Abstract
Background
Serine hydroxymethyltransferase (SHMT) is critical for one-carbon unit metabolism and is increasingly reported to be associated with tumor patients’ outcomes. Thus, we designed and performed this meta-analysis to reveal its prognostic role and relationship with clinicopathological characteristics in human cancer.
Methods
A systematic search of PubMed, Embase, Web of Science and Cochrane Library (CENTRAL) was carried out. Two reviewers independently screened all references for eligibility according to the inclusion criteria. The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality and data was extracted for the meta-analysis.
Results
Ten studies, composed of 1,942 patients in total, were included in this meta-analysis. Higher expression of SHMT2 means an unfavorable prognosis [overall survival: hazard ratio (HR) =2.14, 95% confidence interval (CI): 1.53 to 2.99; progression-free survival (PFS)/disease-free survival (DFS)/recurrence-free survival (RFS): HR =1.90, 95% CI: 1.31 to 2.76]. Furthermore, higher SHMT2 expression is associated with larger tumor size [odds ratio (OR) =2.09, 95% CI: 1.58 to 2.77], more lymph node invasions [OR =2.67, 95% CI: 1.78 to 4.00), and higher tumor node metastasis classification (TNM) stage (OR =2.23, 95% CI: 1.55 to 3.21). Higher expression of SHMT2 is also related to higher histopathological grade (OR =3.46, 95% CI: 1.46 to 8.27) and distant metastasis (OR =1.25, 95% CI: 0.32 to 4.90), however, with significant heterogeneity (I2=61%, P=0.08 for distant metastasis; I2=82%, P<0.001 for histopathological grade). The prognostic clinical role of SHMT1 in clinical patients has not been directly investigated yet.
Discussion
SHMT2 may serve as a promising prognostic biomarker in various cancer, especially in the alimentary system. Further large-scale studies are warranted to verify the possible effect.
Keywords: Serine hydroxymethyltransferase (SHMT), human cancers, clinicopathological features, predictive biomarker
Introduction
One carbon unit is a carbon group containing a carbon atom including methyl, methylene, methylene, hydroxymethyl, formyl, and aminomethyl, produced in the catabolism of serine, glycine, tryptophan, or histidine. Once generated, it binds with tetrahydrofolate (THF) to form the stable form, for instance, N5, N10-methylene-THF (mTHF) or N5-aminomethyl-THF (amTHF), and subsequently engages in nucleotide and methionine synthesis. Serine hydroxymethyltransferase (SHMT) is an enzyme that catalyzes the interconversion reaction of serine to mTHF and glycine, and the latter product of this process, glycine, can likewise be converted into mTHF in the further enzymatic reaction. Therefore, SHMT plays a pivotal role and is even deemed as the core in generating one-carbon units by some researchers (1,2). There are two isoforms of SHMT, namely SHMT1 located in the cytoplasm and SHMT2 that is mainly expressed in mitochondrion (3), to catalyze the same reaction.
Cancer has been a health challenge worldwide. According to the 2020 report from the International Agency for Research on Cancer (IARC) (4), a total of 19.3 million new cases of various types of cancer were diagnosed in the past year. Approximately one in five people would develop cancer in their lifetime. Moreover, 10 million people, about one in eight men or one in eleven women, died from the advanced cancer in the past year, resulting in incalculable family economic loss and social burden. Therefore, it is of paramount importance to early diagnose and explore new therapy. Cancer cells are featured for aberrant proliferation and entail abundant substances, including one carbon unit. As a key enzyme promoting the generation of one carbon unit, cumulative researches have proved the pro-tumor role of SHMT in multiple cancers, making it possible that SHMT can serve as a tumor biomarker and potential target of anticancer agents. We here investigated the prognostic role of SHMT in human cancers and its relationship with clinicopathological characteristics, which had not been previously recapitulated and quantitatively evaluated.
This meta-analysis was designed, performed, and reported following recommendations of PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2485/rc) and Cochrane Handbook guidelines (5).
Methods
Literature searching strategy
We searched for potential studies in PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and web of science before June 2021, using the keywords and their synonyms. Search terms were modified according to each index term in database, such as Medical Subject Heading (MeSH) in PubMed and Emtree in Embase. We focused on clinical studies on humans when searching on Embase, but set no restrictions on other databases. Detailed search terms are shown in Appendix 1.
Inclusion and exclusion criteria
Studies in our review had to meet all of the following criteria: (I) objects were human patients diagnosed as any tumor pathologically; (II) the study reported the association of SHMT expression with survival outcome and/or clinicopathological characteristics.
The exclusion criteria were: (I) duplicate studies; (II) studies with the same data or overlapping data by the same authors; (III) studies without any one of the predetermined outcomes; (IV) studies without access to the full-text.
Quality assessment
The quality of all selected studies was assessed and scored independently by two reviewers according to the Newcastle–Ottawa Quality Assessment Scale (6) in the following regards: selection, comparability, and outcome. Disagreements on the scores would be resolved by consensus or cross-checking with a third author. The final hierarchy of a study was determined by the sum of the score of each item. The corresponding score of 0–3, 4–6, 7–9 was accepted as low quality, medium quality, and high quality, respectively.
Data extraction
Two researchers independently extracted the following data from each study: characters of the study (first author, publication year, number of participants, cancer type, study period), characters of the participants (gender, age, country, expression level ratio, cut-off value, follow-up), and outcomes. If both multivariate analysis and univariate results were reported, hazard ratio (HR) and corresponding 95% confidence interval (CI) of multivariate analysis were preferred. If neither was available, HRs with 95% CIs were extrapolated based on Kaplan-Meier curves using the spreadsheet provided by Tierney et al. (7). During the data extraction, any disagreements between the two authors were resolved by consensus or cross-checking with a third author. Additional information was collected through communication with the principal investigator by email if necessary.
Statistical analysis
Statistical analysis was performed by an independent researcher adept in statistics using RevMan (Version 5.4.0; The Nordic Cochrane Centre, The Cochrane Collaboration) and Stata (Version 16.0; The StataCorp LLC). Continuous variables were reported as mean and standard derivation (SD), while dichotomous variables were shown as frequency or proportion. The results were displayed in forest plots. An initial test for clinical, methodological, and statistical heterogeneities was conducted, and we used the chi-square test with P<0.1 and I2>50% to indicate statistical significance. A random-effects model was applied in the presence of significant heterogeneity and Galbraith radial plot analysis would be performed on the purpose to determine the underlying cause. Otherwise, the fixed-effects model was chosen. HR or odds ratio (OR) with 95% CI was calculated according to the data type.
Results
Study selection
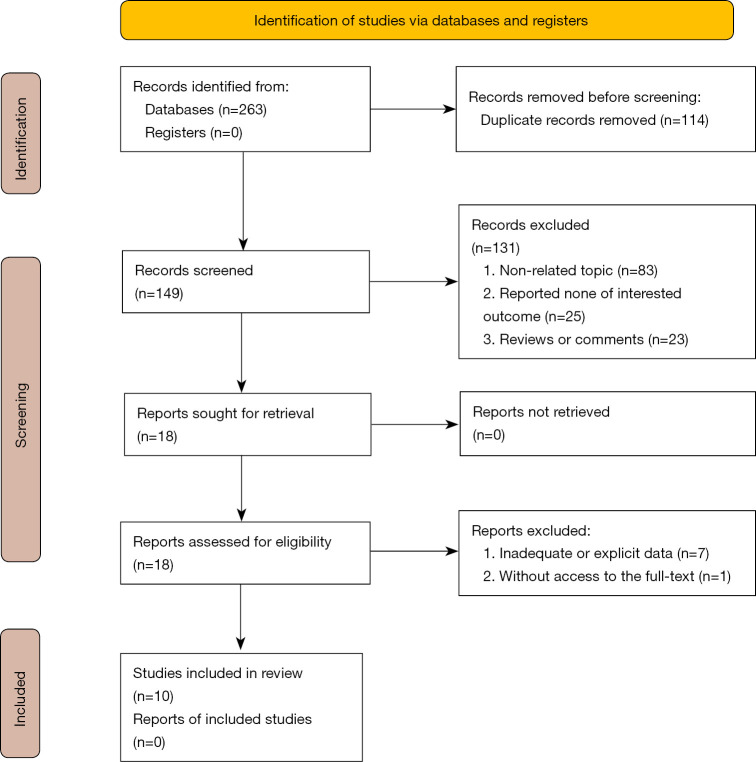
Two hundred sixty-three studies were identified from the primary research, and 114 were omitted for duplication by manual comparing the authors, publication years, and titles. Whereas the remaining were screened by abstract, and 83 articles not relevant to this topic, 25 studies that reported none of the interested outcomes, and 23 reviews or comments, were subsequently excluded. Then, 18 articles were viewed through the whole text for eligibility, of which 7 studies with inadequate or explicit data and one without excess were voted out. Finally, 10 studies were included in the meta-analysis (8-17). The flowchart of the study is shown in Figure 1.
Figure 1.
Study flow.
Characteristics and quality of the enrolled studies
The included studies all focused on SHMT2 and were carried out in China (n=7), Japan (n=2) and Germany (n=1). All chosen studies were published between 2016 and 2019, and involved mainly the alimentary system malignancy including liver carcinoma (n=3), gastric cancer (n=2), colorectal cancer (n=2), esophageal cancer (n=1), pancreatic ductal adenocarcinoma (n=1), and breast cancer (n=2), and human glioma (n=1). All studies utilized immunohistochemistry (IHC) to evaluate the expression level of SHMT2 except the study of Bernhardt et al. (14) that performed the reverse phase protein arrays. Most researchers reported the HR of overall survival (OS) and progression-free survival (PFS)/disease-free survival (DFS)/recurrence-free survival (RFS) with their 95% CI directly while 3 offered the Kaplan-Meier curve and P-value. Characteristics of studies and participants are shown in Tables 1 and 2, respectively.
Table 1. Characteristics of the studies.
| Author | Publication year | Samples | SHMT type | SHMT detection | Cut-off value of SHMT expression | Follow-up (month) | Study period | Country | Source of HR |
|---|---|---|---|---|---|---|---|---|---|
| Shi et al. | 2019 | 130 | SHMT2 | IHC | Staining scores with intensity and proportion (>3) | 46.6 | 2008–2016 | China | Reported |
| Ji et al. | 2019 | 144 | SHMT2 | IHC | Staining scores with intensity and proportion | 36 | 2006–2017 | China | Reported |
| Liu et al._GC | 2019 | 58 | SHMT2 | IHC | Staining scores with intensity and proportion (>4) | Until May 2018 | 2010–2013 | China | Reported |
| Liu et al._EC | 65 | SHMT2 | IHC | Staining scores with intensity and proportion (>4) | Until May 2018 | 2010–2013 | |||
| Liu et al._CC | 60 | SHMT2 | IHC | Staining scores with intensity and proportion (>4) | Until May 2018 | 2010–2013 | |||
| Ning et al. | 2018 | 100 | SHMT2 | IHC | Staining scores including intensity and proportion (>4/12) | 37 | 2005–2015 | China | Reported |
| Noguchi et al. | 2018 | 103 | SHMT2 | IHC | Staining scores with proportion (≥2/2) | 60.9 | 2007–2013 | Japan | Reported |
| Wang et al. | 2017 | 150 | SHMT2 | IHC | Staining scores with intensity (≥2/2) | 36 | 2012–2013 | China | Survival curve |
| Bernhardt et al. | 2017 | 801 | SHMT2 | Reverse phase protein arrays | Median expression level | – | 2009–2011 | Germany | Reported |
| Miyo et al. | 2017 | 117 | SHMT2 | IHC | The samples showing an average staining were used as a positive control | 58.8 | 2006–2009 | Japan | Survival curve |
| Zhang et al. | 2016 | 128 | SHMT2 | IHC | Staining scores with intensity and proportion | Until 30 September 2010 | 2002–2006 | China | Reported |
| Wu et al. | 2016 | 86 | SHMT2 | IHC | Staining scores with intensity (≥3/4) | – | – | China | Survival curve |
GC, gastric cancer; EC, esophageal cancer; CC, colorectal cancer; SHMT, serine hydroxymethyltransferase; IHC, immunohistochemistry; HR, hazard ratio.
Table 2. Characteristics of the participants.
| Author | Expression (high/low) | Gender (male/total) | Age (years, mean ± SD) | Age (percentage of the older) | Cancer type | Control group | Survival outcome | Survival analysis |
|---|---|---|---|---|---|---|---|---|
| Shi et al. | 69/61 | 81/130 | – | 66.2% | Gastric cancer | High vs. low | OS | Multivariate analysis |
| Ji et al. | 80/64 | 89/144 | – | 60.4% | Hepatocellular carcinoma | High vs. low | OS | Multivariate analysis |
| Liu et al._GC | 43/15 | 29/58 | – | 58.6% | Gastric cancer | High vs. low | OS and RFS | Univariate and multivariate analysis |
| Liu et al._EC | 45/20 | 35/65 | – | 49.2% | Esophageal cancer | High vs. low | ||
| Liu et al._CC | 43/17 | 27/60 | – | 53.3% | Colorectal cancer | High vs. low | ||
| Ning et al. | 52/48 | 69/100 | – | 48.0% | Intrahepatic cholangiocarcinoma | High vs. low | OS | Univariate and multivariate analysis |
| Noguchi et al. | 63/40 | 64/103 | 67.2±9.6 | – | Pancreatic ductal adenocarcinoma | High vs. low | OS and DFS | Univariate and multivariate analysis |
| Wang et al. | 68/82 | 76/150 | 48±11 | – | Human glioma | High vs. low | OS and PFS | Univariate analysis |
| Bernhardt et al. | 400/401 | NR | 62.3±13.7 | – | Breast cancer | High vs. low | OS and RFS | Univariate and multivariate analysis |
| Miyo et al. | 89/88 | 71/117 | – | 51.3% | Colorectal cancer | High vs. low | OS and RFS | Univariate and multivariate analysis |
| Zhang et al. | 95/33 | NR | – | 50.0% | Breast cancer | High vs. low | OS and PFS | Univariate and multivariate analysis |
| Wu et al. | 51/35 | 64/86 | – | 66.3% | Hepatocellular carcinoma | High vs. low | OS and PFS | Univariate analysis |
GC, gastric cancer; EC, esophageal cancer; CC, colorectal cancer; SD, standard derivation; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; RFS, recurrence-free survival; NR, not reported.
The quality of the included studies was scored and classified. Nine of the included studies (90%) received no less than 7, which stratified into high-quality studies group, while the left (16) was scored 6 and deemed as the moderate quality study. Detailed Newcastle-Ottawa quality assessment scales of each study are summarized and shown in Table 3.
Table 3. Newcastle-Ottawa quality assessment scales of each study.
| Author | Year | Selection (☆☆☆☆) | Comparability (☆☆) | Outcome (☆☆☆) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||
| Shi et al. | 2019 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 | ||
| Ji et al. | 2019 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | ||
| Liu et al. | 2019 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | – | ☆ | 7 | ||
| Ning et al. | 2018 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | ||
| Noguchi et al. | 2018 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | ||
| Wang et al. | 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | ||
| Bernhardt et al. | 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | – | – | 6 | ||
| Miyo et al. | 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | ||
| Zhang et al. | 2016 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | – | – | 6 | ||
| Wu et al. | 2016 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | – | ☆ | 7 | ||
Analysis of the survival associated with SHMT2 expression
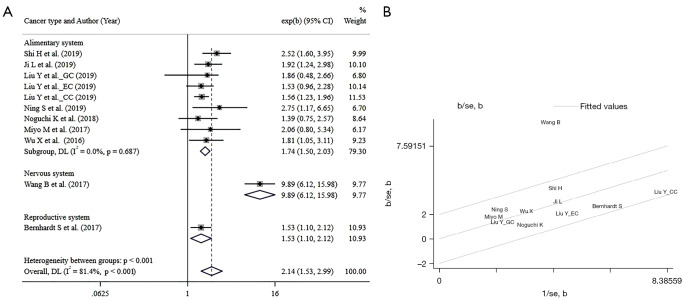
Nine studies (8-15,17), involving 1,814 participants in total, reported the association between SHMT2 expression and overall survival. These studies were subjected to OS analysis, and the pooled HR indicates higher SHMT2 correlated with decreased OS (HR =2.14, 95% CI: 1.53 to 2.99), however, with a significant heterogeneity (I2=81.4%, P<0.001). Subgrouped by the system, the result showed that higher expression of SHMT2 invariably means poorer OS in all involved systems (HR =1.74, 95% CI: 1.50 to 2.03, for the alimentary system; HR =9.89, 95% CI: 6.12 to 15.98 for glioma cancer; HR =1.53, 95% CI: 1.10 to 2.12 for breast cancer) with a decent homogeneity (I2=0%, P=0.678 for the alimentary system). The Forest plot of overall survival is shown in Figure 2.
Figure 2.
The Forest plots and Galbraith radial plot of overall survival analysis: (A) the Forest plots of overall survival analysis; (B) Galbraith radial plot of overall survival analysis. GC, gastric cancer; EC, esophageal cancer; CC, colorectal cancer; CI, confidence interval; DL, DerSimoniar and Laird method; se, standard error.
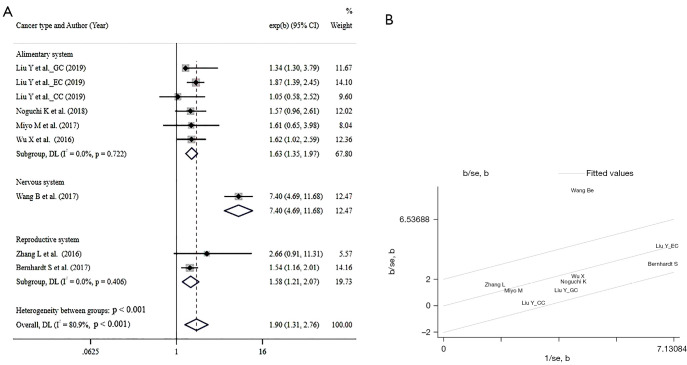
Seven studies (10,12-17), composed of 1,568 participants, included in the PFS/RFS/DFS synthesis also uncover an unfavorable role of SHMT2 (HR =1.90, 95% CI: 1.31 to 2.76, I2=80.9%, P<0.001), when subgrouped by the system, the trend remains consistent (HR =1.63, 95% CI: 1.35 to 1.97 for the alimentary system; HR =7.4, 95% CI: 4.69 to 11.68 for glioma cancer; HR =1.58, 95% CI: 1.21 to 2.07 for breast cancer). The Forest plot of PFS/RFS/DFS is shown in Figure 3.
Figure 3.
The Forest plots and Galbraith radial plot of PFS/RFS/DFS analysis: (A) the Forest plots of PFS/RFS/DFS analysis; (B) Galbraith radial plot of PFS/RFS/DFS analysis. GC, gastric cancer; EC, esophageal cancer; CC, colorectal cancer; CI, confidence interval; PFS, progression-free survival; DFS, disease-free survival; RFS, recurrence-free survival; DL, DerSimoniar and Laird method; se, standard error.
Analysis of the clinicopathological characteristics associated with SHMT2 expression
We also tried to analyze the association between clinicopathological characteristics and SHMT2 expression. The results are encapsulated in Table 4, which reveal that higher expression of SHMT2 was correlated to bigger tumor size (OR =2.09, 95% CI: 1.58 to 2.77), more lymph node invasion (OR =2.67, 95% CI: 1.78 to 4.00), and eventually higher TNM grade (OR =2.23, 95% CI: 1.55 to 3.21). There was also a tendency that showed the unfavorable correlation between expression and distant metastasis (OR =1.25, 95% CI: 0.32 to 4.90) and histopathological grade (OR =3.46, 95% CI: 1.46 to 8.27), but with non-negligible heterogeneity (I2=61%, P=0.08 for distant metastasis; I2=82%, P<0.001 for histopathological grade). Relevant forest plots are shown in Figures S1-S4.
Table 4. summary of the association between clinicopathological characteristics.
| Characteristic | Number of studies | Number of patients | Pooled OR [95% CI] | P value | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | ||||||
| Tumor size | |||||||
| Alimentary system | 5 | 639 | 1.84 [1.32, 2.58] | <0.001 | Fixed | 33 | 0.17 |
| Reproductive system | 1 | 123 | 2.78 [1.18, 6.57] | 0.020 | – | – | – |
| Nervous system | 1 | 150 | 2.95 [1.51, 5.78] | 0.002 | – | – | – |
| Merged result | 7 | 912 | 2.09 [1.58, 2.77] | <0.001 | Fixed | 28 | 0.19 |
| Lymphatic invasion | |||||||
| Alimentary system | 5 | 641 | 2.54 [1.68, 3.84] | <0.001 | Fixed | 0 | 0.64 |
| Reproductive system | 1 | 128 | 5.53 [0.70, 43.81] | 0.110 | – | – | – |
| Merged result | 6 | 769 | 2.67 [1.78, 4.00] | <0.001 | Fixed | 0 | 0.59 |
| Distant metastasis | |||||||
| Alimentary system | 3 | 295 | 1.25 [0.32, 4.90] | 0.08 | Random | 61 | 0.08 |
| TNM grade | |||||||
| Alimentary system | 4 | 559 | 2.23 [1.55, 3.21] | <0.001 | Fixed | 0 | 0.82 |
| Histopathological grade | |||||||
| Alimentary system | 5 | 550 | 2.35 [1.00, 5.51] | 0.001 | Random | 75 | 0.001 |
| Reproductive system | 1 | 150 | 27.73 [9.18, 83.78] | 0.003 | – | – | – |
| Nervous system | 1 | 111 | 4.22 [1.62, 10.98] | <0.001 | – | – | – |
| Merged result | 7 | 811 | 3.46 [1.46, 8.23] | 0.005 | Random | 82 | <0.001 |
OR, odds ratio; TNM, tumor node metastasis classification.
Heterogeneity analysis and sensitivity analysis
The Chi-square test was utilized for heterogeneity analysis. Regrettably, it revealed significant heterogeneity exists for the eligible researchers evaluating OS (I2=81.4%, P<0.001), A following Galbraith radial plot (Figure 2) was generated and indicated heterogeneity was probably caused by the study of Wang et al. (13). As expected, heterogeneity diminished when the mentioned study was removed, and the result remained consistent (HR =1.70, 95% CI: 1.49 to 1.96, heterogeneity: I2=0, P=0.725). Besides, similarity reoccured when we evaluated PFS/RFS/DFS in the eligible studies. There was a significant heterogeneity (I2=80.9%, P<0.001), which was also imputed to the study of Wang et al. (13) in the Galbraith radial plot (Figure 3). Heterogeneity vanished along with the removal of the study of Wang et al. (HR =1.61, 95% CI: 1.38 to 1.88, heterogeneity: I2=0, P=0.826). There was no significant heterogeneity in the subgroup analysis of OS and PFS/RFS/DFS.
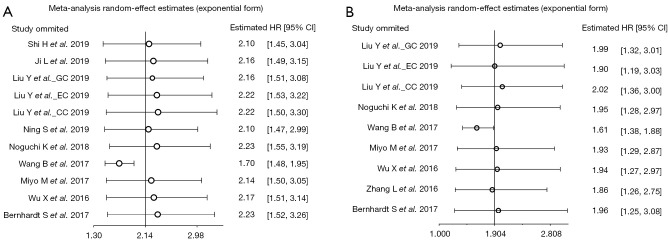
Sensitivity analysis was conducted by omitting the included studies one by one. Expectedly, As shown in Figure 4, no study would influence the pooled HRs and 95% CI except the study of Wang et al. (13), indicating the result possess decent stability both in OS (Figure 4A) and PFS/RFS/DFS (Figure 4B) analysis.
Figure 4.
Sensitivity analysis: (A) sensitivity analysis of studies according to OS; (B) sensitivity analysis of studies according to PFS/RFS/DFS. GC, gastric cancer; EC, esophageal cancer; CC, colorectal cancer; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; RFS, recurrence-free survival.
Discussion
Tumor researches have been in full blossom in recent years and thusly facilitates individualized treatment, which was closely related to the personal genetic pattern. SHMT was first discovered in the 1980s and accepted as a trivial enzyme for catalyzing the so-called futile cycle of serine to glycine. Then it has begun to exhibit various functions in life processes, including energy metabolism, cell growth, and proliferation. Currently, ongoing studies tried to unveil its role in carcinogenesis and cancer development. In this study, we focused on the prognostic role and association with clinicopathological characteristics in human cancer.
In this study, all the included researches examined the expression of SHMT2 in protein level for subsequent analysis. This is important because coding messenger ribonucleic acid (mRNA) expression may not always completely reflect the level of relevant protein. For example, it was reported Spearman's rank correlation coefficient between mRNA and protein translations was less than 0.4 in non-small cell lung cancer, indicating that their expression levels are not highly correlated (18).
We found that patients with higher expression of SHMT2 had a poorer prognosis in any studies included. Classified by the system, the pooled results showed an unfavorable correlation between high SHMT2 expression and patients’ outcomes with decent stability. In line with that, the expression of SHMT2 was naturally supposed to be an adverse factor for patients’ outcomes in the overall combination of different systems, but with significant heterogeneity caused by the study of Wang et al. (13). This could suggest that SHMT2 in different organ derivations may have somewhat varying prognostic values. Notwithstanding, it can’t be ignored that the curves in Wang et al.’s study separated in an early stage and the HR extrapolated from univariate Kaplan-Meier curves, irrespective of effects of other co-factors, was also beyond other studies (HR =9.89 in Wang et al.’s study while HR of others was about 2).
In this study, we also found that higher SHMT2 expression symbolized larger tumor size and more lymph node invasions, which eventually led to a higher TNM stage, revealing that SHMT2 may promote the proliferation and invasion ability of tumor cells. The relationship of the expression level of SHMT2 with histopathological grade was not consistent among the 7 eligible works of literature. 2 studies (11,17) reported higher SHMT2 expression tended to signify lower histopathological grade although the difference did not reach statistical significance. On the contrary, the remaining studies suggest these two matters were negatively related. The merged results of all studies manifested that higher expression increased the risk of higher histopathological grade, however, with significant heterogeneity. In terms of distant metastasis, the final synthetic results also showed heterogeneity which might be caused by the limited studies and small sample.
Concerning the underlying mechanism why SHMT is endowed with the predictive role in cancer patients, the potential reason could be as follows. On the one hand, SHMT is a key enzyme for the production of one carbon unit and is crucial for DNA and methionine synthesis as mentioned previously. On the other hand, SHMT was also implicated in redox homeostasis in the tumor microenvironment and immunology regulation. In the study of Ye et al. (19), SHMT2 was correlated to hypoxia-inducible factor-1α (HIF-1α) in myc-amplified neuroblastoma patient samples and contributed to the survival in an ischemia environment. And it has also been proven that SHMT2 activity weakens pyruvate kinase and consequently lowers oxygen demand and helps to turn into the beneficial metabolic state for survival in poorly vascularized tumor regions (20). Moreover, SHMT1 over-expression can repress the killing effect of natural killer (NK) cells to lung adenocarcinoma cells (21). Accumulative studies have revealed that knockdown of SHMT2 could hinder the proliferation and invasion in tumor cells or animal xenograft models, such as colon cancer cells (22,23), hepatocellular carcinoma (17), breast cancer (24) and so on. While aberrant over-expression of SHMT2 not only reinforces cell proliferation in vitro but potentiates tumor growth in vivo (25).
Considering the incredible importance of SHMT for tumor cells in the growth and reprogramming process, researchers have taken cognizance of the potential of SHMT as a novel drug development target. And several inhibitors have been designed and demonstrated the encouraging anti-tumor efficacy in vitro and xenografts. For example, AGF347, an SHMT2 inhibitor, manifests a significant antitumor efficacy in vivo and both early-stage and upstage MIA PaCa-2 pancreatic tumor xenografts. And 2.12, inhibitor for both the SHMT isoforms, could induce apoptosis in lung cancer cell lines. Additionally, He et al. (26) developed the alanine-scanning-interaction-entropy method that could help calculate protein-ligand binding free energy, identify hot spots and explain the structure-activity relationship beneficial for researchers to design more effective SHMT inhibitors. We are looking forward to furthering studies.
Overall, our study revealed that SHMT could be used as a prognostic factor in clinical practice. Nevertheless, there were a few shortcomings in our study. To begin with, the majority of included studies (9/10) were carried out in Asia and focused on the alimentary system (7/10). Thus, there might be some bias when applying to the rest population. Moreover, some predetermined outcomes were insufficiently reported due to various reasons, and the resultant limited population could mitigate the credibility of the corresponding result, and even caused heterogeneity. Lastly, we did not perform publication bias test for the inadequate number of eligible studies, as it was recommended that the funnel plot was only suitable with no less than 10 studies (5).
Conclusions
SHMT2 may serve as a promising biomarker in various cancer, especially in the alimentary system. Also, it can be a target for drug development. Further large-scale studies are warranted to confirm the possible effect.
Acknowledgments
We would like to extend sincere appreciation to Cindy Li for her help in the grammar and diction revision of this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81802885).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2485/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2485/coif). The authors have no conflicts of interest to declare.
References
- 1.Labuschagne CF, van den Broek NJ, Mackay GM, et al. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep 2014;7:1248-58. 10.1016/j.celrep.2014.04.045 [DOI] [PubMed] [Google Scholar]
- 2.Li AM, Ye J. Reprogramming of serine, glycine and one-carbon metabolism in cancer. Biochim Biophys Acta Mol Basis Dis 2020;1866:165841. 10.1016/j.bbadis.2020.165841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebbring SJ, Chai Y, Ji Y, et al. Serine hydroxymethyltransferase 1 and 2: gene sequence variation and functional genomic characterization. J Neurochem 2012;120:881-90. 10.1111/j.1471-4159.2012.07646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition; Cochrane, 2019. [Google Scholar]
- 6.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 7.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Fang X, Li Y, et al. High Expression of Serine Hydroxymethyltransferase 2 Indicates Poor Prognosis of Gastric Cancer Patients. Med Sci Monit 2019;25:7430-8. 10.12659/MSM.917435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji L, Tang Y, Pang X, et al. Increased Expression of Serine Hydroxymethyltransferase 2 (SHMT2) is a Negative Prognostic Marker in Patients with Hepatocellular Carcinoma and is Associated with Proliferation of HepG2 Cells. Med Sci Monit 2019;25:5823-32. 10.12659/MSM.915754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Yin C, Deng MM, et al. High expression of SHMT2 is correlated with tumor progression and predicts poor prognosis in gastrointestinal tumors. Eur Rev Med Pharmacol Sci 2019;23:9379-92. [DOI] [PubMed] [Google Scholar]
- 11.Ning S, Ma S, Saleh AQ, et al. SHMT2 Overexpression Predicts Poor Prognosis in Intrahepatic Cholangiocarcinoma. Gastroenterol Res Pract 2018;2018:4369253. 10.1155/2018/4369253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi K, Konno M, Koseki J, et al. The mitochondrial one-carbon metabolic pathway is associated with patient survival in pancreatic cancer. Oncol Lett 2018;16:1827-34. 10.3892/ol.2018.8795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B, Wang W, Zhu Z, et al. Mitochondrial serine hydroxymethyltransferase 2 is a potential diagnostic and prognostic biomarker for human glioma. Clin Neurol Neurosurg 2017;154:28-33. 10.1016/j.clineuro.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Bernhardt S, Bayerlová M, Vetter M, et al. Proteomic profiling of breast cancer metabolism identifies SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res 2017;19:112. 10.1186/s13058-017-0905-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyo M, Konno M, Colvin H, et al. The importance of mitochondrial folate enzymes in human colorectal cancer. Oncol Rep 2017;37:417-25. 10.3892/or.2016.5264 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Chen Z, Xue D, et al. Prognostic and therapeutic value of mitochondrial serine hydroxyl-methyltransferase 2 as a breast cancer biomarker. Oncol Rep 2016;36:2489-500. 10.3892/or.2016.5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Deng L, Tang D, et al. miR-615-5p prevents proliferation and migration through negatively regulating serine hydromethyltransferase 2 (SHMT2) in hepatocellular carcinoma. Tumour Biol 2016;37:6813-21. 10.1007/s13277-015-4506-8 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Wei Y, To C, et al. Integrated omic analysis of lung cancer reveals metabolism proteome signatures with prognostic impact. Nat Commun 2014;5:5469. 10.1038/ncomms6469 [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov 2014;4:1406-17. 10.1158/2159-8290.CD-14-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 2015;520:363-7. 10.1038/nature14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Li J, Hu Y, et al. MiR-218-5p Suppresses the Killing Effect of Natural Killer Cell to Lung Adenocarcinoma by Targeting SHMT1. Yonsei Med J 2019;60:500-8. 10.3349/ymj.2019.60.6.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C, Zhang Y, Chen Y, et al. Long noncoding RNA LINC01234 promotes serine hydroxymethyltransferase 2 expression and proliferation by competitively binding miR-642a-5p in colon cancer. Cell Death Dis 2019;10:137. 10.1038/s41419-019-1352-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Wei Z, Song J, Wang G, et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun 2018;9:4468. 10.1038/s41467-018-06812-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li AM, Ducker GS, Li Y, et al. Metabolic Profiling Reveals a Dependency of Human Metastatic Breast Cancer on Mitochondrial Serine and One-Carbon Unit Metabolism. Mol Cancer Res 2020;18:599-611. 10.1158/1541-7786.MCR-19-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong J, Krieger JR, Taylor P, et al. Cancer proteome and metabolite changes linked to SHMT2. PLoS One 2020;15:e0237981. 10.1371/journal.pone.0237981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, Bao J, Yang Y, et al. Study of SHMT2 Inhibitors and Their Binding Mechanism by Computational Alanine Scanning. J Chem Inf Model 2019;59:3871-8. 10.1021/acs.jcim.9b00370 [DOI] [PubMed] [Google Scholar]