Abstract
Recent studies have revealed that aberrant expression of tight junction (TJ) proteins is a hallmark of various solid tumors and it is recognized as a useful therapeutic target. Claudin‐6 (CLDN6), a member of the family of TJ transmembrane proteins, is an ideal therapeutic target because it is not expressed in human adult normal tissues. In this study, we found that CLDN6 is highly expressed in uterine cervical adenocarcinoma (ADC) and that high CLDN6 expression was correlated with lymph node metastasis and lymphovascular infiltration and was an independent prognostic factor. Shotgun proteome analysis revealed that cell‐cell adhesion‐related proteins and drug metabolism‐associated proteins (aldo‐keto reductase [AKR] family proteins) were significantly increased in CLDN6‐overexpressing cells. Furthermore, overexpression of CLDN6 enhanced cell‐cell adhesion properties and attenuated sensitivity to anticancer drugs including doxorubicin, daunorubicin, and cisplatin. Taken together, the results indicate that aberrant expression of CLDN6 enhances malignant potentials and drug resistance of cervical ADC, possibly due to increased cell‐cell adhesion properties and drug metabolism. Our findings provide an insight into a new therapeutic strategy, a CLDN6‐targeting therapy, against cervical ADC.
Keywords: chemoresistance, claudin‐6, prognostic factor, proteomics, uterine cervical adenocarcinoma
High claudin‐6 (CLDN6) expression was correlated with lymph node metastasis and lymphovascular infiltration and was an independent prognostic factor of cervical adenocarcinoma. Shotgun proteome analysis revealed that cell‐cell adhesion‐related proteins and drug metabolism‐associated proteins (aldo‐keto reductase [AKR] family proteins) were significantly increased in CLDN6‐overexpressing cells. Furthermore, overexpression of CLDN6 enhanced cell‐cell adhesion properties and attenuated sensitivity to anticancer drugs including doxorubicin, daunorubicin, and cisplatin.
1. INTRODUCTION
The incidence rates of uterine cervical adenocarcinoma (ADC) have been dramatically increasing worldwide, especially in young women, even though the incidence rates of squamous cell carcinoma (SCC) have been decreasing. 1 , 2 , 3 , 4 , 5 , 6 , 7 It has been shown that adenocarcinoma has a worse prognosis than SCC at the same stage and with the same tumor size. 8 , 9 , 10 , 11 , 12 , 13 The principal reasons for the worse outcome are the high metastatic rate and resistance to chemoradiotherapy. 3 Consequently, a novel therapeutic strategy and an understanding of the malignancy are needed to improve the prognosis of cervical ADC. 14 , 15 , 16 , 17
Tight junctions (TJs) are important cell adhesion structures that are located at the most apical components of intercellular junctional complexes in epithelial and endothelial cells. 18 , 19 , 20 Tight junctions are composed of intracellular scaffold proteins and transmembrane proteins including proteins in the claudin (CLDN) family, junctional adhesion molecule (JAM) family, MAL and related proteins for vesicle trafficking and membrane link (MARVEL) family, and lipolysis‐stimulated lipoprotein receptor (LSR). Claudin family proteins form TJ strands that play a central role in physiological functions of TJs. 19 However, CLDN family proteins have also been reported to be ectopically highly expressed in various cancers and to be involved in cancer malignancy. 14 , 15 , 21 , 22 , 23 , 24 , 25
Claudin‐6, a member of the CLDN family, is expressed in several types of embryonic epithelial cells and germ cell tumors derived from embryonic epithelial cells, such as seminomas, embryonal carcinomas, and yolk sac tumors. 26 , 27 , 28 , 29 , 30 Claudin‐6 is not expressed in normal adult cells but is aberrantly expressed in some cases of gastric adenocarcinomas, lung adenocarcinomas, ovarian adenocarcinomas, and endometrial carcinomas and is involved in malignant potentials of those cancers. 31 , 32 , 33 , 34 , 35 However, the relationship between expression of CLDN6 and malignant potentials of cervical ADC remains unclear.
In this study, we examined the expression of CLDN6 in cervical ADC surgical specimens by immunohistochemistry and investigated the functional relationships between CLDN6 expression and clinicopathologic features. We also investigated how CLDN6 expression contributed to malignant potentials of cervical ADC cells. Our results showed that aberrant expression of CLDN6 enhances not only malignant potentials but also chemoresistance along with overexpression of aldo‐keto reductase (AKR) family proteins.
2. MATERIALS AND METHODS
2.1. Antibodies
Primary Abs used in this study are listed in Table S1.
2.2. Immunohistochemical analysis of surgical specimens
Specimens of 68 cases of cervical ADC including adenocarcinoma in situ (AIS) obtained by surgical resection during the period from 2004 to 2014 were retrieved from the pathology file of Sapporo Medical University Hospital. This study was approved by the Institutional Review Board of Sapporo Medical University (IRB study number 302–197). Written informed consent was obtained from each patient who participated in the investigation. As controls, adjacent nonneoplastic regions were examined as normal tissues (n = 62). Clinicopathologic features of the patients were described previously. 36 Immunohistochemistry was carried out with anti‐CLDN6 Ab (1:100; IBL) as described previously. 37 Surgical specimen staining patterns were scored as follows: score 0, no reactivity or cytosolic reactivity in less than 10% of tumor cells: score 1+, faint/almost no cytosolic reactivity in 10% or more of tumor cells; score 2+, weak to moderate cytosolic reactivity in 10% or more tumor cells; and score 3+, moderate to strong cytosolic reactivity in 10% or more of tumor cells. For statistical purposes, samples with scores 0 and 1+ were considered negative, and those with scores 2+ and 3+ were considered positive. When evaluating the slides, the observers (YI, MM, and AT) were blinded to the clinical data. Discordant cases were discussed, and a consensus was reached.
2.3. Cell culture and treatment
The human cervical ADC cell lines Hela229 and OMC4 were purchased from RIKEN Bio‐Resource Center and HCA1 was from JCRB Cell Bank. The human ADC cell line CAC‐1 and TMCC‐1 were provided by our colleague Dr Hayakawa. 38 , 39 Cells were maintained as described previously. 15 Claudin‐6 cell lines were established by lentiviral transfection as described previously. 40 In brief, CLDN6 lentiviral particles were generated by transfecting HEK293T cells with 10 μg CSII plasmids containing the CLDN6 gene, 5 μg packaging plasmids psPAX2 (#12260; Addgene), and pCMV‐VSV‐G (#8454; Addgene) using PEI Max. Culture media containing recombinant lentiviruses were collected 72 hours after transfection. The lentiviral vectors were added to the cell culture medium of cervical ADC cell lines after filtration. The CLDN6‐overexpressing cells (CLDN6 cells) were screened by western blot analysis. More than 48 hours after transfection, the cells were used for further analysis.
2.4. Cell proliferation, invasion, and migration assay
The colony‐forming assay and the WST‐8 assay (CCK‐8; Dojindo Laboratories) were carried out as described previously. 41 The migration assay and invasion assay were carried out using Transwell (8‐μm pore polycarbonate membrane insert; Corning) and BioCoat Matrigel invasion chamber (#354480; Corning) in 24‐well dishes, respectively, as described previously. 23 Each experiment was independently repeated three times.
2.5. Proteome analysis
Whole cell protein was prepared by using a phase transfer surfactant method and then trypsinized and desalted. 42 Samples were dissolved in 0.1% formic acid and loaded into a nano‐flow UHPLC (Easy‐nLC 1000 system; Thermo Fisher Scientific) online‐coupled to an Orbitrap mass spectrometer equipped with a nanospray ion source (Q‐Exactive Plus; Thermo Fisher Scientific). Samples were separated by using a 75 μm × 20 cm capillary column with a particle size of 3 μm (NTCC‐360; Nikkyo Technos) by applying a linear gradient ranging from 5% to 35% buffer B (100% acetonitrile and 0.1% formic acid) at a flow rate of 300 nL/min for 120 minutes. In mass spectrometry (MS) analysis, survey scan spectra were acquired at a resolution of 70,000 at 200 m/z with a target value of 3e6 ions, ranging from 350 to 2000 m/z with charge states between 1+ and 4+. We applied a data‐dependent top 10 method that generates high‐energy collision dissociation fragments for the 10 most intense precursor ions per survey scan. The tandem mass spectrometry (MS/MS) resolution was 17,500 at 200 m/z with a target value of 1e5 ions.
For MS/MS data analysis, we used the Sequest HT (Thermo Fisher Scientific) and Mascot version 2.5 (Matrix Science) algorithms embedded in the Proteome Discoverer 2.2 platform (Thermo Fisher Scientific), and the peak lists were searched against the UniProt human databases. The tolerance of precursor ions and fragment ions were set to 10 ppm and 0.02 Da, respectively. Gene Ontology (GO) terms enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were carried out on WEB‐based GEne SeT AnaLysis Toolkit (WebGestalt; http://www.webgestalt.org).
2.6. Scanning electron microscopy
Scanning electron microscopy (SEM) was obtained as previously described. 43 In brief, 2.5% glutaraldehyde‐ and then 1% OsO4‐fixed cell specimens were sputter‐coated with platinum and examined under a scanning electron microscope (S4300; Hitachi) operating at 5 kV.
2.7. Transepithelial electrical resistance and paracellular flux
Transepithelial electrical resistance and paracellular flux were measured as previously described. 43 , 44
2.8. Cell adhesion ability assay
Cells were seeded on a collagen‐coated coverslip (5 x 104 cells/coverslip) and incubated in a CO2 incubator for 1 hour. The coverslip was rinsed with PBS three times to remove nonadhered cells and then stained with 0.04% crystal violet to count the number of residual cells.
2.9. Statistical analysis
The measured values are presented as mean ± SD. Data were analyzed and compared using the unpaired two‐tailed Student’s t test, Fisher’s exact test, and Kruskal‐Wallis test. Survival rates were calculated by the Kaplan‐Meier method and compared by the log‐rank test. Multivariate regression analysis Cox proportional hazard model was used to estimate hazard ratios and 95% confidence intervals for each category. Statistical significance was accepted when P < .05 (*P < .05 and **P < .01). All statistical analyses were undertaken with EZR software version 1.27. 45
3. RESULTS
3.1. Expression profiles of CLDN6 in surgical specimens of cervical ADC
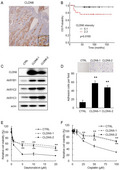
Immunohistochemistry was performed on surgical specimens of uterine cervical ADC using anti‐CLDN6 Abs. No expression of CLDN6 was observed in nonneoplastic cervical glands, being in agreement with the general recognition of negative expression of CLDN6 in most normal adult tissues. 31 In contrast, strong immunostaining of CLDN6 was observed in the whole‐cell membrane and the cytoplasm in ADC cases (Figure 1A). Based on the immunoreactive intensity, the cases were divided into two groups: a CLDN6 positive group (intensity of 2+ or 3+) and a CLDN6‐negative group (intensity of 1+ or 0). Nineteen (33.3%) of 57 ADC cases had CLDN6 positive expression. Those cases included 10 cases (17.5%) and 9 cases (15.8%) with intensities of 3+ and 2+, respectively. Three cases (27.3%) of 11 AIS cases had CLDN6 positive expression. Those cases included 1 case (9.1%) and 2 cases (18.2%) with intensities of 3+ and 2+, respectively. Thus, CLDN6 expression was positive in 22 (32.4%) of the 68 AIS/ADC cases, including 11 cases (16.2%) with an intensity of 3+ and 11 cases (16.2%) with an intensity of 2+ (Figure 1B).
FIGURE 1.
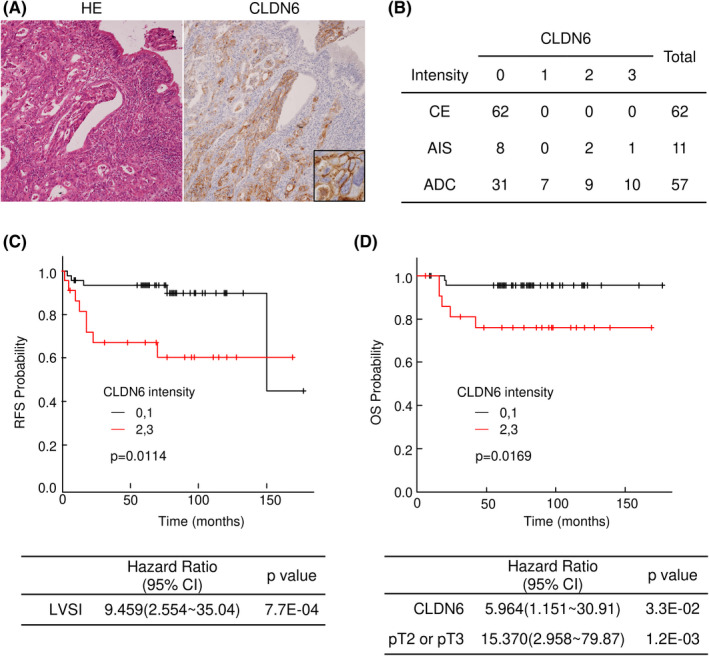
Aberrant expression of claudin‐6 (CLDN6) is correlated with worse prognosis of patients with cervical adenocarcinoma (ADC). (A) Immunohistochemistry of CLDN6 in surgical specimens of human cervical adenocarcinoma. CLDN6 was strongly expressed in cancer nests. (B) Immunoreactive intensities of CLDN6 in the nonneoplastic cervical epithelium (CE), adenocarcinoma in situ (AIS) and ADC. (C,D) Kaplan‐Meier estimates of relapse‐free survival (RFS) and overall survival (OS) of patients with cervical ADC. High expression level of CLDN6 was significantly correlated with worse RFS (P = .0114) and OS (P = .0169). The Cox proportional hazards model revealed that lymphovascular space invasion (LVSI) was an independent predictor for RFS and that CLDN6 positivity and tumor factors (pT2 or pT3) were independent predictors for OS
3.2. Correlations between expression of CLDN6 and clinicopathologic features of cervical ADC
We next examined the possible relationships between the expression status of CLDN6 positivity and clinicopathologic features of cervical ADC. As shown in Table 1, CLDN6 positivity was significantly correlated with lymph node metastasis (P = .0101) and lymphovascular space invasion (LVSI; P = .021); however, there was no significant correlation between CLDN6 positivity and age, histological type, diameter, tumor factor, or UICC stage (Table 1). The relationships between CLDN6 expression with relapse‐free survival (RFS) and overall survival (OS) of patients with cervical ADC were assessed by using the Kaplan‐Meier method. Relapse‐free survival and OS were significantly shorter in the CLDN6 positive group (Figure 1C,D). The Cox proportional hazards model revealed that LVSI was an independent predictor for RFS (Figure 1C) and that CLDN6 positivity and tumor factors (pT2 or pT3) were independent predictors for OS (Figure 1D).
TABLE 1.
Clinicopathologic parameters and immunoreactive intensity of claudin‐6 (CLDN6) in uterine cervical adenocarcinoma (ADC)
| N | CLDN6 intensity | P value | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Age (y) | ||||
| <44 | 33 | 23 | 10 | .7990 |
| ≥44 | 35 | 23 | 12 | |
| Histology | ||||
| AIS | 11 | 8 | 3 | 1.000 |
| ADC | 57 | 38 | 19 | |
| Diameter | ||||
| AIS, ≤40 mm | 51 | 37 | 14 | .1480 |
| >40 mm | 17 | 9 | 8 | |
| Tumor factor | ||||
| pT0 | 10 | 6 | 4 | .5790 |
| pT1 | 46 | 33 | 13 | |
| pT2 | 11 | 6 | 5 | |
| pT3 | 1 | 1 | 0 | |
| Lymph node metastasis | ||||
| Negative | 58 | 43 | 15 | .0101 |
| Positive | 10 | 3 | 7 | |
| UICC stage | ||||
| 0 | 10 | 6 | 4 | .2120 |
| I | 45 | 32 | 13 | |
| II | 7 | 6 | 1 | |
| III | 6 | 2 | 4 | |
| LVSI | ||||
| Negative | 48 | 37 | 11 | .0210 |
| Positive | 20 | 9 | 11 | |
Abbreviations: AIS, adenocarcinoma in situ; LVSI, lymphovascular space invasion.
3.3. Overexpression of CLDN6 promotes malignant transformation of cervical ADC cells
To analyze the significance of CLDN6 expression in cervical ADC, we established cervical ADC cells overexpressing CLDN6. Claudin‐6 was not detectable in any of the parental cervical ADC cell lines examined (Figure 2A), but it was confirmed that CLDN6 was highly expressed in stable clones (Figures 2B and S1A,B). Immunofluorescence microscopy revealed that immunoreactivity of CLDN6 was localized on the plasma membrane of CLDN6‐overexpressing cells (Figure 2C). Among the cell lines, HCA1, a well‐differentiated cervical ADC cell line, was mainly used for subsequent experiments because the cell line retains some epithelial properties, including morphologically intact adherence junctions and TJ, and it does not harbor human herpes viruses. 15
FIGURE 2.
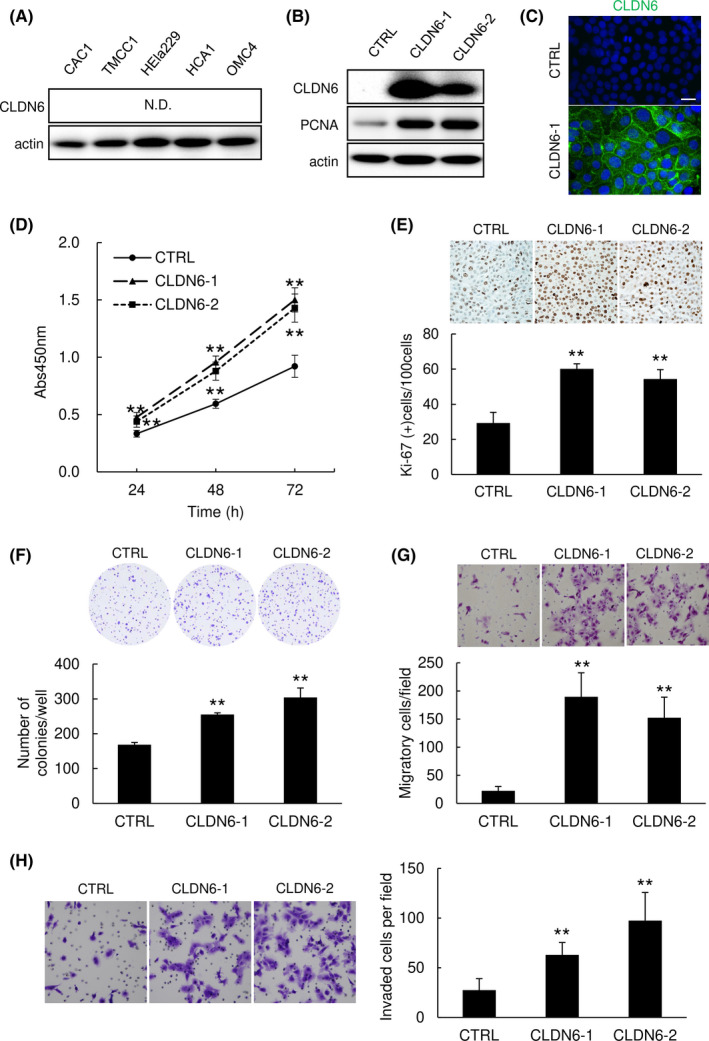
Overexpression of claudin‐6 (CLDN6) promotes the malignant transformation of cervical adenocarcinoma (ADC) cells. (A) Expression of CLDN6 was undetectable in cervical ADC cell lines. N.D., not detected. (B,C) CLDN6‐overexpressing stable clones (CLDN6‐1 and CLDN6‐2 cells) were established from HCA1 cells. Expression of proliferating cell nuclear antigen (PCNA) was induced by CLDN6 overexpression. Western blotting (B) and immunofluorescence (C). Bar = 20 μm. (D‐F) CLDN6 overexpression significantly promoted the proliferation ability of HCA1 cells in a WST‐8 assay (D), immunocytochemistry of Ki‐67 (E), and a colony formation assay (F). (G,H) CLDN6 overexpression significantly promoted the migration ability (G) and invasiveness (H) of HCA1 cells. Graphs represent means ± SD. **P < .01 vs control (CTRL) cells
In a WST‐8 cell proliferation assay, CLDN6 overexpression significantly promoted the proliferation of cells (Figures 2D and S1C,D). In immunocytochemical staining for Ki‐67 in cell block specimens, CLDN6‐overexpressing cells included a significantly larger number of Ki‐67 positive cells than that in control cells (Figure 2E). Expression of proliferating cell nuclear antigen was markedly increased by overexpression of CLDN6 (Figures 2B and S1A,B). In a colony formation assay, the number of colonies was significantly larger for CLDN6‐overexpressing cells than for control cells (Figures 2F and S1E,F). In a migration assay using Transwell membranes, the number of CLDN6‐overexpressing cells migrating through the membrane was significantly larger than that of control cells (Figures 2G and S1G,H). In an invasion assay using a Matrigel matrix‐coated chamber, the number of CLDN6‐overexpressing cells invading through the matrix was significantly larger than that of control cells (Figure 2H). These results indicate that the expression of CLDN6 contributes to the malignant transformation of cervical ADC cells.
3.4. Shotgun proteome analysis of CLDN6‐overexpressing cervical ADC cells
To elucidate the role of CLDN6 expression in the malignant potentials described above, we undertook a comparative MS‐based proteomic analysis. Protein samples from CLDN6‐overexpressing HCA1 (CLDN6‐1 and ‐2) cells and control HCA1 cells were digested by trypsin, and the digested peptides were subjected to MS. A label‐free semiquantitation method was used to compare the protein expression patterns between CLDN6‐overexpressing cells and control cells. To ensure the quality and precision of data, each sample measurement was independently repeated four times. A total of 2862 unique proteins were identified (Data S1); of these, 84 proteins were upregulated (ratio > 1.5, P < .05) and 82 proteins were downregulated in CLDN6‐1 cells (ratio < 0.67, P < .05; Figure 3A,B). To identify the molecular networks affected by CLDN6 overexpression, the upregulated and downregulated proteins were examined by GO analysis and KEGG pathway analysis (Figure 3C,D). As shown in Figure 3C, the enriched GO and KEGG pathway terms for upregulated proteins were predominantly associated with adhesion terms including cell‐cell adhesion (GO:0098609), cell‐cell adherens junction (GO:0005913), focal adhesion (GO:0005925), cadherin binding involved in cell‐cell adhesion (GO:0098641), focal adhesion (hsa04510), and ECM‐receptor interaction (has04512) in CLDN6‐1 cells. Gene Ontology terms involved in metabolism of anticancer drugs, doxorubicin metabolic process (GO:0044598) and daunorubicin metabolic process (GO:0044597), were also enriched for upregulated proteins in CLDN6‐1 cells (Figure 3C). Claudin‐6‐2 cells had almost the same trend (Figure S2). These results suggest that CLDN6 affected the expression of proteins involved in cell adhesion‐related molecular processes (Data S2), and those CLDN6‐dependent alterations could explain the tumor‐promoting roles of CLDN6 in cervical ADC cells as previously reported for other cancer cells. 35
FIGURE 3.
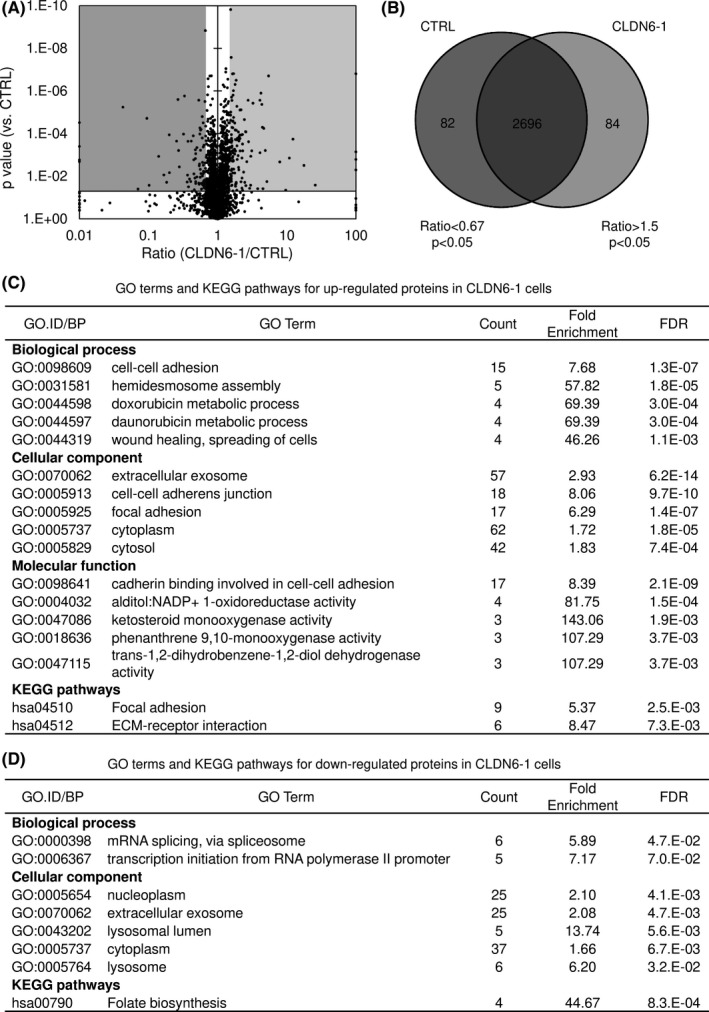
Comparable proteome analysis of claudin‐6 (CLDN6)‐overexpressing cells and control cells. (A,B) Volcano plot (A) and Venn map (B) of proteins that were identified by mass spectrometry‐based proteomic analysis from control HCA1 cells (control; CTRL) and CLDN6‐overexpressing HCA1 cells (CLDN6‐1). A total of 2862 proteins were identified. (C,D) Enriched Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways assigned for upregulated (ratio > 1.5, P < .05 vs CTRL) or downregulated (ratio < 0.67, P < .05 vs CTRL) proteins in CLDN6‐1 cells. False discovery rate (FDR) value was considered to be statistically significant when it was less than 0.05
3.5. Overexpression of CLDN6 contributes to morphological changes and cell adhesion
As the proteome analysis showed an increase in several adhesion‐associated GO terms, we analyzed the changes in cell morphology and cell adhesion, such as cell‐cell adhesion and cell‐substratum adhesion. Morphological changes were observed by phase‐contrast microscopy and SEM. Phase‐contrast microscopy showed that cell‐cell boundaries were indistinct and edges of the cell sheets were smoother in CLDN6‐overexpressing cells than that in control cells (Figures 4A, S3, and S4A). Scanning electron microscopy showed an increase in the number of microvilli due to the high expression level of CLDN6 (Figures 4A and S3A).
FIGURE 4.
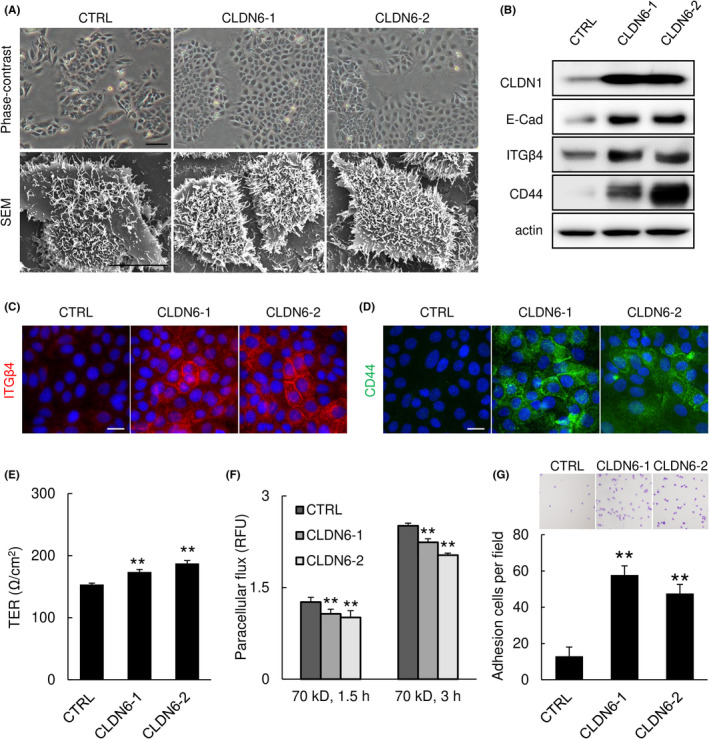
Overexpression of claudin‐6 (CLDN6) enhances cell adhesion‐associated properties of cervical adenocarcinoma cells. (A) Phase‐contrast microscopy (upper panels) and scanning electron microscopy (SEM, lower panels). SEM images showed that microvilli formation was enhanced by overexpression of CLDN6. (B‐D) CLDN6 overexpression increased the expression of cell adhesion‐associated proteins. Western blotting (B) and immunofluorescence (C,D). (E) Transepithelial electric resistance (TER) was increased in CLDN6‐overexpressing cells. (F) Paracellular permeability of FITC‐dextran was suppressed in CLDN6‐overexpressing cells. (G) Adhesion ability was enhanced by CLDN6 overexpression. Bar = 100 μm (upper panels in A), 10 μm (lower panels in A), or 20 μm (C,D). Graphs represent means ±SD. CLDN1, claudin‐1; E‐Cad, E‐cadherin; ITGβ4, integrin beta‐4. **P < .01 vs control (CTRL) cells
In addition to the morphological changes, western blot analysis confirmed that the expression levels of cell adhesion‐associated proteins, including CLDN‐1 (a TJ‐associated protein), E‐cadherin (a major adherens junction resident protein), integrin β4 (a focal adhesion‐associated protein), and CD44, were increased in CLDN6‐overexpressing cells (Figure 4B‐D).
We next examined the effect of CLDN6 expression on TJ barrier function and cell‐substratum adhesion. As expected, trans‐epithelial electric resistance values, an indicator of paracellular movement of ions, were increased in CLDN6‐overexpressing cells and paracellular permeability of fluorescein‐labeled dextran was decreased in CLDN6‐overexpressing cells compared to those in control cells (Figures 4E,F and S3B), indicating that TJ barrier function was strengthened by overexpression of CLDN6. Furthermore, in an adhesion assay for assessing adhesion capacity between the cell and substratum, the number of adhesive cells was significantly increased by a high expression level of CLDN6 (Figure 4G). Taken together, the results indicate that CLDN6 overexpression promotes the cell adhesive property, possibly through induction of cell adhesion‐associated proteins.
3.6. Overexpression of CLDN6 induces AKRs and enhances drug resistance of cervical ADC cells
Interestingly, GO analysis showed that four genes involved in daunorubicin and doxorubicin metabolic processes were commonly upregulated in both CLDN6‐overexpressing clones (Figures 3C and S2). The four genes were AKR1C1, AKR1C2, AKR1C3, and AKR1B1 in the AKR superfamily (Figure 5A). The increase in these proteins was also confirmed in CLDN6‐overexpressing clones by western blot analysis (Figures 5B, S3C, and S4B) and immunofluorescence (Figure 5C). High expression levels of certain AKR superfamily proteins are thought to be involved in resistance to cisplatin as well as daunorubicin and doxorubicin, as a result of increased metabolism of the anticancer drug. 46 Therefore, we assessed whether CLDN6 overexpression affects drug resistance of HCA1 cells. As expected, doxorubicin‐ or daunorubicin‐induced reduction in cell viability was significantly attenuated by CLDN6 overexpression (Figure 5D,E). In the case of exposure to cisplatin, the first‐line drug for treatment of cervical ADC patients, reduction in cell viability was also attenuated by CLDN6 overexpression (Figure 5F). These observations indicate that the high expression level of CLDN6 enhances multidrug resistance, such as resistance to doxorubicin, daunorubicin, and cisplatin. Finally, we examined surgical specimens from uterine cervical ADC patients by immunohistochemistry using anti‐dihydrodiol dehydrogenase Ab, which recognizes AKR1C isoforms of AKR1C1, AKR1C2, AKR1C3, and AKR1C4. We found that ADC lesions were strongly stained with this Ab (Figure 5G). The percentage of AKR1C positive cases (immunoreactive score of 2+ or 3+) tended to be higher in the CLDN6 positive group (10/11, 91%) than in the CLDN6 negative group (6/12, 50%, Figure 5G; P = .0686), suggesting a positive relationship between CLDN6 expression and AKR1C expression in cervical ADC.
FIGURE 5.
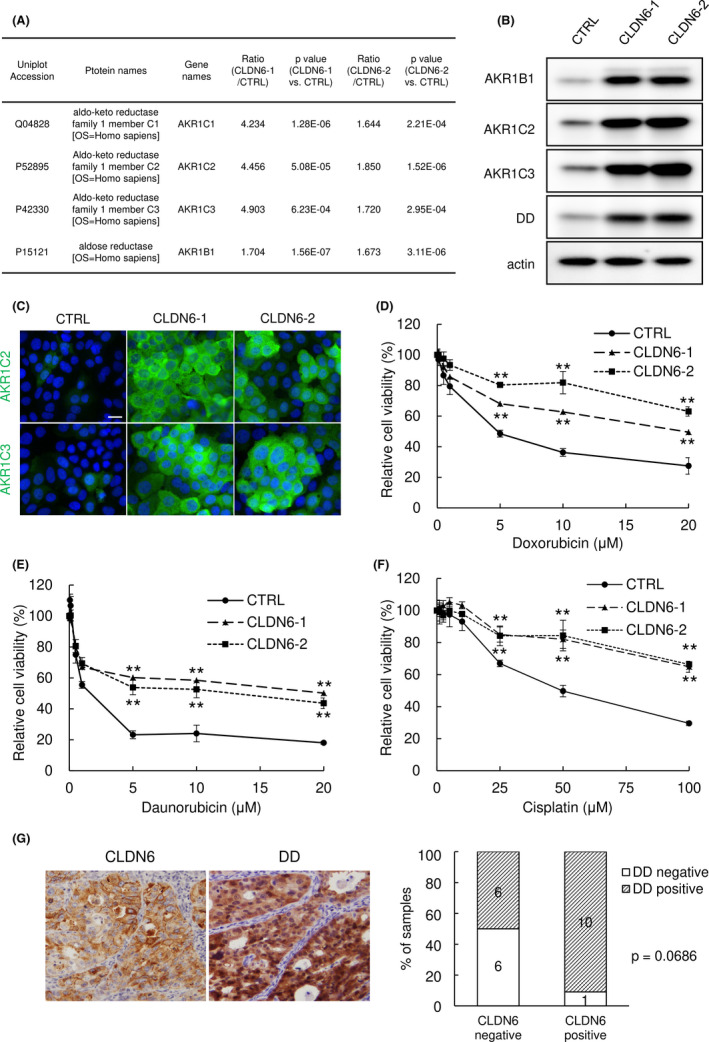
Overexpression of claudin‐6 (CLDN6) enhances chemoresistance through overexpression of the aldo‐keto reductase (AKR) superfamily. (A) Comparative proteome analysis revealed that AKR family members were upregulated in CLDN6‐overexpressing cells. (B,C) Expression of AKR family members was increased in CLDN6‐overexpressing cells. Western blotting (B) and immunofluorescence (C). Bar = 20 μm. (D‐F) CLDN6‐overexpressing cells were less susceptible to anticancer drugs. WST‐8 assay. **P < .01 vs control (CTRL) cells. (G) Immunohistochemistry of dihydrodiol dehydrogenase (DD, an alternative name of AKR1C) in surgical specimens of cervical adenocarcinoma. AKR1C was strongly expressed in cancer nests and the percentage of high AKR1C expression cases tended to be higher in the high CLDN6 expression group than in the low CLDN6 expression group
4. DISCUSSION
In this study, we clearly showed that aberrant expression of CLDN6, one of the transmembrane proteins of TJs, was correlated with poor outcome of uterine cervical ADC. By using CLDN6‐overexpressing model cell lines, we showed that overexpression of CLDN6 strengthened cell adhesion ability and promoted malignant potentials and drug resistance.
In CLDN6‐overexpressing cells, malignant potentials including cell proliferation, colony formation, and migration potentials were significantly increased (Figure 2). In immunohistochemical analysis of surgical specimens, aberrant CLDN6 expression was significantly associated with clinicopathologic parameters including lymph node metastasis and LVSI and was an independent prognostic factor (Figure 1 and Table 1). These results indicate that aberrant expression of CLDN6 contributes to malignancy of cervical ADC, as previously reported for several types of cancers including gastric ADC, lung ADC, ovarian ADC, and endometrial cancer. 32 , 33 , 34 , 35 As CLDN6 is exclusively expressed during the early developmental stage and contributes to fetal epithelial differentiation, 31 cancerous aberrant expression of CLDN6 might also be involved in enhancement of epithelial characteristics that mimic fetal epithelial differentiation. Indeed, our proteomics approach revealed that cell adhesion‐associated GO terms were significantly enriched in CLDN6‐overexpressing cells (Figure 3). Moreover, we found that cell adhesion‐associated properties were enhanced by CLDN6 overexpression (Figure 4). As reported by Kojima et al., 35 CLDN6 expression contributes to malignant potentials possibly through increased cell adhesion properties in uterine cervical ADC cells. Numerous recent studies have revealed that cell adhesion is required for a type of migration called “collective migration,” a relatively new concept of tumor cell migration in which tumor cells maintain adhesion with each other during the migration process. 35 , 40 , 47 , 48 In this study, we found that integrin α6 and integrin β4 were significantly upregulated in CLDN6‐overexpressing cells (Figure 4B and Data S1 and S2). Integrin α6β4, a heterodimer of subunits α6 and β4, has been implicated in the regulation of collective migration, 49 , 50 , 51 suggesting that these subunits play some roles in enhanced migration of CLDN6‐overexpressing cells.
Cervical ADCs are known to frequently develop resistance to anticancer drugs, including the first‐line therapeutic drug cisplatin. 52 Interestingly, we found that CLDN6 overexpression increased viability of cervical ADC cells under the condition of exposure to anticancer drugs including cisplatin (Figure 5), suggesting that aberrant CLDN6 expression plays a role in the development of multidrug resistance of cervical ADC cells. In general, chemoresistance development is due mainly to “resistance to cell death” and/or “resistance to the drug itself” (ie, decreased drug uptake, increased drug efflux, and detoxification of the drug). 53 , 54 We provided evidence that CLDN6 overexpression enhanced some cellular properties evoking “resistance to the drug itself”.
First, CLDN6 overexpression suppressed paracellular flux of small molecules (Figure 4), indicating that CLDN6 overexpression leads to enhanced TJ barrier function in cervical ADC cells. We speculate that aberrantly expressed CLDN6 acts as a suppressor of drug delivery into cancer nests through paracellular routes in some CLDN6 positive cervical ADC cases. Second, CLDN6 overexpression upregulated drug‐metabolizing enzymes, AKR family proteins (Figures 3 and 5). The AKR family of proteins promote the hydroxylation of anthracycline molecules such as daunorubicin and doxorubicin to reduce their activity as anticancer drugs 55 and they have also been reported to be involved in resistance to cisplatin. 56 , 57 Together with CLDN6‐dependent suppression of paracellular permeability described above, cases with a high expression level of CLDN6 could acquire resistance to chemotherapy.
Our findings provide an insight into a new therapeutic strategy, that is, a CLDN6‐targeting therapy, against cervical ADC. The strategy would improve the efficacy of anticancer drugs by attenuating CLDN6‐induced enhancement of barrier function and/or activity of drug‐metabolizing enzymes.
Claudin‐6 is an ideal therapeutic target because it is not expressed in adult human normal tissues, 27 , 58 suggesting a minimal adverse effect on normal adult tissues. Furthermore, CLDN6 is an attractive therapeutic target because its extracellular domain could be easily accessed by Abs, affinity peptides, and small‐molecule inhibitors. Claudin‐affinity peptides and anti‐CLDN Abs have been proposed for CLDN‐targeting therapy against multiple solid tumors. For example, PMTPV was evaluated as a CLDN1‐affinity peptide for CLDN1‐overexpressing lung ADC cells, 59 VPDSM and DSMKF were evaluated for CLDN2, 60 and DFYNP was evaluated for CLDN3 and CLDN4, 61 all of which were derived from amino acid sequences of the second extracellular loop (ECL2) of each CLDN molecule. Another CLDN‐targeting therapy, using an anti‐CLDN Ab, is also designed against ECL2. Among the anti‐CLDN Abs, anti‐CLDN18.2 Ab (zolbetuximab) is the most well‐known and is being used in a phase III clinical trial for gastric and gastroesophageal junction cancer (NCT03504397 and NCT03653507). 62 As in the case of other CLDNs, CLDN6‐affinity peptides and/or an anti‐CLDN6 Ab could enhance the therapeutic efficacy of anticancer drugs in cervical ADC.
More recently, another CLDN6‐targeting therapeutic strategy, CLDN6‐targeting chimeric antigen receptor (CAR)‐T cell therapy, has emerged. 58 , 63 In conjunction with our finding that a high expression level of CLDN6 is associated with poor prognosis of cervical ADC, CLDN6‐targeting CAR‐T therapy seems to be a promising therapy for improving prognosis of cervical ADC in the future.
In conclusion, a high CLDN6 expression level is significantly correlated with clinicopathologic features and is an independent prognostic factor of uterine cervical ADC. Aberrant expression of CLDN6 enhances malignant potentials and drug resistance of cervical ADC, possibly due to increased cell‐cell adhesion properties and drug metabolism. Our findings provide an insight into a new therapeutic strategy, a CLDN6‐targeting therapy, against cervical ADC, including a novel CLDN6‐targeting CAR‐T therapy. Treatment options other than platinum and anthracyclines could be appropriate for cases with aberrant CLDN6 expression.
DISCLOSURE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTIONS
The authors contributed in the following ways: YI, KT, TM, TAk: performed cell biological experiments; YI, KT: drafted the paper; AT: conceived the study, supervised all experiments, and performed the final revision of the manuscript; TM, KSh, KK, TAk: performed and analyzed immunocytochemistry and immunofluorescence; DK, YK, TAo, MOt: performed immunohistochemical experiments; YI, YK, AT, MM: analyzed immunohistochemical experiments; TAk, KSu, HC, TS: provided resources; DK, KK: performed visualization of the work; HC, TS, MOs: supervised experiments, and helped with writing the manuscript. All authors read and approved the final manuscript.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Data S1
Data S2
ACKNOWLEDGMENTS
The authors would like to thank Yui Kawami and Fuminori Daimon for technical assistance with the experiments. This work was supported by Japan Society for the Promotion of Science KAKENHI (grant numbers JP20K07409 and JP20K16196).
Ito Y, Takasawa A, Takasawa K, et al. Aberrant expression of claudin‐6 contributes to malignant potentials and drug resistance of cervical adenocarcinoma. Cancer Sci. 2022;113:1519–1530. doi: 10.1111/cas.15284
Yui Ito and Akira Takasawa contributed equally to this work.
Funding information
Japan Society for the Promotion of Science KAKENHI, Grant/Award Number: JP20K07409, JP20K16196
REFERENCES
- 1. Bray F, Carstensen B, Møller H, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2191‐2199. doi: 10.1158/1055-9965.EPI-05-0231 [DOI] [PubMed] [Google Scholar]
- 2. Bulk S, Visser O, Rozendaal L, et al. Cervical cancer in the Netherlands 1989–1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int J Cancer. 2005;113(6):1005‐1009. doi: 10.1002/ijc.20678 [DOI] [PubMed] [Google Scholar]
- 3. Gien LT, Beauchmin MC, Thomas G. Adenocarcinoma: Aunique cervical cancer. Gynecol Oncol. 2010;116:140‐146. doi: 10.1016/j.ygyno.2009.09.040 [DOI] [PubMed] [Google Scholar]
- 4. Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490‐1493. [DOI] [PubMed] [Google Scholar]
- 5. Smith HO, Tiffany MF, Qualls CR, et al. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States‐A 24‐year population‐based study. Gynecol Oncol. 2000;78:97‐105. doi: 10.1006/gyno.2000.5826 [DOI] [PubMed] [Google Scholar]
- 6. Vizcaino AP, Moreno V, Bosch FX, et al. international trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75(4):536‐545. doi: [DOI] [PubMed] [Google Scholar]
- 7. Ino Y, Akimoto T, Takasawa A, et al. Elevated expression of G protein‐coupled receptor 30 (GPR30) is associated with poor prognosis in patients with uterine cervical adenocarcinoma. Histol Histopathol. 2020;35:351‐359. doi: 10.14670/HH-18-157 [DOI] [PubMed] [Google Scholar]
- 8. Lee J, Kim YT, Kim S, et al. Prognosis of cervical cancer in the era of concurrent chemoradiation from National database in Korea: a comparison between squamous cell carcinoma adenocarcinoma. PLoS One. 2020;10:e0144887. doi: 10.1371/journal.pone.0144887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mabuchi S, Okazawa M, Matsuo K, et al. Impact of histological subtype on survival of patients with surgicallytreated stage IA2‐IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol. 2012;127(1):114‐120. doi: 10.1016/j.ygyno.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 10. Nakanishi T, Ishikawa H, Suzuki Y, et al. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000;79(2):289‐293. doi: 10.1006/gyno.2000.5935 [DOI] [PubMed] [Google Scholar]
- 11. Wright TC, Ferenczy A, Kurman RJ. Carcinoma and other tumors of the cervix. In: Kurman RJ, ed. Blaustein’s Pathology of The Female Genital Tract, 5th ed. Springer‐Verlag; 2002:325‐382. [Google Scholar]
- 12. Yokoi E, Mabuchi S, Takahashi R, et al. Impact of histological subtype on survival in patients with locally advanced cervical cancer that were treated with definitive radiotherapy: adenocarcinoma/ adenosquamous carcinoma versus squamous cell carcinoma. J Gynecol Oncol. 2017;28(2):e19. doi: 10.3802/jgo.2017.28.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yagi A, Ueda Y, Kakuda M, et al. Epidemiological and clinical analyses of cervical cancer using data from the population‐based Osaka cancer registry. Cancer Res. 2019;79:1252‐1259. doi: 10.1158/0008-5472.CAN-18-3109 [DOI] [PubMed] [Google Scholar]
- 14. Akimoto T, Takasawa A, Murata M, et al. Analysis of the expression and localization of tight junction transmembrane proteins, claudin‐1, ‐4, ‐7, occludin and JAM‐A, in human cervical adenocarcinoma. Histol Histopathol. 2016;31:921‐931. doi: 10.1016/j.neo.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 15. Akimoto T, Takasawa A, Takasawa K, et al. Estrogen/GPR30 signaling contributes to the malignant potentials of ER‐negative cervical adenocarcinoma via regulation of claudin‐1 expression. Neoplasia. 2018;20(10):1083‐1093. doi: 10.1016/j.neo.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCluggage WG. New developments in endocervical glandular lesions. Histopathol. 2013;62(1):138‐160. doi: 10.1111/his.12012 [DOI] [PubMed] [Google Scholar]
- 17. Soonthornthum T, Arias‐Pulido H, Joste N, et al. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol. 2011;22(10):2166‐2178. [DOI] [PubMed] [Google Scholar]
- 18. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285‐293. doi: 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- 19. Sawada N. Tight junction‐related human diseases. Pathol Int. 2013;63:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osanai M, Takasawa A, Murata M, et al. Claudins in cancers ‐Bench to Bedside. Pflüg Arch Eur J Physiol. 2017;469:55‐67. doi: 10.1007/s00424-016-1877-7 [DOI] [PubMed] [Google Scholar]
- 21. Tsukita S, Yamazaki Y, Katsuno T, et al. Tight junction‐based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930‐6938. doi: 10.1038/onc.2008.344 [DOI] [PubMed] [Google Scholar]
- 22. Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. doi: 10.1155/2010/541957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takasawa K, Takasawa A, Osanai M, et al. Claudin‐18 coupled with EGFR/ERK signaling contributes to the malignant potentials of bile duct cancer. Cancer Lett. 2017;403:66‐73. doi: 10.1016/j.canlet.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 24. Aoyama T, Takasawa A, Murata M, et al. Immunoreactivity patterns of tight junction proteins are useful for differential diagnosis of human salivary gland tumors. Med Mol Morphol. 2019;52(1):23‐35. doi: 10.1007/s00795-018-0199-6 [DOI] [PubMed] [Google Scholar]
- 25. Magara K, Takasawa A, Osanai M, et al. Elevated expression of JAM‐A promotes neoplastic properties of lung adenocarcinoma. Cancer Sci. 2017;108(11):2306‐2314. doi: 10.1111/cas.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubota H, Chiba H, Takakuwa Y, et al. Retinoid X receptor alpha and retinoic acid receptor gamma mediate expression of genes encoding tight‐junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp Cell Res. 2001;263(1):163‐172. doi: 10.1006/excr.2000.5113 [DOI] [PubMed] [Google Scholar]
- 27. Turksen K, Troy TC. Claudin‐6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dyn. 2001;222(2):292‐300. doi: 10.1002/dvdy.1174 [DOI] [PubMed] [Google Scholar]
- 28. Chiba H, Gotoh T, Satohisa S, et al. Hepatocyte nuclear factor (HNF)‐4alpha triggers formation of functional tight junctions and establishment of polarized epithelial morphology in F9 embryonal carcinoma cells. Exp Cell Res. 2003;286(2):288‐297. doi: 10.1016/s0014-4827(03)00116-2 [DOI] [PubMed] [Google Scholar]
- 29. Anderson WJ, Zhou Q, Alcalde V, et al. Genetic targeting of the endoderm with claudin‐6CreER. Dev Dyn. 2008;237(2):504‐512. doi: 10.1002/dvdy.21437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugimoto K, Ichikawa‐Tomikawa N, et al. The tight‐junction protein claudin‐6 induces epithelial differentiation from mouse F9 and embryonic stem cells. PLoS One. 2013;8(10):e75106. doi: 10.1371/journal.pone.0075106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hashizume A, Ueno T, Furuse M, et al. Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular Gland. Dev Dyn. 2004;231:425‐431. doi: 10.1002/dvdy.20142 [DOI] [PubMed] [Google Scholar]
- 32. Sullivan LM, Yankovich T, Le P, et al. Claudin‐6 is a nonspecific marker for malignant rhabdoid and other pediatric tumors. Am J Surg Pathol. 2012;36(1):73‐80. doi: 10.1097/PAS.0b013e31822cfa7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ushiku T, Shinozaki‐Ushiku A, Maeda D, et al. Distinct expression pattern of claudin‐ 6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology. 2012;61(6):1043‐1056. doi: 10.1111/j.1365-2559.2012.04314.x [DOI] [PubMed] [Google Scholar]
- 34. Kohmoto T, Masuda K, Shoda K, et al. Claudin‐6 is a single prognostic marker and functions as a tumor‐promoting gene in a subgroup of intestinal type gastric cancer. Gastric Cancer. 2020;23:403‐417. doi: 10.1007/s10120-019-01014-x [DOI] [PubMed] [Google Scholar]
- 35. Kojima M, Sugimoto K, Tanaka M, et al. Prognostic significance of aberrant claudin‐6 expression in endometrial cancer. Cancers (Basel). 2020;12(10):2748. doi: 10.3390/cancers12102748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murakami T, Takasawa A, Takasawa K, et al. Aberrant expression of junctional adhesion molecule‐A contributes to the malignancy of cervical adenocarcinoma by interaction with poliovirus receptor/CD155. Cancer Sci. 2021;112(2):906‐917. doi: 10.1111/cas.14734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takasawa A, Murata M, Takasawa K, et al. Nuclear localization of tricellulin promotes the oncogenic property of pancreatic cancer. Sci Rep. 2016;6:33582. doi: 10.1038/srep33582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayakawa O, Kudo R, Koizumi M, et al. Establishment of a human adenocarcinoma cell line, CAC‐1. Sapporo Med J. 1988;57:603‐611. [Google Scholar]
- 39. Zheng P‐S, Iwasaka T, Ouchida M, et al. Growth suppression of a cervical cancer cell line (TMCC‐1) by the human wild‐type p53 gene. Gynecol Oncol. 1996;60:245‐250. doi: 10.1006/gyno.1996.0033 [DOI] [PubMed] [Google Scholar]
- 40. Sugimoto K, Ichikawa‐Tomikawa N, Kashiwagi K, et al. Cell adhesion signals regulate the nuclear receptor activity. Proc Natl Acad Sci USA. 2019;116:24600‐24609. doi: 10.1073/pnas.1913346116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aoyama T, Takasawa A, Takasawa K, et al. Identification of coiled‐coil domain‐containing protein 180 and leucine‐rich repeat‐containing protein 4 as potential immunohistochemical markers for liposarcoma based on proteomic analysis using formalin‐fixed, paraffin‐embedded tissue. Am J Pathol. 2019;189(5):1015‐1028. doi: 10.1016/j.ajpath.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 42. Saito Y, Takasawa A, Takasawa K, et al. Aldolase A promotes epithelial‐mesenchymal transition to increase malignant potentials of cervical adenocarcinoma. Cancer Sci. 2020;111(8):3071‐3081. doi: 10.1111/cas.14524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takasawa K, Takasawa A, Akimoto T, et al. Regulatory roles of claudin‐1 in cell adhesion and microvilli formation. Biochem Biophys Res Commun. 2021;565:36‐42. doi: 10.1016/j.bbrc.2021.05.070 [DOI] [PubMed] [Google Scholar]
- 44. Takasawa A, Kojima T, Ninomiya T, et al. Behavior of tricellulin during destruction and formation of tight junctions under various extracellular calcium conditions. Cell Tissue Res. 2013;351:73‐84. doi: 10.1007/s00441-012-1512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oleg AB, Srinivas MT, Aruni B. The aldo‐Keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40(4):553‐624. doi: 10.1080/03602530802431439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: beyond the migration of single cells. J Biol Chem. 2020;295:2495‐2505. doi: 10.1074/jbc.REV119.007759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445‐457. doi: 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- 49. Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347‐367. doi: 10.1016/j.ccell.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541‐545. doi: 10.1016/S0955-0674(00)00249-0 [DOI] [PubMed] [Google Scholar]
- 51. Colburn ZT, Jones JCR. Α6Β4 integrin regulates the collective migration of epithelial cells. Am J Respir Cell Mol Biol. 2017;56:443‐452. doi: 10.1165/rcmb.2016-0313OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu L, Wang M, Li X, et al. An overview of novel agents for cervical cancer treatment by inducing apoptosis: emerging drugs ongoing clinical trials and preclinical studies. Front Med (Lausanne). 2021;8:682366. doi: 10.3389/fmed.2021.682366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang M, Li Y, Shen X, et al. CLDN6 promotes chemoresistance through GSTP1 in human breast cancer. J Exp Clin Cancer Res. 2017;36:157. doi: 10.1186/s13046-017-0627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yeldag G, Rice A, Hernández AR. Chemoresistance and the self‐maintaining tumor microenvironment. Cancers (Basel). 2018;10(12):471. doi: 10.3390/cancers10120471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hofman J, Malcekova B, Skarka A, et al. Anthracycline resistance mediated by reductive metabolism in cancer cells: the role of aldo‐keto reductase 1C3. Toxicol Appl Pharmacol. 2014;278(3):238‐248. doi: 10.1016/j.taap.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 56. Shirato A, Kikugawa T, Miura N, et al. Cisplatin resistance by induction of aldo‐keto reductase family 1 member C2 in human bladder cancer cells. Oncol Lett. 2014;7(3):674‐678. doi: 10.3892/ol.2013.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou C, Shen G, Yang F, et al. Loss of AKR1C1 is a good prognostic factor in advanced NPC cases and increases chemosensitivity to cisplatin in NPC cells. J Cell Mol Med. 2020;24(11):6438‐6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reinhard K, Rengst B, Oehm P, et al. An RNA vaccine drives expansion and efficacy of claudin‐CAR‐T cells against solid tumors. Science. 2020;367(6476):446‐453. doi: 10.1126/science.aay5967 [DOI] [PubMed] [Google Scholar]
- 59. Nasako H, Takashina Y, Eguchi H, et al. Increase in toxicity of anticancer drugs by PMTPV, a claudin‐1‐binding peptide, mediated via down‐regulation of claudin‐1 in human lung adenocarcinoma A549 cells. Int J Mol Sci. 2020;21(16):5909. doi: 10.3390/ijms21165909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nasako H, Akizuki R, Takashina Y, et al. Claudin‐2 binding peptides, VPDSM and DSMKF, down‐regulate claudin‐2 expression and anticancer resistance in human lung adenocarcinoma A549 cells. Biochim Biophys Acta Mol Cell Res. 2020;1867(4):118642. doi: 10.1016/j.bbamcr.2019.118642 [DOI] [PubMed] [Google Scholar]
- 61. Baumgartner HK, Beeman N, Hodges RS, Neville MC. A d‐peptide analog of the second extracellular loop of claudin‐3 and ‐4 leads to mislocalized claudin and cellular apoptosis in mammary epithelial cells. Chem Biol Drug Des. 2011;77:124‐136. doi: 10.1111/j.1747-0285.2010.01061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lordick F, Al‐Batran SE, Ganguli A, et al. Patient‐reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first‐line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer. 2021;24(3):721‐730. doi: 10.1007/s10120-020-01153-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stadler CR, Mahmud HB, Plum LM, et al. Characterization of the first‐in‐class T‐cell‐engaging bispecific single‐chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. Oncoimmunology. 2016;5(3):e1091555. doi: 10.1080/2162402X.2015.1091555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Data S1
Data S2


