarizes the current understanding of the underlying mechanisms of EMT in fibrogenesis and discusses potential strategies for attenuating EMT to prevent and/or inhibit fibrosis. Despite better understanding the role of EMT in fibrosis development, targeting EMT and beyond in developing therapeutics to tackle fibrosis is challenging but likely feasible.
Keywords: EMT, Fibrosis, TGF-β, Smad, HDAC inhibitors, TGF-β antibody, Organ fibrosis, Senescence, Extracellular matrix
Highlights.
Current understanding of the key roles of EMT in triggering fibrosis development in different organs or tissues is updated.
Cellular signaling pathways that are activated during EMT are extensively discussed.
Drugs or chemicals that can partially inhibit and reverse EMT, providing potential for preventing and inhibiting fibrosis, are reviewed.
Background
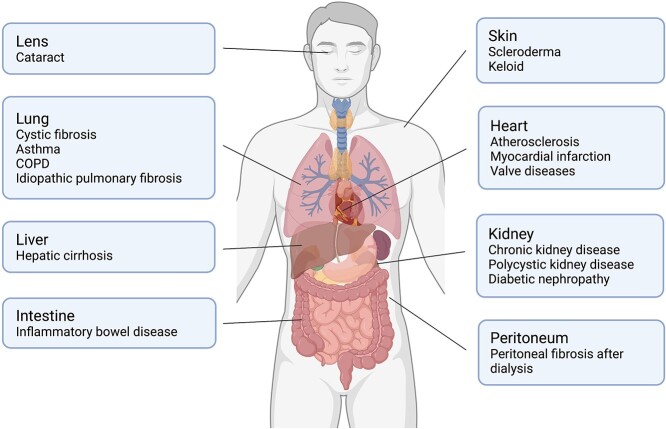
During wound healing, the deposition of extracellular matrix (ECM) produced by myofibroblasts rebuilds the parenchymal tissue architecture. Once ECM production exceeds its degradation, tissue architecture is destructed and subsequently the process of fibrosis is initiated. Fibrosis can occur in vital organs or local tissues, e.g. the kidneys (chronic kidney disease), lungs (idiopathic pulmonary fibrosis (IPF), cystic fibrosis), heart (myocardial fibrosis, atherosclerosis), digestive system (liver cirrhosis, Crohn’s disease), lens (cataract), peritoneum (peritoneal fibrosis) and skin (keloid, scleroderma), leading to organ dysfunction or failure (Figure 1). Developing effective strategies to prevent or even slow down fibrotic pathological processes is urgently needed. To date, 6000 new cases of IPF are diagnosed annually [1], 1.2 million people die worldwide from chronic kidney disease [2] and 6.8 million new cases of irritable bowel disease are reported worldwide [3]. In addition, 300 million people suffer from asthma, which was estimated in 2017. It is predicted that there will be over 100 million people by 2025 [4] who have a high risk of lung fibrosis. In the United States, childhood pulmonary fibrosis costs the health service a total of $1.3 billion per year [5]. Due to the high financial burden of fibrosis and the ever-increasing number of patients, advancements in developing strategies and therapies to prevent fibrosis will bring vast benefits to our society.
Figure 1.
Fibrotic diseases related to multiple organs. Fibrosis is a pathological process that is associated with a variety of diseases. Fibrosis is closely associated with organ dysfunction or failure. COPD chronic obstructive pulmoriary disease
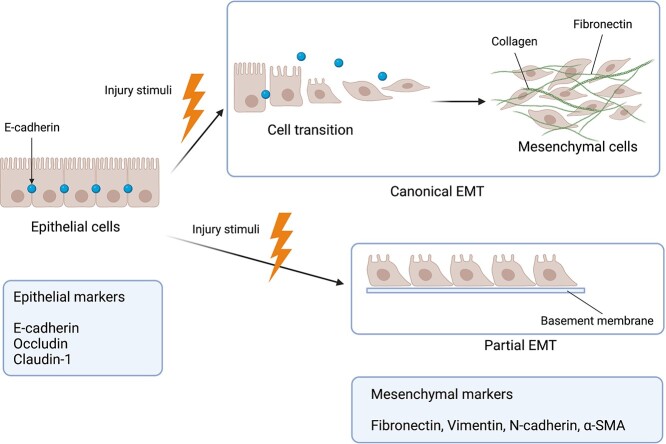
Fibrosis is characterized by loss of cellular homeostasis and excessive ECM deposition [6]. Myofibroblasts are activated in response to tissue injury and subsequently can synthesize ECM during wound healing and can be differentiated from multiple cell types, such as resident fibroblasts, pericytes, bone marrow, adipocytes, endothelial cells and epithelial cells [7,8]. Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells lose their apical–basal polarity and adhesion with downregulated epithelial markers such as E-cadherin, occludin, claudin-1, and acquire mesenchymal features with upregulated mesenchymal markers such as α-smooth muscle actin (α-SMA), vimentin, fibronectin and fibroblast-specific protein 1 (FSP-1) [9,10] (Figure 2). A recent study demonstrated that during EMT, epithelial cells do not cross the glomerular basement membrane to contribute to myofibroblasts, but just acquire mesenchymal features to release signals with upregulated mesenchymal markers [11]. Therefore, a new concept of partial EMT was proposed (Figure 2). EMT is closely related to fibrogenesis in distinct organs, hence targeting EMT can arguably be considered to be a novel anti-fibrotic therapeutic strategy. This review summarizes current understanding of mechanisms underlying EMT and the possible treatments or strategies under development to slow down organ fibrosis through targeting EMT mechanisms.
Figure 2.
The process of epithelial-to-mesenchymal transition (EMT). Normal epithelial cells connect tightly to each other. During wound healing, EMT is a process in which epithelial cells lose their apical–basal polarity and adhesion with downregulated epithelial markers and transform into mesenchymal cells with upregulated mesenchymal markers. Some studies also discovered a new phenomenon called partial EMT, in which epithelial cells do not cross the glomerular basement membrane to contribute to myofibroblasts, but just acquire mesenchymal features with upregulated mesenchymal markers to release signals. α-SMA α-smooth muscle actin
Review
Cellular states triggering EMT
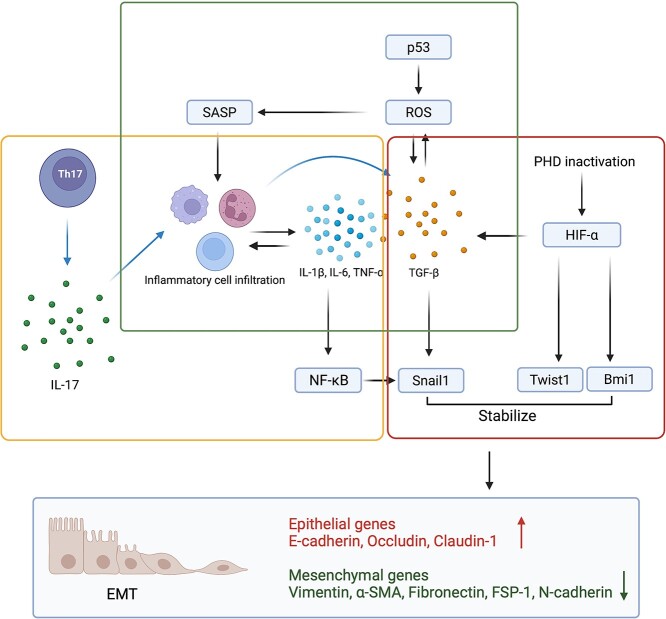
Inflammation, hypoxia and senescence are three cellular conditions closely associated with EMT. They interact and converge at transforming growth factor-β (TGF-β) (Figure 3).
Figure 3.
Cellular conditions triggering EMT. Yellow box (inflammation): Th17 cells generate IL-17 to trigger inflammatory cell infiltration. These inflammatory cells produce pro-inflammatory cytokines, and in turn, pro-inflammatory cytokines stimulate inflammatory cell infiltration. Pro-inflammatory cytokines induce EMT by directly activating Snail1 and indirectly activating the NF-κB pathway to increase Snail1 expression. Red box (hypoxia): under hypoxic conditions, the inactivation of prolyl hydroxylases (PHD) activates hypoxia inducible factor-α (HIF-α). HIF-α triggers the TGF-β/Smad pathway as well as EMT gene expression such as Twist1 and Bm1i to induce the EMT process. Moreover, Twist1 binds to the promoter of Bmi1 to activate the transcription of Bmi1, and Bmi1 further stabilizes Snail1. Green box (senescence): p53 is a well-known gene in senescence. Its activation produces reactive oxygen species (ROS) to trigger cellular senescence. Cells undergoing senescence release cytokines to stimulate inflammatory cell recruitment. Inflammatory cells further generate cytokines to induce EMT. EMT epithelial–mesenchymal transition, SASP senescence associated secretory phenotype, TGF-β transforming growth factor-β, IL interleukin, FSP-1 fibroblast-specific protein-1
Inflammation
Inflammation is widely acknowledged to be closely related to EMT. Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6) and IL-1β activate inflammatory cell infiltration in various organs [12]. In turn, these inflammatory cells (macrophages, neutrophils, lymphocytes, etc.) also release pro-inflammatory cytokines [12,13]. Persistent inflammation exacerbates this inflammatory cascade, which stimulates EMT and causes further excessive ECM accumulation. In addition, TNF-α, IL-6 and IL-1β are inducers of the nuclear factor κB (NF-κB) pathway, the activation of which has been recognized to cause inflammation [14]. The inhibition of the toll-like receptor 4 (TLR4)/NF-κB pathway attenuated renal fibrosis by suppressing inflammation and EMT [15]. Furthermore, it has been reported that inflammation mediated by the NF-κB pathway caused cell cycle arrest and subsequently led to renal EMT [16], indicating that the NF-κB signaling pathway plays a significant role during EMT by mediating inflammation. Pro-inflammatory cytokine IL-17 produced by T helper 17 cells (Th17) activates immune cells to generate TGF-β, a well-known EMT inducer, and further causes pulmonary EMT [17]. However, whether this mechanism occurs in other organs remains to be verified. Collectively, inhibition of the inflammatory response can be considered as an anti-EMT treatment.
Hypoxia
Hypoxia is a critical condition which can trigger EMT through hypoxia inducible factor-α (HIF-α) accumulation [18]. Under hypoxic conditions, the inactivation of prolyl hydroxylases (PHD) caused the accumulation and activation of HIF-α, which further induces EMT-related gene expression such as Snail1, Twist1 and Bmi1 [19]. Upon stimulation of hypoxia, HIF-α activated the TGF-β/Smad and phosphoinositide 3-kinase (PI3K)/Akt pathways to cause renal and pulmonary EMT, respectively [20]. Moreover, in the kidneys, HIF-α induced the expression of Bmi1, which is an EMT-related gene. The expression of Bmi1 activated Twist1 expression both directly and indirectly, which subsequently stabilized the E-cadherin repressor Snail1 [18]. This offers a latent mechanism for hypoxia-induced EMT. In addition, the administration of hyperbaric oxygen therapy (HBOT) was shown to ameliorate pulmonary EMT, indicating that relieving hypoxia was a potential treatment for EMT [21].
Senescence
Senescence is a state in which cells undergo cell-cycle arrest and stop dividing [22]. Nevertheless, senescent cells remain metabolically and secretly active, denoted the senescence associated secretory phenotype (SASP) [22]. Cytokines produced by SASP phenotype cells promoted leukocyte infiltration to cause persistent pro-inflammatory cytokines secretion [22]. The p53 protein triggered senescence by upregulating reactive oxygen species (ROS) [23]. Indoxyl sulfate activated the phosphorylation of p53 in HK-2 cells and renal failure in mice, which further caused cell senescence [24]. In the kidneys, the inhibition of p53 attenuated the ROS level and led to a decrease in the mesenchymal marker α-SMA [24]. p53 knockdown showed a significant reduction of ROS in olaquindox-induced intestinal cells and liver tissue damage [25]. Thus, it was suggested that injury stimuli led to EMT by triggering the p53/ROS axis to promote cell senescence. ROS augmented TGF-β1 expression during lung fibrosis, and in turn, TGF-β1 stimulated NAPDH oxidase (NOX) generation to synthesize ROS [26]. This positive feedback likely contributed to EMT because the increased senescent markers (including p53, p21 and p16) and NOX and mesenchymal markers were found in multiple-organ fibrosis [22,27]. In addition, the activation of peroxisome proliferator-activated receptor alpha (PPAR-α) inhibited ROS levels and further reduced hepatocyte EMT, accompanied by an increase in glutathione (GSH) [28]. Collectively, senescence caused by p53/ROS may be responsible for EMT in distinct organs.
Signaling pathways promoting EMT induction
Several cellular signaling pathways are involved in the EMT process including TGF-β1, Wnt and Notch pathways.
TGF-β1
TGF-β1 activates TGF-β signaling by binding to TGF-β receptors I and II (TGFβRI and TGFβRII). TGF-β1 is an inducer of cell migration and de-differentiation, and a regulator of ECM components such as fibronectin, elastin and collagen [29,30]. Generally, the TGF-β1 signaling pathway can be categorized into Smad-dependent and Smad-independent pathways.
Smad-dependent pathway
TGF-β1/Smad is the most well-studied pathway that induces EMT. The formation of TGFβRI and TGFβRII complexes is activated by TGF-β1, which subsequently leads to Smad2 and/or Smad3 phosphorylation. Phosphorylated Smad2 and/or Smad3 form a complex that heteroligomerizes with Smad4 [30], after which the heterotrimer complex is translocated into the nucleus and then regulates EMT transcription factors such as Snail1, Twist1 and Slug [31].
Smad3 phosphorylation was reported to be critical for EMT induction in a variety of organs [32–34], although this is still debatable. For example, a previous study showed that Smad2 reduced ECM deposition by inducing SnoN (a negative regulator of TGF-β1) expression [35]. In contrast, another group reported that knockout of Smad2 restored EMT [36]. This implies that Smad2 has both pro- and anti-fibrotic functions.
Several studies revealed that Smad4 acted as an EMT facilitator. In kidney, small interfering RNA (siRNA) suppression of Smad4 blocked TGF-β1-induced EMT [37]. The deacetylation of Smad4 inhibited aristolochic acid-induced EMT by regulating the TGF-β pathway in vitro [38]. Smad7 serves as a negative regulator of TGF-β1 signaling via directly interacting with TGFβRI/TGFβRII and competing with Smad4 for interacting with Smad2/3 [39,40]. This implies that Smad4 and Smad7 are targets for reversing organ fibrosis which is mediated through EMT.
Smad-independent pathways
PI3K/Akt and mitogen-activated protein kinase (MAPK) are two Smad-independent pathways induced by TGF-β1 that contribute to EMT.
PI3K/Akt pathway
PI3Ks are a family of lipid kinases that participate in regulating a variety of cellular processes, and Akt is a member of the serine/threonine kinases family. The activation of PI3K induces phosphatidylinositol-3,4,5-trisphosphate (PIP3), a phospholipid membrane protein, to bind to Akt [41]. PI3K/Akt pathway activation was widely detected in EMT in different organs. It was associated with hypoxia or the inflammatory response to induce EMT [42,43]. The specific inhibition of PI3K attenuated EMT in different models [44,45]. Under hypoxic conditions, Bmi1 stabilized Snail1 by triggering the PI3K/Akt pathway to induce EMT [18]. Overexpression of runt-related transcription factor 1 (RUNX1), triggered by unilateral ureteral obstruction (UUO) and folic acid, promoted the transcription of PI3K subunit P110δ to activate Akt and lead to EMT [46]. The PI3K/Akt pathway was reported to trigger EMT by activating mammalian target of rapamycin (mTOR) and inhibiting glycogen synthase kinase 3β (GSK-3β). mTOR is a downstream substrate of the PI3K/Akt pathway, which is essential for cell-type transformation [19]. mTOR inhibition suppressed EMT in diabetic nephropathy [44]. Curcumin-inhibited EMT exhibited a downregulation of the PI3K/Akt/mTOR pathway [47], indicating that this pathway is involved in EMT development. However, this was just an in vitro study and further in vivo studies are required.
GSK-3β is at the intersection of distinct pathways. Its inhibition by Akt resulted in EMT occurrence [48].
High-glucose-induced EMT can be attenuated by zinc through inhibiting PI3K/Akt/GSK-3β to inactivate HIF-1α [49]. Nrf2 activator administration partially attenuated high-glucose-induced EMT by dephosphorylating Akt and GSK-3β at serine-473 and serine-9, respectively [48]. The suppressive effects of Nrf2 on the PI3K/Akt/GSK-3β pathway implies its anti-EMT potential.
MAPK pathways
The MAPK pathway includes p38 MAPK, C-jun N-terminal kinase (JNK) and ERK pathways. The activation of JNK/p38 MAPK/ERK was found in various in vivo studies to increase EMT phenotype acquisition and attenuate EMT by blocking these pathways [50].
The blockage of the p38 MAPK pathway attenuated EMT in diabetic nephropathy both in vivo and in vitro [51,52]. Nevertheless, the mechanism of how p38 MAPK contributes to EMT remains unclear. A recent study reported that inhibition of the MAPK pathway resulted in a decrease in β-catenin [50]. An in vitro study reported that p38 MAPK activation inactivates GSK-3β by phosphorylating the C-terminal of GSK-3β to accumulate β-catenin [53]. Although p38 activation is extensively engaged in renal EMT, whether the TGF-β/p38 MAPK/GSK-3β/β-catenin axis leads to EMT in vivo remains unknown. Connective tissue growth factor (CTGF) release was induced under hypoxic conditions, while p38 inhibition-induced EMT suppression was accompanied by CTGF downregulation [54]. This implies a mechanism in which p38 MAPK is associated with hypoxia-related EMT, but the specific mechanism of CTGF regulation remains elusive.
Several inducers contribute to EMT by phosphorylating JNK, which increases the expression of EMT-related transcription factors such as Snail1 and Slug [55,56]. MAPK phosphatase (MKP2) has been revealed to act as a negative upstream regulator of the JNK pathway, not p38 or ERK [57]. JNKs can be separated into three isoforms, JNK1–3. JNK1 mainly phosphorylates c-Jun, while JNK2 decreases the stability of c-Jun [58]. JNK3 is less studied in the existing literature than JNK1/2. Furthermore, currently used JNK inhibitors nonspecifically suppress all JNK proteins. In order to further investigate the features of each JNK protein, specific JNK inhibitors are needed to suppress the expression of each JNK protein separately.
Blockade of the ERK pathway suppressed renal interstitial fibrosis by regulating EMT [59]. Mesenchymal stem cells (MSCs) released extracellular vesicles containing miR-294/miR-133 which reduced renal EMT [60]. In this case, both Smad and ERK pathways were inhibited [60]. Purine receptors of the purinergic receptor family are inflammatory modulators which reduce renal EMT effectively by releasing calcium ions coupled to the MAPK/ERK pathway [61,62]. Purine receptor A2AR reduced EMT by triggering cAMP-related pathways [63], and specific ERK1/2 inhibition decreased the anti-EMT effect of A2AR [63]. This indicates that the activation of purine receptors may mitigate EMT by regulating the ERK pathway.
Wnt activates EMT through the Wnt/GSK-3β/β-catenin axis
Wnts contain a family of signaling proteins involved in injury and repair [64]. They interact with frizzled (Fzd) receptors and low-density lipoprotein receptor-related protein (LRP5/6) to transmit signals [65]. Without pathological stimulation, β-catenin forms a complex with Axin and adenomatous polyposis coli (APC), and this heterotrimer interacts with GSK-3β [66]. When the Wnt signaling pathway is stimulated, the cytosolic proteins disheveled (Dvl) are recruited to inhibit GSK-3β phosphorylation [67]. Under such conditions, β-catenin accumulates in the cytoplasm and is then transported into nuclei where it interacts with lymphoid enhancer factor 1 (LEF1) and accelerates the transcription of EMT genes [64,65,68].
Notch signaling pathway is involved in fibrosis in several organs
Notch signaling is activated by interaction of Notch receptors (Notch1–4) and multiple ligands including Jagged-1 and Jagged-2 [69]. Notch is a transmembrane receptor which is composed of an extracellular domain (NECD) and an intracellular domain (NICD). γ-Secretase cleaves NICD to promote the translocation of free NICD into the nucleus. Then, the NICD forms a complex with CSL (CBF-1, Suppression of hairless, and Lag), a DNA-binding protein, to stimulate the expression of EMT transcription factors [19,70].
The Notch signaling pathway is engaged in the mechanisms for fibrosis in various organs. Increased expression of Notch1, Jagged-1 and Notch3 was found in pulmonary fibrosis with increased mesenchymal markers and decreased epithelial markers [71]. Hypoxia-induced EMT was promoted in renal tubular epithelial cells by directly targeting Notch1 and Jagged1, and subsequent Notch downstream signaling [72].
A disintegrin and metalloproteinase domain-10 (ADAM10) is a transmembrane protease which was recently found to induce renal fibrosis through EMT by activating the Notch pathway with the complementary help of ADAM17 [73]. However, the specific mechanism of how ADAM10 affects the Notch pathway still remains elusive. A member of TGF-β called Gremlin was found to induce EMT in UUO mice models via interacting with vascular endothelial growth factor receptor 2 and Notch pathway activation was found in this study [74]. Another study found that inhibition of the Notch pathway promoted vascular endothelial growth factor receptor 2 expression [75]. These two findings suggest a potential relationship between the TGF-β pathway and the Notch pathway.
Crosstalk of EMT signaling pathways
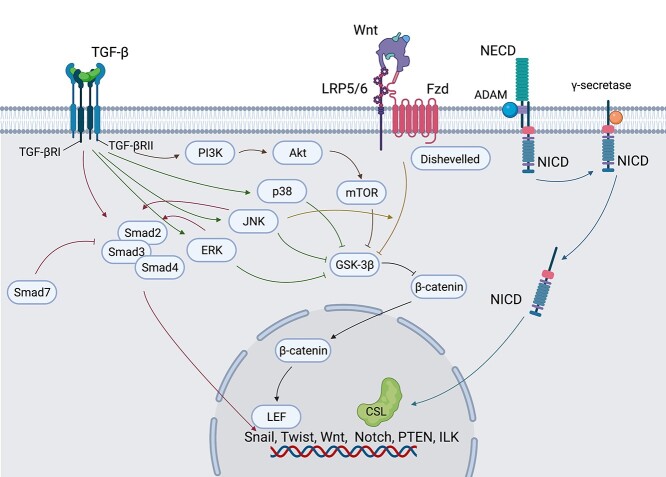
As stated above, EMT is regulated by various pathways. These pathways crosstalk intricately (Figure 4). GSK-3β serves at the intersection between Wnt and non-Smad pathways. Wnt and Smad-independent pathways both inhibit GSK-3β, which is a β-catenin inhibitor, to induce EMT. Some studies have demonstrated that they can regulate each other in regulating EMT. Inhibition of ERK and JNK prevents the phosphorylation of Smad2 and Smad3, respectively [76,77]. The Wnt pathway requires the regulation of the JNK pathway in pulmonary EMT in vitro [78]. Although some studies have found that EMT can be induced by more than one pathway, how these signaling pathways interact and work at different stages requires further investigation.
Figure 4.
The crosstalk of EMT pathways. EMT is regulated by several pathways and these pathways interplay intricately. TGF-β1 stimulates the activation of Smad and non-Smad pathways. All the non-Smad pathways and the Wnt pathway converge at GSK-3β, which is an inhibitor of β-catenin. β-Catenin is transported into nuclei and interacts with lymphoid enhance factor (LEF) to activate EMT gene transcription. The ERK and JNK pathways regulate the phosphorylation of Smad2 and Smad3 respectively. The Wnt pathway is mediated by the JNK pathway. Notch is composed of two domains, an extracellular domain (NECD) and an intracellular domain (NICD). After ADAM cuts the S2 site of the Notch receptor, γ-secretase cleaves NICD to release it. Then the NICD is translocated into the nucleus and bound to a DNA-binding protein called CSL (CBF-1, suppressors of hairless and Lag) to stimulate the expression of EMT transcription factors. EMT epithelial–mesenchymal transition, TGF-β transforming growth factor-β, GSK glycogen synthase kinase, JNK C-jun N-terminal kinase, ADAM10 a disintegrin and metalloproteinase domain-10
Epigenetic mechanisms involved in EMT
Epigenetic modifications such as histone deacetylation, lysine methylation, long noncoding RNAs (lncRNAs) and micro-RNA (miRNA)-induced epigenetic modification have been newly discovered as crucial mechanisms of EMT.
lncRNAs are a group of RNAs with >200 nucleotides that do not code for any proteins [79]. They were demonstrated to affect the EMT process by regulating miRNA. lncRNA nuclear enriched abundant transcript 1 (NEAT1) was shown to promote glucose-induced mouse renal mesangial cell (MMC) EMT and bleomycin-induced pulmonary EMT [79,80]. EMT markers were downregulated by the inhibition of NEAT1 [79,80], but miR-23c depletion partly reversed this effect [79]. This implies that miR-23c may serve as a downstream target of lncRNA NEAT1. Moreover, miR-9-5p was revealed to bind to NEAT1 directly to attenuate pulmonary EMT [80]. lncRNA plasmacytoma variant translocation 1 (PVT1) triggered liver EMT by binding to miR152 competitively [81].
Apart from lncRNAs and miRNAs, several kinds of histone 3 lysine methylations were found to be involved in EMT. Disruptor of telomeric silencing-1 like (DOT1L) is a methyltransferase which can catalyze histone 3 on lysine 79 (H3K79). DOT1L increased by renal damage was found to cause EMT by dimethylating H3K79 [82]. G9a, a Snail-interacting protein, is a histone methyltransferase. Nagaraja et al. demonstrated that upon stimulation by radiation, H3K9 methylation in the E-cadherin promoter region could be modified by ROS to trigger pulmonary EMT via activating G9a [83]. During EMT, decreased H3K9 dimethylation and trimethylation, and increased H3K4 trimethylation on the snail promoter region were discovered [83], but the mechanism remains unknown. Under conditions of hypoxia, histone deacetylase 3 (HDAC3) combined with WD repeat domain 5 (WDR5) to recruit histone methyltransferase, leading to acetylated H3K4 decrease and methylated H3K4 increase [84]. Enhancement of IL-6 was shown to regulate paraquat-induced pulmonary EMT [85]. Furthermore, IL-6 expression was reported to be increased by HDAC inhibitors and decreased by histone acetyltransferase (HAT) [85]. Epigenetically, immunoprecipitation data indicated that H3K4 trimethylation and H3K9 acetylation were promoted at IL-6 promoter regions [85].
EMT in fibrosis in different organs
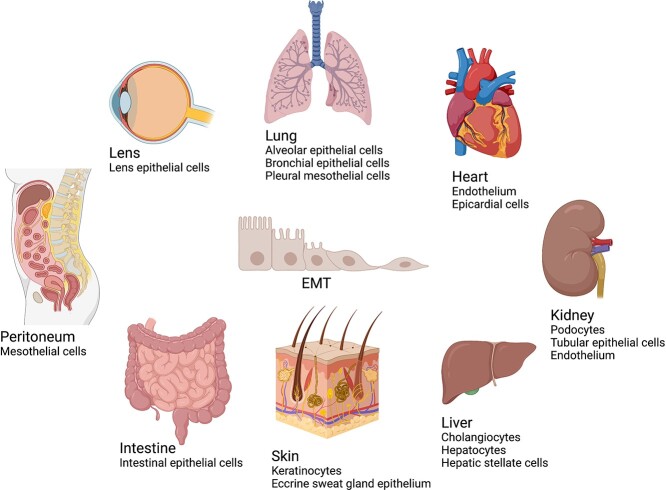
EMT is involved in multiple organs, including lung, liver, kidney, peritoneum, heart, skin and lens. There are multiple cell types that can undergo EMT to acquire myofibroblast features, leading to distinct organ diseases. Targeting these cell types may promote the progress of fibrosis treatment (Figure 5).
Figure 5.
Source of epithelial-to-mesenchymal transition (EMT) in different organ fibrosis. In the lungs, alveolar epithelial cells, bronchial epithelial cells and pleural mesothelial cells are revealed to undergo EMT and cause further airway remodeling. In the liver, after injury, the hepatic stellate cells undergo an EMT-like process with decreased epithelial markers and increased mesenchymal markers. The role of hepatocytes and cholangiocytes in EMT is debatable. In the kidneys, it has been widely accepted that tubular epithelial cells (TECs) are the source of renal EMT. However, some studies demonstrated that TECs undergo partial EMT because they do not cross the renal basement membrane but express mesenchymal and epithelial markers concomitantly. Additionally, studies have shown that podocytes and renal endothelial cells are engaged in renal fibrosis as well. After peritoneal dialysis, peritoneal mesothelial cells tend to undergo EMT and cause peritoneal fibrosis. In the heart, endothelium undergoes endothelial-to-mesenchymal transition (EndMT) in cardiac fibrotic diseases. In the intestines, intestinal epithelial cells undergo EMT to contribute to fibrotic intestinal diseases. In the skin, keratinocytes undergo EMT to cause keloid. Moreover, the eccrine sweat glands epithelium is a potential source of EMT which occurs in scleroderma. In the lens EMT, all the myofibroblasts originate from lens epithelial cells
Lung
EMT is involved in a variety of pulmonary fibrosis diseases [86]. A study revealed that bronchial epithelial cells (BECs) stimulated by TGF-β1 are able to transform into myofibroblasts, and lead to peribronchial fibrosis. This process leads to airway remodeling and is significant in the pathogenesis of asthma [87]. In IPF, alveolar epithelial cells (AECs) that undergo EMT lead to the formation of fibroblastic foci and further destroy the blood flow of alveolar septa [88]. Noteworthily, apart from BECs and AECs that contribute to myofibroblasts during EMT, pleural mesothelial cells also undergo mesothelial-to-mesenchymal transition (MMT), a special type of EMT, during IPF pathogenesis. MMT, termed as mesothelial cells that acquire mesenchymal features, is a special form of EMT in all the serosal membranes. A study revealed that pleural mesothelial cells exhibited mesenchymal features and migrated to the parenchyma of the lung after inhaled fibrogenic stimulation in vivo [89].
Liver
Liver epithelial cells undergo EMT to contribute to myofibroblasts, which has recently attracted more attention. Hepatocyte Snail1 deletion by using the Cre-loxP technique revealed less EMT markers and inflammatory response compared to the WT group in vivo. Rowe et al. also found that in the absence of Snail1, the liver expressed fewer profibrogenic genes such as Col1a1, Col2a1, Col3a1, vimentin, FSP1, PDGFRB and MMP14 [90]. However, the involvement of hepatocytes and cholangiocytes in liver EMT is controversial. Though hepatocytes treated with TGF-β1 were able to undergo EMT in vitro [91], Taura et al. demonstrated that hepatocytes did not express mesenchymal markers in vivo in any phase of liver injury [92]. A study revealed that cholangiocytes isolated from bile duct ligation (BDL) mice expressed fewer epithelial markers but more mesenchymal markers (FSP-1) [93]. However, David et al. failed to detect the EMT process in cholangiocytes in BDL and carbon tetrachloride (CCl4) models using a genetic labeling technique [94]. Hence, the authenticity of the roles of hepatocytes and cholangiocytes in liver EMT requires further study. Hepatic stellate cells (HSCs) are currently considered to be the major source of myofibroblasts after liver injury. In a CCL4 liver injury mouse model, HSCs accounted for 87% of myofibroblasts [95]. During liver development, sub-mesothelial cells underwent EMT to transform into HSCs [96]. Interestingly, in a quiescent state, HSCs expressed more epithelial markers than mesenchymal markers, but they expressed more mesenchymal markers after injury [97]. This implies that although HSCs are not strictly epithelial cells, they can undergo an EMT-like process in the presence of injurious stimulation.
Kidney
It has been widely accepted that tubular epithelial cells (TECs) undergoing EMT are responsible for renal fibrosis development [98]. Iwano et al. reported that one-third of myofibroblasts in renal fibrosis came from epithelial cells through EMT by using lineage-tracing experiments [99]. However, during EMT, the proportion of epithelial cells that transform into myofibroblasts is still controversial. LeBleu et al. later demonstrated that 5 and 10% of myofibroblasts came from tubular epithelial cells and endothelial cells, respectively, by using fate-mapping experiments [7]. Interestingly, Grande et al. demonstrated that TECs did not cross the basement membrane to contribute to myofibroblasts but only co-expressed mesenchymal and epithelial markers in Snail1 reactivation models in vivo [11]. Hence, a new conception of partial EMT (pEMT) was proposed. This pEMT phenomenon indicates that EMT likely contributed to fibrosis not because of fibroblast generation but because of inducing loss of functionality, impairing epithelial regeneration and affecting the inflammatory milieu [11,100]. This conception of pEMT offers us a novel perspective on renal fibrosis treatment. Apart from TECs that have been widely recognized to be the source of renal EMT, podocytes and the endothelium have recently been identified as the origins of EMT in the pathogenesis of renal fibrosis as well [101]. A high glucose-induced diabetic nephropathy (DN) model is highly correlated with podocyte EMT [101]. Therefore, targeting of podocyte EMT may offer a new insight for diabetic nephropathy treatment.
In addition, although studies indicated that the endothelium is able to undergo endothelial-to-mesenchymal transition (EndMT) to acquire mesenchymal phenotypes, the proportion of endothelium that contributes to myofibroblasts in renal fibrosis varies from 10 to 50% [7,102]. This discrepancy may be caused by the distinct endothelial markers that researchers used. Hence, a gold standard is required to enhance the specificity of EndMT detection. Genetic deletion of Twist1 and Snail1 in renal endothelial cells revealed a decreased renal EndMT and fibrosis level [103]. Moreover, their deletion induced the reduction of CD8+ cell infiltration, indicating that a decrease in the inflammatory response may also contribute to the reduction of fibrosis [103].
Aryl hydrocarbon receptor (AhR) activation is associated with chronic kidney disease (CKD) progression. 1-Aminopyrene (AP) was found to be a novel mediator of EMT by upregulating AhR mRNA and the expression of its three target genes [104]. This implied a critical role of aryl-containing metabolites (ACM) in EMT. Recent studies also showed that CKD patients and mice had gut microbes dysbiosis, which resulted in the accumulation of uremic toxins. A UUO and 5/6 nephrectomized rat models revealed the proportion of altered gut microbiota and plasma metabolites, with upregulation of EMT markers such as α-SMA, collagen I and fibronectin [105,106]. Moreover, using plant-derived drugs to restore the dysbiosis of gut microbiota could downregulate the expression of EMT markers. Collectively, these studies imply the potential mechanism by which gut microbes regulates serum metabolites to induce EMT in CKD patients.
Chen et al. discovered that five metabolites including 5-methoxytryptophan (5-MTP) can distinguish all clinical CKD stages. Among them, 5-MTP administration could inhibit the activation of EMT markers, and knockdown of tryptophan hydroxylase-1 (TPH-1) increased the expression of these markers [107]. TPH-1 is an enzyme that is involved in 5-MTP synthesis, and it was downregulated in ischemia reperfusion injury (IRI) and UUO mice. TPH-1 may serve as a critical target of EMT by controlling 5-MTP synthesis. Furthermore, the administration of 5-MTP not only attenuated the expression of pro-inflammatory factor NF-kB and its downstream gene products monocyte chemoattractant protein-1 (MCP-1) and cyclooxygenase-2 (COX-2), but also increased the presence of the anti-inflammatory transcription factor Nrf2 and its target genes Heme oxygenase-1 (HO-1) and NADPH quinone oxidoreductase-1 (NQO-1) [107]. Taken together, this may suggest that TPH-1 may attenuate EMT by targeting NF-kB and Nrf2 pathways.
Intestine
An increasing number of studies showed that EMT was involved in inflammatory bowel disease (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) [108]. In IBD patients, recurrent intestinal inflammatory factors triggered mucosal healing reactions, leading to ECM deposition to form fibrosis [108]. Transgenic mice expressing high levels of tumor necrosis factor-like ligand 1 A (TL1A) were sensitive to dextran sodium sulfate (DSS) and expressed more EMT markers with upregulation of the TGF-b/Smad3 pathway [108]. This indicates the critical role of TL1A in intestinal EMT. EMT also plays a critical role in the formation of fistulas in patients with CD [109]. Recent research revealed that CD patients had increased levels of succinate and its receptor SUCNR1 in fistula tract, accompanied by high levels of EMT markers, and Wnt signaling pathway activation was found to induce intestinal epithelial EMT [110]. Therefore, the Wnt pathway was suggested to contribute to fistula formation. These reports imply that SUCNR1 is a potential therapeutic target for preventing fistula formation in CD.
Peritoneum
Peritoneal dialysis is an alternative to hemodialysis in patients who have suffered from end-stage renal failure. However, during peritoneal dialysis, the persistent exposure to bio-incompatible dialysate and plastic circuits as well as hemoperitoneum can all trigger EMT to induce peritoneal fibrosis [111,112]. MMT is considered an early mechanism underlying peritoneal fibrosis, suggesting early intervention of mesothelial cells (MC). Therefore, EMT may maintain mesothelial barrier integrity [113].
Heart
EMT and its special type, EndMT, are involved in cardiac healing during pathological states such as myocardial infarction, atherosclerosis and valve dysfunction [114,115]. Epicardial EMT was activated after injury to initiate angiogenesis and healing [114]. One-quarter of epicardial cells treated by Wnt1 were found to have mesenchymal phenotypes [116]. Although previous studies revealed that EndMT was involved in cardiac fibrosis, the proportion of endothelial cells that contribute to myofibroblasts is under debate and warrants further study [117]. More in vivo lineage-tracing experiments are required to clarify the proportion of myofibroblasts with endothelial origin. Moreover, since the conception of pEMT in renal fibrosis was proposed [118], the discrepancy of endothelium-derived myofibroblasts measured in different organs may be caused by partial EndMT.
Compared to other organs such as lung, kidney and liver, the understanding of signaling pathways that are involved in cardiac EMT is limited. The activation of PPAR-γ alleviates cardiac EMT by inhibiting the ERK pathway, with the promotion of MMP2 and MMP9 [119]. In addition, a previous study showed that epicardial-specific β-catenin-deletion mice had an ameliorated epicardial EMT after IRI [116], implying the pivotal function of β-catenin-related pathways such as Wnt, MAPK and PI3K/Akt.
However, although EMT plays a role in cardiac healing, it is not sufficient to repair injury completely. The therapeutic strategy for cardiac fibrosis requires the targeting of other mechanisms in addition to EMT [114].
Skin
Evidence suggested that EMT was engaged in cutaneous fibrosis such as keloid and scleroderma. In keloid tissue, EMT markers were found in keratinocytes, and this process was activated by proinflammatory factors [120]. Scleroderma is an autoimmune disease which primarily affects dermis and subcutaneous regions of the skin, with a thick and tight appearance [121]. A study demonstrated that scleroderma was associated with the EMT process in eccrine sweat glands with fibronectin and also affects E-cadherin expression [122]. This study may indicate that the myoepithelium in eccrine glands is a potential source of EMT.
Lens
EMT of lens epithelial cells is vital in the pathogenesis of fibrotic cataract formation after cataract surgery and under high-glucose conditions [123]. It is worth noting that since there are no resident fibroblasts in lens, all the myofibroblasts in lens are derived from lens epithelial cells. This is different from other organs which have diverse potential origins of myofibroblasts [124].
Preventive strategies
Although some drugs have been proven to suppress or partially reverse EMT in laboratory experiments, further clinical trials are needed for their clinical application in the treatment of fibrosis. Unfortunately, in clinical settings, anti-fibrosis drugs are only used to relieve symptoms but not to reverse fibrosis completely.
Naturally derived therapies
Botanical compounds
Multiple herbal extractions were reported to attenuate EMT. Resveratrol (RSV) and its analog pterostilbene are extracted from the red grape. They partially inhibited EMT by regulating Akt and Smad pathways [125,126]. Curcumin extracted from Curcuma longa suppressed EMT by regulating both Smad and non-Smad pathways [43,127]. Inhibition of the PI3K/Akt/mTOR pathway by curcumin was found to attenuate renal and liver EMT in vivo [28,43]. During peritoneal EMT, curcumin inhibited TAK1, and further downregulated the expression of p38 and JNK, indicating its regulatory role in MAPK pathways [127]. Curcumin also regulated the tetratricopeptide repeat domain 3/Smad ubiquitination regulatory factor 2 (SMURF2)/Smad axis [28]. It blunted tetratricopeptide repeat domain 3 which subsequently enhanced SMURF2 expression to prevent Smad2/Smad3 formation in liver EMT [28]. Some RAS inhibitors, such as members from poricoic acid groups extracted from Poria cocos and 25-O-methylalisol F (MAF) extracted from Alismatis rhizoma, were found to suppress EMT [128]. MAF combined with TGF-β receptor I competitively prevented the interaction of Smad3 with TGF-β receptor I [128]. The poricoic acid group contains poricoic acid ZA (PZA), ZC (PZC), ZD (PZD), ZE (PZE), ZF (PZF), ZG (PZG) and ZH (PZH). Of those, PZC, PZD, PZG and PZG belong to the secolanostane tetracyclic triterpenoid group while PZE and PZH belong to lanostane tetracyclic triterpenoid group [129,130]. These poricoic acids, except PZF, were demonstrated to exert anti-EMT function by inhibiting the Wnt/β-catenin and Smad pathways [129,130]. They were found to suppress the interaction of Smad3, but not Smad2, with TGF-β receptor I [129,130], indicating that Smad3, rather than Smad2, was a critical therapeutic target of poricoic acid groups. However, some herbal extractions have been observed to attenuate EMT only in vitro, such as ferulic acid, PZG and PZH [131]. Therefore, more animal experiments should be carried out to test their efficacy. Furthermore, PZF had no effect on anti-EMT, and PZE had a weaker anti-EMT function compared to other secolanostane tetracyclic triterpenoid groups [129,130]. This may be caused by the discrepancy in their chemical structure and the increased number of hydroxyl groups on side chains, whereas carboxyl groups may be more renoprotective [130]. Triptolide (TPL) is a diterpenoid epoxide which is extracted from Tripterygium wilfordii. TPL reduced Snail1 and Twist to inhibit EMT in phosphatase and tensin homolog-induced diabetic kidney disease by interacting with the RING domain of mex-3 RNA binding family number C (MEX3C) to prevent its combination with ubiquitin-conjugating enzyme E2S (UBE2S) [132]. This provides a structural mechanism of how TPL inhibits ubiquitin ligase.
In addition, some plant extractions were shown to mitigate EMT by altering microbial composition. In chronic kidney disease, poricoic acid A and P. cocos were found to decrease the level of glycine-conjugated compounds and polyamine metabolites, which are derived from microbes [105]. Polyporus umbellatus and its extraction, ergone, were revealed to increase plasma tryptophan levels and decrease some tryptophan metabolites to mitigate EMT [106]. Collectively, improving the levels of plasma metabolites that help the recovery of intestinal barrier function, may provide a novel solution to treat EMT.
Compounds derived from living species
Eicosapentaenoic acid (EPA), derived from fish oil has been reported to inhibit inflammatory responses and exert anti-fibrotic activity through regulating Smad3 signaling and activating miR-541 [133,134]. EPA’s function of attenuating renal EMT has only been tested in vitro and further experiments are needed to confirm its in vivo functions. Melittin is the major component of bee venom, which can be applied in anticancer and anti-inflammation therapies. One study indicates that melittin suppressed hepatic EMT via regulating JNK and Smad-dependent pathways both in vitro and in vivo [56]. These reports suggest that EPA and melittin have the potential to attenuate EMT in clinical use.
Epigenetic modification
HDAC modulators
Mammalian HDAC contains 18 subtypes that are classified into four classes. Class I contains HDAC 1–3 and 8; class II contains class IIa (HDAC 4, 5, 7 and 9) and class IIb (HDAC 6, 8 and 10); class IV only contains HDAC 11, and class III contains sirtuins (SIRT) 1–7 [135]. HDACs act as pro-inflammation molecules that are involved in fibrogenesis. HDAC mediators have an effect on EMT by regulating histones epigenetically.
HDAC inhibitors
The inhibition of HDACs restrained E-cadherin reduction and decreased mesenchymal markers as well as ECM components [136], indicating that HDACs inhibitors are potential treatments for renal fibrosis by targeting EMT.
MS-275 is an HDAC 1–3 inhibitor that was proved to attenuate peritoneal and renal EMT [136,137]. The anti-EMT function of MS-275 on the peritoneum depends on H3 acetylation, Snail impairment and rescue acetylation of E-cadherin promoter, which is in accordance with HDAC I gene silencing results [137]. Tubastatin A is an HDAC 6 inhibitor that attenuates peritoneal and renal EMT by inhibiting Smad3 phosphorylation and acetylating histone H4 [138]. However, a study demonstrated that although tubastatin A ameliorated bleomycin-induced pulmonary EMT through targeting the PI3K/Akt pathway, knockdown of HDAC6 did not have this function [139]. Hence, whether the anti-EMT function of tubastatin A depends on inhibiting HDAC6 remains obscure. Valproic acid (VPA) is a selective class I HDAC inhibitor. Pretreatment with VPA attenuated pulmonary EMT and restored lung structure in vivo by downregulating p-Smad2/Smad3 and p-Akt [140]. VPA ameliorated renal EMT by increasing Smad7 and by acetylating histone H3 in mice with diabetic nephropathy, and the promoter enrichment of Fn1 and Col1a1 in H3 was decreased by VPA [141]. Different from tubastatin A and VPA, an HDAC8 specific inhibitor called PCI34051 attenuated renal EMT in vivo with no histone H3 and H4 acetylation [142]. Whether H1 and/or H2 or histone-related protein are mediated by PCI34051 remains unknown and requires further study. Trichostatin A (TSA) is a class HDAC I and II inhibitor, and it was found to attenuate EMT in UUO-induced renal fibrosis by promoting M1-to-M2 macrophage transition and increasing the number of anti-inflammatory M2c instead of proinflammatory M2a macrophages [143]. However, the specific mechanism of how TSA affects macrophages is unclear. A recent study indicated that only the JNK signaling pathway was involved in the anti-EMT function of TSA, which led to the inactivation of Notch-2 [144]. Suberoylanilide hydroxamic acid (SAHA) is an HDAC inhibitor approved by the food and drug administration (FDA) to treat cutaneous T-cell lymphoma. SAHA was shown to inhibit the CKD process in col4a3−/− mice with decreased α-SMA and fibronectin [145]. The administration of SAHA decreased mesenchymal markers and increased epithelial markers in HSCs [146]. Interestingly, like TSA, only JNK signaling was found to be inhibited by SAHA [145], indicating that the JNK pathway may be promising for reversing EMT. RSV is a well-studied plant-derived drug which can relieve fibrotic EMT in the lungs and kidneys by inhibiting the ROS and Smad pathways [126,147]. However, research on how RSV affects HDAC epigenetically is limited. Its metabolite, piceatannol, was revealed to suppress EMT protein expression by inhibiting HDAC4 and 5 [148]. The inhibition of the p38 MAPK pathway, rather than the Smad pathway, was also detected under the administration of piceatannol [148], indicating the potential relationship between the p38 pathway and class IIa HDAC. A specific HDAC class IIa inhibitor, MC1568, attenuated renal EMT through blocking the TGF-β/Smad pathway [149]. Collectively, HDAC 7 and/or 9 may have an important impact on EMT through activating TGF-β-related pathways.
On this basis, epigenetic regulation plays a potential role in ameliorating EMT. However, whether these HDAC inhibitors affect histone acetylation by modulating histone chaperones or binding to histones directly is unclear. No HDAC inhibitors are currently approved for clinical use in fibrosis treatment.
SIRT activators
SIRT are a series of Class III HDAC which require adenine dinucleotide (NAD+). They play significant roles in physiology and pathology [150]. Based on their roles in fibrosis, several studies found SIRT activators have an anti-EMT effect in different organs.
RSV activated SIRT1 to inhibit EMT dose-dependently. The underlying mechanism of RSV is to decrease Smad3 phosphorylation and further impede Smad3 and Smad4 complex formation [151]. Astragaloside IV (AS-IV) and salvianolic acid B (Sal B) impair EMT by activating SIRT1 to inhibit NF-κB p65 acetylation and phosphorylation [101,152]. Honokiol (HKL) is a kind of SIRT3 activator which can reduce the level of α-SMA and ECM components in renal fibrosis induced by UUO. Since SIRT3 suppression is associated with EMT emergence, HKL was suspected of preventing fibrosis by inhibiting the EMT process. Therefore, epithelial markers need to be studied to confirm HKL’s role in inhibiting EMT. HKL also increased NF-κB p65 phosphorylation, indicating that it exerts anti-EMT function probably by anti-inflammatory processes [153]. Decreased Smad2 and Smad3 phosphorylation were also found in kidneys treated with HKL, but the specific mechanism is unknown.
SIRT6 interacts with the promoters of β-catenin target genes to induce histone H3K56 deacetylation to prevent the transcription of fibrosis-related genes. Hence, SIRT6 reduced the transcription of genes associated with β-catenin through epigenetic regulation [154]. However, to date no SIRT6 activator is currently being investigated for the attenuation of EMT. Since several EMT-related signaling pathways converge at β-catenin, finding a SIRT6 activator may be promising for EMT inhibition.
miRNA and lncRNA
Some lncRNAs and miRNAs were reported to regulate EMT in some models. lncRNA zinc finger E-box binding homeobox 1 antisense (ZEB1-AS1) was downregulated upon high-glucose treatment. Overexpression of lncRNA ZEB1-AS1 was shown to attenuate renal EMT by targeting miR-216a-5p directly assessed with a luciferase reporter assay [155]. In CCl4-treated hepatic EMT models, Salvianolic acid B upregulated miR-152, which further contributed to DNA methyltransferase 1 (DNMT1) inhibition and DNA demethylation [156].
Histone methylation
EPZ5676 is a DOT1L inhibitor. DOT1L was shown to methylate H3K79 during renal EMT [82]. Inhibition of methyltransferase DOT1L reduced Snail, Twist and Notch1 expression and reversed the reduction of renoprotective factors such as Klotho, Smad2 and Smad7 [82]. Hence, using EPZ5676 to block H3K79 methylation may have therapeutic potential. KDM6A is a histone lysine demethylase which can regulate E-cadherin expression. Its inhibitor, GSK-J4, was found to restore EMT in streptozotocin (STZ)-induced renal injury mice [157]. In addition, miR-199b-3p overexpression targeted KDM6A to prevent EMT [157]. However, which lysine of histone is targeted by KDM6A to regulate EMT remains unclear. As a large number of EMT epigenetic mechanisms have been found, blocking histone methylation is a prospective therapeutic strategy.
Therapies related to TGF-β
The activation of TGF-β signaling contributes to EMT in various scenarios. Hence, targeting TGF-β signaling to reverse or stop EMT may be worth developing for clinical use.
Bone morphogenetic protein-7
Bone morphogenetic protein-7 (BMP-7) is a member of the TGF-β superfamily and its administration alleviates EMT in pulmonary, intestine and renal cells [108,158]. The binding of BMP-7 and BMP receptors (BMPRs) stimulated Smad1/5/8 phosphorylation. Subsequently, phosphorylated Smad1/5/8 can compete with Smad2/Smad3 to form a heteromeric complex with Smad4 [159]. BMP-7 was found to suppress EMT in renal HK-2 cells by inhibiting Wnt3a/b-catenin and TGF-β1/Smad2/3 pathways [160]. In RLE-6TN cells, BMP-7 induced mesenchymal-to-epithelial transition (MET) and blunted the EMT process by inhibiting the p38 MAPK pathway [161]. A recent study used protein transduction domain (PTD)-fused BMP-7 to treat A549 cells and UUO mice [158]. It has been demonstrated that the protein transduction domain fused BMP-7 in micelle (mPTD-BMP-7) was transduced into cells via an endosomal pathway and secreted to exosomes containing BMP-7. EMT was successfully ameliorated by mPTD-BMP-7 through activating the Smad1/5/8 pathway [158]. Furthermore, the protein transduction domain fused BMP-7 in micelle (mPTD-BMP-7) was found in Bowman’s space after its intra-arterial administration to porcine renal artery, and the fibrosis caused by UUO was alleviated [158]. However, whether this effect was related to EMT has not yet been identified. Interestingly, one study showed that BMP-7 treatment in diabetic nephropathy almost completely reverses the expression of E-cadherin and α-SMA [162].
TGF-β monoclonal antibody
TGF-β is a well-known fibrosis inducer that has been found in multiple organs. TGF-β activation can induce EMT by stimulating its downstream signaling pathways, such as Smad-dependent and Smad-nondependent pathways, as previously described. Antagonizing TGF-β by using its monoclonal antibody (mAb) was reported to reduce ECM deposition and attenuate fibrosis in various scenarios, suggesting its role in anti-EMT. Hence, several studies investigated whether TGF-β mAb can ameliorate EMT after tissue damage. 1D11 is a murine antibody which targets all three TGF-β isoforms and reversed pre-existing thioacetamide-induced liver fibrosis by decreasing ECM production and increasing ECM degradation [163]. In addition, the administration of TGF-β mAb reduced PAI-1 expression [163], a plasminogen activator inhibitor whose interaction with the urokinase-type plasminogen activator receptor (uPAR) caused ECM accumulation by triggering TGF-β-related signaling pathways [164]. This indicates that TGF-β antibody has a therapeutic impact on hepatic fibrosis. Notably, the expression of TGF-β2 and TGF-β3 was not changed by 1D11 [163]. It is unclear whether this was due to 1D11 affinity or the intracellular distribution of TGF-β2 and TGF-β3.
TGF-β neutralizing antibody injection reduced collagen deposition and expression of fibronectin extra domain A (FnEDA) in UUO mice [165], indicating the anti-EMT role of TGF-β neutralization antibody during renal injury. A bispecific antibody containing both FnEDA and TGF-β domains was also constructed to reverse EMT during renal injury. Nevertheless, injecting TGF-β mAb alone demonstrated a diffused distribution in contrast to the bispecific antibody in the kidney [165]. This indicates that TGF-β mAb therapeutic efficiency can be improved by bispecific antibody construction. Further experiments are required to investigate the effectiveness of TGF-β mAb in pre-existing renal fibrosis.
Collectively, the use of TGF-β mAb provides a novel approach for anti-EMT and subsequent fibrosis development in different organs. However, TGF-β is a critical cytokine with multiple functions such as regulating the adaptive immune system and T cell differentiation [166]. Therefore, the risk/benefit ratio of TGF-β mAb therapy needs to be fully assessed before clinical use can be implemented.
Stem cells
Stem cell transplantation is emerging as a novel approach for EMT inhibition. The administration of adipose-derived stem cells (ADSCs) attenuated cigarette smoke extract-induced pulmonary EMT in vitro [167], and renal EMT in vivo [167]. This process was suggested to be associated with the TGF-β pathway, but which specific downstream signaling pathway is involved remains unknown. Human umbilical mesenchymal stem cells (usMSCs) inhibited aristolochic acid-induced renal EMT by regulating the TGF-β/Smad pathway [168]. Vesicles containing miRNA derived from MSCs were proved to be essential in preventing EMT in renal fibrosis. Vesicles which carried miR-294 and miR-133 inhibited EMT and renal fibrosis by regulating the Smad and/or ERK pathway [60]. Anti-miR-let-7 carried by MSC vesicles increased tuberous sclerosis complex 1 (TSC1) expression level and reduced the phosphorylation of mTOR/p70S6K [169]. However, the exact mechanism is unclear. Let-7i-5p, a regulator of fibrogenesis EMT, was found to bind to TSC1 [169]. In normal physiological conditions, TSC1 and TSC2 form a complex. Akt phosphorylation can inactivate TSC2 and impede its interaction with TSC1 [170]. Hence, MSC vesicles may carry anti-miR-let-7 through targeting the Akt/TSC2/mTOR axis.
Erythropoietin (EPO) is a glycoprotein secreted by kidney which attenuates renal fibrosis in different models by inhibiting EMT [171]. However, it is unfeasible to inject low-dose EPO continuously in clinical events. Therefore, MSCs transfected with EPO genes (named hEPO-MSCs) were produced to tackle this concern. Interestingly, microparticles released by hEPO-MSCs were proved to attenuate renal fibrosis by targeting Smad and p38 MAPK EMT pathways [171].
Extracellular vesicles released by bone marrow stem cells (BMSC-EVs) were demonstrated to attenuate EMT in vivo by releasing milk fat globule-epidermal growth factor 8 (MFG-E8) [172]. Klotho is an anti-aging protein mainly expressed in renal tubular cells [173] and it has been shown to be essential for anti-EMT in renal fibrosis in a variety of studies. Using adenovirus to build Klotho-overexpressing BMSC had an anti-EMT effect and inhibited renal fibrosis by targeting the Wnt pathway in vivo [173]. However, stem cells and/or their derivative therapies are still not ready to be applied clinically.
Clinical medication
Unfortunately, no medication can cure or completely reverse fibrosis currently; all drugs in clinical settings focus on relieving symptoms.
Pirfenidone and nintedanib are anti-fibrotic drugs used for IPF clinically [174,175]. However, their severe gastrointestinal and skin adverse effects require mitigation [175]. Both nintedanib- and pirfenidone-treated patients have decreased liver function [175]. Further investigations such as dosage use should be carried out to prevent these side effects.
Losartan is an angiotensin II receptor antagonist that has been widely used in hypertension and end-stage renal disease treatment [176]. Previous studies have demonstrated that losartan reduced EMT in mitral valve remodeling after myocadiac infarction and renal fibrosis through regulating the TGF-β1 pathway [176,177]. Nevertheless, the administration of losartan is not sufficient to reverse the fibrotic clinical manifestations [178]. Noteworthily, other renin-angiotensin-aldosterone system (RAAS) intervention drugs such as eplerenone, telmisartan and spironolactone are proved to prevent EMT in vitro. Hence, this implies that RAAS intervention has the potential to be administrated in clinical events.
Chinese medicine recipes were reported to be used clinically for patients who suffered from fibrosis. [179–181]. Although these herbal recipes are capable of ameliorating EMT in experiments and are used for treating fibrosis in some cases, the side effects of herbal remedies such as nephrotoxicity and hepatotoxicity make their clinical use debatable.
Conclusions
EMT is a key mechanism during fibrogenesis in various organs. It is regulated by several pathways which crosstalk intricately. Recent studies have also explored the significance of epigenetic mechanisms during EMT. Several treatments were shown to inhibit or reverse EMT partially but not completely. Despite better understanding of the role of EMT in fibrosis development, targeting EMT and beyond in developing therapeutics to tackle fibrosis is challenging but feasible.
Abbreviations
α-SMA: α-smooth muscle actin; ADAM: A disintegrin and metalloproteinase domain; AEC: Alveolar epithelial cell; BDL: Bile duct ligation; BEC: Bronchial epithelial cells; BMP-7: Bone morphogenetic protein-7; CCl4: carbon tetrachloride ; CD: Crohn's disease; CKD: Chronic kidney disease; COX-2: Cyclooxygenase-2; CSL: CBF, Hairless, and Lag; CTGF: Connective tissue growth factor; DOT1L: Disruptor of telomeric silencing-1 like; ECM: Extracellular matrix; EndMT: Endothelial-to-mesenchymal transition; EMT: Epithelial-to-mesenchymal transition; EPA: Eicosapentaenoic acid; EPO: Erythropoietin; FDA: Food and drug administration; FnEDA: Fibronectin extra domain A; FSP: Fibroblast-specific protein 1; GSH: Glutathione; GSK-3: Glycogen synthase kinase 3; H3K79: histone 3 on lysine 79; HDAC3: Histone deacetylase 3;HKL:Honokiol;HSC:Hepatic stellate cells; HIF-α: Hypoxia inducible factor-α; IBD: Inflammatory bowel disease; IL: Interleukin; IPF: Idiopathic pulmonary fibrosis; JNK: C-jun N-terminal kinase; HO-1: Heme oxygenase-1; lncRNA: Long noncoding RNA; mAb: Monoclonal antibody; MAPK: Mitogen-activated protein kinase; MC: mesothelial cells; MCP-1: Monocyte chemoattractant protein-1; MET: Mesenchymal-to-epithelial transition; miRNA: Micro-RNA; MMT: Mesothelial-to-mesenchymal transition; 5-MPT: 5-Methoxytryptophan; mTOR: Mammalian target of rapamycin; MEX3C: mex-3 RNA binding family number C; mPTD-BMP7: protein transduction domain fused BMP-7 in micelle; NEAT1: Nuclear enriched abundant transcript 1; NF-κB: Nuclear factor-κB; NICD: Notch intracellular domain; NQO-1: NADPH quinone oxidoredctase-1; pEMT: partial epithelial–mesenchymal transition; PIP3: Phosphatidylinositol-3,4,5-trisphosphate; PPAR-α Peroxisome proliferator-activated receptor alpha; RAAS: Renin-angiotensin-aldosterone system; ROS: reactive oxygen species; RSV: Resveratrol; SAHA: Suberoylanilide hydroxamic acid; siRNA: Small interfering RNA; SMURF2: Smad ubiquitination regulatory factor 2; TEC: Tubular epithelial cells; TGF-β: Transforming growth factor-β; TL1A: Tumor necrosis factor-like ligand 1 A; TLR4: Toll-like receptor 4; TNF-α: Tumor necrosis factor-α; TPH-1: Tryptophan hydroxylase-1; TPL: Triptolide; TSA: Trichostatin A; TSC1: Tuberous sclerosis complex 1; UBE2S: Ubiquitin-conjugating enzyme E2S; UUO: Unilateral ureteral obstruction; VPA: Valproic acid; WDR5: WD repeat domain 5.
Authors’ contributions
DM conceived the project. LL wrote the first draft. All authors read, revised, edited and approved the final manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Acknowledgment
All figures were created with http://biorender.com
Funding
This study was supported by the BOC chair grant, the Westminster Medical School Research Trust and BJA/RCoA project grant.
Competing interests
None declared.
References
- 1. Olson AL, Gifford AH, Inase N, Fernandez Perez ER, Suda T. The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev. 2018;27:180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng JK, Li PK. Chronic kidney disease epidemic: how do we deal with it? Nephrology (Carlton). 2018;23:116–20. [DOI] [PubMed] [Google Scholar]
- 3. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duarte VM, McGrath CL, Shapiro NL, Bhattacharrya N. Healthcare costs of acute and chronic tonsillar conditions in the pediatric population in the United States. Int J Pediatr Otorhinolaryngol. 2015;79:921–5. [DOI] [PubMed] [Google Scholar]
- 6. Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–49. [DOI] [PubMed] [Google Scholar]
- 7. LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, et al. . Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, et al. . Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, et al. . Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–67. [DOI] [PubMed] [Google Scholar]
- 10. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. [DOI] [PubMed] [Google Scholar]
- 11. Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, et al. . Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–97. [DOI] [PubMed] [Google Scholar]
- 12. Di Gregorio J, Robuffo I, Spalletta S, Giambuzzi G, De Iuliis V, Toniato E, et al. . The epithelial-to-mesenchymal transition as a possible therapeutic target in fibrotic disorders. Front Cell Dev Biol. 2020;8:607483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan B, Liu G, Jiang Z, Zheng D. Regulation of renal fibrosis by macrophage polarization. Cell Physiol Biochem. 2015;35:1062–9. [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu B, Ding F, Hu D, Zhou Y, Long C, Shen L, et al. . Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi R, Wang J, Jiang Y, Qiu Y, Xu M, Rong R, et al. . Snai1-induced partial epithelial-mesenchymal transition orchestrates p53-p21-mediated G2/M arrest in the progression of renal fibrosis via NF-kappaB-mediated inflammation. Cell Death Dis. 2021;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cong LH, Li T, Wang H, Wu YN, Wang SP, Zhao YY, et al. . IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J Cell Mol Med. 2020;24:8532–44. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Du R, Xia L, Ning X, Liu L, Sun W, Huang C, et al. . Hypoxia-induced Bmi1 promotes renal tubular epithelial cell-mesenchymal transition and renal fibrosis via PI3K/Akt signal. Mol Biol Cell. 2014;25:2650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei X, Zhu X, Jiang L, Huang X, Zhang Y, Zhao D, et al. . Recent advances in understanding the role of hypoxia-inducible factor 1alpha in renal fibrosis. Int Urol Nephrol. 2020;52:1287–95. [DOI] [PubMed] [Google Scholar]
- 21. Zhang M, Liu S, Guan E, Liu H, Dong X, Hao Y, et al. . Hyperbaric oxygen therapy can ameliorate the EMT phenomenon in keloid tissue. Medicine (Baltimore). 2018;97:e11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sosa Peña MP, Lopez-Soler R, Melendez JA. Senescence in chronic allograft nephropathy. Am J Physiol Renal Physiol. 2018;315:F880–9. [DOI] [PubMed] [Google Scholar]
- 23. Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY). 2010;2:471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimizu H, Bolati D, Adijiang A, Enomoto A, Nishijima F, Dateki M, et al. . Senescence and dysfunction of proximal tubular cells are associated with activated p53 expression by indoxyl sulfate. Am J Physiol Cell Physiol. 2010;299:C1110–7. [DOI] [PubMed] [Google Scholar]
- 25. Li D, Pei X, Qin X, Liu X, Li C, Li L, et al. . Olaquindox-induced liver damage involved the crosstalk of oxidative stress and p53 in vivo and in vitro. Oxidative Med Cell Longev. 2020;2020:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez-Gonzalez FJ, Chandel NS, Jain M, Budinger GRS. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res. 2017;190:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muthuramalingam K, Cho M, Kim Y. Cellular senescence and EMT crosstalk in bleomycin-induced pathogenesis of pulmonary fibrosis-an in vitro analysis. Cell Biol Int. 2020;44:477–87. [DOI] [PubMed] [Google Scholar]
- 28. Kong D, Zhang Z, Chen L, Huang W, Zhang F, Wang L, et al. . Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020;36:101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jolly MK, Ward C, Eapen MS, Myers S, Hallgren O, Levine H, et al. . Epithelial-mesenchymal transition, a spectrum of states: role in lung development, homeostasis, and disease. Dev Dyn. 2018;247:346–58. [DOI] [PubMed] [Google Scholar]
- 30. Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. . New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, et al. . Central role of dysregulation of TGF-beta/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–81. [DOI] [PubMed] [Google Scholar]
- 32. Yang F, Huang XR, Chung AC, Hou CC, Lai KN, Lan HY. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol. 2010;221:390–401. [DOI] [PubMed] [Google Scholar]
- 33. Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–84. [DOI] [PubMed] [Google Scholar]
- 34. Wang YC, Liu JS, Tang HK, Nie J, Zhu JX, Wen LL, et al. . miR221 targets HMGA2 to inhibit bleomycininduced pulmonary fibrosis by regulating TGFbeta1/Smad3-induced EMT. Int J Mol Med. 2016;38:1208–16. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Zhang X, Mao Y, Liang L, Liu L, Peng W, et al. . Smad2 and Smad3 play antagonistic roles in high glucose-induced renal tubular fibrosis via the regulation of SnoN. Exp Mol Pathol. 2020;113:104375. [DOI] [PubMed] [Google Scholar]
- 36. Loeffler I, Liebisch M, Allert S, Kunisch E, Kinne RW, Wolf G. FSP1-specific SMAD2 knockout in renal tubular, endothelial, and interstitial cells reduces fibrosis and epithelial-to-mesenchymal transition in murine STZ-induced diabetic nephropathy. Cell Tissue Res. 2018;372:115–33. [DOI] [PubMed] [Google Scholar]
- 37. Gervasi M, Bianchi-Smiraglia A, Cummings M, Zheng Q, Wang D, Liu S, et al. . JunB contributes to Id2 repression and the epithelial-mesenchymal transition in response to transforming growth factor-beta. J Cell Biol. 2012;196:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiao Z, Chen C, Meng T, Zhang W, Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-beta pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood). 2016;241:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan X, Chen YG. Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J. 2011;434:1–10. [DOI] [PubMed] [Google Scholar]
- 40. Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, et al. . Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-beta (TGF-beta)/Smad Signaling. J Biol Chem. 2016;291:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (review). Mol Med Rep. 2018;18:3547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Z, Chen Z, Li B, Zhang B, Du Y, Liu Y, et al. . Curcumin attenuates renal interstitial fibrosis of obstructive nephropathy by suppressing epithelial-mesenchymal transition through inhibition of the TLR4/NF-small ka, CyrillicB and PI3K/AKT signaling pathways. Pharm Biol. 2020;58:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu Q, Wang WW, Zhang MZ, Ma ZX, Qiu XR, Shen M, et al. . ROS induces epithelial-mesenchymal transition via the TGF-beta1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp Ther Med. 2019;17:835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang J, Zhu H, Huang L, Zhu X, Sha J, Li G, et al. . Nrf2 signaling attenuates epithelial-to-mesenchymal transition and renal interstitial fibrosis via PI3K/Akt signaling pathways. Exp Mol Pathol. 2019;111:104296. [DOI] [PubMed] [Google Scholar]
- 46. Zhou T, Luo M, Cai W, Zhou S, Feng D, Xu C, et al. . Runt-related transcription factor 1 (RUNX1) promotes TGF-β-induced renal tubular epithelial-to-mesenchymal transition (EMT) and renal fibrosis through the PI3K subunit p110δ. EBioMedicine. 2018;31:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu FQ, Chen MJ, Zhu M, Zhao RS, Qiu W, Xu X, et al. . Curcumin suppresses epithelial-mesenchymal transition of renal tubular epithelial cells through the inhibition of Akt/mTOR pathway. Biol Pharm Bull. 2017;40:17–24. [DOI] [PubMed] [Google Scholar]
- 48. Shin JH, Kim KM, Jeong JU, Shin JM, Kang JH, Bang K, et al. . Nrf2-Heme Oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3βSignaling. J Diabetes Res. 2019;2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang X, Liang D, Fan J, Lian X, Zhao Y, Wang X, et al. . Zinc attenuates Tubulointerstitial fibrosis in diabetic nephropathy via inhibition of HIF through PI-3K Signaling. Biol Trace Elem Res. 2016;173:372–83. [DOI] [PubMed] [Google Scholar]
- 50. Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY, et al. . Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin. 2020;41:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Zhou Y, Zhang Y, He X, Zhao X, Zhao H, et al. . Roscovitine attenuates renal interstitial fibrosis in diabetic mice through the TGF-β1/p38 MAPK pathway. Biomed Pharmacother. 2019;115:108895. [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, Liang D, Chi ZH, Chu Q, Zhao C, Ma RZ, et al. . Effect of zinc on high glucose-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells. Int J Mol Med. 2015;35:1747–54. [DOI] [PubMed] [Google Scholar]
- 53. Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, et al. . Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao F, Wang Y, Li S, Wang Z, Liu C, Sun D. Inhibition of p38 mitogen-activated protein kinases attenuates renal interstitial fibrosis in a murine unilateral ureteral occlusion model. Life Sci. 2016;167:78–84. [DOI] [PubMed] [Google Scholar]
- 55. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park JH, Park B, Park KK. Suppression of hepatic epithelial-to-mesenchymal transition by Melittin via blocking of TGFβ/Smad and MAPK-JNK Signaling pathways. Toxins (Basel). 2017;9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Z, Liu X, Tian F, Li J, Wang Q, Gu C. MKP2 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells through a JNK-dependent pathway. Clin Sci (Lond). 2018;132:2339–55. [DOI] [PubMed] [Google Scholar]
- 58. Ebelt ND, Cantrell MA, Van Den Berg CL. C-Jun N-terminal kinases mediate a wide range of targets in the metastatic Cascade. Genes Cancer. 2013;4:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Y, Wang L, Deng D, Zhang Q, Liu W. Renalase protects against renal fibrosis by inhibiting the activation of the ERK Signaling pathways. Int J Mol Sci. 2017;18:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Guo YF, Fu GP, Guan C, Zhang X, Yang DG, et al. . Protective effect of miRNA-containing extracellular vesicles derived from mesenchymal stromal cells of old rats on renal function in chronic kidney disease. Stem Cell Res Ther. 2020;11:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiao H, Shen HY, Liu W, Xiong RP, Li P, Meng G, et al. . Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One. 2013;8:e60173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–57. [DOI] [PubMed] [Google Scholar]
- 63. Zuccarini M, Giuliani P, Buccella S, Di Liberto V, Mudo G, Belluardo N, et al. . Modulation of the TGF-β1-induced epithelial to mesenchymal transition (EMT) mediated by P1 and P2 purine receptors in MDCK cells. Purinergic Signal. 2017;13:429–42. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011). 2014;4:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwang I, Seo EY, Ha H. Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease. Arch Pharm Res. 2009;32:1653–62. [DOI] [PubMed] [Google Scholar]
- 67. Schinner S, Willenberg HS, Schott M, Scherbaum WA. Pathophysiological aspects of Wnt-signaling in endocrine disease. Eur J Endocrinol. 2009;160:731–7. [DOI] [PubMed] [Google Scholar]
- 68. Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB, Yoon GS, et al. . The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol. 2013;6:1747–58. [PMC free article] [PubMed] [Google Scholar]
- 69. Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, et al. . Convergence of notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jeong MH, Kim HR, Park YJ, Chung KH. Akt and notch pathways mediate polyhexamethylene guanidine phosphate-induced epithelial-mesenchymal transition via ZEB2. Toxicol Appl Pharmacol. 2019;380:114691. [DOI] [PubMed] [Google Scholar]
- 71. Li T, Yang X, Xin S, Cao Y, Wang N. Paraquat poisoning induced pulmonary epithelial mesenchymal transition through Notch1 pathway. Sci Rep. 2017;7:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, et al. . Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7:e30771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li B, Zhu C, Dong L, Qin J, Xiang W, Davidson AJ, et al. . ADAM10 mediates ectopic proximal tubule development and renal fibrosis through notch signaling. J Pathol. 2020;252:274–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marquez-Exposito L, Lavoz C, Rodrigues-Diez RR, Rayego-Mateos S, Orejudo M, Cantero-Navarro E, et al. . Gremlin regulates tubular epithelial to mesenchymal transition via VEGFR2: potential role in renal fibrosis. Front Pharmacol. 2018;9:1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hultgren NW, Fang JS, Ziegler ME, Ramirez RN, Phan DTT, Hatch MMS, et al. . Slug regulates the Dll4-notch-VEGFR2 axis to control endothelial cell activation and angiogenesis. Nat Commun. 2020;11:5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. . Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–75. [DOI] [PubMed] [Google Scholar]
- 77. Liu Q, Zhang Y, Mao H, Chen W, Luo N, Zhou Q, et al. . A crosstalk between the Smad and JNK signaling in the TGF-beta-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. PLoS One. 2012;7:e32009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Velden JL, Guala AS, Leggett SE, Sluimer J, Badura EC, Janssen-Heininger YM. Induction of a mesenchymal expression program in lung epithelial cells by wingless protein (Wnt)/β-catenin requires the presence of c-Jun N-terminal kinase–1 (JNK1). Am J Respir Cell Mol Biol. 2012;47:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li N, Jia T, Li YR. LncRNA NEAT1 accelerates the occurrence and development of diabetic nephropathy by sponging miR-23c. Eur Rev Med Pharmacol Sci. 2020;24:1325–37. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Y, Yao XH, Wu Y, Cao GK, Han D. LncRNA NEAT1 regulates pulmonary fibrosis through miR-9-5p and TGF-β signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:8483–92. [DOI] [PubMed] [Google Scholar]
- 81. Zheng J, Yu F, Dong P, Wu L, Zhang Y, Hu Y, et al. . Long non-coding RNA PVT1 activates hepatic stellate cells through competitively binding microRNA-152. Oncotarget. 2016;7:62886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu L, Zou J, Guan Y, Zhang Y, Zhang W, Zhou X, et al. . Blocking the histone lysine 79 methyltransferase DOT1L alleviates renal fibrosis through inhibition of renal fibroblast activation and epithelial-mesenchymal transition. FASEB J. 2019;33:11941–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nagaraja SS, Subramanian U, Nagarajan D. Radiation-induced H3K9 methylation on E-cadherin promoter mediated by ROS/snail axis : role of G9a signaling during lung epithelial-mesenchymal transition. Toxicol in Vitro. 2021;70:105037. [DOI] [PubMed] [Google Scholar]
- 84. Wu MZ, Tsai YP, Yang MH, Huang CH, Chang SY, Chang CC, et al. . Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol Cell. 2011;43:811–22. [DOI] [PubMed] [Google Scholar]
- 85. Hu L, Yu Y, Huang H, Fan H, Hu L, Yin C, et al. . Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in Paraquat-induced pulmonary fibrosis. Front Immunol. 2016;7:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res. 2018;19:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng XY, et al. . Transforming growth factor-beta1 induces bronchial epithelial cells to mesenchymal transition by activating the snail pathway and promotes airway remodeling in asthma. Mol Med Rep. 2013;8:1663–8. [DOI] [PubMed] [Google Scholar]
- 88. Yamaguchi M, Hirai S, Tanaka Y, Sumi T, Miyajima M, Mishina T, et al. . Fibroblastic foci, covered with alveolar epithelia exhibiting epithelial-mesenchymal transition, destroy alveolar septa by disrupting blood flow in idiopathic pulmonary fibrosis. Lab Investig. 2017;97:232–42. [DOI] [PubMed] [Google Scholar]
- 89. Zolak JS, Jagirdar R, Surolia R, Karki S, Oliva O, Hock T, et al. . Pleural mesothelial cell differentiation and invasion in fibrogenic lung injury. Am J Pathol. 2013;182:1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, Greenson JK, et al. . Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol. 2011;31:2392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Park JH, Yoon J, Lee KY, Park B. Effects of geniposide on hepatocytes undergoing epithelial-mesenchymal transition in hepatic fibrosis by targeting TGFbeta/Smad and ERK-MAPK signaling pathways. Biochimie. 2015;113:26–34. [DOI] [PubMed] [Google Scholar]
- 92. Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. . Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, et al. . Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, et al. . Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. . Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:E3297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, et al. . Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Luo GH, Lu YP, Yang L, Song J, Shi YJ, Li YP. Epithelial to mesenchymal transformation in tubular epithelial cells undergoing anoxia. Transplant Proc. 2008;40:2800–3. [DOI] [PubMed] [Google Scholar]
- 99. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, et al. . Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J, et al. . Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT–NF-κB p65 axis. Sci Rep. 2019;9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lovisa S, Fletcher-Sananikone E, Sugimoto H, Hensel J, Lahiri S, Hertig A, et al. . Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal. 2020;13, eaaz2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, et al. . Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177:3415–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, et al. . Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76:4961–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen L, Chen DQ, Liu JR, Zhang J, Vaziri ND, Zhuang S, et al. . Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu H, Su W, et al. . Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun. 2019;10:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wenxiu J, Mingyue Y, Fei H, Yuxin L, Mengyao W, Chenyang L, et al. . Effect and mechanism of TL1A expression on epithelial-mesenchymal transition during chronic colitis-related intestinal fibrosis. Mediat Inflamm. 2021;2021:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Scharl M, Weber A, Furst A, Farkas S, Jehle E, Pesch T, et al. . Potential role for SNAIL family transcription factors in the etiology of Crohn's disease-associated fistulae. Inflamm Bowel Dis. 2011;17:1907–16. [DOI] [PubMed] [Google Scholar]
- 110. Ortiz-Masia D, Gisbert-Ferrandiz L, Bauset C, Coll S, Mamie C, Scharl M, et al. . Succinate activates EMT in intestinal epithelial cells through SUCNR1: a novel protagonist in fistula development. Cell. 2020;9:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Strippoli R, Moreno-Vicente R, Battistelli C, Cicchini C, Noce V, Amicone L, et al. . Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int. 2016;2016:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, Peso G, Jimenez-Heffernan JA, et al. . Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–13. [DOI] [PubMed] [Google Scholar]
- 113. Kang DH. Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis. Kidney Res Clin Pract. 2020;39:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Blom JN, Feng Q. Cardiac repair by epicardial EMT: current targets and a potential role for the primary cilium. Pharmacol Ther. 2018;186:114–29. [DOI] [PubMed] [Google Scholar]
- 115. Hao YM, Yuan HQ, Ren Z, Qu SL, Liu LS, Dang H, et al. . Endothelial to mesenchymal transition in atherosclerotic vascular remodeling. Clin Chim Acta. 2019;490:34–8. [DOI] [PubMed] [Google Scholar]
- 116. Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, et al. . Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012;31:429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li Y, Lui KO, Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat Rev Cardiol. 2018;15:445–56. [DOI] [PubMed] [Google Scholar]
- 118. Lovisa S, Zeisberg M, Kalluri R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab. 2016;27:681–95. [DOI] [PubMed] [Google Scholar]
- 119. Yan XL, Wang YY, Yu ZF, Tian MM, Li H. Peroxisome proliferator-activated receptor-gamma activation attenuates diabetic cardiomyopathy via regulation of the TGF-β/ERK pathway and epithelial-to-mesenchymal transition. Life Sci. 2018;213:269–78. [DOI] [PubMed] [Google Scholar]
- 120. Kuwahara H, Tosa M, Egawa S, Murakami M, Mohammad G, Ogawa R. Examination of epithelial mesenchymal transition in keloid tissues and possibility of keloid therapy target. Plast Reconstr Surg Glob Open. 2016;4:e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bale S, Pulivendala G, Godugu C. Withaferin a attenuates bleomycin‐induced scleroderma by targeting FoxO3a and NF‐κβ signaling: connecting fibrosis and inflammation. Biofactors. 2018;44:507–17. [DOI] [PubMed] [Google Scholar]
- 122. Takahashi M, Akamatsu H, Yagami A, Hasegawa S, Ohgo S, Abe M, et al. . Epithelial-mesenchymal transition of the eccrine glands is involved in skin fibrosis in morphea. J Dermatol. 2013;40:720–5. [DOI] [PubMed] [Google Scholar]
- 123. Du L, Hao M, Li C, Wu W, Wang W, Ma Z, et al. . Quercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and SRA01/04 cells through transforming growth factor-β2/phosphoinositide 3-kinase/Akt pathway. Mol Cell Endocrinol. 2017;452:44–56. [DOI] [PubMed] [Google Scholar]
- 124. Saika S, Yamanaka O, Flanders KC, Okada Y, Miyamoto T, Sumioka T, et al. . Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr Metab Immune Disord Drug Targets. 2008;8:69–76. [DOI] [PubMed] [Google Scholar]
- 125. Gu TT, Chen TY, Yang YZ, Zhao XJ, Sun Y, Li TS, et al. . Pterostilbene alleviates fructose-induced renal fibrosis by suppressing TGF-β1/TGF-β type I receptor/Smads signaling in proximal tubular epithelial cells. Eur J Pharmacol. 2019;842:70–8. [DOI] [PubMed] [Google Scholar]
- 126. Zhang X, Lu H, Xie S, Wu C, Guo Y, Xiao Y, et al. . Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in kidneys via proliferation-related signaling pathways. Br J Pharmacol. 2019;176:4745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhao JL, Zhang T, Shao X, Zhu JJ, Guo MZ. Curcumin ameliorates peritoneal fibrosis via inhibition of transforming growth factor-activated kinase 1 (TAK1) pathway in a rat model of peritoneal dialysis. BMC Complement Altern Med. 2019;19:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen H, Yang T, Wang MC, Chen DQ, Yang Y, Zhao YY. Novel RAS inhibitor 25-O-methylalisol F attenuates epithelial-to-mesenchymal transition and tubulo-interstitial fibrosis by selectively inhibiting TGF-β-mediated Smad3 phosphorylation. Phytomedicine. 2018;42:207–18. [DOI] [PubMed] [Google Scholar]
- 129. Wang M, Chen DQ, Chen L, Liu D, Zhao H, Zhang ZH, et al. . Novel RAS inhibitors Poricoic acid ZG and Poricoic acid ZH attenuate renal fibrosis via a Wnt/β-catenin pathway and targeted phosphorylation of smad3 Signaling. J Agric Food Chem. 2018;66:1828–42. [DOI] [PubMed] [Google Scholar]
- 130. Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, et al. . Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175:2689–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wei MG, Sun W, He WM, Ni L, Yang YY. Ferulic acid attenuates TGF-β1-induced renal cellular fibrosis in NRK-52E cells by inhibiting Smad/ILK/snail pathway. Evid Based Complement Alternat Med. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Li Y, Hu Q, Li C, Liang K, Xiang Y, Hsiao H, et al. . PTEN-induced partial epithelial-mesenchymal transition drives diabetic kidney disease. J Clin Invest. 2019;129:1129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. . EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–74. [DOI] [PubMed] [Google Scholar]
- 134. Zhiqiang W, Juan C, Xu Z, Di Y, Deyu X, Guoyuan L. EPA attenuates epithelial-mesenchymal transition and fibrosis through the TGF-β1/Smad3/ILK pathway in renal tubular epithelial HK-2 cells by up-regulating miR-541. Int J Clin Exp Pathol. 2019;12:2516–25. [PMC free article] [PubMed] [Google Scholar]
- 135. Lyu X, Hu M, Peng J, Zhang X, Sanders YY. HDAC inhibitors as antifibrotic drugs in cardiac and pulmonary fibrosis. Ther Adv Chronic Dis. 2019;10:204062231986269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Choi SY, Kee HJ, Kurz T, Hansen FK, Ryu Y, Kim GR, et al. . Class I HDACs specifically regulate E-cadherin expression in human renal epithelial cells. J Cell Mol Med. 2016;20:2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rossi L, Battistelli C, Turris V, Noce V, Zwergel C, Valente S, et al. . HDAC1 inhibition by MS-275 in mesothelial cells limits cellular invasion and promotes MMT reversal. Sci Rep. 2018;8:8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Choi SY, Ryu Y, Kee HJ, Cho SN, Kim GR, Cho JY, et al. . Tubastatin a suppresses renal fibrosis via regulation of epigenetic histone modification and Smad3-dependent fibrotic genes. Vasc Pharmacol. 2015;72:130–40. [DOI] [PubMed] [Google Scholar]
- 139. Saito S, Zhuang Y, Shan B, Danchuk S, Luo F, Korfei M, et al. . Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta-PI3K-Akt pathway. PLoS One. 2017;12:e0186615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chen L, Alam A, Pac-Soo A, Chen Q, Shang Y, Zhao H, et al. . Pretreatment with valproic acid alleviates pulmonary fibrosis through epithelial-mesenchymal transition inhibition in vitro and in vivo. Lab Investig. 2021;101:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nguyen-Thanh T, Kim D, Lee S, Kim W, Park SK, Kang KP. Inhibition of histone deacetylase 1 ameliorates renal tubulointerstitial fibrosis via modulation of inflammation and extracellular matrix gene transcription in mice. Int J Mol Med. 2018;41:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang Y, Zou J, Tolbert E, Zhao TC, Bayliss G, Zhuang S. Identification of histone deacetylase 8 as a novel therapeutic target for renal fibrosis. FASEB J. 2020;34:7295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tseng WC, Tsai MT, Chen NJ, Tarng DC. Trichostatin a alleviates renal interstitial fibrosis through modulation of the M2 macrophage subpopulation. Int J Mol Sci. 2020;21, 5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tung CW, Hsu YC, Cai CJ, Shih YH, Wang CJ, Chang PJ, et al. . Trichostatin a ameliorates renal tubulointerstitial fibrosis through modulation of the JNK-dependent Notch-2 signaling pathway. Sci Rep. 2017;7:14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Williams VR, Konvalinka A, Song X, Zhou X, John R, Pei Y, et al. . Connectivity mapping of a chronic kidney disease progression signature identified lysine deacetylases as novel therapeutic targets. Kidney Int. 2020;98:116–32. [DOI] [PubMed] [Google Scholar]
- 146. Ozel M, Baskol M, Akalin H, Baskol G. Suberoylanilide Hydroxamic acid (SAHA) reduces fibrosis markers and deactivates human stellate cells via the epithelial-mesenchymal transition (EMT). Cell Biochem Biophys. 2021;79:349–57. [DOI] [PubMed] [Google Scholar]
- 147. Zhang YQ, Liu YJ, Mao YF, Dong WW, Zhu XY, Jiang L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clin Nutr. 2015;34:752–60. [DOI] [PubMed] [Google Scholar]
- 148. Choi SY, Piao ZH, Jin L, Kim JH, Kim GR, Ryu Y, et al. . Piceatannol attenuates renal fibrosis induced by unilateral ureteral obstruction via downregulation of histone deacetylase 4/5 or p38-MAPK Signaling. PLoS One. 2016;11:e0167340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Xiong C, Guan Y, Zhou X, Liu L, Zhuang MA, Zhang W, et al. . Selective inhibition of class IIa histone deacetylases alleviates renal fibrosis. FASEB J. 2019;33:8249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Morigi M, Perico L, Benigni A. Sirtuins in renal health and disease. J Am Soc Nephrol. 2018;29:1799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu S, Zhao M, Zhou Y, Wang C, Yuan Y, Li L, et al. . Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: a potential risk to individuals with impaired kidney function. Phytomedicine. 2019;57:223–35. [DOI] [PubMed] [Google Scholar]
- 152. He Y, Lu R, Wu J, Pang Y, Li J, Chen J, et al. . Salvianolic acid B attenuates epithelial-mesenchymal transition in renal fibrosis rats through activating Sirt1-mediated autophagy. Biomed Pharmacother. 2020;128:110241. [DOI] [PubMed] [Google Scholar]
- 153. Quan Y, Park W, Jin J, Kim W, Park SK, Kang KP. Sirtuin 3 activation by Honokiol decreases unilateral ureteral obstruction-induced renal inflammation and fibrosis via regulation of mitochondrial dynamics and the renal NF-κB-TGF-β1/Smad Signaling pathway. Int J Mol Sci. 2020;21:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cai J, Liu Z, Huang X, Shu S, Hu X, Zheng M, et al. . The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2020;97:106–18. [DOI] [PubMed] [Google Scholar]
- 155. Meng Q, Zhai X, Yuan Y, Ji Q, Zhang P. lncRNA ZEB1-AS1 inhibits high glucose-induced EMT and fibrogenesis by regulating the miR-216a-5p/BMP7 axis in diabetic nephropathy. Braz J Med Biol Res. 2020;53:e9288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156. Yu F, Lu Z, Chen B, Wu X, Dong P, Zheng J. Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis by attenuating DNMT1-mediated Patched1 methylation. J Cell Mol Med. 2015;19:2617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bai S, Xiong X, Tang B, Ji T, Li X, Qu X, et al. . Hsa-miR-199b-3p prevents the epithelial-mesenchymal transition and dysfunction of the renal tubule by regulating E-cadherin through targeting KDM6A in diabetic nephropathy. Oxidative Med Cell Longev. 2021;2021:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim S, Jeong CH, Song SH, Um JE, Kim HS, Yun JS, et al. . Micellized protein transduction domain-bone morphogenetic Protein-7 efficiently blocks renal fibrosis via inhibition of transforming growth factor-Beta-mediated epithelial-mesenchymal transition. Front Pharmacol. 2020;11:591275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond). 2013;124:243–54. [DOI] [PubMed] [Google Scholar]
- 160. Song Y, Lv S, Wang F, Liu X, Cheng J, Liu S, et al. . Overexpression of BMP‑7 reverses TGF‑β1‑induced epithelial‑mesenchymal transition by attenuating the Wnt3/β‑catenin and TGF-β1/Smad2/3 signaling pathways in HK‑2 cells. Mol Med Rep. 2020;21:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wang Y, Liang D, Zhu Z, Li X, An G, Niu P, et al. . Bone morphogenetic protein-7 prevented epithelial-mesenchymal transition in RLE-6TN cells. Toxicol Res (Camb). 2016;5:931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Liu L, Wang Y, Yan R, Liang L, Zhou X, Liu H, et al. . BMP-7 inhibits renal fibrosis in diabetic nephropathy via miR-21 downregulation. Life Sci. 2019;238:116957. [DOI] [PubMed] [Google Scholar]
- 163. Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. . Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in Thioacetamide-treated rats. PLoS One. 2013;8:e54499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yamagami Y, Kawami M, Ojima T, Futatsugi S, Yumoto R, Takano M. Role of plasminogen activator inhibitor-1 in methotrexate-induced epithelial-mesenchymal transition in alveolar epithelial A549 cells. Biochem Biophys Res Commun. 2020;525:543–8. [DOI] [PubMed] [Google Scholar]
- 165. McGaraughty S, Davis-Taber RA, Zhu CZ, Cole TB, Nikkel AL, Chhaya M, et al. . Targeting anti-TGF-beta therapy to fibrotic kidneys with a dual specificity antibody approach. J Am Soc Nephrol. 2017;28:3616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Song Y, Peng C, Lv S, Cheng J, Liu S, Wen Q, et al. . Adipose-derived stem cells ameliorate renal interstitial fibrosis through inhibition of EMT and inflammatory response via TGF-β1 signaling pathway. Int Immunopharmacol. 2017;44:115–22. [DOI] [PubMed] [Google Scholar]
- 168. Yu Y, Hu D, Zhou Y, Xiang H, Liu B, Shen L, et al. . Human umbilical cord mesenchymal stem cell attenuates renal fibrosis via TGF-β/Smad signaling pathways in vivo and in vitro. Eur J Pharmacol. 2020;883:173343. [DOI] [PubMed] [Google Scholar]
- 169. Jin J, Qian F, Zheng D, He W, Gong J, He Q. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA let-7i-5p Antagomir. Int J Nanomedicine. 2021;16:3565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol. 2002;4:648–57. [DOI] [PubMed] [Google Scholar]
- 171. Lee M, Kim SH, Jhee JH, Kim TY, Choi HY, Kim HJ, et al. . Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis. Stem Cell Res Ther. 2020;11:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shi Z, Wang Q, Zhang Y, Jiang D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res Ther. 2020;11:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang F, Wan X, Cao YZ, Sun D, Cao CC. Klotho gene-modified BMSCs showed elevated antifibrotic effects by inhibiting the Wnt/β-catenin pathway in kidneys after acute injury. Cell Biol Int. 2018;42:1670–9. [DOI] [PubMed] [Google Scholar]
- 174. Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, et al. . Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5:33–41. [DOI] [PubMed] [Google Scholar]
- 175. Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology. 2017;22:1171–8. [DOI] [PubMed] [Google Scholar]
- 176. Yao Y, Li Y, Zeng X, Ye Z, Li X, Zhang L. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (EMT) under high-fat diet-induced Hyperglycemia. Front Pharmacol. 2018;9:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bartko PE, Dal-Bianco JP, Guerrero JL, Beaudoin J, Szymanski C, Kim DH, et al. . Effect of losartan on mitral valve changes after myocardial infarction. J Am Coll Cardiol. 2017;70:1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Arnoni CP, Maquigussa E, Passos CS, Pereira LG, Boim MA. Inhibition of cellular transdifferentiation by losartan minimizes but does not reverse type 2 diabetes-induced renal fibrosis. J Renin-Angiotensin-Aldosterone Syst. 2015;16:469–80. [DOI] [PubMed] [Google Scholar]
- 179. Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, et al. . Qinggan Huoxue recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J Gastroenterol. 2016;22:4695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Meng L, Zhang X, Wang H, Dong H, Gu X, Yu X, et al. . Yangyin Yiqi mixture ameliorates bleomycin-induced pulmonary fibrosis in rats through inhibiting TGF-β1/Smad pathway and epithelial to mesenchymal transition. Evid Based Complement Alternat Med. 2019;2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yin ZF, Wei YL, Wang X, Wang LN, Li X. Buyang Huanwu Tang inhibits cellular epithelial-to-mesenchymal transition by inhibiting TGF-β1 activation of PI3K/Akt signaling pathway in pulmonary fibrosis model in vitro. BMC Complement Med Ther. 2020;20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]