Abstract
A metallo-β-lactamase determinant was cloned from a genomic library of Legionella (Fluoribacter) gormanii ATCC 33297T constructed in the plasmid vector pACYC184 and transformed into Escherichia coli DH5α, by screening for clones showing a reduced susceptibility to imipenem. The product of the cloned determinant, named FEZ-1, contains a 30-kDa polypeptide and exhibits an isoelectric pH of 7.6. Sequencing revealed that FEZ-1 is a molecular-class B β-lactamase which shares the closest structural similarity (29.7% of identical residues) with the L1 enzyme of Stenotrophomonas maltophilia, being a new member of the highly divergent subclass B3 lineage. All the residues that in L1 are known to be directly or indirectly involved in coordination of the zinc ions were found to be conserved also in FEZ-1, suggesting that the geometry of zinc coordination in the active site of the latter enzyme is identical to that of L1. Unlike L1, however, FEZ-1 appeared to be monomeric in gel permeation chromatography experiments and exhibited a distinctive substrate specificity with a marked preference for cephalosporins and meropenem. The properties of FEZ-1 overall resembled those of a β-lactamase previously purified from the same strain of L. gormanii (T. Fujii, K. Sato, K. Miyata, M. Inoue, and S. Mitsuhashi, Antimicrob. Agents Chemother. 29:925–926, 1986) and are as yet unique among class B enzymes, reinforcing the notion that considerable functional heterogeneity can be encountered among members of this class. A system for overexpression of the blaFEZ-1 gene in E. coli, based on the T7 phage promoter, was also developed.
Metallo-β-lactamases (molecular-class B, group 3 of the functional classification [7]) are monomeric or oligomeric proteins that require a metal cofactor (Zn2+ in native enzymes) for activity and are structurally and evolutionarily unrelated to active-site serine β-lactamases (6, 13). The relevance of metallo-β-lactamases as resistance effectors is mostly due to their substrate specificity, which always includes carbapenems and often also cephalosporins and penicillins, and to their resistance to mechanism-based serine β-lactamase inhibitors (6, 12, 19, 20, 26).
Several metallo-β-lactamases have been sequenced (4, 18, 26, 27, 29, 33). From a structural standpoint these enzymes appear to be clustered into three different evolutionary lineages: subclass B1 (including most known metallo-β-lactamases), subclass B2 (including the Aeromonas enzymes), and subclass B3 (including the highly divergent L1 enzyme from Stenotrophomonas maltophilia) (26). From a functional standpoint most of these enzymes exhibit a broad substrate specificity (subgroup 3a in the functional classification), whereas the Aeromonas enzymes behave as rather specific carbapenemases (subgroup 3b in the functional classification) (6, 26).
Additional metallo-β-lactamases have been purified and partially characterized from Myroides odoratus (formerly Flavobacterium odoratum) (30), Legionella (Fluoribacter) gormanii (14), and Burkholderia cepacia (3), but their definitive attribution to molecular-class B awaits the confirmation of sequence data. Among these, the enzyme of L. gormanii exhibits distinctive biochemical properties compared to other metallo-β-lactamases, with preferential activity for cephalosporin compounds (14). This warranted its inclusion in a further subgroup (subgroup 3c) of the functional classification (6, 26).
In this study we cloned and characterized a metallo-β-lactamase determinant from L. gormanii ATCC 33297T, whose product overall resembles the enzyme that was previously purified from this strain (14). Sequencing revealed that the L. gormanii metallo-β-lactamase is a new member of the highly divergent subclass B3 lineage of class B β-lactamases.
(These results were presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1999.)
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
L. gormanii ATCC 33297T was used as the donor strain for construction of the genomic library. Escherichia coli DH5α (Gibco-BRL, Bethesda, Md.) was used as the host for genetic vectors and recombinant plasmids. E. coli BL21(DE3)(pLysS) (Novagen Inc., Madison, Wis.) was used as a host for overexpression of the L. gormanii blaFEZ-1 gene cloned in the expression vector pET-24. Plasmid pACYC184 (10) was used as the vector for construction of the L. gormanii genomic library. Plasmid pBC-SK (Stratagene, La Jolla, Calif.) was used for some subcloning steps. Plasmid pET-24 (Novagen) was used as a T7-based vector for tightly regulated overexpression of the blaFEZ-1 gene in E. coli.
Antibiotics and β-lactamase substrates.
Antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. Imipenem and cefoxitin were from Merck Research Laboratories (Rahway, N.J.), meropenem was from Zeneca Pharmaceuticals (Macclesfield, Cheshire, United Kingdom), cefuroxime and ceftazidime were from Glaxo-Wellcome (Verona, Italy), cefotaxime was from Hoechst-Marion-Roussel (Frankfurt, Germany), and aztreonam was from Bristol-Myers Squibb Co. (Wallingford, Conn.). Nitrocefin was from Unipath (Milan, Italy).
In vitro susceptibility testing.
In vitro susceptibility of the E. coli clone carrying the cloned blaFEZ-1 metallo-β-lactamase determinant was determined by a broth macrodilution method (22), using Mueller-Hinton medium (Difco Laboratories, Detroit, Mich.) and a bacterial inoculum of 105 CFU per tube. Results were recorded after incubation at 28°C for 24 h.
β-Lactamase assays.
β-Lactamase activity in crude E. coli cell extracts was assayed spectrophotometrically. Reactions were performed in phosphate-buffered saline (PBS), pH 7.3, at 25°C, in a total volume of 0.75 ml. The initial substrate concentrations were 100 μM for cephalosporins, 150 μM for carbapenems, and 1 mM for penicillins. For the indicated compounds, wavelengths and changes in the extinction coefficient, respectively, were as follows: imipenem, 299 nm and −9,000 M−1 cm−1; meropenem, 297 nm and −6,500 M−1 cm−1; nitrocefin, 482 nm and +15,000 M−1 cm−1; cefuroxime, 260 nm and −7,600 M−1 cm−1; cefotaxime, 260 nm and −7,500 M−1 cm−1; cefoxitin, 260 nm and −7,800 M−1 cm−1; penicillin G, 235 nm and −800 M−1 cm−1; and ampicillin, 235 nm and −820 M−1 cm−1. Inhibition of enzymatic activity by EDTA or dipicolinic acid was determined by measuring the residual activity after incubation of the crude extract, for 15 min at 25°C, in the presence of variable concentrations of each chelating agent. Crude cell extracts were prepared as follows. Cells were grown in Luria-Bertani broth (28) aerobically at 28°C, collected by centrifugation, resuspended in PBS (1/10 of the original culture volume), and disrupted by sonication (six times for 15 s each, at 50 W). The supernatant obtained after centrifugation at 10,000 × g for 10 min, to remove cell debris, represented the crude extract. Protein concentration in solution was determined with a commercial protein assay kit (Bio-Rad, Richmond, Calif.) with bovine serum albumin as a standard.
Protein analysis techniques.
Analytical isoelectric focusing (IEF) of crude cell extracts was performed in precast 5% polyacrylamide gels containing ampholites (pH range, 3.5 to 9.5) (Ampholine PAGplate; Amersham Pharmacia Biotech, Uppsala, Sweden) using a Multiphor II Apparatus (Pharmacia). Gels were focused at 0.1 W/cm2 for 2 h at 10°C. β-Lactamases were detected as pink bands after overlaying the gel with filter paper soaked with a 0.25 mM nitrocefin solution in 30 mM ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]–NaOH buffer, pH 7.0, supplemented with 2 mM ZnCl2. Zymogram detection of β-lactamase activity after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of protein preparations was performed essentially as previously described (21). After the initial renaturation treatment, the gel was equilibrated in PBS for 20 min at 37°C, and β-lactamase activity was revealed by the appearance of pink bands after overlaying the gel with filter paper soaked with a 0.25 mM nitrocefin solution in PBS.
Recombinant DNA methodology.
Basic recombinant DNA procedures were performed essentially as described by Sambrook et al. (28). Extraction of the genomic DNA from L. gormanii was performed as described previously (16) from cells grown on BCYEα agar plates (Oxoid Ltd., Basingstoke, United Kingdom) at 37°C in an aerobic atmosphere enriched with 5% CO2. For construction of the library, genomic DNA was partially digested with Sau3AI, and fragments in the 4- to 12-kb size range were purified by agarose gel electrophoresis using the Geneclean II kit (Bio-101, La Jolla, Calif.). The purified restriction fragments were ligated to BamHI-linearized and dephosphorylated pACYC184 vector, and the ligation mixture was used to transform E. coli DH5α by electroporation using a Gene Pulser apparatus (Bio-Rad) according to the manufacturer's instructions. The ratio of recombinant clones to those carrying an empty religated vector was >5, as shown by replica plating of transformants onto plates containing both chloramphenicol (85 μg/ml) and tetracycline (20 μg/ml). The system for overexpression of the blaFEZ-1 gene in E. coli was prepared as follows. The blaFEZ-1 open reading frame (ORF) and some downstream region were amplified by PCR using primers BLAFEZ-FN (5′-GGAATTCCATATGAAAAAAGTATTAAGTTTAAC), which added an NdeI linker overlapping the blaFEZ-1 start codon (in boldface type), and BLAFEZ-RB (5′-GCGGATCCTTTGACCAATATG), which was designed 0.35 kb downstream of the blaFEZ-1 stop codon and contained a BamHI linker. The 1.2-kb amplimer was digested with BamHI, cloned in plasmid pBC-SK digested with BamHI and EcoRV (the resulting recombinant plasmid was named pBLL/FEZ1), and subjected to confirmatory sequencing. The 1.2-kb NdeI-BamHI fragment of pBLL/FEZ1 was then subcloned in pET-24, and the resulting recombinant plasmid, named pET-24/FEZ1, was transformed into E. coli BL21(DE3)(pLysS). The expression experiments with E. coli BL21(DE3)(pLysS)(pET-24/FEZ1) were performed in duplicate. In these experiments the strain was grown aerobically at 28°C in Luria-Bertani medium containing chloramphenicol (70 μg/ml) and kanamycin (50 μg/ml). For induction, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a 0.5 mM final concentration when the culture turbidity achieved an A600 of 0.8 to 0.9.
DNA sequencing and computer analysis of sequence data.
DNA sequences were determined by the dideoxy-chain termination method using an automatic DNA sequencer (LICOR 4000; LI-COR Inc., Lincoln, Nebr.), the Thermosequenase DNA sequencing kit (Amersham), and IRD 800-labeled custom sequencing primers (MWG-Biotech, Munich, Germany). Sequences were determined on both strands, using denatured double-stranded DNA templates. Similarity searches against sequence databases were performed using an updated version of the BLAST program (1). Computer analysis of sequence data was performed using an updated version (8.1) of the Wisconsin Package (version 8.1; Genetics Computer Group Inc., Madison, Wis.) available at the Italian EMBL node of Bari. Multiple sequence alignments were generated with the help of the PILEUP program of the Genetics Computer Group package and manually refined considering the information available on the three-dimensional structures of the Bc-II, CcrA, and L1 enzymes (8, 9, 11, 31).
Gel permeation chromatography.
Gel permeation chromatography was performed on a Superdex-75 column (1 by 30 cm; Pharmacia). The column was equilibrated and eluted in 10 mM sodium cacodylate buffer, pH 6.5, containing 0.2 M NaCl, and calibrated by determining the retention volume of different protein standards. The proteins were eluted at a flow rate of 1 ml/min, and the absorbance at 280 nm was recorded. A crude extract containing the FEZ-1 protein (300 μl), prepared from an induced culture of E. coli BL21(DE3)(pLysS)(pET-24/FEZ1), was eluted at the same flow rate. Fractions of 1 ml were collected, and the presence of the β-lactamase activity was monitored by testing for cefuroxime hydrolysis.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank/DDBJ sequence databases and assigned the accession no. Y17896.
RESULTS
Cloning of a metallo-β-lactamase determinant from L. gormanii ATCC 33297T.
A metallo-β-lactamase determinant was isolated from a genomic library of L. gormanii ATCC 33297T constructed in the pACYC184 plasmid vector and transformed in E. coli DH5α, by screening for clones showing a reduced susceptibility to imipenem. For this purpose, individual clones grown on a medium containing only chloramphenicol (for vector selection) were replica plated onto a medium containing chloramphenicol plus imipenem (5 μg/ml). One clone growing on this medium (clone 8AI) was isolated out of approximately 15,000 screened recombinants. Growth of this clone on the imipenem-containing medium was very slow, with colonies 1 mm in diameter being visible only after 4 to 5 days of incubation at 28°C.
Measurement of the β-lactamase activity present in the crude extract of clone 8AI showed an increased hydrolytic activity against carbapenems and nitrocefin compared to the basal levels observed with the parent E. coli strain. Specific activity with meropenem was higher than that with imipenem (Table 1). The carbapenemase activity present in the crude extract was inhibited by EDTA and dipicolinic acid, with 50% inhibitory concentrations of 1 and 0.01 mM, respectively, suggesting a metal-dependent nature for the enzyme.
TABLE 1.
β-Lactamase activity in the crude extract of clone 8AI (DH5α[pLLB-8AI]) with nitrocefin (NCF), imipenem (IMI), and meropenem (MEM)a
| Strain | Sp act (pmol/min/μg of protein)
|
||
|---|---|---|---|
| NCF | IMI | MEM | |
| DH5α(pLLB-8AI) | 386 ± 20 | 27 ± 2 | 132 ± 7 |
| DH5α(pACYC184) | <10 | <5 | <5 |
The basal activity of E. coli DH5α carrying an empty vector is also shown for comparison. Crude extracts were prepared from cells collected in the late-exponential phase of growth (A600, 1.5 to 1.8). Specific activities are the mean values of three measurements ± standard deviations.
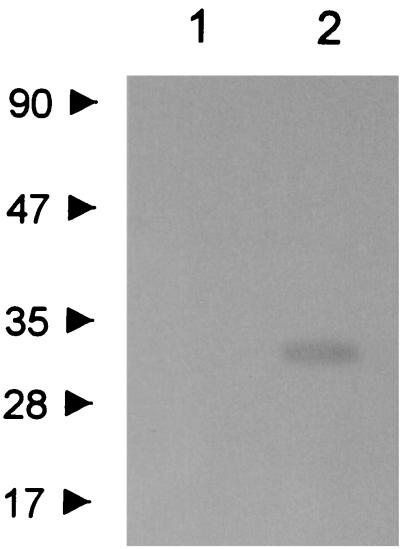
IEF analysis of the crude extract of clone 8AI yielded a band of β-lactamase activity focusing at pH 7.6, which was not detectable in the parent E. coli strain (results not shown). A zymogram analysis of the crude extract, performed after renaturing SDS-PAGE, yielded a band of β-lactamase activity at approximately 30 kDa which was not detectable in the parent E. coli strain (Fig. 1).
FIG. 1.
Results of zymogram analysis performed after renaturing SDS-PAGE using the chromogenic cephalosporin nitrocefin as the substrate for detection of β-lactamase activity. Protein size standards (in kilodaltons) are indicated on the left. Lanes: 1, crude extract from E. coli DH5α(pACYC184); 2, crude extract from E. coli DH5α(pLLB-8AI).
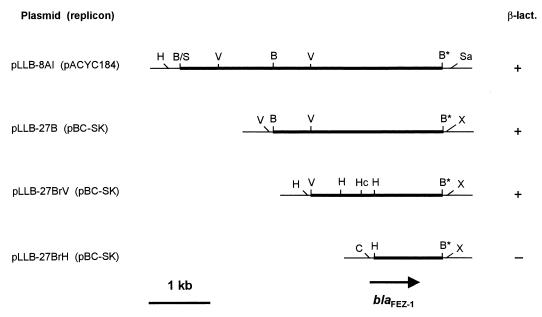
Restriction mapping of the recombinant plasmid harbored by clone 8AI, named pLLB-8AI, revealed the presence of a 4.3-kb DNA insert. Subcloning analysis showed that the β-lactamase determinant was apparently located within a 2.2-kb BamHI-EcoRV fragment (Fig. 2). The origin of the cloned β-lactamase determinant from the chromosome of L. gormanii ATCC 33297T was confirmed by Southern blot experiments in which the 2.7-kb BamHI insert of plasmid pLLB-27B (Fig. 2) was probed against the Legionella genomic DNA. The probe hybridized with the band of undigested chromosomal DNA, with a single restriction fragment of 7.9 kb after digestion with BamHI and with two restriction fragments of 8.8 and 1.5 kb, respectively, after digestion with EcoRV (data not shown).
FIG. 2.
Physical map of the insert of plasmid pLLB-8AI and subcloning strategy. Thick lines represent cloned DNA, while thin lines correspond to vector sequences. Production of metallo-β-lactamase activity (β-lact.) was assayed on crude extracts prepared from cells collected in the late-exponential phase of growth (A600, 1.5 to 1.8), using meropenem as the substrate. The location of the blaFEZ-1 ORF is also indicated. The BamHI site labeled with an asterisk was generated after cloning of the Sau3AI genomic DNA fragment in the BamHI site of the plasmid vector and is not present in the Legionella chromosomal DNA, as indicated by the results of Southern blot experiments (see text). Abbreviations for restriction enzymes: B, BamHI; B/S, BamHI/Sau3AI junction; H, HindIII; Hc, HincII; Sa, SalI; V, EcoRV; X, XbaI.
Sequence of the cloned metallo-β-lactamase determinant.
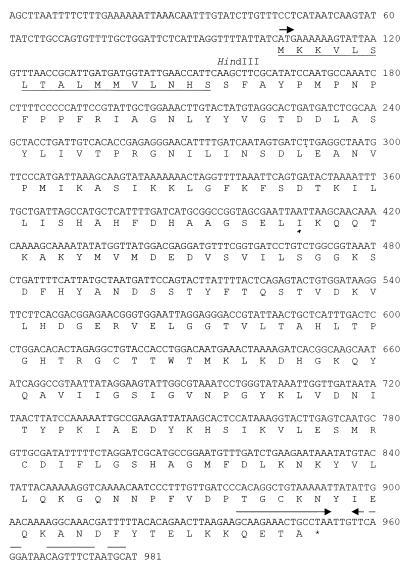
The 2.7-kb insert of plasmid pLLB-27B (Fig. 2) was sequenced. Analysis of sequence data revealed the presence of an 849-bp ORF (Fig. 3) encoding a protein which, in a BLAST search, showed the highest sequence similarity (29.3% identical residues) with the L1 enzyme of S. maltophilia and weaker similarities with other class B β-lactamases. Results of subcloning experiments (Fig. 2) were consistent with the identification of this ORF as the metallo-β-lactamase determinant, which was named blaFEZ-1. The blaFEZ-1 ORF encodes a 282-amino-acid polypeptide whose amino-terminal sequence exhibits features typical of procaryotic signal peptides targeting protein secretion into the periplasmic space via the general secretory pathway (Fig. 3). According to known patterns (24), the cleavage site could be located after the Ser-17 residue. In this case the calculated molecular mass and pI value of the mature protein would be 29,567 Da and 7.71, respectively, which are in agreement with the results of analytical IEF and of zymograms performed after renaturing SDS-PAGE (see above). The low G+C content of the blaFEZ-1 ORF (37.8%) is consistent with the average values reported for the genomes of Legionellaceae (5).
FIG. 3.
Nucleotide sequence of the blaFEZ-1 ORF and flanking regions. The initiation codon of the blaFEZ-1 ORF is indicated, and protein translation is reported below the sequence. The putative signal peptide for protein secretion is underlined. An inverted repeat overlapping the termination codon of the blaFEZ-1 gene, possibly functioning as a transcriptional terminator, is overlined by arrows.
Comparison of the FEZ-1 enzyme with other metallo-β-lactamases.
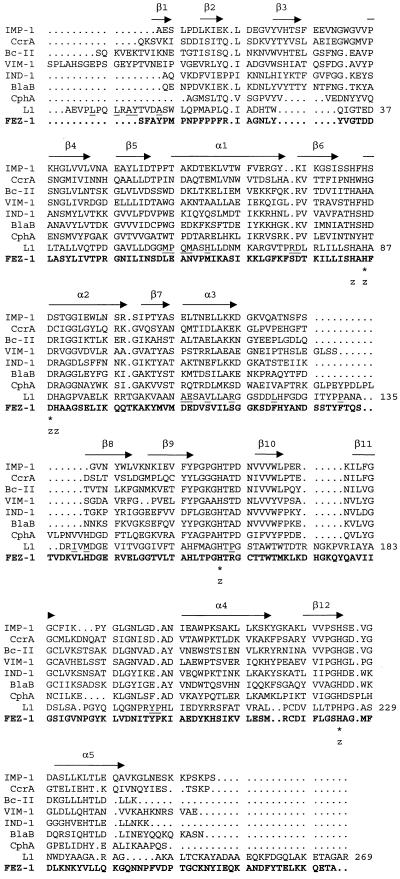
A multiple sequence alignment analysis with other class B β-lactamases confirmed the closest similarity of the L. gormanii FEZ-1 enzyme with the S. maltophilia metallo-β-lactamase. FEZ-1 and L1 could be aligned over the entire sequence without introducing major gaps (Fig. 4), and the percent identity between the two (29.7%) was considerably higher than those between FEZ-1 and the other class B enzymes (Table 2). Compared to L1, the major differences of FEZ-1 are represented by the absence of the N-terminal 310 helix region that, in L1, is positioned prior to β1, and by an insertion within the C-terminal α5 helix which, in L1, is already considerably elongated in comparison with Bc-II and CcrA (31) (Fig. 4).
FIG. 4.
Comparison of the FEZ-1 amino acid sequence (in boldface type) with those of other molecular-class B β-lactamases. The numbering scheme refers to the L1 enzyme (31). Identical residues are indicated by an asterisk. Residues of the L1 enzyme involved in binding of Zn2+ are indicated by a z, and those involved in inter-subunit interactions are underlined (31). Secondary structure elements of Bc-II (9) are also indicated, above the sequences. Abbreviations: IMP-1, IMP-1 enzyme encoded by the blaIMP gene cassette found in Serratia marcescens TN9106 (23) and in other gram-negative strains (2, 17); CcrA, CcrA enzyme of Bacteroides fragilis TAL3636 (25); Bc-II, β-lactamase II of Bacillus cereus 569/H (15); VIM-1, VIM-1 enzyme encoded by the blaVIM gene cassette found in Pseudomonas aeruginosa VR-143/97 (18); IND-1, IND-1 enzyme of Chryseobacterium indologenes 001 (4); BlaB, BlaB enzyme of Chryseobacterium meningosepticum CCUG4310 (27); CphA, CphA enzyme of Aeromonas hydrophila AE036 (21); L1, L1 enzyme of S. maltophilia IID 1275 (32).
TABLE 2.
Pairwise comparison of percent amino acid sequence identity between class B β-lactamasesa
| Enzyme | % Amino acid identity with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CcrA | Bc-II | VIM-1 | IND-1 | BlaB | CphA | L1 | FEZ-1 | |
| IMP-1 | 37.7 | 37.1 | 31.4 | 32.2 | 31.1 | 24.2 | 15.7 | 13.9 |
| CcrA | 33.6 | 31.6 | 29.8 | 28.6 | 27.6 | 15.9 | 8.2 | |
| Bc-II | 38.7 | 35.0 | 26.4 | 30.1 | 18.9 | 13.3 | ||
| VIM-1 | 28.1 | 26.3 | 26.2 | 16.0 | 7.7 | |||
| IND-1 | 45.2 | 26.8 | 13.4 | 14.9 | ||||
| BlaB | 28.4 | 11.5 | 14.8 | |||||
| CphA | 16.1 | 13.7 | ||||||
| L1 | 29.7 | |||||||
Enzyme names are explained in the legend to Fig. 4.
Of the six invariant residues shared by the other class B enzymes (His-86, Asp-88, Gly-91, His-160, Gly-195, and His-225, in the numbering of the L1 enzyme of S. maltophilia IID 1275 [31]), four (His-86, Asp-88, His-160, and His-225) are conserved also in FEZ-1, while Gly-91 and Gly-195 are replaced by an alanine and an aspartate residue, respectively. All the residues known to be involved in Zn2+ binding in the L1 enzyme (His-84, His-86, Asp-88, His-89, His-160, and His-225 [31]) are conserved in the Legionella enzyme. At position 185, FEZ-1 contains a serine residue, similar to the L1 enzyme but unlike the enzymes of molecular subclasses B1 and B2 (Fig. 4).
Patterns of β-lactam susceptibility of E. coli producing the L. gormanii metallo-β-lactamase.
The susceptibility to several β-lactams of E. coli DH5α(pLLB-8AI), which carries the cloned blaFEZ-1 determinant and produces the enzyme (Fig. 2), was compared to that of the same E. coli host carrying an empty vector.
Production of the FEZ-1 enzyme was associated with a decrease of the in vitro susceptibility to various cephalosporins (including cephalothin, cefoxitin, cefuroxime, cefotaxime, and ceftazidime) and meropenem. The susceptibility to imipenem was only slightly affected, and that to penicillins and aztreonam was apparently unaffected (Table 3). The above results suggested a marked preferential activity of the FEZ-1 enzyme for cephalosporins and meropenem. The ability of DH5α(pLLB-8AI) to grow (albeit very slowly) on the medium used to screen the library, containing imipenem at a higher concentration (5 μg/ml) than the MIC, was likely due to the larger bacterial inoculum used in the replica plating procedure and to the intrinsic poor stability of imipenem contained in the medium.
TABLE 3.
In vitro susceptibility to various β-lactams of E. coli DH5α(pLLB-8AI), which contains the cloned blaFEZ-1 determinant, and of E. coli DH5α carrying the empty vector
| β-Lactam | MIC (μg/ml) for:
|
|
|---|---|---|
| DH5α(pLLB-8AI) | DH5α(pACYC184) | |
| Ampicillin | 2 | 2 |
| Carbenicillin | 4 | 4 |
| Piperacillin | 1 | 1 |
| Cephalothin | 32 | 4 |
| Cefoxitin | 32 | 2 |
| Cefuroxime | 32 | 4 |
| Cefotaxime | 4 | 0.06 |
| Ceftazidime | 0.50 | 0.12 |
| Imipenem | 0.25 | 0.12 |
| Meropenem | 0.25 | 0.03 |
| Aztreonam | 0.12 | 0.12 |
Overexpression of the blaFEZ-1 determinant in E. coli.
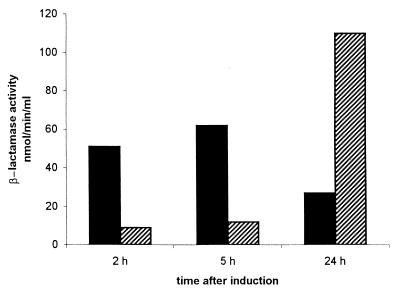
A system for overexpression of the blaFEZ-1 gene in E. coli was developed. In preliminary experiments, the use of expression systems based on the strong T7 phage promoter but characterized by relatively high basal levels of expression of the heterologous gene yielded unstable clones that eventually contained rearranged vectors and did not overproduce the enzyme. Therefore, a tightly regulated expression system was used in which the blaFEZ-1 gene was cloned in the pET-24 vector and the resulting plasmid, pET-24/FEZ1, was introduced into the E. coli host BL21(DE3)(pLysS). Using this system, production of the FEZ-1 enzyme was detectable only upon induction with IPTG. In cell extracts prepared from induced cultures, the meropenem-hydrolyzing activity achieved a value of 377 ± 19 pmol/min/μg of protein at 2 h after induction, retained a similar value (383 ± 21 pmol/min/μg of protein) at 5 h after induction, and was found to be consistently decreased (107 ± 6 pmol/min/μg of protein) after 24 h, whereas in cell extracts prepared from uninduced cultures it remained lower than 5 pmol/min/μg of protein during the same time course. Interestingly, at 24 h after induction, a consistent amount of β-lactamase activity was detected in the culture supernatant, revealing that a progressive leakage of the enzyme in the medium occurred during prolonged growth. In fact, the global amount of activity present in the culture was found to be maximal at 24 h after induction (Fig. 5). In analytical IEF and renaturing SDS-PAGE experiments, the β-lactamase activity produced by E. coli BL21(DE3)(pLysS)(pET-24/FEZ1) was apparently identical to that produced by DH5α(pLLB-8AI) (data not shown).
FIG. 5.
β-Lactamase activity measured in different fractions of a culture of E. coli BL21(DE3)(pLysS)(pET-24/FEZ1) at different times after induction with IPTG. Activities were assayed using meropenem as the substrate and are relative to culture volumes. ▄, activity in the cell fraction; , activity in the culture supernatant.
Relative hydrolysis rates, measured with a crude cell extract prepared from an induced culture of E. coli BL21(DE3)(pLysS)(pET-24/FEZ1), were as follows: nitrocefin, 100; cefuroxime, 206; cefoxitin, 105; cefotaxime, 71; meropenem, 33; imipenem, 7; penicillin G, 9; and ampicillin, 3.
In experiments of gel permeation chromatography performed with a crude FEZ-1 preparation from E. coli BL21(DE3)(pLysS)(pET-24/FEZ1), an Mr of 30,000 was calculated for the metallo-β-lactamase activity, suggesting that the native FEZ-1 enzyme is found in a monomeric form (data not shown).
DISCUSSION
Results of this study showed that L. gormanii ATCC 33297T carries an apparently resident class B β-lactamase determinant whose product exhibits a distinctive preference for cephalosporin substrates. A similar feature remains as yet unique among class B enzymes (4, 6, 18, 26, 27) and reinforces the notion that considerable functional heterogeneity can be encountered among members of this class.
Sequencing of the cloned determinant revealed that its product is a new member of the highly divergent subclass B3 lineage, which thus far has included only one member, namely, the L1 enzyme of S. maltophilia (6, 26). The degree of sequence similarity between FEZ-1 and L1, which approaches 30% identical residues, suggests that the three-dimensional folding of the FEZ-1 molecule is probably very similar to that recently described for L1 (31). Moreover, the complete conservation of the residues that in L1 are known to be directly or indirectly involved in coordination of the two zinc ions suggests that the geometry of zinc coordination in the active site of FEZ-1 is the same as that in L1, being different from that observed in Bc-II and CcrA (8, 9, 11, 31). Since the two cysteine residues that in L1 (at positions 218 and 246) contribute an intramolecular disulfide bridge unique among class B enzymes (31) are conserved in FEZ-1, a similar disulfide bridge is likely to be retained also in the latter molecule. One of the major differences between FEZ-1 and L1 is represented by the absence, in the former, of the long N-terminal region which, in L1, plays a prominent role in providing the interactions between the A and C and between the B and D subunits (31). This difference could be responsible for the monomeric rather than oligomeric structure of the native FEZ-1 enzyme, although other differences found at positions that in L1 are known to participate in the subunit assembly (31) (Fig. 4) could also provide a contribution in this sense.
Results of this work also indicated that the blaFEZ-1 gene can be expressed in E. coli, resulting in a functional product. Overproduction of the enzyme in E. coli, however, was found to be difficult using expression systems that are characterized by a high-level basal expression of the heterologous gene, and such overproduction could be obtained only with a tightly regulated expression system. This system could facilitate production of consistent amounts of the FEZ-1 enzyme, which, owing to its unique features, appears to be an interesting candidate for further structural and enzymological investigations. An analysis of the kinetic parameters of FEZ-1 with a broad array of β-lactam substrates and with various chelating agents and other inhibitors is currently under way.
ACKNOWLEDGMENTS
This work was supported by the European research network on metallo-β-lactamases within the Training and Mobility of Researchers program (contract FMRX-CT98-0232) and by grant 98.00510.CT04 from the Italian National Research Council (CNR).
We also thank Jean Denis Docquier for helpful discussions and acknowledge the excellent technical support of Michela Cappelli and Tiziana Di Maggio.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter I A, Lambert P A. Isolation and partial purification of a carbapenem-hydrolysing metallo-β-lactamase from Pseudomonas cepacia. FEMS Microbiol Lett. 1994;122:251–256. doi: 10.1111/j.1574-6968.1994.tb07176.x. [DOI] [PubMed] [Google Scholar]
- 4.Bellais S, Leotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolysing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. doi: 10.1111/j.1574-6968.1999.tb13422.x. [DOI] [PubMed] [Google Scholar]
- 5.Brenner D J, Feeley J C, Weaver R E. Family VII. Legionellaceae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The William & Wilkins Co.; 1984. p. 279. [Google Scholar]
- 6.Bush K. Metallo-β-lactamases: a class apart. Clin Infect Dis. 1998;27:S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 7.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfi A, Duée E, Galleni M, Frère J-M, Dideberg O. 1.85 Å resolution structure of the zinc(II) β-lactamase from B. cereus. Acta Crystallog Sect D. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 9.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J-M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereusreveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concha N, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 12.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuel L, Frère J-M. An overview of the kinetic parameters of class B β-lactamases. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frère J M. Beta-lactamases and bacterial resistance to antibiotics. Mol Microbiol. 1995;16:385–395. doi: 10.1111/j.1365-2958.1995.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujii T, Sato K, Miyata K, Inoue M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Legionella gormanii. Antimicrob Agents Chemother. 1986;29:925–926. doi: 10.1128/aac.29.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain M, Carlino A, Madonna M J, Lampen J O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J L. Similarity analysis of DNAs. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 655–682. [Google Scholar]
- 17.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosaintegron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauretti L, Riccio M L, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini G M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosaclinical isolate. Antimicrob Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore D M, Williams J D. β-Lactams: mode of action and mechanisms of bacterial resistance. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The William & Wilkins Co.; 1996. pp. 502–578. [Google Scholar]
- 21.Massidda O, Rossolini G M, Satta G. The Aeromonas hydrophyla cphAgene: molecular heterogeneity among metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 23.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescensthat shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilisTAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J-M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticumcarbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sanschagrin F, Dufresne J, Levesque R C. Molecular heterogeneity of the L-1 metallo-β-lactamase family from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:1245–1248. doi: 10.1128/aac.42.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato K, Fujii T, Okamoto R, Inoue M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Flavobacterium odoratum. Antimicrob Agents Chemother. 1985;27:612–614. doi: 10.1128/aac.27.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullah J H, Walsh T R, Taylor I A, Emery D C, Verma C S, Gamblin S J, Spencer J. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophiliaat 1.7 Å resolution. J Mol Biol. 1998;284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T R, Hall L, Assinder S J, Nichols W W, Cartwright S J, MacGowan A P, Bennet P M. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Walsh T R, Neville W A, Haran M H, Tolson D, Payne D J, Bateson J H, MacGowan A P, Bennett P M. Nucleotide and amino acid sequences of the metallo-β-lactamase, ImiS, from Aeromonas veroniibv. sobria. Antimicrob Agents Chemother. 1998;42:436–439. doi: 10.1128/aac.42.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]