Abstract
Background
Nutritional rickets is a disease of growing children leading to bone deformities, bone pain, convulsions or delayed motor development. Today, high‐incidence of nutritional rickets is mainly found in low‐income countries.
Objectives
To assess the effects of various interventions on the prevention of nutritional rickets in term born children.
Search methods
Studies were obtained from computerised searches of The Cochrane Library, MEDLINE, EMBASE, LILACS and reference lists of relevant articles. We contacted authors of studies or reviews to obtain further studies.
Selection criteria
Studies were included if they were randomised controlled clinical trials, controlled clinical trials or prospective cohort studies comparing any intervention for the prevention of nutritional rickets in term born children with placebo or no intervention. Minimum duration of the intervention was three months for children under 12 months or six months for children over 12 months.
Data collection and analysis
Two authors independently extracted data and assessed study quality. Authors of studies were contacted to obtain missing information.
Main results
Four studies enrolled approximately 1700 participants. Trials lasted between nine months to two years. Three studies were randomised controlled trials, two of which showed a cluster randomised design; one trial probably was a controlled trial with researcher controlled group assignment. In children up to three years of age in Turkey, Vitamin D compared to no intervention showed a relative risk of 0.04 (95% confidence interval (CI) 0 to 0.71). Despite a marked non‐compliance, a Chinese trial in children up to three years of age comparing a combined intervention of supplementation of vitamin D, calcium and nutritional counselling showed a relative risk of 0.76 (95% CI 0.61 to 0.95) compared to no intervention. In two studies conducted in older children in China and in France no rickets occurred in both the intervention and control group.
Authors' conclusions
There a only few studies on the prevention of nutritional rickets in term born children. Until new data become available, it appears sound to offer preventive measures (vitamin D or calcium) to groups of high risk, like infants and toddlers; children living in Africa, Asia or the Middle East or migrated children from these regions into areas where rickets is not frequent. Due to a marked clinical heterogeneity and the scarcity of data, the main and adverse effects of preventive measures against nutritional rickets should be investigated in different countries, different age groups and in children of different ethnic origin.
Plain language summary
Interventions for the prevention of nutritional rickets in term born children
Although only a few studies with different results exist, preventive measures against nutritional rickets appear reasonable in high risk groups until new data become available.
Rickets is a disease which affects the bone of growing children. Calcium and phosphate are important elements which form the bone. In nutritional rickets, initially the availability of calcium is diminished, later disturbances in phosphate occur. The shortage of calcium may be caused by limited intake or limited resorption in the gut. The latter is highly regulated by vitamin D, which can be synthesised from the skin after sun exposure or can be acquired from dietary sources, for example cod liver. Therefore, shortage of calcium, vitamin D or both may lead to nutritional rickets, which is mainly characterized by deformed bones, bone pain, convulsions or delayed development. Since the 1930s supplementation of vitamin D is used for the prevention of rickets in children, mainly in high‐income countries; several other measures like supplementation of calcium or longer exposure of the skin to sunlight are also used. Over the time many factors have changed, for example nutrition, which provides calcium, air pollution, through which sun light is absorbed leading to a diminished synthesis of vitamin D in the skin, or social issues, for example child labour, again leading to limited sun exposure. Because of these changes we looked for studies conducted in the last 50 years which investigated patient‐relevant outcomes. As patient‐relevant outcomes we defined the occurrence of rickets, adverse effects of the intervention, mortality, health‐related quality of life and costs. Four trials enrolled approximately 1700 participants and lasted between nine months and two years. Study participants were aged from one month to 15 years. There were different results on the occurrence of nutritional rickets in different settings. Adverse effects were investigated in one study only. Considering the partial high frequency of nutritional rickets, the obvious way of action of supplementation of vitamin D or calcium and the favourable risk‐benefit ratio, preventive measures are reasonable in high risk groups like infants and toddlers. New studies investigating main and side effects of preventive measures against nutritional rickets in different age groups and in different countries are indicated.
Background
Description of the condition
Rickets comprises a group of disorders characterised by defective mineralization and disorganisation of the epiphyseal growth plates. Therefore, rickets is a disease limited to growing children. Mineralisation of the bone matrix is also defective, called osteomalacia. The main components of the bone mineral matrix are calcium and phosphate. Rickets is classified depending on the lacking mineral. Nutritional rickets is the main form of calcipenic rickets, nevertheless alterations in phosphate also occur in the course of the disease. The clinical presentation of nutritional rickets depends on the age of the child, it includes soft skull bone, called craniotabes; hypocalcaemic convulsions; typical bone deformities like deformation of the weight‐bearing limbs; swelling of the wrist, knee or ankle; swelling of the costochondral junction of the ribs, called rachitic rosary or the deformity of the soft rib cage due to pulling of the diaphragm, called Harrison's sulcus. Furthermore, muscular hypotonia or delayed motor development may occur. The biochemical findings of nutritional rickets include a normal or decreased blood level of calcium, a normal, decreased or increased blood level of phosphate as well as elevated blood levels of alkaline phosphatase, parathyroid hormone or both. In vitamin D‐deficiency rickets 25‐hydroxyvitamin D blood levels are decreased. Radiologically, there is an irregular metaphyseal outline like cupping, widening or fraying due to diminished calcification of the growth plate. In younger children the radiological changes can best be visualised in the wrist, in older children the area above and below the knee is most useful (Pettifor 2005; Shaw 2004; Thacher).
Vitamin D and calcium In general, nutritional rickets is the result of either calcium or vitamin D deficiency, or both. The exposure of the skin to ultraviolet light (wavelengths from 290 to 315 nm, called UV‐B) is crucial for the beginning of the endogenous syntheses of vitamin D. Provitamin D3 (7‐dehydrocholesterol), stored in the skin, is photolysed by the absorbed energy of UV‐B to previtamin D3. Previtamin D3 converts to vitamin D3 (cholecalciferol) rapidly. Thus, low UV‐B irradiation due to northern or southern latitude, high air pollution, short outdoors exposure time, veiling of the skin, the use of sunscreens or high amounts of the skin pigment melanin lower the production of vitamin D3. As vitamin D3 is synthesised due to sunlight exposure, it is not a vitamin in the strict sense of definition. Exogenous sources of vitamin D provide vitamin D2 (ergocalciferol) and vitamin D3. Very few foods contain a reasonable amount of vitamin D. Human breast milk has only a low concentration of vitamin D, which is an important factor for infants being solely breast fed for a long time. Vitamin D, which denotes both vitamin D2 and vitamin D3, is hydroxylated in the liver to 25‐hydroxyvitamin D (calcidiol). A further hydroxylation, mainly in the kidneys, leads to 1,25‐dihydroxyvitamin D (calcitriol), the final active form of vitamin D. 1,25‐dihydroxyvitamin D acts via the vitamin D receptor (VDR), an intracellular protein which belongs to the steroid‐hormone‐thyroid hormone‐retinoic acid receptor gene superfamily. Conflicting data exist for the role of polymorphisms of VDR for the pathogenesis of rickets. The target of VDR is the vitamin D response element regulating gene transcription which evokes in the intestine the synthesis of proteins necessary for the transfer of calcium from the intestinal lumen to the capillaries. It is assumed that only ten percent of the calcium entry is vitamin D independent. The availability of calcium is lowered by complexation with oxalate or phytate. Inadequate low intake of calcium leads to inactivation of calcidiol. A high‐fibre diet results in degradation of calcitriol, as does elevated parathyroid hormone resulting from hypocalcaemia. In state of dietary calcium deficiency, vitamin D also interacts with VDR in bone tissue. The thereby induced maturation of osteoclasts leads to dissolving of bone tissue and thus, release of calcium. It is not clear whether there is a direct effect of vitamin D on bone formation, too. There are many other actions of vitamin D besides the regulation of calcium homeostasis, for example regulation of cell growth and apoptosis being associated with several forms of cancer. Furthermore, the modulation of immune reactions is associated with autoimmune diseases like type 1 diabetes mellitus or multiple sclerosis. There are influences on the functions of muscles and the nervous system, too (Bronner 2003; Dusso 2005; Pettifor 2003; Thacher 2003).
Frequency of nutritional rickets To describe the frequency of nutritional rickets in term infants three aspects have to be considered. First, due to different diagnostic criteria reported frequencies are not directly comparable (Peach 1984). Secondly, frequency is extremely time dependent. In the 1920s and 1930s the prevalence of nutritional rickets was between 75% to 98% in Europe and the United States of America based on autopsies, clinical examination or radiographs (Chesney 2001). Thirdly, three groups have to be distinguished:
Infants with fair skin
Infants with intermediate or dark skin living in their indigenous area
Infants with intermediate or dark skin living in an area with lower UV‐B irradiation than in their indigenous area
In the first group nutritional rickets mostly is due to pure vitamin D deficiency. Only few recent data for incidence or prevalence are known. For example, in a study in the West Midlands, United Kingdom, in which the overall incidence was 7.5 per 100,000 children under five years, only 1 of 24 children with rickets (defined by radiological changes or hypocalcaemic convulsions) was classified as 'white' which approximates an incidence of 0.4 per annum per 100,000 children under five years (Callaghan 2006). In Turkey, 10% of children from 3 to 36 months had clinical and biochemical changes consistent with rickets (Beser 1994). Fifteen per cent of children under one year showed biochemical changes in Greece (Lapatsanis 1968), nine per cent of children between 12 and 24 months showed radiological changes in the United Kingdom (Richards 1968). In the second group, nutritional rickets may be due to calcium or vitamin D deficiency. For example, in Nigeria nine per cent of children between six months and three years showed clinical signs of rickets (Pfitzner 1988). In Tibet, 66% of children over 24 months showed clinical features of rickets (Harris 2001). Children of immigrants or immigrated infants represent the third group. The study in the West Midlands, United Kingdom mentioned above showed an incidence for children of south Asian ethnic origin of 38 and for children of black African or African‐Caribbean ethnic origin of 95 per annum per 100,000 children under the age of 5 five years (Callaghan 2006).
Development of prevention Although early descriptions of rickets reach back to antiquity, rickets became more frequent in the 17th century. During and after the industrialisation in Europe and North America, rickets was a disease of high frequency, presumably because of the changing socio‐economic and nutritional circumstances. Cod liver oil was first used traditionally, in the 19th century the specific action against rickets was noted. After its discovery vitamin D was used in different formes for the treatment and later the prevention of rickets (Rajakumar 2003). In the 20th century rickets was considered to be a disease which affects children up to approximately five years of age. After the recognition of rickets in older immigrant children in the United Kingdom, cross‐sectional studies showed that native children were also affected. Therefore, preventive measures need had to be considered (Cooke 1973). Because of the relationship of sun light and synthesis of vitamin D, rickets were considered not be prevalent in tropical areas. Reports starting in the 1930s showed that rickets was a frequent disease in these regions and still is today. That is why preventive measures need to be considered in these areas as well (Jelliffe 1968; Thacher 2006).
Diagnostic criteria used for the review There are no generally accepted diagnostic criteria for nutritional rickets. Therefore, we extracted data according to authors' definition of rickets. The different diagnostic criteria may produce significant variability in the clinical characteristics of the people with rickets included as well as in the results obtained. We planned to explore these differences in a sensitivity analysis. Ideally, three diagnostic criteria would be encountered: 1. Clinical signs or radiological findings of rickets. 2. Elevated blood levels of alkaline phosphatase, parathyroid hormone or both. 3. Exclusion of disorders mimicking nutritional rickets (like phosphopenic rickets, vitamin D deficiency or lack of calcium due to gastrointestinal or renal diseases, inborn disorders of vitamin D or calcium metabolism).
Description of the intervention
Interventions for the prevention of nutritional rickets include supplementation of vitamin D, for example on a daily basis, as a "stossprophylaxis" (intermittent application of large amounts) or in fortified food, especially milk; calcium supplementation or advice on sun exposure.
How the intervention might work
Since paucity of calcium is crucial in nutritional rickets it is essential to increase the resorption of calcium. Therefore, there are three possibilities of intervention: First, increasing the calcium intake; secondly, increasing the endogenous syntheses of vitamin D; thirdly, increasing the vitamin D intake. Adverse effects of the intervention Reported adverse effects of vitamin D supplementation are hypercalcaemia or nephrocalcinosis (Markestad 1987; Rönnefarth 2000). Overexposure of the skin to sunlight may lead to skin cancer.
Why it is important to do this review
There are still many countries in Africa, Asia and the Middle East with high frequency of nutritional rickets. Furthermore, the incidence of rickets seems to be reincreasing in countries with a formerly low incidence. The latter might be linked to reduced acceptance of preventive measures. Considering the worldwide high burden of nutritional rickets leads to the question whether there are any effective measures for the prevention of nutritional rickets. As far as we know there is no systematic review, meta‐analysis or health‐technology assessment report about the prevention of nutritional rickets.
Objectives
To assess the effects of various interventions on the prevention of nutritional rickets in term born children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, quasi‐randomised and non‐randomised controlled clinical trials and prospective cohort studies.
Types of participants
Healthy term born children or children with diseases not increasing the risk of developing rickets.
Types of interventions
Intervention Any intervention used for the prevention of nutritional rickets like vitamin D supplementation via tablets, liquids or fortified food; calcium supplementation, advice to get more sunlight or combinations of these interventions.
Comparison Placebo or no intervention.
Types of outcome measures
Primary outcomes
occurrence of rickets;
adverse effects.
Secondary outcomes
all‐cause mortality;
quality of life (ideally measured by a validated instrument);
costs.
Covariates, effect modifiers and confounders
different groups of children (mentioned under 'Frequency of nutritional rickets');
compliance;
nutrition (for example phytate or oxalate intake);
age.
Timing of outcome assessment
We had planned to include prospective studies only if observation lasted longer than three years. This observation period was considered to be long enough to definitely exclude rickets clinically, radiologically, or both. As we failed to identify these, we adapted the criteria: for children younger than 12 months of age, observation had to last three months or longer; for children older than 12 months of age, observation had to last six months or longer.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
The Cochrane Library (issue 3, 2006);
MEDLINE (via OVID interface, until August 2006);
EMBASE (via OVID interface, until August 2006);
LILACS (until August 2006).
We also searched the metaRegister of Clinical Trials (www.controlled‐trials.com/mrct) to identify ongoing studies.
Studies published in any language were included. For details on the search strategy see Appendix 1. This strategy was used for MEDLINE and adapted for the other databases.
Reference lists We tried to identify additional studies by searching the reference lists of included trials and reviews identified.
Correspondence We contacted authors of studies and reviews as experts in the field to obtain additional references or unpublished trials.
Data collection and analysis
Selection of studies
First, one author (CL) scanned the title or abstract, or both sections of every record retrieved to eliminate all records not dealing with nutritional rickets or unequivocally not reporting comparative studies. Secondly, both authors scanned the title or abstract, or both sections of the selected records to determine which studies require further assessment. All potentially relevant articles were investigated as full text. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Consensus about variation in rated records was reached by discussion. An adapted QUOROM (quality of reporting of meta‐analyses) flow chart of study selection (Moher 1999) is attached (see (Figure 1).
1.
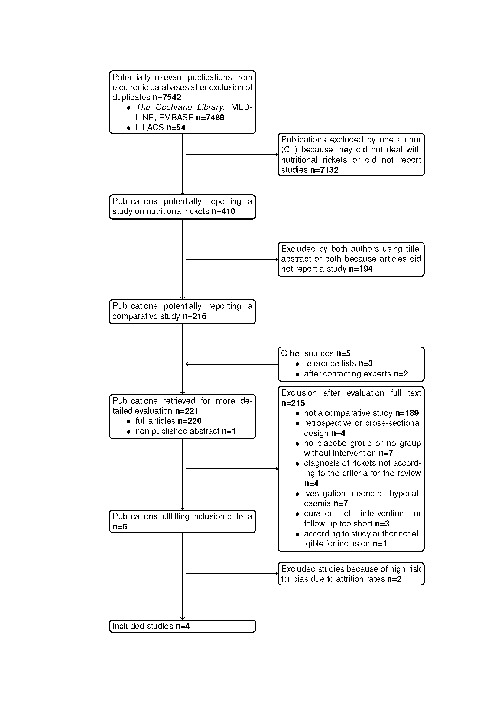
QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Data extraction and management
For studies fulfilling the inclusion criteria, both authors independently extracted relevant population and intervention characteristics using standard data extraction sheets. Consensus about differently extracted data was reached by discussion. Any relevant missing information on the study was sought from the authors of the study. For details, please see 'Characteristics of included studies' and Table 1, Appendix 2,Appendix 3 and Appendix 4.
1. Risk of bias.
| Characteristic | Beser 2004 | Du 2004 | Duhamel 2000 | Strand 2002/2003 |
| Intervention 1 (I1) / intervention 2 (I2) / control 1 (C1) | I1. vitamin D 400 IU/day per os C1. no placebo or other intervention | I1: fortified milk, averaged calcium 245 mg/day per os I2: fortified milk, averaged calcium 245 mg/day per os, vitamin D3 133 IU/day per so C1: no placebo or other intervention | I1. vitamin D3 100,000 IU every 3 months per os C1. placebo | I1: promoting of exclusive breastfeeding from birth, supplementation of solid foods at age 5 months, weaning at 12 to 18 months, vitamin D 300 IU/day per os during the first 12 months, calcium 378 mg/day per os from age 5 months to 24 months C1: no placebo or other intervention. |
| Randomised controlled clinical trial (RCT) | N | Y | Y | Y |
| Controlled clinical trial | ? (presumed Y) | N | N | N |
| Prospective cohort study | N | N | N | N |
| Method of randomisation (specify) | N/A | ? | ? | ? |
| Unit of randomisation (individuals, cluster ‐ specify) | N/A | cluster (pupils of matched 3 x 3 schools) | individuals | cluster (children of 16 matched villages ) |
| Randomisation stratified for centres | N/A | N/A | ? | N/A |
| Randomisation ratio | N/A | ? | ? | ? (intervention group: 14 villages, control group: 2 villages) |
| Concealment of allocation (specify) | N/A | ? | ? | ? |
| Study population representative of population | Y | ? | ? (recruited from hospital) | Y |
| Method of selection of controls (specify) (*) | N/A | N/A | N/A | N/A |
| Patients in different groups form same population (*) | N/A | N/A | N/A | N/A |
| Exposed/unexposed over same period of time (*) | N/A | N/A | N/A | N/A |
| Ascertainment of group determination (specify) (*) | N/A | N/A | N/A | N/A |
| Ascertainment of outcome (specify) | direct measurement | direct measurement | direct measurement | direct measurement |
| Stated blinding (open; single, double, triple blind) | ? | ? | double | ? |
| Actual blinding: participant/parents | N/A | ? | Y | N/A |
| Actual blinding: caregiver / treatment administrator | N/A | ? | ? | N/A |
| Actual blinding: outcome assessor | ? | ? | ? | N |
| Actual blinding: others | ? | ? | ? | ? |
| Blinding checked: participant | ? | ? | ? | ? |
| Blinding checked: caregiver / treatment administrator | ? | ? | ? | ? |
| Blinding checked: outcome assessor | ? | ? | ? | ? |
| Primary endpoint defined | Y | N | N | Y |
| [n] of primary endpoint(s) | 1 | ? | ? | 1 |
| [n] of secondary endpoints | 0 | ? | ? | 2 |
| Total [n] of endpoints | 1 | 12 (no distinction made between primary and secondary outcomes) | 8 (no distinction made between primary and secondary outcomes) | 3 |
| Prior publication of study design | ? | ? | ? | ? |
| Power calculation | ? | ? | ? | ? |
| [n] participants per group calculated | ? | ? | ? | ? |
| Intention‐to‐treat analysis (ITT) | Y (no summary statistic in analysis) | Y (no summary statistic for rickets in analysis) | Y (no summary statistic for rickets in analysis) | N (based on analysed patients) |
| Per‐protocol‐analysis | N | N | N | N (based on analysed patients) |
| ITT defined | N | N | N | N |
| Dealing with missing data | ? | ? | ? | ? (imputing for birth weight which is not relevant for this review) |
| [n] of screened participants (I1/ I2 / C1 / total) | ? | ? | ? | ? |
| [n] of randomised/included participants (for primary endpoint) | I1: 302 C1: 374 | I1: 238 I2: 260 C1: 259 | I1: 32 C1: 34 | Total: 259 |
| [n] of participants finishing the study | I1: 293 C1: 369 | I1: 209 I2: 242 C1: 247 | I1: 32 C1: 31 | Total: 245 |
| [n] of patients analysed (for primary endpoint) | no summary statistic provided | no summary statistic for rickets provided | no summary statistic for rickets provided | I1: 183 C1: 46 Total: 229 |
| Description of discontinuing participants | N | Y | N | N |
| Drop‐outs (reasons explained) | N | Y | N | N |
| Withdrawals (reasons explained) | N | Y | N | N |
| Losses‐to‐follow‐up (reasons explained) | N | Y | N | N |
| [n] of participants who discontinued | 14 | 59 | 3 | 14 |
| [%] discontinuation rate | 2 | 8 | 5 | 5 |
| Discontinuation rate similar between groups | Y | Y | Y | Y |
| Differences [n] calculated to analysed patients | N/A | N/A | N/A | N/A |
| Adjustment for multiple outcomes / repeated measurements | N/A no summary statistic provided | Y no summary statistic for rickets provided | N/A no summary statistic provided | Y |
| Baseline characteristics: clinically relevant differences | ? | ? | ? | Y (more preterm born children in intervention group) |
| Treatment identical (apart from intervention) | ? | ? | ? | ? |
| Compliance measured | ? | Y | Y | Y |
| Other important covariates measured (specify) | ? | ? | ? | ? |
| Co‐morbidities measured | ? | ? | ? | ? |
| Co‐medications measured | ? | ? | ? | ? |
| Different length of follow‐up (taken into account) (*) | N/A | N/A | N/A | N/A |
| Loss of study patients taken into account | Y | Y | Y | Y |
| Specific doubts about study quality | N | N | N | N |
| Funding: commercial | ? | N | ? | N |
| Funding: non‐commercial | ? | Y | ? | Y |
| Publication status: peer review journal | Y | Y | Y | Y |
| Publication status: journal supplement | N | N | N | N |
| Publication status: abstract | N | N | N | N |
| Publication status: other | N | N | N | N |
| Symbols & abbreviations: Y = yes; N = no; ? = unclear; N/A = not applicable; I = intervention; C = control (*) for prospective cohort studies only | ||||
Assessment of risk of bias in included studies
Both authors independently assessed the methodological quality of each included study, differences were resolved by discussion. For randomised controlled trials we used quality criteria specified by Schulz 1995 and by Jadad 1996, for other prospective comparative studies quality criteria specified by Downs 1998 and by Deeks 2003. We planned to explore the influence of individual quality criteria in a sensitivity analysis (see under 'sensitivity analyses'). Inter‐rater agreement was calculated using the kappa statistic (Cohen 1960).
Measures of treatment effect
We decided to base the analysis on dichotomous data (here, rickets yes/no) expressed as relative risks (RR) with 95% confidence intervals (CI).
Unit of analysis issues
We planned to address cluster‐randomised studies or studies with multiple interventions for meta‐analyses specifically.
Dealing with missing data
Relevant missing data were obtained or tried to obtain from authors. Evaluation of important numerical data such as screened, eligible and randomised children as well as intention‐to‐treat and per‐protocol population was carefully performed. Drop‐outs, misses to follow up and withdrawn study participants were investigated.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we planned to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) was planned to obtain priority.
Assessment of heterogeneity
In case of relevant heterogeneity due to clinical, methodological or statistical issues, study result were not planned to be combined in a meta‐analysis. Heterogeneity was planned to be identified and quantified using the χ²‐test with significance being set α = 0.1 and the I2 statistic (Higgins 2002; Higgins 2003), the latter describing the percentage of total variation across studies that is due to heterogeneity rather than chance. I2‐values of 50% or more indicate relevant heterogeneity. We planned to identify possible sources of heterogeneity by evaluating individual study characteristics and subgroup and sensitivity analyses.
Assessment of reporting biases
We planned to use funnel plots to assess small study bias.
Data synthesis
Data were planned to be summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis was planned to be performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). Pooled results were planned to be analysed using primarily the DerSimonian and Laird random‐effects method (DerSimonian 1986). For preventive measures, the relative risk of adverse outcome, here occurrence of rickets, is the appropriate summary statistic (Deeks 2002).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to be only performed if one of the primary outcome parameters demonstrated statistically significant differences between treatment groups. The following subgroup analyses were planned:
using the three groups described in the section 'Description of the disease';
breast milk nutrition during infancy;
veiling of the mother during pregnancy or nursing;
mode of administration of the intervention.
Subgroup analyses were planned to be mainly used to explore heterogeneity due to clinical, methodological or statistical issues and as a hypothesis generating exercise.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of study quality, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country, no primary consensus regarding study selection.
The robustness of the results was planned to be tested by repeating the analysis using different measures of effects size (risk difference, odds ratio etc.) and different statistic models (fixed‐ and random‐effects models).
Results
Description of studies
Results of the search The initial search identified 7542 records, from these, 221 full papers were identified for further examination. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study (see Figure 1 for details of the amended QUOROM (quality of reporting of meta‐analyses) statement). After screening the full text of the selected papers, four studies finally met the inclusion criteria.
Assessment of inter‐rater agreement Inter‐rater agreement for study selection, that is qualifying a study as 'potentially relevant' was 74%.
Missing data We contacted or tried to contact all authors of included studies: Beser 1994 was contacted for clarification of the mode of group allocation. We received no response. Du 2004 was contacted for clarification if there was a thorough clinical examination regarding the clinical signs of rickets and if participants with rickets were discovered. Authors provided additional data. Duhamel 2000 was contacted for clarification if the two participants who showed radiological signs of rickets at the beginning of the study were the same as the two participants showed radiological signs of rickets at the end of the study. We received no response. Strand 2002/2003 was contacted for providing the number of participants in each group and the number of participants with rickets in each group. Authors provided additional data. Specific data for participants who were compliant with vitamin D and calcium supplementation were not available.
Excluded studies Two studies had to be excluded after careful evaluation of the full publication. Reasons for exclusion was high risk for bias due to high or marked different attrition rates (for details see table Characteristics of excluded studies).
Characteristics of included studies For details see Table 1 and Appendix 2, Appendix 3, Appendix 4.
Interventions Comparisons Beser 1994 investigated vitamin D versus no intervention. Du 2004 studied milk fortified with calcium versus milk fortified with calcium and cholecalciferol versus no intervention. Duhamel 2000 investigated vitamin D versus placebo. Strand 2002/2003 studied a combined intervention of supplementation of vitamin D and calcium plus parents' nutritional counselling.
Number of study centres Three of the four included studies had one study centre only, Duhamel 2000 reported four study centres.
Country and location Du 2004 and Strand 2002/2003 were performed in China, Beser 1994 in Turkey and Duhamel 2000 in France.
Setting Beser 1994 recruited children from the community. Du 2004 was a cluster study with recruitment from schools. Duhamel 2000 recruited from hospital patients. Strand 2002/2003 was also a cluster study, recruiting from different villages. Treatment before study No publication informed about treatment before the intervention.
Methods Duration of the intervention Included studies had a treatment duration ranging from six months to two years.
Duration of follow up The follow up in Strand 2002/2003 lasted 6 to 30 months, including a post‐intervention follow up up to 6 months. In the remaining studies duration of treatment and follow up was identical, there was no post‐intervention follow up.
Language of publication Three of the four included studies were published in English, Duhamel 2000 in French.
Participants Who participated Study participants were aged from one months to 15 years, Du 2004 recruited only girls.
Inclusion and exclusion criteria In three of four included studies, participants were described as 'healthy'. Duhamel 2000 recruited from hospital patients, but excluded patients with gastrointestinal or nephrological diseases or other conditions involved in the metabolism of vitamin D or phosphorus. Beser 1994 excluded all children with clinical signs of nutritional rickets, Strand 2002/2003 excluded children with heart malformations.
Relevant diagnostic criteria Beser 1994 used a stepwise approach with clinical signs leading to radiological and biochemical assessment. Duhamel 2000 used radiological criteria. Du 2004 and Strand 2002/2003 used clinical signs only.
Co‐morbidities No study described co‐morbidities, although Duhamel 2000 recruited hospital patients.
Co‐medications No study described co‐medications. Outcomes Primary, secondary and additional outcomes Beser 1994 and Strand 2002/2003 used the occurrence of rickets as the only outcome parameter. Duhamel 2000 measured calcium, phosphorus, 25‐hydroxyvitamin D, intact parathyroid hormone and alkaline phosphatases in blood and radiographed wrists without distinction of primary or secondary outcomes. Du 2004 investigated bone mineral content, bone area, bone mineral density, total body composition, plasma 25‐hydroxyvitamin D, intact parathyroid hormone, blood calcium, calcium/creatinine ratio in urine, weight, length, sitting height, clinical signs of rickets without distinction of primary or secondary outcomes.
Risk of bias in included studies
For details on methodological quality of included studies see Table 1.
Overview All included studies were of parallel design. Interrater agreement for the key quality indicators was 100%.
Randomisation and allocation concealment Beser 1994 was probably as a controlled clinical trial with researcher determined group assignment. The other three studies were randomised controlled clinical trials, two of which used a cluster randomisation design (Du 2004; Strand 2002/2003). None of the randomised controlled clinical trials provided details about the method of randomisation or the concealment of allocation. Blinding Beser 1994 and Strand 2002/2003 used no placebo, so participants were not blinded. Duhamel 2000 was described as 'double‐blind' without further details. Du 2004 gave no details regarding blinding. No publications reported checking of blinding.
Definition of primary endpoint and secondary endpoints No study provided an explicit definition of the primary endpoint. However, Beser 1994 and Strand 2002/2003 investigated one endpoint only .
Power calculation None of the included studies reported power calculation.
Intention‐to‐treat and per‐protocol analyses, missing data Beser 1994 did not report summary statistics, therefore we calculated relative risks on an intention‐to‐treat basis. Strand 2002/2003 provided count data, relative risk was based on analysed patients. In the remaining studies, there were no quantitative data for rickets in both, intervention and control groups. For missing data, imputation was not used for relevant outcomes in any of the included studies.
Screened and randomised patients None of the included studies reported number of screened patients.
Discontinuing participants and attrition rates All included studies reported the number of discontinuing participants, only Du 2004 provided details of the reasons for discontinuation.
Compliance measures Du 2004 and Duhamel 2000 controlled adherence by direct observation of intake of the interventional medication.
Funding Two of the four studies reported funding. Strand 2002/2003 was funded by an non‐governmental organisation, Du 2004 was funded by an organisation which receives money from diary levies and the Australian government.
Publication status All included studies were published in regular issues of journal with a peer review system.
Effects of interventions
Baseline characteristics For details of baseline characteristics see Appendix 2.
Primary outcomes
For details of primary outcomes see Appendix 3.
Occurrence of rickets In two of the four included studies, rickets neither occurred in the intervention nor the control group (Du 2004; Duhamel 2000). Beser 1994 recruited children aged 3 to 36 months at inclusion in a rural community in Turkey aiming for a complete survey. After exclusion of children with clinical signs of rickets, the remaining children were divided in two groups with similar socio‐economic and cultural background and nourishment levels. The intervention group received oral vitamin D 400 IU per day for 12 months, the control group received no intervention. At the end of the study a stepwise approach was used for the diagnosis of nutritional rickets: clinical signs led to biochemical and radiological assessment. In the intervention group rickets was not observed in any of the 302 children. In the control group 14 children out of 374 developed rickets. The relative risk (RR) was 0.04 (95% confidence interval (CI) 0 to 0.71). Strand 2002/2003 was a cluster‐randomised study in rural China. The intervention consisted of the promotion of exclusive breastfeeding from birth, supplementation of solid foods at the age of five months, weaning at 12 to 18 months, oral vitamin D 300 IU/day during the first 12 months, oral calcium 378 mg/day from age five months to 24 months. At the timing of outcome assessment, children were aged 6 to 30 months meaning that some children still received the intervention. Outcome assessment used clinical parameters. In the intervention group 100 out of 183 children showed clinical signs of nutritional rickets, in the control group 13 out of 46 children. The RR was 0.76 (95% CI 0.61 to 0.95). There was a pronounced non‐compliance to the recommended supplementation of vitamin D and calcium.
Adverse effects of the interventions Duhamel 2000 stated, that no hypercalcaemia was observed. The remaining studies did not investigate adverse effects.
Secondary outcomes For details of secondary outcomes see Appendix 4.
Mortality No study investigated mortality.
Quality of life No study investigated health‐related quality of life.
Costs No study investigated costs.
Heterogeneity Due to obvious clinical heterogeneity and only a few included studies, we did not perform a meta‐analysis.
Subgroup analyses Not performed due to lack of data.
Sensitivity analyses Not performed due to lack of data.
Publication and small study bias Not performed due to insufficient amount of data.
Discussion
Summary of the main findings This systematic review shows that there are only few studies on the prevention of clinical or radiological diagnosed nutritional rickets in term born children. Because of the clinical heterogeneity in nutritional rickets itself as well as between the included studies neither a quantitative nor a qualitative data synthesis is reasonable. Vitamin D prevented rickets in children up to three years of age in Turkey. In China, a combined intervention of vitamin D, calcium and nutritional counselling led to a decreased risk of rickets in children up to three years of age, although there was a marked non‐compliance. In a study conducted in prepubertal girls in China rickets did not occur in both control or intervention groups who received calcium or calcium plus vitamin D. In France, rickets were not observed in children of about twelve years of age who received vitamin D or placebo. Adverse effects were only addressed in one study, in which no hypercalcaemia was observed after administration of vitamin D. No study investigated health‐related quality of life or economic costs of the interventions.
Limitations of the review We focused on a minimum duration of intervention of three months for children under twelve months of age and of six months for children over twelve months of age. Theoretically, studies of a shorter duration could demonstrate a significant impact, but this is thought to be highly unlikely. Although the prevention of nutritional rickets has a long history and was studied many years ago, we decided to include studies conducted in the last 50 years only because of the substantial changes of life circumstances like nutritional issues, sun exposure or environmental factors such as air pollution. Since preventive measures were introduced to prevent nutritional rickets with its 'classical' clinical and radiological features we used this definition for our review. We did not include rickets diagnosed solely on biochemical data.
Authors' conclusions
Implications for practice.
We discovered only a few published studies of interventions for the prevention of nutritional rickets in term born children. Considering pathophysiological aspects, the high frequency of nutritional rickets and the favourable risk‐benefit ratio we conclude that it is reasonable to offer preventive measures (vitamin D or calcium) to all children up to two years of age. Further groups of high risk are children living in Africa, Asia or the Middle East and migrants from these regions into areas where rickets is not frequent.
Implications for research.
Due to a marked clinical heterogeneity and scarcity of data, the current indication for prevention of nutritional rickets should be investigated in different countries, different age groups and in children of different ethnic origin. Besides patient‐oriented outcomes on efficacy, adverse effects of the chosen intervention should be studied. Especially with reference to vitamin D, controlled prospective studies should investigate both short‐time effects on occurrence of nutritional rickets and long‐term effects on occurrence of autoimmune diseases or cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the Department of General Pediatrics, University Children's Hospital, Moorenstr. 5, 40225 Duesseldorf, GERMANY (Dr Thomas Meissner) for its support to finish this review.
For help with translations we thank (in alphabetical order) Markéta Benesová, Daniel Bereczki, Barbara and Frank Brüderle, Monica Durosca, Hanna Kauffmann, Marta Kollenda, Morgane Legendre, Regina Miltner, Andrey Solodarenko, translation bureau Semantik and Maria Zangmeister. We thank the following experts and authors of studies for their responses to our enquiries (in alphabetical order): Uri Alon, Annie Anderson, Christopher Bates, Abdullah Bereket, Xueqin Du, Ghada El‐Hajj Fuleihan, Philip Fischer, Catherine Gordon, Frank Greer, Zeev Hochberg, Marjo Lehtonen‐Veromaa, Michael Levine, John Pettifor, Willem Proesmans, Frank Rauch, Eckhard Schönau, Nicolas Shaw, Mark Strand, Tom Thacher, Jan‐Maarten Wit, Zvi Zadik and Kathy Zu.
Appendices
Appendix 1. Search strategy
| Search strategy |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical Subject Heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. The following MEDLINE search strategy using the OVID database will be adapted for use with the other databases. (1) exp RICKETS/ (2) rickets.tw. (3) rachiti$.tw. (4) exp OSTEOMALACIA/ (5) osteomalac$.tw. (6) exp VITAMIN D Deficiency/ (7) (vitamin$‐D adj defic$).tw. (8) (defic$ adj vitamin$‐D).tw. (9) or/1‐8 (10) exp CALCIUM/ (11) exp Vitamin D/ (12) exp Ergocalciferols/ (13) exp CHOLECALCIFEROL/ (14) (calcium or vitamin$ D).tw. (15) sunlight$.tw. (16) exp SUNLIGHT/ (17) or/10‐16 (18) exp Dietary Supplements/ (19) supplement$.tw. (20) (defic$ or poor$ or poverty or lack$ or limit$).tw. (21) 18 or 19 or 20 (22) 17 and 21 (23) 22 or 9 (24) exp Infant, Newborn/ (25) exp INFANT/ (26) exp CHILD/ (27) (infant$ or newborn$ or child$).tw. (28) or/24‐27 (29) 23 and 28 (30) exp Infant, Premature/ (31) preterm$ or prematur$) adj infant$).tw. (32) 30 or 31 (33) 29 not 32 (34) limit 33 to human |
Appendix 2. Baseline characteristics
| Characteristic | Beser 1994 | Du 2004 | Duhamel 2000 | Strand 2002/2003 |
| Intervention 1 (I1) / intervention 2 (I2) / control 1 (C1) | I1: vitamin D 400 IU/day per os C1: no placebo or other intervention | I1: fortified milk, averaged calcium 245 mg/day per os I2: fortified milk, averaged calcium 245 mg/day per os, vitamin D3 133 IU/day per so C1: no placebo or other intervention | I1: vitamin D3 100,000 IU every three months per os C1: placebo | I1: promoting of exclusive breastfeeding from birth, supplementation of solid foods at age 5 months, weaning at 12 to 18 months, vitamin D 300 IU/day per os during the first 12 months, calcium 378 mg/day per os from age 5 months to 24 months C1: no placebo or other intervention |
| [n] (I1/ I2 / C1 / total) | I1: 302 I2: 374 Total: 676 | I1: 238 I2: 260 C1: 259 Total: 757 | I1: 32 C1: 34 Total: 66 | I1: 183 C1: 46 Total: 229 |
| Age at inclusion [mean (SD)] | Total: 3 months to 36 months (range) | Total: 10 years | Total: 12.5 years (1.5) | Total: approximately 1 month |
| Sex [n,%] | ? | female: 100 | male: 51 female: 49 | I1: male 50 female 50 C1: male 63 female 37 |
| Ethnic groups [%] | ? | ? | ? | ? |
| Socioeconomic status | ? | ? | ? | ? |
| Location | Turkey, villages around Akcaabat | China, Beijing | France, northern Loire | China, Shanxi Province |
| Start of study [year] | 1990 | 1999 | 1996 | 1997 |
| Length of follow‐up | 12 months | 24 months | 9 months | 6 to 30 months (mean age at outcome assessment: I1: 18.2 months C1: 15.6 months) |
| Outcome assessment | first clinical, than biochemical and radiological assessment | clinical assessment | radiological assessment | clinical assessment |
| Notes | Number of participants: Two children who showed radiological changes at the beginning of the study and at the end of the study were excluded for this review. Thus, number of participants was corrected. | Participants: included a small amount of preterm born children (which is assumed as a risk factor for rickets), proportion in intervention group higher than on control group (8.2% vs. 4.3%) Number of participants: based on analysed participants, total of enrolled 259, total of study completed 245 | ||
| Symbols & abbreviations: ? = unclear I = intervention; C = control |
Appendix 3. Primary outcome data
| Study | Occurence of rickets | Adverse effects |
| Beser 1994 I1: vitamin D 400 IU/day per os C1: no placebo or other intervention | I1: 0/302 C1: 14/374 | not investigated |
| Du 2004 I1: fortified milk, averaged calcium 245 mg/day per os I2: fortified milk, averaged calcium 245 mg/day per os, vitamin D3 133 IU/day per so C1: no placebo or other intervention | I1: 0/238 I2: 0/260 C1: 0/259 | not investigated |
| Duhamel 2000 I1: vitamin D3 100,000 IU every 3 months per os C1: placebo | I1: 0/32 C1: 0/34 | hypercalcaemia: I1: 0/32 C1: 0/34 |
| Strand 2002/2003 I1: promoting of exclusive breastfeeding from birth, supplementation of solid foods at age 5 months, weaning at 12 to 18 months, vitamin D 300 IU/day per os during the first 12 months, calcium 378 mg/day per os from age 5 months to 24 months C1: no placebo or other intervention | I1: 100/183 C1: 33/46 based on analysed patients | not investigated |
| Symbols & abbreviations: I = intervention; C = control |
Appendix 4. Secondary outcome data
| Characteristic | Mortality | Quality of life | Costs |
| Beser 1994 I1: vitamin D 400 IU/day per os C1: no placebo or other intervention | not investigated | not investigated | not investigated |
| Du 2004 I1: fortified milk, averaged calcium 245 mg/day per os I2: fortified milk, averaged calcium 245 mg/day per os, vitamin D3 133 IU/day per so C1: no placebo or other intervention | not investigated | not investigated | not investigated |
| Duhamel 2000 I1: vitamin D3 100,000 IU every 3 months per os C1: placebo | not investigated | not investigated | not investigated |
| Strand 2002/2003 I1: promoting of exclusive breastfeeding from birth, supplementation of solid foods at age 5 months, weaning at 12 to 18 months, vitamin D 300 IU/day per os during the first 12 months, calcium 378 mg/day per os from age 5 months to 24 months C1: no placebo or other intervention | not investigated | not investigated | not investigated |
| Abbreviations: I = intervention; C = control |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beser 1994.
| Methods | DURATION OF INTERVENTION: 12 months DURATION OF FOLLOW‐UP: 12 months LANGUAGE OF PUBLICATION: English | |
| Participants | WHO PARTICIPATED: Turkish children, aged 3 to 36 months at inclusion INCLUSION CRITERIA: prior exclusion of rickets EXCLUSION CRITERIA: see above DIAGNOSTIC CRITERIA: stepwise approach: clinical signs led to radiological and biochemical assessment CO‐MORBIDITIES: not stated CO‐MEDICATIONS: not stated | |
| Interventions | NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: Turkey, rural SETTING: community INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Vitamin D 400 IU/day per os CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): none TREATMENT BEFORE STUDY: not stated TITRATION PERIOD: not stated | |
| Outcomes | PRIMARY OUTCOME(S): occurrence of rickets SECONDARY OUTCOMES: none | |
| Notes | STATED AIM OF STUDY: to assess primary prevention of rickets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Du 2004.
| Methods | DURATION OF INTERVENTION: 2 years DURATION OF FOLLOW‐UP: 2 years LANGUAGE OF PUBLICATION: English | |
| Participants | WHO PARTICIPATED: Chinese girls, aged 10 years at inclusion INCLUSION CRITERIA: free of any disease that might affect bone development EXCLUSION CRITERIA: see above DIAGNOSTIC CRITERIA: clinical assessment CO‐MORBIDITIES: not stated CO‐MEDICATIONS: not stated | |
| Interventions | NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: China, urban SETTING: cluster study with primary schools as cluster INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): I1: fortified milk with calcium 560 mg/school day, averaged 245 mg/day I2: fortified milk with calcium 560 mg plus vitamin D3 200 (during first third of study) or 320 (during second and third third of study) IU/school day, averaged calcium 245 mg plus vitamin D3 133 IU/day CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): none TREATMENT BEFORE STUDY: not stated | |
| Outcomes | PRIMARY OUTCOME(S): bone mineral content, bone area, bone mineral density, total body composition, 25‐hydroxyvitamin D in blood, intact parathyroid hormone in blood, blood calcium in blood, calcium/creatinine ratio in urine, weight, length, sitting height, clinical signs of rickets SECONDARY OUTCOMES: no distinction made between primary and secondary outcomes | |
| Notes | STATED AIM OF STUDY: to examine the effects of providing a dietary supplement of a small volume of milk to Chinese pre‐pubertal girls in Beijing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Duhamel 2000.
| Methods | DURATION OF INTERVENTION: 9 months DURATION OF FOLLOW‐UP: 9 months LANGUAGE OF PUBLICATION: French | |
| Participants | WHO PARTICIPATED: French children, aged 10 to 15 years INCLUSION CRITERIA: hospital admission EXCLUSION CRITERIA: gastrointestinal or nephrological diseases or other conditions involved in the metabolism of vitamin D or phosphate DIAGNOSTIC CRITERIA: clinical, radiological and biochemical assessment CO‐MORBIDITIES: not stated CO‐MEDICATIONS: not stated | |
| Interventions | NUMBER OF STUDY CENTRES: 4 COUNTRY/ LOCATION: France, urban SETTING: hospital based INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): vitamin D3 100,000 IU at beginning, after 3 and after 6 month, per os CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): placebo TREATMENT BEFORE STUDY: not stated | |
| Outcomes | PRIMARY OUTCOME(S): calcium in blood and urine, phosphorus in blood and urine, alkaline phosphatases in blood, intact parathyroid hormone in blood, 25‐hydroxyvitamin D in blood, radiographs of the wrist SECONDARY OUTCOMES: no distinction made between primary and secondary outcomes | |
| Notes | STATED AIM OF STUDY: to follow the changes in calcium status and 25‐hydroxyvitamin D and parathyroid hormone levels under repeated dosis of vitamin D in winter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Strand 2002/2003.
| Methods | DURATION OF INTERVENTION: 2 years DURATION OF FOLLOW‐UP: up to 2.5 years LANGUAGE OF PUBLICATION: English | |
| Participants | WHO PARTICIPATED: Chinese children, age at inclusion approximately 1 month INCLUSION CRITERIA: not stated EXCLUSION CRITERIA: not stated (however, children with congenital heart defects were excluded) DIAGNOSTIC CRITERIA: clinical assessment CO‐MORBIDITIES: not stated CO‐MEDICATIONS: not stated | |
| Interventions | NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: China, rural SETTING: cluster study with villages as clusters INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): promoting of exclusive breastfeeding from birth, supplementation of solid foods at age 5 months, weaning at 12 to 18 months, vitamin D 300 IU/day per os during the first 12 months, calcium 378 mg/day per os from age 5 months to 24 months CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): none TREATMENT BEFORE STUDY: not stated | |
| Outcomes | PRIMARY OUTCOME(S): occurrence of rickets SECONDARY OUTCOMES: none stated | |
| Notes | STATED AIM OF STUDY: to determine the effectiveness of this rickets prevention programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Nguema‐Asseko 2005 | high risk for bias due to marked differences in discontinuation rates between groups after three months (intervention 9%, comparison 29%, overall 19%), after six months discontinuation rate 34% |
| Thacher | high risk for bias due to discontinuation 40% |
Differences between protocol and review
| Background | details added, arrangement changed | ||
| Types of studies | prospective cohort studies, controlled clinical trials, randomised controlled clinical trials | randomised controlled trials preferred, if not identified also controlled clinical trials, cohort studies, case‐control studies | to minimise bias |
| Time of outcome assessment | intervention or follow‐up for children under 12 months of age three months at least, for children over 12 months of age six months at least | minimum duration of prospective studies three years | we did not identify studies lasting three years |
| Selection of studies | in the first step, only one author (CL) checked title, abstract or both to determine publications dealing with nutritional rickets and obviously not reporting non‐comparative data | every step of studies selection was planned to be done by two authors | approximately 8000 records retrieved, efforts to reduce work load |
Contributions of authors
CHRISTIAN LERCH: protocol development, searching for trials, quality assessment of trials, data extraction, data analysis, review development THOMAS MEISSNER: protocol development, quality assessment of trials, data extraction, review development
Sources of support
Internal sources
Heinrich‐Heine University, Germany.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Beser 1994 {published data only}
- Beser E, Cakmakci T. Factors affecting the morbidity of vitamin D deficiency and primary protection. East African Medical Journal 1994;71:358‐62. [PubMed] [Google Scholar]
Du 2004 {published and unpublished data}
- Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, et al. School‐milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10‐12 years in Beijing. British Jounral of Nutrition 2004;92:159‐68. [DOI] [PubMed] [Google Scholar]
Duhamel 2000 {published data only}
- Duhamel JF, Zeghoud F, Sempé M, Boudailliez B, Odièvre M, Laurans M, et al. Prevention of vitamin D deficiency during adolescence: interventional multicenter study on the biological effects of repeated dosis of 100,000 IU of vitamin D3 [Prophylaxie de la carence en vitamine D chez l'adolscent et le préadolscent. Étude interventionnelle multicentrique sur les effets biologiques d'un apport répété de 100 000 UI de vitamine D3]. Archives de Pédiatrie 2000;7:148‐53. [DOI] [PubMed] [Google Scholar]
Strand 2002/2003 {published and unpublished data}
- Strand MA, Peng G, Zhang P, Lee G. Preventing rickets in locally appropriate ways: a case report from north China. International Quarterly of Community Health Education 2002/2003;21:297‐322. [Google Scholar]
References to studies excluded from this review
Nguema‐Asseko 2005 {published data only}
- Nguema‐Asseko B, Ganga‐Zandzou PS, Ovono F, Lendoye E, Lemamy GJ, Akendengue B, et al. Vitamin D status in gabonese children [Statut et besoins en vitamine D chez le nourisson au Gabon]. Archives de pédiatrie 2005;12:1587‐90. [DOI] [PubMed] [Google Scholar]
Thacher {unpublished data only}
Additional references
Bronner 2003
- Bronner F. Mechanisms of intestinal calcium absorption. Journal of Cellular Biochemistry 2003;88:387‐93. [DOI] [PubMed] [Google Scholar]
Callaghan 2006
- Callaghan AL, Moy RJD, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Archive of Diseases in Childhood 2006;91:606‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chesney 2001
- Chesney RW. Vitamin D deficiency and rickets. Reviews in Endocrine & Metabolic Disorders 2001;2:145‐51. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Cooke 1973
- Cooke WT, Swan CH, Asquith P, Melikian V, McFeely WE. Serum alkaline phosphatase and rickets in urban schoolchildren. British Medical Journal 1973;1(5849):324‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21:1575‐600. [DOI] [PubMed] [Google Scholar]
Deeks 2003
- Deeks JJ, Dines J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):iii‐x, 1‐173. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health 1998;52:377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusso 2005
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. American Journal of Physiology: Renal Physiology 2005;289:F8‐28. [DOI] [PubMed] [Google Scholar]
Harris 2001
- Harris NS, Crawford PB, Yangzom Y, Pinzo L, Gyaltsen P, Hudes M. Nutritional and health status of Tibetan children living at high altitude. New England Journal of Medicine 2001;344:341‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4. Chichester, UK: John Wiley & Sons, Ltd, 2006.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jelliffe 1968
- Jelliffe DB. Infant nutrition in the subtropics and tropics. 2nd Edition. Geneva: World Health Organization, 1968. [PubMed] [Google Scholar]
Lapatsanis 1968
- Lapatsanis P, Deliyanni V, Doxiadis S. Vitamin D deficiency rickets in Greece. The Journal of Pediatrics 1968;73:195‐202. [DOI] [PubMed] [Google Scholar]
Markestad 1987
- Markestad T, Hesse V, Siebenhuner M, Jahreis G, Aksnes L, Plenert W, et al. Intermittent high‐dose vitamin D prophylaxis during infancy: effect on vitamin D metabolites, calcium, and phosphorus. American Journal of Clinical Nutrition 1987;46:652‐8. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Peach 1984
- Peach H. A critique of survey methods used to measure the occurence of osteomalacia and rickets in the United Kingdom. Community Medicine 1984;6:20‐8. [PubMed] [Google Scholar]
Pettifor 2003
- Pettifor JM. Nutritional rickets. In: Glorieux FH, Pettifor JM, Jüppner H editor(s). Pediatric Bone. Amsterdam: Academic Press, 2003:541‐65. [Google Scholar]
Pettifor 2005
- Pettifor JM. Rickets and vitamin D deficiency in children and adolescents. Endocrinology and metabolism clinics of North America 2005;34:537‐53. [DOI] [PubMed] [Google Scholar]
Pfitzner 1988
- Pfitzner MA, Thacher TF, Pettifor JM, Zoakah AI, Lawson JO, Isichei CO, et al. Absence of vitamin D deficiency in young Nigerian children. The Journal of Pediatrics 1998;133:740‐4. [DOI] [PubMed] [Google Scholar]
Rajakumar 2003
- Rajakumar K. Vitamin D, cod‐liver oil, sunlight and rickets: a historical perspective. Pediatrics 2003;112:e132‐5. [DOI] [PubMed] [Google Scholar]
Richards 1968
- Richards IDG, Sweet EM, Arneil GC. Infantile rickets persists in Glasgow. Lancet 1968;1:803‐5. [DOI] [PubMed] [Google Scholar]
Rönnefarth 2000
- Rönnefarth G, Misselwitz J, Members of the Arbeitsgemeinschaft für pädiatrische Nephrologie. Nephrocalcinosis in children: a retrospective survey. Pediatric Nephrology 2000;14:1016‐21. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shaw 2004
- Shaw NJ, Kershaw M. Vitamin D deficiency in children. In: David TJ editor(s). Recent Advances in Paediatrics 21. London: Royal Society of Medicine Press, 2004:85‐100. [Google Scholar]
Thacher 2003
- Thacher TD. Calcium‐deficiency rickets. Endocrine Development 2003;6:105‐25. [DOI] [PubMed] [Google Scholar]
Thacher 2006
- Thacher TD, Fisher PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Annals of Tropical Paediatrics 2006;26:1‐16. [DOI] [PubMed] [Google Scholar]


