Abstract
Context
Conflicting practice-based dietary recommendations are sometimes given to patients with inflammatory bowel disease (IBD); whereas intake of fiber should increase during remission, it should be avoided during relapse. Moreover, European countries set daily requirements of total fiber and do not specify any types.
Objective
This systematic review appraised data from randomized clinical trials (RCTs) of the types of fibers beneficial for patients in the treatment of IBD to guide dietary fiber advice.
Data Sources
The PubMED database was searched following PRISMA guidelines.
Data Extraction
RCTs evaluating the effects of any type of fiber on clinical and physiological outcomes in patients with IBD were assessed. Quality assessment of the selected full-text articles was conducted using the Cochrane Risk of Bias Tool.
Data Analysis
Eight studies were included reporting on 5 types of fibers. In 2 RCTs, germinated barley foodstuff (GBF) was shown to lower pro-inflammatory cytokines and clinical disease activity scores. Fructo-oligosaccharides (FOS) were demonstrated to lower IBD Questionnaire scores (lower well-being), in contrast to inulin, which decreased disease activity scores. An RCT could not find lower remission rates in the psyllium treatment group, while another RCT reported that administration led to less symptoms in patients. In RCTs, no concrete evidence was found that wheat bran improves disease course.
Conclusions
Although the evidence is sparse, GBF and inulin seem propitious and merit further exploration. Evidence on wheat bran and psyllium is still too limited. Adequately powered long-term human RCTs with objective outcomes are needed to improve dietary advice on types of fiber in IBD.
Keywords: dietary fibers, dietary recommendations, Inflammatory Bowel Disease (IBD)
INTRODUCTION
Increasing dietary fiber intake is considered to be beneficial for maintaining periods of remission in patients with inflammatory bowel disease (IBD).1 IBD is a chronic-relapsing disorder with an incapacitating nature and includes Crohn’s disease (CD)2 and ulcerative colitis (UC).3 When patients are experiencing a flare-up of their disease, avoidance of dietary fiber intake is often recommended in practice, as it is to patients with stricturing disease.4,5 However, these practice-based recommendations are hardly evidence-based and have previously been questioned.4,6,7 In addition, remission rates have not been found to differ between exclusive enteral nutrition (EEN) formulas with or without fiber.8 Furthermore, most European countries only make recommendations about the total daily amount of fiber that should be taken, and do not advise on the specific types of fiber that should preferably be consumed.9 Therefore, it remains unclear for patients, physicians, and dietitians which specific fiber-rich food items should be recommended or avoided.
Furthermore, the pathophysiology of developing IBD and the factors related to a more complicated disease course have not been fully elucidated, making the process of developing evidence-based dietary guidelines even more complex.10 This lack of dietary guidelines encourages the IBD patient to start experimenting with their habitual dietary intake (guided or unguided by an expert). When dietary experiments are not properly supported, they can have a detrimental effect on disease course, with unintended side effects, eg, micronutrient deficiencies.11 This underscores the need for evidence-based dietary guidelines for patients with IBD.
Although not always defined as such, dietary fiber, ie, indigestible plant components, has been consumed for centuries.12 Thousands of years ago, when people were still gathering foods, dietary fiber intake was estimated to be 100–120 g/day. This intake has dropped, in recent times, with the increasing intake of processed foods – nowadays only an average of 10–20 g/day is consumed.13 Dietary fibers can be divided into two basic groups: insoluble fibers – including cellulose, hemicellulose, and lignin, and soluble fibers – such as pectins, gums, and mucilages, which become gummy when added to water.12 Furthermore, these fibers can be classified based on function. Bulking fibers such as cellulose, hemicellulose, and psyllium absorb water and therewith promote bowel peristalsis.14 Viscous fibers such as psyllium and β-glucans thicken the fecal mass and are believed to have an influence at the metabolic level by decreasing blood cholesterol or glucose levels.15,16 Fermentable fibers such as resistant starch and inulin are metabolized into short-chain fatty acids (SCFAs), and those SCFAs potentially affect the large intestine via microbiome-dependent or -independent mechanisms.17 Butyrate, for example, plays a role in IBD; impaired butyrate metabolism is linked with mucosal damage and inflammation in IBD patients.18 The proposed advantages of consuming dietary fibers depend on the type of fiber and its properties.
Beneficial effects of fibers have already been demonstrated in a number of animal studies. For example, mice were less affected by colitis if they ingested a wheat bran (WB)-enriched diet compared with a resistant starch diet, or a control group.19 Furthermore, Araki et al20 evaluated the effect of administration of germinated barley foodstuff (GBF) to rats with dextran sulfate sodium –induced colitis and found that it effectively prevented bloody diarrhea and mucosal damage when compared with control rats. Moreover, psyllium supplementation has been shown to reduce the severity of colitis in specific pathogen-free mice via microbiota-dependent and microbiota-independent mechanisms.21 In other studies, it was found that both fructo-oligosaccharides (FOSs) and inulin can influence the microbiome; inulin was demonstrated to 2.3-fold increase the number of Bifidobacteria22as were FOSs.23 Prebiotic fermentation of these dietary fibers resulted in the production of short-chain fatty acids such as butyrate, a decrease in colonic pH, and stimulation of lactate production.24,25 These prebiotic properties of FOSs are thought to be necessary to reduce trinitrobenzenesulfonic acid (TNBS)- induced colitis in rats.24
Building on learnings from animal studies, it is believed that specific dietary fibers play a role in the treatment of IBD in humans as well. Nevertheless, currently, dietary guidelines lack recommendations on the specific types of fibers that should preferably be consumed.9 Therefore, this systematic review aims to appraise the available data from randomized clinical trials on the use of any type of fibers in the treatment of IBD patients.
MATERIALS AND METHODS
Definition of fiber
The definition of fiber has been subject of much discussion over the years. Stephen et al9 made an effort to summarize all of the different definitions used around the world. The Codex Alimentarius26 defines dietary fiber as not being digested or absorbed in the human intestine, and made up of carbohydrate polymers, including (1) nonstarch polysaccharides (NSPs), (2) resistant (nondigestible) oligosaccharides (ROs) and (3) resistant starch (RS).
Search strategy
PRISMA guidelines27 were followed. The American Association of Cereal Chemists provides an overview of the constituents of dietary fiber, and these were used as search terms.28 The PUBMed database was searched combining the following search areas: disease type, types of fibers, and disease status. To define disease type, the following search terms were used: inflammatory bowel disease, Crohn’s disease, and ulcerative colitis. To identify as many different types of fibers as possible, the abovementioned descriptions of dietary fiber were supplemented with commonly used terms found by a manual search of literature. Eventually, the following search terms were included: β-glucans, (poly-)fructans, psyllium, wheat bran, oat bran, germinated barley, non-digestible oligosaccharides, non-starch polysaccharides, (hemi-/methyl-/hydroxypropylmethyl-) cellulose, arabinoxylans, arabinogalactans, inulin, fructo-oligosaccharides, galacto-oligosaccharides, gum, mucilages, pectins, analogous carbohydrates, indigestible dextrins, resistant maltodextrins, resistant potato dextrins, synthesized carbohydrate compounds, polydextrose, indigestible starch, lignin, wax, phytate, cutin, saponin, suberin, tannin, and dietary fiber. The following search terms were used to identify articles discussing disease status as clinically relevant end points: exacerbations, flares, remission, clinical parameters, Mayo, C(D)AI, SCCAI, HBI, fecal calprotectin, disease activity, CRP, ESR, endoscopy, sigmoidoscopy, and colonoscopy. The search terms, according to PICOS structure (Participants, Intervention, Comparators, Outcomes, Study design), for disease type (“Participants”), types of fibers (“Intervention”) and disease status (“Outcomes”) were combined using the Boolean operator “AND”, and the search terms within those 3 categories were combined by using “OR”. The search was restricted to human studies with a randomized controlled trial (RCT) design (“Study design”) in the English, German, or Dutch language. The last literature search was completed in June 2020, Table 1.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Criterion |
|---|---|
| Participants | Patients with disease type: “Inflammatory bowel disease” OR “Crohn’s disease” OR “Ulcerative colitis” |
| Intervention | Fiber under study: {(“β-glucan” [Title/Abstract]) OR (“β -glucans” [Title/Abstract])} OR
|
| Comparators | |
| Outcomes | Disease status: “Exacerbation” [Title/Abstract] OR “Flare” [Title/Abstract] OR “Flares” [Title/Abstract] OR “Clinical remission” [Title/Abstract] OR “Remission” [Title/Abstract] OR “Clinical outcome” [Title/Abstract] OR “Clinical parameter” [Title/Abstract] OR “Mayo” [Title/Abstract] OR “CDAI” [Title/Abstract] OR “Crohn’s Disease Activity Index” [Title/Abstract] OR “CAI” [Title/Abstract] OR “Colitis Activity Index” [Title/Abstract] OR “SCCAI” [Title/Abstract] OR “Simple Clinical Colitis Activity Index” [Title/Abstract] OR “HBI” [Title/Abstract] OR “Harvey Bradshaw Index” [Title/Abstract] OR “Fecal calprotectin” [Title/Abstract] OR “Faecal calprotectin” [Title/Abstract] OR “Disease activity” [Title/Abstract] OR “CRP” [Title/Abstract] OR “ESR” [Title/Abstract] OR Endoscop* [Title/Abstract] OR Sigmoidoscop* [Title/Abstract] OR Colonoscop* [Title/Abstract] |
| Study design | Randomized controlled trial and report in English, German, or Dutch language. |
The PubMed search terms have been used to describe the inclusion criteria.
Selection of articles
No review protocol is available online, and therefore the procedure will be described in detail here. The following predetermined criteria were used to evaluate each study for inclusion: participants previously diagnosed with IBD (CD or UC); participants undergoing a clinical intervention with a specific type of fiber; and evaluation of disease status or clinical outcomes, fecal microbiome, or immunological parameters before and after intervention. Studies in which the effects of types of fibers could not be determined (eg, when interventions were combined with other nonfiber interventions) were excluded. However, due to the low number of studies that could be included, interventions combining multiple fibers were included. All studies were independently reviewed by two authors (V.P. and M.C.).
Data extraction
From all articles that met the inclusion criteria, data was extracted; information on the PICOS topics were gathered using a standardized collection form. In the context of the “Participants”, the following items were subtracted from the full-text articles: location, clinical setting (inpatients or outpatients), disease type, disease stage or severity, number of patients recruited, number of patients evaluated, age, and gender. For “Intervention”, the following items are reported: fiber source, type of intervention, dose, period of administration, duration of follow-up, type of blinding, presence of co-administered medication, and compliance (method). For “Comparators” we collected: comparison method (ie, placebo, standard therapy) and number of patients in the comparator group. For “Outcome”, the form was structured as follow: nutritional intake, clinical symptoms, quality of life, clinical disease activity scores, fecal calprotectin, microbiome or stool parameters, biomarkers, histopathological and immunological parameters, endoscopy scores, and disease activity status (relapse/remission). In the context of “Study design”, the following items were pooled: study design and language.
Quality assessment
The quality assessment was conducted using the Cochrane Collaboration’s tool29 for assessing risk of bias in randomized trials (Risk of Bias 2 – ROB230) Studies examined by ROB2 were classified into 3 categories: low risk, some concerns, or high risk. The results were visualized using the ROBvis31 tool. Each article was assessed for bias in 5 domains: bias arising from the randomization process, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results.
RESULTS AND DISCUSSION PER FIBER TYPE
Study retrieval
A total of 186 articles were retrieved from the PUBMed database. After screening of titles and abstracts, 19 articles were selected for full-text evaluation. Next, 11 articles were excluded because they either combined multiple interventions (fiber with nonfibers), the study design was not eligible (nonrandomized or noncontrolled set-up), the full-text reading revealed that the article did not fit the PICO question, or the full text was not available (see Figure 1). Eventually, 8 RCTs were included (1 in CD and 7 in UC).
Figure 1.
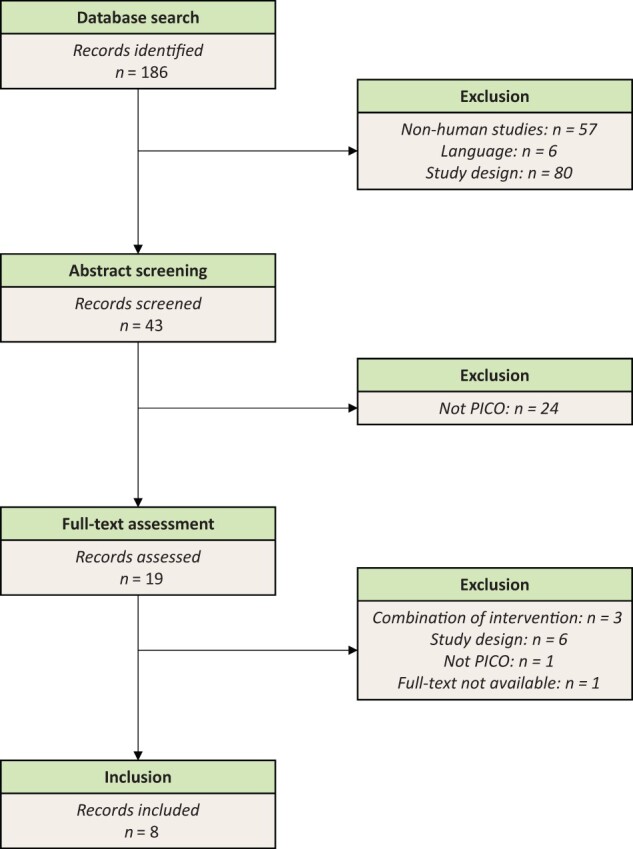
Flow diagram of the literature search process. Language = other than English, German, or Dutch. PICOS = Participants, Intervention, Comparators, Outcomes, Study design
Assessment of the study quality
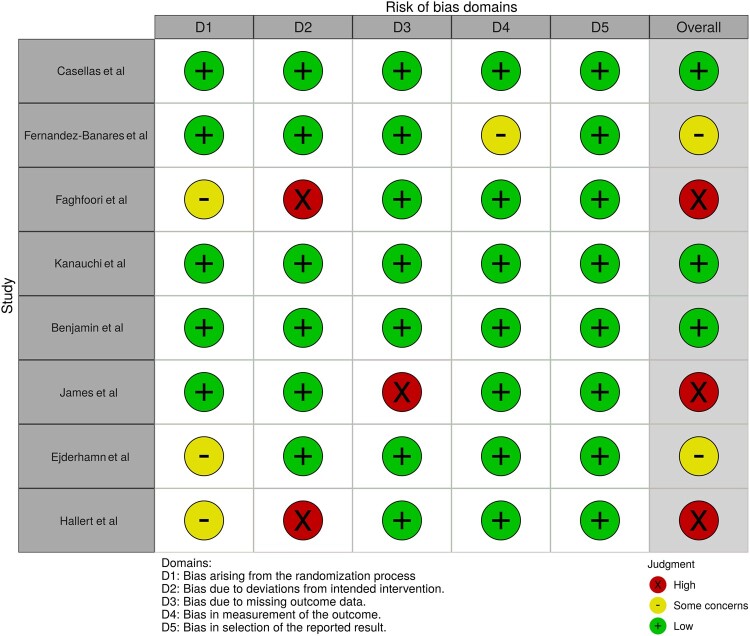
Only one study was assessed as having low risk of bias (green crossed circle symbol);32 some concerns (orange crossed circle symbol)25,33,34 were reported on another 3; and another 4 were evaluated as having high risk of bias (red crossed circle symbol).35–38 A detailed quality assessment is shown for each study in Figure 2 (or colorblind-friendly, see Figure S1 in the Supporting Information online) and see Table S1 in the Supporting Information online.
Figure 2.
Risk of bias assessment
Characteristics of the included studies
The selected RCTs were classified according to the type of fiber they reported on; WB (2 studies), GBF (2 studies), psyllium (3 studies), FOSs (1 study), and inulin (1 study). These studies will be discussed under the headings of the fiber type being researched, as summarized in Table 2.25,32–38 A general discussion will follow.
Table 2.
Summary of findings for randomized controlled trials
|
Study details
|
Patient details
|
Intervention details
|
Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference, year, country | Type of fiber | Design | CCRBT | Number recruited and evaluated | Male:Female | Age | Disease type and status | Intervention | Duration | Concomitant medication | Compliance check | Clinical, microbiome, and immunological outcomes |
| James et al, 2014,37 Australia | WB-associated fiber and amylose-associated RS | Single-blinded, randomized, controlled cross-over | Red crossed circle symbol |
|
|
>18 | Ulcerative colitis patients in remission and healthy controls |
|
48 days: 2 × 17 days; doses were increased incrementally over 3 days to increase tolerance, then full doses were given for 14 days, and 14-day wash-out period between periods | Only standard therapy with mesalazine was allowed | Yes, combination of returned foods, checklists and diary entries |
|
| Ejderhamn et al, 1991,34 Sweden | Wheat fiber and ispaghula | Double-blinded, randomized, placebo (wash-out) cross-over | Yellow crossed circle symbol |
|
NS | 10–18 | Ulcerative colitis patients in remission |
|
|
Standard therapy (sulfasalazine 2–3 g/d) | Yes, dietary records and unpredicted counts of sachets at irregular times during the course of the study |
|
| Hallert et al, 1991,38 Sweden | Ispaghula husks | Double-blinded, randomized, placebo cross-over | Red crossed circle symbol |
Recruited: 36 Evaluated: 29 |
14:22 | 20–75 | Ulcerative colitis patients in remission |
|
|
Standard therapy: 25% were receiving medication regularly (70% sulfasalazine) | NS | Symptoms: during treatment, the intervention group reported generally less symptoms while taking ispaghula compared with patients taking placebo (P < 0.001). At baseline, patients reported 5.9 (SEM, 0.20) symptoms (out of a possible 8). Both treatments caused a reduction: 26% by ispaghula (4.3, SEM 0.39, P < 0.001), 16% by placebo (5.0, SEM 0.36, P < 0.05), with no significant difference between intervention and placebo. |
| Fernández-Bañares et al, 1999,35 Spain | PO seeds | Open-label, randomized, controlled (parallel group) | Red crossed circle symbol |
|
|
18–70 | Ulcerative colitis patients in remission |
|
12 mo | NA | Yes, unused sachet count at each study visit |
|
| Faghfoori et al, 2011,36 Iran | GBFs | Open-label, randomized, controlled (parallel group) | Red crossed circle symbol | Evaluated:
|
26:25 | >18 | Ulcerative colitis patients in remission |
|
2 mo | Standard therapy; not specified | NS | Immunological parameters: between the pretreatment serum values of TNF-α (P = 0.28), IL-6 (P = 0.19) and IL-8 (P = 0.6) there was no significant difference, when comparing patients in the treatment arm and the control arm. TNF-α: decreased after intervention in the treatment group (P = 0.18) and increased in the control group (P = 0.08). IL-6: a significant reduction was seen after the intervention in the treatment group (P = 0.034), but there was an increase in the control group (P = 0.46). IL-8: decreased in the treatment group (P = 0.013) after intervention, but there was an increase in the control group (P = 0.35). |
| Kanauchi et al, 2002,32 Japan | GBFs | Open-label, randomized, controlled (parallel group) | Green crossed circle symbol |
|
NS | >18 | Ulcerative colitis patients with mild to moderate active disease |
|
4 wks | NS | NS |
|
| Benjamin et al, 2011,25 UK | FOSs | Double-blinded, randomized, placebo | Yellow crossed circle symbol |
|
|
>18 | Crohn’s disease patients with active disease |
|
4 wks | The maximum permissible steroid dose was 20 mg/d. | Yes, sachet count at end of study period |
|
| Casellas et al, 2007,33 Spain | Inulin | Double-blinded, randomized, placebo, pilot | Yellow crossed circle symbol |
|
|
18–75 | Ulcerative colitis patients with mild to moderate active disease |
|
2 wks | Mesalazine (3 g/d) | Yes, bringing study medication to visits |
|
The Cochrane Risk-of-Bias Tool (CCRBT) was used to assess the risk of bias in studies – overall judgment for randomized trials (ROB2): green crossed circle symbol = low risk, orange crossed circle symbol = some concerns, red crossed circle symbol = high risk; see Figure S1 in the Supporting Information online for detailed quality assessment. FOSs, fructo-oligosaccharides; GBF, germinated barley foodstuff; G1, Group 1; G2, Group 2; G3, Group 3; NS, not specified; PO, Plantago ovata; RS, resistant starch; SCFAs, short-chain fatty acids; WB, wheat bran.
Wheat bran. WB is a by-product of milling wheat flour. WB contains soluble polysaccharides and a high proportion of water-insoluble arabinoxylans and lignin,39 and it reduces the intestinal transit time and increases the fecal bulk.
In our systematic review, two RCTs report on WB. Both studied WB but in combination with another element: respectively, amylose-associated resistant starch (RS) and psyllium, both of which are also dietary fibers.
James et al37 evaluated 14 UC patients in remission and 10 healthy controls in a single-blinded randomized controlled cross-over study. In both of the parallel groups (UC patients and healthy controls), the intervention consisted of a “low RS/WB” diet containing 2–5 g RS and 2–5 g WB per day , alternated with a “high RS/WB” diet containing 15 g RS and 12 g WB per day, or the diet as described in reverse order. The RS and WB were added to bread, cereals, and muffins. The dose was increased incrementally over 3 days to build up tolerance, then full doses were given for 14 days. There was a wash-out period of 14 days between both interventions. Other than the supplementation, patients continued their habitual intake during the study period. During the intervention, only standard therapy with mesalazine was allowed. Compliance was checked by returned foods, checklists, and food diaries. Disease activity was measured by the Clinical Activity Index (CAI), and scores were compared between the two treatment arms both at baseline and during the intervention with both high and low RS/WB. The UC group reported numerically more clinical symptoms than controls, but no difference was found between the treatment arms. During the high RS/WB dietary intervention, the fecal output of starch (P = 0.002) more than doubled in healthy controls but did not change in patients with UC, while the output of non-starch polysaccharides was increased in both the healthy (P = 0.015) and UC (P = 0.008) subjects. In healthy controls, whole gut transit time was shorter during the high WB/RS compared with during the low WB/RS (P = 0.024), whereas no difference was observed in patients with UC. Furthermore, the microbiota composition was different between patients and controls; UC patients had more diverse microbiota in their Clostridium cluster XIVa (irrespective of the treatment arm) and a lower proportion of Akkermansia muciniphila. No relative or absolute change in abundance was demonstrated in either patients or controls after increasing the intake of RS/WB. To conclude, James et al found that gut fermentation of nonstarch polysaccharides and starch is diminished in patients with UC. Nevertheless, they could not explain this finding by abnormal gut transit, and it was not corrected by increasing RS/WB intake. Hence, it may be due to abnormal functioning of the gut microbiota in UC patients.
Ejderhamn et al34 studied 10 children (age 10–18) with UC in remission in a randomized double-blind cross-over study for 18 months. The patients received a dietary intervention with either 16 g wheat fiber or 16.1 g psyllium for 6 months. This period was followed by a wash-out period of 6 months and then followed by another 6-month intervention with the opposite intervention. Compliance was checked using dietary records and unpredicted sachet counts. Standard therapy (sulfasalazine 2–3 g/d) was co-administered during the trial. The study showed that fecal mass did not change significantly after daily supplementation with either wheat fiber or psyllium. Some decreases in microbiota strings were reported: Bacteroides (P < 0.05) and Enterococcus faecium (P < 0.05) after wheat fiber supplementation, and Bacillus (P < 0.05) after psyllium supplementation.
To summarize, although in two RCTs reporting on WB no improvement in objective clinical end points was shown, some changes in the microbiota string were demonstrated in those studies. Despite no concrete treatment strategies being discovered (yet), these finding are potential leads for future research.
Germinated barley foodstuff. GBF is made from malt (by a process of sieving and milling to extract the endosperm of barley after germination), and it consists mainly of water-insoluble (hemicellulose-rich) dietary fiber and glutamine-rich protein, and has prebiotic properties.40 The fiber fraction has a high water-holding capacity and hence modulates stool water content.41 Barley ranks fourth of cereal production worldwide, with 144 million tons (in 2013), and it is used as cattle feed or (after malting) as basis for alcoholic and non-alcoholic drinks.42 Germination of barley is a natural, biological processing strategy that is utilized to enhance the nutritional, functional, and sensory characteristics of cereal grains and increase micronutrients.43
In our systematic review, two RCTs reported on GBF. Faghfoori et al36 evaluated the effect of GBF by oral administration to 41 patients with UC in remission. For 2 months, 20 patients received 30 g/day GBF plus standard therapy, and 21 patients received standard therapy only. After the intervention, IL-6 and IL-8 were significantly reduced in the treatment group (IL-6: P = 0.034, IL-8: P = 0.013), whereas in the control group a nonsignificant increase was reported (IL-6: P = 0.46, IL-8: P = 0.35).
Kanauchi et al32 studied 18 patients with mild to moderately active UC. In this 4-week trial, patients were randomized to receive either 20–30 g GBF plus baseline treatment or baseline therapy only. Whereas at baseline no significant difference was seen between groups, after 4 weeks of intervention the clinical activity index score44 (which summarizes: [a] the number of episodes of diarrhea, [b] the presence of nocturnal diarrhea, [c] the degree of visible blood in the stools, [d] the presence of fecal incontinence, [e] the degree of abdominal pain or cramping, [f] general well-being, [g] the degree of abdominal tenderness, and [h] the need for antidiarrheal drugs)) was significantly lower in the treatment group compared with the control group (P < 0.05). Furthermore, endoscopy after 4 weeks of GBF supplementation showed that erythema, granularity, and erosion were dramatically attenuated compared with endoscopy at baseline.
To conclude, in two RCTs, GBF was shown to lower pro-inflammatory cytokines (IL-6 and IL-8) and clinical disease activity scores. Although the evidence is sparse, GBF might be of interest for further investigation in larger trials.
Psyllium. Psyllium is a medicinal plant that is scientifically known as Plantago ovata. Psyllium is a commonly used name for several members of the plant genus Plantago. In addition, psyllium husk and ispaghula husk are other names used to describe this plant.45 Psyllium stimulates large bowel regulation and is sold (over the counter) as a natural laxative. It contains a mixture of 65% insoluble (cellulose, hemicellulose, and lignin) and 35% soluble fibers.46 Psyllium is predominantly a soluble fiber; due to its powerful ability to form a gel in water, psyllium is classified as a mucilaginous fiber.46
In our systematic review, three RCTs reported on psyllium. Moreover, Ejderhamn et al34 combined researching psyllium with wheat fiber in a cross-over trial, and this study is therefore already described under the “Wheat bran” heading.
Hallert et al38 investigated psyllium in a double-blind randomized placebo-controlled cross-over study in 29 UC patients in remission, in which patients received a dietary intervention with psyllium (ispaghula husk) supplementation (4 g rough granules containing 3.52 g psyllium, 88% gel-forming dietary fiber) for 2 months and afterwards a placebo (crushed crispbread with a low content [17.3%] of non-gel-forming dietary fiber) twice daily for 2 months (or in reverse order). Standard therapy was allowed and reported. Patients reported generally less symptoms38 (lower scores on all 8 scales measuring [1] abdominal pain, [2] diarrhea, [3] loose stools when pain was present, [4] urgency when pain was present, [5] bloating, [6] incomplete evacuation, [7] mucus discharge, and [8] constipation) during the treatment with ispaghula husk compared with placebo (P < 0.001).
Fernández-Bañares et al35 evaluated the effect of psyllium in a 12-months open-label randomized controlled trial with 94 UC patients in remission. The first group (n = 32) received 20 g/day psyllium (Plantago ovata seeds), the second group (n = 35) 1.5 g/day mesalazine, and the third group (n = 27) both, meaning 20 g/day psyllium plus 1.5 g/day mesalazine. Compliance was checked by counting unused sachets at each study visit. There was no difference in the maintenance of remission over 1 year between the 3 intervention groups (Mantel-Cox test P = 0.67, Breslow test P = 0.58). Furthermore, butyrate concentrations were significantly increased, comparing baseline concentrations with post-intervention concentrations of patients in the psyllium group, and there was a trend towards increasing acetate and total SCFA concentrations.
To conclude, while an RCT reported that administration of psyllium led to less clinical symptoms in patients, another RCT could not find lower remission rates in the psyllium treatment group. These findings require further research.
Fructans. Fructo-oligosaccharides are inulin-type fructans with a short chain length and are mainly produced by partial enzymatic hydrolysis of inulin.47 Fructans are often used as functional ingredients by the food industry to modify the texture and taste due to their properties as gelling agents, fat substitutes, soluble dietary fiber, and low-calorie sweeteners.48 Both inulin and FOSs are among the most widely used prebiotic fibres in the food industry.47 Inulin is delivered largely intact into the colon and is thought to have a bifidogenic effect there, meaning that fecal and mucosal bifodobacteria and fecal Faecalibacterium prausnitzii are selectively stimulated by oligofructose.22,49–51 Natural sources of fructans are plant foods such as agave, artichokes (16–20 g/100 g), asparagus, chicory root (15–20 g/100 g), garlic (10–16 g/100 g), leeks, onions (including spring onions), and wheat (1–4 g/100 g).52 Most of our dietary inulin intake (>90%) originates from wheat and onions.53
Fructo-oligosaccharides. In our systematic review, 1 RCT was included reporting on FOSs. Benjamin et al25 studied supplementation with either 15 g/day of FOSs or placebo in 103 CD patients with active disease (Crohn’s Disease Activity Index [CDAI] ≥220) with one additional marker of inflammation (elevated C reactive protein [CRP]/erythrocyte sedimentation rate/platelet count) for 4 weeks. Compliance was checked by a sachet count at the end of the study period. The maximum permissible steroid dose was settled at 20 mg/day during the trial. When comparing both groups after the intervention, no significant difference was seen in CDAI scores (P = 0.112). Moreover, in the intention-to-treat population, the number of patients achieving clinical response (a fall in CDAI of ≥70 points) after the intervention did not differ from that of the placebo group (P = 0.067). In addition, the baseline IBD Questionnaire scores did not differ between groups, whereas after the 4-week intervention, the treatment group reported significantly lower IBD Questionnaire scores (reflecting a lower well-being) than in the placebo group (P = 0.0004).
Inulin. In our systematic review, one RCT was included that reported on inulin. Casellas et al33 studied 15 UC patients with mild to moderate disease activity in a 2-week trial comparing the outcomes for supplementation with 12 g/day oligofructose-enriched inulin with those of supplementation with 12 g/day placebo. Compliance was checked by letting patients bring the study supplements to visits. Medical therapy with mesalazine 3 g/day was allowed during the trial period. Disease activity scores (Rachmilewitz scores54) decreased in both groups after 14 days (P < 0.05). At the end of the study, almost all patients showed a decline in disease activity, and scores were lower than at baseline in both groups (P < 0.05); however, there was no difference between groups. Furthermore, on day 7 an early significant reduction of calprotectin was observed in the treatment group but not in the placebo group; this, however, was not significant after 14 days.
To summarize, FOS was demonstrated to lower IBD Questionnaire scores (reflecting lower well-being), but, in contrast, inulin decreased disease activity scores.
DISCUSSION
In our systematic review, 8 studies (1 with low risk of bias, 3 with some concerns, and 4 with high risk of bias) were included, reporting on WB, GBF, psyllium, and fructans (FOS and inulin) in patients with CD (n = 1) and UC (n = 7). The best evidence is available for GBF. A good quality study32 demonstrated that, compared with controls, supplementation with GBF decreased disease activity and improved endoscopy scores, whereas another study36 showed immunological improvement (lower TNF-α, IL-6, and IL-8). The results for inulin seem propitious as disease activity scores, dyspeptic symptoms, and fecal calprotectin are enhanced after supplementation.33 The evidence on psyllium or WB was too limited to drawn firm conclusions. Although the abovementioned RCTs confirmed that some of these types of fiber might have an ameliorative effect, findings still need to be confirmed in larger human trials.
Other studies, eg, nonrandomized studies in humans, animal studies, or in vitro studies, also supported the found beneficial effect of fibers. Nevertheless, these studies have a lower position in the hierarchy of the evidence pyramid,55 as do studies reporting on multiple elements combined. Furthermore, hard clinical end points are often lacking, such as (objective) disease activity scores, endoscopy scores, or blood, urine, and/or fecal biomarkers, making translation to practice difficult.
The development of guidelines is also hampered by low numbers of included patients, short duration of interventions, and heterogeneity: huge variation in patient populations, disease type and status, dose and exposure time to the types of fiber, and measurement of the outcomes, make it difficult to conclude what types of fiber should be recommended. Moreover, the normal diet contains a wide variety of fiber, and even food products often contain multiple types of fiber. This makes it complex to study the effects of fiber types in whole foods, let alone their effect in the long term. The knowledge gathered from animal studies should provide a basis for well-conducted, urgently needed future RCTs in humans. Nevertheless, RCTs will also have to deal with issues concerning dietary blinding, compliance, and foods consumed next to a dietary intervention, and should therefore be designed carefully. Until guidelines are developed on what type of fiber to take in, we advise physicians to support their patients properly (where possible) in their search to relieve symptoms via nutritional therapy. Especially when fiber intake is first increased, symptoms of bloating can occur. Patients should be made aware of this fact, as symptoms should cease over time.
A limitation of our study is that only a small number of studies fitted our PICOS search question. A meta-analysis could not be conducted due to paucity of data and too much heterogeneity among studies. A limitation of some studies included in this systematic review is that they did not evaluate or correct for habitual dietary intake. The strengths of this study are that the review was conducted in a systematic way, thereby limiting the risk on reporting bias. A structured system was used to assess potential bias. In addition, types of fibers were analyzed, rather than dietary fiber in general.
CONCLUSION
Of the 186 studies, 8 RCTs could be included in this systematic review reporting on 5 types of fibers. Whereas evidence on GBF and inulin is still sparse, these types of fibers seem propitious, as both were demonstrated to decrease disease activity scores. Evidence on WB, FOS and psyllium is still too limited. This systematic review contributes to enabling clinicians and dietitians to make evidence-based decisions when advising patients on what types of fiber should be increased in their habitual diet. However, due to paucity of the data, the results of the reported studies should be interpreted with care. To enable proper dietary advice on the use of specific types of fibers in the future, more adequately powered human long-term randomized trials with objective outcomes are urgently needed.
Funding. This work was not supported by external funding.
Declaration of interest. V.P. reports no conflicts of interest. G.D. reports speakers’ fees (outside the submitted work) from Janssen Pharmaceuticals, Takeda, and Pfizer. M.J.E.C.-K. received invited speaking fees (outside the submitted work) from Baxter and Takeda.
Supporting information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Figure S1. Risk of Bias Assessment (colorblind-friendly figure)
Table S1. Quality assessment of studies with parallel-group or cross-over design
Supplementary Material
Acknowledgments
Author contributions. Conceptualization: V.P. and M.J.E.C.-K., methodology: V.P. and M.J.E.C.-K., formal analysis: V.P. and M.J.E.C.-K., investigation: V.P. and M.J.E.C.-K., resources: V.P., G.D., and M.J.E.C.-K., data curation: V.P. and M.J.E.C.-K., writing – original draft preparation: V.P. and M.J.E.C.-K., writing – review and editing: V.P., G.D., and M.J.E.C.-K., visualization: V.P., supervision: G.D. and M.J.E.C.-K.
REFERENCES
- 1. Wedlake L, Slack N, Andreyev HJN, et al. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014;20:576–586. [DOI] [PubMed] [Google Scholar]
- 2. Van Assche G, Dignass A, Panes J, et al. ; European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 3. Stange EF, Travis SPL, Vermeire S, et al. ; European Crohn’s and Colitis Organisation (ECCO). European evidence-based Consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. [DOI] [PubMed] [Google Scholar]
- 4. Meier R, Gassull MA.. Consensus recommendations on the effects and benefits of fibre in clinical practice. Clin Nutr suppl. 2004;1:73–80. [Google Scholar]
- 5. Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18:1381–1392. [DOI] [PubMed] [Google Scholar]
- 6. Brotherton CS, Martin CA, Long MD, et al. Avoidance of fiber is associated with greater risk of Crohn’s disease flare in a 6 month period. Clin Gastroenterol Hepatol. 2016;14:1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Allen R, Ashley S, et al. ; Gastroenterology Specialist Group of the British Dietetic Association. British Dietetic Association evidence-based guidelines for the dietary management of Crohn’s disease in adults. J Hum Nutr Diet. 2014;27:207–218. [DOI] [PubMed] [Google Scholar]
- 8. Logan M, Gkikas K, Svolos V, et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease—new insights into dietary disease triggers. Aliment Pharmacol Ther. 2020;51:935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephen AM, Champ MM-J, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30:149–190. [DOI] [PubMed] [Google Scholar]
- 10. Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2019;2:CD012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scaldaferri F, Pizzoferrato M, Lopetuso LR, et al. Nutrition and IBD: malnutrition and/or sarcopenia? A practical guide. Gastroenterol Res Pract. 2017;2017:8646495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhingra D, Michael M, Rajput H, et al. Dietary fibre in foods: a review. J Food Sci Technol. 2012;49:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliver A, Chase AB, Weihe C, et al. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. mSystems 2021;6:e00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan W, Lee G, Chen J-H, et al. Relationships between distention-, butyrate- and pellet-induced stimulation of peristalsis in the mouse colon. Front Physiol. 2020;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer MH, Yu N, Gray GR, et al. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr Res. 2004;339:2009–2017. [DOI] [PubMed] [Google Scholar]
- 16. Vuksan V, Jenkins AL, Jenkins DJA, et al. Using cereal to increase dietary fiber intake to the recommended level and the effect of fiber on bowel function in healthy persons consuming North American diets. Am J Clin Nutr. 2008;88:1256–1262. [DOI] [PubMed] [Google Scholar]
- 17. Schmoldt A, Benthe HF, Haberland G.. Towards a food pharmacy: immunologic modulation through diet. Biochem Pharmacol. 1975;24:1639–1641. [PubMed] [Google Scholar]
- 18. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiminez JA, Uwiera TC, Abbott DW, et al. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Araki Y, Andoh A, Koyama S, et al. Effects of germinated barley foodstuff on microflora and short chain fatty acid production in dextran sulfate sodium–induced colitis in rats. Biosci Biotechnol Biochem. 2000;64:1794–1800. [DOI] [PubMed] [Google Scholar]
- 21. Llewellyn SR, Britton GJ, Contijoch EJ, et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology. 2018;154:1037–1046.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harmsen HJM, Raangs GC, Franks AH, et al. The effect of the prebiotic inulin and the probiotic Bifidobacterium longum on the fecal microflora of healthy volunteers measured by FISH and DGGE. Microb Ecol Health Dis. 2002;14:212–220. [Google Scholar]
- 23. Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cherbut C, Michel C, Lecannu G.. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr. 2003;133:21–27. [DOI] [PubMed] [Google Scholar]
- 25. Benjamin JL, Hedin CRH, Koutsoumpas A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60:923–929. [DOI] [PubMed] [Google Scholar]
- 26.Codex Alimentarius Commission. Report of the 30th session of the Codex Committee on Nutrition and Foods for Special Dietary Uses – ALINORM 09/32/26. Vol. 2. Rome, Italy: Food and Agriculture Organization of the United Nations and World Health Organization; 2009. Available at: http://www.fao.org/input/download/report/710/al32_26e.pdf. Accessed August 16, 2021.
- 27. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 28. DeVries JW. On defining dietary fibre. Proc Nutr Soc. 2003;62:37–43. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:L4898. [DOI] [PubMed] [Google Scholar]
- 31. McGuinness LA, Higgins JPT.. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- 32. Kanauchi O, Suga T, Tochihara M, et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37:67–72. [DOI] [PubMed] [Google Scholar]
- 33. Casellas F, Borruel N, Torrejón A, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061–1067. [DOI] [PubMed] [Google Scholar]
- 34. Ejderhamn J, Nord CE, Strandvik B.. Absence of effect on faecal microflora of long-term supplementation of dietary fibres in juvenile ulcerative colitis. Int J Clin Pharm Res. 1991;11:247–252. [PubMed] [Google Scholar]
- 35. Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Am J Gastroenterol. 1999;94:427–433. [DOI] [PubMed] [Google Scholar]
- 36. Faghfoori Z, Navai L, Shakerhosseini R, et al. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-α, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48:233–237. [DOI] [PubMed] [Google Scholar]
- 37. James SL, Christophersen CT, Bird AR, et al. Abnormal fibre usage in UC in remission. Gut. 2015;64:562–570. [DOI] [PubMed] [Google Scholar]
- 38. Hallert C, Kaldma M, Petersson BG.. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol. 1991;26:747–750. [DOI] [PubMed] [Google Scholar]
- 39. Watson RR, Preedy VR, Zibadi S. Wheat and Rice in Disease Prevention and Health: Benefits, Risks, and Mechanisms of Whole Grains in Health Promotion. Burlington, MA: Elsevier Science; 2014.
- 40. Triantafillidis JK, Triantafyllidi A, Vagianos C, et al. Favorable results from the use of herbal and plant products in inflammatory bowel disease: evidence from experimental animal studies. Ann Gastroenterol. 2016;29:268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanauchi O, Iwanaga T, Mitsuyama K.. Germinated barley foodstuff feeding. Digestion. 2001:63;60–67. [DOI] [PubMed] [Google Scholar]
- 42. Gorzolka K, Kölling J, Nattkemper TW, et al. Spatio-temporal metabolite profiling of the barley germination process by MALDI MS imaging. PLoS One. 2016;11:e0150208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaur H, Gill BS.. Changes in physicochemical, nutritional characteristics and ATR–FTIR molecular interactions of cereal grains during germination. J Food Sci Technol. 2021;58:2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. [DOI] [PubMed] [Google Scholar]
- 45. Masood R, Miraftab M.. Psyllium: current and future applications. In: Medical and Healthcare Textiles. Cambridge, UK: Elsevier Ltd; 2010:244–253. [Google Scholar]
- 46.Health Canada. Plantago ovata (Psyllium) Monograph. 2018
- 47. Singh V, Yeoh BS, Walker RE, et al. Microbiota fermentation–NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68:1801–1812. [DOI] [PubMed] [Google Scholar]
- 48. Shalini R, Antony U.. Fructan distribution in banana cultivars and effect of ripening and processing on Nendran banana. J Food Sci Technol. 2015;52:8244–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995;108:975–982. [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Gibson GR.. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Bacteriol. 1993;75:373–380. [DOI] [PubMed] [Google Scholar]
- 51. Ramirez-Farias C, Slezak K, Fuller Z, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2008;101:541–550. [DOI] [PubMed] [Google Scholar]
- 52. van Loo J, Coussement P, de Leenheer L, et al. On the presence of inulin and oligofructose as natural ingredients in the Western diet. Crit Rev Food Sci Nutr. 1995;35:525–552. [DOI] [PubMed] [Google Scholar]
- 53. Schaafsma G, Slavin JL.. Significance of inulin fructans in the human diet. Compr Rev Food Sci Food Saf. 2015;14:37–47. [DOI] [PubMed] [Google Scholar]
- 54. Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yetley EA, MacFarlane AJ, Greene-Finestone LS, et al. Options for basing Dietary Reference Intakes (DRIs) on chronic disease endpoints: report from a joint US-/Canadian-sponsored working group. Am J Clin Nutr. 2017;105:249S–285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.