Abstract
Invertases (INVs) and pectin methylesterases (PMEs) are essential enzymes coordinating carbohydrate metabolism, stress responses, and sugar signaling. INVs catalyzes the cleavage of sucrose into glucose and fructose, exerting a pivotal role in sucrose metabolism, cellulose biosynthesis, nitrogen uptake, reactive oxygen species scavenging as well as osmotic stress adaptation. PMEs exert a dynamic control of pectin methylesterification to manage cell adhesion, cell wall porosity, and elasticity, as well as perception and signaling of stresses. INV and PME activities can be regulated by specific proteinaceous inhibitors, named INV inhibitors (INVIs) and PME Inhibitors (PMEIs). Despite targeting different enzymes, INVIs and PMEIs belong to the same large protein family named “Plant Invertase/Pectin Methylesterase Inhibitor Superfamily.” INVIs and PMEIs, while showing a low aa sequence identity, they share several structural properties. The two inhibitors showed mainly alpha-helices in their secondary structure and both form a non-covalent 1:1 complex with their enzymatic counterpart. Some PMEI members are organized in a gene cluster with specific PMEs. Although the most important physiological information was obtained in Arabidopsis thaliana, there are now several characterized INVI/PMEIs in different plant species. This review provides an integrated and updated overview of this fascinating superfamily, from the specific activity of characterized isoforms to their specific functions in plant physiology. We also highlight INVI/PMEIs as biotechnological tools to control different aspects of plant growth and defense. Some isoforms are discussed in view of their potential applications to improve industrial processes. A review of the nomenclature of some isoforms is carried out to eliminate confusion about the identity and the names of some INVI/PMEI member. Open questions, shortcoming, and opportunities for future research are also presented.
Keywords: pectin methylesterase inhibitors, invertase inhibitors, sucrose metabolism, CW integrity, degree of methylesterification, plant growth and defence, biotechnological applications
Introduction
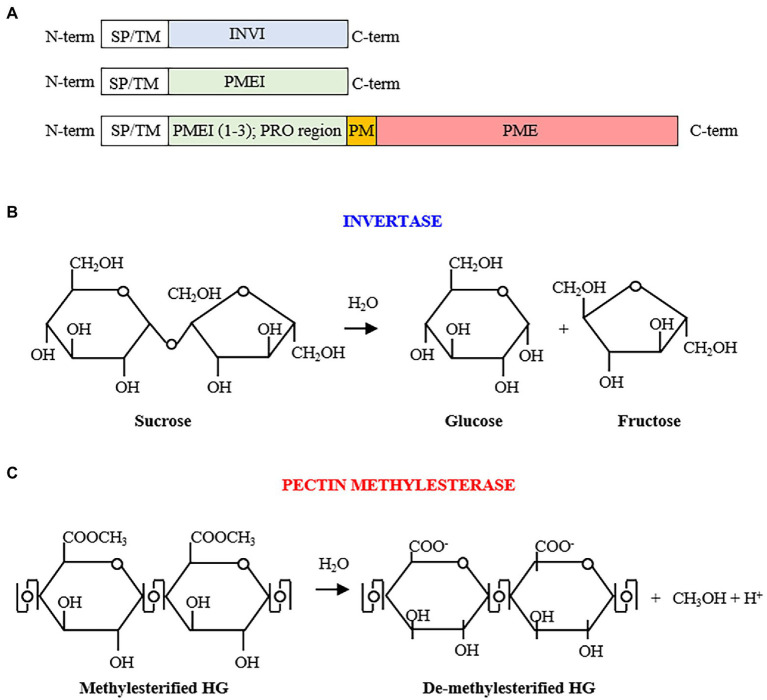
Plant Invertase/Pectin Methyl Esterase Inhibitors (INV/PMEIs; PF04043)1 belong to a large protein superfamily acting in the tight post-transcriptional regulation of Invertases (INVs) and Pectin methylesterases (PMEs), two classes of enzymes with distinct enzymatic activities in carbohydrate metabolism (Figure 1; Gough et al., 2001). INVI/PMEIs are highly represented in different plant species (Table 1). Plant INVs (also known as β-fructosidases), convert the sucrose into its building blocks, fructose and glucose, central molecules for carbohydrate translocation, metabolism, and sensing in higher plants (Figure 1B; Roitsch and González, 2004). INVs play different roles in organ development, carbohydrate partitioning, sugar signaling, and response to biotic and abiotic stresses (Ruan et al., 2010; Tauzin and Giardina, 2014; Liao et al., 2020). Acid INVs and neutral/alkaline INVs were identified, showing different pH optima and subcellular compartments. The Acid INVs, belonging to gH32 (glycoside hydrolase family 32),2 shows an optimum pH of 3.5–5.0 and can be divided in cell wall (CW) and vacuolar (V) INVs. Neutral/alkaline INVs show an optimum pH of 6.8–9.0, belong to gH100, and appear to be localized to the cytosol, mitochondrion, plastids, and nucleus. The INV activity can be post-transcriptionally controlled by INV Inhibitor (INVI; Figures 1A, 2A,B, 3). Based on the subcellular site where their activity is exerted, CW- and V-INVIs (previously named also Inhibitor of β-Fructosidases; C/VIFs) can be distinguished (Rausch and Greiner, 2004; Figure 3). The first INVI was described more than 40 years ago in potato plants (Solanum tuberosum; Pressey, 1966). Although INVIs have been reported for various plant species, little is known about their roles in plant physiology (Bate et al., 2004; Raiola et al., 2004; Reca et al., 2008; Zhang et al., 2010; Lionetti et al., 2015b).
Figure 1.
(A) Invertase inhibitor (INVI) and pectin methylesterase inhibitor (PMEI) structural organizations. The INVI/PMEIs domain are preceded by a signal peptide (SP) or a transmembrane domain (TM) for the targeting to the endomembrane system leading to the different subcellular localization. PMEI/INVIs can possess one, both, and neither of these motifs. Some PMEIs (from 1 to 3 isoforms; also named PRO region) can be clustered with a C-terminal PME. (B) Sucrose hydrolysis by invertase activity yielding glucose and fructose. (C) De-methylesterification of homogalacturonan (HG) by pectin methylesterases, with consequent production of negatively charged carboxyl groups, methanol, and protons.
Table 1.
INVI/PMEIs assignments in different plant species genomes.
| Species | Common name | Number of proteins |
|---|---|---|
| Actinidia chinensis Hongyang | 109 | |
| Aegilops tauschii | 94 | |
| Amborella trichopoda | 40 | |
| Aquilegia coerulea | 63 | |
| Arabidopsis lyrata | Lyrate rockcress | 132 |
| Arabidopsis thaliana | Thale cress | 125 |
| Brachypodium distachyon | Stiff brome | 60 |
| Brassica rapa | Field mustard | 167 |
| Capsella rubella | 130 | |
| Carica papaya | Papaya | 54 |
| Citrus clementina | 75 | |
| Citrus sinensis | Sweet orange | 78 |
| Cucumis sativus | Cucumber | 62 |
| Eucalyptus grandis | Rose gum | 46 |
| Fragaria vesca | Wild strawberry | 82 |
| Glycine max | Soybean | 163 |
| Gossypium raimondii | 152 | |
| Hordeum vulgare | Domesticated barley | 68 |
| Linum usitatissimum | Flax | 160 |
| Lotus japonicus | 69 | |
| Malus domestica | Apple | 144 |
| Manihot esculenta | Cassava | 100 |
| Medicago truncatula | Barrel medic | 171 |
| Mimulus guttatus | Spotted monkey flower | 115 |
| Musa acuminata | Wild Malaysian banana | 58 |
| Musa balbisiana | Balbis banana | 88 |
| Nicotiana benthamiana | 160 | |
| Oryza sativa | Rice | 81 |
| Panicum virgatum | Switchgrass | 131 |
| Phaseolus vulgaris | French bean | 104 |
| Phoenix dactylifera | Date palm | 13 |
| Phyllostachys heterocyclavar | Kikko-chiku | 30 |
| Physcomitrella patens | 12 | |
| Picea abies | Norway spruce | 53 |
| Picea sitchensis | Sitka spruce | 5 |
| Pinus taeda | Loblolly pine | 82 |
| Populus trichocarpa | Black cottonwood | 118 |
| Prunus persica | Peach | 70 |
| Ricinus communis | Castor bean | 71 |
| Selaginella moellendorffii | 13 | |
| Setaria italica | Foxtail millet | 67 |
| Solanum lycopersicum | Tomato | 86 |
| Solanum pimpinellifolium | Currant tomato | 86 |
| Solanum tuberosum | Potato | 113 |
| Sorghum bicolor | Sorghum | 73 |
| Thellungiella halophila | 105 | |
| Theobroma cacao | Cacao | 72 |
| Triticum aestivum | Bread wheat | 95 |
| Triticum urartu | 85 | |
| Vitis vinifera | Wine grape | 21 |
| Zea mays | Maize | 76 |
| Zea mays | Maize | 79 |
Figure 2.
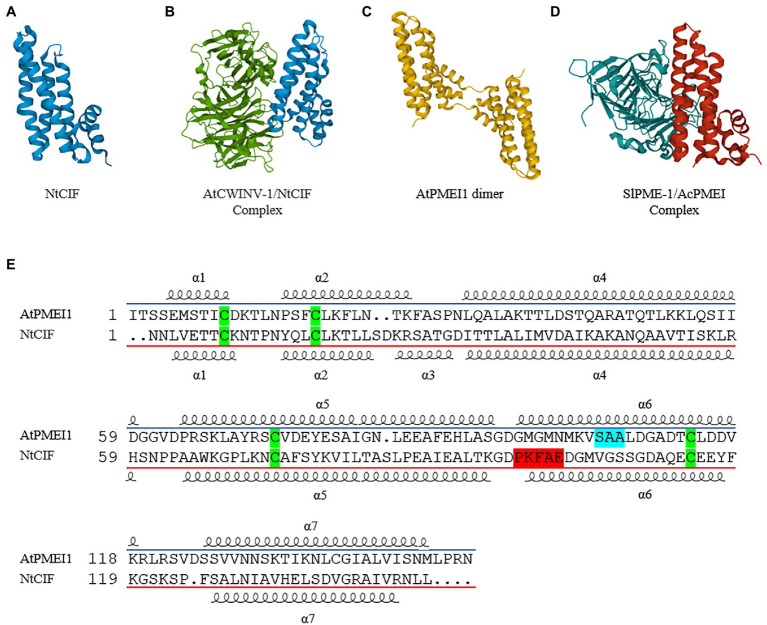
Crystal structure of different INVI/PMEI isoforms alone or in complex with their enzymatic counterpart. (A) NtCIF (PDB ID; 1RJ1) and (B) NtCIF in complex with AtCWINV-1 (PDB ID; 2XQR). (C) AtPMEI1 dimer (PDB ID; IX8Z) and (D) AcPMEI in complex with SlPME-1 (PDB ID; 1Xg2). (E) Sequence Comparison of AtPMEI1 and NtCIF. The most conserved motifs are highlighted in light blue for PMEI and in red for INVI. The four cysteine conserved in both inhibitors are highlighted in green. The α symbols followed by numbers indicates the different alpha-helices.
Figure 3.
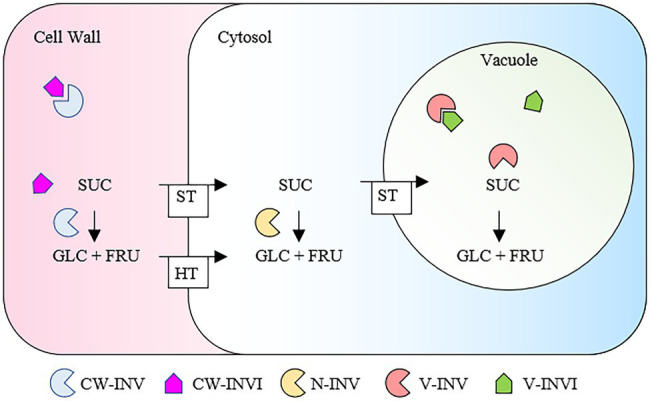
Subcellular localization and activities of INVs and INVIs. Sucrose can be cleaved in the apoplast by a CW-INV in glucose and fructose, which are transported into the cytoplasm by a hexose transporter. Sucrose can also be directly loaded by specific sucrose transporters into the cytosol or in the vacuole where it is cleaved by neutral invertase (N-INV; or sucrose synthase) or V-INV, respectively. The hexoses generated by INV activity can serve as substrate for growth as well as can regulate gene expression during growth and defense. Modulations of INVs by INVIs are dependent from different environmental stimuli and can influence different physiological processes. FRU, fructose; glc, glucose; SUC, Sucrose. ST, Sucrose Transporter; HT, Hexose Transporter.
A member of INVI/PMEI family can be classified as PME Inhibitor (PMEI) if it is able to inhibit a PME activity (Figures 1A–C, 2C,D, 4). The first PMEI, named AcPMEI, was discovered in ripe fruit of kiwi (Actinidia chinensis; Balestrieri et al., 1990). PMEs (CE8, Carbohydrate Esterase) catalyze the de-methylesterification of pectin, releasing free carboxyl ester groups, protons, and methanol (Figure 1C). PMEs are encoded by large multigene families in many plant species (Pelloux et al., 2007; Harholt et al., 2010). Until now, these enzymes were linked to the modulation of the degree and pattern of methylesterification of homogalacturonan (HG), the major component of pectin secreted in a highly methylesterified form to the CW (Figure 4). The degree of methylesterification constitutes an important factor influencing stiffness and hydration status of the pectic matrix (Catoire et al., 1998; Willats et al., 2001). The current knowledge on the mode of de-methylesterification of the single plant PME isoforms remains scarce. The existence of different methylester distributions on HG in vivo suggests the involvement of multiple PME isoforms with different action patterns. A blockwise de-methylesterification results in the production of adjacent free galacturonic acid units that can form calcium crosslinks between HG chains, known as “egg-box” structures, resulting in pectin stiffening (Limberg et al., 2000; Wu et al., 2018). Instead, the random de-methylesterification results in the removal of one methylester group at a time from various non-contiguous residues on the HG chains exposing the polymer to the activity of pectinolytic enzymes (Limberg et al., 2000). While this latter mechanism has been demonstrated for PMEs of microbial origin, plant PMEs with a random de-methylesterification have not been identified so far. PME isoforms finely tune the degree and pattern of methylesterification during multiple developmental processes, such as stomata function (Amsbury et al., 2016; Huang et al., 2017), cell adhesion (Lionetti et al., 2014a; Daher and Braybrook, 2015), organ development, and phyllotactic patterning (Peaucelle et al., 2011b; Senechal et al., 2014). Plant PMEs also play a critical role in multiple plant–microbe interactions and stress responses (Lionetti et al., 2012). An immunity triggered PME activity, driven by specific PME isoforms, is exploited against pathogens (Bethke et al., 2014; Del Corpo et al., 2020). This activity is triggered to modulate pectin methylesterification in Arabidopsis thaliana against fungi, such as Botrytis cinerea and Alternaria brassicicola, bacteria, such as Pseudomonas syringae, and viruses like turnip vein clearing virus (TVCV; Bethke et al., 2014; Lionetti et al., 2014b, 2015a, 2017). Moreover, PMEI activity and pectin methylesterification status play important roles during plant resistance to abiotic stresses (Wu et al., 2018).
Figure 4.
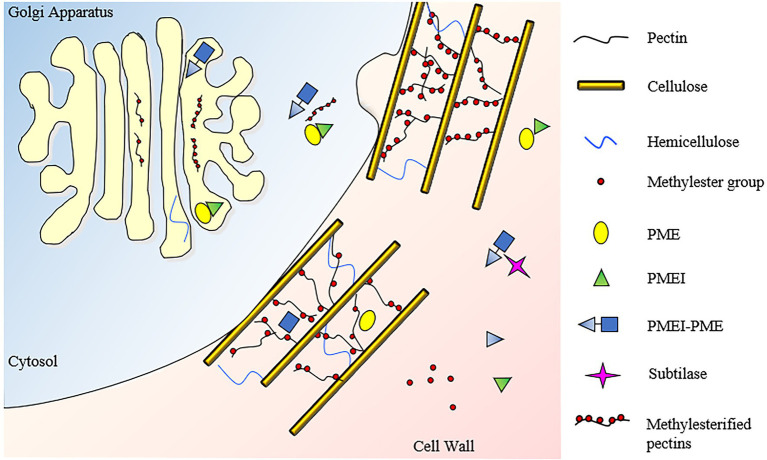
Subcellular localization and activities of PMEI-PMEs and PMEIs. HG is methylesterified in the golgi apparatus, where PMEIs can avoid a premature pectin de-methylesterification by PME, which could cause a pectin jellification. Pectin is secreted in the apoplast in a high methylesterified form. In this compartment, a fine-tuning of PME activity is exerted by independent and clustered PMEIs to regulate the degree and pattern of pectin methylesterification in various plant physiology processes. Subtilisin-like proteases (Subtilases) can degrade the processing motif of PMEI-PME catalyzing the separation of the inhibitor from the PME domain.
A PMEI can be transcribed independently or in pairs with a Type I PME as one polycistronic messenger RNA which resembles an operon-like gene cluster (Figure 1A; Boycheva et al., 2014). In these PMEI-PMEs clusters, PMEI region (also referred to as PRO region, PRO domain, or PMEI-like region) can be separated from the PME domain by specific subtilisin-like serine proteases (subtilases) before excretion of PME domain or later into the apoplast during different physiological processes (Figueiredo et al., 2018; Schaller et al., 2018). Different evidence indicates that in these clusters, PMEI domain acts as an intramolecular inhibitor of PME enzymatic activity (Bosch et al., 2005; Wolf et al., 2009; Del Corpo et al., 2020). Other works indicate that PMEI domain can be required for the targeting of PMEs toward the CW (Wolf et al., 2009) or that it could work as intramolecular chaperone in the regulation of PME folding (Micheli, 2001). Further investigation is needed to understand the subcellular action of both independent and PME-clustered PMEIs.
Gene structure and sequence analyses show that the origin of the independently expressed PMEIs may be derived from the neofunctionalization of the PMEI domain from the PMEI-PME genes (Wang et al., 2013). PMEI-PME clusters evolved during the divergence of moss from charophytes, while independent PMEIs appear later in land plants. The physiological reasons which prompted plants to initially evolve a coordinated expression of the enzyme with its inhibitor counterpart and, later, to involve also a PME inhibition using independent PMEIs, deserves further investigation. Most likely, both PMEI-PMEs clusters and independent PMEIs were fundamental factors finely tuning the pectin methyl-esterification in the CW remodeling that emerged as necessary in land plants. The existence of specific pairs between PMEIs and PMEs has been hypothesized based on data obtained in vitro (Table 2). A single PMEI can inhibit multiple plant PMEs and the PMEI characterized up to now are unable to inhibit microbial PMEs (Lionetti, 2015; Lionetti et al., 2015b). The bacterial enzymes show much longer turns that protrude out of the β-helix making its putative active site cleft deeper and narrower than that of plant PMEs, a feature that could prevent the approach of the inhibitor to the active site of the enzyme (D’Avino et al., 2003).
Table 2.
Arabidopsis INVI-PMEI independent protein isoforms.
| Gene ID | AGI code | Symbol | Possible interactor | Function | Literature | |
|---|---|---|---|---|---|---|
| 1 | 837879 | At1g02550 | ||||
| 2 | 837458 | At1g09360 | ||||
| 3 | 837459 | At1g09370 | ||||
| 4 | 837620 | At1g10770 | ||||
| 5 | 6240451 | At1g11362 | ||||
| 6 | 7922417 | At1g11593 | ||||
| 7 | 838054 | At1g14890 | ||||
| 8 | 838929 | At1g23205 | ||||
| 9 | 838944 | At1g23350 | Plant stresses | Coolen et al., 2019 | ||
| 10 | 841214 | At1g47960 | AtCW-INVI1, AtC/VIF1 | Seed germination, Root length, Plant-pathogen interaction, Salt susceptibility | Link et al., 2004; Siemens et al., 2011; Su et al., 2016; Yang et al., 2020 | |
| 11 | 841219 | At1g48010 | ||||
| 12 | 841220 | At1g48020 | AtPMEI1 | ATPPME1, AtPMEI-PME17, AtPMEI-PME3, AtPMEI-PME16 | Plant growth, Pavement cells morphogenesis, Plant-pathogen interaction | Wolf et al., 2003; Raiola et al., 2004; Lionetti et al., 2007; Lin et al., 2021 |
| 13 | 6240492 | At1g50325 | ||||
| 14 | 841456 | At1g50340 | ||||
| 15 | 841904 | At1g54620 | ||||
| 16 | 841939 | At1g54980 | ||||
| 17 | 842026 | At1g55770 | ||||
| 18 | 842062 | At1g56100 | AtPMEI14 | Mucilage release | Shi et al., 2018; Ding et al., 2021 | |
| 19 | 842117 | At1g56620 | AtPMEI16 | Mucilage release | Shi et al., 2018; Ding et al., 2021 | |
| 20 | 842370 | At1g60760 | ||||
| 21 | 842574 | At1g62760 | AtPMEI10 | Salt susceptibility, Plant-pathogen interaction, | Jithesh et al., 2012; Lionetti et al., 2017 | |
| 22 | 842576 | At1g62770 | AtPMEI9 | AtPME3 | CW integrity, Root growth | Sorek et al., 2015; Hocq et al., 2017b |
| 23 | 843391 | At1g70540 | EDA24 | Embryo sac | Pagnussat et al., 2005 | |
| 24 | 843409 | At1g70720 | ||||
| 25 | 814690 | At2g01610 | ||||
| 26 | 815562 | At2g10970 | ||||
| 27 | 816026 | At2g15345 | ||||
| 28 | 3768435 | At2g31425 | ||||
| 29 | 817701 | At2g31430 | AtPMEI5 | Seed germination, Seedling emergence, Plant growth | Wolf et al., 2012; Müller et al., 2013; Jonsson et al., 2021 | |
| 30 | 6241279 | At2g31432 | ||||
| 31 | 819319 | At2g47050 | ||||
| 32 | 819347 | At2g47340 | ||||
| 33 | 819380 | At2g47670 | AtPMEI6 | Mucilage release | Saez-Aguayo et al., 2013 | |
| 34 | 3768856 | At3g05741 | AtPMEI15 | Mucilage release | Shi et al., 2018; Ding et al., 2021 | |
| 35 | 820471 | At3g12880 | ||||
| 36 | 820970 | At3g17130 | AtPMEI8 | CW integrity, Root growth | Sorek et al., 2015, p. 16 | |
| 37 | 820971 | At3g17140 | ||||
| 38 | 820972 | At3g17150 | ||||
| 49 | 5008004 | At3g17152 | ||||
| 40 | 820981 | At3g17220 | AtPMEI2 | ATPPME1, AtPMEI-PME3, AtPMEI-PME16 |
Plant growth, Plant-pathogen interaction | Wolf et al., 2003; Raiola et al., 2004; Tian et al., 2006; Lionetti et al., 2007 |
| 41 | 820982 | At3g17225 | ||||
| 42 | 28719277 | At3g17227 | ||||
| 43 | 820983 | At3g17230 | ||||
| 44 | 6240965 | At3g27999 | ||||
| 45 | 819850 | At3g36659 | ||||
| 46 | 823892 | At3g47380 | AtPMEI11 | Plant-pathogen interaction | Lionetti et al., 2017 | |
| 47 | 823921 | At3g47670 | ||||
| 48 | 824095 | At3g49330 | ||||
| 49 | 824734 | At3g55680 | ||||
| 50 | 825391 | At3g62180 | ||||
| 51 | 825457 | At3g62820 | ||||
| 52 | 828192 | At4g00080 | UNE11 | Embryo sac | Pagnussat et al., 2005 | |
| 53 | 7922364 | At4g00872 | ||||
| 54 | 827589 | At4g02250 | ||||
| 55 | 828628 | At4g25250 | AtPMEI4 | AtPMEI-PME3; AtPMEI-PME17 | Root growth | Pelletier et al., 2010 |
| 56 | 6240679 | At4g03945 | ||||
| 57 | 827253 | At4g15750 | AtPMEI13 | Mucilage release | Shi et al., 2018; Ding et al., 2021 | |
| 58 | 828566 | At4g24640 | APPB1 | Holmes-Davis et al., 2005 | ||
| 59 | 828628 | At4g25250 | ||||
| 60 | 828629 | At4g25260 | AtPMEI7 | AtPMEI-PME3 | Sénéchal et al., 2015 | |
| 61 | 832197 | At5g20740 | AtPMEI3 | Phyllotaxis, Rhyzotaxis, Pavement cells morphogenesis | Peaucelle et al., 2008; Haas et al., 2020; Wachsman et al., 2020 | |
| 62 | 832508 | At5g24370 | ||||
| 63 | 833851 | At5g38610 | ||||
| 64 | 834739 | At5g46930 | ||||
| 65 | 834740 | At5g46940 | ||||
| 66 | 834741 | At5g46950 | InvINH2 | Zuma et al., 2018 | ||
| 67 | 834742 | At5g46960 | AtPMEI12 | Plant-pathogen interaction | Lionetti et al., 2017; Zuma et al., 2018 | |
| 68 | 834743 | At5g46970 | ||||
| 69 | 834744 | At5g46980 | ||||
| 70 | 834745 | At5g46990 | ||||
| 71 | 835067 | At5g50030 | ||||
| 72 | 835068 | At5g50040 | ||||
| 73 | 835069 | At5g50050 | ||||
| 74 | 835070 | At5g50060 | ||||
| 75 | 835071 | At5g50070 | ||||
| 76 | 835226 | At5g51520 | ||||
| 77 | 836355 | At5g62340 | ||||
| 78 | 836356 | At5g62350 | ||||
| 79 | 836357 | At5g62360 | AtPMEI17 | Salt and Aphid tolerance, Freezing susceptibility | Chen et al., 2018; Silva-Sanzana et al., 2019 | |
| 80 | 836583 | At5g64620 | AtCW/V-INVI2; AtC/VIF2 | Plant-pathogen interaction | Link et al., 2004; Siemens et al., 2011 |
From a structural point of view, PMEIs and INVIs, despite having a low aa sequence identity (20–30%), they share several structural properties. The three-dimensional structures of AtPMEI1 from Arabidopsis and of a CW-INVI from tobacco (Nicotiana tabacum; NtCIF) were elucidated (Hothorn et al., 2004a,b; Figures 2A,C). Both fold in a four-helix bundle structure preceded by an N-terminal extension thought to play an important role during the enzymatic inhibition. Both inhibitors hold four conserved cysteine residues typically engaged in the formation of two disulfide bridges, important to stabilize both two α helices of the hairpin loop and two α helices of the four-helical bundle structure. The structure of AtPMEI1 (Figure 2C) is composed of a four-helix bundle that arranges the helical components in an up-down–up-down topology with disulfide bridges. The N-terminal region, composed of two short and distorted helices, extends outside the central domain, forming a hairpin. The orientation of this hairpin allows the extensive contacts with the α-hairpin of a neighboring molecule forming an active dimer in solution. The N-terminal region of AtPMEI1 was proposed to be crucial for the interaction with a PME from Carrot (Daucus carota; Hothorn et al., 2004b). Although this model does not fit with the crystallographic data regarding the complex between AcPMEI and SlPME-1 of tomato (Solanum lycopercsicum; Figure 2D; Di Matteo et al., 2005), where the N-terminal region of AcPMEI does not establish contact with PME, it cannot be excluded that the two inhibitors use two different modes of interaction. AcPMEI interacts with PME at the level of the active site by forming a stoichiometric 1:1 complex in which the inhibitor covers the shallow cleft of the enzyme where the putative active site is located (Di Matteo et al., 2005; Ciardiello et al., 2008). The four-helix bundle of AcPMEI packs roughly perpendicular to the parallel β-helix of SlPME-1, and three of these helices (and not an helix of the N-terminal extension as proposed for AtPMEI1) interact with SlPME-1 in proximity of the putative active site (Di Matteo et al., 2005). The crystal structure of the complex between the AtCWINV-1 from Arabidopsis and the NtCIF was also elucidated (Figures 2A,B; Hothorn et al., 2010). The structure revealed that the four-helix bundle of NtCIF binds primarily in the substrate-binding cleft of the five-bladed β-propeller module of invertase. PME and INV activities and their complexes with their respective inhibitors are pH sensitive (Hothorn et al., 2010; Bonavita et al., 2016; Hocq et al., 2017b). There is no information available on the three-dimensional structure of PMEI-PME clusters.
A PMEI or an INVI can hold specific features in their amino acid sequence (Figure 2E). PMEI has a conserved Threonine residue, previously demonstrated to strengthen the interaction with PME at the acidic apoplastic pH, a typical Serine, Alanine, Alanine (SAA) amino acid motif in α6 helix, and a C-terminal hydrophobic region of six amino acids involved in the stabilization of the four-helical bundle structure of the protein (Di Matteo et al., 2005). Instead, the amino acid motif Proline, Lysine, Phenylalanine (PKF) in α6 helix, as well as the contiguous Alanine and glutamic Acid (AE) residues are the sequence fingerprints highly conserved in INVIs and critical for INVI-INV interaction. Both INVIs residues, important for the formation of the complex with INVs, and the distortion of INVI α2 helix could be responsible for the lack of interaction between INVI and PME (Di Matteo et al., 2005). Unfortunately, these features do not always help to understand the identity of an isoform. For example, PKF motif is absent in AtC/VIF2 (Link et al., 2004).
The Arabidopsis INVI/PMEI Superfamily
Most of the knowledge on the role of INVI/PMEIs in plant physiology has so far been gained by studying A. thaliana, the annual dicotyledonous plant, served as a model for physiological studies in many laboratories. For this reason, this review will deal specifically with all Arabidopsis isoforms, integrating them with data obtained in other species. We identified in silico 125 INVI/PMEIs in Arabidopsis, 80 independent INVI/PMEIs, and 45 PMEI-PME clusters (Tables 1–3). Given the need to verify the type of inhibitory activity, the INVI-PMEIs members cannot be pre-numbered as for other families. The name of various members was assigned by the scientific community based on the chronological order they were characterized. However, confusion about the identity and the names of some INVI/PMEI member begins to appear in literature. For example, both PMEI and INVI functions have been proposed for the same AgI code. At5g46960, although named InvINH1, its INVI activity has never been demonstrated (Zuma et al., 2018). Rather, At5g46960 is the AtPMEI12, which possess the SAA amino acid motif and influences PME activity, the degree of pectin methylesterification, and the HG integrity in Arabidopsis during B. cinerea infection (Lionetti et al., 2017). Also, two different isoforms were named with the same name. This is the case of At4g15750 and At5g62360 isoforms both called AtPMEI13 by different authors (Chen et al., 2018; Shi et al., 2018; Silva-Sanzana et al., 2019). To avoid confusion and respecting the chronology of publications, we rename At5g62360 as AtPMEI17. Moreover, the lack of symbol for At3g60730 and At2g26450 led us to name them, respectively, AtPMEI-PME65 and AtPMEI-PME66. Also unfortunately, the AtPMEI-PME17 isoform was considered an independent PMEI in different papers (Leyva-González et al., 2012; Takahashi et al., 2019). We propose to rename Type I PMEs, by adding the PMEI-tag in front of PME (PMEI-PMEs; Table 3) to allow the scientific community to immediately recognize a PMEI co-expressed with a PME. We will begin to discuss the findings on PMEIs, given the greater amount of data available compared to INVIs.
Table 3.
Arabidopsis INVI-PMEI protein isoforms clustered with a PME.
| Gene ID | AGI code | PME Symbol | New symbol | Function | Paper | |
|---|---|---|---|---|---|---|
| 1 | 838078 | At1g02810 | AtPME7 | AtPMEI-PME7 | Probable pseudogene | Dedeurwaerder et al., 2008 |
| 2 | 837701 | At1g11580 | AtPME18; AtPME-PCRA | AtPMEI-PME18 | Root growth, Plant- pathogen interaction | Micheli et al., 1998, p. 3; De-la-Peña et al., 2008; Lionetti et al., 2017; Stefanowicz et al., 2021 |
| 3 | 837702 | At1g11590 | AtPME19 | AtPMEI-PME19 | ||
| 4 | 838928 | At1g23200 | AtPME6, HIgHLY METHYL ESTERIFIED SEEDS (HMS) |
AtPMEI-PME6 | Stomatal function, Embryo development, Mucilage release | Levesque-Tremblay et al., 2015; Amsbury et al., 2016 |
| 5 | 841820 | At1g53830 | AtPME2 | AtPMEI-PME2 | Callus formation | Xu et al., 2018 |
| 6 | 841821 | At1g53840 | AtPME1 | AtPMEI-PME1 | ||
| 7 | 817184 | At2g26440 | AtPME12 | AtPMEI-PME12 | ||
| 8 | 817185 | At2g26450 | No number | AtPMEI-PME66 | ||
| 9 | 818907 | At2g43050 | AtPME16; ATPMEPCRD | AtPMEI-PME16 | ||
| 10 | 819130 | At2g45220 | AtPME17 | AtPMEI-PME17 | Root growth, Plant-pathogen interaction | Senechal et al., 2014; Del Corpo et al., 2020 |
| 11 | 819317 | At2g47030 | AtPME4; VgDH1 | AtPMEI-PME4 | Pollen tube growth | Jiang et al., 2005 |
| 12 | 819318 | At2g47040 | AtPME5; VgD1 | AtPMEI-PME5 | Pollen tube growth | Jiang et al., 2005 |
| 13 | 819368 | At2g47550 | AtPME20 | AtPMEI-PME20 | ||
| 14 | 819727 | At3g05610 | AtPME21 | AtPMEI-PME21 | ||
| 15 | 819728 | At3g05620 | AtPME22 | AtPMEI-PME22 | ||
| 16 | 819867 | At3g06830 | AtPME23 | AtPMEI-PME23 | ||
| 17 | 820240 | At3g10710 | AtPME24; RHS12 | AtPMEI-PME24 | Root hair development | Won et al., 2009; Cheong et al., 2019 |
| 18 | 820241 | At3g10720 | AtPME25 | AtPMEI-PME25 | ||
| 19 | 820650 | At3g14300 | AtPME26; ATPMEPCRC | AtPMEI-PME26 | ||
| 20 | 820651 | At3g14310 | AtPME3 | AtPMEI-PME3 | Seed germination, Root development, Pavement cells morphogenesis, Plant-pathogen interactions, Metal tolerance | Micheli et al., 1998; Hewezi et al., 2008; Raiola et al., 2011; Weber et al., 2013; Guénin et al., 2017; Lin et al., 2021 |
| 21 | 822422 | At3g27980 | AtPME30 | AtPMEI-PME30 | Beneficial bacterial recruitment, Plant-pathogen interaction | Lakshmanan et al., 2013; Zehra et al., 2021 |
| 22 | 823402 | At3g43270 | AtPME32 | AtPMEI-PME32 | ||
| 23 | 823894 | At3g47400 | AtPME33 | AtPMEI-PME33 | ||
| 24 | 824083 | At3g49220 | AtPME34 | AtPMEI-PME34 | Transpiration, Heat tolerance | Huang et al., 2017; Wu et al., 2017 |
| 25 | 825070 | At3g59010 | AtPME35 | AtPMEI-PME35 | Mechanical strength of stem | Hongo et al., 2012, p. 35 |
| 26 | 825244 | At3g60730 | No number | AtPMEI-PME65 | ||
| 27 | 825390 | At3g62170 | AtPME37; VgDH2 | AtPMEI-PME37 | Pollen tube growth | Jiang et al., 2005 |
| 28 | 828218 | At4g00190 | PME38 | AtPMEI-PME38 | Probable pseudogene | Dedeurwaerder et al., 2008 |
| 29 | 827708 | At4g02300 | AtPME39 | AtPMEI-PME39 | ||
| 30 | 828067 | At4g02320 | AtPME40 | AtPMEI-PME40 | ||
| 31 | 828064 | At4g02330 | AtPME41; ATPMEPCRB | AtPMEI-PME41 | Chilling tolerance | Qu et al., 2011 |
| 32 | 825703 | At4g03930 | AtPME42 | AtPMEI-PME42 | ||
| 33 | 827282 | At4g15980 | AtPME43 | AtPMEI-PME43 | ||
| 34 | 829458 | At4g33220 | AtPME44 | AtPMEI-PME44 | ||
| 35 | 829459 | At4g33230 | AtPME45 | AtPMEI-PME45 | ||
| 36 | 830378 | At5g04960 | AtPME46 | AtPMEI-PME46 | Metal tolerance | Geng et al., 2017 |
| 37 | 830379 | At5g04970 | AtPME47 | AtPMEI-PME47 | ||
| 38 | 830836 | At5g09760 | AtPME51 | AtPMEI-PME51 | ||
| 39 | 832209 | At5g20860 | AtPME54 | AtPMEI-PME54 | ||
| 40 | 832850 | At5g27870 | AtPME28 | AtPMEI-PME28 | ||
| 41 | 834977 | At5g49180 | AtPME58 | AtPMEI-PME58 | Mucilage release | Turbant et al., 2016 |
| 42 | 835223 | At5g51490 | AtPME59 | AtPMEI-PME59 | ||
| 43 | 835224 | At5g51500 | AtPME60 | AtPMEI-PME60 | ||
| 44 | 835418 | At5g53370 | AtPME61; ATPMEPCRB | AtPMEI-PME61 | ||
| 45 | 836585 | At5g64640 | AtPME64 | AtPMEI-PME64 |
PME Inhibitors
To date, 17 INVI/PMEI isoforms were already identified as independent PMEI in Arabidopsis, although only AtPMEI1, AtPMEI2, and AtPMEI7 were purified and their activities verified. The inhibitory activity of the PMEI region in the PMEI-PME cluster was demonstrated for AtPMEI-PME17 (Del Corpo et al., 2020). The remaining isoforms were considered PMEIs because their transgenic overexpression or mutation leads to an alteration of PME activity and/or of the pectin methylesterification, with consequences in different plant physiology processes (Figures 5, 6). From here on, considerations on the role of PMEI-PMEs will be understood as actions of the enzyme controlled by the inhibitor in the specific cluster.
Figure 5.
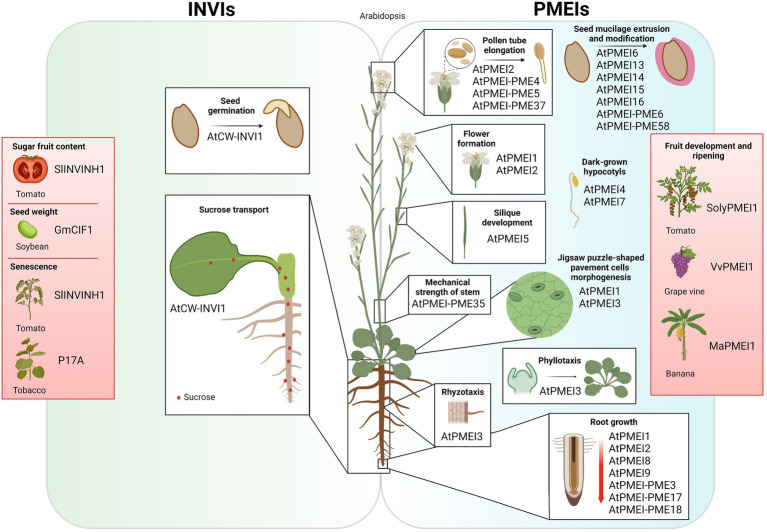
Overview of INVIs and PMEIs functions in plant growth and development. INVIs control seed germination, sugar transport in roots; senescence and sugar fruit content. PMEIs play multiple roles in several physiological processes, such as pollen tube elongation, seed mucilage extrusion, and modification, flowering transition, silique development, mechanical strength of stem, phyllotaxis, rhyzotaxis, pavement cells morphogenesis, hypocotyl growth in the dark, and root growth, and they are also involved in fruit development and ripening.
Figure 6.
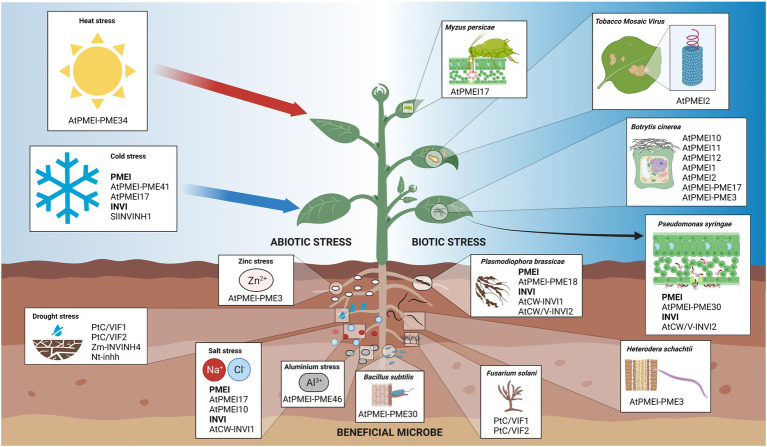
Schematic representation of the involvement of INVI/PMEI members in plant-environment interactions. Plants face different pathogens and pests, such as bacteria, nematodes, fungi, viruses, and insects as well as multiple abiotic stresses. Different INVI/PMEI members have a pivotal role plant-microbe associations beneficial to the host plant like also during multiple plant-pathogen interactions. PMEIs and INVIs are also involved in plant responsiveness to different abiotic stresses and in heavy metal tolerance.
PMEIs Assist Pollen Tube Growth During Fertilization and Embryogenesis
Pollen tube tip growth in the transmitting tract is crucial for reproductive success of plants. Pollen tube elongation is driven by secretion of pectic material and a gradient of degree of pectin methylesterification along the pollen tube axis provides the plasticity and rigidity requested for pollen tube growth (Parre and geitmann, 2005). PME and PMEI can play multiple roles in this process in different species (Bosch and Hepler, 2005; Zhang et al., 2010; Rocchi et al., 2011). AtPMEI1 and AtPMEI2 transcripts are particularly expressed in flower tissues suggesting a role of these inhibitors during flower formation or during the reproductive process. Both proteins interact with, and inhibit in vitro, different PMEs indicating a large spectrum of recognition for the PMEIs (Table 2; Raiola et al., 2004; Lionetti et al., 2007; Röckel et al., 2008). An interesting role in the regulation of the dynamics of pectin metabolism in polar pollen cell growth was demonstrated for AtPMEI2 (Röckel et al., 2008). AtPMEI2 showed a polarized accumulation at the pollen tube apex favored by a local PMEI endocytosis at the flanks of the tip. By reducing blockwise de-methylesterification and Ca2+-mediated pectin crosslinks, the localized AtPMEI2 accumulation at pollen tube apex favors CW extensibility and polar growth. Also, AtPMEI-PME5, AtPMEI-PME4, and AtPMEI-PME37 clusters are highly expressed during pollen tube growth (Jiang et al., 2005). A controlled AtPMEI-PME5 activity by PMEI domain could modulate pectin methylesterification of the transmitting tract cells to assist pollen tube movement toward the ovules.
The remodeling of pectin methylesterification play important roles in embryogenesis (Cruz-Valderrama et al., 2018; Pérez-Pérez et al., 2019). AtPMEI12 is expressed in the micropylar endosperm that surrounds the embryo and its prolonged expression suppressed embryo growth in Arabidopsis, most likely, by disturbing pectin methylesterification homeostasis (Zuma et al., 2018). AgL62 and FIS2 are required to regulate the expression of AtPMEI12 in the syncytial endosperm (Hoffmann et al., 2022). AtPMEI-PME6 is required for cell wall loosening in the embryo to facilitate cell expansion (Levesque-Tremblay et al., 2015).
PMEIs Regulate Pectin Extensibility During Emergence, Formation, and Growth of Different Organs
Precise spatiotemporal modifications of CW composition and structure are critical for cell expansion and shape (Somerville et al., 2004). Pectin remodeling underlie changes in CW elasticity in organ initiation and differentiation (Hocq et al., 2017a; Shin et al., 2021). The phyllotaxis in the Arabidopsis shoot apical meristem is accompanied by a pectic de-methylesterification in subepidermal tissue layers which is strictly controlled by AtPMEI3 (Peaucelle et al., 2008, 2011a). The AtPMEI3 overexpression in Arabidopsis produced, throughout the meristem dome, a significant reduction of “egg-box” structures, the development of shorter cells, and loss of growth asymmetry leading to an altered primordia outgrowth. Also in rhyzotaxis, AtPMEI3 expression, acting on pectin methylesterification, influences the functionality of the root clock for lateral root formation (Wachsman et al., 2020).
The jigsaw puzzle-shaped pavement cells of the leaf epidermis serve as an attractive model to investigate the mechanisms for cell–cell coordination of cell shapes (Yang, 2008). Pectin nanofilament expansion drives morphogenesis in plant epidermal cells (Haas et al., 2020). Plant overexpressing AtPMEI1 or AtPMEI3 showed defects in interdigitation and lobe formation in the pavement cells of the leaf epidermis indicating that the pectin methylesterification can influence plant cell morphogenesis (Haas et al., 2020; Lin et al., 2021). Auxin-induced callus formation is considered as a cell reprogramming process for in vitro regeneration of plants. Transgenic plants overexpressing AtPMEI-PME2 developed callus-like structures in the roots when grown on medium without exogenous auxin indicating that this cluster participates in the cell reprogramming during callus formation (Xu et al., 2018).
The Arabidopsis dark-grown hypocotyls and root growth were extensively used as models to study pectin modifications during organ elongation (Hocq et al., 2017b). The growth of hypocotyls in the dark is biphasic, with an initial slow and synchronous growth and a subsequent growth acceleration that propagates rapidly from the base to the top of the hypocotyls. AtPMEI4 controls the timing of the growth acceleration modulating the PME activity and pectin de-methylesterification (Pelletier et al., 2010). AtPMEI4 overexpression showed an increased concentrations of methylesterified pectins and a delay of growth acceleration. AtPMEI7 was also detected in apoplastic proteins extracted from dark-grown hypocotyl although its role in this process remain to be demonstrated (Sénéchal et al., 2015).
Modulation of PMEIs expression can have different effects on root and root hair growth. A decrease in PME activity correlated with an increased root length in atpmei-pme17 mutants and in plant overexpressing AtPMEI1, AtPMEI2, or AtPMEI9 compared to controls (Lionetti et al., 2007, 2010; Wolf et al., 2012; Senechal et al., 2014, p. 17; Hocq et al., 2017b). On the contrary, a pmei4 mutant and a AtPMEI-PME3 overexpressor, both expressing an elevated PME activity in Arabidopsis root CWs, showed an increased root length (Hewezi et al., 2008; Senechal et al., 2015). Specific PMEI-PME interactions and their regulation could underlie the contrasting root phenotypes observed in the transgenic plants. AtPMEI1 can inhibit the AtPMEI-PME17 activity in vitro (Del Corpo et al., 2020). AtPMEI7 expressed in Escherichia coli can form in vitro a pH-dependent reversible complex with AtPMEI-PME3 (Sénéchal et al., 2017). Indirect evidence indicates that AtPMEI4/AtPMEI-PME17, AtPMEI4/AtPMEI-PME3 and AtPMEI9/AtPMEI-PME3 interactions are likely to occur in vivo (Senechal et al., 2015; Hocq et al., 2017b). The inhibition capacity of AtPMEI4 was predicted to be highly pH-dependent, for the presence of key protonatable amino acids interacting with AtPMEI-PME3. AtPMEI5 overexpression in Arabidopsis caused root waiving in seedlings and strong defects in adult plants like fusion of cauline leaf and shoot as well as strongly impaired silique development (Wolf et al., 2012; Müller et al., 2013). These effects were related to brassinosteroids as part of a compensatory response against the loss of CW integrity, triggered by an imbalance in pectin methylesterification. Moreover, the AtPMEI5 overexpressors germinate earlier and faster compared to control suggesting that pectin methylesterification is essential for the temporal regulation of radicle emergence in endospermic seeds by altering the mechanical properties of the CWs (Muller et al., 2013). Interestingly, AtPMEI5 overexpression also revealed a correlation between HG methylesterification and auxin distribution in cell elongation that induce hypocotyl bending required for seedling emergence (Jonsson et al., 2021). AtPMEI8 and AtPMEI9 were identified during a suppressor screen for genetic suppressors of cobra, an Arabidopsis mutant with a reduced root length associated to defects in cellulose formation and an increased ratio of unesterified/esterified pectin (Sorek et al., 2015). AtPMEI8 and AtPMEI9 expression is exaggerated in mutants with CW defects. The overexpression of AtPMEI8 and AtPMEI9 increases the amount of pectin methylesterification in the cob-6 mutant at the wild-type levels and partially restore the cobra root growth suggesting that pectin methylesterification is a significant factor for CW integrity. AtPMEI-PME3 is ubiquitous in Arabidopsis tissues and it was involved in multiple physiological processes like adventitious rooting, root hair production, and seed germination (Guénin et al., 2011, 2017). AtPMEI-PME24 plays a role in root hair development and it can be inhibited by the non-proteinaceous PME inhibitor phenylephrine (Won et al., 2009; Cheong et al., 2019).
Lignin in secondary CW is considered the main component influencing the mechanical strength of the stem. Interestingly, the de-methylesterification of the primary CW can play a role in CW stiffening for mechanical support of the Arabidopsis inflorescence stem (Hongo et al., 2012). A loss-of-function mutant of AtPMEI-PME35 showed a pendant stem phenotype and an increased deformation rate of the stem.
PMEI in Fruit Development, Ripening, and Postharvest Fruit Processes
Fruit development and ripening require a combined, sequential, and synergistic action of a range of CW degrading enzymes (CWDEs; Wang et al., 2018). Advanced softening during ripening is a limiting factor in fruit shelf life and storage (Brummell and Harpster, 2001). The role of PME in fruit ripening was intensively examined in tomato. PME activity can affect pectin structure during ripening and fruit processing and it can also be a potential enhancers of ascorbic acid production (Tieman et al., 1992; Gaffe et al., 1994; Tieman and Handa, 1994; Thakur et al., 1996; Rigano et al., 2018). PMEIs can modulate PME activity and pectin methylesterification in different stages of fruit life. Several inhibitors were identified and characterized from the fruits of different species, like AcPMEI in Kiwi (Balestrieri et al., 1990), SolyPMEI1 in Tomato (Reca et al., 2012), MaPMEI1 in Banana (Musa acuminata; Srivastava et al., 2012) and VvPMEI1 in grapevine (Vitis vinifera; Lionetti et al., 2015b). The AcPMEI, SolyPMEI, and MaPMEI1 expressions increase as the fruits ripen to finely control pectin de-methylesterification in softening during ripening. Differently, VvPMEI1 control PME activity at early phases of grape berry development to assist a rapid cell growth and to maintain pulp firmness, by preventing precocious pectin degradation and grape berry softening.
Different evidence indicates PMEI activity as a valid tool in food processes (Sørensen et al., 2004). PME is physiologically released into the juice during processing, and it is considered a juice clarifying enzyme. PME activity, by triggering the formation of “egg-box” structures, causes the precipitation of pectins and cloud loss in juice, one of the major problems in fruit juice manufacturing industries (Bazaraa et al., 2020). The thermal inhibition of PME activity might be a solution but it can negatively affect the nutritional quality of the juice. The addition of PMEI during the process was demonstrated to reduce phase separation improving juice quality (Castaldo et al., 1991; Sørensen et al., 2004; Bellincampi et al., 2005). PME activity can represent also a postharvest problem in grape fermentation and distillation processes, inducing a high methanol content in spirits (Botelho et al., 2020). PMEI can reduce methanol formation in grape must and marc as well as in products derived by fermentation and distillation (Lante et al., 2008; Zocca et al., 2008).
PMEIs Modulate Seed Mucilage Extrusion and the Mucilage Degree of Methylesterification
The mucilage secretory cells present in the epidermal layer of the seed coat are responsible for mucilage production and release (Francoz et al., 2015). The release and function of mucilage are affected by PME activity (Rautengarten et al., 2008), and several evidence indicate that this activity requires a fine regulation by PMEIs. AtPMEI6 is specifically expressed in seed coat epidermal cells and pmei6 mutants showed a delayed mucilage release (Saez-Aguayo et al., 2013). The analysis of PME activity in soluble mucilage from pmei6 and 35S:PMEI6 transgenic plants indicates that AtPMEI6 inhibits endogenous PME activities. The level of AtPMEI6 expression in transformants correlated with the level of methylesterified HG revealed using antibodies recognizing HG methylesterification status. This evidence leads to conclude that AtPMEI6 controls CW integrity of seed coat epidermal cells by preventing HG de-methylesterification for a correct seed mucilage release. Mechanistic insights indicate that the AtPMEI6-dependent partially methylesterified HG pattern represents an amphiphilic polysaccharidic platform necessary for PEROXIDASE36-specific anchoring, useful to loosen the outer periclinal wall domains of mucilage secretory cells, necessary for mucilage extrusion (Kunieda et al., 2013; Francoz et al., 2019).
AtPMEI13, AtPMEI14, AtPMEI15, and AtPMEI16 are other four independent mucilage-related PMEIs (Shi et al., 2018; Ding et al., 2021). pmei13 and pmei14 mutants but not pmei15 mutant showed an increased PME activity and a reduced degree of methylesterification in the seed mucilage. AtPMEI15 might play only a minimal role in HG de-methylesterification or it could also be an INVI. AtPMEI14 protein seems dedicated to the modulation of pectin de-methylesterification in the mucilage after its release because any discernible mucilage extrusion defects were detected in the pmei14 mutant. The expression of AtPMEI6, AtPMEI14, and AtPMEI16, like also other pectin modifying enzymes can be activated by the transcriptional factors GLABRA2 (GL2), LEUNIG_HOMOLOG/MUCILAGE MODIFIED1 (LUH/MUM1), SEEDSTICK (STK), and MYELOBLASTOSIS 52 (MYB52; Saez-Aguayo et al., 2013; Ezquer et al., 2016; Ding et al., 2021). The Arabidopsis ETHYLENE RESPONSIVE ELEMENT BINDING FACTOR 4 (ERF4) and the MYB52 transcription factors interact and play antagonistic roles in the regulation of pectin de-methylesterification in seed mucilage. ERF4 directly suppresses the expression of AtPMEI13, AtPMEI14, ATPMEI15 and suppresses AtPMEI6 indirectly by antagonizing MYB52 function, giving rise to positive regulation of pectin de-methylesterification during seed development (Ding et al., 2021). Also the clusters AtPMEI-PME6 and AtPMEI-PME58 were associated to HG modification during mucilage release (Levesque-Tremblay et al., 2015; Turbant et al., 2016). AtPMEI-PME6 is required for mucilage extrusion while AtPMEI-PME58 activity participates in the regulation of interactions between HG and other polymers (probably rhamnogalacturonan I) during the formation of the mucilage adherent layer.
PMEIs Are Involved in Multiple Biotic and Abiotic Stresses
A fine modulation of PME activity and pectin methylesterification is exerted to face biotic and abiotic stresses (Lionetti et al., 2012; Bellincampi et al., 2014). Plants involve a spatiotemporal modulation of PME activity against multiple pathogens to trigger defense response in several ways (Bethke et al., 2014; Lionetti, 2015; Del Corpo et al., 2020). PMEs can induce the formation of the “egg-box” structures, resulting in pectin stiffening (Limberg et al., 2000). Moreover, PME activity can favor the production of damage-associated molecular patterns. For instance, PMEs can promote the release or perception of de-methylesterified oligogalacturonides, able to trigger plant immunity (Osorio et al., 2008, 2011; Ferrari et al., 2013; Kohorn et al., 2014). De-methylesterification of pectin by PMEs can also generate the alarm signal methanol (Hann et al., 2014). Methanol and oligogalacturonides are able to trigger a defensive priming in plants (Dorokhov et al., 2012; Komarova et al., 2014; Gamir et al., 2021; Giovannoni et al., 2021). Moreover, the pathogen recognition receptors Wall Associated Kinase 1 (WAK1), WAK2, and FERONIA (FER) preferentially bind to de-methylesterified pectins (Decreux and Messiaen, 2005; Kohorn et al., 2014; Feng et al., 2018; Guo et al., 2018; Lin et al., 2021). Independent and clustered PMEIs were involved in plant immunity, at different times during microbial infection. Intriguingly, INVI/PMEIs families show gene duplications, which are frequent in stress-related genes and are beneficial for survival in challenging environments (Oh et al., 2012; Kalunke et al., 2015). atpmei-pme17 mutants exhibited increased susceptibility to B. cinerea indicating that AtPMEI-PME17 cluster contributes to trigger PME activity against B. cinerea (Del Corpo et al., 2020). AtPMEI-PME17 expression is regulated by defense signaling pathways suggesting its involvement for early defense response. At later stages of infection, an extensive PME mediated de-methylesterification of pectin could favor pectin degradation by microbial CWDE. This effect can be restrained by the expression of independent PMEIs (Lionetti et al., 2017). atpmei10, atpmei11, and atpmei12 mutants showed increased PME activity, decreased pectin degree of methylesterification, and increased susceptibility to infection indicating that AtPMEI10, AtPMEI11, and AtPMEI12 can be exploited late during Botrytis infection to lock an extensive decrease of pectin methylesterification to defend pectin integrity. Intriguingly, the evidence that AtPMEI11 is induced by oligogalacturonides suggests a system of amplification of the pectin protection during immunity. Consistently, the overexpression of AtPMEI1 and AtPMEI2 in Arabidopsis induced a high degree of pectin methylesterification that correlated with a low susceptibility to B. cinerea (Lionetti et al., 2007). Similar results were obtained also in other pathosystems and also in monocots where the pectin level is low (An et al., 2008; Volpi et al., 2011; Liu et al., 2018). It must be emphasized that PMEI overexpression is free of disease resistance/developmental growth trade-offs observed in plants with engineered CWs (Pontiggia et al., 2020; Ha et al., 2021). Rather, PMEI overexpressors had a higher biomass yield and can improve tissue saccharification in bioconversion (Lionetti et al., 2010, 2014a; Francocci et al., 2013). Early aphid infestation induces an increase in PME activity, methanol emissions, and HG de-methylesterification (Silva-Sanzana et al., 2019). atpmei17 mutants (named pmei13 mutants in the original article) are significantly more susceptible to the green peach aphid (Myzus persicae) compared to control in terms of settling preference, phloem access, and phloem sap drainage. AtPMEI17 seems particularly effective in plant–aphid interaction, since aphid feeding activities were not altered in AtPMEI6 overexpressors.
Induced Systemic Resistance (ISR) triggered by microbial bio-agents showed strong potential for biocontrol against phytopathogens (Zehra et al., 2021). Interestingly, the beneficial microbe Bacillus subtilis manipulate PME activity in root to improve own colonization while promoting plant resistance to leaf microbes (Lakshmanan et al., 2013). Roots of atpmei-pme30 mutant inoculated with showed increased bacterial root colonization and foliar protection against the pathogen P. syringae.
Also, pathogens can exploit plant PMEI-PMEs to create an optimal cellular environment for their own survival. The ubiquitous AtPMEI-PME3 seems particularly targeted. AtPMEI-PME3 is exploited by B. cinerea and Pectobacterium carotovorum as susceptibility factor required for tissue degradation and colonization (Raiola et al., 2011). Cyst nematodes use own CWDEs, such as cellulases and pectinases to breach the CW for their root penetration and migration (Bohlmann and Sobczak, 2014). A cellulose binding domain-containing protein released by the sugar beet cyst nematode Heterodera schachtii into Arabidopsis tissues interacts with AtPMEI-PME3 to aid cyst nematode parasitism (Hewezi et al., 2008). PME activity, by reducing the level of pectin methylesterification can improve the accessibility of other CWDEs to CW polymers, assisting syncytium development. The cellulose binding domain-containing protein could interact with AtPMEI-PME3 in the cytoplasm followed by a potential joint export into the CW. The PMEI region of AtPMEI-PME3 could protect pectin in the golgi apparatus from premature de-methylesterification. CW remodeling of Arabidopsis root cells is also exploited by the obligate biotrophic Plasmodiophora brassicae, a protist pathogen that causes clubroot disease in brassica species (Stefanowicz et al., 2021). A pectin de-methylesterification mediated by AtPMEI-PME18 can favor the release of resting spores of the fungus. Intriguingly, AtPMEI-PME18 showed both PME and ribosome-inactivating proteins activity (De-la-Peña et al., 2008). Intriguingly, AtPMEI-PME18 activity could be manipulated by P. brassicae to kill host cells to its advantage since ribosome-inactivating proteins were previously considered as “suicidal agent,” exploited from plant to contain the spread of pathogens (Bonness et al., 1994; Park et al., 2004). Also, the viruses like Tobacco Mosaic Virus (TMV), Turnip vein clearing virus (TVCV), Cauliflower mosaic virus, and Chinese wheat mosaic virus can exploit the interaction between a own movement protein and a PME for cell-to-cell movement (Chen et al., 2000; Chen and Citovsky, 2003). PME-dependent formation of methanol and PME-dependent enhancement of RNA silencing also influences viral cell-to-cell movement (Dorokhov et al., 2006, 2012). A tobacco PMEI (FN432040) is a methanol-inducible gene involved in defense reactions. The overexpression of AtPMEI2 in Arabidopsis and AcPMEI in tobacco contrasts the cell-to-cell and systemic movement of tobamoviruses (Lionetti et al., 2014b, 2015a).
The ability of plants to sense and maintain pectin integrity is important for salt tolerance (Yan et al., 2018; Liu et al., 2021). A fine control of different PME isoforms could modulate the ion-binding capacities of CWs to cope with salt stress (Pilling et al., 2004). AtPMEI17 positively contributes to salt tolerance in Arabidopsis (Chen et al., 2018; Liu et al., 2021). AtPMEI17 overexpression showed decreased PME activity, increased pectin methylesterification, and an improved seeds germination, root growth and survival rate under salt stress compared to control. Instead, AtPMEI10 is a negative regulator of salinity tolerance (Jithesh et al., 2012). Atpmei10 mutants upon NaCl treatment showed enhanced root growth and biomass yield and a reduced salt stress.
The ubiquitous AtPMEI-PME3 was also involved in basal metal tolerance to Zinc (Weber et al., 2013). A defective proteolytic cleavage of PMEI domain from catalytic part of AtPMEI-PME3 cluster in ozs2 (overly zinc sensitive 2) mutant, causes a root hypersensitivity to zinc. The PME activity, by producing free carboxylic groups, could potentially favor the binding of metal cations to CW thereby lowering their uptake into the symplast. Similarly, AtPMEI-PME46 mediated de-methylesterification reduces aluminum binding to CWs and hence alleviating aluminum-induced root growth inhibition (Geng et al., 2017).
Pectin contents, PME activity, and pectin methylesterification are dynamically regulated during plant acclimation to temperature stresses (Solecka et al., 2008; Baldwin et al., 2014). Under chilling stress PME activity can increase the stiffness of CWs, increasing cold and freezing tolerance for the plant (Qu et al., 2011). AtPMEI-PME41 is proposed to modulate the chilling tolerance by modifying the mechanical properties of CW though the brassinosteroid signaling. AtPMEI17 (named AtPMEI13 in the original article) negatively contributes to Arabidopsis freezing tolerance (Chen et al., 2018). However, AtPMEI17 overexpressors showed longer roots under less severe cold conditions, suggesting a role of this inhibitor in balancing the trade-off between freezing tolerance and growth maintenance under low-temperature conditions. AtPMEI-PME34 can regulate the rate of transpiration during the heat response (Huang et al., 2017; Wu et al., 2017). This cluster is highly expressed in guard cell where it contributes to regulate CW flexibility and heat tolerance, promoting stomatal movement.
INV Inhibitors
The first INVI was identified and biochemically characterized in potato (Schwimmer et al., 1961). Later, INVI isoforms were also identified in tobacco, maize (Zea mays), tomato, potato, soybean (glycine max), and Arabidopsis (Greiner et al., 1998; Bate et al., 2004; Reca et al., 2008; Liu et al., 2010; Su et al., 2016; Tang et al., 2017). To date, only two INVI/PMEI genes were annotated to encode INVIs in A. thaliana. These genes, originally termed AtC/VIF1 and AtC/VIF2, were cloned in E. coli, their activity characterized in vitro and identified as INVIs (Link et al., 2004). AtC/VIF1 exhibited an apoplastic localization and inhibited a large proportion of CW-INV activity in Arabidopsis (Su et al., 2016). The situation is less clear for AtC/VIF2, which inhibited both V-INV and CW-INV activity, but the affinity for V-INV activity was about 10-fold higher than that for CW-INV activity (Link et al., 2004). However, AtC/VIF2 clearly localized in the cell wall. To standardize the nomenclature of the INVI/PMEI superfamily and enjoying a broader view of information, we rename these two proteins as AtCW-INVI1 and AtCW/V-INVI2, respectively. Unlike PMEIs, the physiological information on INVIs in Arabidopsis is scarce. The contribution to understanding their roles comes mainly from experiments carried out in other plant species (Figures 5, 6).
INVIs Affect Seed Germination, Seedling Growth, Senescence, and Sugar Content in Fruits
Some INVIs can regulate cell elongation and division. In Arabidopsis, AtCW-INVI1 can influence sugar metabolism and transport in seeds and roots. atcw-invi1 mutant showed a faster seed germination and an increased root length in seedlings compared to control (Su et al., 2016). CW-INV activity can support cell division of the endosperm and embryo during early kernel development (Bate et al., 2004). Zm-INVINH1 is a maize CW-INVI that localizes specifically to the embryo surrounding regions. Zm-INVINH1 activity could compartmentalize invertase activity within the early kernel to allow the endosperm and embryo to follow distinct developmental programs.
CW-INVs play roles also in seed filling and fruit set in a wide range of plant species (Zanor et al., 2009; Wang and Ruan, 2012; Li et al., 2013; Liao et al., 2020). The control of INV activity by CW-INVIs is part of sugar unloading from phloem to fruits. In tomato, a knock-down expression of the apoplastic SlINVINH1 by RNAi technology caused an improvement in seed filling and sugar content in fruits (Jin et al., 2009). Later, a tomato knock-out for SlINVINH1 obtained using genome editing technology increases sugar content of tomato fruit without decrease fruit weight (Kawaguchi et al., 2021). Also in soybean, the suppression of the apoplastic GmCIF1 improves seed weight (Tang et al., 2017). Also, fruit ripening seems affected by the balance between CW and V-INV activity. The repression of the vacuolar SlVIF by RNA interference delayed tomato fruit ripening and its overexpression increased ethylene production and led to a precocious color shift, due to elevated lycopene accumulation (Qin et al., 2016). Manipulation of INVI-INV interactions represents a promising strategy to increase crop production and to produce crops with a high sugar content in fruits.
Leaf senescence is characterized by a nutrient relocation from leaves to other parts of the plant and the cytokinins delay senescence affecting source–sink relations (Guo et al., 2021). Different CW-INVIs were correlated to senescence. Silencing the SlINVINH1 expression in tomato increases apoplastic INV activity and delays leaf senescence (Jin et al., 2009). Consistently, the expression of the INVI P17A in tobacco under control of a cytokinin-inducible promoter causes the loss of cytokinin-induced delay in senescence (Balibrea Lara et al., 2004).
INVIs Play Roles During Biotic and Abiotic Stresses
Invertase activity and its post-translational modulation by INVIs are part of immune responses against microbes, especially in the apoplast (Tauzin and Giardina, 2014). The downregulation of AtCW/V-INVI2 expression and activity in Arabidopsis source leaves in response to infection by P. syringae, de-represses invertase activity as part of the plant defense response (Bonfig et al., 2010). However, invertase activity can also be exploited by pathogens in the root. Invertase gene expression is upregulated in root galls developed by P. brassicae in Arabidopsis (Siemens et al., 2011). The overproduction of AtCW-INVI1 and AtCW/V-INVI2 in Arabidopsis transgenic lines caused a reduced invertase activity in the root, a lower sucrose import into infected cells, leading to a reduced clubroot symptoms. The expressions of the two apoplastic PtC/VIF1 and PtC/VIF2 are strongly induced in the root of Populus trichocarpa against different stress cues including fusarium wilt (Fusarium solani), drought, abscisic acid, wound, and senescence (Su et al., 2020). A Nicotiana attenuata CW-INVI, named NaCWII, is strongly upregulated in a JA-dependent manner to increase secondary metabolite biosynthesis in Manduca sexta-attacked plants (Ferrieri et al., 2015).
Cold stress limits productivity and adversely affects plant growth and development. The content of sugars with an osmoprotective function increased during cold treatment (Janská et al., 2010). During chilling stress, tomato plants de-repress INV activity in the apoplast by controlling SlINVINH1expression (Xu et al., 2017). Cold storage of potato tubers prevent sprouting and pathogenesis favoring the maintenance of supply throughout the year. However, cold induces a breakdown of starch to sucrose that is ultimately cleaved into glucose and fructose by acid invertases, leading to a tuber sweetening (Mckenzie et al., 2013). Cold-induced sweetening is a serious postharvest problem for potato compromising tuber quality. The ectopic expression of different V-INVIs in potato tubers prevents cold-induced sweetening by capping the activities of V-INVs (Greiner et al., 1999; Brummell et al., 2011; Liu et al., 2013; Mckenzie et al., 2013).
Stomatal movement is critical in plant response to drought and V-INV activity is correlated with stomatal aperture under normal and drought conditions (Ni, 2012). The ectopic expression of the tobacco V-INVI, Nt-inhh, under the control of an ABA-sensitive and guard cell-specific promoter AtRab18 conferred enhanced drought tolerance in Arabidopsis and tomato (Chen et al., 2016). More recently, the drought-responsive apoplastic Zm-INVINH4 was identified and characterized in maize (Chen et al., 2019). Moreover, Arabidopsis salt tolerance can be influenced by AtCW-INVI1 (Yang et al., 2020). Transgenic plant overexpressing AtCW-INVI1 showed enhanced sensitivity to ABA and reduced tolerance to salt.
Undefined INVI/PMEIs and Their Possible Roles
Some knowledge was collected for INVI/PMEI members from now on presented. However, new experiments are needed to define their PMEI or INVI activity and to elucidate their roles in plant physiology. A INVI/PMEI isoforms, named AppB1 (At4g24640) was previously associated to pollen development (Holmes-Davis et al., 2005). A large-scale mutant screen in A. thaliana led to the identification of two INVI/PMEI mutants with defects in female gametophyte development and function (Pagnussat et al., 2005). The mutant embryo sac development arrest 24 (EDA 24) fails in polar nuclei fusion during the embryo sac development while the mutant unfertilized embryo sac 11 (UNE11) is affected in embryo sac fertilization. At5g46950 (named InvINH2) is an endosperm-specific INVI-PMEI, but its specific activity remain still to be characterized (Zuma et al., 2018). A genome wide association study supported by quantitative trait loci mapping identified the INVI/PMEI At1g23350 as a candidate gene for the response to drought/B. cinerea sequential double-stress combination (Coolen et al., 2019).
Conclusion and Perspective
Data obtained on the pattern of expression, specific activity, and related physiological effects deepen our knowledge of INVI/PMEI roles in plant growth and defense and allow to engineer precise biotechnological applications. A stage-specific manipulation of INVI/PMEIs in planta could be used as biotechnological strategy to control the fruit growth and postharvest fruit softening. INVI/PMEI represent also genetic sources to generate crop varieties, either by traditional breeding or by genetic engineering, with a durable resistance to stresses and/or with a high crop yield. The revision allowed to eliminate various inaccuracies in the nomenclature of INVI/PMEI members, providing a clear tool for future studies. We emphasize the importance of verifying the inhibitory activity of new members of the superfamily not yet characterized, before assigning them an identity. Some shortcomings on the family certainly emerge, especially on the physiological role of INVIs, and several questions remain unanswered. Although a vacuolar, cytosolic or apoplastic function was suggested for some INVI/PMEI isoforms, further investigation is needed to understand their subcellular action and processing. Future research could also try to reveal the dynamics of inhibition as well as the fate of the inhibitors after performing their function. Moreover, PMEIs were linked to the modulation of the degree and pattern of methylesterification of HG. However, an interesting and not yet tested hypothesis is that some PME and PMEI isoforms could be dedicated to xylogalacturonan or rhamnogalacturonans, which are other pectic polysaccharides showing some degree of methylesterification. PMEIs, like also other apoplastic factor as pH and calcium concentration could influence not only the degree but also the pattern of methylesterification. Studies aimed at identifying the three-dimensional structure of PMEI-PMEs could provide important information useful to clarify the role of PMEIs in these clusters. New knowledge on the interactions between specific PMEI and PME isoforms and on the inhibition features in PMEI-PMEs isoforms will be necessary to understand the dynamics of the control of PME activity in plant physiology.
Author Contributions
DC and VL collected data from literature and prepared figures and revised the paper. VL designed and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by Sapienza University of Rome, Grants RM120172B78CFDF2 and RM11916B7A142CF1 to VL and AR12117A8A4A1ADC to DC and LV.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figures 5, 6 were created with biorender.com.
Glossary
Abbreviations
- Ac
Actinidia chinensis
- At
Arabidopsis thaliana
- CIF1
Cell Wall Inhibitor of β-Fructosidase
- C/VIFs
Cell Wall/Vacuolar Inhibitor of β-Fructosidases
- CW
Cell Wall
- CWDEs
Cell Wall Degrading Enzymes
- CW-INV
Cell Wall Invertase
- CW-INVI
Cell Wall Invertase Inhibitor
- EDA
embryo sac development arrest
- ERF
Ethylene Responsive Element Binding Factor
- FER
FERONIA
- GH
Glycoside Hydrolase family
- GL
GLABRA
- Gm
Glycine max
- HG
Homogalacturonan
- HMS
Highly Methyl Esterified Seeds
- INV
Invertase
- INVI
Invertase Inhibitor
- ISR
Induced Systemic Resistance
- JA
Jasmonic Acid
- LUH/MUM
Leunig Homolog/Mucilage Modified
- Ma
Musa acuminata
- MYB
Myeloblastosis
- ozs2
overly Zinc sensitive 2
- PME
Pectin Methylesterase
- PMEI
Pectin Methylesterase Inhibitor
- Sl
Solanum lycopercsicum
- STK
Seedstick
- TMV
Tobacco Mosaic Virus
- TVCV
Turnip Vein Clearing Virus
- UNE
Unfertilized Embryo Sac
- V-INV
Vacuolar Invertase
- Vv
Vitis vinifera
- WAK
Wall Associated Kinase
- Zm
Zea mays
Footnotes
References
- Amsbury S., Hunt L., Elhaddad N., Baillie A., Lundgren M., Verhertbruggen Y., et al. (2016). Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr. Biol. 26, 2899–2906. doi: 10.1016/j.cub.2016.08.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S. H., Sohn K. H., Choi H. W., Hwang I. S., Lee S. C., Hwang B. K. (2008). Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228, 61–78. doi: 10.1007/s00425-008-0719-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin L., Domon J.-M., Klimek J. F., Fournet F., Sellier H., Gillet F., et al. (2014). Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104, 37–47. doi: 10.1016/j.phytochem.2014.04.011, PMID: [DOI] [PubMed] [Google Scholar]
- Balestrieri C., Castaldo D., Giovane A., Quagliuolo L., Servillo L. (1990). A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis). Eur. J. Biochem. 193, 183–187. doi: 10.1111/j.1432-1033.1990.tb19321.x, PMID: [DOI] [PubMed] [Google Scholar]
- Balibrea Lara M. E., Gonzalez Garcia M.-C., Fatima T., Ehness R., Lee T. K., Proels R., et al. (2004). Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16, 1276–1287. doi: 10.1105/tpc.018929, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate N. J., Niu X., Wang Y., Reimann K. S., Helentjaris T. G. (2004). An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiol. 134, 246–254. doi: 10.1104/pp.103.027466, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaraa W. A., Ammar A. S., Aqlan A. M. (2020). Effects of kiwi’s pectin methylesterase inhibitor, nanomilling and pasteurization on orange juice quality. Food Sci. Nutr. 8, 6367–6379. doi: 10.1002/fsn3.1886, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D., Cervone F., Lionetti V. (2014). Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 5:228. doi: 10.3389/fpls.2014.00228, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D., Cervone F., Raiola A., Camardella L., Balestrieri C., De Lorenzo G., et al. (2005). Pectin methylesterase inhibitors for the preparation of fruit juices and derivative. Patent No. WO2005005470A2. Available at: https://patents.google.com/patent/WO2005005470A2/en
- Bethke G., Grundman R. E., Sreekanta S., Truman W., Katagiri F., Glazebrook J. (2014). Arabidopsis PECTIN METHYLESTERASES contribute to immunity against Pseudomonas syringae. Plant Physiol. 164, 1093–1107. doi: 10.1104/pp.113.227637, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann H., Sobczak M. (2014). The plant cell wall in the feeding sites of cyst nematodes. Front. Plant Sci. 5:89. doi: 10.3389/fpls.2014.00089, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavita A., Carratore V., Ciardiello M. A., Giovane A., Servillo L., D’Avino R. (2016). Influence of pH on the structure and function of kiwi pectin methylesterase inhibitor. J. Agric. Food Chem. 64, 5866–5876. doi: 10.1021/acs.jafc.6b01718, PMID: [DOI] [PubMed] [Google Scholar]
- Bonfig K. B., Gabler A., Simon U. K., Luschin-Ebengreuth N., Hatz M., Berger S., et al. (2010). Post-translational derepression of invertase activity in source leaves via down-regulation of invertase inhibitor expression is part of the plant defense response. Mol. Plant 3, 1037–1048. doi: 10.1093/mp/ssq053, PMID: [DOI] [PubMed] [Google Scholar]
- Bonness M. S., Ready M. P., Irvin J. D., Mabry T. J. (1994). Pokeweed antiviral protein inactivates pokeweed ribosomes; implications for the antiviral mechanism. Plant J. 5, 173–183. doi: 10.1046/j.1365-313X.1994.05020173.x, PMID: [DOI] [PubMed] [Google Scholar]
- Bosch M., Cheung A. Y., Hepler P. K. (2005). Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 138, 1334–1346. doi: 10.1104/pp.105.059865, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Hepler P. (2005). Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17, 3219–3226. doi: 10.1105/tpc.105.037473, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho G., Anjos O., Estevinho L. M., Caldeira I. (2020). Methanol in grape derived, fruit and honey spirits: a critical review on source, quality control, and legal limits. PRO 8:1609. doi: 10.3390/pr8121609 [DOI] [Google Scholar]
- Boycheva S., Daviet L., Wolfender J.-L., Fitzpatrick T. B. (2014). The rise of operon-like gene clusters in plants. Trends Plant Sci. 19, 447–459. doi: 10.1016/j.tplants.2014.01.013, PMID: [DOI] [PubMed] [Google Scholar]
- Brummell D. A., Chen R. K. Y., Harris J. C., Zhang H., Hamiaux C., Kralicek A. V., et al. (2011). Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J. Exp. Bot. 62, 3519–3534. doi: 10.1093/jxb/err043, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell D. A., Harpster M. H. (2001). Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 47, 311–339. doi: 10.1023/A:1010656104304, PMID: [DOI] [PubMed] [Google Scholar]
- Castaldo D., Lovoi A., Quagliuolo L., Servillo L., Balestrieri C., Giovane A. (1991). Orange juices and concentrates stabilization by a proteic inhibitor of pectin methylesterase. J. Food Sci. 56, 1632–1634. doi: 10.1111/j.1365-2621.1991.tb08658.x [DOI] [Google Scholar]
- Catoire L., Pierron M., Morvan C., du Penhoat C. H., Goldberg R. (1998). Investigation of the action patterns of pectinmethylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications In cell wall expansion. J. Biol. Chem. 273, 33150–33156. doi: 10.1074/jbc.273.50.33150, PMID: [DOI] [PubMed] [Google Scholar]
- Chen J., Chen X., Zhang Q., Zhang Y., Ou X., An L., et al. (2018). A cold-induced pectin methyl-esterase inhibitor gene contributes negatively to freezing tolerance but positively to salt tolerance in Arabidopsis. J. Plant Physiol. 222, 67–78. doi: 10.1016/j.jplph.2018.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- Chen M.-H., Citovsky V. (2003). Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J. 35, 386–392. doi: 10.1046/j.1365-313X.2003.01818.x, PMID: [DOI] [PubMed] [Google Scholar]
- Chen S.-F., Liang K., Yin D.-M., Ni D.-A., Zhang Z.-G., Ruan Y.-L. (2016). Ectopic expression of a tobacco vacuolar invertase inhibitor in guard cells confers drought tolerance in Arabidopsis. J. Enzyme Inhib. Med. Chem. 31, 1381–1385. doi: 10.3109/14756366.2016.1142981, PMID: [DOI] [PubMed] [Google Scholar]
- Chen L., Liu X., Huang X., Luo W., Long Y., Greiner S., et al. (2019). Functional characterization of a drought-responsive invertase inhibitor from maize (Zea mays L.). Int. J. Mol. Sci. 20:4081. doi: 10.3390/ijms20174081, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. H., Sheng J., Hind G., Handa A. K., Citovsky V. (2000). Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 19, 913–920. doi: 10.1093/emboj/19.5.913, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong M. S., Lee D. Y., Seo K. H., Choi G.-H., Song Y. H., Park K. H., et al. (2019). Phenylephrine, a small molecule, inhibits pectin methylesterases. Biochem. Biophys. Res. Commun. 508, 320–325. doi: 10.1016/j.bbrc.2018.11.117, PMID: [DOI] [PubMed] [Google Scholar]
- Ciardiello M. A., D’Avino R., Amoresano A., Tuppo L., Carpentieri A., Carratore V., et al. (2008). The peculiar structural features of kiwi fruit pectin methylesterase: amino acid sequence, oligosaccharides structure, and modeling of the interaction with its natural proteinaceous inhibitor. Proteins 71, 195–206. doi: 10.1002/prot.21681, PMID: [DOI] [PubMed] [Google Scholar]
- Coolen S., Van Pelt J. A., Van Wees S. C. M., Pieterse C. M. J. (2019). Mining the natural genetic variation in Arabidopsis thaliana for adaptation to sequential abiotic and biotic stresses. Planta 249, 1087–1105. doi: 10.1007/s00425-018-3065-9, PMID: [DOI] [PubMed] [Google Scholar]
- Cruz-Valderrama J. E., Jiménez-Durán K., Zúñiga-Sánchez E., Salazar-Iribe A., Márquez-Guzmán J., Gamboa-deBuen A. (2018). Degree of pectin methyl esterification in endosperm cell walls is involved in embryo bending in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 495, 639–645. doi: 10.1016/j.bbrc.2017.11.077, PMID: [DOI] [PubMed] [Google Scholar]
- D’Avino R., Camardella L., Christensen T. M. I. E., Giovane A., Servillo L. (2003). Tomato pectin methylesterase: Modeling, fluorescence, and inhibitor interaction studies—comparison with the bacterial (Erwinia chrysanthemi) enzyme. Proteins 53, 830–839. doi: 10.1002/prot.10487, PMID: [DOI] [PubMed] [Google Scholar]
- Daher F. B., Braybrook S. A. (2015). How to let go: pectin and plant cell adhesion. Front. Plant Sci. 6:523. doi: 10.3389/fpls.2015.00523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decreux A., Messiaen J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46, 268–278. doi: 10.1093/pcp/pci026, PMID: [DOI] [PubMed] [Google Scholar]
- Dedeurwaerder S., Menu-Bouaouiche L., Mareck A., Lerouge P., Guerineau F. (2008). Activity of an atypical Arabidopsis thaliana pectin methylesterase. Planta 229, 311–321. doi: 10.1007/s00425-008-0831-0, PMID: [DOI] [PubMed] [Google Scholar]
- Del Corpo D., Fullone M. R., Miele R., Lafond M., Pontiggia D., Grisel S., et al. (2020). AtPME17 is a functional Arabidopsis thaliana pectin methylesterase regulated by its PRO region that triggers PME activity in the resistance to Botrytis cinerea. Mol. Plant Pathol. 21, 1620–1633. doi: 10.1111/mpp.13002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-la-Peña C., Badri D. V., Vivanco J. M. (2008). Novel role for pectin methylesterase in Arabidopsis: a new function showing ribosome-inactivating protein (RIP) activity. Biochim. Biophys. Acta Gen. Subj. 1780, 773–783. doi: 10.1016/j.bbagen.2007.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- Di Matteo A., Giovane A., Raiola A., Camardella L., Bonivento D., Lorenzo G. D., et al. (2005). Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 17, 849–858. doi: 10.1105/tpc.104.028886, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A., Tang X., Yang D., Wang M., Ren A., Xu Z., et al. (2021). ERF4 and MYB52 transcription factors play antagonistic roles in regulating homogalacturonan de-methylesterification in Arabidopsis seed coat mucilage. Plant Cell 33, 381–403. doi: 10.1093/plcell/koaa031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorokhov Y. L., Frolova O. Y., Skurat E. V., Ivanov P. A., Gasanova T. V., Sheveleva A. A., et al. (2006). A novel function for a ubiquitous plant enzyme pectin methylesterase: the enhancer of RNA silencing. FEBS Lett. 580, 3872–3878. doi: 10.1016/j.febslet.2006.06.013, PMID: [DOI] [PubMed] [Google Scholar]
- Dorokhov Y. L., Komarova T. V., Petrunia I. V., Frolova O. Y., Pozdyshev D. V., Gleba Y. Y. (2012). Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 8:e1002640. doi: 10.1371/journal.ppat.1002640, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquer I., Mizzotti C., Nguema-Ona E., Gotté M., Beauzamy L., Viana V. E., et al. (2016). The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. Plant Cell 28, 2478–2492. doi: 10.1105/tpc.16.00454, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Kita D., Peaucelle A., Cartwright H. N., Doan V., Duan Q., et al. (2018). The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666.e5–675.e5. doi: 10.1016/j.cub.2018.01.023, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Savatin D. V., Sicilia F., Gramegna G., Cervone F., De Lorenzo G. (2013). Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4:49. doi: 10.3389/fpls.2013.00049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrieri A. P., Arce C. C. M., Machado R. A. R., Meza-Canales I. D., Lima E., Baldwin I. T., et al. (2015). A Nicotiana attenuata cell wall invertase inhibitor (NaCWII) reduces growth and increases secondary metabolite biosynthesis in herbivore-attacked plants. New Phytol. 208, 519–530. doi: 10.1111/nph.13475, PMID: [DOI] [PubMed] [Google Scholar]
- Figueiredo J., Silva M. S., Figueiredo A. (2018). Subtilisin-like proteases in plant defence: the past, the present and beyond. Mol. Plant Pathol. 19, 1017–1028. doi: 10.1111/mpp.12567, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francocci F., Bastianelli E., Lionetti V., Ferrari S., De Lorenzo G., Bellincampi D., et al. (2013). Analysis of pectin mutants and natural accessions of Arabidopsis highlights the impact of de-methyl-esterified homogalacturonan on tissue saccharification. Biotechnol. Biofuels 6:163. doi: 10.1186/1754-6834-6-163, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoz E., Ranocha P., Burlat V., Dunand C. (2015). Arabidopsis seed mucilage secretory cells: regulation and dynamics. Trends Plant Sci. 20, 515–524. doi: 10.1016/j.tplants.2015.04.008, PMID: [DOI] [PubMed] [Google Scholar]
- Francoz E., Ranocha P., Le Ru A., Martinez Y., Fourquaux I., Jauneau A., et al. (2019). Pectin demethylesterification generates platforms that anchor peroxidases to remodel plant cell wall domains. Dev. Cell 48, 261.e8–276.e8. doi: 10.1016/j.devcel.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Gaffe J., Tieman D. M., Handa A. K. (1994). Pectin methylesterase isoforms in tomato (Lycopersicon esculentum) tissues (effects of expression of a pectin methylesterase antisense gene). Plant Physiol. 105, 199–203. doi: 10.1104/pp.105.1.199, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamir J., Minchev Z., Berrio E., García J. M., De Lorenzo G., Pozo M. J. (2021). Roots drive oligogalacturonide-induced systemic immunity in tomato. Plant Cell Environ. 44, 275–289. doi: 10.1111/pce.13917, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Horst W. J., Golz J. F., Lee J. E., Ding Z., Yang Z.-B. (2017). LEUNIG_HOMOLOG transcriptional co-repressor mediates aluminium sensitivity through PECTIN METHYLESTERASE46-modulated root cell wall pectin methylesterification in Arabidopsis. Plant J. 90, 491–504. doi: 10.1111/tpj.13506, PMID: [DOI] [PubMed] [Google Scholar]
- Giovannoni M., Lironi D., Marti L., Paparella C., Vecchi V., Gust A. A., et al. (2021). The Arabidopsis thaliana LysM-containing receptor-Like kinase 2 is required for elicitor-induced resistance to pathogens. Plant Cell Environ. 44, 3775–3792. doi: 10.1111/pce.14192, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J., Karplus K., Hughey R., Chothia C. (2001). Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 313, 903–919. doi: 10.1006/jmbi.2001.5080, PMID: [DOI] [PubMed] [Google Scholar]
- Greiner S., Krausgrill S., Rausch T. (1998). Cloning of a tobacco apoplasmic invertase inhibitor. Proof of function of the recombinant protein and expression analysis during plant development. Plant Physiol. 116, 733–742. doi: 10.1104/pp.116.2.733, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S., Rausch T., Sonnewald U., Herbers K. (1999). Ectopic expression of a tobacco invertase inhibitor homolog prevents cold-induced sweetening of potato tubers. Nat. Biotechnol. 17, 708–711. doi: 10.1038/10924, PMID: [DOI] [PubMed] [Google Scholar]
- Guénin S., Hardouin J., Paynel F., Müller K., Mongelard G., Driouich A., et al. (2017). AtPME3, a ubiquitous cell wall pectin methylesterase of Arabidopsis thaliana, alters the metabolism of cruciferin seed storage proteins during post-germinative growth of seedlings. J. Exp. Bot. 68, 1083–1095. doi: 10.1093/jxb/erx023, PMID: [DOI] [PubMed] [Google Scholar]
- Guénin S., Mareck A., Rayon C., Lamour R., Assoumou Ndong Y., Domon J.-M., et al. (2011). Identification of pectin methylesterase 3 as a basic pectin methylesterase isoform involved in adventitious rooting in Arabidopsis thaliana. New Phytol. 192, 114–126. doi: 10.1111/j.1469-8137.2011.03797.x, PMID: [DOI] [PubMed] [Google Scholar]
- Guo H., Nolan T. M., Song G., Liu S., Xie Z., Chen J., et al. (2018). FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol. 28, 3316.e6–3324.e6. doi: 10.1016/j.cub.2018.07.078 [DOI] [PubMed] [Google Scholar]
- Guo Y., Ren G., Zhang K., Li Z., Miao Y., Guo H. (2021). Leaf senescence: progression, regulation, and application. Mol. Hortic. 1:5. doi: 10.1186/s43897-021-00006-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C. M., Rao X., Saxena G., Dixon R. A. (2021). Growth–defense trade-offs and yield loss in plants with engineered cell walls. New Phytol. 231, 60–74. doi: 10.1111/nph.17383, PMID: [DOI] [PubMed] [Google Scholar]
- Haas K. T., Wightman R., Meyerowitz E. M., Peaucelle A. (2020). Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367, 1003–1007. doi: 10.1126/science.aaz5103, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann C. T., Bequette C. J., Dombrowski J. E., Stratmann J. W. (2014). Methanol and ethanol modulate responses to danger- and microbe-associated molecular patterns. Front. Plant Sci. 5:550. doi: 10.3389/fpls.2014.00550, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Suttangkakul A., Scheller H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395. doi: 10.1104/pp.110.156588, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi T., Howe P., Maier T. R., Hussey R. S., Mitchum M. G., Davis E. L., et al. (2008). Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell 20, 3080–3093. doi: 10.1105/tpc.108.063065, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocq L., Pelloux J., Lefebvre V. (2017a). Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci. 22, 20–29. doi: 10.1016/j.tplants.2016.10.009, PMID: [DOI] [PubMed] [Google Scholar]
- Hocq L., Sénéchal F., Lefebvre V., Lehner A., Domon J.-M., Mollet J.-C., et al. (2017b). Combined experimental and computational approaches reveal distinct pH dependence of pectin methylesterase inhibitors. Plant Physiol. 173, 1075–1093. doi: 10.1104/pp.16.01790, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T., Shi X., Hsu C.-Y., Brown A., Knight Q., Courtney L. S., et al. (2022). The identification of type I MADS box genes as the upstream activators of an endosperm-specific invertase inhibitor in Arabidopsis. BMC Plant Biol. 22:18. doi: 10.1186/s12870-021-03399-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes-Davis R., Tanaka C. K., Vensel W. H., Hurkman W. J., McCormick S. (2005). Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5, 4864–4884. doi: 10.1002/pmic.200402011, PMID: [DOI] [PubMed] [Google Scholar]
- Hongo S., Sato K., Yokoyama R., Nishitani K. (2012). Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell 24, 2624–2634. doi: 10.1105/tpc.112.099325, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn M., D’Angelo I., Márquez J. A., Greiner S., Scheffzek K. (2004a). The invertase inhibitor Nt-CIF from tobacco: a highly thermostable four-helix bundle with an unusual N-terminal extension. J. Mol. Biol. 335, 987–995. doi: 10.1016/j.jmb.2003.10.066, PMID: [DOI] [PubMed] [Google Scholar]
- Hothorn M., Van den Ende W., Lammens W., Rybin V., Scheffzek K. (2010). Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc. Natl. Acad. Sci. U. S. A. 107, 17427–17432. doi: 10.1073/pnas.1004481107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn M., Wolf S., Aloy P., Greiner S., Scheffzek K. (2004b). Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 16, 3437–3447. doi: 10.1105/tpc.104.025684, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-C., Wu H.-C., Wang Y.-D., Liu C.-H., Lin C.-C., Luo D.-L., et al. (2017). PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 174, 748–763. doi: 10.1104/pp.17.00335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janská A., Maršík P., Zelenková S., Ovesná J. (2010). Cold stress and acclimation – what is important for metabolic adjustment? Plant Biol. 12, 395–405. doi: 10.1111/j.1438-8677.2009.00299.x, PMID: [DOI] [PubMed] [Google Scholar]
- Jiang L., Yang S.-L., Xie L.-F., Puah C. S., Zhang X.-Q., Yang W.-C., et al. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17, 584–596. doi: 10.1105/tpc.104.027631, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Ni D.-A., Ruan Y.-L. (2009). Posttranslational elevation of Cell Wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 21, 2072–2089. doi: 10.1105/tpc.108.063719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jithesh M. N., Wally O. S. D., Manfield I., Critchley A. T., Hiltz D., Prithiviraj B. (2012). Analysis of seaweed extract-induced transcriptome leads to identification of a negative regulator of salt tolerance in Arabidopsis. HortScience 47, 704–709. doi: 10.21273/HORTSCI.47.6.704 [DOI] [Google Scholar]
- Jonsson K., Lathe R. S., Kierzkowski D., Routier-Kierzkowska A.-L., Hamant O., Bhalerao R. P. (2021). Mechanochemical feedback mediates tissue bending required for seedling emergence. Curr. Biol. 31, 1154–1164.e3. doi: 10.1016/j.cub.2020.12.016, PMID: [DOI] [PubMed] [Google Scholar]
- Kalunke R. M., Tundo S., Benedetti M., Cervone F., De Lorenzo G., D’Ovidio R. (2015). An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 20:146. doi: 10.3389/fpls.2015.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Takei-Hoshi R., Yoshikawa I., Nishida K., Kobayashi M., Kusano M., et al. (2021). Functional disruption of cell wall invertase inhibitor by genome editing increases sugar content of tomato fruit without decrease fruit weight. Sci. Rep. 11:21534. doi: 10.1038/s41598-021-00966-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Kohorn S. L., Saba N. J., Martinez V. M. (2014). Requirement for pectin methyl esterase and preference for fragmented over native Pectins for wall-associated kinase-activated, EDS1/PAD4-dependent stress response in Arabidopsis. J. Biol. Chem. 289, 18978–18986. doi: 10.1074/jbc.M114.567545, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova T. V., Sheshukova E. V., Dorokhov Y. L. (2014). Cell wall methanol as a signal in plant immunity. Front. Plant Sci. 5:101. doi: 10.3389/fpls.2014.00101, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunieda T., Shimada T., Kondo M., Nishimura M., Nishitani K., Hara-Nishimura I. (2013). Spatiotemporal secretion of PEROXIDASE36 is required for seed coat mucilage extrusion in Arabidopsis. Plant Cell 25, 1355–1367. doi: 10.1105/tpc.113.110072, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan V., Castaneda R., Rudrappa T., Bais H. P. (2013). Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 238, 657–668. doi: 10.1007/s00425-013-1920-2, PMID: [DOI] [PubMed] [Google Scholar]
- Lante A., Zocca F., Spettoli P., Lomolino G., Raiola A., Bellincampi D., et al. (2008). Use of a protein inhibitor of pectin methylesterase for reducing methanol formation in grape must and marc, and process therefor. Patent No. WO2008104555A1. Available at: https://patents.google.com/patent/WO2008104555A1/en:Method
- Levesque-Tremblay G., Müller K., Mansfield S. D., Haughn G. W. (2015). HIGHLY METHYL ESTERIFIED SEEDS is a pectin methyl esterase involved in embryo development. Plant Physiol. 167, 725–737. doi: 10.1104/pp.114.255604, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-González M. A., Ibarra-Laclette E., Cruz-Ramírez A., Herrera-Estrella L. (2012). Functional and transcriptome analysis reveals an acclimatization strategy for abiotic stress tolerance mediated by Arabidopsis NF-YA family members. PLoS One 7:e48138. doi: 10.1371/journal.pone.0048138, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Liu H., Zhang Y., Kang T., Zhang L., Tong J., et al. (2013). Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol. J. 11, 1080–1091. doi: 10.1111/pbi.12102, PMID: [DOI] [PubMed] [Google Scholar]
- Liao S., Wang L., Li J., Ruan Y.-L. (2020). Cell wall invertase is essential for ovule development through sugar signaling rather Than provision of carbon nutrients. Plant Physiol. 183, 1126–1144. doi: 10.1104/pp.20.00400, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg G., Korner R., Buchholt H. C., Christensen T. M., Roepstorff P., Mikkelsen J. D. (2000). Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr. Res. 327, 293–307. doi: 10.1016/S0008-6215(00)00067-7, PMID: [DOI] [PubMed] [Google Scholar]
- Lin W., Tang W., Pan X., Huang A., Gao X., Anderson C. T., et al. (2021). Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Curr. Biol. 32, 497.e4–507.e4. doi: 10.1016/j.cub.2021.11.030, PMID: [DOI] [PubMed] [Google Scholar]
- Link M., Rausch T., Greiner S. (2004). In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett. 573, 105–109. doi: 10.1016/j.febslet.2004.07.062, PMID: [DOI] [PubMed] [Google Scholar]
- Lionetti V. (2015). PECTOPLATE: the simultaneous phenotyping of pectin methylesterases, pectinases, and oligogalacturonides in plants during biotic stresses. Front. Plant Sci. 6:331. doi: 10.3389/fpls.2015.00331, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Cervone F., Bellincampi D. (2012). Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 169, 1623–1630. doi: 10.1016/j.jplph.2012.05.006, PMID: [DOI] [PubMed] [Google Scholar]
- Lionetti V., Cervone F., De Lorenzo G. (2014a). A lower content of de-methylesterified homogalacturonan improves enzymatic cell separation and isolation of mesophyll protoplasts in Arabidopsis. Phytochemistry 112, 188–194. doi: 10.1016/j.phytochem.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Lionetti V., Fabri E., De Caroli M., Hansen A. R., Willats W. G., Piro G., et al. (2017). Three pectin methyl esterase inhibitors protect cell wall integrity for immunity to Botrytis. Plant Physiol. 173, 1844–1863. doi: 10.1104/pp.16.01185, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Francocci F., Ferrari S., Volpi C., Bellincampi D., Galletti R., et al. (2010). Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc. Natl. Acad. Sci. U. S. A. 107, 616–621. doi: 10.1073/pnas.0907549107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Camardella L., Giovane A., Obel N., Pauly M., et al. (2007). Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 143, 1871–1880. doi: 10.1104/pp.106.090803, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Cervone F., Bellincampi D. (2014b). Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol. Plant Pathol. 15, 265–274. doi: 10.1111/mpp.12090, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Cervone F., Bellincampi D. (2015a). How do pectin methylesterases and their inhibitors affect the spreading of tobamovirus? Plant Signal. Behav. 9:e972863. doi: 10.4161/15592316.2014.972863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V., Raiola A., Mattei B., Bellincampi D. (2015b). The grapevine VvPMEI1 gene encodes a novel functional pectin Methylesterase inhibitor associated to grape berry development. PLoS One 10:e0133810. doi: 10.1371/journal.pone.0133810, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin Y., Liu J., Song B., Ou Y., Zhang H., et al. (2013). StInvInh2 as an inhibitor of StvacINV1 regulates the cold-induced sweetening of potato tubers by specifically capping vacuolar invertase activity. Plant Biotechnol. J. 11, 640–647. doi: 10.1111/pbi.12054, PMID: [DOI] [PubMed] [Google Scholar]
- Liu X., Song B., Zhang H., Li X.-Q., Xie C., Liu J. (2010). Cloning and molecular characterization of putative invertase inhibitor genes and their possible contributions to cold-induced sweetening of potato tubers. Mol. Gen. Genomics. 284, 147–159. doi: 10.1007/s00438-010-0554-3, PMID: [DOI] [PubMed] [Google Scholar]
- Liu N., Sun Y., Pei Y., Zhang X., Wang P., Li X., et al. (2018). A pectin Methylesterase inhibitor enhances resistance to verticillium Wilt1[OPEN]. Plant Physiol. 176, 2202–2220. doi: 10.1104/pp.17.01399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang W., Long S., Zhao C. (2021). Maintenance of Cell Wall integrity under high salinity. Int. J. Mol. Sci. 22:3260. doi: 10.3390/ijms22063260, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie M. J., Chen R. K. Y., Harris J. C., Ashworth M. J., Brummell D. A. (2013). Post-translational regulation of acid invertase activity by vacuolar invertase inhibitor affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ. 36, 176–185. doi: 10.1111/j.1365-3040.2012.02565.x, PMID: [DOI] [PubMed] [Google Scholar]
- Micheli F. (2001). Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. doi: 10.1016/S1360-1385(01)02045-3, PMID: [DOI] [PubMed] [Google Scholar]
- Micheli F., Holliger C., Goldberg R., Richard L. (1998). Characterization of the pectin methylesterase-like gene AtPME3: a new member of a gene family comprising at least 12 genes in Arabidopsis thaliana. Gene 220, 13–20. doi: 10.1016/S0378-1119(98)00431-4, PMID: [DOI] [PubMed] [Google Scholar]
- Muller K., Levesque-Tremblay G., Bartels S., Weitbrecht K., Wormit A., Usadel B., et al. (2013). Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 161, 305–316. doi: 10.1104/pp.112.205724, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Levesque-Tremblay G., Fernandes A., Wormit A., Bartels S., Usadel B., et al. (2013). Overexpression of a pectin methylesterase inhibitor in Arabidopsis thaliana leads to altered growth morphology of the stem and defective organ separation. Plant Signal. Behav. 8:e26464. doi: 10.4161/psb.26464, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D. A. (2012). Role of vacuolar invertase in regulating Arabidopsis stomatal opening. Acta Physiol. Plant. 34, 2449–2452. doi: 10.1007/s11738-012-1036-5 [DOI] [Google Scholar]
- Oh D.-H., Dassanayake M., Bohnert H. J., Cheeseman J. M. (2012). Life at the extreme: lessons from the genome. Genome Biol. 13:241. doi: 10.1186/gb4003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S., Bombarely A., Giavalisco P., Usadel B., Stephens C., Araguez I., et al. (2011). Demethylation of oligogalacturonides by FaPE1 in the fruits of the wild strawberry Fragaria vesca triggers metabolic and transcriptional changes associated with defence and development of the fruit. J. Exp. Bot. 62, 2855–2873. doi: 10.1093/jxb/erq465, PMID: [DOI] [PubMed] [Google Scholar]
- Osorio S., Castillejo C., Quesada M. A., Medina-Escobar N., Brownsey G. J., Suau R., et al. (2008). Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J. 54, 43–55. doi: 10.1111/j.1365-313X.2007.03398.x, PMID: [DOI] [PubMed] [Google Scholar]
- Pagnussat G. C., Yu H.-J., Ngo Q. A., Rajani S., Mayalagu S., Johnson C. S., et al. (2005). Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132, 603–614. doi: 10.1242/dev.01595, PMID: [DOI] [PubMed] [Google Scholar]
- Park S.-W., Vepachedu R., Sharma N., Vivanco J. M. (2004). Ribosome-inactivating proteins in plant biology. Planta 219, 1093–1096. doi: 10.1007/s00425-004-1357-8, PMID: [DOI] [PubMed] [Google Scholar]
- Parre E., Geitmann A. (2005). Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220, 582–592. doi: 10.1007/s00425-004-1368-5, PMID: [DOI] [PubMed] [Google Scholar]
- Peaucelle A., Braybrook S. A., Le Guillou L., Bron E., Kuhlemeier C., Höfte H. (2011a). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21, 1720–1726. doi: 10.1016/j.cub.2011.08.057, PMID: [DOI] [PubMed] [Google Scholar]
- Peaucelle A., Louvet R., Johansen J. N., Höfte H., Laufs P., Pelloux J., et al. (2008). Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18, 1943–1948. doi: 10.1016/j.cub.2008.10.065, PMID: [DOI] [PubMed] [Google Scholar]
- Peaucelle A., Louvet R., Johansen J. N., Salsac F., Morin H., Fournet F., et al. (2011b). The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 138, 4733–4741. doi: 10.1242/dev.072496, PMID: [DOI] [PubMed] [Google Scholar]
- Pelletier S., Van Orden J., Wolf S., Vissenberg K., Delacourt J., Ndong Y. A., et al. (2010). A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 188, 726–739. doi: 10.1111/j.1469-8137.2010.03409.x [DOI] [PubMed] [Google Scholar]
- Pelloux J., Rustérucci C., Mellerowicz E. J. (2007). New insights into pectin methylesterase structure and function. Trends Plant Sci. 12, 267–277. doi: 10.1016/j.tplants.2007.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez Y., Carneros E., Berenguer E., Solís M.-T., Bárány I., Pintos B., et al. (2019). Pectin de-methylesterification and AGP increase promote Cell Wall Remodeling and are required During somatic embryogenesis of Quercus suber. Front. Plant Sci. 9:1915. doi: 10.3389/fpls.2018.01915, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling J., Willmitzer L., Bücking H., Fisahn J. (2004). Inhibition of a ubiquitously expressed pectin methyl esterase in Solanum tuberosum L. affects plant growth, leaf growth polarity, and ion partitioning. Planta 219, 32–40. doi: 10.1007/s00425-004-1204-y, PMID: [DOI] [PubMed] [Google Scholar]
- Pontiggia D., Benedetti M., Costantini S., De Lorenzo G., Cervone F. (2020). Dampening the DAMPs: how plants maintain the homeostasis of cell wall molecular patterns and avoid hyper-immunity. Front. Plant Sci. 11:2010. doi: 10.3389/fpls.2020.613259, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. (1966). Separation and properties of potato invertase and invertase inhibitor. Arch. Biochem. Biophys. 113, 667–674. doi: 10.1016/0003-9861(66)90246-3, PMID: [DOI] [PubMed] [Google Scholar]
- Qin G., Zhu Z., Wang W., Cai J., Chen Y., Li L., et al. (2016). A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiol. 172, 1596–1611. doi: 10.1104/pp.16.01269, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T., Liu R. F., Wang W., An L. Z., Chen T., Liu G. X., et al. (2011). Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology 63, 111–117. doi: 10.1016/j.cryobiol.2011.07.003, PMID: [DOI] [PubMed] [Google Scholar]
- Raiola A., Camardella L., Giovane A., Mattei B., De Lorenzo G., Cervone F., et al. (2004). Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors11The industrial utilization of Arabidopsis and kiwi PMEIs is patent pending it, no. RM2003A000346. FEBS Lett. 557, 199–203. doi: 10.1016/S0014-5793(03)01491-1, PMID: [DOI] [PubMed] [Google Scholar]
- Raiola A., Lionetti V., Elmaghraby I., Immerzeel P., Mellerowicz E. J., Salvi G., et al. (2011). Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant-Microbe Interact. 24, 432–440. doi: 10.1094/MPMI-07-10-0157, PMID: [DOI] [PubMed] [Google Scholar]
- Rausch T., Greiner S. (2004). Plant protein inhibitors of invertases. Biochim. Biophys. Acta 1696, 253–261. doi: 10.1016/j.bbapap.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Rautengarten C., Usadel B., Neumetzler L., Hartmann J., Bussis D., Altmann T. (2008). A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J. 54, 466–480. doi: 10.1111/j.1365-313X.2008.03437.x, PMID: [DOI] [PubMed] [Google Scholar]
- Reca I. B., Brutus A., D’Avino R., Villard C., Bellincampi D., Giardina T. (2008). Molecular cloning, expression and characterization of a novel apoplastic invertase inhibitor from tomato (Solanum lycopersicum) and its use to purify a vacuolar invertase. Biochimie 90, 1611–1623. doi: 10.1016/j.biochi.2008.04.019, PMID: [DOI] [PubMed] [Google Scholar]
- Reca I. B., Lionetti V., Camardella L., D’Avino R., Giardina T., Cervone F., et al. (2012). A functional pectin methylesterase inhibitor protein (SolyPMEI) is expressed during tomato fruit ripening and interacts with PME-1. Plant Mol. Biol. 79, 429–442. doi: 10.1007/s11103-012-9921-2, PMID: [DOI] [PubMed] [Google Scholar]
- Rigano M. M., Lionetti V., Raiola A., Bellincampi D., Barone A. (2018). Pectic enzymes as potential enhancers of ascorbic acid production through the D-galacturonate pathway in Solanaceae. Plant Sci. 266, 55–63. doi: 10.1016/j.plantsci.2017.10.013, PMID: [DOI] [PubMed] [Google Scholar]
- Rocchi V., Janni M., Bellincampi D., Giardina T., D’Ovidio R. (2011). Intron retention regulates the expression of pectin methylesterase inhibitor (Pmei) genes during wheat growth and development. Plant Biol. 14, 365–373. doi: 10.1111/j.1438-8677.2011.00508.x [DOI] [PubMed] [Google Scholar]
- Röckel N., Wolf S., Kost B., Rausch T., Greiner S. (2008). Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 53, 133–143. doi: 10.1111/j.1365-313X.2007.03325.x, PMID: [DOI] [PubMed] [Google Scholar]
- Roitsch T., González M.-C. (2004). Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 9, 606–613. doi: 10.1016/j.tplants.2004.10.009, PMID: [DOI] [PubMed] [Google Scholar]
- Ruan Y.-L., Jin Y., Yang Y.-J., Li G.-J., Boyer J. S. (2010). Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol. Plant 3, 942–955. doi: 10.1093/mp/ssq044, PMID: [DOI] [PubMed] [Google Scholar]
- Saez-Aguayo S., Ralet M.-C., Berger A., Botran L., Ropartz D., Marion-Poll A., et al. (2013). PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25, 308–323. doi: 10.1105/tpc.112.106575, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A., Stintzi A., Rivas S., Serrano I., Chichkova N. V., Vartapetian A. B., et al. (2018). From structure to function - a family portrait of plant subtilases. New Phytol. 218, 901–915. doi: 10.1111/nph.14582, PMID: [DOI] [PubMed] [Google Scholar]
- Schwimmer S., Makower R. U., Rorem E. S. (1961). Invertase & invertase inhibitor in potato. Plant Physiol. 36, 313–316. doi: 10.1104/pp.36.3.313, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senechal F., Graff L., Surcouf O., Marcelo P., Rayon C., Bouton S., et al. (2014). Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann. Bot. 114, 1161–1175. doi: 10.1093/aob/mcu035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F., Habrylo O., Hocq L., Domon J.-M., Marcelo P., Lefebvre V., et al. (2017). Structural and dynamical characterization of the pH-dependence of the pectin methylesterase–pectin methylesterase inhibitor complex. J. Biol. Chem. 292, 21538–21547. doi: 10.1074/jbc.RA117.000197, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F., L’Enfant M., Domon J.-M., Rosiau E., Crépeau M.-J., Surcouf O., et al. (2015). Tuning of pectin methylesterification: pectin methylesterase inhibitor 7 modulates the processive activity of co-expressed pectin methylesterase 3 in a pH-dependent manner. J. Biol. Chem. 290, 23320–23335. doi: 10.1074/jbc.M115.639534, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senechal F., Mareck A., Marcelo P., Lerouge P., Pelloux J. (2015). Arabidopsis PME17 activity can be controlled by pectin methylesterase inhibitor4. Plant Signal. Behav. 10:e983351. doi: 10.4161/15592324.2014.983351, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Ren A., Tang X., Qi G., Xu Z., Chai G., et al. (2018). MYB52 negatively regulates pectin Demethylesterification in seed coat mucilage. Plant Physiol. 176, 2737–2749. doi: 10.1104/pp.17.01771, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y., Chane A., Jung M., Lee Y. (2021). Recent advances in understanding the roles of pectin as an active participant in plant Signaling networks. Plants 10:1712. doi: 10.3390/plants10081712, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J., González M.-C., Wolf S., Hofmann C., Greiner S., Du Y., et al. (2011). Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Pathol. 12, 247–262. doi: 10.1111/j.1364-3703.2010.00667.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Sanzana C., Celiz-Balboa J., Garzo E., Marcus S. E., Parra-Rojas J. P., Rojas B., et al. (2019). Pectin methylesterases modulate plant homogalacturonan status in defenses against the aphid Myzus persicae. Plant Cell 31, 1913–1929. doi: 10.1105/tpc.19.00136, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecka D., Żebrowski J., Kacperska A. (2008). Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 101, 521–530. doi: 10.1093/aob/mcm329, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J., et al. (2004). Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211. doi: 10.1126/science.1102765, PMID: [DOI] [PubMed] [Google Scholar]
- Sorek N., Szemenyei H., Sorek H., Landers A., Knight H., Bauer S., et al. (2015). Identification of MEDIATOR16 as the Arabidopsis COBRA suppressor MONGOOSE1. Proc. Natl. Acad. Sci. U. S. A. 112, 16048–16053. doi: 10.1073/pnas.1521675112, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. F., Kragh K. M., Sibbesen O., Delcour J., Goesaert H., Svensson B., et al. (2004). Potential role of glycosidase inhibitors in industrial biotechnological applications. Biochim. Biophys. Acta Proteins Proteom. 1696, 275–287. doi: 10.1016/j.bbapap.2003.09.016, PMID: [DOI] [PubMed] [Google Scholar]
- Srivastava S., Gupta S. M., Sane A. P., Nath P. (2012). Isolation and characterization of ripening related pectin methylesterase inhibitor gene from banana fruit. Physiol. Mol. Biol. Plants 18, 191–195. doi: 10.1007/s12298-012-0102-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanowicz K., Szymanska-Chargot M., Truman W., Walerowski P., Olszak M., Augustyniak A., et al. (2021). Plasmodiophora brassicae-triggered cell enlargement and loss of cellular integrity in root systems are mediated by pectin demethylation. Front. Plant Sci. 12:1569. doi: 10.3389/fpls.2021.711838, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Han M., Min J., Zhou H., Zhang Q., Zhao J., et al. (2020). Functional characterization of invertase inhibitors PtC/VIF1 and 2 revealed their involvements in the defense response to fungal pathogen in Populus trichocarpa. Front. Plant Sci. 10:1654. doi: 10.3389/fpls.2019.01654, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Wolf S., Han M., Zhao H., Wei H., Greiner S., et al. (2016). Reassessment of an Arabidopsis cell wall invertase inhibitor AtCIF1 reveals its role in seed germination and early seedling growth. Plant Mol. Biol. 90, 137–155. doi: 10.1007/s11103-015-0402-2, PMID: [DOI] [PubMed] [Google Scholar]
- Takahashi D., Gorka M., Erban A., Graf A., Kopka J., Zuther E., et al. (2019). Both cold and sub-zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana. Sci. Rep. 9:2289. doi: 10.1038/s41598-019-38688-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Su T., Han M., Wei L., Wang W., Yu Z., et al. (2017). Suppression of extracellular invertase inhibitor gene expression improves seed weight in soybean (Glycine max). J. Exp. Bot. 68, 469–482. doi: 10.1093/jxb/erw425, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin A. S., Giardina T. (2014). Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 5:293. doi: 10.3389/fpls.2014.00293, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur B. R., Singh R. K., Tieman D. M., Handa A. K. (1996). Tomato product quality from transgenic fruits with reduced pectin methylesterase. J. Food Sci. 61, 85–87. doi: 10.1111/j.1365-2621.1996.tb14731.x [DOI] [Google Scholar]
- Tian G.-W., Chen M.-H., Zaltsman A., Citovsky V. (2006). Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 294, 83–91. doi: 10.1016/j.ydbio.2006.02.026, PMID: [DOI] [PubMed] [Google Scholar]
- Tieman D. M., Handa A. K. (1994). Reduction in pectin Methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 106, 429–436. doi: 10.1104/pp.106.2.429, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman D. M., Harriman R. W., Ramamohan G., Handa A. K. (1992). An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell 4, 667–679. doi: 10.2307/3869525, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbant A., Fournet F., Lequart M., Zabijak L., Pageau K., Bouton S., et al. (2016). PME58 plays a role in pectin distribution during seed coat mucilage extrusion through homogalacturonan modification. J. Exp. Bot. 67, 2177–2190. doi: 10.1093/jxb/erw025, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi C., Janni M., Lionetti V., Bellincampi D., Favaron F., D’Ovidio R. (2011). The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol. Plant-Microbe Interact. 24, 1012–1019. doi: 10.1094/MPMI-01-11-0021, PMID: [DOI] [PubMed] [Google Scholar]
- Wachsman G., Zhang J., Moreno-Risueno M. A., Anderson C. T., Benfey P. N. (2020). Cell wall remodeling and vesicle trafficking mediate the root clock in Arabidopsis. Science 370, 819–823. doi: 10.1126/science.abb7250, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ruan Y.-L. (2012). New insights into roles of cell wall invertase in early seed development revealed by comprehensive spatial and temporal expression patterns of GhCWIN1 in cotton. Plant Physiol. 160, 777–787. doi: 10.1104/pp.112.203893, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yeats T. H., Uluisik S., Rose J. K. C., Seymour G. B. (2018). Fruit softening: revisiting the role of pectin. Trends Plant Sci. 23, 302–310. doi: 10.1016/j.tplants.2018.01.006, PMID: [DOI] [PubMed] [Google Scholar]
- Wang M., Yuan D. J., Gao W. H., Li Y., Tan J. F., Zhang X. L. (2013). A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS One 8:e72082. doi: 10.1371/journal.pone.0085650, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Deinlein U., Fischer S., Rogowski M., Geimer S., Tenhaken R., et al. (2013). A mutation in the Arabidopsis thaliana cell wall biosynthesis gene pectin methylesterase 3 as well as its aberrant expression cause hypersensitivity specifically to Zn. Plant J. 76, 151–164. doi: 10.1111/tpj.12279, PMID: [DOI] [PubMed] [Google Scholar]
- Willats W. G., Orfila C., Limberg G., Buchholt H. C., van Alebeek G. J., Voragen A. G., et al. (2001). Modulation of the degree and pattern of methyl esterification of pectic homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties and cell adhesion. J. Biol. Chem. 276, 19404–19413. doi: 10.1074/jbc.M011242200, PMID: [DOI] [PubMed] [Google Scholar]
- Wolf S., Grsic-Rausch S., Rausch T., Greiner S. (2003). Identification of pollen-expressed pectin methylesterase inhibitors in Arabidopsis. FEBS Lett. 555, 551–555. doi: 10.1016/S0014-5793(03)01344-9, PMID: [DOI] [PubMed] [Google Scholar]
- Wolf S., Mravec J., Greiner S., Mouille G., Hofte H. (2012). Plant cell wall homeostasis is mediated by Brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737. doi: 10.1016/j.cub.2012.07.036, PMID: [DOI] [PubMed] [Google Scholar]
- Wolf S., Rausch T., Greiner S. (2009). The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 58, 361–375. doi: 10.1111/j.1365-313X.2009.03784.x, PMID: [DOI] [PubMed] [Google Scholar]
- Won S.-K., Lee Y.-J., Lee H.-Y., Heo Y.-K., Cho M., Cho H.-T. (2009). cis-Element- and transcriptome-based screening of root hair-specific genes and their functional characterization in Arabidopsis. Plant Physiol. 150, 1459–1473. doi: 10.1104/pp.109.140905, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-C., Bulgakov V. P., Jinn T.-L. (2018). Pectin methylesterases: cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 9:1612. doi: 10.3389/fpls.2018.01612, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-C., Huang Y.-C., Stracovsky L., Jinn T.-L. (2017). Pectin methylesterase is required for guard cell function in response to heat. Plant Signal. Behav. 12:e1338227. doi: 10.1080/15592324.2017.1338227, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Cao H., Xu E., Zhang S., Hu Y. (2018). Genome-wide identification of Arabidopsis LBD29 target genes reveals the molecular events behind auxin-induced cell reprogramming during callus formation. Plant Cell Physiol. 59, 749–760. doi: 10.1093/pcp/pcx168, PMID: [DOI] [PubMed] [Google Scholar]
- Xu X., Hu Q., Yang W., Jin Y. (2017). The roles of cell wall invertase inhibitor in regulating chilling tolerance in tomato. BMC Plant Biol. 17:195. doi: 10.1186/s12870-017-1145-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., He H., Fang L., Zhang A. (2018). Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 496, 497–501. doi: 10.1016/j.bbrc.2018.01.025, PMID: [DOI] [PubMed] [Google Scholar]
- Yang Z. (2008). Cell polarity Signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol. 24, 551–575. doi: 10.1146/annurev.cellbio.23.090506.123233, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Chen S., Cheng Y., Zhang N., Ma Y., Wang W., et al. (2020). Cell wall/vacuolar inhibitor of fructosidase 1 regulates ABA response and salt tolerance in Arabidopsis. Plant Signal. Behav. 15:1744293. doi: 10.1080/15592324.2020.1744293, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanor M. I., Osorio S., Nunes-Nesi A., Carrari F., Lohse M., Usadel B., et al. (2009). RNA interference of LIN5 in tomato confirms its role in controlling brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for Normal fruit development and fertility. Plant Physiol. 150, 1204–1218. doi: 10.1104/pp.109.136598, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehra A., Raytekar N. A., Meena M., Swapnil P. (2021). Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: a review. Curr. Res. Microb. Sci. 2:100054. doi: 10.1016/j.crmicr.2021.100054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. Y., Feng J., Wu J., Wang X. W. (2010). BoPMEI1, a pollen-specific pectin methylesterase inhibitor, has an essential role in pollen tube growth. Planta 231, 1323–1334. doi: 10.1007/s00425-010-1136-7, PMID: [DOI] [PubMed] [Google Scholar]
- Zocca F., Lomolino G., Spettoli P., Lante A. (2008). A study on the relationship between the volatile composition of moscato and prosecco grappa and enzymatic activities involved in its production. J. Federat. Inst. Brew. 114, 262–269. doi: 10.1002/j.2050-0416.2008.tb00337.x [DOI] [Google Scholar]
- Zuma B., Dana M. B., Wang D. (2018). Prolonged expression of a putative invertase inhibitor in micropylar endosperm suppressed embryo growth in Arabidopsis. Front. Plant Sci. 9:61. doi: 10.3389/fpls.2018.00061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]