Abstract
The AU‐rich binding factor 1 (AUF1) is one of the well known adenylate‐uridylate‐rich element (ARE)‐specific RNA‐binding proteins (ARE‐BPs) for which dysregulation has been reported in various human cancers. However, the involvement of AUF1 in the initiation and progression of hepatocellular carcinoma (HCC) is still elusive. In this study, we aimed at exploring the clinical significance, function, and mechanism of the abnormal expression of AUF1 in HCC. Using a bioinformatics analysis of The Cancer Genome Atlas (TCGA) and Liver Cancer Institute (LCI) database, we identified that AUF1 was abnormally highly expressed in HCC tissues and that the high expression of AUF1 was correlated with poor prognosis in patients with HCC. We also confirmed the increased AUF1 expression and its prognostic value in our HBV‐related HCC cohorts. AUF1 overexpression in hepatoma cells promoted cell proliferation and increased the resistance of hepatoma cells toward doxorubicin, whereas knockdown of AUF1 exerted the opposite effects. Mechanistically, we demonstrated that AKR1B10 was a critical target of AUF1 and was essential for sustaining the AUF1‐induced proliferation and drug resistance of hepatoma cells. AUF1 increased AKR1B10 expression by binding to the 3′UTR region of AKR1B10 mRNA and stabilizing AKR1B10 mRNA. Additionally, we demonstrated that E2F1 enhanced AUF1 expression in HCC at the transcription level. Our study revealed a novel role of AUF1 in promoting the development and drug resistance of HCC via the post‐transcriptional regulation of AKR1B10 expression. The E2F1/AUF1/AKR1B10 axis can serve as a potential therapeutic target in HCC.
Keywords: AKR1B10, AUF1, E2F1, hepatocellular carcinoma, RNA‐binding protein
Our study revealed a novel role for AUF1 in promoting hepatocarcinogenesis and drug resistance via the post‐transcriptional regulation of AKR1B10 expression. The E2F1/AUF1/AKR1B10 axis can serve as a potential therapeutic target in HCC.
Abbreviations
- ARE‐BPs
ARE‐binding proteins
- AREs
adenine/uridine‐rich elements
- AUF1
AU‐rich binding factor 1
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- RA
retinoic acid
- RBPs
RNA‐binding proteins
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most aggressive human cancers and currently ranks as the third leading cause of cancer‐related deaths worldwide. 1 Despite recent progress in clinical treatment, the 5‐y overall patient survival rate for HCC is less than 15%. 2 The poor prognosis of patients with HCC is mainly because of delayed diagnosis, limited therapeutic options, frequent cancer metastasis, and high cancer recurrence rates. 3 , 4 Therefore, a better understanding of the molecular mechanisms underlying the development of HCC is crucial for the development of novel therapeutic drugs.
Recent transcriptomic studies have revealed that transcriptome dysregulation plays driving roles during the development and progression of HCC, 5 , 6 and that abnormal post‐transcriptional control of mRNA metabolism is related to the significant transcriptomic imbalance in HCC tumors. 7 , 8 , 9
The post‐transcriptional regulation of mRNA is mediated by the so‐called RBPs. Among RBPs, the ARE‐BPs are the best known regulators of post‐transcription in eukaryotes. 10 , 11 Typically, AREs are short linear motifs with a high content of complementary A and U nucleotides located in the 3′ untranslated region (3'UTR) of mRNAs. ARE‐BPs can recognize and bind to hundreds of cellular mRNA through interactions with the ARE motif and regulate the mRNA metabolism of their targets, including mRNA processing, turn‐over, localization, and translational control. 12 , 13 Numerous studies have demonstrated that mRNAs of many oncogenes are regulated by ARE‐BPs and that dysregulated ARE‐BP expression plays important roles in the development and progression of human cancers. 14 , 15
AU‐rich binding factor 1 (AUF1), also called heterogeneous nuclear ribonucleoprotein D (hnRNPD), is one of the well known ARE‐BPs that is predominantly associated with mRNA‐destabilizing activity. The target genes of AUF1 are involved in many physiological processes such as cell proliferation, 15 apoptosis, 16 senescence and metastasis, 17 and dysregulation of AUF1 has been reported in breast, skin, thyroid, and liver cancers. Recent studies have reported that AUF1 can also stabilize mRNA, 15 , 17 , 18 suggesting that AUF1 plays a critical and complex role in tumorigenesis. However, the involvement of AUF1 in HCC initiation and progression is still elusive.
In the present study, we investigated the expression pattern and role of AUF1 in HCC. We found that AUF1 was significantly upregulated in tumor tissues, and the higher expression level of AUF1 was associated with poor prognoses in patients with HCC. Interestingly, we demonstrated that AUF1 promoted HCC development and drug resistance by upregulating AKR1B10, an independent risk factor for HCC. 19 , 20 Moreover, we identified that AUF1 was a direct transcriptional target of E2F1. Collectively, these data support a model in which the E2F1/AUF1/AKR1B10 axis plays an important role in the development of HCC and can serve as a potential therapeutic target in HCC.
2. MATERIALS AND METHODS
Detailed Materials and Methods are shown in Supporting information.
3. RESULTS
3.1. AUF1 is highly expressed in HCC and predicts poor prognosis
To evaluate the importance of ARE‐BPs in HCC, we explored the expression profiles of 8 ARE‐BPs in 214 patients with HBV‐related HCC from the LCI database; these included AUF1, HUR, ZFP36, KSRP, TIA1, BRF1, HUD, and Hsp70, which have been frequently dysregulated in tumors and shown in previous studies to affect the occurrence and development of tumors. 7 , 13 , 21 The results showed that expression of ZFP36, BRF1, and HUD was downregulated, while other ARE‐BPs were upregulated in HCC tissues compared with that in the paired non‐tumor tissues (Figures 1A,B and S1A). Survival analysis of patients showed that only AUF1 expression level in tumor tissues was negatively associated with the overall survival time of patients with HCC (P = .035; Figures 1C and S1B). The upregulation of AUF1 in HCC tissues was also observed in TCGA RNA‐seq data set that enrolled 371 patients with HCC (P < .001; Figure 1D,E). The survival analysis in this cohort also showed that patients with high AUF1 expression had a worse prognosis, although the difference was not statistically significant (P = .059; Figure 1F). It is well known that elevated Alpha fetoprotein (AFP) levels are associated with the poor prognosis of patients with HCC. 22 In line with this, the AUF1 expression levels in patients with serum AFP levels ≥300 ng/mL were significantly higher than for patients with serum AFP levels <300 ng/mL in both the LCI (P < .001; Figure S1C) and TCGA databases (P < .001; Figure S1D). Additionally, the expression levels of AUF1 mRNA were significantly higher in the tumor tissues derived from patients with HCC who had Barcelona Clinic Liver Cancer (BCLC) stage C or larger tumors than that from patients who had BCLC stage A (P = .011; Figure S1E) or smaller tumors (P = .047; Figure S1F).
FIGURE 1.
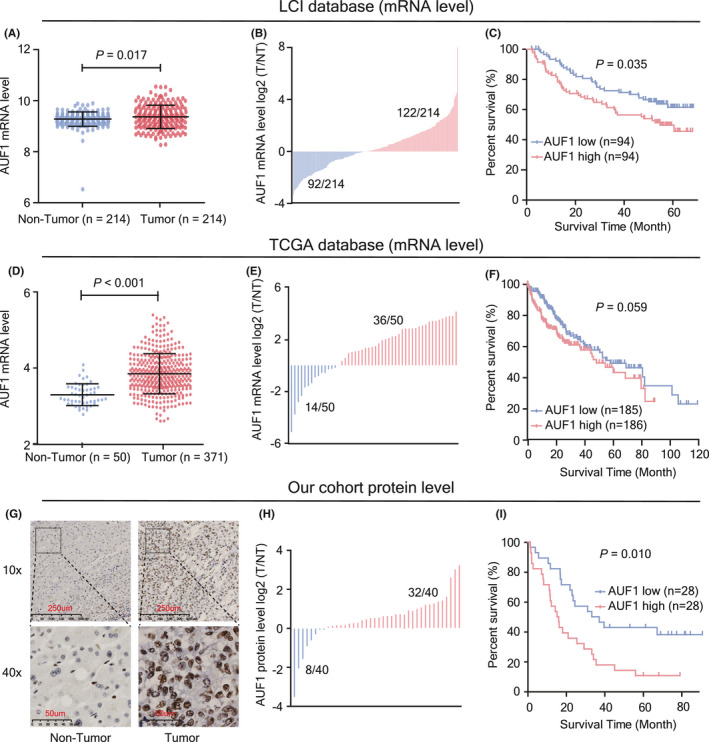
Expression and prognostic value of AUF1 in patients with HCC. A, AUF1 mRNA levels in HCC tissues and matched non‐tumor tissues from the LCI database (n = 214). B, Abnormal expression of AUF1 mRNA in 214 HCC tissues from the LCI database. T/NT indicates the expression level ratio of tumor tissues and their corresponding non‐tumor tissues. C, Kaplan‐Meier analysis of AUF1 mRNA and overall survival from 188 HBV‐HCC tissues from the LCI database. D, AUF1 mRNA level in 371 HCC tissues and 50 non‐tumor tissues from TCGA database. E, Abnormal expression of AUF1 mRNA in 50 HCC tissues from TCGA database. F, Kaplan‐Meier analysis of AUF1 mRNA and overall survival from 371 HCC tissues from TCGA database. G, H, AUF1 protein levels in paired HBV‐HCC tissues detected using immunohistochemistry (IHC) staining (n = 66) and western blot assay (n = 40). I, Kaplan‐Meier analysis of AUF1 IHC score and overall survival from 56 HBV‐HCC tissues
To further confirm the expression and the prognostic value of AUF1, an immunohistochemistry (IHC) assay was performed to determine the expression of AUF1 at the protein level in our 66 HBV‐related HCC samples. Overall, we observed positive staining of AUF1 protein in 95.5% (63/66) of HCC tumor tissues, including 22 tissues with very strong AUF1 staining (IHC score ≥3), 27 with moderate staining (2 ≤ IHC score <3), and 14 with weak positive staining (IHC score = 1). In contrast, AUF1 staining in the adjacent non‐tumor liver tissues was very weak (Figure 1G and Table S1). Consistent with the IHC results, western blot assay showed that 72% (32/40) of tumor tissues had marked AUF1 upregulation (Figures 1H and S2A). Further survival analysis confirmed the poor prognosis of patients in this HBV‐HCC cohort who had higher AUF1 expression in tumor tissues (P = .010; Figure 1I). In addition, the upregulation of AUF1 was also observed in multiple human hepatoma cell lines (Figure S2B). These data demonstrated that the expression of AUF1 was frequently upregulated in HCC tumor tissues and higher AUF1 expression always predicted a poor prognosis for patients with HCC.
3.2. AUF1 promotes the growth of hepatoma cells in vitro
The above results suggested that AUF1 might promote HCC progress. To address this issue, we established stable AUF1 knockdown models in HepG2 and Huh‐1 cells using 2 independent shRNA expression plasmids (Figure 2A). Silencing of AUF1 inhibited the proliferation rates of both HepG2 and Huh‐1 cells (Figure 2B). Correspondingly, AUF1 knockdown significantly repressed the colony formation capability of HepG2 and Huh‐1 cells (Figure 2C) and induced a G2/M cell cycle arrest in hepatoma cells (Figure 2D). We further performed a gain‐of‐function study (Figure 2E) and investigated the influence of AUF1 overexpression on cell proliferation. When trying to construct AUF1 overexpression cell lines, we selected HepG2 and Huh‐7 cells due to their higher infection efficiency for lentivirus. As anticipated, stable overexpression of AUF1 in both HepG2 and Huh‐7 cells significantly promoted cell proliferation (Figure 2F) and colony formation (Figure 2G), and triggered more cells to enter S phase (Figure 2H). These results demonstrate that AUF1 could promote the growth of hepatoma cells in vitro.
FIGURE 2.
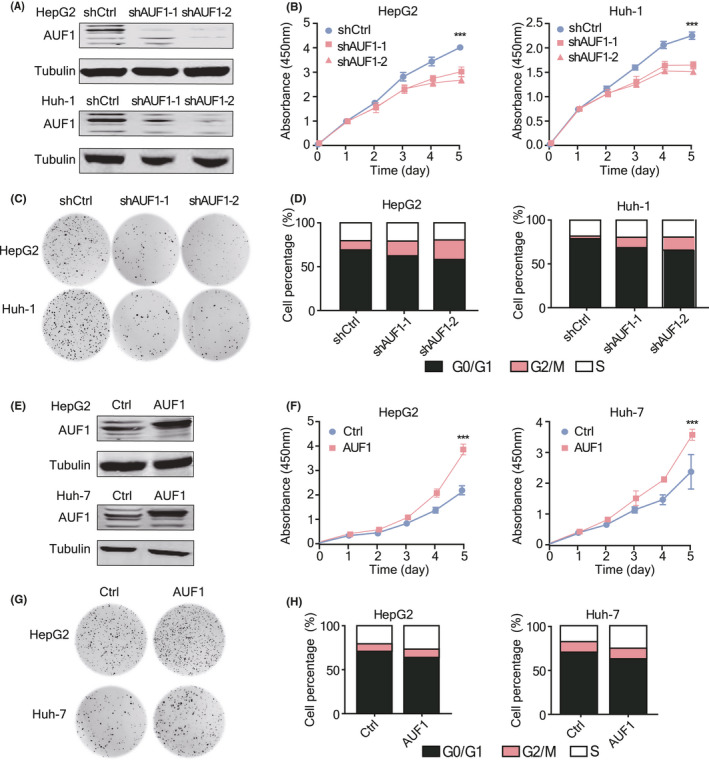
AUF1 promotes the growth of HCC cells in vitro. A, AUF1 stable knockdown cell models were established. The knockdown efficiency of 2 shRNA were verified using western blot assay. B‐D, Cell proliferation rate (B), colony formation ability (C), and cell cycle (D) were detected in AUF1‐knockdown HCC cell lines. E, AUF1 stable overexpression cell models were established. Protein levels of AUF1 were measured using western blot assay. F‐H, Cell proliferation rate (F), colony formation ability (G), and cell cycle (H) were detected in AUF1‐overexpression HCC cell lines. ***P < .001
3.3. AUF1 promotes the development of hepatoma cells in vivo
To further validate the involvement of AUF1 in the progression of HCC, Huh‐1 cells with stable knockdown of AUF1, or control cells, were utilized for tumor formation in NPG/Vst mice. Silencing of AUF1 expression resulted in a decreased tumor size and tumor weight in NPG/Vst mice (Figure 3A‐C). Ki‐67 staining in tumors subjected to AUF1 knockdown was also weaker than that in the control tumors (Figure 3D). In contrast, overexpression of AUF1 in Huh‐7 cells resulted in a significant increase in tumor growth (Figure 3E‐G) and Ki‐67‐positive cells in tumor tissues (Figure 3H). Taken together, these data further confirmed that AUF1 markedly promoted HCC development.
FIGURE 3.
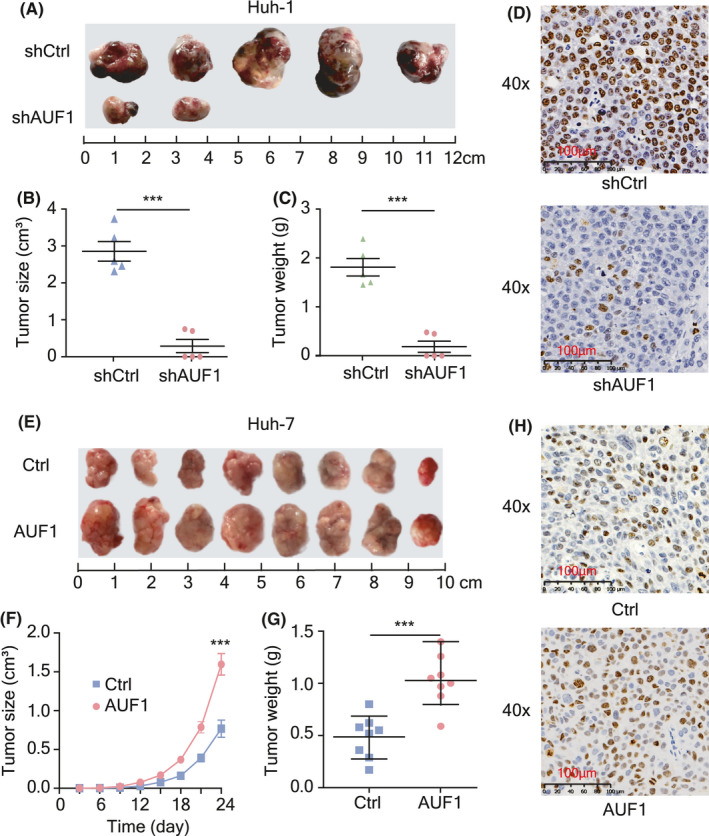
AUF1 promotes HCC tumorigenicity in vivo. A‐C, Subcutaneous injection of Huh‐1 cells with stable knockdown of AUF1 or control cells into NPG/Vst mice (n = 5). Images of neoplasms from each group of NPG/Vst mice (A), measurement of tumor volumes (B), and tumor weight (C). D, Immunohistochemistry staining of proliferation marker Ki‐67 in tumor tissues. Scale bars, 100 μm. E‐G, Subcutaneous tumor model of Huh‐7 cells with AUF1 overexpression or control cells injected into NPG/Vst mice (n = 8). Images of neoplasms from each group of NPG/Vst mice (E), measurement of tumor volumes (F), and tumor weight (G). H, Immunohistochemistry staining images of Ki67in tumor tissues. Scale bars, 100 μm. ***P < .001
3.4. AUF1 enhances the chemotherapeutic drug resistance of hepatoma cells
To evaluate whether AUF1 elevates the chemotherapeutic drug resistance of hepatoma cells, hepatoma cells with AUF1 silencing or overexpression were treated with different doses of doxorubicin (Dox) and the cell viability was detected. The results showed that AUF1 silencing led to more susceptible cells to Dox than the control cells in a drug concentration‐dependent manner in HepG2 and Huh‐1 cells (Figure 4A). Moreover, silencing of AUF1 expression significantly increased Dox‐induced apoptosis in HCC cells in a dose‐dependent manner (Figure 4B), which was also demonstrated by increased expression level of Bax, cleaved‐PARP proteins and decreased expression level of Bcl2 (Figure 4C). In contrast, AUF1 overexpression significantly reduced the cell susceptibility to Dox and the Dox‐induced apoptosis in Dox‐treated HepG2 and Huh‐7 cells (Figure 4D‐F). These data demonstrated that AUF1 protected hepatoma cells against Dox‐induced apoptosis and enhanced the chemotherapeutic drug resistance.
FIGURE 4.
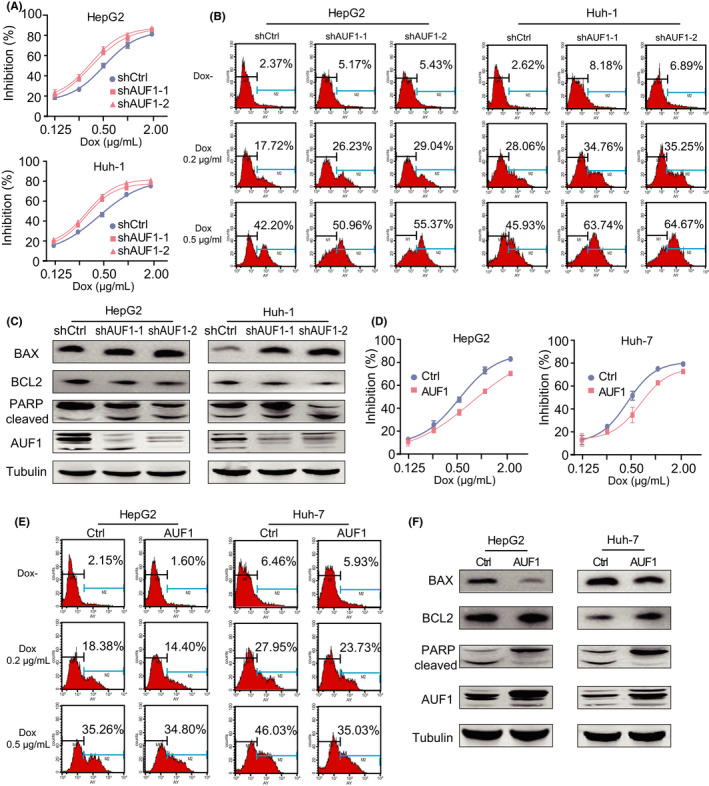
AUF1 expression in hepatoma cells with Dox resistance. A, Inhibitory effects of Dox toward AUF1‐knockdown HCC cell lines. B, Percentage of annexin V‐positive cells in AUF1 knockdown cells after Dox treatment for 48 h. C, Western blot analysis shows the apoptotic cascade protein expression levels upon AUF1 knockdown in hepatoma cells after Dox (0.5 μg/mL) treatment for 48 h. D, E, Incidence of resistance (D) and apoptosis (E) were detected in AUF1‐overexpressed HCC cell lines. F, Western blot analysis showed the apoptotic cascade protein expression levels upon AUF1 overexpression in hepatoma cells after Dox (0.5 μg/mL) treatment for 48 h
3.5. AUF1 preferentially affects the expression of metabolism‐related genes in HCC
To gain insights into the molecular mechanism underlying the pro‐tumorigenic role of AUF1, RNA‐seq assay was performed to investigate the transcriptome changes caused by AUF1 silencing in HepG2 cells. In total, 1495 transcripts that were significantly altered upon AUF1 knockdown were identified (Table S2), consistent with the notion that AUF1 is an ARE‐BP that can lead to transcriptomic imbalance. Among these genes, 658 transcripts were found to be differentially expressed between HCC and non‐tumor samples in both the LCI and TCGA data sets (Figure 5A). As AUF1 was upregulated in HCC tissues, theoretically, the downregulated genes after AUF1 knockdown should be upregulated in HCC, or vice versa. Accordingly, 210 genes expressed in a reciprocal pattern were identified among these 658 HCC‐specific genes (Table S3). We found that these 210 AUF1‐dependent HCC‐specific genes could be used to discriminate accurately the tumor from non‐tumor, with accuracies of more than 97.4% in TCGA and 97.7% in the LCI data sets (Figure 5B). Therefore, we reasoned that these 210 transcripts might accurately represent AUF1‐dependent transcripts that acted as key drivers of HCC.
FIGURE 5.
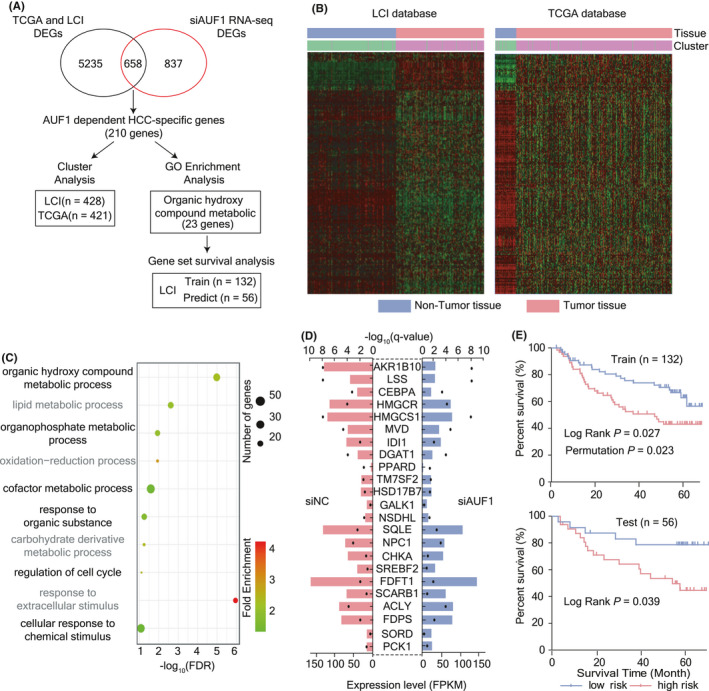
Screening of AUF1‐dependent HCC‐related genes. A, Analysis flow chart. DEGs indicates differentially expressed genes. B, Cluster analysis of HCC tissues and non‐tumor tissues using 210 AUF1‐dependent HCC‐specific genes for the LCI and TCGA HCC samples. C, Gene Ontology enrichment analysis of 210 AUF1‐dependent HCC‐specific genes. The color of the dot represents the fold enrichment, and the size of the dot represents the number of genes enriched in the pathway. D, Differential expression analysis of 23 AUF1‐dependent organic hydroxy compound metabolism genes in siAUF1 RNA‐seq. The length of bar represents fold change, and the black dot is used to indicate the q‐value. E, Survival gene set analysis of 23 AUF1‐dependent organic hydroxy compound metabolism genes for HCC in the LCI database. Log‐Rank indicates the log‐rank test P‐value, and permutation indicates the permutation test P‐value; in total 1000 random trials were conducted
To further analyze the functional importance of the AUF1‐dependent HCC‐specific genes, we performed Gene Ontology (GO) analysis. The results showed that the functions of these genes were mainly enriched in metabolism‐associated signatures including organic hydroxy compound metabolism, lipid metabolism, organophosphate metabolism, and oxidation‐reduction processes, of which organic hydroxy compound metabolism was ranked as the most enriched pathway (Figure 5C and Table S4). We named a total of 23 genes as AUF1‐dependent organic hydroxy compound metabolism genes (Figure 5D). To evaluate the prognosis predictive value of these 23 genes for HCC, we conducted a gene set survival analysis. In total, the results for 188 patients with HCC in the LCI database with BCLC staging information available were selected and randomly divided into training set and test set with a ratio of 7:3, and analyzed using BRB‐array tools. We found that in the training set, the log‐rank P‐value and permutation P‐value of the survival risk prediction model constructed by these 23 genes reached .027 and .023, respectively. In the test set, the log‐rank P‐value also reached .039. The above results suggested that AUF1 preferentially affected the expression of metabolism‐related genes and that AUF1‐dependent organic hydroxy compound metabolism genes are important prognostic risk factors for HCC (Figure 5E).
3.6. AUF1 is physically associated with AKR1B10 in HCC and upregulates AKR1B10 expression by increasing its mRNA stability
Among the genes affected by AUF1 knockdown, AKR1B10 is the top one with the most significant difference. As our previous studies and other studies have proved that dysregulation of AKR1B10 expression is involved in HCC development, 20 we considered that AKR1B10 might be a target gene of AUF1. To further confirm the regulation of AUF1 on AKR1B10 expression, we detected AKR1B10 expression at mRNA and protein levels in hepatoma cells with AUF1 overexpression or knockdown. As shown in Figure 6A,B, AUF1 knockdown significantly decreased AKR1B10 expression at both the protein and mRNA levels, while AUF1 overexpression increased AKR1B10 expression. Next, we analyzed the correlation between AKR1B10 and AUF1 levels in 40 pairs of HCC tissue specimens in which both AKR1B10 and AUF1 protein expression had been detected using western blot assays. We found that AKR1B10 expression was upregulated in 71.0% (27/40) of HCC tumor tissues, and the protein levels of AKR1B10 and AUF1 were correlated with each other in these 40 HCC tissues (r = .406, P < .001; Figure 6C). Additionally, co‐localization analysis of the IHC results showed that, in adjacent sections of HCC tissues, most regions that stained for AUF1 were also positive for AKR1B10 and vice versa (Figure S3). Furthermore, using another 40 HCC tissues in which AUF1 protein had been determined through IHC and AKR1B10 mRNA was tested using a qRT‐PCR assay, we also observed a marked positive correlation between AKR1B10 mRNA levels and the AUF1 protein level (r = .425, P < .001; Figure 6D).
FIGURE 6.
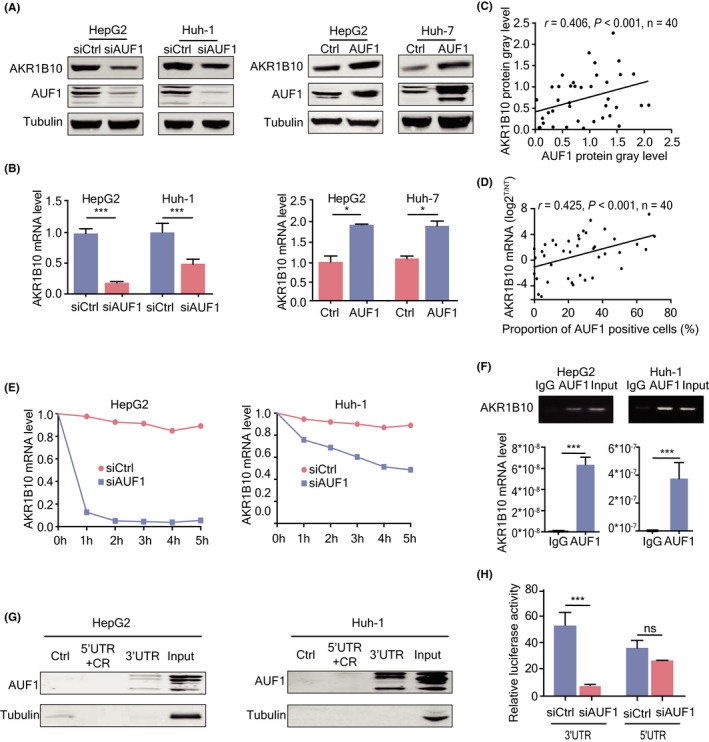
AUF1‐enhanced AKR1B10 expression through post‐transcriptional regulation. A, B, Western blot and RT‐qPCR analyses of AKR1B10 protein (A) and mRNA (B) in hepatoma cells with AUF1 overexpression or knockdown. C, Expression levels of AUF1 protein were positively correlated with AKR1B10 in 40 pairs of human HCC samples determined by western blot. Data were digitalized using ImageJ software statistical analysis. D, mRNA expression levels of AKR1B10 in 40 pairs of HCC samples were positively correlated with protein expression of AUF1. E, Half‐life of AKR1B10 mRNA in HepG2 and Huh‐1 cells with AUF1 knocked down. The levels of AKR1B10 mRNA was tested using qRT‐PCR at the indicated time followed by actinomycin D treatment (5 mg/mL). F, Binding of AUF1 to AKR1B10 mRNA detected using ribonucleoprotein immunoprecipitation (RNP‐IP) assays. AKR1B10 mRNA abundance in immune precipitates was tested using RT‐PCR and qRT‐PCR. G, Motif in AKR1B10 mRNA interacted with AUF1 identified using RNA pulldown assay. Ctrl indicates the negative control, and Input indicates total RNA extracted. 5′UTR, CR, and 3′UTR, respectively, indicate the corresponding regions of AUF1 mRNA. H, Luciferase activity of the reporter containing the 3′UTR or 5′UTR of the AKR1B10 transcript detected using luciferase activity assays. *P < .05; ***P < .001
Next, we analyzed whether there existed potential AREs in AKR1B10 mRNA. Using AREsite we identified conserved AU‐rich element clusters in the 3′UTR of AKR1B10 mRNA (Figure S4). RNA turn‐over assay showed that the half‐life of AKR1B10 mRNA was significantly shorter in AUF1 knockdown cells than that in control cells (Figure 6E). RNA immunoprecipitation (RIP) assays showed that AUF1 could strongly bind to AKR1B10 mRNA (Figure 6F). Further RNA pulldown assay demonstrated that only the AKR1B10 3′UTR region could combine with AUF1 (Figure 6G). Consistent with this result, knockdown of AUF1 significantly decreased luciferase activity of the reporter containing the 3′UTR region of the AKR1B10 transcript, but had no effect on the 5′UTR region of the AKR1B10 transcript (Figure 6H). Taken together, these data indicated that AUF1 enhanced AKR1B10 expression by increasing its mRNA stability following binding to its 3′UTR region.
3.7. AUF1‐promoted HCC development and drug resistance are AKR1B10 dependent
It has been reported that AKR1B10 functions as an efficient retinal reductase that may reduce RA levels and therefore promoting cell proliferation. 23 , 24 Among the differentially expressed genes described in the AUF1 knockdown RNA‐seq data, only 2 RA metabolism‐related genes, AKR1B10 and AKR1C2, were downregulated in AUF1 knockdown cells. Compared with AKR1C2, AKR1B10 had a higher activity to metabolize RA and more significant downregulation of expression in the AUF1 knockdown cells. Therefore, we speculated that AUF1 could promote the expression of AKR1B10, thereby promoting RA metabolism and HCC development. As expected, RA levels were significantly higher in tumors from mice bearing AUF1‐knockdown cells, but decreased in tumors from mice bearing AUF1‐overexpression cells. In addition, the increase of RA levels induced by AUF1 overexpression was almost completely reversed by AKR1B10 knockout (Figure 7A). These results suggested that the tumor‐promoting function of AUF1 in HCC was at least partially mediated by the catalytic efficiency of AKR1B10.
FIGURE 7.
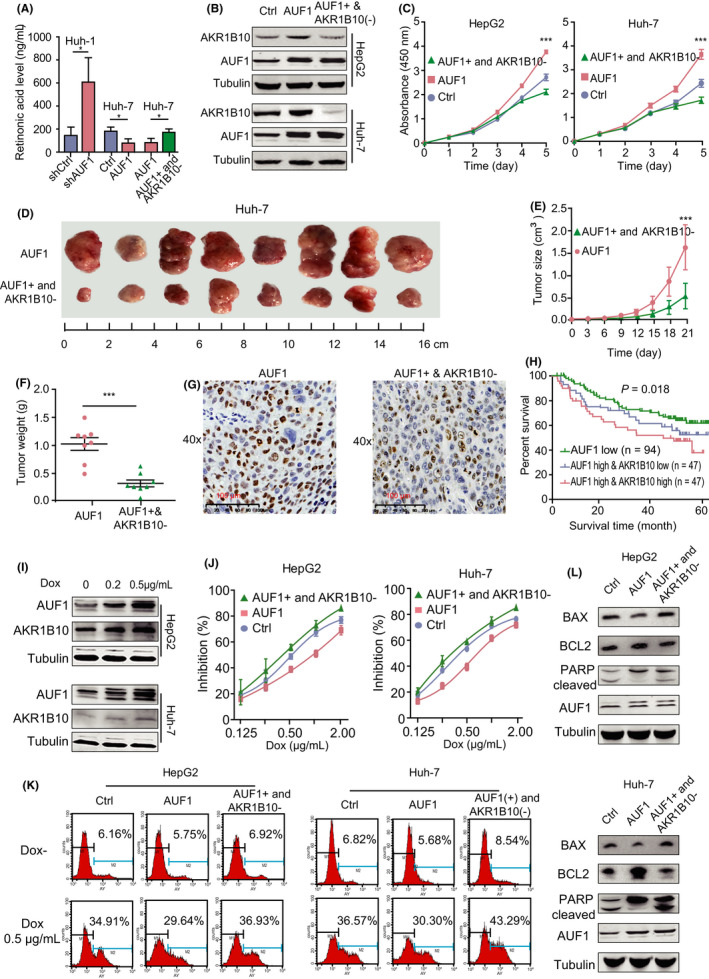
AKR1B10 contributed to the oncogenic function of AUF1. A, RA levels in tumors from the indicated tumor‐bearing mice were measured. B, AKR1B10 stable knockout cell models were established based on AUF1‐overexpression cells. Knockout efficiency was verified using western blot assay. C, AKR1B10 knockout rescued the AUF1 overexpression‐induced malignant phenotypes of HCC cells in vitro. D‐F, AKR1B10 knockout rescued the AUF1‐enhanced tumor formation in vivo. G, Representative micrographs of immunohistochemistry staining of proliferation marker Ki‐67 in tumors. Scale bars, 100 μm. H, Kaplan‐Meier analysis of the LCI cohorts using the expression levels of AUF1 and AKR1B10. I, Western blot analysis shows AUF1 and AKR1B10 protein expression in HCC cells after Dox treatment. J‐L, AKR1B10 knockout rescues AUF1 overexpression‐induced incidence of resistance and apoptosis. *P < .05; ***P < .001
Next, we set out to determine whether AUF1 exerted its functions depending on AKR1B10 in HCC. For this purpose, we established stable HepG2 and Huh‐7 cells expressing the AUF1, as well a CRISPR/Cas9 system that targeted AKR1B10 (Figure 7B). As anticipated, silencing of AKR1B10 expression rescued cell proliferation (Figure 7C) and colony formation (Figure S5A), and induced a G2/M cell cycle arrest in HepG2 and Huh‐7 cells that overexpressed AUF1 (Figure S5B). Furthermore, our results also showed that AUF1‐enhanced tumor formations of Huh‐7 cells in NPG/Vst mice were attenuated by AKR1B10 knockout, both in the weight and volume of the tumor (P < .001; Figure 7D‐F). Simultaneously, the percentage of Ki‐67‐positive cells in tumors from AUF1‐overexpressing cells was decreased significantly by AKR1B10 knockout (Figure 7G). In addition, we jointly analyzed the power of AUF1 and AKR1B10 mRNA expression levels on the prognosis of HCC. The results showed that the survival rates of patients with HCC with only AUF1 low expression showed the best overall survival, while patients with both high AUF1 and AKR1B10 expression was the worst of those in the LCI database (P = .018; Figure 7H), which suggested that high levels of AKR1B10 worsened the poor prognosis of patients with HCC who exhibited higher AUF1 expression. These data altogether indicated that AKR1B10 is a major target of AUF1 in HCC and that the upregulation of AKR1B10 by AUF1 is vital for maintaining the oncogenic phenotype of HCC.
It has been reported that AKR1B10 participates in the cellular metabolism of Dox and idarubicin, resulting in drug resistance. In light of our observation that AUF1 enhanced the drug resistance to Dox in hepatoma cells, we reasoned that AKR1B10 might be required for AUF1‐induced drug resistance. To test this hypothesis, we firstly evaluated the effects of Dox on the expression of AUF1 and AKR1B10. The results showed that, upon Dox treatment, both AUF1 and AKR1B10 protein expression in HepG2 and Huh‐7 cells was significantly increased (Figure 7I). Furthermore, we found that the AKR1B10 deficiency completely abrogated the AUF1‐mediated changes in cell viability, cell apoptosis, and apoptosis markers, such as Bax, Bcl2, and cleaved PARP. (Figure 7J‐L). These data indicated that AUF1‐induced drug resistance to Dox also relied on the upregulation of AKR1B10.
3.8. E2F1 triggers the transcriptional activation of AUF1 in HCC
Transcriptional regulation is responsible for the upregulation of many oncogenes. In an effort to better understand the upstream regulation mechanism of AUF1 in HCC, we analyzed the cis‐elements in the AUF1 promoter region using the LASAGNA database. Notably, a potential E2F1 binding site was identified in AUF1 promoter region (Figure 8A). We then evaluated whether E2F1 could regulate AUF1 expression in HCC. To start with, an E2F1 expressing plasmid was transiently transfected into Huh‐7 and HEK 293T cells due to their relatively higher transfection efficiency and the expression of AUF1 was detected. The results showed that E2F1 significantly increased both mRNA and protein levels of AUF1 in these cells (Figure 8B,C). Consistently, overexpression of E2F1 increased the promoter activity of the AUF1 gene (Figure 8D). Next, as shown in Figure 8E, ChIP‐PCR assay showed that E2F1 was able to specifically bind to the AUF1 promoter region. Furthermore, the expression of E2F1 was upregulated in HCC tissues from TCGA data sets and the expression levels of AUF1 and E2F1 were positively correlated (r = .449, P < .001; Figure 8F). Taken together, these data suggested that E2F1 is an important transcription factor of the AUF1 gene and that overexpression of E2F1 may contribute to the AUF1 upregulation in HCC.
FIGURE 8.
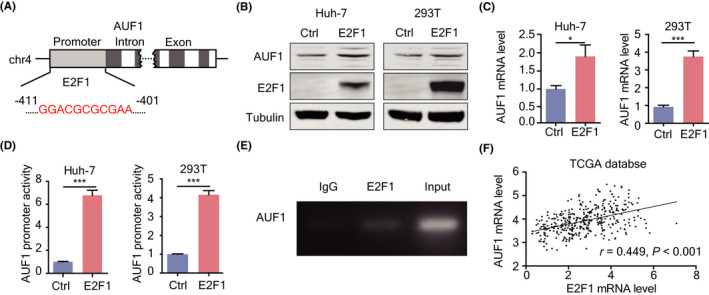
Transcriptional regulation of AUF1 by E2F1 in HCC. A, Scheme of putative E2F1 binding sites in the AUF1 promoter region. B, C, E2F1‐regulated AUF1 expression in Huh‐7 and 293T cells at both protein and mRNA levels. D, E2F1 increases the transcriptional activity of the AUF1 promoter. E, ChIP‐qPCR was performed using chromatin from Huh‐7 cells and anti‐E2F1 antibody. Non‐specific IgG antibody was used as the control. F, Correlation analysis between the expression levels of AUF1 and E2F1 in HCC tissues from TCGA database
4. DISCUSSION
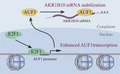
Dysregulation of AUF1 has been proposed to have a role in various type of cancers during cancer initiation, progression, and chemoresistance. 15 , 25 However, functions and mechanisms of AUF1 in the development and progression of HCC are still obscure. In this study, we demonstrated that the expression of AUF1 was significantly increased in HCC and correlated with the poor prognosis of patients with HCC. By regulating the mRNA stability of its functional target AKR1B10, AUF1 contributed to HCC development and chemoresistance. Moreover, this is also the first publication to report that E2F1 regulates AUF1 transcription and increases AUF1 expression (Figure 9).
FIGURE 9.
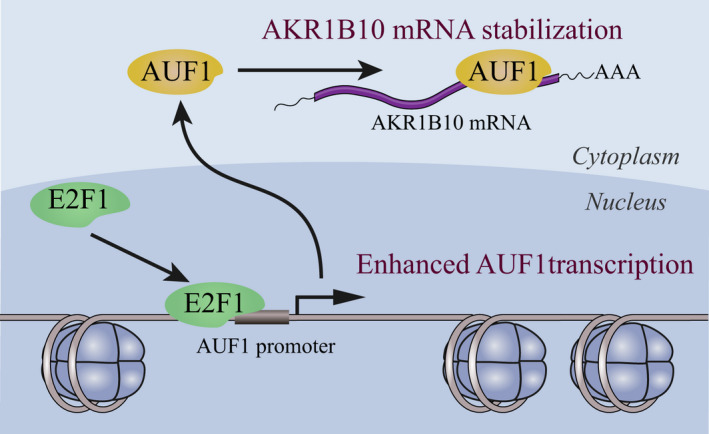
Scheme of the E2F1/AUF1/AKR1B10 axis
To date, the limited reports addressing AUF1 expression in HCC revealed that AUF1 was highly expressed in the tumor tissues and AUF1 high expression was closely associated with hepatitis B surface antigen status. 26 , 27 However, its abundance and biological activity in HCC are completely unknown. In this study, we demonstrated that AUF1 is frequently overexpressed in HCC tissues, not only from the LCI and TCGA databases, but also in our HBV‐related HCC cohorts. AUF1 promoted cell growth, colony formation, and tumor formation of hepatoma cells, and enhanced the Dox resistance of these cells. Furthermore, we demonstrated that AUF1 expression levels in HCC tissues were closely associated with the poor survival of patients with HCC in both the LCI database, TCGA database, and our HCC cohort. Therefore, we concluded that AUF1 may play an important role in promoting HCC progression.
Changes in metabolic processes represent key drivers of tumorigenesis and malignancy in the liver. 28 However, whether AUF1 could regulate liver metabolism favoring HCC progression is still poorly understood. In this study, we demonstrated that organic hydroxy compound metabolism was the most significantly enriched pathway altered by AUF1 ablation, which enriched 23 AUF1‐dependent genes. Most importantly, expression of these organic hydroxy compound metabolism genes was associated with the overall survival of patients with HCC. Therefore, the abnormal organic hydroxy compound metabolism induced by AUF1 overexpression in HCC may be associated with HCC aggressiveness.
AKR1B10 is a NADPH‐dependent reductase that metabolizes a variety of endogenous compounds such as aromatic and aliphatic aldehydes and dicarbonyl compounds, and some drug ketones. 29 Increasing evidence has demonstrated that AKR1B10 is highly expressed in several solid tumors including HCC and is considered as a relevant biomarker for the development of HCC. 23 In addition, AKR1B10 has been recently reported to be significantly upregulated in some cancer cell lines acquiring resistance toward chemotherapeutic agents, suggesting the validity of the enzyme as a chemoresistance marker. 30 Our data here revealed that AKR1B10 is the one of the most significant genes among the 23 AUF1‐dependent organic hydroxy compound metabolism genes. We demonstrated that expression of AKR1B10 and AUF1 was positively correlated in an expanded cohort of clinical HCC samples. AUF1 could bind to and stabilize AKR1B10 transcripts by interacting with its 3′UTR region. In addition, AKR1B10 silencing was sufficient to revert to the oncogenic phenotype and chemoresistance induced by AUF1. Therefore, we concluded that AKR1B10 is indispensable for the regulation of HCC development and drug sensitivity by AUF1 in HCC.
It has been reported that AUF1 upregulation can be driven by activation of the ERK pathway. 31 However, mechanisms of AUF1 upregulation in HCC are still obscure. Through analysis of the published comparative genomic hybridization (aCGH) data and the MEXPRESS database from TCGA, we found that no copy number increase of AUF1 was identified in HCC tissues. In addition, we did not find any methylation sites significantly related to the expression levels of AUF1 (data not shown). These analyses suggested that the abnormal expression of AUF1 may be caused by upregulation of AUF1 transcription. E2F1 is a transcription factor that contributes to the regulation of many cellular processes including cell proliferation. 32 , 33 , 34 Because a growing body of evidence has indicated that the upregulation of E2F1 expression is intimately associated with HCC and that there are potential E2F1 binding sites in the AUF1 promoter region, it is not surprising to find that E2F1 promotes AUF1 transcription, thereby upregulating AUF1 expression. Interestingly, E2F1 levels also affected growth factor‐induced ERK phosphorylation. 35 , 36 Whether the ERK pathway is involved in E2F1‐induced AUF1 upregulation deserves further exploration.
In summary, we propose a model that explains the possible mechanism for AUF1 in HCC development. In the model, E2F1 promotes the expression of AUF1 by upregulating its transcription. Then, AUF1 binds to and stabilizes AKR1B10 mRNA, leading to upregulation of AKR1B10 expression. AKR1B10 is required for the promoting role of AUF1 in HCC development and chemoresistance. Therefore, specifically targeting hepatocyte AUF1 or the E2F1/AUF1/AKR1B10 axis may be a potential therapeutic strategy for HCC treatment in the future.
DISCLOSURE
The authors have declared that no competing interest exists.
AUTHOR CONTRIBUTIONS
Xiangmei Chen conceived and supervised the study. Ting Zhang, Guiwen Guan, and Jing Zhang performed experiments and analyzed data. Huiling Zheng, Deyao Li, and Wengong Wang provided scientific suggestions. Xiangmei Chen, Fengmin Lu, Ting Zhang, and Guiwen Guan wrote the manuscript. All authors discussed the results and commented on the manuscript.
Supporting information
Fig S1‐S5
Table S1‐S5
Supplementary Material
ACKNOWLEDGMENTS
We thank Professor Juhua Ni (Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Peking University Health Science Center) for supporting the E2F1 expression plasmids.
Zhang T, Guan G, Zhang J, et al. E2F1‐mediated AUF1 upregulation promotes HCC development and enhances drug resistance via stabilization of AKR1B10. Cancer Sci. 2022;113:1154–1167. doi: 10.1111/cas.15272
Ting Zhang, Guiwen Guan, and Jing Zhang contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China (grant numbers 81372603 and 81572366), Beijing Natural Science Foundation (7182079), National Key Research and Development Program of China (2017YFA0504302), Peking University Medicine Seed Fund for Interdisciplinary Research (BMU2021MX007) and the Fundamental Research Funds for the Central Universities.
Contributor Information
Fengmin Lu, Email: lu.fengmin@hsc.pku.edu.cn.
Xiangmei Chen, Email: xm_chen6176@bjmu.edu.cn.
DATA AVAILABILITY STATEMENT
Complete RNA‐seq files and gene expression values have been deposited in NCBI's Gene Expression Omnibus and are accessible through Gene Expression Omnibus (GEO) Series accession number GSE162706.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7‐33. [DOI] [PubMed] [Google Scholar]
- 2. Carlessi R, Kohn‐Gaone J, Olynyk JK, Tirnitz‐Parker JEE. Mouse models of hepatocellular carcinoma. In: Tirnitz‐Parker JEE, ed. Hepatocellular Carcinoma. Codon Publications; 2019. [PubMed] [Google Scholar]
- 3. Mak LY, Cruz‐Ramon V, Chinchilla‐Lopez P, et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262‐279. [DOI] [PubMed] [Google Scholar]
- 4. Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dang H, Takai A, Forgues M, et al. Oncogenic activation of the RNA binding protein NELFE and MYC signaling in hepatocellular carcinoma. Cancer Cell. 2017;32:101‐114.e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang X, Liu Y, Wang G, et al. Up‐regulation of CLIC1 activates MYC signaling and forms a positive feedback regulatory loop with MYC in Hepatocellular carcinoma. Am J Cancer Res. 2020;10:2355‐2370. [PMC free article] [PubMed] [Google Scholar]
- 7. Dolicka D, Sobolewski C, Correia de Sousa M, Gjorgjieva M, Foti M. mRNA post‐transcriptional regulation by AU‐Rich element‐binding proteins in liver inflammation and cancer. Int J Mol Sci. 2020;21:6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han L, Huang C, Zhang S. The RNA‐binding protein SORBS2 suppresses hepatocellular carcinoma tumourigenesis and metastasis by stabilizing RORA mRNA. Liver Int. 2019;39:2190‐2203. [DOI] [PubMed] [Google Scholar]
- 9. Vazquez‐Chantada M, Fernandez‐Ramos D, Embade N, et al. HuR/methyl‐HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology. 2010;138:1943‐1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lykke‐Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE‐mediated decay activation domains in the proteins TTP and BRF‐1. Genes Dev. 2005;19:351‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gherzi R, Lee KY, Briata P, et al. A KH domain RNA binding protein, KSRP, promotes ARE‐directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14:571‐583. [DOI] [PubMed] [Google Scholar]
- 12. Chen CY, Shyu AB. AU‐rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465‐470. [DOI] [PubMed] [Google Scholar]
- 13. Barreau C, Paillard L, Osborne HB. AU‐rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138‐7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franks TM, Lykke‐Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU‐rich elements. Genes Dev. 2007;21:719‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Y, Wang W, Cao J, et al. Upregulation of AUF1 is involved in the proliferation of esophageal squamous cell carcinoma through GCH1. Int J Oncol. 2016;49:2001‐2010. [DOI] [PubMed] [Google Scholar]
- 16. Dai W, Mu L, Cui Y, et al. Berberine promotes apoptosis of colorectal cancer via regulation of the long non‐coding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU‐binding factor 1 (AUF1)/B‐Cell CLL/Lymphoma 2 (Bcl‐2) axis. Med Sci Monit. 2019;25:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xia B, Hong T, He X, Hu X, Gao Y. A circular RNA derived from MMP9 facilitates oral squamous cell carcinoma metastasis through regulation of MMP9 mRNA stability. Cell Transplant. 2019;28:1614‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. AlAhmari MM, Al‐Khalaf HH, Al‐Mohanna FH, Ghebeh H, Aboussekhra A. AUF1 promotes stemness in human mammary epithelial cells through stabilization of the EMT transcription factors TWIST1 and SNAIL1. Oncogenesis. 2020;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiStefano JK, Davis B. Diagnostic and prognostic potential of AKR1B10 in human hepatocellular carcinoma. Cancers (Basel). 2019;11:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Zhang J, Liu H, et al. Compensatory upregulation of aldo‐keto reductase 1B10 to protect hepatocytes against oxidative stress during hepatocarcinogenesis. Am J Cancer Res. 2019;9:2730‐2748. [PMC free article] [PubMed] [Google Scholar]
- 21. Henics T, Nagy E, Oh HJ, Csermely P, von Gabain A, Subjeck JR. Mammalian Hsp70 and Hsp110 proteins bind to RNA motifs involved in mRNA stability. J Biol Chem. 1999;274:17318‐17324. [DOI] [PubMed] [Google Scholar]
- 22. Liang L, Wang MD, Zhang YM, et al. Association of postoperative biomarker response with recurrence and survival in patients with hepatocellular carcinoma and high alpha‐fetoprotein expressions (>400 ng/ml). J Hepatocell Carcinoma. 2021;8:103‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porte S, Xavier Ruiz F, Gimenez J, et al. Aldo‐keto reductases in retinoid metabolism: search for substrate specificity and inhibitor selectivity. Chem Biol Interact. 2013;202:186‐194. [DOI] [PubMed] [Google Scholar]
- 24. Liu TA, Jan YJ, Ko BS, et al. Regulation of aldo‐keto‐reductase family 1 B10 by 14‐3‐3epsilon and their prognostic impact of hepatocellular carcinoma. Oncotarget. 2015;6:38967‐38982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian XY, Li J, Liu TH, et al. The overexpression of AUF1 in colorectal cancer predicts a poor prognosis and promotes cancer progression by activating ERK and AKT pathways. Cancer Med. 2020;9:8612‐8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frau M, Feo F, Pascale RM. Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J Hepatol. 2013;59:830‐841. [DOI] [PubMed] [Google Scholar]
- 27. Yang Y, Kang P, Gao J, et al. AU‐binding factor 1 expression was correlated with metadherin expression and progression of hepatocellular carcinoma. Tumour Biol. 2014;35:2747‐2751. [DOI] [PubMed] [Google Scholar]
- 28. Berndt N, Eckstein J, Heucke N, et al. Metabolic heterogeneity of human hepatocellular carcinoma: implications for personalized pharmacological treatment. FEBS J. 2021;288:2332‐2346. [DOI] [PubMed] [Google Scholar]
- 29. Gallego O, Ruiz FX, Ardevol A, et al. Structural basis for the high all‐trans‐retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci USA. 2007;104:20764‐20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng BY, Lau EY, Leung HW, et al. IRAK1 augments cancer stemness and drug resistance via the AP‐1/AKR1B10 signaling cascade in hepatocellular carcinoma. Cancer Res. 2018;78:2332‐2342. [DOI] [PubMed] [Google Scholar]
- 31. Guo Y, Zhang T, Shi Y, et al. Helicobacter pylori inhibits GKN1 expression via the CagA/p‐ERK/AUF1 pathway. Helicobacter. 2020;25:e12665. [DOI] [PubMed] [Google Scholar]
- 32. Clijsters L, Hoencamp C, Calis JJA, et al. Cyclin F controls cell‐cycle transcriptional outputs by directing the degradation of the three activator E2Fs. Mol Cell. 2019;74:1264‐1277. e1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dos Reis VL, Pujiz RS, Strauss BE, Krieger JE. Knockdown of E2f1 by RNA interference impairs proliferation of rat cells in vitro. Genet Mol Biol. 2010;33:17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farra R, Grassi G, Tonon F, et al. The role of the transcription factor E2F1 in hepatocellular carcinoma. Curr Drug Deliv. 2017;14:272‐281. [DOI] [PubMed] [Google Scholar]
- 35. Korotayev K, Chaussepied M, Ginsberg D. ERK activation is regulated by E2F1 and is essential for E2F1‐induced S phase entry. Cell Signal. 2008;20:1221‐1226. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Wang R, Hu X, et al. Activated MEK/ERK pathway drives widespread and coordinated overexpression of UHRF1 and DNMT1 in cancer cells. Sci Rep. 2019;9:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S5
Table S1‐S5
Supplementary Material
Data Availability Statement
Complete RNA‐seq files and gene expression values have been deposited in NCBI's Gene Expression Omnibus and are accessible through Gene Expression Omnibus (GEO) Series accession number GSE162706.


