Abstract
Radioresistance is the potential cause of cancer metastasis and recurrence. Radiation‐induced changes in exosomes can partially explain the undesirable prognosis of radiotherapy (RT). Exosomes, newly discovered ways of cell communication, carry the characteristics of their origin, resulting in their diversity. Various exosomes in the tumor microenvironment exert different function in immune response. In this review, the dual effect of RT on the immune system was described, and the effect of radiotherapy on tumors via exosomes was explored. The molecules in exosomes after RT were described to play immunosuppressive and immunocompetent roles: immune‐related receptors and cell signaling molecules involved in both adaptive and innate immune system were present. CD69, TIGIT, TIM‐3, LAG‐3 and the tumor necrosis factor (TNF) family that signal to T cells were shown to be regulated by exosomes after irradiation. The change in innate immunity‐derived like receptors, Leukocyte Immunoglobin‐Like Receptors (LILR) was described, as well as B7‐H3, V‐domain containing Ig suppressor of T cell activation (VISTA), and CD155 on tumor cells. These changed molecules inhibit and activate the immune system through different mechanisms. By analyzing the relationship between exosome‐derived molecules and immunity, this review shows that radiotherapy can induce immunosuppression and immune clearance through exosomes, thereby treating tumors and improving patient prognosis.
Keywords: cancer, exosome, immunomodulation, radiotherapy
In this review, we mainly focus on the bipolar effect of radiotherapy in the immune system, and illustrate the influences of radiotherapy on tumor treatment through the changes in exosomes. By analyzing the relationship between exosome‐derived molecules and immunity, it is believed that radiotherapy has activity in both immunosuppression and immune clearance, which can largely enhance the effects of tumor treatments and improves the prognosis.
1. INTRODUCTION
There is nearly a century of history behind radiotherapy (RT), which has evolved from discovery to widespread use in clinically significant tumors. Currently, with an estimated 50% of cancer patients receiving RT, radioactive rays such as X‐rays are commonly used to weaken the viability of tumor cells before surgery and facilitate radical cure after tumor resection, potentially lowering the risk of cancer recurrence and metastasis. 1 The damage effects of radiation are accomplished by ionizing reactions and initiating biochemical responses in the body, which occur first in the cell, the nucleus, and then causes necrosis and apoptosis. The cellular response to radiation is separated into physical, chemical, and biological reaction stages. This is followed by the production of reactive oxygen species (ROS), which contributes to the chemical changes in DNA materials in the nucleus including base destruction, enzyme damage, single‐stranded and double‐stranded breaks (DSB) or crosslinking. Despite the repair mechanism of cells, complicated DSBs are beyond correction, which results in the death of cells and the increased discernability of tumor antigen.
Because of its potent cytotoxic effects, RT appears to make a significant difference in the radical cure of cancer therapy. Patients undergoing RT, conversely, have experienced several negative prognostic outcomes, such as radioresistance, tumor recurrence, and metastasis. The presence of a poor clinical outcome raises awareness of the recognition of RT bipolarity in cancer treatment. Given that RT exerts a dominant effect in cancer therapy with undesirable secondary effects, the adjustment of patients following RT is thoroughly examined to maximize the antitumor response strength. It is permissible to correlate newly discovered exosomes implicated in the tumor microenvironment (TME) with the prognosis of RT. Exosomes are a subtype of microvesicles found in cells. They contain a variety of bioorganic macromolecules and micromolecules, most notably complex RNA and protein. Recent studies have focused on the association between tumors and exosomes, and numerous researchers have attempted to use exosomes to illustrate the specific mechanisms in the TME. Studies have reported that not only tumor cells but also the composition of exosomes secreted by those cells change before and after radiation treatment. This review presents the background of exosomes in normal immune cells and their modification following radiation as well as the possible mechanisms underlying RT duality: immunosuppression and immunocompetence. The improvement in radiotherapy's immune‐enhancing effects, as well as the reduction in its immunosuppressive effects, offer hope for expanding the benefits of radiation to a greater number of patients.
2. EXOSOMES
2.1. Structures of exosomes
Exosomes are produced by a wide variety of cells, including innate and adaptive immune cells such as dendritic cells (DCs), macrophages, natural killer (NK) cells, T lymphocytes, and B lymphocytes. Exosomes with a diverse array of cell content provide insight into their biological origins and formation. In general, they are composed primarily of proteins generated from cells, lipids, glycoconjugates, and nucleic acids. 2 The exosome membrane comprises transmembrane proteins such as integrins and tetraspanins that are involved in cell growth, motility, signaling, and adhesion, all of which contribute to cell homeostasis. 3 Because exosomes control active metabolism, a greater number of exosomes must be released to facilitate complex intercellular communication in metabolically hyperactive cells such as cancer cells.
This is due to a variety of molecular pathways. Tetraspanins have been shown to interact with other transmembrane proteins, for example, integrins and MHC proteins, as well as with cytosolic proteins. 3 , 4 The universally cytosolic proteins found in exosomes that are most likely to be involved in exosome biogenesis, intercellular communication, and signal transduction include (1) cytoskeletal proteins: tubulin, actin, annexin, vimentin, and talin; (2) a few metabolic enzymes: PGK1, GAPDH, protein kinases, and G proteins; and (3) a few heat shock proteins (chaperones), such as HSP70, and HSP90. 3 , 4 In addition, cell‐specific proteins are also involved in the cellular characteristics of exosomes, enabling them to perform a variety of roles. Given that exosomes are implicated in a range of tumor‐associated immune responses, their diverse composition provides a new direction for clinical research on radiation therapy. (Figure 1).
FIGURE 1.
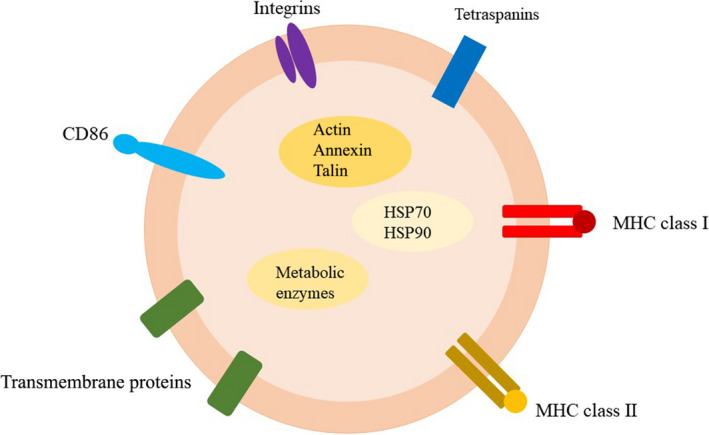
Schematic diagram of the structures of exosomes. Exosome membrane contain MHC proteins, tetraspanins, and other transmembrane proteins for cellular communication. Exosomes contain cytosolic proteins such as heat shock proteins, metabolic enzymes, and cytoskeletal proteins
2.2. The dual characteristics of exosomes in immunity
Radiotherapy affects both immunological suppression and activation, which has been associated with the function of exosomes. Exosomes play a critical role in the immune system because they are capable of expressing and processing antigens, particularly those derived from antigen‐presenting cells (APCs). 5 In addition, exosomes transfer MHC proteins and other proteins and RNAs between cells; therefore, genetic information is transferred by exosomes. 6
Exosomes provide a dual purpose in immune system regulation. For years, the debate on the procancer or anticancer effects of exosomes has been controversial. On the one hand, exosomes released by normal cells have been shown to enhance the immune response to cancer. In addition, tumor‐derived exosomes (TEXs) may be used by the immune system to kill cancer cells. It has been demonstrated that TEXs can present antigens to DCs, activating CD8+ T cells to kill cancer cells and eventually achieve antitumor effects. 7 TEXs, in conjunction with DCs, play an important role in anticancer immune responses. Exosomes have been shown to induce tumor‐antigen‐specific cytotoxic T cell (CTL) responses and to influence leukocyte proliferation via phosphorylation when cooperating with DCs. 8
On the other hand, numerous studies have demonstrated that exosomes can promote cancer growth, rendering both innate and adaptive immunity insensitive and reducing the ability of pathological cells such as cancer to proliferate. For example, a previous study has established that glioblastoma exosomes transfer microRNAs that induce tumor formation in a benign cell line. 9 Additionally, several studies have demonstrated that TEXs can suppress the immune system by reducing T cell immune responses and transforming immune cells into tumor‐supporting phenotypes. 10 For example, breast TEXs, brain‐TEXs, and melanoma cell‐derived exosomes all have different effects on the proliferation and functions of CD4+ T cells and thereby inhibit cytotoxic immunity through different mechanisms. 11
In general, TEXs may act as stimulatory and/or inhibitory mediators on immune cells. Because the functions and composition of exosomes can vary, it is natural for their functions to shift as cancer progresses, implying that they can have protumor or antitumor effects.
2.3. Exosomes variants involved in the immune system
2.3.1. Dendritic cell‐derived exosomes
Dendritic cells are the most powerful APCs in the immune system, acting as a link between the innate and adaptive immune systems. Dendritic cell‐derived exosomes (DEX) secreted by DCs share many similar components such as MHC protein complexes, costimulatory molecules, and other components that participate in the immune process. 12 Proteomic studies have demonstrated that DEX has potent immunostimulatory properties. 13 DEX contains MHC I and MHC II protein complexes, as well as CD86 and HSP70‐90 chaperones, all of which contribute to the activation of CD4+ and CD8+ T cells. 14 In summary, DEX facilitates T cell responses via MHC complexes. 15 Moreover, DEX contains mRNA and microRNA that are transferred between cells, and which is thought to be a method of communication and posttranscriptional modification between DCs. 16
Despite their comparable compositions, DCs and DEXs play distinct roles in immunity. For example, DCs may not express enough NK cell receptor ligands and are easily influenced by the TME and immunosuppression and can be converted into negative modulators of immune responses, whereas DEXs cannot respond to immunosuppressive molecules. 17 , 18 In conclusion, DEXs are more effective against tumors compared with DCs because they induce greater immunological immune responses.
2.3.2. T lymphocyte cell‐derived exosomes
T cells are broadly classified into CD8+cytotoxic T cells (CTLs) and CD4+ helper T cells, the latter of which includes numerous subtypes such as regulatory T cells (Tregs) and Th17 cells. CTLs have the ability to not only kill tumor cells directly but also induce tumor apoptosis and regulate immune responses via the release of exosomes. Exosomes can elicit robust immunological responses; for example, CTL‐derived exosomes activate bystander CD8+ T cells under the influence of IL‐12. 19 However, another study has established that CD8+ T cell‐derived exosomes carrying Fas ligand (FasL) can promote tumor invasion and metastasis by activating the c‐FLIPL, and extracellular signal‐regulated kinase (ERK) and nuclear factor kappa B (NF‐κB), signaling pathways. Activation of these pathways results in the accumulation of MMP9 expression in tumor cells, which is required for tumor invasion. 20 Similar to Tregs, Treg‐derived exosomes perform immunosuppressive functions in the TME, including suppressing CTL‐mediated antitumor immunity. 21 These findings provide compelling evidence that T cells can influence tumor immune responses and development through the release of exosomes.
2.3.3. Natural killer cell‐derived exosomes
Natural killer cells are important components of innate immunity because they contribute to immunosurveillance and protect against a variety of disorders, including pathogen infections and tumors. NK cells are capable of recognizing abnormal cells that lack class I MHC antigen expression and killing them with cytotoxic effector molecules contained in lytic granules. 22 Interestingly, NK cells can also release exosomes in response to immunological stimuli. Furthermore, these NK‐derived exosomes contain typical NK markers (e.g., NKG2D and CD56) and lytic proteins (e.g., perforin, granzymes, granulysin, and FasL/CD178). 23 NK‐derived exosomes utilize these lytic proteins to exert cytotoxic effects and kill tumor cells via a variety of mechanisms, including the combined action of perforins and granzymes and the interaction between ligand FasL and receptor Fas. 24 Although it has been demonstrated that NK‐derived exosomes can kill cancer cells, TEX‐containing NK ligands can reduce the expression of NKG2D receptors on NK cells, therefore decreasing their cytotoxic effects. 25
2.4. Exosomes as biomarkers
With the deepening of exosome research, many studies have demonstrated that exosomes can be used as biomarkers for early diagnosis of diseases. First, exosomes are widely distributed in body fluids, such as blood, urine, semen, amniotic fluid, and saliva, so they can be easily isolated. Second, as mentioned above, different derived exosomes vary in composition, expressing specific miRNAs, mRNAs, and proteins. Abnormal changes in these components can used to diagnose diseases and predict the prognosis of patients. For example, PCA3 mRNA and TMPRSS2:ERG fusion transcripts can be detected in the urinary exosomes of patients with prostate cancer. 26 In addition, large numbers of miRNAs are considered as new biomarkers. (Table 1).
TABLE 1.
Potential exosomal miRNAs as biomarkers
| Cancers | miRNAs | Ref |
|---|---|---|
| Lung cancers |
Increased serum miR‐96 levels in lung cancer patients. Increased miR‐21 and miR‐4257 levels in patients with non–small‐cell lung cancer (NSCLC) with recurrence. |
27 |
| Breast cancers |
miR‐301 is a prognostic maker. High levels of miR‐155, miR‐21 and miR‐1246 predict poor prognosis. |
28 |
| Colorectal cancer | The serum exosomal levels of let‐7a, miR‐1229, miR‐1246, miR‐150, miR‐21, miR‐223, and miR‐23a were increased in patients and were decreased after surgery. | 29 |
| Prostate cancers | The urinary exosomal levels of miR‐26a‐5p, miR‐532‐5p, and miR‐99b‐3p were upregulated in prostate patients with recurrence and metastasis. | 30 |
| Hepatocellular carcinoma | The serum exosomal levels of miR‐93, miR‐21, miR‐122, miR‐125b were increased but miR‐9‐3p were decreased in patients. | 31 |
However, exosomes as biomarkers have some defects. First, exosomes from cancer patients and healthy people may not have detectable differences in composition. In the above study, TMPRSS2:ERG fusion transcripts could be detected in patients with high levels of malignancy, but not in patients with low levels of malignancy. 26 Second, miRNAs in exosomes are not specific enough to distinguish between different types of cancers. For example, miR‐21 is upregulated in almost all carcinomas such as melanomas, hepatocellular carcinomas, breast, brain, gastric, and colon cancers; 32 miR‐125b is upregulated in acute myeloid leukemia (AML) but downregulated in chronic lymphocytic leukemia (CLL). 33 Third, considering the accuracy, cost, and efficiency of clinical practice, most exosome biomarkers are not clinically feasible.
3. EXOSOMES RESULT IN RT DUALITY IMMUNOLOGICALLY
3.1. Exosomes and associated circuits in relation to RT immunosuppressive activity
Exosomes discovered following RT contain a vast number of biomolecules that are implicated in both innate and adaptive immunity. Immunity is activated in response to the appearance of deviant cells such as cancer cells to selectively root out aberrant cells; this serves as a self‐protective mechanism for the human body. It is well established that RT has the potential to enhance the sensitivity of the immune system against cancer cells. However, a decreased immunological state has been observed in a variety of cancers following radiation. Changed exosomes are described to modulate the immune system and maintain a balance between activating the immune response and immunosuppressive reaction. 34
3.1.1. Exosomes in adaptive immunity
Multiple exosomal molecules changed by radiation exert a critical impact on the inhibition of specific immunity. For example, PD‐L1 (one of the membrane‐spanning proteins) is demonstrated to be associated with the inhibition of T cell proliferation and activation. In a diverse mouse model, a low dosage of radiation has been demonstrated to cause an increase in PD‐L1. 35 A potential mechanism in patients with head and neck squamous cell carcinoma (HNSCC) who present chemoradiotherapy resistance and poor prognosis is demonstrated to be partially related to the immunosuppressive response induced by TEX, which has been discovered to transport PD‐L1 in the TME. Elevated PD‐L1 levels are demonstrated to be related to decreased CD69 levels, which results in the inactivation of cytotoxic T cells. 34 Tu X, et al. used the antibody H1A to demonstrate that RNA‐binding protein PD‐L1 competes with catalytic RNA exosomes to suppress the degradation of target RNA, which is involved in DNA damage responses, to decrease the sensitivity of cancer cells to RT in human breast cancer cells. H1A is a developed PD‐L1 antibody that can expose PD‐L1 in the reactive lysosome by its negative effect on protective CMTM6. 36 Another anti‐PD‐L1 antibody, cemiplimab, can be administered with RT with alleviative immune‐related Adverse Events (irAEs). In the Papadopoulos study, 10% patients with advanced cancer (6 in 60) who were treated with cemiplimab and RT displayed antitumor benefit and prolonged responses. 37
Nevertheless, cancer treated with anti‐PD‐1 therapy develops resistance, most notably in melanoma, as a result of inhibitory immune components being immersed in the TME. The upregulated expression of Lymphocyte Activation Gene 3 (LAG‐3) transmembrane protein and exosomal T cell Immunoglobulin‐ and Mucin‐domain‐containing molecule 3 (TIM‐3), which both bind to galectin‐3 or galectin‐9, may be the underlying mechanism. 38 , 39 Increased TIM‐3 expression is observed in patients with HNSCC who were receiving RT and PD‐L1 blockade therapy, which has been demonstrated to act as an offsetting mechanism to immune checkpoint blockade (ICB). Anti‐TIM‐3, ICB, and Treg depletion all work in concert. 40 Clinically, the newly produced antibody FS118, which binds both LAG‐3 and PD‐L1, has been described as a promising method for treating cancers that are resistant to anti‐PD‐L1 therapy. The dual restriction of FS118, by preferentially associating with LAG‐3 and PD‐L1, has been used to reverse T cell anergy in mice. The interaction between FS118 and LAG‐3 and PD‐L1 results in an increase in soluble LAG‐3 (sLAG‐3), which enhances the antigen presentation capacity of APC and the immunogenicity of CTL when combined with MHC II. 41
As previously stated, the antigen presentation pathway is influenced, and includes costimulatory and coinhibitory factors. 42 , 43 Irradiation increases the expression of costimulatory molecules, resulting in immunological activation, whereas coinhibitory factors have the reverse effect. In melanoma, it has been demonstrated that the T cell immunoglobulin and ITIM domain (TIGIT), which binds to the coinhibitory factors CD155 and B7‐H3 and V‐domain containing Ig suppressor of T cell activation (VISTA), inhibits the production of IFN‐γ and TNF‐α, which initiate cell apoptosis to eliminate abnormal cells. TIGIT expression is decreased with a radiation dosage of 18 × 2 Gy and increased with a dose of 3 × 8 Gy. The ratio of CD8 + T cells in 18 × 2 Gy are ~2.5 times higher (p < 0.05). 44 B7‐H3 is found in medulloblastoma exosomes to aid in the progression of immune evasion via PI3K/AKT activation and subsequent activation of STAT3, which prefers to undergo oxidative phosphorylation reprogramming to meet the metabolic needs of excessive growth rather than the aerobic glycolysis. 38 , 44 , 45 , 46
3.1.2. Exosomes in innate immunity
Nonspecific immunity is primarily mediated by the following types of innate immune cells, including phagocytes and NK cells, whose ability to eliminate abnormal cells is skewed in the TME. In particular, as with phagocytes, it was discovered that integrin‐associated protein (IAP) also known as CD47 can bind to signal‐regulatory protein α(SIRPα) on macrophages, contributing to the prevention of phagocytic reactions and thereby protecting normal red blood cells from digestion. Nevertheless, in cancer, an elevated level of CD47 is present in the environment infiltrated by immune cells, allowing cancer cells to escape immune clearance more quickly. It has been observed that classic radiation therapy for mouse breast cancer increases both CD47 and HER2 expression simultaneously. Breast cancer is capable of developing radioresistance when it is accompanied by decreased phagocytic ability induced by CD47 and increased clonogenic ability induced by HER2. Candas‐Green D, et al. demonstrated that anti‐CD47 and anti‐HER2 double blocking are superior in eradicating radioresistant cancer cell in BALB/c mice injected with 4T1 cells. 47 Similarly, the inhibition of CD47 has been shown to provide significant relief for T cells that are hypersensitive to irradiation during RT in lymphomas. CD47‐containing exosomes have been linked to cancer metastasis. 48 miRNAs included in exosomes also participate in the inherent immune pathway to potentially promote tumor growth. As an illustration, upregulated miRNA expression in RT is associated with radioresistance and a poor prognosis in the treatment of colorectal cancer (CRC). miR‐590‐3p from cancer‐associated fibroblasts (CAFs) promotes radiation resistance in CRC cells by downregulating the expression of CLCA4, which exerts an inhibitory effect on the PI3K/AKT pathway to facilitate M2 polarization. miR‐590‐3p overexpression can be utilized as a measure of radiation resistance in patients with CRC. 49 Additionally, it has been found that RT enhances MHC I expression. 50 The interaction between MHC I and immunoglobulin‐like receptors, Leukocyte Immunoglobin‐Like Receptor (LILR), is critical for bridging innate and acquired immunity through the process of antigen presentation, which has been demonstrated to be recognized by immunogenic receptors via exosome transportation in the form of a dimer. The LILRB (LILRB1/2/4) family is abundantly expressed in immune cells, particularly those of patients with cancer. LILRB are described to disrupt the clearance of phagocytes via the recognition of MHC I to induce M2 polarization of macrophages, which is the inverted response to combat cancer. 51 As for NK cell, miRNA‐378a‐3p engendered by RT has been shown to be a suppressor of its antitumor activity. 52
In general, the inhibitory response dominates in the management of the TME in patients receiving RT, establishing a trend toward better cancer management with the combination of immunotherapy and RT. (Figure 2).
FIGURE 2.
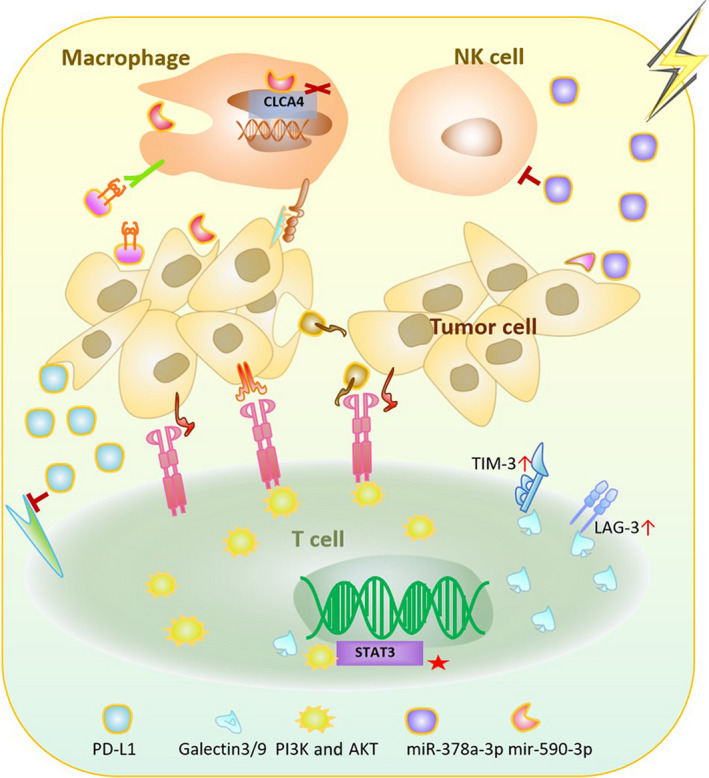
Schematic diagram of the immunosuppressive effect of radiotherapy (RT) with associated exosomes. Tumor cells and immune cells in acquired and congenital pathways are capable of releasing or receiving exosomes generated by RT to adjust the immune system into a depressed state. Exosomal MHC Ⅰ, miR‐590‐3p, SIRPα, and miR‐378a‐3p are increased in association with innate immunity. Exosomal PD‐L1, B7‐H3, TIGIT, TIM‐3, and LAG‐3 levels are increased in association with adaptive immunity
3.2. Exosomes and circuits correlate with the immunocompetent activity of RT
In contrast, in addition to the cancer cell ability to circumvent the immune system, RT is capable of activating an antitumor response to address infinitely abnormal proliferation. With a thorough understanding of exosomes, radiation‐induced immune activation is reintroduced. Since the destruction of immunogenic cancer cells, there has been an increase in the production of activated immune molecules, the majority of which are involved in adaptive immunity and the activation of T cells. Reactivation and reinforcement of these molecules may represent a potential strategy in supervising cancer patients.
T cells are the terminal targets for RT‐associated exosomes. For example, radiation stress induces an enhanced phagocytosis signal, which has been reported to be initiated by calreticulin (CRT) and may be regulated by exosomes. The death of immunogenic cells caused by RT contributes to the boosted translocation of CRT to cancer cells and the reactivated immune stress state, which initiates CTL accompanied by the production of HMGB1 (nuclear DNA binding factor) and ATP. 53
Costimulating molecules involved in antigen presentation are also changed after irradiation via the transport of exosomes such as CD86, CD28, and CD47. 54 , 55 , 56 During the treatment of pancreatic ductal adenocarcinoma, RT has been shown to target sensitive T cells and induce the exhaustion of T cells during the early treatment phase. However, studies have shown that the removal of intratumoral T cells upregulates CD86, thereby potentiating the effects of immunotherapy. 57 Normally, APCs express costimulatory molecules such as B7/CD28, which communicate with T cells to initiate the immune response. 38 Liu SZ, et al. presented that in male Kunming mice low‐dose irradiation can boost CD28 expression on lymphocytes. Activation of CD28 may therefore be an effective approach for predicting the prognosis of patients after receiving chemoradiotherapy. 58 As we mentioned earlier, CD47 is an immunosuppressive factor that increased with radiation dose in a mouse model of breast cancer. However, in HPV+ HNSCC C57Bl/6 mice model, the expression of CD47 decreased with RT in a dose‐dependent manner (from 2 Gy to 40 Gy). The decrease in CD47 levels following radiation treatment further amplified the effects of RT. 59
Tumor necrosis factor ligand superfamily 4‐1BBL, OX40L, and glucocorticoid‐induced TNFR‐related receptor (GITRL) are also upregulated after RT. Radiation increases 4‐1BBL expression, which further activates T cells. TEXs from cancer cells infected by oncolytic viruses express 4‐1BBL. 60 , 61 OX40L has been reported to be expressed on cytotoxic T cells, Tregs, and exosomes originating from mast cells, and its expression was increased by RT. 62 , 63 Kumari A, et al. found that 5/6 CRC cell lines presented an OX40L and 4‐1BBL increase after RT treatment, which can be the potential cause of enhanced RT antitumor response 64 Similarly, irradiation upregulates the expression of GITRL. Exosomes from Treg cells contain GITRL, which disrupts the structure of Tregs and decreases their immunosuppressive activity. 63 , 65
Taken together, these findings demonstrated that, in addition to the inhibitory effect of exosomes in the immune system, exosomes generated after RT still play a role in the antitumor response. This can be the mechanism for the abscopal effect. The abscopal effect suggests that, under the control of focused RT on primary lesions, there is remission of untreated distal metastatic tumors. However, the abscopal effect is rare and transient. 66 The suppression of the immune system caused by exosomes eclipses their activation, resulting in the development of radioresistance. The duality of these molecules in immune function contributes to the bipolar effects of RT (Figure 3).
FIGURE 3.
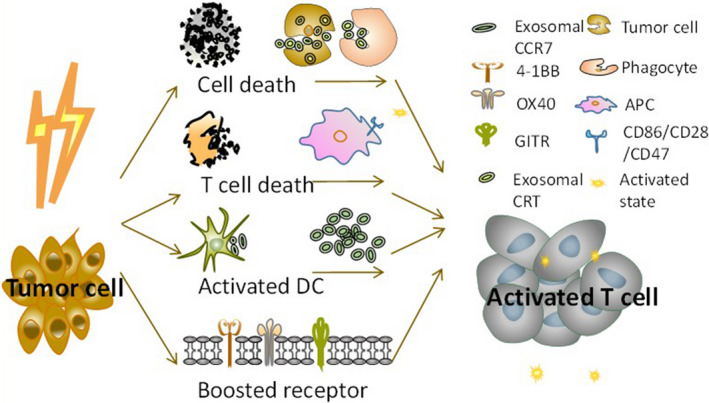
Schematic diagram showing the immunocompetent aspects of radiotherapy (RT) and exosomes involved. The cell damage caused by RT increases the number of immunogenic molecules that then activate T cells to exert an antitumor response. The cell death caused by radiation activates T cells through exosomal calreticulin (CRT) and CCR7 and increases CD86/CD28/CD47 levels and the expression of receptors 4‐1BB, OX40, and GITR
4. CONCLUSION
To our knowledge, the effects of RT on the prognosis of cancer patients are inextricably linked to the immune system through the mediating role of exosomes. The results from our study demonstrated that RT has immunosuppressive and immune activating effects, which are achieved through exosomes. Cancer cells often develop radioresistance, which limits the efficacy of radiotherapy. Exosomes derived from tumor and immune cells mediate the dual effects of RT on immune function. Here, we illustrate that exosomes participate in TME activities after RT. These exosomes can not only transport immunosuppressive molecules that dampen the antitumor response, but also carry immunocompetent substances that create an immune‐stimulated state, which also demonstrated the existence of the abscopal effect This provides a clear direction for the combination of immunotherapy and radiotherapy in the future to reduce immunosuppression and enhance the immune promotion of RT. Future studies should investigate the benefits of combining RT and immune checkpoint therapy (ICT) to improve the advantages of RT, that is, boost its immune activating effects and immunosuppressive effects.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (nos. 82060526 and 81860540).
Hu X, Qiu Y, Zeng X, Wang H. Exosomes reveal the dual nature of radiotherapy in tumor immunology. Cancer Sci. 2022;113:1105–1112. doi: 10.1111/cas.15314
Xinru Hu and Yuyue Qiu contributed equally to this work.
REFERENCES
- 1. Hughes JR, Parsons JL. FLASH radiotherapy: current knowledge and future insights using proton‐beam therapy. Int J Mol Sci. 2020;21(18):6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487‐514. [DOI] [PubMed] [Google Scholar]
- 3. Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol. 2018;28(8):R435‐R444. [DOI] [PubMed] [Google Scholar]
- 4. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569‐579. [DOI] [PubMed] [Google Scholar]
- 5. Duijvesz D, Luider T, Bangma CH, Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59(5):823‐831. [DOI] [PubMed] [Google Scholar]
- 6. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 7. Wolfers J, Lozier A, Raposo G, et al. Tumor‐derived exosomes are a source of shared tumor rejection antigens for CTL cross‐priming. Nat Med. 2001;7(3):297‐303. [DOI] [PubMed] [Google Scholar]
- 8. Zech D, Rana S, Büchler MW, Zöller M. Tumor‐exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iero M, Valenti R, Huber V, et al. Tumour‐released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80‐88. [DOI] [PubMed] [Google Scholar]
- 11. Xu Z, Zeng S, Gong Z, Yan Y. Exosome‐based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitt JM, Andre F, Amigorena S, et al. Dendritic cell‐derived exosomes for cancer therapy. J Clin Invest. 2016;126(4):1224‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell‐derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309‐7318. [DOI] [PubMed] [Google Scholar]
- 14. Viaud S, Théry C, Ploix S, et al. Dendritic cell‐derived exosomes for cancer immunotherapy: what's next? Cancer Res. 2010;70(4):1281‐1285. [DOI] [PubMed] [Google Scholar]
- 15. Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176(5):2722‐2729. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor‐bearing hosts. Cell Immunol. 1996;170(1):101‐110. [DOI] [PubMed] [Google Scholar]
- 18. Gabrilovich D. Mechanisms and functional significance of tumour‐induced dendritic‐cell defects. Nat Rev Immunol. 2004;4(12):941‐952. [DOI] [PubMed] [Google Scholar]
- 19. Li L, Jay SM, Wang Y, Wu S‐W, Xiao Z. IL‐12 stimulates CTLs to secrete exosomes capable of activating bystander CD8+ T cells. Sci Rep. 2017;7(1):13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Z, Yang F, Yu L, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188(12):5954‐5961. [DOI] [PubMed] [Google Scholar]
- 21. Xie Y, Zhang X, Zhao T, Li W, Xiang J. Natural CD8⁺25⁺ regulatory T cell‐secreted exosomes capable of suppressing cytotoxic T lymphocyte‐mediated immunity against B16 melanoma. Biochem Biophys Res Comm. 2013;438(1):152‐155. [DOI] [PubMed] [Google Scholar]
- 22. Wu J, Gao FX, Wang C, et al. IL‐6 and IL‐8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2019;38(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jong AY, Wu CH, Li J, et al. Large‐scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6(1):1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY. Biological roles and potential applications of immune cell‐derived extracellular vesicles. J Extracell Vesicles. 2017;6(1):1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundholm M, Schroder M, Nagaeva O, et al. Prostate tumor‐derived exosomes down‐regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9(9):e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer‐derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen R, Xu X, Qian Z, et al. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci. 2019;76(23):4613‐4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jabbari N, Akbariazar E, Feqhhi M, Rahbarghazi R, Rezaie J. Breast cancer‐derived exosomes: tumor progression and therapeutic agents. J Cell Physiol. 2020;235(10):6345‐6356. [DOI] [PubMed] [Google Scholar]
- 29. Ogata‐Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9(4):e92921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim MY, Shin H, Moon HW, Park YH, Park J, Lee JY. Urinary exosomal microRNA profiling in intermediate‐risk prostate cancer. Sci Rep. 2021;11(1):7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Xu X. Biological functions and clinical applications of exosomal non‐coding RNAs in hepatocellular carcinoma. Cell Mol Life Sci. 2019;76(21):4203‐4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paranjape T, Slack FJ, Weidhaas JB. MicroRNAs: tools for cancer diagnostics. Gut. 2009;58(11):1546‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev. 2013;253(1):167‐184. [DOI] [PubMed] [Google Scholar]
- 34. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD‐L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res. 2014;74(19):5458‐5468. [DOI] [PubMed] [Google Scholar]
- 36. Tu X, Qin B, Zhang Y, et al. PD‐L1 (B7–H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol Cell. 2019;74(6):1215‐1226.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papadopoulos KP, Johnson ML, Lockhart AC, et al. First‐in‐human study of cemiplimab alone or in combination with radiotherapy and/or low‐dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025‐1033. [DOI] [PubMed] [Google Scholar]
- 38. Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52(Pt 2):39‐52. [DOI] [PubMed] [Google Scholar]
- 39. Gao J, Qiu X, Li X, et al. Expression profiles and clinical value of plasma exosomal Tim‐3 and Galectin‐9 in non‐small cell lung cancer. Biochem Biophys Res Comm. 2018;498(3):409‐415. [DOI] [PubMed] [Google Scholar]
- 40. Oweida A, Hararah MK, Phan A, et al. Resistance to radiotherapy and PD‐L1 blockade is mediated by TIM‐3 upregulation and regulatory T‐cell infiltration. Clin Cancer Res. 2018;24(21):5368‐5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kraman M, Faroudi M, Allen NL, et al. FS118, a bispecific antibody targeting LAG‐3 and PD‐L1, enhances T‐cell activation resulting in potent antitumor activity. Clin Cancer Res. 2020;26(13):3333‐3344. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Luo Y, Qin SL, et al. The clinical impact of ICOS signal in colorectal cancer patients. Oncoimmunology. 2016;5(5):e1141857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fan X, Wang J, Qin T, et al. Exosome miR‐27a‐3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac Cancer. 2020;11(6):1453‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grapin M, Richard C, Limagne E, et al. Optimized fractionated radiotherapy with anti‐PD‐L1 and anti‐TIGIT: a promising new combination. J Immunother Cancer. 2019;7(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Purvis IJ, Velpula KK, Guda MR, Nguyen D, Tsung AJ, Asuthkar S. B7–H3 in medulloblastoma‐derived exosomes; a novel tumorigenic role. Int J Mol Sci. 2020;21(19):7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee M, Hirpara JL, Eu JQ, et al. Targeting STAT3 and oxidative phosphorylation in oncogene‐addicted tumors. Redox Biol. 2019;25:101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Candas‐Green D, Xie B, Huang J, et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun. 2020;11(1):4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lian S, Xie X, Lu Y, Jia L. Checkpoint CD47 function on tumor metastasis and immune therapy. Onco Targets Ther. 2019;12:9105‐9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen X, Liu Y, Zhang Q, et al. Exosomal miR‐590‐3p derived from cancer‐associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids. 2021;24:113‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Briand J, Garnier D, Nadaradjane A, et al. Radiotherapy‐induced overexpression of exosomal miRNA‐378a‐3p in cancer cells limits natural killer cells cytotoxicity. Epigenomics. 2020;12(5):397‐408. [DOI] [PubMed] [Google Scholar]
- 53. Sato H, Okonogi N, Nakano T. Rationale of combination of anti‐PD‐1/PD‐L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol. 2020;25(5):801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969‐1978. [DOI] [PubMed] [Google Scholar]
- 55. Tucci M, Mannavola F, Passarelli A, Stucci LS, Cives M, Silvestris F. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget. 2018;9(29):20826‐20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rech AJ, Dada H, Kotzin JJ, et al. Radiotherapy and CD40 activation separately augment immunity to checkpoint blockade in cancer. Cancer Res. 2018;78(15):4282‐4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL‐12/IL‐10 interaction in the radiation‐induced immune changes. BMC Immunol. 2001;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation‐induced loss of cell surface CD47 enhances immune‐mediated clearance of human papillomavirus‐positive cancer. Int J Cancer. 2013;133(1):120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Labani‐Motlagh A, Naseri S, Wenthe J, Eriksson E, Loskog A. Systemic immunity upon local oncolytic virotherapy armed with immunostimulatory genes may be supported by tumor‐derived exosomes. Mol Ther Oncolytics. 2021;20:508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4–1BB activation and CTLA‐4 blockade yields long‐term survival and a protective antigen‐specific memory response in a murine glioma model. PLoS One. 2014;9(7):e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chan M, Wu L, Yun Z, et al. Blocking the GITR‐GITRL pathway to overcome resistance to therapy in sarcomatoid malignant pleural mesothelioma. Commun Biol. 2021;4(1):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li F, Wang Y, Lin L, et al. Mast cell‐derived exosomes promote Th2 cell differentiation via OX40L‐OX40 ligation. J Immunol Res. 2016;2016:3623898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumari A, Garnett‐Benson C. Effector function of CTLs is increased by irradiated colorectal tumor cells that modulate OX‐40L and 4–1BBL and is reversed following dual blockade. BMC Res Notes. 2016;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amoozgar Z, Kloepper J, Ren J, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun. 2021;12(1):2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25(6):1709‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]


