Abstract
Purpose
Ankylosing spondylitis (AS) is a risk factor for acute coronary syndrome (ACS). However, the influence of infectious insults, such as endophthalmitis, on the risk of ACS among AS patients has not been studied yet. In this study, we aimed to investigate the relationship between endophthalmitis in patients with AS and the incidence of ACS.
Methods
This retrospective cohort study extracted medical records from the Taiwan Longitudinal Health Insurance Database (LHID) from January 1, 2000, to December 31, 2015. The primary outcome was the incidence of ACS. Univariate and multivariate Cox regression analyses with and without Fine and Gray’s competing risk model and Kaplan–Meier survival curve were used for the analyses. Spearman’s rank correlation coefficient was performed for sensitivity analysis.
Results
We identified 530 AS patients with endophthalmitis and 2,120 AS patients without endophthalmitis for comparison. The incidence rate of endophthalmitis in our study population was 2.66%. The overall incidence rate of ACS was 1,595.96 per 100,000 person-years in AS patients with endophthalmitis and 953.96 per 100,000 person-years in AS patients without endophthalmitis (adjusted HR = 1.787; 95% CI: 1.594–2.104, p < 0.001). In comparison to those without comorbidities, higher adjusted HRs were found in AS patients with endophthalmitis and comorbidities such as diabetes mellitus, hyperlipidemia, hypertension, cerebrovascular accident, congestive heart failure, chronic obstructive pulmonary disease, asthma, and coronary artery disease. Besides, the age ≥ 60 years revealed a high risk for ACS in AS patients with endophthalmitis.
Conclusion
Endophthalmitis was found to be an independent risk factor for ACS in patients with AS. Further clinical studies are required to elucidate the underlying mechanisms and status of systemic inflammation during endophthalmitis.
Keywords: ankylosing spondylitis, acute coronary syndrome, endophthalmitis, inflammation, epidemiology
Introduction
Ankylosing spondylitis (AS), a chronic inflammatory disorder, is a subset of spondyloarthritis. AS patients typically experience symptoms such as inflammatory back pain, morning stiffness lasting for more than 30 minutes, dull and insidious back, and buttock pain, as well as the improvement in pain with exercise and its return when inactive (1). Human leukocyte antigen (HLA)-B27 was found to be strongly associated with AS, contributing to approximately 20% of AS heritability (2). The most common extra-articular manifestations of AS are uveitis, bowel disease, lung, heart, skin, bone, and kidney involvement (3). Previous studies have reported that AS patients have an increased risk of acute coronary syndrome (ACS) (4–6).
Clinically, ACS is one of cardiovascular diseases and includes unstable angina (UA) and acute myocardial infarction (AMI). The incidence rates (IRs) of ACS were 160 to 417 per 100,000 person-years (7–10), whereas the IRs of AMI were 100 to 251 per 100,000 person-years in different countries (7, 8, 10–12). In Taiwan, the age- and gender-adjusted IR of AMI was 50.7 per 100,000 person-years in 2015 (13). ACS is potentially life-threatening and causes major healthcare and economic burden (14). The complicated pathophysiology of ACS originated rupture or erosion of coronary atherosclerotic plaques and then formed acute thrombus with intracoronary stenosis or obstruction. In addition, several studies have reported that inflammation also plays an important role in the pathogenesis of ACS (15, 16).
Endophthalmitis, an intraocular inflammatory disorder, is a severe panuveitis that can lead to persistent vision impairment or visual loss. Previous studies have reported that HLA B27-associated panuveitis could mimic endophthalmitis (17, 18). Additionally, several case reports have shown that AS patients develop endophthalmitis as a complication of systemic infection (19, 20). Furthermore, previous case reports also demonstrated the relation between endophthalmitis and cardiovascular diseases, such as myocarditis and endocarditis (21–23). However, data regarding the prevalence of endophthalmitis in AS patients are scarce. In addition, it is unclear whether endophthalmitis could influence ACS risk among patients with AS. Therefore, the epidemiological study focuses on the endophthalmitis and ACS in AS patients is an important issue. The aim of this study was to investigate the association between endophthalmitis and the incidence of ACS in patients with AS using a million-level database in Taiwan.
Materials and Methods
Data Source
This retrospective population-based cohort study used reimbursement data from the Longitudinal Health Insurance Database (LHID), which randomly sampled 1 million beneficiaries from the original National Health Insurance Research Database (NHIRD) in Taiwan. The representativeness of LHIDs has been validated by the National Health Research Institutes (24). More than 99% of Taiwan’s population (including foreigners) were enrolled in the single-payer National Health Insurance program. The use of NHIRD is limited to research purposes only, and researchers must follow the related laws and regulations in Taiwan. In this study, we extracted fully anonymized medical records from the LHID of 1,914,201 registered beneficiaries from January 1, 2000, to December 31, 2015. All patient demographics (including gender, age, related comorbidities, and index date) were recorded for further analyses.
Ethical Considerations
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB No.: B-110-56). The need for written informed consent from the study participants was waived owing to the fully anonymized data of the NHIRD.
Patient Selection
The study population was identified using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes from the LHID from 2000 to 2015. Patients who had received diagnosis of AS (ICD-9-CM code 720.0) once during hospitalization or at least three times during their outpatient visits were included. The exclusion criteria of this study were age less than 20 years; AS diagnosed before January 1, 2000; ACS before tracking; patients without tracking; or patients with incomplete medical records, defined as those having incomplete insurance status or unrelated or incorrect given codes.
Among the identified AS patients, those who had medical codes of endophthalmitis (ICD-9-CM codes 360.0, 360.00-360.04, 360.1) were further extracted as the study cohort. A comparison cohort that was four-fold the number of the study cohort and matched by gender, age, comorbidities, and index date was selected from the remaining AS patients without endophthalmitis. All patients were followed from the index date until the incidence of ACS (ICD-9-CM code of AMI: 410, and ICD-9-CM code of unstable angina: 411.1, 411.8), the date of withdrawal from the insurance system, or the end of the study period (December 31, 2015).
Comorbidities
Patient comorbidities, including diabetes mellitus (DM), hyperlipidemia, hypertension (HTN), cerebrovascular accident (CVA), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), asthma, coronary artery disease (CAD), cardiomegaly, and metabolic syndrome (MetS) were identified in this cohort study. Comorbidities at baseline were recorded if the patients received medical codes within 1 year before the index date with at least three medical visits. Comorbidities at the endpoint were recorded if patients received medical codes within 1 year before the incidence of ACS with at least three medical visits. The Charlson comorbidity index revised (CCI_R) score, which has been widely used to assess the presence of chronic diseases, was also recorded. The ICD-9-CM codes and definitions used in this study for data extraction and analysis are listed in Table S1.
Statistical Analyses
The characteristics of AS patients with/without endophthalmitis at baseline and at the end of this study were analyzed. Continuous variables are reported as means ± standard deviations, and categorical variables are reported as numbers and percentages. The independent student t-test was used to compare continuous variables between the two study groups. Pearson chi-square and Fisher exact tests were used to evaluate the differences in the categorical variables. The cumulative risk of ACS in AS patients with/without endophthalmitis in the study period of this cohort was illustrated using a Kaplan–Meier survival curve and compared using the log-rank test. Hazard ratios (HRs) of the association between ACS and endophthalmitis, gender, age, CCI_R score, and patient comorbidities were evaluated using the univariate and multivariate Cox regression analyses. The competing-risks data are inherent to medical research in which the occurrence of the event of interest is precluded by another event. For analyzing data with competing events, we chose the measure of a proportional hazards model for the sub-distribution of a competing risk. The Fine and Gray’s competing risk model was applied to investigate the influence of all-cause mortality as a competing risk factor (25). Furthermore, sensitivity analysis with Spearman’s rank correlation coefficient was conducted to evaluate the possible multicollinearity between variables. The analyses with group stratification based on related clinical variables were also conducted. The results were reported as crude and adjusted HRs, 95% confidence intervals, and p-values. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
Results
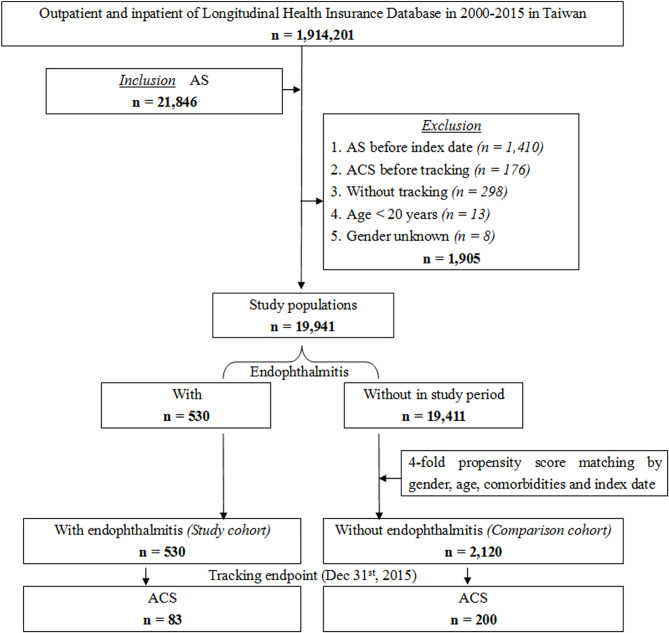
A total of 21,846 patients with AS were identified from the LHID of 1,914,201 registered beneficiaries between 2000 and 2015 in Taiwan. Several patients were excluded from the study for the following reasons: they were diagnosed with AS before the index date (n=1,410), ACS before tracking (n=176), without tracking (n=298), age less than 20 years (n=13), and gender unknown (n=8). Among the remaining 19,941 patients in the study population, 530 patients with endophthalmitis were selected as the study cohort. The incidence rate of endophthalmitis in our study population was 2.66%. We then retrieved a fourfold group (2,120 individuals) matched by gender, age, comorbidities, and index date from 19,411 patients without endophthalmitis as a comparison cohort (Figure 1).
Figure 1.
Flowchart of the patient selection in this cohort.
The baseline demographic characteristics of the enrolled patients are summarized in Table 1. All the listed variables, namely gender, age, DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, CAD, cardiomegaly, MetS, and CCI_R score were not significantly different between the two study groups. Table 2 shows the demographic characteristics of the enrolled patients at the study endpoint. A total of 83 (15.66%) and 200 (9.43%) patients developed ACS in AS patients with and without endophthalmitis, respectively (p < 0.001). Comparing AS patients without endophthalmitis, ACS, CCI_R score, and all-cause mortality were significantly higher in AS patients with endophthalmitis. The mean follow-up period in all patients was 9.85 ± 8.52 years, and no difference was noted between the groups (Table S2). The mean time for ACS development after enrollment was 2.98 ± 3.27 years in the AS with endophthalmitis group, and 3.75 ± 4.16 years in AS without endophthalmitis group (p < 0.001; Table S3).
Table 1.
The baseline demographic characteristics of the enrolled patients.
| Endophthalmitis | Total | With Endophthalmitis | Without Endophthalmitis | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 2,650 | 530 | 20.00 | 2,120 | 80.00 | ||
| Gender | 0.999 | ||||||
| Male | 1,480 | 55.85 | 296 | 55.85 | 1,184 | 55.85 | |
| Female | 1,170 | 44.15 | 234 | 44.15 | 936 | 44.15 | |
| Age (years) | 37.72 ± 19.80 | 37.69 ± 19.17 | 37.78 ± 19.99 | 0.915 | |||
| Age group (yrs) | 0.999 | ||||||
| 20-39 | 1,580 | 59.62 | 316 | 59.62 | 1,264 | 59.62 | |
| 40-59 | 740 | 27.92 | 148 | 27.92 | 592 | 27.92 | |
| ≧60 | 330 | 12.45 | 66 | 12.45 | 264 | 12.45 | |
| DM | 0.630 | ||||||
| Without | 1,984 | 74.87 | 396 | 74.72 | 1,588 | 74.91 | |
| With | 666 | 25.13 | 134 | 25.28 | 532 | 25.09 | |
| Hyperlipidemia | 0.912 | ||||||
| Without | 2,496 | 94.19 | 500 | 94.34 | 1,996 | 94.15 | |
| With | 154 | 5.81 | 30 | 5.66 | 124 | 5.85 | |
| HTN | 0.892 | ||||||
| Without | 2,105 | 79.43 | 420 | 79.25 | 1,685 | 79.48 | |
| With | 545 | 20.57 | 110 | 20.75 | 435 | 20.52 | |
| CVA | 0.946 | ||||||
| Without | 2,505 | 94.53 | 500 | 94.34 | 2,005 | 94.58 | |
| With | 145 | 5.47 | 30 | 5.66 | 115 | 5.42 | |
| CHF | 0.743 | ||||||
| Without | 2,577 | 97.25 | 515 | 97.17 | 2,062 | 97.26 | |
| With | 73 | 2.75 | 15 | 2.83 | 58 | 2.74 | |
| COPD | 0.932 | ||||||
| Without | 2,230 | 84.15 | 446 | 84.15 | 1,784 | 84.15 | |
| With | 420 | 15.85 | 84 | 15.85 | 336 | 15.85 | |
| Asthma | 0.890 | ||||||
| Without | 2,153 | 81.25 | 431 | 81.32 | 1,722 | 81.23 | |
| With | 497 | 18.75 | 99 | 18.68 | 398 | 18.77 | |
| CAD | 0.927 | ||||||
| Without | 2,486 | 93.81 | 497 | 93.77 | 1,989 | 93.82 | |
| With | 164 | 6.19 | 33 | 6.23 | 131 | 6.18 | |
| Cardiomegaly | 0.878 | ||||||
| Without | 2,624 | 99.02 | 525 | 99.06 | 2,099 | 99.01 | |
| With | 26 | 0.98 | 5 | 0.94 | 21 | 0.99 | |
| MetS | 0.946 | ||||||
| Without | 2,560 | 96.60 | 512 | 96.60 | 2,048 | 96.60 | |
| With | 90 | 3.40 | 18 | 3.40 | 72 | 3.40 | |
| CCI_R | 0.93 ± 1.11 | 0.96 ± 1.13 | 0.92 ± 1.11 | 0.175 | |||
CAD, Coronary artery disease; CCI_R, Charlson comorbidity index revised; CHF, Congestive heart failure; COPD, Chronic obstructive pulmonary disease; CVA, Cerebrovascular accident; DM, Diabetes Mellitus; HTN, Hypertension, MetS, Metabolic syndrome.
Table 2.
Demographic characteristics of the enrolled patients at the study endpoint.
| Endophthalmitis | Total | With Endophthalmitis | Without Endophthalmitis | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 2,650 | 530 | 20.00 | 2,120 | 80.00 | ||
| ACS | <0.001** | ||||||
| Without | 2,367 | 89.32 | 447 | 84.34 | 1,920 | 90.57 | |
| With | 283 | 10.68 | 83 | 15.66 | 200 | 9.43 | |
| Gender | 0.999 | ||||||
| Male | 1,480 | 55.85 | 296 | 55.85 | 1,184 | 55.85 | |
| Female | 1,170 | 44.15 | 234 | 44.15 | 936 | 44.15 | |
| Age (yrs) | 41.34 ± 20.47 | 40.66 ± 20.23 | 41.50 ± 20.57 | 0.135 | |||
| Age group (yrs) | 0.842 | ||||||
| 20-39 | 1,481 | 55.89 | 295 | 55.66 | 1,186 | 55.94 | |
| 40-59 | 754 | 28.45 | 148 | 27.92 | 606 | 28.58 | |
| ≧60 | 415 | 15.66 | 87 | 16.42 | 328 | 15.47 | |
| CCI_R | 0.91 ± 1.10 | 0.96 ± 1.17 | 0.90 ± 1.10 | 0.011* | |||
| All-cause mortality | <0.001** | ||||||
| Without | 2,417 | 91.21 | 468 | 88.30 | 1,949 | 91.93 | |
| With | 233 | 8.79 | 62 | 11.70 | 171 | 8.07 | |
ACS, Acute coronary syndrome, CCI_R, Charlson comorbidity index revised.
*p < 0.05. **p< 0.001.
The Kaplan–Meier survival curve of the cumulative risk of ACS in AS patients with/without endophthalmitis is shown in Figure 2. A significantly higher cumulative risk of ACS was noted in AS patients with endophthalmitis than in those without endophthalmitis (p < 0.001; log-rank test).
Figure 2.
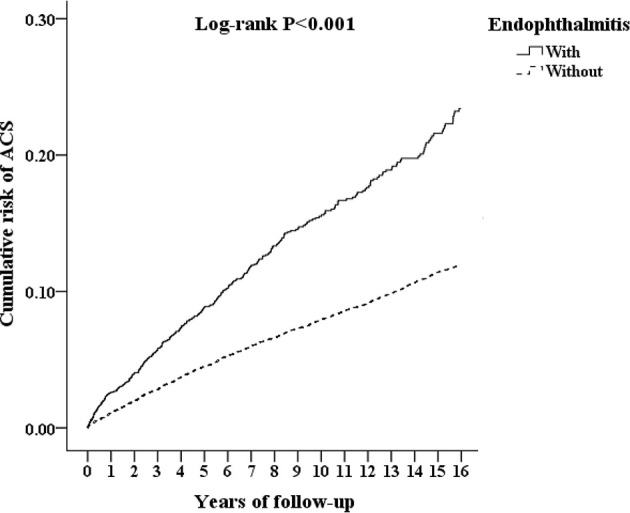
Kaplan–Meier survival curve of the cumulative risk of ACS in AS patients with and without endophthalmitis.
Table 3 shows the risk analysis of ACS using univariate and multivariate Cox regression with and without Fine & Gray’s competing risk model. MetS has severe multicollinearity with DM, Hyperlipidemia, or HTN (Spearman’s rank correlation coefficient r = 0.901, 0.815, and 0.832, respectively; all p < 0.001). This indicated that MetS has high correlation coefficients with DM, Hyperlipidemia, or HTN. Therefore, we excluded MetS from the analysis of adjusted HR in Table 3 and 4. After adjusting for listed variables except MetS, patients with endophthalmitis; those aged 40–59 years and ≥ 60 years, those with DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, CAD, and those with a higher CCI_R score had a significantly higher risk of ACS (all p < 0.001). The statistical significance of all these risk factors persisted with or without the competing risks in the model.
Table 3.
Risk analysis of ACS by Cox regression with/without Fine & Gray’s competing risk model.
| Variables | Without competing risk model | With competing risk model | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P | Adjusted HR (95% CI) | P | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Endophthalmitis | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 1.927 (1.617-2.147) | <0.001** | 1.683 (1.497-1.935) | <0.001** | 2.023 (1.733-2.188) | <0.001** | 1.787 (1.594-2.104) | <0.001** |
| Gender | ||||||||
| Male | 1.239 (0.836-1.657) | 0.319 | 1.234 (0.819-1.638) | 0.320 | 1.280 (0.905-1.824) | 0.204 | 1.249 (0.867-1.810) | 0.250 |
| Female | Reference | Reference | Reference | Reference | ||||
| Age (yrs) | ||||||||
| 20-39 | Reference | Reference | Reference | Reference | ||||
| 40-59 | 1.171 (1.104-1.384) | <0.001** | 1.139 (1.065-1.349) | <0.001** | 1.186 (1.118-1.483) | <0.001** | 1.179 (1.080-1.435) | <0.001** |
| ≧60 | 1.712 (1.275-1.868) | <0.001** | 1.603 (1.217-1.842) | <0.001** | 1.839 (1.295-1.995) | <0.001** | 1.822 (1.313-1.990) | <0.001** |
| DM | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 3.138 (2.161-5.286) | <0.001** | 3.245 (2.201-5.287) | <0.001** | 3.341 (2.515-5.691) | <0.001** | 3.299 (2.311-5.674) | <0.001** |
| Hyperlipidemia | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 2.872 (1.652-4.566) | <0.001** | 2.814 (1.606-4.488) | <0.001** | 3.127 (2.030-5.284) | <0.001** | 3.145 (1.967-5.218) | <0.001** |
| HTN | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 3.341 (2.191-5.399) | <0.001** | 3.293 (2.188-5.396) | <0.001** | 3.636 (2.639-6.125) | <0.001** | 3.571 (2.482-5.860) | <0.001** |
| CVA | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 2.357 (1.447-2.896) | <0.001** | 2.268 (1.319-2.807) | <0.001** | 2.418 (1.489-2.994) | <0.001** | 2.360 (1.452-2.900) | <0.001** |
| CHF | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 2.487 (1.622-3.120) | <0.001** | 2.472 (1.573-3.066) | <0.001** | 2.589 (1.689 -3.160) | <0.001** | 2.501 (1.639-3.172) | <0.001** |
| COPD | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 1.567 (1.116-2.592) | <0.001** | 1.538 (1.101-2.544) | <0.001** | 1.643 (1.167-2.748) | <0.001** | 1.617 (1.142-2.683) | <0.001** |
| Asthma | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 2.044 (1.138-2.814) | <0.001** | 2.045 (1.107-2.762) | <0.001** | 2.126 (1.211-2.915) | <0.001** | 2.059 (1.197-2.899) | <0.001** |
| CAD | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 4.434 (2.425-7.512) | <0.001** | 4.341 (2.329-7.435) | <0.001** | 4.588 (2.540-7.689) | <0.001** | 4.425 (2.358-7.452) | <0.001** |
| Cardiomegaly | ||||||||
| Without | Reference | Reference | Reference | Reference | ||||
| With | 0.000 | 0.999 | 0.000 | 0.999 | 0.000 | 0.999 | 0.000 | 0.999 |
| MetS | ||||||||
| Without | Reference | Multicollinearity with DM, Hyperlipidemia, and HTN | Reference | Multicollinearity with DM, Hyperlipidemia, and HTN | ||||
| With | 2.179 (2.048-2.270) | <0.001** | 2.293 (2.169-2.563) | <0.001** | ||||
| CCI_R | 1.281 (1.211-1.299) | <0.001** | 1.246 (1.201-1.283) | <0.001** | 1.294 (1.226-1.320) | <0.001** | 1.276 (1.203-1.286) | <0.001** |
Adjusted HR means adjusted for variables listed in the table except MetS. Competing risk: All-cause mortality.
ACS, Acute coronary syndrome; CAD, Coronary artery disease; CCI_R, Charlson comorbidity index revised; CHF, Congestive heart failure; CI, confidence interval; COPD, Chronic obstructive pulmonary disease; CVA, Cerebrovascular accident; DM, Diabetes Mellitus; HR, hazard ratio; HTN, Hypertension; MetS, Metabolic syndrome.
**p< 0.001.
Table 4.
Risk analysis of ACS after stratification with variables listed in the table.
| Endophthalmitis | With | Without (Reference) | Without competing risk model | With competing risk model | P for interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | Rate (per 105 PYs) | Events | Rate (per 105 PYs) | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |||||
| Total | 83 | 1,595.96 | 200 | 953.96 | 1.683 (1.497-1.935) | <0.001** | 1.787 (1.594-2.104) | <0.001** | |||||
| Gender | 0.861 | ||||||||||||
| Male | 47 | 1,618.32 | 109 | 930.65 | 1.717 (1.543-2.001) | <0.001** | 1.812 (1.625-2.108) | <0.001** | |||||
| Female | 36 | 1,567.68 | 91 | 983.45 | 1.589 (1.428-1.852) | <0.001** | 1.677 (1.507-1.952) | <0.001** | |||||
| Age (yrs) | <0.001** | ||||||||||||
| 20-39 | 44 | 1,514.57 | 129 | 1,100.94 | 1.373 (1.235-1.601) | <0.001** | 1.453 (1.304-1.685) | <0.001** | |||||
| 40-59 | 23 | 1,568.65 | 56 | 938.44 | 1.561 (1.404-1.818) | <0.001** | 1.648 (1.486-1.917) | <0.001** | |||||
| ≧60 | 16 | 1,929.36 | 15 | 457.21 | 4.139 (3.720-4.822) | <0.001** | 4.369 (3.918-5.084) | <0.001** | |||||
| DM | 0.072 | ||||||||||||
| Without | 64 | 1,647.19 | 183 | 1,067.61 | 1.529 (1.376-1.785) | <0.001** | 1.618 (1.452-1.879) | <0.001** | |||||
| With | 19 | 1,444.63 | 17 | 444.54 | 3.052 (2.744-3.557) | <0.001** | 3.225 (2.889-3.747) | <0.001** | |||||
| Hyperlipidemia | 0.135 | ||||||||||||
| Without | 74 | 1,510.10 | 190 | 945.74 | 1.562 (1.405-1.820) | <0.001** | 1.652 (1.480-1.921) | <0.001** | |||||
| With | 9 | 2,997.10 | 10 | 1,142.53 | 2.980 (2.678-3.473) | <0.001** | 3.148 (2.823-3.659) | <0.001** | |||||
| HTN | 0.097 | ||||||||||||
| Without | 65 | 1,574.88 | 188 | 1,057.77 | 1.505 (1.349-1.751) | <0.001** | 1.588 (1.427-1.846) | <0.001** | |||||
| With | 18 | 1,677.01 | 12 | 375.93 | 3.606 (3.238-4.203) | <0.001** | 3.809 (3.415-4.429) | <0.001** | |||||
| CVA | 0.064 | ||||||||||||
| Without | 77 | 1,567.38 | 195 | 985.69 | 1.583 (1.420-1.844) | <0.001** | 1.682 (1.495-1.942) | <0.001** | |||||
| With | 6 | 2,083.62 | 5 | 422.93 | 4.189 (3.765-4.881) | <0.001** | 4.425 (3.967-5.141) | <0.001** | |||||
| CHF | 0.732 | ||||||||||||
| Without | 81 | 2,164.96 | 199 | 1,101.38 | 1.665 (1.495-1.938) | <0.001** | 1.755 (1.576-2.041) | <0.001** | |||||
| With | 2 | 137.06 | 1 | 34.52 | 1.939 (1.742-2.259) | <0.001** | 2.047 (1.836-2.383) | <0.001** | |||||
| COPD | 0.674 | ||||||||||||
| Without | 75 | 1,717.69 | 189 | 1,044.03 | 1.634 (1.469-1.905) | <0.001** | 1.730 (1.547-2.005) | <0.001** | |||||
| With | 8 | 958.90 | 11 | 384.30 | 2.335 (2.097-2.720) | <0.001** | 2.468 (2.211-2.868) | <0.001** | |||||
| Asthma | 0.583 | ||||||||||||
| Without | 73 | 1,733.32 | 184 | 1,040.22 | 1.672 (1.507-1.950) | <0.001** | 1.768 (1.587-2.052) | <0.001** | |||||
| With | 10 | 1,011.07 | 16 | 488.30 | 1.809 (1.626-2.110) | <0.001** | 1.913 (1.711-2.219) | <0.001** | |||||
| CAD | 0.061 | ||||||||||||
| Without | 76 | 1,556.53 | 193 | 973.29 | 1.586 (1.417-1.839) | <0.001** | 1.679 (1.497-1.935) | <0.001** | |||||
| With | 7 | 2,201.47 | 7 | 616.39 | 3.939 (3.542-4.590) | <0.001** | 4.160 (3.728-4.838) | <0.001** | |||||
| Cardiomegaly | – | ||||||||||||
| Without | 83 | 1,614.04 | 200 | 957.58 | 1.683 (1.497-1.935) | <0.001** | 1.787 (1.594-2.104) | <0.001** | |||||
| With | 0 | 0.00 | 0 | 0.00 | – | – | – | – | |||||
Adjusted HR means adjusted for variables listed in the table. Competing risk: All-cause mortality.
ACS, Acute coronary syndrome; AMI, Acute myocardial infarction; CAD, Coronary artery disease; CCI_R, Charlson comorbidity index revised; CHF, Congestive heart failure; CI, confidence interval; COPD, Chronic obstructive pulmonary disease; CVA, Cerebrovascular accident; DM, Diabetes Mellitus; HR, hazard ratio; HTN, Hypertension. **p< 0.001.
A subgroup analysis of ACS risk between AS patients with/without endophthalmitis stratified by the listed variables was further conducted using the Cox regression with/without Fine & Gray’s competing risk model. The results are presented in Table 4. The overall incidence rate of ACS was 1,595.96 per 100,000 person-years in AS patients with endophthalmitis, and 953.96 per 100,000 person-years in AS patients without endophthalmitis (adjusted HR with competing risk model = 1.787; 95% CI: 1.594–2.104, p < 0.001). Irrespective of the patients with or without any of the stratified variables (gender, age, DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, CAD, and cardiomegaly) and with/without competing risk in the model, the adjusted HRs were significantly elevated in AS patients with endophthalmitis than in AS patients without endophthalmitis (all p < 0.001 for all stratification). With respect to comorbidities, AS patients with endophthalmitis without DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, and CAD had the following adjusted HRs for developing ACS: 1.618, 1.652, 1.588, 1.682, 1.755, 1.730, 1.768, and 1.679, respectively (all p < 0.001). In comparison, AS patients with endophthalmitis combined with these comorbidities had higher adjusted HRs. The adjusted HRs of AS patients with endophthalmitis and comorbidities of DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, and CAD were 3.225, 3.148, 3.809, 4.425, 2.047, 2.468, 1.913, and 4.160, respectively (all p < 0.001). Among all the listed variables, the p for interaction showed a statistical significance between different age groups (p < 0.001).
Discussion
In this study, we found a significantly higher risk of ACS in AS patients with endophthalmitis than in those without endophthalmitis after adjusting for all possible confounding factors and performing stratification analysis. According to the Kaplan–Meier analysis, endophthalmitis significantly increased the cumulative risk of developing ACS in AS patients. Besides, being in the age group of 40–59 years and ≥ 60 years; having DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, and CAD; and having a higher CCI_R score were significant risk factors for the development of ACS in AS patients. In addition, comorbidities, including DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, and CAD were associated with higher adjusted HRs in AS patients with endophthalmitis. Furthermore, the age ≥ 60 years was an important risk factor of ACS in AS patients with endophthalmitis.
The incidence rate of endophthalmitis was 2.66% in AS patients in our study population, which was composed of AS patients without ACS, age ≥ 20 years, and had regular follow-up medical records. Previous studies have reported that the incidence rate of endophthalmitis ranges from 0% to 16.5%, which varies among studies due to the differences in factors such as the definition of endophthalmitis, underlying etiology (post-operation, post-trauma, or dissemination from a systemic or local infection), type of surgical procedure, status of underlying disease, and whether there was illicit IV drug use (26–32). To the best of our knowledge, this is the first study to report the incidence rate of endophthalmitis among patients with AS. However, the results of our study should be interpreted carefully when comparing other incidence studies. Further prospective cohort studies are required to thoroughly investigate the incidence of endophthalmitis among patients with AS.
In this study, we obtained the data of all -cause mortality to exclude the influence of case mortality by using the Fine & Gray’s competing risk model in the study. Interestingly, the mortality rate at the study endpoint increased in AS patient with endophthalmitis. In our previous study, we also found that the mortality rate increased in endophthalmitis comorbid with renal disease, septicemia, pneumonia, and tumors (33). Thus, a further study might be needed to investigate the influence of endophthalmitis on the mortality rates in AS patients.
Age is one of important risk factors of ACS (34). In Table 3, significantly higher adjusted HRs of ACS were noted in age groups of 40–59 years and ≥ 60 years comparing age group of 20–39 years among AS patients (adjusted HRs = 1.179 and 1.822, respectively). Besides, Table 4 shows that the adjusted HRs of ACS in AS patients with endophthalmitis were 1.453, 1.648, and 4.369 in 20–39 years, 40-59 years, and ≥ 60 years age groups, respectively. Furthermore, the p value for interaction has statistical significance between different age groups. This result indicated that the ≥ 60 years age group has higher risk of ACS than other age groups (age 20-39 and age 40-59) in AS patients with endophthalmitis. Overall, these findings might suggest that increased age could increase the risk of ACS in AS patients with endophthalmitis.
Although the etiopathogenesis of how infectious diseases increase ACS risk is unclear, many infectious etiologies have been found to have an association with ACS, including periodontitis (35), cholangitis (36), Helicobacter pylori (37), scrub typhus (38), syphilis (39), human immunodeficiency virus (40), herpes zoster (41), cytomegalovirus (42), hepatitis C virus (43), and coronavirus disease 2019 (44). However, none of the previous studies have investigated the relationship between endophthalmitis and ACS and the combined effect of AS and endophthalmitis on ACS risk. According to our results, the infectious insult caused by endophthalmitis was found to be an independent risk factor for ACS in AS patients. However, this result was established based on a prerequisite that correct ICD coding for ACS. Previous studies demonstrated that the positive predictive values (PPV) was 88% for the diagnosis of AMI and 47.6% for the diagnosis of CAD by using ICD coding in Taiwan’s NHIRD (45, 46). Other supporting factors [coronary intervention, stenting, antiplatelet prescription, and ATC code (C01, C07-C10)] could increase the rate of PPV by using ICD coding (45, 46). In this study, we used ICD-9-CM codes for identification of patients with ACS. Therefore, supporting factors could be used to increase the validity of the reported findings in further study.
The underlying mechanisms of atherosclerotic plaque formation and rupture are multifaceted, and involve both innate and adaptive immune responses. Lipoprotein-driven chronic inflammation of the vascular wall plays a key role in the pathophysiology of ACS (47). Many inflammatory markers, such as interleukin-1 (IL-1), IL-6, monocyte chemoattractant protein-1 (MCP-1), high-sensitivity C-reactive protein, and tumor necrosis factor (TNF)-α were identified as a surrogate of disease severity or a promising therapeutic target of ACS (48–51). In addition, the cytokine levels were found to be elevated during endophthalmitis, including the levels of granulocyte colony stimulating factor, growth-regulated oncogene, interferon gamma (IFN-γ), IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-8, IFN-γ-induced protein 10, MCP-1, MCP-3, macrophage inflammatory protein 1 alpha, IL-1β, transforming growth factor alpha, and TNF-α (52).
Endophthalmitis may increase the risk of ACS in AS patients through multiple mechanisms. Both infectious and non-infectious uveitis have been found to induce elevation of cytokine levels both systemically and locally (53–55). Circulating inflammatory cytokines may contribute to atherosclerosis, plaque rupture, erosion, and thrombosis by inflammatory cell recruitment, production of reactive oxygen species and proteolytic enzymes, and incitement of vascular wall damage and further inflammation (37, 47, 56). An infectious insult may, therefore, accelerate and exacerbate the condition of the cardiovascular disease, especially among susceptible individuals, such as in patients with an underlying chronic inflammatory disease. There is a scarcity of literature regarding the analyses of the systemic response to endophthalmitis. Further clinical studies are required to investigate the mechanisms by which these underlying responses contribute to an increased risk of ACS in patients with AS.
This study had several strengths. First, this study was conducted using a validated nationwide population-based million-level database in Taiwan with a 16-year follow-up period, which provides a good statistical power due to its large sample size and long follow-up period. Second, variables such as gender, age, and comorbidities were matched between the study groups. Additionally, patient comorbidities, including DM, hyperlipidemia, HTN, CVA, CHF, COPD, asthma, CAD, and cardiomegaly were adjusted during the statistical analysis. Third, univariate and multivariate Cox regression analyses were performed and Fine and Gray’s competing risk model was used to adjust for possible confounding factors in this study.
However, this study has several limitations. The causal relationship between endophthalmitis in patients with AS and ACS development was difficult to extrapolate due to the study’s retrospective cohort design. Another limitation is that all study participants were from LHID, which is largely composed of the Taiwanese population; consequently, the influence of different countries and ethnicities was not considered in this study. In our previous study, we have used ICD coding as the basis for a diagnosis of endophthalmitis to evaluate the epidemiology and mortality-related prognostic factors in endophthalmitis (33). However, these ICD codes have not been validated in National Health Insurance Research Database (NHIRD) in Taiwan. Thus, the accuracy of ICD coding for a diagnosis of endophthalmitis is one of our limitations. In addition, the disease severity, clinical features, and underlying etiologies of both AS and endophthalmitis could not be obtained from this fully anonymized database. In present study, we considered endophthalmitis as infectious events regardless of the etiologies or subtypes. However, endophthalmitis can be divided into endogenous and exogenous subtype according to the etiologies. Therefore, one limitation is that the study did not analyze the influence of endogenous and exogenous endophthalmitis on the risk of ACS in AS patients. Moreover, the medications that were prescribed were not stratified in this study, which may have influenced the incidence of ACS and endophthalmitis (either protective or exacerbating). Finally, data from laboratory examinations and medical imaging, such as X-ray, magnetic resonance imaging, or fundus images, were lacking in the NHIRD database. All study participants were identified using the ICD-9-CM codes. Misdiagnosis or coding errors cannot be completely ruled out in the current study design.
In conclusion, endophthalmitis is associated with an increased risk of ACS in patients with AS, irrespective of the listed clinical variables. Owing to increased potential cardiovascular risk, physicians should keep an eye on AS patients with a history of endophthalmitis, especially for those with underlying comorbidities. Further clinical studies are required to elucidate the underlying mechanisms and status of systemic inflammation during endophthalmitis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Tri-Service General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization: T-YL, W-CC, and C-LC. Methodology: W-CC, Y-FL, and C-HC. Software: W-CC, C-AS, and C-HC. Literature search: T-YL, and C-LC. Data acquisition and curation: W-CC, Y-FL, and C-HC. Data analysis: T-YL, W-CC, C-AS, C-HC, and C-LC. Validation: T-YL, W-CC, C-HC, and C-LC. Investigation: T-YL, Y-HC, and J-TC. Manuscript preparation: T-YL. Manuscript editing: T-YL and C-LC.Visualization: W-CC, Y-FL, and C-HC. Supervision: W-CC, Y-HC, C-AS, C-HC, J-TC, and C-LC. Project administration: W-CC and C-LC. Funding acquisition: C-LC. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-D-109110, TSGH-D-110112, and VTA111-V1-1-2). The sponsor has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW), Taiwan, for providing the National Health Insurance Research Database (NHIRD).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.843796/full#supplementary-material
The ICD-9-CM codes, and definitions used in this study for data extraction and analysis.
Years of follow-up.
Years to ACS.
References
- 1. Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med (2016) 374(26):2563–74. doi: 10.1056/NEJMra1406182 [DOI] [PubMed] [Google Scholar]
- 2. Chen B, Li J, He C, Li D, Tong W, Zou Y, et al. Role of Hla-B27 in the Pathogenesis of Ankylosing Spondylitis (Review). Mol Med Rep (2017) 15(4):1943–51. doi: 10.3892/mmr.2017.6248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Maghraoui A. Extra-Articular Manifestations of Ankylosing Spondylitis: Prevalence, Characteristics and Therapeutic Implications. Eur J Intern Med (2011) 22(6):554–60. doi: 10.1016/j.ejim.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 4. Chou CH, Lin MC, Peng CL, Wu YC, Sung FC, Kao CH, et al. A Nationwide Population-Based Retrospective Cohort Study: Increased Risk of Acute Coronary Syndrome in Patients With Ankylosing Spondylitis. Scand J Rheumatol (2014) 43(2):132–6. doi: 10.3109/03009742.2013.822097 [DOI] [PubMed] [Google Scholar]
- 5. Eriksson JK, Jacobsson L, Bengtsson K, Askling J. Is Ankylosing Spondylitis a Risk Factor for Cardiovascular Disease, and How Do These Risks Compare With Those in Rheumatoid Arthritis? Ann Rheum Dis (2017) 76(2):364–70. doi: 10.1136/annrheumdis-2016-209315 [DOI] [PubMed] [Google Scholar]
- 6. Bengtsson K, Forsblad-d’Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, et al. Are Ankylosing Spondylitis, Psoriatic Arthritis and Undifferentiated Spondyloarthritis Associated With an Increased Risk of Cardiovascular Events? A Prospective Nationwide Population-Based Cohort Study. Arthritis Res Ther (2017) 19(1):102. doi: 10.1186/s13075-017-1315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen KM, Foldspang A, Larsen ML, Gerdes LU, Rasmussen S, Faergeman O. Estimating the Incidence of the Acute Coronary Syndrome: Data From a Danish Cohort of 138 290 Persons. Eur J Cardiovasc Prev Rehabil (2007) 14(5):608–14. doi: 10.1097/HJR.0b013e328278522f [DOI] [PubMed] [Google Scholar]
- 8. Taylor MJ, Scuffham PA, McCollam PL, Newby DE. Acute Coronary Syndromes in Europe: 1-Year Costs and Outcomes. Curr Med Res Opin (2007) 23(3):495–503. doi: 10.1185/030079906x167462 [DOI] [PubMed] [Google Scholar]
- 9. Antoniades L, Christodoulides T, Georgiou P, Hadjilouca C, Christodoulou E, Papasavas E, et al. Epidemiology of Acute Coronary Syndromes in the Mediterranean Island of Cyprus (Cypacs Study, Cyprus Study of Acute Coronary Syndromes). Hellenic J Cardiol (2014) 55(2):139–49. [PubMed] [Google Scholar]
- 10. Ogata S, Marume K, Nakai M, Kaichi R, Ishii M, Ikebe S, et al. Incidence Rate of Acute Coronary Syndrome Including Acute Myocardial Infarction, Unstable Angina, and Sudden Cardiac Death in Nobeoka City for the Super-Aged Society of Japan. Circ J (2021) 85(10):1722–30. doi: 10.1253/circj.CJ-20-1207 [DOI] [PubMed] [Google Scholar]
- 11. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population Trends in the Incidence and Outcomes of Acute Myocardial Infarction. N Engl J Med (2010) 362(23):2155–65. doi: 10.1056/NEJMoa0908610 [DOI] [PubMed] [Google Scholar]
- 12. Wong CX, Sun MT, Lau DH, Brooks AG, Sullivan T, Worthley MI, et al. Nationwide Trends in the Incidence of Acute Myocardial Infarction in Australia, 1993-2010. Am J Cardiol (2013) 112(2):169–73. doi: 10.1016/j.amjcard.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 13. Lee CH, Fang CC, Tsai LM, Gan ST, Lin SH, Li YH. Patterns of Acute Myocardial Infarction in Taiwan From 2009 to 2015. Am J Cardiol (2018) 122(12):1996–2004. doi: 10.1016/j.amjcard.2018.08.047 [DOI] [PubMed] [Google Scholar]
- 14. Makki N, Brennan TM, Girotra S. Acute Coronary Syndrome. J Intensive Care Med (2015) 30(4):186–200. doi: 10.1177/0885066613503294 [DOI] [PubMed] [Google Scholar]
- 15. Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. New Engl J Med (2005) 352(16):1685–95. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Liu Z, Shao J, Lin L, Jiang M, Wang L, et al. Immune and Inflammation in Acute Coronary Syndrome: Molecular Mechanisms and Therapeutic Implications. J Immunol Res (2020) 2020:4904217. doi: 10.1155/2020/4904217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanghvi C, Mercieca K, Jones NP. Very Severe Hla B27-Associated Panuveitis Mimicking Endophthalmitis: A Case Series. Ocul Immunol Inflammation (2010) 18(2):139–41. doi: 10.3109/09273940903560236 [DOI] [PubMed] [Google Scholar]
- 18. Toh ZH, Agrawal R. Diagnostic Dilemma: Unilateral Panuveitis Mimicking Endophthalmitis in Very Severe Hla B27-Associated Uveitis. Am J Ophthalmol Case Rep (2020) 17:100589. doi: 10.1016/j.ajoc.2020.100589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gopalamurugan AB, Wheatcroft S, Hunter P, Thomas MR. Bilateral Endophthalmitis and Ards Complicating Group G Streptococcal Endocarditis. Lancet (2005) 366(9502):2062. doi: 10.1016/S0140-6736(05)67817-8 [DOI] [PubMed] [Google Scholar]
- 20. Yin W, Zhou H, Li C. Endogenous Klebsiella Pneumoniae Endophthalmitis. Am J Emergency Med (2014) 32(10):1300.e3–.e5. doi: 10.1016/j.ajem.2014.03.038 [DOI] [PubMed] [Google Scholar]
- 21. Filipowicz A, Coca MN, Blair BM, Chang PY. Acute Myocarditis With Cardiogenic Shock and Multiple Organ Failure, Followed by Bilateral Panuveitis Masquerading as Endogenous Endophthalmitis, Due to Toxoplasma Gondii in an Immunocompetent Patient. Retin Cases Brief Rep (2021) 15(5):575–80. doi: 10.1097/icb.0000000000000855 [DOI] [PubMed] [Google Scholar]
- 22. Nakata M, Mashidori T, Higa N, Manita M, Chibana N, Tabata K. Infective Endocarditis With No Underlying Disease for Which Bacterial Endophthalmitis Have Been the First Symptom. Intern Med (2020) 59(16):2061–5. doi: 10.2169/internalmedicine.6083-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitaka H, Gomez T, Perlman DC. Scleritis and Endophthalmitis Due to Streptococcus Pyogenes Infective Endocarditis. Am J Med (2020) 133(1):e15–e6. doi: 10.1016/j.amjmed.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 24. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s National Health Insurance Research Database: Past and Future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/clep.S196293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fine JP. Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 26. Ahmed Y, Schimel AM, Pathengay A, Colyer MH, Flynn HW., Jr. Endophthalmitis Following Open-Globe Injuries. Eye (Lond) (2012) 26(2):212–7. doi: 10.1038/eye.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Incidence of Endophthalmitis After Vitrectomy: A Systematic Review and Meta-Analysis. Retina (2019) 39(5):844–52. doi: 10.1097/iae.0000000000002055 [DOI] [PubMed] [Google Scholar]
- 28. Hou CH, Lee JS, Lin KK, Chang SH, Huang WK, Kuo CF, et al. Endophthalmitis Incidence of Cancer Patients After Cataract Surgery: A Nationwide Matched Cohort Study in Taiwan. Am J Ophthalmol (2019) 199:246–54. doi: 10.1016/j.ajo.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 29. Gounder PA, Hille DM, Khoo YJ, Phagura RS, Chen FK. Endogenous Endophthalmitis in Western Australia: A Sixteen-Year Retrospective Study. RETINA (2020) 40(5):908–18. doi: 10.1097/iae.0000000000002512 [DOI] [PubMed] [Google Scholar]
- 30. Gondhale H, Jaichandran VV, Jambulingam M, Anand AR, Srinivasan S, Raman R, et al. Distribution and Risk Factors of Postoperative Endophthalmitis in People With Diabetes. Indian J Ophthalmol (2021) 69(11):3329–34. doi: 10.4103/ijo.IJO_1485_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malmin A, Syre H, Ushakova A, Utheim TP, Forsaa VA. Twenty Years of Endophthalmitis: Incidence, Aetiology and Clinical Outcome. Acta Ophthalmol (2021) 99(1):e62–e9. doi: 10.1111/aos.14511 [DOI] [PubMed] [Google Scholar]
- 32. Mir TA, Papudesu C, Fang W, Hinkle DM. Incidence of Drug Use–Related Endogenous Endophthalmitis Hospitalizations in the United States, 2003 to 2016. JAMA Ophthalmol (2021) 139(1):18–26. doi: 10.1001/jamaophthalmol.2020.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weng TH, Chang HC, Chung CH, Lin FH, Tai MC, Tsao CH, et al. Epidemiology and Mortality-Related Prognostic Factors in Endophthalmitis. Invest Ophthalmol Vis Sci (2018) 59(6):2487–94. doi: 10.1167/iovs.18-23783 [DOI] [PubMed] [Google Scholar]
- 34. Majeed F, Kelemen MD. Acute Coronary Syndromes in the Elderly. Clin Geriatr Med (2007) 23(2):425–40, viii. doi: 10.1016/j.cger.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 35. Gwon JG, Choi J, Kim SH, Kim SH, Ryu JJ, Cho DH, et al. Risk of Acute and Chronic Coronary Syndrome in a Population With Periodontitis: A Cohort Study. Oral Dis (2021) 00:1–8. doi: 10.1111/odi.13816 [DOI] [PubMed] [Google Scholar]
- 36. Tsai MS, Li YF, Lin CL, Hsu YC, Lee PH, Sung FC, et al. Long-Term Risk of Acute Coronary Syndrome in Patients With Cholangitis: A 13-Year Nationwide Cohort Study. Eur J Intern Med (2014) 25(5):444–8. doi: 10.1016/j.ejim.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 37. Fang Y, Fan C, Xie H. Effect of Helicobacter Pylori Infection on the Risk of Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Med (Baltimore) (2019) 98(50):e18348. doi: 10.1097/md.0000000000018348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung WS, Lin CL, Hsu WH, Kao CH. Scrub Typhus Increases the Risk of Developing Acute Coronary Syndrome: A Nationwide Cohort Study. Heart (2014) 100(23):1844–50. doi: 10.1136/heartjnl-2014-306181 [DOI] [PubMed] [Google Scholar]
- 39. Barbosa-Barros R, Pérez-Riera AR, Koivula K, de Carvalho Santos J, de Abreu LC, Nikus K. Acute Coronary Syndrome of Very Unusual Etiology. Ann Noninvasive Electrocardiol (2018) 23(5):e12531. doi: 10.1111/anec.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boccara F, Mary-Krause M, Potard V, Teiger E, Lang S, Hammoudi N, et al. Hiv Infection and Long-Term Residual Cardiovascular Risk After Acute Coronary Syndrome. J Am Heart Assoc (2020) 9(17):e017578. doi: 10.1161/jaha.119.017578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang CC, Lin CL, Chang YJ, Wang GJ, Sung FC, Kao CH. Herpes Zoster Infection Associated With Acute Coronary Syndrome: A Population-Based Retrospective Cohort Study. Br J Dermatol (2014) 170(5):1122–9. doi: 10.1111/bjd.12768 [DOI] [PubMed] [Google Scholar]
- 42. Nikitskaya E, Lebedeva A, Ivanova O, Maryukhnich E, Shpektor A, Grivel JC, et al. Cytomegalovirus-Productive Infection Is Associated With Acute Coronary Syndrome. J Am Heart Assoc (2016) 5(8):e003759. doi: 10.1161/jaha.116.003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, Bagchi S. Risk of Cardiovascular Disease Due to Chronic Hepatitis C Infection: A Review. J Clin Transl Hepatol (2017) 5(4):343–62. doi: 10.14218/jcth.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA. Bohula EA. A Current Review of Covid-19 for the Cardiovascular Specialist. Am Heart J (2020) 226:29–44. doi: 10.1016/j.ahj.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of Algorithms to Identify Stroke Risk Factors in Patients With Acute Ischemic Stroke, Transient Ischemic Attack, or Intracerebral Hemorrhage in an Administrative Claims Database. Int J Cardiol (2016) 215:277–82. doi: 10.1016/j.ijcard.2016.04.069 [DOI] [PubMed] [Google Scholar]
- 46. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of Acute Myocardial Infarction Cases in the National Health Insurance Research Database in Taiwan. J Epidemiol (2014) 24(6):500–7. doi: 10.2188/jea.je20140076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of Plaque Formation and Rupture. Circ Res (2014) 114(12):1852–66. doi: 10.1161/circresaha.114.302721 [DOI] [PubMed] [Google Scholar]
- 48. Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory Biomarkers in Acute Coronary Syndromes: Part I: Introduction and Cytokines. Circulation (2006) 113(6):e72–5. doi: 10.1161/circulationaha.105.595520 [DOI] [PubMed] [Google Scholar]
- 49. Oudi ME, Aouni Z, Mazigh C, Khochkar R, Gazoueni E, Haouela H, et al. Homocysteine and Markers of Inflammation in Acute Coronary Syndrome. Exp Clin Cardiol (2010) 15(2):e25–8. [PMC free article] [PubMed] [Google Scholar]
- 50. Crea F, Liuzzo G. Anti-Inflammatory Treatment of Acute Coronary Syndromes: The Need for Precision Medicine. Eur Heart J (2016) 37(30):2414–6. doi: 10.1093/eurheartj/ehw207 [DOI] [PubMed] [Google Scholar]
- 51. Nanchen D, Klingenberg R, Gencer B, Räber L, Carballo D, von Eckardstein A, et al. Inflammation During Acute Coronary Syndromes - Risk of Cardiovascular Events and Bleeding. Int J Cardiol (2019) 287:13–8. doi: 10.1016/j.ijcard.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 52. Deshmukh D, Chakrabarti M, Jayasudha R, Hasnat Ali M, Tyagi M, Sharma S, et al. Elevated Cytokine Levels in Vitreous as Biomarkers of Disease Severity in Infectious Endophthalmitis. PloS One (2018) 13(10):e0205292. doi: 10.1371/journal.pone.0205292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lacomba MS, Martin CM, Chamond RR, Galera JMG, Omar M, Estevez EC. Aqueous and Serum Interferon Γ, Interleukin (Il) 2, Il-4, and Il-10 in Patients With Uveitis. Arch Ophthalmol (2000) 118(6):768–72. doi: 10.1001/archopht.118.6.768 [DOI] [PubMed] [Google Scholar]
- 54. Takase H, Futagami Y, Yoshida T, Kamoi K, Sugita S, Imai Y, et al. Cytokine Profile in Aqueous Humor and Sera of Patients With Infectious or Noninfectious Uveitis. Invest Ophthalmol Visual Sci (2006) 47(4):1557–61. doi: 10.1167/iovs.05-0836 [DOI] [PubMed] [Google Scholar]
- 55. Lahmar I, Abou-Bacar A, Abdelrahman T, Guinard M, Babba H, Ben Yahia S, et al. Cytokine Profiles in Toxoplasmic and Viral Uveitis. J Infect Dis (2009) 199(8):1239–49. doi: 10.1086/597478 [DOI] [PubMed] [Google Scholar]
- 56. Pober JS. Warner-Lambert/Parke-Davis Award Lecture. Cytokine-Mediated Activation of Vascular Endothelium. Physiology and Pathology. Am J Pathol (1988) 133(3):426–33. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The ICD-9-CM codes, and definitions used in this study for data extraction and analysis.
Years of follow-up.
Years to ACS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.