Abstract
Axial spondyloarthritis is a chronic inflammatory rheumatic disease that affects the axial skeleton and causes severe pain and disability. It may be also associated with extra-articular manifestations. Early diagnosis and appropriate treatment can reduce the severity of the disease and the risk of progression. The biological disease-modifying antirheumatic drugs (bDMARDs) tumor necrosis factor alpha (TNFα) inhibitors (TNFi) and the anti-interleukin (IL)-17A antibodies secukinumab and ixekizumab are effective agents to reduce disease activity and minimize the inflammation that damages the joints. New alternatives such as Janus kinase (JAK) inhibitors are also available. Unfortunately, response rates to bDMARDs are far from optimal, and many patients experience so-called treatment failure. The definition of treatment failure definition is often vague and may depend on the rigorousness of the therapeutic goal, the inclusion or not of peripheral symptoms/extra-articular manifestations, or patients’ overall health. After an exhaustive bibliographic review, we propose a definition based on loss of the following status: low disease activity assessed by Ankylosing Spondylitis Disease Activity Score (ASDAS)-CRP, absence of extra-articular manifestations, and low disease impact on the patients’ general health. Apart from discontinuing the therapy because of safety or intolerance reasons, two types of treatment failure can be differentiated depending on when it occurs: primary failure (no response within 6 months after treatment initiation, or lack of efficacy) and secondary failure (response within 6 months but lost thereafter, or loss of efficacy over time). Physicians should carefully consider the moment and the reason for the treatment failure to decide the next therapeutic step. In the case of primary failure on a first TNFi, it seems reasonable to switch to another class of drugs, i.e., an anti-IL-17 agent, as phase III trials showed that the response to IL-17 blockade was higher than to placebo in patients previously exposed to TNFi. When secondary failure occurs, and loss of efficacy is suspected to be caused by antidrug antibodies (ADAs), it is advisable to analyze serum TNFi and ADAs concentrations, if possible; in the presence of ADAs and low TNFi levels, changing the TNFi is rational as it may restore the TNFα blocking capacity. If ADAs are absent/low with adequate drug therapeutic levels, switching to another target might be the best strategy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02064-x.
Keywords: Axial spondyloarthritis, Treatment failure, TNF inhibitors, Secukinumab, Ixekizumab, Disease activity
Key Summary Points
| Axial spondyloarthritis is a chronic inflammatory disease that causes severe pain and disability. |
| The biologic agents TNFα inhibitors (TNFi) and anti-interleukin (IL)-17A antibodies have demonstrated efficacy to reduce disease activity and risk of progression, but some patients experiment lack (primary treatment failure) or loss (secondary treatment failure) of response. |
| As the definition of treatment failure is often vague, here we propose a definition based on loss of the following status: low disease activity according to Ankylosing Spondylitis Disease Activity Score (ASDAS)-CRP, absence of extra-articular manifestations, and low disease impact on the patients’ general health. |
| Physicians should carefully consider the moment and the reason for the treatment failure to decide the next therapeutic step. The main options are targeting the same biologic pathway (changing between TNFi) or switching to another class of drug (anti-IL-17). |
Introduction
The term spondyloarthritis (SpA) designates a group of chronic inflammatory conditions that share pathophysiological, genetic, and clinical characteristics. The global prevalence of SpA ranges from 0.2% to 1.61% in the general population, with higher rates in North America and Europe which may correspond to the prevalence of the HLA-B27 allele, the most important genetic predisposition factor in SpA [1, 2]. The Assessment of Spondyloarthritis International Society (ASAS) classified SpA as either axial or peripheral depending on the predominant involvement: while axial SpA mostly affects the spine and sacroiliac joints (axial skeleton), peripheral SpA predominantly affects the peripheral skeleton (arthritis, enthesitis, or dactylitis). In line with the ASAS classification, axial SpA encompasses two subsets of patients: those with radiographic sacroiliitis visible on X-rays (ankylosing spondylitis [AS] or radiographic axial SpA [r-axSpA]) and those without evidence of radiographic damage of the sacroiliac joints (non-radiographic axial SpA [nr-axSpA]) [3]. Additionally, axial SpA may be associated with extra-articular manifestations, including uveitis, psoriasis, and inflammatory bowel disease [4].
Axial SpA has a significant impact on a patient’s life, leading to reduction of physical function and health-related quality of life. In addition, it generates substantial societal and economic burden for the healthcare systems because of the high direct costs derived from the frequent use of health resources and the indirect costs associated with the loss of work productivity [5, 6]. Thus, early diagnosis and treatment to prevent progressive structural damage and disability are crucial for managing patients with axial SpA. Nonsteroidal anti-inflammatory drugs (NSAIDs), regular exercise, and physical therapy are the recommended first-line interventions for patients with active disease [7, 8]; however, not all patients achieve adequate control of the disease with this strategy or tolerate high and/or prolonged doses of NSAIDs, so a significant number of them will require therapy escalation. The most effective agents currently available are biological disease-modifying antirheumatic drugs (bDMARDs): tumor necrosis factor alpha inhibitors (TNFi) and the monoclonal antibodies against interleukin (IL)-17A secukinumab and ixekizumab. A new therapeutic class for the treatment of axial SpA, Janus kinase inhibitors (JAKi), has also been approved. Unfortunately, response rates to bDMARDs are far from optimal, and many patients (about 40%) experience treatment failure [9]. Subsequent management is challenging, and the practicing clinician should carefully consider the moment and the reason for discontinuing the first bDMARD to decide the next therapeutic step [10].
In order to facilitate this process, we provide a narrative review based on a focused literature search (see the appendix in the electronic supplementary material) of available therapies and treatment strategies used in the management of axial SpA, and we propose a definition of treatment failure according to disease activity. We also discuss the different approaches to address treatment failure and areas of uncertainty related to this matter. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Second-Line Therapy in Axial SpA: bDMARDs and JAK1i
The TNFi approved for patients with axial SpA are infliximab (and its biosimilars, although this one is not approved for the use in nr-axSpA), etanercept (and its biosimilars), adalimumab (and its biosimilars), golimumab, and certolizumab pegol. As shown in Table 1, all the TNFi currently authorized for the indication of active axial SpA with inadequate response to NSAIDs showed a significant superiority compared to placebo in terms of ASAS20 response rates and other outcomes in clinical trials of 12–24 weeks of duration [11–16]. Additional studies indicated that TNFi maintain their safety and efficacy for several years [17–19], and delayed structural progression [20, 21], whereas the rates of discontinuing the first TNFi owing to lack of or loss of efficacy range from 13% to 68% [22].
Table 1.
Characteristics of clinical trials with bDMARDs and JAKi currently approved for active axial SpA
| Patient sample | Primary endpoint | Primary endpoint (vs placebo) | |
|---|---|---|---|
| Radiographic axial SpA | |||
| Infliximab | N = 279 | ASAS20 at week 24 | 61.2% (vs 19.2%) |
| Etanercept | N = 277 | ASAS20 at week 12 | 59% (vs 28.0%) |
| Adalimumab | N = 315 | ASAS20 at week 12 | 58.2% (vs 20.6%) |
| Golimumab | N = 216 | ASAS20 at week 14 | 59.4% (vs 21.8%) |
| Certolizumab pegol | N = 178 (20.2% with prior TNFi exposure) | ASAS20 at week 12 |
200 mg every 2 weeks: 56.9% 400 mg every 4 weeks: 64.3% (vs 36.8%) |
| Secukinumab | N = 590 (26.0–39.0% of patients had inadequate responses/intolerance to TNFi) | ASAS20 at week 16 |
61% (vs 29.0%) TNFi-naïve: 68.2% (vs 31.1%) TNFi-exposed: 50.0% (vs 24.1%) |
| Ixekizumab |
N = 251 (not previously been treated with bDMARDs) N = 316 (previously treated with TNFi) |
ASAS40 at week 16 |
48.0% (vs 18.0%) 25.4% (vs 12.5%) |
| Upadacitinib | N = 187 | ASAS40 at week 14 | 52% (vs 26.0%) |
| Non-radiographic axial SpA | |||
| Adalimumab | N = 185 | ASAS40 at week 12 | 36% (vs 15.0%) |
| Etanercept | N = 215 | ASAS40 at week 12 | 32% (vs 16.0%) |
| Golimumab | N = 197 | ASAS20 at week 16 | 71.1% (vs 40.0%) |
| Certolizumab pegol | N = 147 (10.9% with prior TNFi exposure) | ASAS20 at week 12 |
200 mg every 2 weeks: 58.7% 400 mg every 4 weeks: 62.7% (vs 40.0%) |
| Secukinumab | N = 371 (9.7% patients previously exposed to TNFi) | ASAS40 at week 16 naïve population | 41.5% (vs 29.2%) |
| Ixekizumab | N = 201 | ASAS40 at week 16 | 35% (vs 19.0%) |
Data correspond to the approved dosing regimen of each agent
ASAS Assessment of Spondyloarthritis International Society, SpA spondyloarthritis, TNFi TNFα inhibitor
With an alternative mechanism for disrupting inflammation, anti-IL-17 agents extended the therapeutic options for patients with both radiographic and non-radiographic axial SpA. The efficacy of the IL-17 blockade in r-axSpA was demonstrated in several phase III clinical trials (Table 1). Firstly secukinumab [23], and later ixekizumab [24], showed efficacy for the treatment of axial SpA, with similar magnitude to that observed with TNFi. In the first studies exploring the anti-IL-17 effect after TNFi failure, higher response rates were obtained with anti-IL-17 drugs than with placebo [25, 26]. Furthermore, absence of radiographic progression was observed in 79–89% of patients treated with anti-IL-17 agents [27, 28]. Recent studies in patients with nr-axSpA showed that secukinumab [29] and ixekizumab [30] demonstrated similar efficacy to that in patients with r-axSpA (Table 1).
Upadacitinib, a selective JAK1i, is currently the only agent of this therapeutic class approved for r-axSpA, on the basis of a randomized, double-blind, placebo-controlled phase II/III study [31]. Significantly more patients treated with upadacitinib (vs placebo) achieved an ASAS40 response (Table 1), showing a rapid onset of benefit. The trial did not include biologic-exposed patients nor patients with nr-axSpA [31], although preliminary data of the SELECT-AXIS 2 (NCT04169373) supports its efficacy in this latest population.
The ASAS/European League Against Rheumatism (EULAR) [7] and the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network (ACR/SAA/SPARTAN) [8] guidelines recommended TNFi as first-line bDMARDs, mainly owing to longer clinical experience and greater familiarity with their safety profile. However, subsequent guidelines updates should consider the latest evidence, and this advice may change when new real-life data and long-term results of clinical trials on anti-IL-17 agents and JAKi are taken into account. There is also a lack of data regarding the use of TNFi after anti-IL-17 blockade.
Disease Activity Measures in Axial SpA
The drug efficacy evaluated in the aforementioned clinical trials described was based on ASAS20 or ASAS40 response rates, but these endpoints do not reflect the final disease states of the patients after a period of treatment. Ideally, the measures used to assess disease activity in ax-SpA should be easily implementable in clinical practice and relevant for both patients and physicians. A classic composite measure of disease activity is the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which collects six patient-reported variables evaluating clinical symptoms of inflammation. Historically, active disease has been defined by a BASDAI level of at least 4 [32]. The more recent Ankylosing Spondylitis Disease Activity Score (ASDAS) incorporates laboratory evaluation of inflammation (using C-reactive protein [CRP] or erythrocyte sedimentation rate [ESR]), with good discriminatory properties and sensitivity to change [33]. ASDAS may be a surrogate marker of spinal inflammation and it has been shown that is longitudinally linked to progression of the structural damage [34, 35]. Moreover, ASDAS has validated cutoff levels for disease activity states: a value below 1.3 is considered inactive disease or remission, between 1.3 and 2 as low disease activity, between 2.1 and 3.5 as high disease activity, and above 3.5 as very high disease activity [36, 37].
On the basis of this scale, several international and national guidelines advocate treatment to the target of achieving clinical remission (ASDAS < 1.3) or at least low disease activity (ASDAS < 2.1) [7, 38, 39]. The ASDAS cutoff for minimal clinically important improvement between examinations is 1.1 or higher, and a change at least 2.0 units is considered a major improvement [36]. On the other hand, an increase of at least 0.9 points is the ASAS definition for clinically important worsening [40]. In general terms, treatment should be individualized considering other symptoms and signs of the disease (axial, peripheral, extra-articular), comorbidities, psychosocial factors, and patients’ opinions.
Biological Treatment Failure: When, Why, and Controversies
Failure on bDMARDs can be detected or revealed both by objective measures (e.g., the presence of active manifestations of the disease on examination, raised CRP levels attributable to disease activity, or inflammatory lesions detected by magnetic resonance imaging [MRI]) or by the results of the patient-reported outcomes (PROs). However, sometimes there is discordance between the results of the objective measures and the results of the PROs. This is probably due to factors such as persistence of pain in some patients without evidence of inflammation and the influence of other aspects affecting the patient’s well-being and pain perception such as sleep disturbances or fatigue, as has been observed in patients with rheumatoid arthritis [41, 42]. The fact that some factors such as age, education level, gender, radiographic damage, or comorbidities influence the way in which the patients handle, or report, their disease process cannot be dismissed [43, 44]. Since treatment compliance and continuation is essential for its success, it must be verified that the patient’s adherence to therapy is adequate before definitively establishing the failure. An important issue to consider is that one drug can cause adverse events or undesirable effects (even when it is effective), so the treatment failure and drug discontinuation are not exclusively based on disease activity.
Apart from stopping the therapy because of safety or intolerance reasons, two types of treatment failure are usually differentiated depending on when it occurs: primary failure (no response within 6 months after treatment initiation, or lack of efficacy) and secondary failure (response within 6 months but lost thereafter, or loss of efficacy over time) [8]. The distinction between lack or loss of response is often vague and is conditioned by the rigorousness of the therapeutic goal (remission, low disease activity, ASAS response as used in clinical trials) and the drug used and its speed of action/effect (Table 2). Other factors that can modify the treatment failure definition are the inclusion or not of peripheral symptoms/extra-musculoskeletal manifestations or the health status in the assessment of the response. In fact, ASAS recommended a validated tool for evaluating the health of patients with axial SpA, the ASAS Health Index (ASAS-HI), to test real-life functioning both in clinical trials and daily practice [45]. The TICOSPA trial (Tight Control in Spondyloarthritis), the first study evaluating the potential benefits of tight control and a treat to target approach in patients with axial SpA, has shown a favorable effect in terms of improvement of ASAS-HI, compared to usual care, although it was not statistically significant [46].
Table 2.
Time of response assessment according to each summary of product characteristics
| Drug | |
|---|---|
| Infliximab | If a patient does not respond by 6 weeks (i.e., after 2 doses), no additional treatment with infliximab should be given |
| Etanercept | Available data suggest that a clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period |
| Adalimumab | Available data suggest that a clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period |
| Golimumab | Available data suggest that clinical response is usually achieved within 12–14 weeks of treatment (after 3–4 doses). Continued therapy should be reconsidered in patients who show no evidence of therapeutic benefit within this time period |
| Certolizumab pegol | Available data suggest that clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in patients who show no evidence of therapeutic benefit within the first 12 weeks of treatment |
| Secukinumab | Available data suggest that a clinical response is usually achieved within 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response by 16 weeks of treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks |
| Ixekizumab | Consideration should be given to discontinuing treatment in patients who have shown no response after 16–20 weeks of treatment. Some patients with initially partial response may subsequently improve with continued treatment beyond 20 weeks |
| Upadacitinib | Consideration should be given to discontinuing treatment in patients with ankylosing spondylitis who have shown no clinical response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks |
Another aspect to take into account to determine treatment failure is the possible occurrence of radiographic progression. Although a 2-year period is required before changes can be reliably detected with the modified Stoke Ankylosing Spondylitis Spine Scoring (mSASSS) [47], imaging methods other than plain radiographs, such as low-dose computed tomography (CT), have the potential to identify earlier vertebral and/or sacroiliac progression in axial SpA [48, 49]. On the other hand, CT or MRI may help to support the decision whether the appearance or worsening of symptoms reflects the failure of therapy or an alternate source of pain such as degenerative or prolapsed disc pain [50]. In certain clinical scenarios the findings of active lesions on MRI could reinforce the treatment failure suspicion.
Therapeutic Strategies After Treatment Failure
One of the dilemmas faced by physicians caring for patients with axial SpA is what to do in the event of a biological treatment failure. Although it is great news, the introduction of more alternatives to TNFi has made this decision even more complicated to make. Usually, population-based studies indicate that clinical response after switching to a second bDMARD (either a TNFi or anti-IL-17) is lower than the one experienced by patients naïve to biologic therapies [51, 52]. Nonetheless, drug switching is required (and recommended) when there is treatment failure or intolerance. According to the ASAS-EULAR recommendations, in patients with a primary failure on the first TNFi, it is more reasonable to switch to another class of drugs, i.e., an anti-IL-17, always after reconsidering if the diagnosis and the indication for the start of the first TNFi were correct [7]. Supporting this suggestion, the results of the phase III trials with secukinumab and ixekizumab showed that, among patients previously exposed to TNFi, the response to the anti-IL-17 agents was higher than to placebo [25, 26]. However, at least one study has shown that secukinumab has comparable effectiveness versus an alternative TNFi after prior TNFi failure [53]. In the case of failure on IL-17 blockade, there is no solid data regarding the switch from anti-IL-17 to TNF inhibition.
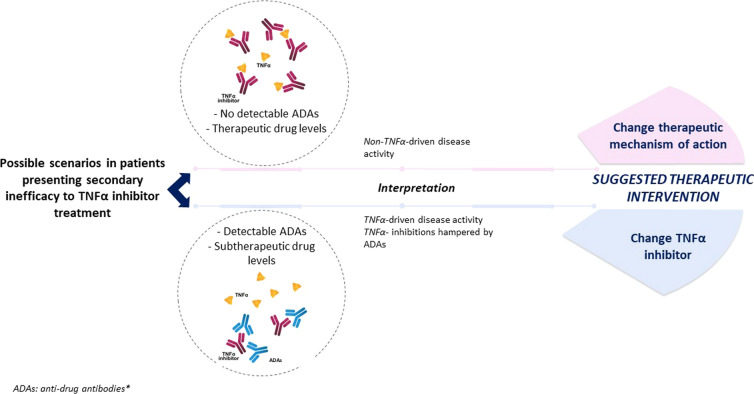
Of note, biological agents may induce an unwanted immune response (immunogenicity), which may alter the bioavailability of the drug causing a loss of efficacy. The development of antidrug antibodies (ADAs) on treatment with TNFi may represent one of the main causes for secondary treatment failure [54]. Thus, determination of serum ADAs or drug levels could identify the reason for poor response and assist in deciding the selection of the subsequent treatment [55]. In the presence of ADAs and low TNFi levels, cycling between TNFi is rational as it may restore the TNFα blocking capacity. In fact, the failure to respond to a first TNFi as a result of the development of ADAs seems to be predictive for a better clinical response to a second TNFi in SpA [56]. If ADAs are absent/low with adequate therapeutic levels of the TNFi, inefficacy is probably not due to the neutralization of the therapeutic effect, but because TNFα is not the main cytokine instigating disease activity. In this case, switching to another target might be the best strategy (Fig. 1) [57]. However, it is not always feasible to perform ADAs determinations and its use in clinical practice is yet limited. Stronger evidence from larger series is still lacking to support the systematic implementation of this measure in clinical practice.
Fig. 1.
Possible scenarios for management of secondary treatment failure on TNFi. *ADAs determination is recommended, if available
Discussion and Perspectives
Despite the difficulty of establishing a concise definition of treatment failure, it is necessary to set a general criterion that allows decisions to be made on the basis of objective parameters. We propose a definition based on Ankylosing Spondylitis Disease Activity Score (ASDAS)-CRP, absence of extra-articular manifestations and low disease impact on the patients’ general health (ASAS-HI). However, this standard definition must be individualized to each patient’s condition, ruling out additional causes of treatment failure, such as comorbidities deteriorating patients’ function and well-being [43] and other causes of chronic pain unrelated to the SpA, such as fractures and degenerative conditions. In the future, and based on the most advanced imaging techniques, findings of rapid radiographic progression may support a definitive failure, but the current (lack of) evidence precludes the ability to establish a precise definition or cutoff point. In this regard, what has been established is a clear longitudinal relationship between disease activity and radiographic progression, and that reinforces the need to achieve a level of low disease activity/inactive disease [20, 34, 35]. Thus, in patients with a primary failure on a first TNFi, switching to a drug with a different mechanism of action seems reasonable, trying to avoid long-lasting active disease. This may be the best option also when secondary failure occurs in the absence of ADAs; if loss of efficacy is confirmed to be caused by immunogenicity, it is advisable to use a different TNFi, although the lack of clinical trials comparing TNF blockers makes it difficult to decide which is the optimal therapeutic step. Secukinumab dose escalation is being evaluated in those patients not achieving inactive disease at week 16 according to ASDAS (NCT03350815), but the results are pending. Patients with obesity/overweight usually present higher disease activity and reduced response to TNFi, and may benefit from dose intensification, if ADAs are absent [58, 59], similar to the weight-based dosing of secukinumab proposed for patients with psoriasis [60]; however, weight reduction should be always advised in all patients with obesity. Most importantly, we should also remind the patients that exercise is a cornerstone of the treatment, and it is indicated in all stages of the disease. Finally, the concomitant use of conventional DMARDs or the combination of bDMARDs with JAKi has been scarcely investigated in the setting of biologic treatment failure, so future studies should address this gap.
In conclusion, there are still many unknowns to resolve in the event of a treatment failure. More clinical trials and real-life studies are needed, as well as updated guidelines or consensus algorithms to optimize patient care. The final objective must be to improve patients’ quality of life and avoid harm, and this is only achieved with an informed decision in the case of treatment failure, making the consequent change of drug or therapeutic target.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Novartis Pharmaceuticals provided economic funding for this work. The sponsor had no role in the design, the analysis and the interpretation of the data, the wording of the article or the decision to send the article for publication. The journal’s Rapid Service Fee was funded by Springer Healthcare Ibérica.
Medical Writing Assistance
The authors would like to acknowledge Anabel Herrero PhD., who provided medical writing support on behalf of Springer Healthcare, with funding from Novartis Pharmaceuticals.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors (XJ, MJMR, JMB, CFC, and JG) contributed to the design of the bibliographic research and revised the literature. All authors (XJ, MJMR, JMB, CFC, and JG) critically revised all versions of the manuscript, and read and approved the final manuscript.
Disclosures
Xavier Juanola has received unrelated honoraria or research grants from Abbvie, Lilly, Janssen, Novartis, Pfizer, and UCB. Manuel J. Moreno Ramos has received unrelated honoraria or research grants from Abbvie, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer and UCB, and advisor’s honoraria from Abbvie, Celgene, Janssen, Lilly, Novartis, Pfizer and UCB unrelated to the present work. Joaquin Maria Belzunegui has received unrelated honoraria from Janssen, Lilly, Novartis, Abbvie, BMS and UCB. Cristina Fernández-Carballido has received payments or speaker’s honoraria from Abbvie, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB and advisor’s honoraria from Abbvie, Celgene, Janssen, Lilly, Novartis, Pfizer and UCB unrelated to the present work. Jordi Gratacós has received unrelated honoraria or research grants from Abbvie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, and UCB.
Compliance with Ethical Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res. 2016;68:1320–1331. doi: 10.1002/acr.22831. [DOI] [PubMed] [Google Scholar]
- 2.Kavadichanda CG, Geng J, Bulusu SN, Negi VS, Raghavan M. Spondyloarthritis and the human leukocyte antigen (HLA)-B*27 connection. Front Immunol. 2021;12:601518. doi: 10.3389/fimmu.2021.601518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 4.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 5.Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther. 2020;7:65–87. doi: 10.1007/s40744-020-00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido-Cumbrera M, Gratacós J, Collantes-Estevez E, et al. A benchmarking study evaluating axial spondyloarthritis burden in Spain and other European countries. Results from the Spanish Atlas and the European Map of Axial Spondyloarthritis (EMAS) studies. Int J Rheum Dis. 2021;24:1127–1136. doi: 10.1111/1756-185X.14173. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71:1599–1613. doi: 10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moral E, Plasencia C, Navarro-Compán V, et al. Discontinuation of anti-TNF therapy in patients with axial spondyloarthritis in clinical practice: prevalence and causes. Ann Rheum Dis. 2016;75:1129. [Google Scholar]
- 10.Manica SR, Sepriano A, Pimentel-Santos F, et al. Effectiveness of switching between TNF inhibitors in patients with axial spondyloarthritis: is the reason to switch relevant? Arthritis Res Ther. 2020;22:195. doi: 10.1186/s13075-020-02288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JCJ, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–3236. doi: 10.1002/art.11325. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–591. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 13.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 14.Inman RD, Davis JCJ, van der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–3412. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 15.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015;67:2702–2712. doi: 10.1002/art.39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JC, van der Heijde DM, Braun J, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis. 2007;67:346–352. doi: 10.1136/ard.2007.078139. [DOI] [PubMed] [Google Scholar]
- 18.van der Heijde D, Schiff MH, Sieper J, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68:922–929. doi: 10.1136/ard.2007.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun J, Baraliakos X, Listing J, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis. 2008;67:340–345. doi: 10.1136/ard.2007.075879. [DOI] [PubMed] [Google Scholar]
- 20.Molnar C, Scherer A, Baraliakos X, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2018;77:63–69. doi: 10.1136/annrheumdis-2017-211544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajrawat P, Touma Z, Sari I, Taheri C, Diaz Martinez JP, Haroon N. Effect of TNF-inhibitor therapy on spinal structural progression in ankylosing spondylitis patients: a systematic review and meta-analysis. Int J Rheum Dis. 2020;23:728–743. doi: 10.1111/1756-185X.13829. [DOI] [PubMed] [Google Scholar]
- 22.Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum. 2017;47:343–350. doi: 10.1016/j.semarthrit.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 25.Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 study. Ann Rheum Dis. 2017;76:571–592. doi: 10.1136/annrheumdis-2016-210023. [DOI] [PubMed] [Google Scholar]
- 26.Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor nec. Arthritis Rheumatol. 2019;71:599–611. doi: 10.1002/art.40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology. 2019;58:859–868. doi: 10.1093/rheumatology/key375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Heijde D, Østergaard M, Reveille JD, et al. POS0918|evaluation of spinal radiographic progression in patients with radiographic axial spondyloarthritis receiving ixekizumab therapy over 2 years. Ann Rheum Dis. 2021;80:720. [Google Scholar]
- 29.Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73:110–120. doi: 10.1002/art.41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395:53–64. doi: 10.1016/S0140-6736(19)32971-X. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D, Song I-H, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394:2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 32.Landewé R, van Tubergen A. Clinical tools to assess and monitor spondyloarthritis. Curr Rheumatol Rep. 2015;17:47. doi: 10.1007/s11926-015-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Heijde D, Braun J, Dougados M, et al. Sensitivity and discriminatory ability of the Ankylosing Spondylitis Disease Activity Score in patients treated with etanercept or sulphasalazine in the ASCEND trial. Rheumatology. 2012;51:1894–1905. doi: 10.1093/rheumatology/kes142. [DOI] [PubMed] [Google Scholar]
- 34.Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis. 2014;73:1455–1461. doi: 10.1136/annrheumdis-2014-205178. [DOI] [PubMed] [Google Scholar]
- 35.Llop M, Moreno M, Navarro-Compán V, et al. Sustained low disease activity measured by ASDAS slow radiographic spinal progression in axial spondyloarthritis patients treated with TNF-inhibitors. Data from REGISPONSERBIO. Arthritis Res Ther. 2021 doi: 10.21203/rs.3.rs-705421/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado P, Landewé R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70:47–53. doi: 10.1136/ard.2010.138594. [DOI] [PubMed] [Google Scholar]
- 37.Marzo-Ortega H, Gaffney KM, Gaffney K. Defining the target: clinical aims in axial spondyloarthritis. Rheumatology. 2018;57:vi18–22. doi: 10.1093/rheumatology/key176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73:6–16. doi: 10.1136/annrheumdis-2013-203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gratacós J, Del Campo D, Fontecha P, et al. Recommendations by the Spanish Society of Rheumatology on the use of biological therapies in axial spondyloarthritis. Reumatol Clin. 2018;14:320–333. doi: 10.1016/j.reuma.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Molto A, Gossec L, Meghnathi B, et al. An Assessment in SpondyloArthritis International Society (ASAS)-endorsed definition of clinically important worsening in axial spondyloarthritis based on ASDAS. Ann Rheum Dis. 2018;77:124–127. doi: 10.1136/annrheumdis-2017-212178. [DOI] [PubMed] [Google Scholar]
- 41.Lee YC, Cui J, Lu B, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. 2011;13:R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turk SA, Rasch LA, van Schaardenburg D, et al. Pain, sleep and emotional well-being explain the lack of agreement between physician- and patient-perceived remission in early rheumatoid arthritis. BMC Rheumatol. 2018;2:16. doi: 10.1186/s41927-018-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikiphorou E, Ramiro S, van der Heijde D, et al. Association of comorbidities in spondyloarthritis with poor function, work disability, and quality of life: results from the Assessment of Spondyloarthritis International Society Comorbidities in Spondyloarthritis study. Arthritis Care Res. 2018;70:1257–1262. doi: 10.1002/acr.23468. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Carballido C, Martín-Martínez MA, García-Gómez C, et al. Impact of comorbidity on physical function in patients with ankylosing spondylitis and psoriatic arthritis attending rheumatology clinics: results from a cross-sectional study. Arthritis Care Res. 2020;72:822–828. doi: 10.1002/acr.23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiltz U, van der Heijde D, Boonen A, et al. Measurement properties of the ASAS Health Index: results of a global study in patients with axial and peripheral spondyloarthritis. Ann Rheum Dis. 2018;77:1311–1317. doi: 10.1136/annrheumdis-2017-212076. [DOI] [PubMed] [Google Scholar]
- 46.Molto A, López-Medina C, Van den Bosch FE, et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann Rheum Dis. 2021;80:1436–1444. doi: 10.1136/annrheumdis-2020-219585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology. 2019;58:388–400. doi: 10.1093/rheumatology/key128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Koning A, de Bruin F, van den Berg R, et al. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis. 2018;77:293–299. doi: 10.1136/annrheumdis-2017-211989. [DOI] [PubMed] [Google Scholar]
- 49.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Development and preliminary validation of the spondyloarthritis research consortium of canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. 2015;42:79–86. doi: 10.3899/jrheum.140519. [DOI] [PubMed] [Google Scholar]
- 50.de Hooge M, de Bruin F, de Beer L, et al. Is the site of back pain related to the location of magnetic resonance imaging lesions in patients with chronic back pain? Results from the Spondyloarthritis Caught Early Cohort. Arthritis Care Res. 2017;69:717–723. doi: 10.1002/acr.22999. [DOI] [PubMed] [Google Scholar]
- 51.Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor α inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis. 2013;72:1149–1155. doi: 10.1136/annrheumdis-2012-201933. [DOI] [PubMed] [Google Scholar]
- 52.Ciurea A, Exer P, Weber U, et al. Does the reason for discontinuation of a first TNF inhibitor influence the effectiveness of a second TNF inhibitor in axial spondyloarthritis? Results from the Swiss Clinical Quality Management Cohort. Arthritis Res Ther. 2016;18:71. doi: 10.1186/s13075-016-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micheroli R, Tellenbach C, Scherer A, et al. Effectiveness of secukinumab versus an alternative TNF inhibitor in patients with axial spondyloarthritis previously exposed to TNF inhibitors in the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2020;79:1203–1209. doi: 10.1136/annrheumdis-2019-215934. [DOI] [PubMed] [Google Scholar]
- 54.Bandrés Ciga S, Salvatierra J, López-Sidro M, et al. An examination of the mechanisms involved in secondary clinical failure to adalimumab or etanercept in inflammatory arthropathies. J Clin Rheumatol. 2015;21:115–119. doi: 10.1097/RHU.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 55.Bornstein G, Lidar M, Langevitz P, Fardman A, Ben-Zvi I, Grossman C. The prevalence and clinical effect of immunogenicity of TNF-α blockers in patients with axial spondyloarthritis. Clin Exp Rheumatol. 2018;36:228–232. [PubMed] [Google Scholar]
- 56.Plasencia C, Pascual-Salcedo D, García-Carazo S, et al. The immunogenicity to the first anti-TNF therapy determines the outcome of switching to a second anti-TNF therapy in spondyloarthritis patients. Arthritis Res Ther. 2013;15:R79. doi: 10.1186/ar4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenzin M, Ometto F, Ortolan A, et al. An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. Ther Adv Musculoskelet Dis. 2020;12:17–59720X20934277. doi: 10.1177/1759720X20934277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One. 2018;13:e0195123. doi: 10.1371/journal.pone.0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liew JW, Huang IJ, Louden DN, Singh N, Gensler LS. Association of body mass index on disease activity in axial spondyloarthritis: systematic review and meta-analysis. RMD Open. 2020;6:e001225. doi: 10.1136/rmdopen-2020-001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412–421. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.