Abstract
Background
Worldwide, the prevalence of obesity and overweight in industrialized countries and in a substantial number of developing countries is increasing at an alarming rate. Rimonabant is a selective cannabinoid‐1 receptor antagonist that has been investigated for its efficacy in reducing body weight and associated risk factors in obese people. Phase III trials are now under way to test the use of rimonabant for long‐term weight‐loss. Given the prevalence of overweight and obesity, it is important to establish the efficacy and safety of rimonabant.
Objectives
To assess the effects of rimonabant in overweight and obese people.
Search methods
MEDLINE, EMBASE, The Cochrane Library, LILACS, databases of ongoing trials and reference lists were used to identify relevant trials.
Selection criteria
Randomised controlled trials comparing rimonabant with placebo or other weight loss interventions in overweight or obese adults.
Data collection and analysis
Two reviewers independently assessed all potentially relevant citations for inclusion and methodological quality. The primary outcome measures were weight loss change, morbidity and adverse effects occurrence.
Main results
Four studies evaluating rimonabant 20 mg versus rimonabant 5 mg versus placebo in addition to a hypocaloric diet lasting at least one year were included. Compared with placebo, rimonabant 20 mg produced a 4.9 kg greater reduction in body weight in trials with one‐year results. Improvements in waist circumference, high‐density lipoprotein cholesterol, triglyceride levels and systolic and diastolic blood pressure were also seen. However, the results with rimonabant 5 mg demonstrated a weight reduction which was only 1.3 kg greater when compared with placebo. No clinically relevant effects on plasma lipids and blood pressure were found. Rimonabant 20 mg caused significant more adverse effects both of general and serious nature, especially of nervous system, psychiatric or gastro‐intestinal origin. Attrition rates were approximately 40% at the end of one year.
Authors' conclusions
The use of rimonabant after one year produces modest weight loss of approximately 5%. Even modest amounts of weight loss may be potentially beneficial. The observed results should be interpreted with some caution, though, since the evaluated studies presented some deficiencies in methodological quality. Studies with longer follow‐ups after the end of treatment and of more rigorous quality should be done before definitive recommendations can be made regarding the role of this new medication in the management of overweight or obese patients.
Plain language summary
Rimonabant for overweight or obesity
Rimonabant is the first drug of a new class of medications that seems to reduce body weight and improve risk factors for diseases of the blood vessels and heart in people who are overweight or obese. We found four studies which evaluated weight loss, occurrence of disorders and adverse effects of treatment. The four studies involved 6625 people comparing rimonabant 20 mg with rimonabant 5 mg and placebo, in combination with a hypocaloric diet after one or two years of treatment. Greater weight loss and improvement in risk factors were seen after 20 mg of rimonabant. These results have to be interpreted with caution though, due to high discontinuation rates of study participants and the overall low quality of the included studies. We conclude that: 1. average weight loss with rimonabant appears modest, and 2. more rigorous studies examining the efficacy and safety of rimonabant are required to fully evaluate the benefit risk ratio of this new drug. In Europe, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications. Rimonabant is furthermore not recommended for patients with other untreated psychiatric conditions.
Background
Description of the condition
Worldwide, the prevalence of obesity and overweight in industrialized countries and in a substantial number of developing countries is increasing at an alarming rate (WHO 2000). This problem is associated with a large variety of health consequences representing an enormous burden on health care systems and, most importantly, the quality of life of the affected individuals might be substantially lowered. Obesity refers to an excess of body fat or adiposity. Body mass index (BMI; in kg/m2) is widely recognized as a weight‐for‐height index that has a high correlation with adiposity. In clinical terms, a BMI of 25‐29 kg/m2 is classified as overweight and a higher BMI (greater than 30 kg/m2) reflects obesity (WHO 2000). Overweight and obesity are associated with a large variety of health consequences, including hypertension (Brown 2000; Dyer 1989), type 2 diabetes mellitus (Medalie 1974; Ohlson 1985), heart diseases (NIHCD 1985; Willett 1995), stroke (Rexrode 1997; Walker 1996), osteoarthritis (Cicuttini 1996; Hart 1993), sleep apnoea and respiratory difficulties (Chua 1994) and also several common cancers (Bergstrom 2001; Chu 1991; Schottenfeld 1996). The presence of excess fat in the abdomen out of proportion to total body fat is an independent predictor of type 2 diabetes, dyslipidaemia, hypertension and cardiovascular disease (NHLBI 1998). Waist circumference and waist‐to‐hip circumference ratios (WHR) are simple and convenient for epidemiological studies and provide a useful estimation of the proportion of abdominal or upper‐body fat (Björntorp 1984; Björntorp 1985; Kissebah 1985). Several studies have investigated the correlation of abdominal fat and increased mortality and risk for disorders such as diabetes, hyperlipidaemia, hypertension and cardiovascular diseases (Donahue 1987; Ducimetiere 1986; Lapidus 1984; Larsson 1984; Ohlson 1985; Stokes 1985).
Description of the intervention
Obesity is seen as a disease, which requires treatment. Treatment means, first of all, weight reduction. The general goals of weight loss and management are: (1) to reduce body weight; and (2) to maintain a lower body weight over the long term; or (3) at a minimum, to prevent further weight gain. There is strong evidence that weight loss (5% to 15% of the body weight) in obese individuals reduces risk factors associated with obesity (NHLBI 1998).
Traditional methods to promote weight loss focus on reducing energy intake through low‐calorie or low‐fat diets, increasing energy expenditure by increase in physical activity, and behavioural modification. The inclusion of exercise in a weight reduction program is supposed to make the weight loss easier, compared with food restriction alone. However, the effectiveness of these weight reduction methods is limited, with an overall pattern of moderate weight loss followed by gradual weight regain (Curioni 2005). Numerous other weight loss interventions are available including pharmacotherapy, surgery to reduce food consumption and alternative therapies. Surgical procedures have greater long‐term success rates but are currently indicated for the very obese only (BMI greater than 40 kg/m2 or BMI 35 to 40 kg/m2 together with an obesity‐related disorder). Operative mortality rates are reported to average below one percent, but long‐term complications such as malabsorption syndromes may occur (Greenway 1996). Pharmacotherapy should be considered in overweight and obese patients with a BMI greater than 27 kg/m2, particularly in the presence of comorbidities or an increased waist circumference, when conservative measures such as behaviour therapy, diet and exercise have not resulted in the desired weight loss (NHLBI 1998). Drugs should always be used in conjunction with non‐pharmacological therapy, though.
Approved anti‐obesity medications can be divided into two broad categories: (1) Inhibitors of intestinal fat absorption, where the only agent currently available compound in this class is orlistat, a drug that inhibits pancreatic and other lipases. Side effects are related to malabsorption of fat within the gastrointestinal tract and include steatorhea, bloating, and oily discharge. Fecal incontinence and malabsorption of fat‐soluble vitamins, such as vitamin A, D, E, and K, have also been reported (McNeely 1998). (2) Drugs that act to suppress appetite. An example of this category includes sibutramine, which inhibits re‐uptake of serotonin and norepinephrine. The most common adverse effects are related to increased adrenergic activity and include dry mouth, headache, insomnia, and constipation. Sibutramine may also cause increases in blood pressure and heart rate (Luque 1999). Orlistat and sibutramine are the only medications approved for long‐term use.
A number of anti‐obesity drugs are currently undergoing clinical development. These include: (1) Centrally‐acting drugs, such as the noradrenergic and dopaminergic re‐uptake inhibitor rodafaxine, the endocannabinoid antagonist rimonabant, the selective serotonin 5‐HT2C agonist APD‐356, and oleoyl‐estrone; (2) Drugs that target peripheral episodic satiety signals, such as glucagon‐like peptide‐1, peptide YY and amylin; (3) Drugs that block fat absorption, such as the novel lipase inhibitors cetilistat and GT‐389255; and (4) A human growth hormone fragment (AOD‐9604) that increases adipose tissue breakdown (Halford 2006 ). Most of these investigational drugs are in the early or mid‐stages of development. Rimonabant is the only drug in the late‐stage of investigations.
How the intervention might work
Rimonabant is a selective cannabinoid‐1 receptor antagonist that has been investigated for its efficacy in reducing body weight and associated risk factors in obese people (Bonner 2005). The cannabinoid‐1 receptor plays a role in the regulation of appetite and body weight. The endocannabinoid system (EC) consists of cannabinoids receptors (CB1 receptors) and endocannabinoids, as well as enzymes catalysing their biosynthesis and degradation (Di Marzo 2005). CB1 receptors are expressed predominantly in several areas of the brain and in peripheral organs, including the autonomic nervous system, liver, muscle, gastrointestinal tract, and adipose tissue (Di Marzo 2004). Pharmacological stimulation of CB1 receptors by systemic administration of plant or endogenous cannabinoids stimulates eating, even in satiated animals (Colombo 1998; Rowland 2001; Simiand 1998). The administration of the first selective CB1 antagonist (rimonabant) attenuated agonists' stimulatory effects on food intake and strongly reduced both the consumption of palatable food (such as sweet food) and the intake of normal food by animals deprived of food (Rinaldi‐Carmona 2004; Werner 2003). The EC system may regulate food intake by modulating actions or expressions of many anorectic and orexigenic mediators in the brain, particularly hypothalamic peptides such as corticotrophin‐releasing hormone (CRH) and melanin‐concentrating hormone (MCH). Of particular importance are the effects of endocannabinoids on the mesolimbic system that modulates reward behaviours (such as food intake as a reward after managing stressful situations). Endocannabinoids enhance dopamine release in the nucleus accumbens, thus increase the drive to eat (Di Marzo 2005). Rimonabant has been shown to reduce food intake, hunger and body weight in overweight or obese men after seven days of treatment (Vickers 2005).
Phase III trials are now under way to test the use of rimonabant for long‐term weight‐loss. Four clinical trials (RIO (rimonabant in obesity) RIO‐North America, RIO‐Europe, RIO‐Diabetes and RIO‐Lipids) involving 6000 participants suggest that rimonabant 20 mg produces a placebo‐subtracted weight loss of approximately five kg. No major side effects were reported; however, given the wide distribution of CB1 receptors within the body, it is possible that the drug could affect a number of systems unrelated to eating (Halford 2006). A Cochrane systematic review is being developed to analyse the efficacy of cannabinoid type 1 receptor antagonists for smoking cessation (Hey 2005)
Important adverse effects
In Europe, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications. Rimonabant is furthermore not recommended for patients with other untreated psychiatric conditions.
Why it is important to do this review
There is no systematic review evaluating the efficacy and also the possible adverse effects of rimonabant in overweight or obese people. Given the prevalence of overweight and obesity, it is important to establish the possible impact of rimonabant in people suffering from these conditions.
Objectives
To assess the effects of rimonabant in overweight and obese people.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were considered for inclusion.
Types of participants
Individuals aged 18 years and older defined as overweight or obese at baseline. Criteria for defining overweight or obesity include BMI cut‐off points (WHO 2000). Ideally, diagnostic criteria should have been described. If necessary, authors' definition of overweight or obesity were used and eventually subjected to sensitivity analysis. Studies including children, pregnant women, or patients with serious medical conditions would have been excluded.
Types of interventions
Interventions eligible for inclusion in the review included:
Rimonabant versus placebo;
Rimonabant plus other interventions such as diet or exercise versus placebo plus the same intervention;
Rimonabant versus any other pharmacological intervention;
Rimonabant versus a non‐pharmacological intervention.
Types of outcome measures
Primary outcomes
To be eligible for inclusion in the review, trials had to report one or more of the following outcomes:
change in weight measures (for example body weight, body mass index (BMI), waist circumference or hip/waist circumference, other anthropometric measures);
morbidity (such as cardiovascular disorders, gastrointestinal disorders, nervous system disorders, psychiatric disorders, renal and urinary disorders);
adverse effects of treatment (such as headache, nausea, anxiety, insomnia, gastrointestinal symptoms)
Secondary outcomes
all‐cause mortality;
change in risk factors (such as blood pressure, lipid profile and HbA1c);
health‐related quality of life (ideally using a validated instrument);
Costs.
Specific patient covariates thought to be effect modifiers
compliance with the treatment;
initial overweight or obesity;
duration of the intervention.
Timing of outcome assessment (duration of the intervention)
short‐term (four weeks to 24 weeks of treatment);
medium‐term (more than 24 weeks to 12 months of treatment);
long‐term (more than 12 months of treatment).
Search methods for identification of studies
Electronic searches
The following electronic databases were searched to identify relevant trials, reviews, meta‐analyses, and economic analyses:
The Cochrane Library (issue 3, 2006);
MEDLINE (up to June 2006);
EMBASE (up to June 2006);
LILACS (up to June 2006);
There were no language restrictions for either searching or trial inclusion. The search strategy using a combination of MeSH terms and text words was used for MEDLINE and was adapted to suit the other databases. For a detailed search strategy, please see under Appendix 1.
Databases of ongoing trials
Current Controlled Trials (http://www.controlled‐trials.com);
National Research Register (http://www.update‐software.com/National/nrr‐frame.html);
National Institutes of Health (http://clinicalstudies.info.nih.gov/)
Searching other resources
Efforts were made to identify additional studies by searching the references lists of relevant trials and reviews identified.
Authors of relevant identified studies and other experts were contacted to obtain additional references, unpublished trials, ongoing trials and missing data not reported in the published trials.
Data collection and analysis
Selection of studies
Two reviewers (CC and CA) independently scanned the titles, abstracts and keywords of trials identified through the respective search strategies. The articles were evaluated for inclusion whenever there was a suggestion that they:
Included patients with overweight or obesity;
Compared rimonabant with placebo or another active intervention;
Articles were rejected on initial screen if both reviewers could positively determine from the title and abstract that they did not meet the inclusion criteria. All potentially relevant articles were investigated as full text. Agreement between the two reviewers was expressed using the kappa statistic (Cohen 1960). Eventual disagreement was resolved by discussion between the reviewers. Excluded studies and the reasons for exclusion were clearly reported in a specific table.
Data extraction and management
Two reviewers (CC and CA) independently extracted data from each study using standard data extraction forms (for details see Characteristics of included studies and Table 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5). Differences between reviewers' extraction results were resolved by discussion. The standard data extraction forms included the following items:
1. Study quality (included studies).
| Characteristic | RIO‐Lipids | RIO‐Europe | RIO‐North America | RIO‐Diabetes |
| Randomised controlled clinical trial | Y | Y | Y | Y |
| Non‐inferiority / equivalence trial | N | N | N | N |
| Controlled clinical trial | NA | NA | NA | NA |
| Design: Parallel study | Y | Y | Y | Y |
| Design: Crossover study | NA | NA | NA | NA |
| Design: Factorial study | N | N | N | N |
| Crossover study: Wash‐out phase | NA | NA | NA | NA |
| Crossover study: Carryover effect tested | NA | NA | NA | NA |
| Crossover study: Period effect tested | NA | NA | NA | NA |
| Method of randomisation | ? | Y | Y | ? |
| Unit of randomisation (individuals, cluster ‐ specify) | ? | individuals | individuals | ? |
| Randomisation stratified for centres | ? | ? | Y | ? |
| Concealment of allocation | double | double | double | double |
| Stated blinding (open; single, double, triple blind) | ? | ? | ? | ? |
| Actual blinding: Patient | ? | ? | ? | ? |
| Actual blinding: Carer / treatment administrator | ? | ? | ? | ? |
| Actual blinding: Outcome assessor | ? | ? | ? | ? |
| Actual blinding: Others | ? | ? | ? | ? |
| Blinding checked: Patient | ? | ? | ? | ? |
| Blinding checked: Carer / treatment administrator | ? | ? | ? | ? |
| Primary endpoint defined (power calculation) | ? | ? | Y | ? |
| Total number of outcomes | 22 | 12 | 10 | 6 |
| Prior publication on design of study | N | N | N | N |
| Outcomes of prior / current publication identical | N | N | N | N |
| Power calculation | ? | ? | Y | ? |
| [n] patients per group calculated | ? | ? | Y | ? |
| Non‐inferiority trial: Interval for equivalence specified | NA | NA | NA | NA |
| Intention‐to‐treat analysis | Y | Y | Y | Y |
| Per‐protocol‐analysis | ? | ? | ? | ? |
| Analysis stratified for centres | N | N | N | N |
| Missing data: Last‐observation‐carried‐forward (LOCF) | Y | Y | Y | Y |
| Missing data: Other methods | N | N | Y: baseline imputed | N |
| Treatment Y (T1) / treatment 2 (T2) / control Y (C1) | ||||
| [n] of screened patients (T1/ T2 / C1 / total) | ? | 2168 | 4604 | ? |
| [n] of eligible patients (T1/ T2 / C1 / total) | ? | 1676 | 3500 | ? |
| [n] of randomised patients (T1/ T2 / C1 / total) | 346 / 345 / 342 / 1033 | 599 / 603 / 305 / 1507 | 1222 / 1216 / 607 / 3045 | 339 / 358 / 348 / 1045 |
| [n] of patients finishing the study (T1/ T2 / C1 / total) | 221 / 208 / 214 / 643 | 363 / 379 / 178 / 920 | 673 / 620 / 309 / 1602 | ? |
| [n] of ITT patients (T1/ T2 / C1 / total) | 344 / 340 / 334 / 1018 | 599 / 603 / 305 / 1507 | 1189 / 1191 / 590 / 2970 | 339 / 358 / 348 / 1045 |
| [n] of patients analysed (T1/ T2 / C1 / total) | 344 / 340 / 334 / 1018 | 599 / 603 / 305 / 1507 | 1189 / 1191 / 590 / 2970 | 339 / 358 / 348 / 1045 |
| Drop‐outs described | Y | Y | Y | N |
| Withdrawals described | Y | Y | Y | N |
| Losses‐to‐follow‐up described | Y | Y | Y | N |
| [n] of drop‐outs (T1/ T2 / C1 / total) | 125 / 137 / 128 / 390 | 236 / 224 / 127 / 587 | 549 / 596 / 298 / 1443 | ? |
| [%] attrition rate (T1/ T2 / C1 / total) | 36 / 40 / 37 / 38 | 40 / 37 / 42 / 39 | 45 / 49 / 49 / 47 | ? |
| Differences to [n] / power calculation | ? | ? | ? | ? |
| [n] of subgroups | ? | ? | ? | ? |
| Subgroups: Pre‐defined | ? | ? | ? | ? |
| Subgroups: Post‐hoc | ? | ? | ? | ? |
| Adjustment for multiple outcomes / repeated measurements | Y | Y | Y | ? |
| Baseline characteristics: Clinically relevant differences | N | N | N | N |
| Treatment identical (apart from intervention) | Y | Y | Y | Y |
| Timing of outcomes' measurement comparable between groups | Y | Y | Y | Y |
| Compliance measured | ? | ? | ? | ? |
| Other important covariates measured | N | N | N | N |
| Co‐morbidities measured | Y | Y | Y | Y |
| Co‐medications measured | Y | ? | ? | ? |
| Specific doubts about study quality | N | N | N | N |
| Funding: Commercial | Y | Y | Y | Y |
| Funding: Non‐commercial | N | N | N | N |
| Publication status: Peer review journal | Y | Y | Y | N |
| Publication status: Journal supplement | N | N | N | N |
| Publication status: Abstract | N | N | N | N |
| Publication status: Other | N | N | N | Y (presentation in congress) |
| Notes | ||||
| Symbols: yes=Y; no=N; unclear=? | ||||
| Abbreviations: T=treatment; C=control; NA=not applied | ||||
General information: published or unpublished, title, authors, source, contact address, country, language and year of publication, duplicate publications, sponsoring, and setting;
Participants: sampling (random or convenience), exclusion criteria, total number and number in the compared groups; age; gender; initial BMI, assessment of compliance, similarity of groups at baseline (including any co‐morbidity), withdrawals or losses to follow‐up (reasons or description), subgroups;
Trial characteristics: design, duration, randomisation (and its method), allocation concealment (and its method), blinding (patients, people administering treatment, outcome assessors), and check of blinding;
Interventions: types, duration, description (schedule, dose, route, timing, etc.);
Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes;
Results: measures of effect specified above, use of intention‐to‐treat analysis.
Assessment of risk of bias in included studies
Two reviewers evaluated independently methodological quality of trials by means of individual quality component analysis (CC and CA). Inter‐rater agreement was planned to be calculated using the kappa statistic (Cohen 1960). Studies were not excluded only on the basis of low quality.
Measures of treatment effect
Continuous data
Continuous outcomes (for example weight loss measure by weight) were expressed, if possible, as weighted mean differences with 95% confidence intervals (CI). If results for continuous outcomes were presented on different scales, we planned to use the standardised mean differences (SMD).
Dichotomous data
Dichotomous outcomes (for example stroke yes or no) were expressed as odds ratios (OR) or relative risks (RR) with 95% CI.
Time‐to‐event data
Time‐to‐event outcomes (for example time until death) were expressed as hazard ratios (HR) with 95% CI.
Unit of analysis issues
Different units of analysis (for example OR and RR) were planned to be subjected to a sensitivity analysis.
Dealing with missing data
When necessary, relevant missing data were sought by correspondence with the main authors of the studies. Evaluation of important numerical data such as screened, eligible and randomised patients as well as intention‐to‐treat and per‐protocol population were carefully performed. Drop‐outs, misses to follow‐up and withdrawn study participants were investigated.
Dealing with duplicate publications
Multiple publications were planned to be collated and assessed as one study to try maximise yield of information by simultaneous evaluation of all available data.
Assessment of heterogeneity
Heterogeneity between trial results were tested for using a standard chi‐squared test. Tests of heterogeneity are used for examining whether the observed variation in study results is compatible with the variation expected by chance alone. A significance level of alpha set at 0.1 was used for the test of heterogeneity in view of the low power of such tests. Heterogeneity was also examined with I2, where I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When found, we attempted to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
Assessment of reporting biases
Funnel plot asymmetry were planned to be assessed statistically to explore publication bias if sufficient randomised clinical trials were identified (Egger 1997).
Data synthesis
Data were summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analyses were be performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Pooled results were analysed using primarily a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to explore whether there were any systematic differences between groups of patients. If the results of at least one of the main outcomes were significant, in order to explore effect size differences and if the amount of data permitted, subgroup analyses were planned to be conducted according to the following:
Weight level (BMI) at baseline;
Age;
Gender;
Different comparison interventions;
Duration of intervention.
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking study quality, as specified above, into account;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also be tested by repeating the analysis using different measures of effects size (risk difference, odds ratio etc) and different statistical models (fixed and random‐effects models).
Results
Description of studies
From the initial search, 326 records were identified, and from these, 75 full papers were identified for further examination. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study. See Figure 1 for details of the amended QUOROM (quality of reporting of meta‐analyses) statement.
1.
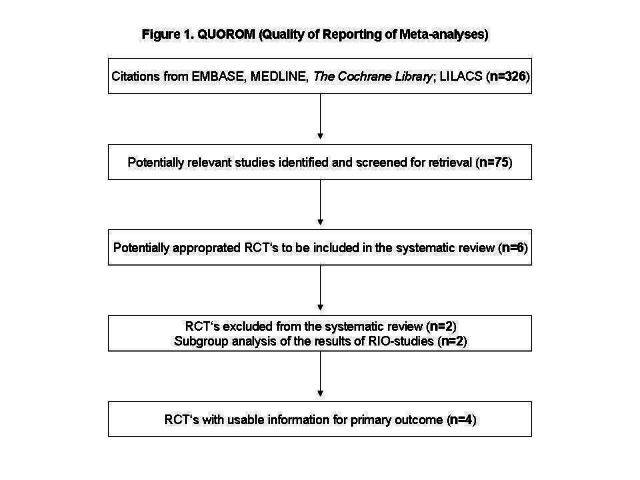
QUOROM (quality of reporting of meta‐analyses) flow chart for study selection
After screening the full text of the selected papers, six studies finally met the inclusion criteria. Two studies were only presented as abstracts of congresses. One of these was a subgroup analysis of the RIO‐studies and it was not included in the results. This study is included in Characteristics of excluded studies. One of the six studies is an one ongoing trial, with an expected end of study in August, 2007 (see Characteristics of ongoing studies for details). One additional title was selected, but it was not retrieved because the reference was wrong. The other three articles were published in peer review journals.
Assessment of publication bias and inter‐rater agreement
Two authors (CC and CA) reviewed the studies. There was agreement on the studies to be extracted for closer inspection from the searches. The full papers were obtained, and from these, the studies eligible for the review were selected. The authors completely agreed on the final papers chosen for the review.
Missing data
Some authors were contacted for further information. The authors of the RIO‐North America study (Dr Pi‐Sunyer and Dr Aronne) were contacted by e‐mail to ask whether they could send details about the results, which were only published as placebo subtracted changes from baseline or changes from baseline only presented in figures, but unfortunately we received no answer. Professor Hollander, who presented the results of the RIO‐Diabetes trial in the '1st International Congress on "Prediabetes" and the Metabolic Syndrome, 2005', was contacted by e‐mail for particulars about the methodology and results of the study, but also did not reply. Dr Jensen was contact by e‐mail to ask about more information regarding the study entitled "The selective CB1‐receptor antagonist rimonabant reduces body weight and waist circumference in obese subjects" which was presented only as an abstract. He replied, and stated that the presented study constituted a subgroup analysis of the results of three RIO‐studies (RIO‐Europe, RIO‐Lipids and RIO‐North America); the abstract publication therefore was not included in the present analysis. With regards to the selected title where the reference was wrong entitled "SR141716, a selective cannabinoid CB1 receptor antagonist, reduces hunger, caloric intake and body weight in over weight or obese men", we got an answer from Dr Belisse, one of authors, informing that the study was part of a series of trials done by the Sanofi company on this product, and that the methods and results are the property of Sanofi. Unfortunately, we got no answer from Sanofi when we asked for unpublished data.
Excluded studies
Excluded studies and the reasons for exclusion are given in the table Characteristics of excluded studies.
Included studies
Details of the characteristics of the included studies are given in the table Characteristics of included studies. The following gives a brief overview.
Characteristics of included studies
Methods
All four studies finally selected for the review were randomised controlled trials published in English. The duration of the intervention was 24 months for the RIO‐North America and 12 months for the RIO‐Diabetes, RIO‐Lipids and RIO‐Europe study. Although the last two described a period of 24 months during which they were conducted, only the first 12‐months results are provided. All trials had a run‐in, as a single blind period before the randomisation.
Participants
The included studies involved 6625 participants. The main inclusion criteria entailed adults (18 years or older), with a body mass index greater than 27 kg/m² and less than 5 kg variation in body weight within the three months before study entry.
Intervention
All trials were multicentric. The RIO‐North America was conducted in the USA and Canada, RIO‐Europe in Europe and the USA, RIO‐Diabetes in the USA and 10 other different countries not specified, and RIO‐Lipids in eight unspecified different countries. The intervention received was placebo, 5 mg of rimonabant or 20 mg of rimonabant once daily in addition to a mild hypocaloric diet (600 kcal/day deficit).
Outcomes
Primary In all studies the primary outcome assessed was weight change from baseline after one year of treatment and the RIO‐North America study also evaluated the prevention of weight regain between the first and second year. All studies evaluated adverse effects, including those of any kind and serious events. Quality of life was measured in only one study, but the results were not described (RIO‐Europe).
Secondary and additional outcomes These included prevalence of metabolic syndrome after one year and change in cardiometabolic risk factors such as blood pressure, lipid profile, etc.
No study included mortality and costs as outcome.
The timing of outcome measures was variable and could include monthly investigations, evaluations every three months or a single final evaluation after one year.
Risk of bias in included studies
The methodological characteristics of the included studies are summarised in Table 1. There was complete agreement between the two authors regarding quality assessment; therefore a kappa‐statistic was not calculated.
Overview
All four studies had some methodological weaknesses according to the methodological quality criteria evaluated. None fulfilled all quality criteria.
Randomisation and allocation concealment
All selected trials were described as randomised. However, only two studies reported an appropriate method of randomisation (RIO‐Europe and RIO‐North America). Most studies did not mention any procedure for allocation concealment, with only one reporting adequate allocation concealment (RIO‐Europe). The main characteristics of the participants in the three groups were similar at baseline in all selected studies.
Blinding
The selected trials were described as double‐blind. However, no details of the methods employed or at which stage of the process blinding was performed, were reported. No trial reported blinding of outcome assessors (those taking samples or carrying out laboratory tests). None of the included studies specifically reported blinding of either the study participants or the providers of treatment.
Power calculation
Power calculations were reported as incorporated in the design of one of the four studies included in this review. The sample size of the RIO‐North America study was based on 99% power to detect a 3‐kg difference between one dose of rimonabant and placebo after one year with P < 0.025. No other included study reported power calculations.
Descriptions of losses to follow‐up
Three of the selected trials described losses to follow‐up (RIO‐Europe, RIO‐Lipids and RIO‐North America). Only one, RIO‐Diabetes, described the number of patients who discontinued treatment because of adverse events.
Intent‐to‐treat (ITT) population analyses
All studies reported an intention‐to‐treat analysis. The method used for missing data was mainly last‐observation‐carried‐forward. No study described the ITT population in detail.
Effects of interventions
Results of the meta‐analyses are reported below. Data from the studies were pooled for meta‐analysis at one year.
Primary outcomes
Weight (kg) change
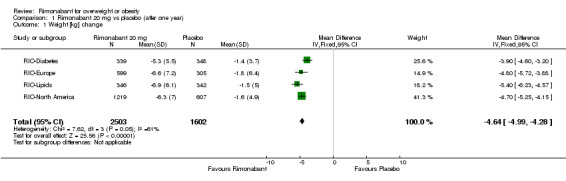
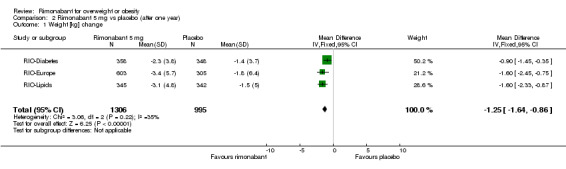
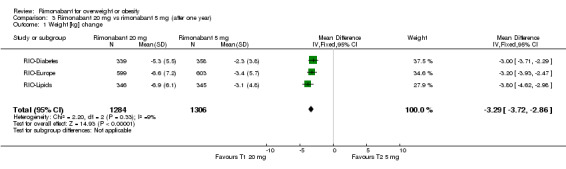
Comparing rimonabant 20 mg with placebo, meta‐analysis of all included studies showed evidence of heterogeneity with a reduction in mean weight of 4.6 kg for the rimonabant group but this result could not be considered (I² = 60.6%). When we performed the analysis excluding the RIO‐Diabetes study which was only published as an abstract of congress we observed that the heterogeneity was significantly reduced and the pooled data showed a reduction in mean weight of 4.9 kg (‐4.9 weighted mean difference [WMD], 95% confidence interval [CI] ‐5.3 to ‐4.5). In the comparison between rimonabant 5 mg and placebo, the pooled effect was a weight reduction of 1.3 kg for the rimonabant group excluding the RIO‐North America study due to lack of data (‐1.3 kg WMD, 95% CI ‐1.6 to ‐0.9). Comparing the two intervention groups, there was a pooled effect of weight reduction of 3.3 kg for the rimonabant 20 mg group, also excluding the RIO‐North America study (‐3.3 kg WMD, 95% CI ‐3.7 to ‐2.9). All results were statistically significant (P < 0.0001).
Waist circumference (cm) change
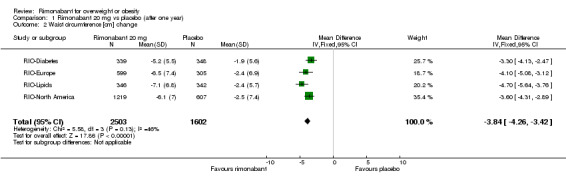
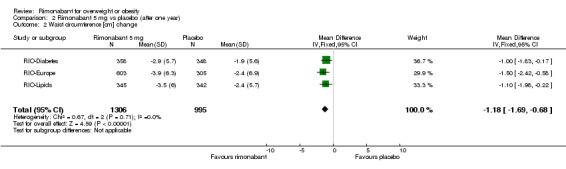
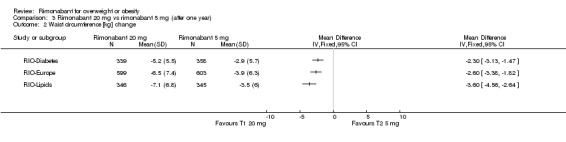
The pooled meta‐analysis of all included studies showed evidence of a reduction in waist circumference of 3.8 cm for the rimonabant 20 mg group when compared with placebo (‐3.8 cm WMD, 95% CI ‐4.3 to ‐3.4). In the rimonabant 5 mg versus placebo comparison, the pooled effect was a reduction of 1.2 cm for the rimonabant group excluding the RIO‐North America study due to lack of data (‐1.2 cm WMD, 95% CI ‐1.7 to ‐0.7). Comparing the two intervention groups, the pooled data showed substantial heterogeneity (I² = 53.5%). We performed several sensitivity analysis, but even excluding the RIO‐Diabetes study which was only published as an abstract of congress, we could not explain the observed heterogeneity. For this reason the pooled data are not reliable. All results were statistically significant (P < 0.0001).
Adverse effects (general)
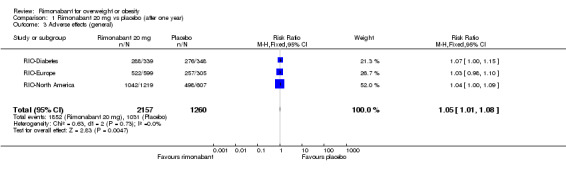
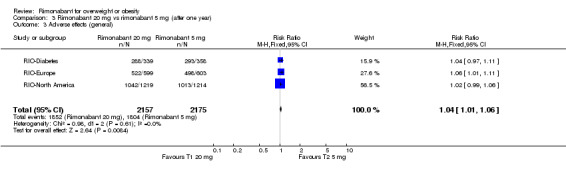
The combined results of the three trials (excluding the RIO‐Lipids study) indicated that compared to placebo, patients treated with rimonabant 20 mg reported significantly more general adverse effects: RR = 1.05, 95% CI 1.01 to 1.08 (P = 0.005). There were no statistically significant differences for adverse effects in the meta‐analysis comparing the results of rimonabant 5 mg with placebo (also excluding the RIO‐Lipids study). Comparing the two intervention groups, patients treated with rimonabant 20 mg reported significantly more adverse effects than the rimonabant 5 mg group: RR = 1.04, 95% CI 1.01 to 1.06 (P = 0.008). The RIO‐Lipids study was not included because it did not provide overall numbers for adverse effects but for single events only (multiple episodes were possible for each patient).
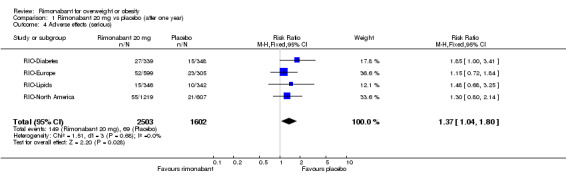
Adverse effects (serious)
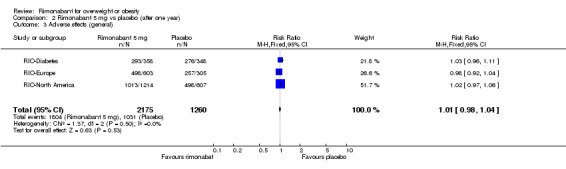
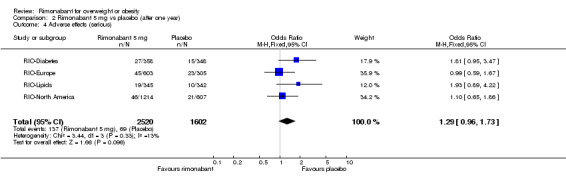
The pooled meta‐analysis of all included studies showed that compared to placebo, patients treated with rimonabant 20 mg reported significantly more serious adverse effects: RR = 1.37, 95% CI 1.04 to 1.80 (P = 0.03). There were no statistically significant differences for serious adverse effects in the meta‐analysis comparing the results of rimonabant 5 mg with placebo and also for the comparison between the two intervention groups.
Discontinuation due to adverse effects
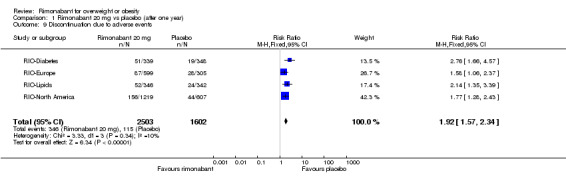
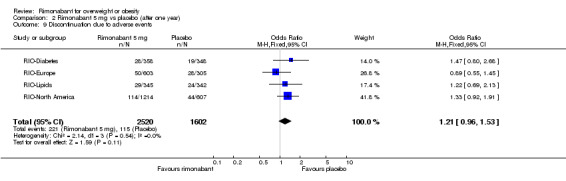
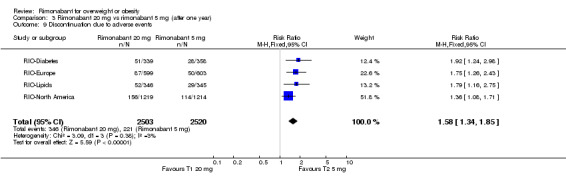
The pooled meta‐analysis of all included studies showed that compared to placebo, patients treated with rimonabant 20 mg reported a significantly greater rate of discontinuation due to adverse effects: RR = 1.92, 95% CI 1.57 to 2.34 (P < 0.00001). There were no statistically significant differences comparing the results of rimonabant 5 mg with placebo. For the comparison between the two intervention groups patients treated with rimonabant 20 mg also reported a significantly greater rate of discontinuation due to adverse effects: RR = 1.58, 95% CI 1.34 to 1.85 (P < 0.00001).
Secondary outcomes
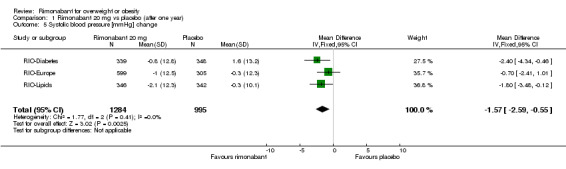
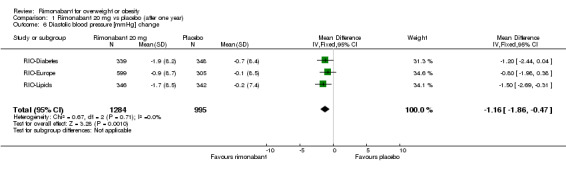
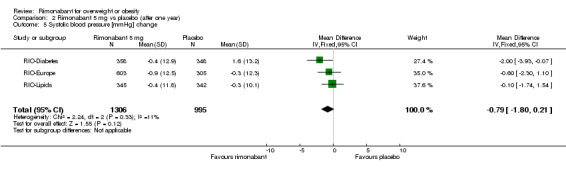
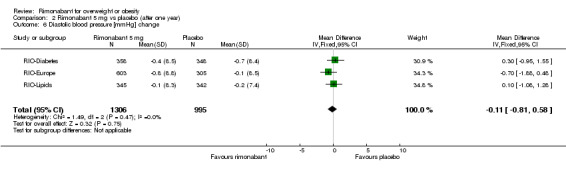
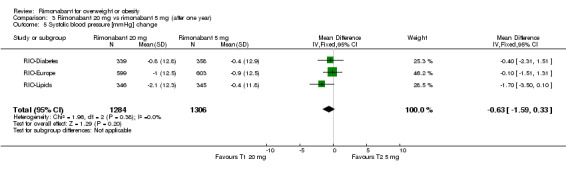
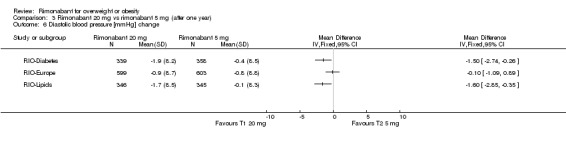
Blood pressure change
Comparing rimonabant 20 mg with placebo, the meta‐analysis of the included studies, excluding the RIO‐North America study due to lack of data, showed evidence of an average reduction in systolic and diastolic blood pressure of 2 mm Hg (‐2 mm Hg WMD, 95% CI ‐3 to ‐1) and 1 mm Hg (‐1 mm Hg WMD, 95% CI ‐2 to ‐0.5) respectively in the rimonabant group. There were no statistically significant differences for the rimonabant 5 mg versus placebo comparison in the same three studies, considering both systolic and diastolic blood pressures. Comparing the two intervention groups, the pooled data showed substantial heterogeneity (I² = 55.6%). We performed sensitivity analyses, but even excluding the RIO‐Diabetes study which was only published as an abstract of congress, we could not explain the observed heterogeneity. For this reason the pooled data are not reliable.
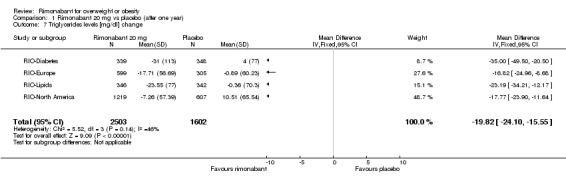
Lipid profile change
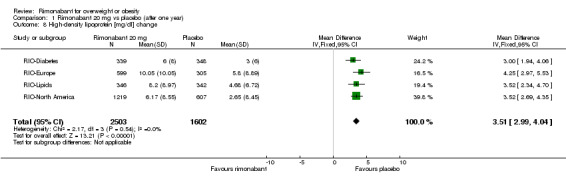
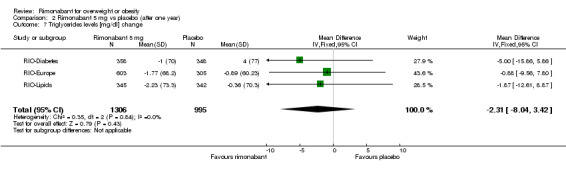
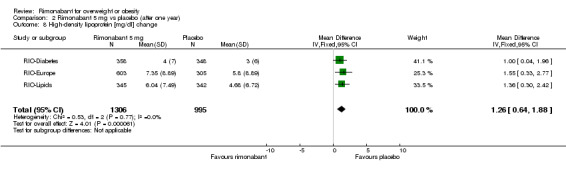
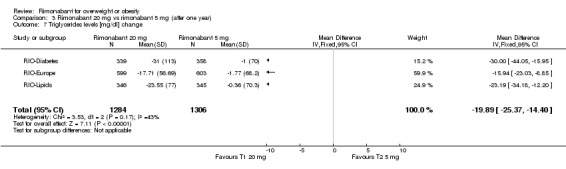
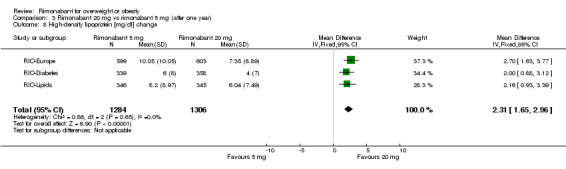
Serum triglyceride levels were reported in all studies as well as high density lipoprotein cholesterol and a meta‐analysis of these data was carried out. The pooled data of the all studies showed a significant lowering of plasma triglycerides in the rimonabant 20 mg group compared with placebo of 19.8 mg/dl (‐19.8 mg/dl WMD, 95% CI ‐24.1 to ‐15.6; P < 0.00001). There was no statistically significant reduction of plasma triglycerides in the rimonabant 5 mg and placebo comparison in three studies (excluding the RIO‐North America study due to lack of data). In the rimonabant 20 mg versus 5 mg comparison the meta‐analysis of same three studies showed a statistically significant average reduction of 19.9 mg/dl (‐19.9 mg/dl WMD, 95% CI ‐25.4 to ‐14.4; P < 0.00001) for the 20 mg group. For the high density lipoprotein cholesterol analysis, the pooled data of all studies of the rimonabant 20 mg versus placebo comparison showed an increase of 3.5 mg/dl in the rimonabant group (3.5 mg/dl WMD, 95% CI 3.0 to 4.0). For the rimonabant 5 mg versus placebo comparison, excluding the RIO‐North America study, there was an increase of 1.3 mg/dl in the rimonabant group (1.3 mg/dl WMD, 95% CI 0.3 to 1.9). Comparing the two intervention groups, an average increase of 2.3 mg/dl (2.3 mg/dl WMD, 95% CI 1.7 to 3.0) was found favouring the rimonabant group. All analyses were statistically significant (P < 0.00001).
Health‐related quality of life
This was measured in only one study, but the results were not described (RIO‐Europe).
Mortality and costs
No study evaluated mortality or costs as an outcome. The RIO‐Lipids study described in the results section that there were no deaths in any of the three groups. The RIO‐Europe study mentioned in the results section that two deaths occurred, one in the placebo group (haemorraghic cerebrovascular accident, about 2.5 months after randomisation) and one in the rimonabant 20 mg group (uterine adenocarcinoma, two months after randomisation). The other two studies did not describe mortality.
Heterogeneity
Statistical tests for heterogeneity yielded statistically significant results in weight change in the rimonabant 20 mg with placebo comparison, waist circumference change in the rimonabant 20 mg with rimonabant 5 mg comparison and in diastolic blood pressure comparing the two intervention groups. In general, the studies seemed to be homogeneous, but one of them (RIO‐Diabetes) was only published as an abstract of congress. This possible source for heterogeneity was investigated in the sensitivity analyses and is described below.
Subgroup analyses
We did not have sufficient data to perform subgroup analyses for initial level of weight, age, sex or duration of treatment.
Sensitivity analyses
Since we observed statistical heterogeneity for some pooled estimates, we performed several sensitivity analyses. Excluding the RIO‐Diabetes study, the only trial which was not published in a peer review journal, appeared to significantly reduce the observed heterogeneity in the weight change outcome for the rimonabant 20 mg versus rimonabant 5 mg comparison. In the other two results with substantial heterogeneity (waist circumference change and diastolic blood pressure change in the rimonabant 20 mg versus rimonabant 5 mg comparison), even excluding the same study could not relevantly reduce heterogeneity. The studies appeared to be similar, but exploring the influence of other factors on effect size did not seem to be appropriate.
Assessment of publication bias
Funnel plots were not carried out due to the small number of included studies.
Discussion
Summary of main results
In this systematic review, we found a statistically significant effect of rimonabant on body weight, blood pressure and plasma lipids. Compared with placebo, rimonabant 20 mg produced a 4.9 kg greater reduction in body weight in trials with one‐year results. Improvements in waist circumference, high‐density lipoprotein cholesterol, triglyceride levels and systolic and diastolic blood pressure also were seen with rimonabant 20 mg. However, the results with rimonabant 5 mg were not the same, and even being statistically significant the weight reduction was only 1.3 kg greater when compared with placebo. No clinically relevant effects on plasma lipids and blood pressure were found with 5 mg. Comparing the two interventions groups the results were similar to the rimonabant 20 mg versus placebo comparison, favouring the first group. Rimonabant 20 mg caused significant more adverse effects both of general and serious type, especially of nervous system, psychiatric or gastro‐intestinal origin. Although the studies were large trials, we detected deficiencies in the methodological quality of all included studies in this review. Methods for concealment of allocation were described in only one study, and the randomisation method was described in only two. Most studies were described as double‐blind, but it was unclear which two parties were blinded (patient, treatment administrator or outcome assessor). In spite of the reported deficiencies in the methodological quality, the data were too homogeneous to explore the effects of allocation concealment and blinding on outcomes. Only one study described power calculation, and in the intention‐to‐treat (ITT) analysis part, no study described the ITT population. The quality of descriptive information on study population and the intervention were generally adequate.
Potential biases in the review process
This is the first systematic review and meta‐analysis on the topic of rimonabant related to overweight and obesity. It offers an up‐to‐date and complete overview of all randomised trials concerning the topic as it is based on an extensive search, including unpublished studies. In addition, strong efforts have been done to minimise missing or incomplete data by attempting to contact all authors. Even if this review presents a possibly confusing amount of data and figures, completeness is one of the strengths of a Cochrane systematic review. The way we presented these data, subdivided in subheadings in different tables, makes it possible for the reader to find every specific piece of information obtained from the individuals trials. This review will be regularly updated, allowing eventual addition of information or correction of possible errors.
The major methodological limitation of this review is attributed to the quality of the included studies. We observed a high attrition rate in both treatment and placebo groups (approximately 40% in all studies). Authors tried to address this by carrying forward the last observation on record to the end of the study. When the data are analysed in this way, biased results may occur in either direction, depending on the reasons for withdrawal and the differential dropout rates in treatment and placebo groups. If we consider, for example, that patients in the placebo group may drop out of the study early because of weight gain due to lack of efficacy, measuring their weight at the point of withdrawal will likely underestimate their weight at the end of the study period supposing that they should slowly gain weight during the rest of the intervention period. This would underestimate the degree of weight gain in the placebo group and suggest a falsely lower overall treatment effect. On the other hand, if non‐responders in the treatment arm drop out early leaving only responders to complete the trial, the treatment effect may be overestimated. Such high attrition rates are difficult to compensate by any form of analysis. Considerable bias may be introduced into the results of these studies, and should be kept in mind when interpreting the results of this review. The RIO‐North America study reported outcomes in a way that could not contribute to meta‐analyses. This problem was not solved by asking authors for additional data, because unfortunately we got no answer. Research funded by pharmaceutical companies could be more likely to produce results favouring the tested drug. Consistent evidence also demonstrated that industry ties are associated with both publication delays and data withholding. These restrictions serve to compound bias in biomedical research. Rather, such behaviour appears to arise when investigators are involved in the process of bringing their research results to market (Bekelman 2003). In this review all studies were sponsored by the pharmaceutical company, in which the sponsor was the producer of the evaluated drug. The results should be interpreted with caution since conflict of interests could have influence on the results. Health related quality of life is an important question that was not considered in the included studies. The effect of treatment on patient‐reported outcomes, including any report coming directly from affected persons concerning their life, health conditions and treatment should be considered in clinical trials. Despite a thorough search, including requests to manufacturers, we still cannot rule out publication bias. The included studies are phase III clinical trials evaluating the effects, tolerability and safety of rimonabant. Others studies have probably been done primarily to evaluate the short‐term effects of the drug, as indicated by one of the authors contacted. We tried to obtain unpublished studies from the manufacturer, but unfortunately got no answer. Still, we welcome unpublished data for future updates.
Agreements and disagreements with other studies or reviews
Although this is the first systematic review concerning rimonabant in overweight and obesity, some reviews have been published recently (Boyd 2005; Cox 2005; Gelfand 2006; Tonstad 2006). They only focused on describing the clinical results in the published studies. The quality of these reviews is limited: selection criteria for the studies were insufficiently specified and there was no mentioning of the criteria used to assess the validity of individual trials. Further, these reviews did not present explicit methods on data extraction, assessment of quality or heterogeneity analyses. Presently the approved anti‐obesity pharmacotherapy options are limited to orlistat and sibutramine. Padwall (Padwall 2003) carried out a systematic review and meta‐analysis of long term pharmacotherapy for overweight and obesity. Compared with placebo, those receiving orlistat had a 2.7 kg decrease in body weight, and the treatment with sibutramine produced a weight loss of 4.3 kg. Orlistat‐treated patients displayed improvements in total cholesterol, low‐density lipoprotein cholesterol, blood pressure, and glycaemic control but had increased rates of gastrointestinal side effects and slightly lower high‐density lipoprotein levels. Sibutramine produced small improvements in high‐density lipoprotein cholesterol and triglyceride levels, but was associated with a net increase in blood pressure. Comparing the results obtained in this review with the results of the review evaluating the other two cited drugs it seems that the weight loss associated with rimonabant use was slightly greater compared to that related to sibutramine use, with more positive impact on cardiometabolic risk factors. The effects compared with orlistat seem to be greater weight loss and less frequent adverse effects. However, in the rimonabant studies the number of discontinuation due to adverse effects was greater when compared with orlistat studies. The authors of the rimonabant studies cited that the adverse effects were transient, based on the occurrence in the first months of the study. Although the number of patients discontinuing therapy due to adverse effects was approximately 10% in all studies, high study attrition rates raise the possibility that some of these events remained uncaptured. Considering the figures presented in all studies related to weight loss change during the entire period, the pattern of weight loss observed indicated that approximately after the 36th week, the level of weight loss decreased and the body weight was maintained practically until the end of the studies. The RIO‐North America evaluated the data after two years, and the patients who remained on rimonabant 20 mg seemed to maintain their weight loss, while those who were re‐randomised to placebo gained significant weight. Since only the RIO‐North America study reported results after two years where the patients were re‐randomised after the end of the first year, and the results were only presented as placebo subtracted changes from baseline, we did not include the data of the second year. Both the American Food and Drugs Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) demand that any anti‐obesity drug should produce significantly greater weight loss compared to placebo. The FDA specifically demands that placebo‐subtracted weight loss is at least 5%. Moreover, significantly more individuals in the drug treated group should lose 5% or more of their initial body weight compared to placebo. On the other hand, the EMEA demands a weight loss of at least 10% from baseline, which should also be greater than placebo. The secondary outcome of anti‐obesity drug trials is to ensure that this weight loss is sustained and able to induce a significant reduction in risk factors for a number of obesity related co‐morbidities. Moreover, drug induced weight loss should have a positive impact on health related quality of life.
Authors' conclusions
Implications for practice.
This review suggests that the use of rimonabant after one year could produce a modest weight loss of approximately 5%. Even modest amounts of weight loss may be potentially beneficial. Some caution regarding the observed results should be taken into account since the studies presented some deficiencies in the methodological quality. Studies with longer follow‐up after the end of treatment and with more rigor in the quality of methodology should be done before definitive recommendations can be made regarding the role of this medication in the management of obese patients. Drug therapy in obesity should always be considered in connection with non‐pharmacological interventions, on an individual basis, with stronger consideration given to those individuals with greater degrees of obesity and associated co‐morbidities. Efforts focusing on the prevention of obesity in non‐obese people and non‐pharmacological management in obese people should remain the cornerstone of obesity therapy. With regard to adverse effects, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications in Europe. Rimonabant is furthermore not recommended for patients with other untreated psychiatric conditions.
Implications for research.
Further high quality research is needed to assess the efficacy and safety of rimonabant over longer follow‐up periods. Future adequately‐powered trials should incorporate appropriate design principles including methods of allocation concealment, randomisation, blinding, minimization of attrition and follow‐up of drop‐outs. Whilst drop‐outs and withdrawals can not always be avoided, every effort should be made to ascertain the reasons for withdrawals so that factors affecting adherence can be further elucidated. Future studies should evaluate the use of medication associated with exercise and behavioural modification, identify subgroups of patients that may derive greater benefit from drug therapy, incorporate health‐related quality outcomes and also assess the economic costs of treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2008 | Amended | Converted to new review format. |
Notes
On October 23rd 2008 the European Medicines Agency (EMEA) recommended the suspension of the marketing authorisation for rimonabant from the manufacturer. The EMEA concluded that the benefits of rimonabant no longer outweigh its risk and the marketing authorisation should be suspended across the European Union.
Acknowledgements
We are grateful to Bernd Richter for his valuable contribution and suggestions for this review
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. MEDLINE: Obesity 1. exp Obesity/ 2. exp Weight Gain/ 3. exp Weight Loss/ 4. body mass index/ 5. (overweight or over weight).tw. 6. fat overload syndrom$.tw. 7. (overeat or over eat).tw. 8. (overfeed or over feed).tw. 9. (adipos$ or obes$).tw. 10. (weight adj (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control$ or gain or chang$)).tw. 11. body mass inde$.tw. 12. or/1‐11 Rimonabant 13. rimonabant.tw. 14. SR141716A.ti,ab. 15. acomplia.tw. 16. 13 or 14 or 15 Obesity and Rimonabant 17. 12 and 16 Controlled Clinical Trials 18. limit 17 to humans [Limit not valid in: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations; records were retained] 19. randomized controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized controlled trials.sh. 22. random allocation.sh. 23. double‐blind method.sh. 24. single‐blind method.sh. 25. or/19‐24 26. limit 25 to animal 27. limit 25 to human [Limit not valid in: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations; records were retained] 28. 26 not 27 29. 25 not 28 30. clinical trial.pt. 31. exp clinical trials/ 32. (clinic$ adj25 trial$).tw. 33. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. 34. placebos.sh. 35. placebo$.tw. 36. random$.tw. 37. research design.sh. 38. (latin adj square).tw. 39. or/30‐38 40. limit 39 to animal 41. limit 39 to human [Limit not valid in: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations; records were retained] 42. 40 not 41 43. 39 not 42 44. comparative study.sh. 45. exp evaluation studies/ 46. follow‐up studies.sh. 47. prospective studies.sh. 48. (control$ or prospectiv$ or volunteer$).tw. 49. cross‐over studies.sh. 50. or/ 44‐49 51. limit 50 to animal 52. limit 50 to human [Limit not valid in: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations; records were retained] 53. 51 not 52 54. 50 not 53 55. 29 or 43 or 54 Obesity and Rimonabant and Controlled Clinical Trials 56.18 and 55 |
Appendix 2. Baseline characteristics
| Characteristic | RIO‐Lipids | RIO‐Europe | RIO‐North America | RIO‐Diabetes |
| [n] (T1/ T2 / C1 / total) | 346 / 345 / 342 / 1033 | 599 / 603 / 305 / 1507 | 1219 / 1214 / 607 / 3040 | 339 / 358 / 348 / 1045 |
| Sex [n,%] (T1/ T2 / C1 / total) | T1 M ‐ 133, 38.4%, F ‐ 213, 61.6% T2 M ‐ 130, 37.7%, F ‐ 215, 62.3% C M ‐ 144, 42.1%; F ‐ 198, 57.9% | T1 M ‐ 121, 20.2%, F ‐ 478, 79.8% T2 M ‐ 127, 21.1%, F ‐ 476, 78.9% C M ‐ 61, 20%; F ‐ 244, 80% | T1 M ‐ 230, 18.9%, F ‐ 989, 81.1% T2 M ‐ 245, 20.2%, F ‐ 969, 79.8% C M ‐ 113, 18.6%; F ‐ 494, 81.4% | T1 M ‐ 169, 50%, F ‐ 170, 50% T2 M ‐ 172, 48%, F ‐ 186, 52% C M ‐ 188, 54%; F ‐ 160, 46%" |
| Age, years [mean,SD] (T1/ T2 / C1 / total) | T1 48,4 (10,0) T2 48,1 (10,2) C 47,0 (10,1)" | T1 44,6 (11,9) T2 45,4 (11,2) C 45,0 (11,6) | T1 45,6 (11,8) T2 44,4 (11,3) C 44,8 (11,6) | T1 56,0 (8,5) T2 55,9 (8,6) C 54,8 (8,6) |
| Ethnic groups (T1/ T2 / C1 / total) | ? | T1 white 555 (92,7%) T2 white 565 (93,7%) C1 white 290 (95,1%) | T1 white 1027 (84.2%) black 132 (10,8%) T2 white 1010 (11,5%) black 140 (11,5%) C1 white 516 (85,0%) black 67 (11.0%)" | T1 caucasian 302 (89,1%) T2 caucasian 315 (88,0%) C1 caucasian 308 (88,5%)" |
| Duration of disease [mean,SD] (T1/ T2 / C1 / total) | ? | ? | ? | ? |
| Weigh [mean,SD] (T1/ T2 / C1 / total) | T1 ‐ 95.3 (15.1) T2 ‐ 96.0 (14.6) C ‐ 97.0 (15.4) | T1 ‐ 101.7 (19.5)T2 ‐ 100.9 (19.8)C ‐ 100.0 (20.3) | T1 ‐ 103.0 (20.3) T2 ‐ 105.5 (21.9) C ‐ 105.0 (21.8) | ? |
| Body mass index [mean,SD] (T1/ T2 / C1 / total) | T1 ‐ 33.9 (3.3) T2 ‐ 34.1 (3.5) C ‐ 34.0 (3.5) | T1 ‐ 36,0 (5,8) T2 ‐ 36,0 (5,9) C ‐ 35,7 (5,9) | T1 ‐ 37.2 (6.2) T2 ‐ 38.0 (6.7) C ‐ 37.6 (6.4) | T1 ‐ 34.1 (3.6) T2 ‐ 34.4 (3.6) C ‐ 34.2 (3.6) |
| Pharmaco‐naive patients [n,%] (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| Co‐morbidity (T1/ T2 / C1 / total) | Metabolic syndrom T1 ‐ 183 (52,9%) T2 ‐ 193 (55,9%) C ‐ 178 (51,9%) | Metabolic syndrom T1 ‐ 251 (42,4%) T2 ‐ 243 (40,8%) C ‐ 121 (40,6%) Hypertension T1 ‐ 237 (39,6%) T2 ‐ 264 (43,8%) C ‐ 116 (38,0%) | Metabolic syndrom T1 ‐ 419 (34,6%) T2 ‐ 438 (36,3%) C ‐ 192 (31,8%) Hypertension T1 ‐ 390 (32,0%) T2 ‐ 367 (30,2%) C ‐ 168 (63,9%) | Metabolic syndrom T1 ‐ 269 (79,2%) T2 ‐ 285 (79,5%) C ‐ 276 (79,2%) Hypertension T1 ‐ 216 (63,7%) T2 ‐ 218 (60.9%) C ‐ 206 (59,2%) |
| Co‐medication (T1/ T2 / C1 / total) | ? | ? | ? | Anti‐diabetic treatment Metformin T1 ‐ 218 (64,3%) T2 ‐ 230 (64,2%) C ‐ 230 (66,1%) Sulfonylureas T1 ‐ 121 (35,7%) T2 ‐ 128 (35.8%) C ‐ 118 (33,9%) |
| HbA1c [mean,SD] (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| Smoker status | T1 ‐ 53 (15,3%) T2 ‐ 58 (16,8%) C ‐ 61 (17,8%) | T1 ‐ 102 (17,0%) T2 ‐ 136 (22,6%) C ‐ 60 (19,7%) | T1 ‐ 118 (9,7%) T2 ‐ 102 (8,4%) C ‐ 64 (10,5%) | ? |
| Blood pressure systolic mm Hg [mean,SD] (T1/ T2 / C1 / total) | T1 ‐ 124.9 (12.7) T2 ‐ 123.8 (13.5) C ‐ 124.0 (13.8) | T1 ‐ 127.0 (14.1) T2 ‐ 127.0 (14.8) C ‐ 126.0 (13.7) | T1 ‐ 121.7 (12.7) T2 ‐ 121.9 (12.7) C ‐ 121.7 (12.4) | ? |
| Blood pressure diastolic mm Hg [mean,SD] (T1/ T2 / C1 / total) | T1 ‐ 78.2 (7.7) T2 ‐ 78.1 (8.9)C ‐ 78.2 (8.4) | T1 ‐ 79.4 (8.8) T2 ‐ 79.6 (9.1) C ‐ 79.6 (8.5) | T1 ‐ 77.7 (8.2) T2 ‐ 78.2 (8.1) C ‐ 78.1 (7.8) | ? |
| Note | ||||
| Symbols: unclear=? | ||||
| Abbreviations: T=treatment; C=control; NA=not applied |
Appendix 3. Adverse events
| Characteristic | RIO‐Lipids | RIO‐Europe | RIO‐North America | RIO‐Diabetes |
| [n] adverse events (T1/ T2 / C1 / total) | 407 / 367 / 340 / 1114 | 522 / 498 / 257 / 1277 | 1042 / 1013 / 498 / 2553 | 288 / 293 / 276 / 857 |
| [%] adverse events (T1/ T2 / C1 / total) | 117.6 / 106.4 / 99.4 / 107.8 | 87.1 / 82.6 / 84.3 / 84.7 | 85.5 / 83.4 / 82.0 / 84.0 | 85 / 81.8 / 79.3 / 82 |
| [n] nausea (T1/ T2 / C1 / total) | 44/ 25 / 11 / 80 | 77 / 31 / 13 / 121 | 117 / 69 / 29 / 215 | 41 / 22 / 20 / 83 |
| [%] nausea (T1/ T2 / C1 / total) | 12.7 / 7.2 / 3.2 / 7.7 | 12.9 / 5.1 / 4.3 / 8.0 | 11.2 / 6.8 / 5.8 / 7.1 | 12.1 / 6.1 / 5.7 / 7.9 |
| [n] dizziness (T1/ T2 / C1 / total) | 36 / 29 / 23 / 88 | 52 / 42 / 15 / 109 | 58 / 46 / 20 / 114 | 31 / 11 / 17 / 59 |
| [%] dizziness (T1/ T2 / C1 / total) | 10.4 / 8.4 / 6.7 / 8.5 | 8.7 / 7.0 / 4.9 / 7.2 | 5.6 / 4.5 / 4.0 / 4.1 | 9.1 / 3.1 / 4.9 / 5.6 |
| [n] serious adverse events (T1/ T2 / C1 / total) | 15 / 19 / 10 / 44 | 52 / 45 / 23 / 120 | 55 / 46 / 21 / 122 | 27 / 27 / 15 / 69 |
| [%] serious adverse events (T1/ T2 / C1 / total) | 4.3 / 5.5 / 2.9 / 4.3 | 8.7 / 7.5 / 7.5 / 8.0 | 4.5 / 3.8 / 3.5 / 4.0 | 8.0 / 7.5 / 4.3 / 6.6 |
| [n] psychiatric disorders (T1/ T2 / C1 / total) | 1/ 1 / 1 / 3 | 9 / 2 / 1 / 12 | 76 / 44 / 14 / 134 | ? |
| [%] psychiatric disorders (T1/ T2 / C1 / total) | 0.3 / 0.3 / 0.3 / 0.3 | 1.5 / 0.3 / 0.3 / 0.8 | 6.2 / 3.6 / 2.3 / 4.4 | ? |
| [n] nervous system disorders (T1/ T2 / C1 / total) | 2 / 0 / 2 / 4 | 3 / 7 / 3 / 13 | 27 / 14 / 6 / 47 | ? |
| [%] nervous system disorders (T1/ T2 / C1 / total) | 0.6 / 0 / 0.6 / 0.4 | 0.5 / 1.2 / 1.0 / 0.9 | 2.2 / 1.2 / 1.0 / 1.6 | ? |
| [n] neoplasms (T1/ T2 / C1 / total) | 2 / 3 / 0 / 5 | 7 / 5 / 2 / 14 | ? | ? |
| [%] neoplasms (T1/ T2 / C1 / total) | 0.6 / 0.9 / 0 / 0.5 | 1.2 / 0.8 / 0.7 / 0.9 | ? | ? |
| [n] discontinuation due to adverse events (T1/ T2 / C1 / total) | 52 / 29 / 24 / 105 | 87 / 50 / 28 / 165 | 156 / 114 / 44 / 314 | 51 / 28 / 19 / 98 |
| [%] discontinuation due to adverse events (T1/ T2 / C1 / total) | 15.0 / 8.4 / 7.0 / 10.2 | 14.5 / 8.3 / 9.2 / 10.9 | 12.8 / 9.4 / 7.2 / 10.3 | 15.0 / 7.8 / 5.5 / 9.4 |
| [n] hospitalisation (T1/ T2 / C1 / total) | ? | ? | ? | ? |
| [%] hospitalisation (T1/ T2 / C1 / total) | ? | ? | ? | ? |
| [n] out‐patient treatment (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [%] out‐patient treatment (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [n] hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [%] hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [n] severe hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [%] severe hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [n] nocturnal hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [%] nocturnal hypoglycaemic episodes (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [n] with symptoms (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| [%] with symptoms (T1/ T2 / C1 / total) | NA | NA | NA | NA |
| Notes | ||||
| Symbols: unclear=? | ||||
| Abbreviations: T=treatment, C=control, NA=not applied |
Appendix 4. Primary outcome data
| Characteristic | RIO‐Lipids | RIO‐Europe | RIO‐North America | RIO‐Diabetes |
| Weight change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐6.9 (6.1) T2 ‐3.1 (4.8) C ‐1.5 (5.0) | T1 ‐6.6 (7.2) T2 ‐3.4 (5.7) C ‐1.8 (6.4) | T1 ‐6.3 (?) T2 ‐2.9 (?) C ‐1.6 (?) | T1 ‐5.3 (0.3) T2 ‐2.3 (0.2) C ‐1.4 (0.2) |
| Waist circumference change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐7.1 (6.8) T2 ‐3.5 (6.0) C ‐2.4 (5.7) | T1 ‐6.5 (7.4) T2 ‐3.9 (6.3) C ‐2.4 (6.9) | T1 ‐6.1 (?) T2 ‐3.1 (?) C ‐2.5 (?) | T1 ‐5.2 (0.3) T2 ‐2.9 (0.3) C ‐1.9 (0.3) |
| Adverse effects ‐ see under Additional table ‐ Table 04 | ||||
| Morbidity ‐ see under Additional table ‐ Table 04 | ||||
| Notes | ||||
| Symbols: unclear=? | ||||
| Abbreviations: T=treatment; C=control |
Appendix 5. Secondary outcome data
| Characteristic | RIO‐Lipids | RIO‐Europe | RIO‐North America | RIO‐Diabetes |
| Total cholesterol / HDL ratio change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐0.72 (0.93) T2 ‐0.47 (0.82) C ‐0.40 (0.90) | T1 ‐0.71 (0.78) T2 ‐0.52 (0.80) C ‐0.42 (0.83) | ? | ? |
| Triglycerides change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐12.6 (41.2) T2 +1.2 (39.4) C ‐0.2 (38.7) | T1 ‐0.20 (0.64) T2 ‐0.02 (0.77) C ‐0.20 (0.64) | T1 ‐5.3 (?) T2 ‐3.7 (?) C +7.9 (?) | T1 ‐31.0 (113.0) T2 ‐1.0 (70.0) C +4.0 (77.0) |
| HDL change [mean,SD] (T1/ T2 / C1 / total) | T1 +19.1 (20.9) T2 +14.2 (17.6) C +11.0 (15.8) | T1 +0.26 (0.26) T2 +0.19 (0.23) C +0.15 (0.23) | T1 +12.6 (?) T2 +7.6 (?) C +5.4 (?) | T1 +6.0 (8.0) T2 +4.0 (7.0) C +3.0 (6.0) |
| LDL change [mean,SD] (T1/ T2 / C1 / total) | T1 +7.2 (28.4) T2 +6.6 (21.4) C +7.0 (22.4) | T1 +0.08 (0.63) T2 +0.13 (0.62) C +0.17 (0.70) | ? | ? |
| Blood pressure systolic change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐2.1 (12.3) T2 ‐0.4 (11.8) C ‐0.3 (10.1) | T1 ‐1.0 (12.5) T2 ‐0.9 (12.5) C ‐0.3 (12.3) | ? | T1 ‐0.8 (12.8) T2 ‐0.4 (12.9) C +1.6 (13.2) |
| Blood pressure diastolic change [mean,SD] (T1/ T2 / C1 / total) | T1 ‐1.7 (8.5) T2 ‐0.1 (8.3) C ‐0.2 (7.4) | T1 ‐0.9 (8.7) T2 ‐0.8 (8.8) C ‐0.1 (8.5) | ? | T1 ‐1.9 (8.2) T2 ‐0.4 (8.5) C ‐0.7 (8.4) |
| Notes | results by last observation carried forward | results by last observation carried forward | results by last observation carried forward results published as placebo subtract changes from baseline for 1 year, this results were obtained from results published in American Hearth Association 2004 Scientific Sessions ‐ http://www.medscape.com/viewarticle/493901 | results by last observation carried forward |
| Symbols: unclear=? | ||||
| Abbreviations: T=treatment; C=control |
Data and analyses
Comparison 1. Rimonabant 20 mg vs placebo (after one year).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight [kg] change | 4 | 4105 | Mean Difference (IV, Fixed, 95% CI) | ‐4.64 [‐4.99, ‐4.28] |
| 2 Waist circumference [cm] change | 4 | 4105 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐4.26, ‐3.42] |
| 3 Adverse effects (general) | 3 | 3417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.08] |
| 4 Adverse effects (serious) | 4 | 4105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.04, 1.80] |
| 5 Systolic blood pressure [mmHg] change | 3 | 2279 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐2.59, ‐0.55] |
| 6 Diastolic blood pressure [mmHg] change | 3 | 2279 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.86, ‐0.47] |
| 7 Triglycerides levels [mg/dl] change | 4 | 4105 | Mean Difference (IV, Fixed, 95% CI) | ‐19.82 [‐24.10, ‐15.55] |
| 8 High‐density lipoprotein [mg/dl] change | 4 | 4105 | Mean Difference (IV, Fixed, 95% CI) | 3.51 [2.99, 4.04] |
| 9 Discontinuation due to adverse events | 4 | 4105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.57, 2.34] |
1.1. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 1 Weight [kg] change.
1.2. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 2 Waist circumference [cm] change.
1.3. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 3 Adverse effects (general).
1.4. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 4 Adverse effects (serious).
1.5. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 5 Systolic blood pressure [mmHg] change.
1.6. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 6 Diastolic blood pressure [mmHg] change.
1.7. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 7 Triglycerides levels [mg/dl] change.
1.8. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 8 High‐density lipoprotein [mg/dl] change.
1.9. Analysis.
Comparison 1 Rimonabant 20 mg vs placebo (after one year), Outcome 9 Discontinuation due to adverse events.
Comparison 2. Rimonabant 5 mg vs placebo (after one year).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight [kg] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐1.64, ‐0.86] |
| 2 Waist circumference [cm] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐1.69, ‐0.68] |
| 3 Adverse effects (general) | 3 | 3435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| 4 Adverse effects (serious) | 4 | 4122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.96, 1.73] |
| 5 Systolic blood pressure [mmHg] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.80, 0.21] |
| 6 Diastolic blood pressure [mmHg] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.81, 0.58] |
| 7 Triglycerides levels [mg/dl] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | ‐2.31 [‐8.04, 3.42] |
| 8 High‐density lipoprotein [mg/dl] change | 3 | 2301 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.64, 1.88] |
| 9 Discontinuation due to adverse events | 4 | 4122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.96, 1.53] |
2.1. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 1 Weight [kg] change.
2.2. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 2 Waist circumference [cm] change.
2.3. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 3 Adverse effects (general).
2.4. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 4 Adverse effects (serious).
2.5. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 5 Systolic blood pressure [mmHg] change.
2.6. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 6 Diastolic blood pressure [mmHg] change.
2.7. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 7 Triglycerides levels [mg/dl] change.
2.8. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 8 High‐density lipoprotein [mg/dl] change.
2.9. Analysis.
Comparison 2 Rimonabant 5 mg vs placebo (after one year), Outcome 9 Discontinuation due to adverse events.
Comparison 3. Rimonabant 20 mg vs rimonabant 5 mg (after one year).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight [kg] change | 3 | 2590 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐3.72, ‐2.86] |
| 2 Waist circumference [kg] change | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (general) | 3 | 4332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.06] |
| 4 Adverse effects (serious) | 4 | 5023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 5 Systolic blood pressure [mmHg] change | 3 | 2590 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.59, 0.33] |
| 6 Diastolic blood pressure [mmHg] change | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Triglycerides levels [mg/dl] change | 3 | 2590 | Mean Difference (IV, Fixed, 95% CI) | ‐19.89 [‐25.37, ‐14.40] |
| 8 High‐density lipoprotein [mg/dl] change | 3 | 2590 | Mean Difference (IV, Fixed, 95% CI) | 2.31 [1.65, 2.96] |
| 9 Discontinuation due to adverse events | 4 | 5023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.34, 1.85] |
3.1. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 1 Weight [kg] change.
3.2. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 2 Waist circumference [kg] change.
3.3. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 3 Adverse effects (general).
3.4. Analysis.

Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 4 Adverse effects (serious).
3.5. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 5 Systolic blood pressure [mmHg] change.
3.6. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 6 Diastolic blood pressure [mmHg] change.
3.7. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 7 Triglycerides levels [mg/dl] change.
3.8. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 8 High‐density lipoprotein [mg/dl] change.
3.9. Analysis.
Comparison 3 Rimonabant 20 mg vs rimonabant 5 mg (after one year), Outcome 9 Discontinuation due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
RIO‐Diabetes.
| Methods | DURATION OF INTERVENTION:
12 months DURATION OF FOLLOW‐UP: 0 months RUN‐IN PERIOD: 1‐ ? LANGUAGE OF PUBLICATION: English |
|
| Participants | WHO PARTCIPATED: INCLUSION CRITERIA: Age: 18 to 70 years old; BMI of 27 to 40 kg/m2; type 2 diabetes that had been treated with metformin or various sulfonylurea monotherapy for at least 6 months. hgA1c between 6.5% and 10%, fasting plasma glucose between 100 mg/dL and 271 mg/dL, variation in body weight within the previous three months < 5kg EXCLUSION CRITERIA: ? |
|
| Interventions | NUMBER OF STUDY CENTRES:
151 COUNTRY/LOCATION: USA and 10 other countries (?) SETTING: ? INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo or rimonabant 20 mg or rimonabant 5 mg ‐ daily in addition to a hypocaloric diet |
|
| Outcomes | PRIMARY:
weight change in 1 year SECONDARY: change in hgA1c, waist circumference, fasting lipid levels with emphasis on HDL and TG, and change in metabolic syndrome prevalence as defined by the ATP III criteria. TIMING OF OUTCOME MEASURES: body weigh, BP, waist circumference ‐ monthly, the other measures every 3 months ALL‐CAUSE MORTALITY. 0 DISEASE SPECIFIC MORTALITY: 0 MORBIDITY/COMPLICATIONS: 1 QUALITY OF LIFE: 0 ADVERSE EFFECTS: 1 COSTS: 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RIO‐Europe.
| Methods | DURATION OF INTERVENTION:
12 months DURATION OF FOLLOW‐UP: 0 RUN‐IN PERIOD: 1 ‐ 4 weeks LANGUAGE OF PUBLICATION: English |
|
| Participants | WHO PARTCIPATED: INCLUSION CRITERIA: BMI > 30 or 27 with hypertension or dyslipidaemia patients had less than 5 Kg variation within 3 months before EXCLUSION CRITERIA: clinical disorders (endocrine disease, diabetes, cardiovascular, pulmonary, hepatic, renal, neurological, psychological, severe depression); previous history of surgical procedures for weight loss; concomitant use of medications known to alter body weight or appetite was not allowed; marijuana or hashish use. DIAGNOSTIC CRITERIA: |
|
| Interventions | NUMBER OF STUDY CENTRES:
60 (40 ‐ Europe and 20 ‐ USA) COUNTRY/LOCATION: Europe and USA SETTING: ? INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo or rimonabant 20 mg or rimonabant 5 mg ‐ once/day in addition to a hypocaloric diet and stimulus for exercising |
|
| Outcomes | PRIMARY:
weight change SECONDARY: waist circumference, glucose and insuline in serum when fasting, HDL and TG and the prevalence of metabolic syndrome ADDICIONAL changes in total cholesterol and LDL, changes in insulin resistance TIMING OF OUTCOME MEASURES: body weight, waist circumference, BP ‐ monthly fasting glucose and insulin ‐ every 3 months other measures ‐ 12 months ALL‐CAUSE MORTALITY. 0 DISEASE SPECIFIC MORTALITY: 0 MORBIDITY/COMPLICATIONS: 1 QUALITY OF LIFE: 1, but not analysed ADVERSE EFFECTS: 1 COSTS: 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
RIO‐Lipids.
| Methods | DURATION OF INTERVENTION:
12 months DURATION OF FOLLOW‐UP: 0 months RUN‐IN PERIOD: 1 LANGUAGE OF PUBLICATION: English |
|
| Participants | WHO PARTCIPATED: INCLUSION CRITERIA: Age: 18 to 70 years old; BMI of 27 to 40 kg/m2; TG of 1,7 to 7,9 mmol/L chol/HDL >5 (men) and >4,5 (women) variation in body weight within the previous three months < 5kg EXCLUSION CRITERIA: history of pharmacologic treatment for dyslipidemia 6 weeks before or treatment with VLCD 6 months before; type 1 or 2 diabetes; clinically significant findings indicating cardiovascular disease, endocrine, pulmonary, neurologic, psychiatric, gastrointestinal, hepatic, hematologic, renal or dermatologic disease; + test for hepatitis B surface antigen or hepatitis C antibody, abnormal thyrotropin level; levels of alanine aminotransferase or aspartate aminotransferase > 2,5 times the upper limit; haemoglobin levels < 11g/dl; neutrophil levels < 1500 ml3; platelet levels < 100,000 ml3; creatinine levels > 150mmol/L; a history of marijuana or hashish use; severe depression; treatment for epilepsy, an eating disorder; malignant disease; BP > than 165 or 105 mm Hg; pregnancy; or lactation; < 80% compliance with a hypocaloric diet and placebo during the post‐screening four week single blind run in period. DIAGNOSTIC CRITERIA: ? |
|
| Interventions | NUMBER OF STUDY CENTRES:
67 COUNTRY/LOCATION: 8 countries ‐ ? SETTING: ? INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo or rimonabant 20 mg or rimonabant 5 mg ‐ daily in addition to a hypocaloric diet |
|
| Outcomes | PRIMARY:
weight change SECONDARY: changes in HDL, TG, Glucose and insulin during an oral glucose‐tolerance test and the prevalence of metabolic syndrom ADDITIONAL: waist circumference, leptin and adiponectin levels and relevant biochemical cardiovascular markers TIMING OF OUTCOME MEASURES: body weigh, BP, waist circumference ‐ monthly, the other measures only 12 months ALL‐CAUSE MORTALITY. 0 DISEASE SPECIFIC MORTALITY: 0 MORBIDITY/COMPLICATIONS: 1 QUALITY OF LIFE: 0 ADVERSE EFFECTS: 1 COSTS: 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RIO‐North America.
| Methods | DURATION OF INTERVENTION:
24 months DURATION OF FOLLOW‐UP: 0 RUN‐IN PERIOD: 1 ‐ 4 weeks LANGUAGE OF PUBLICATION: English |
|
| Participants | WHO PARTCIPATED: INCLUSION CRITERIA: 18 years or older; BMI of 30 or 27 with treated or untreated dyslipidemia or hypertension EXCLUSION CRITERIA: weight fluctuation of more than 5 Kg in the previous 3 months; clinically significant cardiac, renal, hepatic, gastrointestinal tract, neuropsychiatric or endocrine disorders, drug‐treated or diagnosed type 1 or 2 diabetes, use of medications that alter body weight or appetite, a history of current substance abuse; changes in smoking habits or smoking cessation within the past 6 months DIAGNOSTIC CRITERIA: |
|
| Interventions | NUMBER OF STUDY CENTRES:
72 (64 ‐ USA and 8 ‐ Canada) COUNTRY/LOCATION: USA and Canada SETTING: INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Placebo or rimonabant 20 mg or rimonabant 5 mg ‐ once/day in addition to a hypocaloric diet and stimulus for exercising |
|
| Outcomes | PRIMARY:
weight change in 1 year
prevention of weight regain between the first and second years SECONDARY: changes in HDL in 1 year and the prevalence of metabolic syndrom ADDITIONAL: changes in BP, levels of glucose and insulin, lipids, and insulin resistence TIMING OF OUTCOME MEASURES: in the end of year 1 and 2 ALL‐CAUSE MORTALITY. 0 DISEASE SPECIFIC MORTALITY: 0 MORBIDITY/COMPLICATIONS: 1 QUALITY OF LIFE: 0 ADVERSE EFFECTS: 1 COSTS: 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BMI ‐ body mass index; hgA1c ‐ hemoglobinaA1c; HDL ‐ high density lipoprotein; TG ‐ triglycerides; BP ‐ blood pressure; LDL ‐ low density lipoprotein; chol/HDL ‐ ratio total cholesterol / HDL; VLCD ‐ very low calory diet
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jensen 2004 | Subgroup analysis of the results of the RIO‐studies |
Characteristics of ongoing studies [ordered by study ID]
STRADIVARIUS.
| Trial name or title | STRADIVARIUS (Strategy To Reduce Atherosclerosis Development InVolving Administration of Rimonabant ‐ the Intravascular Ultrasound Study) |
| Methods | |
| Participants | Ages Eligible for Study: 18 Years and above, Genders Eligible for Study: Both
Criteria
Inclusion Criteria: Written and signed informed consent Indication for coronary angiography Abdominal obesity defined by waist circumference > 88 cm in women or > 102 cm in men At least one of the two following conditions: *a) Metabolic syndrome as defined by the presence of at least two of the following additional risk factors: 1. Triglyceride level >= 150 mg/dL (1.69 mmol/L); 2. HDL cholesterol < 40 mg/dL (1.03 mmol/L) [men] or 50 mg/dL (1.28 mmol/L) [women]; 3. Fasting glucose >= 110 mg/dL (6.1 mmol/L); 4. High blood pressure (>= 140 mmHg systolic and/or >= 90 mmHg diastolic) at Screening visit, or current treatment by anti‐hypertensive medication; *b) Currently smoking (> 10 cigarettes /day) and willing to stop Angiographic evidence of coronary heart disease as defined by at least 1 lesion in a native coronary artery that has >= 20% reduction in lumen diameter by angiographic visual estimation Presence of at least one coronary artery complying with the definition of “target vessel” for IVUS assessment Acceptation of the Baseline IVUS tape by the IVUS Core Laboratory Exclusion Criteria: Age < 18 years Pregnant or breast‐feeding women History of very low‐calorie diet or surgical procedures for weight loss (eg, stomach stapling, bypass) within 6 months prior to screening visit Obesity of known endocrine origin Uncontrolled diabetes with HBA1c >10% Presence of any severe medical or psychological condition, that in the opinion of the Investigator would compromise the subject’s safety or successful participation in the study Severe congestive heart failure (New York Heart Association [NYHA] Class III or IV) Clinically significant heart disease which in the opinion of the Investigator is likely to require coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI), cardiac transplantation, surgical repair and/or replacement during the course of the study Angioplasty of a non‐qualifying artery which is considered at high risk of acute complication or restenosis, during baseline catheterization >50% reduction in lumen diameter of the left main coronary artery by visual angiographic estimation Recent ST‐elevation myocardial infarction (MI) <= 72 hours prior to randomization |
| Interventions | Study Type: Interventional Study Design: Treatment, Randomized, Double‐Blind, Placebo Control, Parallel Assignment, Safety/Efficacy Study |
| Outcomes | Inhibition of Atherosclerosis Progression Assessed by IVUS (IntraVascular UltraSounds) |
| Starting date | Study start: January 2005; Study completion: August 2007 |
| Contact information | Sponsored by: Sanofi‐Aventis Information provided by: Sanofi‐Aventis ClinicalTrials.gov Identifier: NCT00124332 |
| Notes | Data from "www.clinicaltrials.gov" The purpose of this study is to determine if rimonabant 20 mg once daily (od) administered during 18‐20 months will reduce progression of coronary atherosclerosis as assessed by intravascular ultrasound (IVUS) when administered on top of standard behavioral and pharmacological therapy given as needed, in patients with abdominal obesity associated with current smoking and/or metabolic syndrome. Study ID Numbers: EFC5827; SR141716 Location Information ‐ Ohio: The Cleveland Clinic Foundation, Cleveland, Ohio, 44195, United States This study is no longer recruiting patients. |
Differences between protocol and review
In the protocol quality assessment according to the criteria by Schulz and Jadad were planned. We did not persue bias categorisation but preferred an individual component analysis approach instead.
Contributions of authors
CINTIA CURIONI: Protocol and review development, searching for trials, selection of studies, quality assessment, data extraction, data analysis, review development.
CHARLES ANDRÉ: Selection of studies, quality assessment, data extraction, data analysis, review development.
Sources of support
Internal sources
Instituto de Medicina Social ‐ UERJ, Brazil.
Universidade Federal do Rio de Janeiro, Brazil.
External sources
FAPERJ, Brazil.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
RIO‐Diabetes {unpublished data only}
- Hollander P. Endocannabinoid Blockade for Improving Glycemic Control and Lipids in Patients With Type 2 Diabetes. 1st International Congress on "Prediabetes" and the Metabolic Syndrome; April 13‐16, 2005; Berlin, Germany. Available via <http://www.medscape.com/viewarticle/515508> (last accessed 04/08/2006).
RIO‐Europe {published data only}
- Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effect of the cannabinoid‐1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1‐year experience from the RIO‐Europe study. The Lancet 2005;365:1389‐97. [DOI] [PubMed] [Google Scholar]
RIO‐Lipids {published data only}
- Depres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. The New England Journal of Medicine 2005;353:2121‐34. [DOI] [PubMed] [Google Scholar]
RIO‐North America {published data only}
- Pi‐Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid‐1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA 2006;295:761‐75. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Jensen 2004 {published data only}
- Jesen M, Abu‐Lebdeh H, Geohas J, Brazg R, Block M, Noveck R. The selective cb1‐receptor antagonist rimonabant reduces body weight and waist circumference in obese subjects. International Journal of Obesity 2004;28 (Suppl. 1):S27. [Google Scholar]
References to ongoing studies
STRADIVARIUS {published data only}
- STRADIVARIUS (Strategy To Reduce Atherosclerosis Development InVolving Administration of Rimonabant ‐ the Intravascular Ultrasound Study). Ongoing study Study start: January 2005; Study completion: August 2007.
Additional references
Bekelman 2003
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454‐65. [DOI] [PubMed] [Google Scholar]
Bergstrom 2001
- Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. International Journal of Cancer 2001;1:421‐30. [DOI] [PubMed] [Google Scholar]
Björntorp 1984
- Björntorp P. Hazards in subgroups of human obesity. European Journal of Clinical Investigation 1984;14:239‐41. [DOI] [PubMed] [Google Scholar]
Björntorp 1985
- Björntorp P. Obesity and the risk of cardiovascular disease. Annals of Clinical Research 1985;17:3‐9. [PubMed] [Google Scholar]
Bonner 2005
- Bonner TI. Structure of a cannabinoid receptor and functional antagonist. Annals of Pharmacotherapy 2005;39:684‐90. [Google Scholar]
Boyd 2005
- Boyd ST, Fremming BA. Rimonabant ‐ a selective CB1 antagonist. The Annals of Pharmacotherapy 2005;39:684‐90. [DOI] [PubMed] [Google Scholar]
Brown 2000
- Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, et al. Body mass index and prevalence of risk factors for cardiovascular disease. Obesity Research 2000;8:676‐7. [DOI] [PubMed] [Google Scholar]
Chu 1991
- Chu SY, Lee NC, Wingo PA, Senie RT, Greenberg RS, Peterson HB. The relationship between body mass and breast cancer among women enrolled in the Cancer and Steroid Hormone Study. Journal of Clinical Epidemiology 1991;44:1197‐206. [DOI] [PubMed] [Google Scholar]
Chua 1994
- Chua W, Chediak AD. Obstructive sleep apnea. Treatment improves quality of life and may prevent death. Postgraduate Medicine 1994;95:123‐38. [PubMed] [Google Scholar]
Cicuttini 1996
- Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. Journal of Rheumatology 1996;23:1221‐6. [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Colombo 1998
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sciences 1998;63:PL113‐7. [DOI] [PubMed] [Google Scholar]
Cox 2005
- Cox SL. Rimonabant hydrochloride: an investigational agent for the management of cardiovascular risk factors. Drugs of Today 2005;41:499‐508. [DOI] [PubMed] [Google Scholar]
Curioni 2005
- Curioni CC, Lourenço PM. Long‐term weight loss after diet and exercise ‐ a systematic review. International Journal of Obesity and related metabolic disorders 2005;29:1168‐74. [DOI] [PubMed] [Google Scholar]
Di Marzo 2004
- Marzo V, Bifulco M, Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nature reviews. Drug discovery 2004;3:771‐84. [DOI] [PubMed] [Google Scholar]
Di Marzo 2005
- Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nature Neuroscience 2005;8:585‐9. [DOI] [PubMed] [Google Scholar]
Donahue 1987
- Donahue RP, Abbott RD, Bloom E, Reed DM, Yano K. Central obesity and coronary heart disease in men. Lancet 1987;1:821‐4. [DOI] [PubMed] [Google Scholar]
Ducimetiere 1986
- Ducimetiere P, Richard J, Cambien F. The pattern of subcutaneous fat distribution in middle‐aged men and risk of coronary heart disease: The Paris Prospective Study. International Journal of Obesity 1986;10:229‐40. [PubMed] [Google Scholar]
Dyer 1989
- Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co‐operative Research Group. Journal of Human Hypertension 1989;3:299‐308. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelfand 2006
- Gelfand EV, Cannon CP. Rimonabant: a cannabinoid receptor type 1 blocker for management of multiple cardiometabolic risk factors. Journal of the American College of cardiology 2006;47:1919–26. [DOI] [PubMed] [Google Scholar]
Greenway 1996
- Greenway FL. Obesity surgery. Endocrinology and Metabolism Clinics of North America 1996;25:1005‐27. [DOI] [PubMed] [Google Scholar]
Halford 2006
- Halford JC. Obesity drugs in clinical development. Current Opinion in Investigational Drugs 2006;7:312‐8. [PubMed] [Google Scholar]
Hart 1993
- Hart DJ, Spector TD. The relationship of obesity, fat distribution, and osteoarthritis in women in the general population: the Chingford Study. The Journal of Rheumatololy 1993;20:331‐5. [PubMed] [Google Scholar]
Hey 2005
- Hey k, Ussher MH, Lancaster T. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation. The Cochrane Database of Systematic Reviews 2005, Issue 3. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005] In: The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Kissebah 1985
- Kissebah AH, Evans DJ, Peiris A, Wilson CR. Endrocrine characteristics in regional obesities:role of sex steroids. In: Vague J, Björntorp P, Guy Grand B, Rebuffé‐Scrive M, Vague P editor(s). Metabolic Complications of Human Obesities. Elsevier Science Publishers, Amsterdam, 1985:115‐30. [Google Scholar]
Lapidus 1984
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow‐up of participants in the population study of women in Gothenburg, Sweden. British Medical Journal (Clinical Research Ed.) 1984;289:1257‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 1984
- Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, TibblinG. Abdominal adipose tissue distribution, obesity and risk of cardiovascular disease and death: 13 year follow‐up of participants in the study of men born in 1913. British Medical Journal (Clinical Research Ed.) 1984;288:1401‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luque 1999
- Luque CA, Rey JA. Sibutramine: a serotonin‐norepinephrine reuptake‐inhibitor for the treatment of obesity. Annals of Pharmacotherapy 1999;33:968‐78. [DOI] [PubMed] [Google Scholar]
McNeely 1998
- McNeely W, Benfield P. Orlistat. Drugs 1998;52:241‐9. [DOI] [PubMed] [Google Scholar]
Medalie 1974
- Medalie JH, Papier C, Herman JB, Goldbourt U, Tamir S, Neufeld HN, et al. Diabetes mellitus among 10,000 adult men. I. 5‐year incidence and associated variables. Israel Journal of Medical Sciences 1974;10:681‐97. [PubMed] [Google Scholar]
NHLBI 1998
- National Institutes of Health. National Heart, Lung, and Blood Institute (NIHLBI). Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults ‐ the evidence report (Clinical Gdlns). Obesity Research 1998;6:51S‐209S. [PubMed] [Google Scholar]
NIHCD 1985
- National Institutes of Health Consensus Development Conference Statement (NIHCD). Health implications of obesity. Annals of Internal Medicine 1985;103:1073‐7. [PubMed] [Google Scholar]
Ohlson 1985
- Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow‐up of the participants in the study of men born in 1913. Diabetes 1985;34:1055‐8. [DOI] [PubMed] [Google Scholar]
Padwall 2003
- Padwal R, Li SK, Lau DCW. Long‐term pharmacotherapy for obesity and overweight. The Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Rexrode 1997
- Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich‐Edwards JW, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA 1997;277:1539‐45. [DOI] [PubMed] [Google Scholar]
Rinaldi‐Carmona 2004
- Rinaldi‐Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, et al. SR147778 [5‐(4‐bromophenyl)‐1‐(2,4‐dichlorophenyl)‐4‐ethyl‐N‐(1‐piperidinyl)‐1H‐pyrazole‐3‐carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. The Journal of Pharmacology and Experimental Therapeutics 2004;310:905‐14. [DOI] [PubMed] [Google Scholar]
Rowland 2001
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology 2001;159:111‐6. [DOI] [PubMed] [Google Scholar]
Schottenfeld 1996
- Schottenfeld D, Fraumeni JF. Cancer Epidemiology and Prevention. New York: Oxford University Press, 1996. [Google Scholar]
Simiand 1998
- Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behavioural Pharmacology 1998;9:179‐81. [PubMed] [Google Scholar]
Stokes 1985
- Stokes III J, Garrison RJ, Kannel WB. The independent contributions of various indices of obesity to the 22‐year incidence of coronary heart disease: The Framingham Heart Study. In: Vague J, Björntorp P, Guy‐Grand B, Rebuffe‐Scrive M, Vague editor(s). Metabolic Complications of Human Obesities. Amsterdam: Elsevier Science Publishers, 1985. [Google Scholar]
Tonstad 2006
- Tonstad S. Rimonabant: a cannabinoid receptor blocker for the treatment of metabolic and cardiovascular risk factors. Nutrition, Metabolism & Cardiovascular Diseases 2006;16:156‐62. [DOI] [PubMed] [Google Scholar]
Vickers 2005
- Vickers SP, Kennett GA. Cannabinoids and the regulation of ingestive behaviour. Current Drug Targets 2005;6:215‐23. [DOI] [PubMed] [Google Scholar]
Walker 1996
- Walker SP, Rimm EB, Ascherio A, Kawachi I, Stampfer MJ, Willett WC. Body size and fat distribution as predictors of stroke among US men. American Journal of Epidemiology 1996;144:1143‐50. [DOI] [PubMed] [Google Scholar]
Werner 2003
- Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation‐induced intake in Lewis rats. Brain Research 2003;28:290‐2. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic. Technical Report Series 894, Geneva: WHO 2000. [PubMed]
Willett 1995
- Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. JAMA 1995;273:461‐5. [DOI] [PubMed] [Google Scholar]


