Abstract
Natural systems transfer chiral information across multiple length scales through dynamic supramolecular interaction to accomplish various functions. Inspired by nature, many exquisite artificial supramolecular systems have been developed, in which controlling the supramolecular chirality holds the key to completing specific tasks. However, to achieve precise and non-invasive control and modulation of chirality in these systems remains challenging. As a non-invasive stimulus, light can be used to remotely control the chirality with high spatiotemporal precision. In contrast to common molecular switches, a synthetic molecular motor can act as a multistate chiroptical switch with unidirectional rotation, offering major potential to regulate more complex functions. Here, we present a light-driven molecular motor-based supramolecular polymer, in which the intrinsic chirality is transferred to the nanofibers, and the rotation of molecular motors governs the chirality and morphology of the supramolecular polymer. The resulting supramolecular polymer also exhibits light-controlled multistate aggregation-induced emission. These findings present a photochemically tunable multistate dynamic supramolecular system in water and pave the way for developing molecular motor-driven chiroptical materials.
Introduction
Chirality is one of the most essential and fundamental features of nature.1−8 How chirality transfers from the molecular level to multiple length scales draws extensive attention and remains far from fully understood.1−6,9−11 Supramolecular interactions are crucial for building nanostructures and amplifying the asymmetry from the molecular level to the macroscopic scale.12−17 Controlling the supramolecular chirality in a self-assembled system requires a delicate match of many parameters, such as the distance between the chiral centers in the assembly and the interaction strength.2−5,18 As highly ordered assemblies are constructed through the use of a directional non-covalent bond, supramolecular polymers have a distinct advantage in transferring the chirality from monomers or environments (solvents and guests) to supramolecular handedness,2 playing an essential role in various areas19−23 such as amplification of asymmetry16,24,25 and optical and electronic devices.26−28
Controlling the chirality of supramolecular polymers using external stimuli, for example, heat,29 solvent,30,31 and light,10,32−34 holds enormous potential in smart and responsive materials. Among diverse stimuli, light exhibits superiority in controlling a system with high spatiotemporal precision,35−38 thus attracting increasing attention to the tuning of supramolecular chirality.5,39 However, most studies are based on two-stage photoswitches covalently linked to chiral moieties10,32−34 or coassembled with chiral molecules,40,41 and relatively few systems can be operated in water,41 a unique medium for assembly in nature. A multistate photoswitch with different inherent chiralities and distinct geometries would significantly enrich the opportunities of this field and allow more complex responsive functions in the future.42−44
Light-driven molecular motors undergo unidirectional rotation with interconvertible inherent chirality and a distinct helical geometry.45−48 Taking advantage of multistate chirality, they have been used to dynamically control metal–ligand helicate oligomers,42 cholesteric liquid crystal materials,43 spin selectivity,49 and asymmetric catalysis.50−52 However, the amplification of the asymmetry of molecular motors to the nanoscale and macroscopic scale still has massive untapped potential and has not been achieved in aqueous media so far.42,43
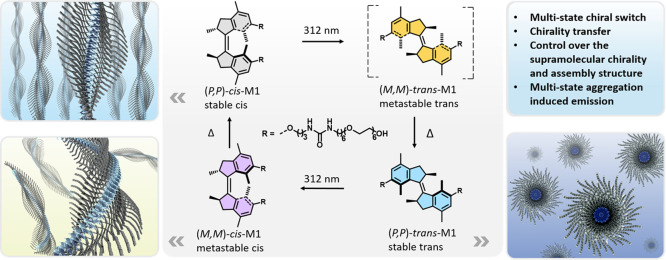
Here, we report water-soluble supramolecular polymers formed by molecular motors, including a racemic stable cis-motor (cis-M1) and the corresponding homochiral compound (P,P-cis-M1) (Figure 1). The chirality of the molecular motor was transmitted to the supramolecular polymer resulting in helical fibers in water. During the unidirectional rotation, the molecular motor displayed four states with different inherent chiralities and geometries, which allowed us to regulate the morphology and the chirality of the supramolecular polymer (Figure 1). Remarkably, the polymer exhibited light-tuneable multistate aggregation-induced emission (AIE), competing with the excited-state molecular motor rotation.
Figure 1.
Molecular structures of molecular motor M1 and illustration of multistate rotation, isomers with distinct chirality, and the corresponding assembly structures.
Results and Discussion
Molecular Design and Synthesis of Molecular Motors
Monomers were designed with an overcrowded alkene, first generation molecular motor45−48 core, and two urea groups bearing hexaethylene glycol and chains to ensure water solubility (Figure 1). The tight hydrogen bonding between urea groups is beneficial for constructing supramolecular polymers.53−59 C6 alkyl-linkers are positioned between the hexaethylene glycol chains and the urea groups, providing a hydrophobic pocket to facilitate hydrogen bonding. This bioinspired strategy of protecting hydrogen bonds in water has been shown by Meijer and others to favor the development of synthetic supramolecular polymers in aqueous media.60−64 The synthesis of racemic stable cis-M1 and stable (R,R)-(P,P)-cis-M1 is summarized in Supporting Information, Section S3 (Figure S1). The name of (R,R)-(P,P)-cis-M1 is shortened as (P,P)-cis-M1 in the present study. All the novel structures were characterized by 1H, 13C NMR, and high-resolution ESI-MS (Figures S25–S36).
The four-step rotary cycle process of M1 features two photoisomerization and two thermal steps. Each photoisomerization is followed by a thermal helix inversion (THI): stable (P,P)-cis-M1 photochemically converts to metastable (M,M)-trans-M1, which subsequently forms stable (P,P)-trans-M1 during THI; then stable (P,P)-trans-M1 photochemically converts to metastable (M,M)-cis-M1, followed by THI to recover stable (P,P)-cis-M1 (Figure 1).
Rotary Motion of Molecular Motors and Their Chirality Changes in Solution
Before studying the assembly and property of motors in water, we investigated the rotation of monomeric M1 in MeOH using UV–vis, circular dichroism (CD) absorption, and 1H NMR spectroscopy. The monomeric state of M1 in MeOH was confirmed by dynamic light scattering (DLS) measurements, showing an average hydrodynamic diameter (Dh) around 2 nm (Figure S13). Upon 312 nm light irradiation at −15 °C, a characteristic absorption band of stable cis-M1 at 270–330 nm decreased with the formation of an absorption band at 330–390 nm, showing an isosbestic point at 326 nm. This phenomenon indicated the selective photochemical interconversion of stable cis-M1 to metastable trans-M1 (Figure S2a). Keeping the molecule in the dark at −15 °C, the absorption band at 330–390 nm diminished with an increase in absorption at 260–330 nm, as a consequence of the THI interconversion of metastable trans to stable trans (Figure S2b).
Subsequent irradiation with 312 nm light led to a decrease of the absorption band at 260–330 nm and an increase of the one at 330–400 nm, indicating the selective photoisomerization of stable trans-M1 to metastable cis-M1 (Figure S2c). After warming the sample at 45 °C in the dark, the absorption band at 330–400 nm diminished with an increase at 270–330 nm, suggesting the helix inversion of metastable cis-M1 to stable cis-M1 (Figure S2d). The rotation behavior of M1 is comparable with the one observed in a previous study in our group on first generation molecular motor-based double-stranded helicates.42 Eyring analysis of the THI data of metastable trans-M1 to stable trans-M1 and metastable cis-M1 to stable cis-M1 revealed a standard Gibbs free energy of activation (Δ⧧G°) of 78.8 and 100.4 kJ mol–1 at 20 °C, respectively (Figures S3 and S4). The determined half-life of metastable cis-M1 is 24.8 h, while the one of metastable trans-M1 is 12.4 s, indicating the challenge to capture metastable trans-M1 in solution at room temperature.
Due to the short half-life of metastable trans-M1, irradiation of stable (P,P)-cis-M1 at 20 °C resulted in the generation of metastable cis-M1 at the photostationary state (PSS), indicated by the decrease of the absorption band at 270–330 nm, accompanied by the increase of the absorption band at 330–400 nm (Figure 2a). Stable (P,P)-cis-M1 displayed a characteristic negative signal at 290–350 nm in the CD spectrum, which disappeared with the generation of a positive band at 320–400 nm upon irradiation, characteristic of the opposite inherent chirality of (M,M)-cis-M1 and (P,P)-cis-M1. Nearly identical CD and absorption spectra of (P,P)-cis-M1 were recovered after keeping the sample in the dark at 45 °C for 5 h, indicating a THI process of metastable (M,M)-cis to stable (P,P)-cis-M1. The same isomerization process was also demonstrated by 1H NMR (Figure 2b). Proton signals of Hd (δ = 1.05 ppm) shift downfield to 1.46 ppm, while Ha, Hb, and Hc shift upfield upon 312 nm light irradiation for 30 min [Figure 2b(i,ii)], in accordance with the conversion from stable cis-M1 to metastable cis-M1. The ratio of metastable cis to stable trans-M1 is 76:24 at the PSS, established by integrating the NMR signals. Subsequently, warming the sample at 55 °C in the dark for 1.5 h resulted in the recovery of the initial 1H NMR spectrum of stable cis-M1 [Figure 2b(iii)].
Figure 2.
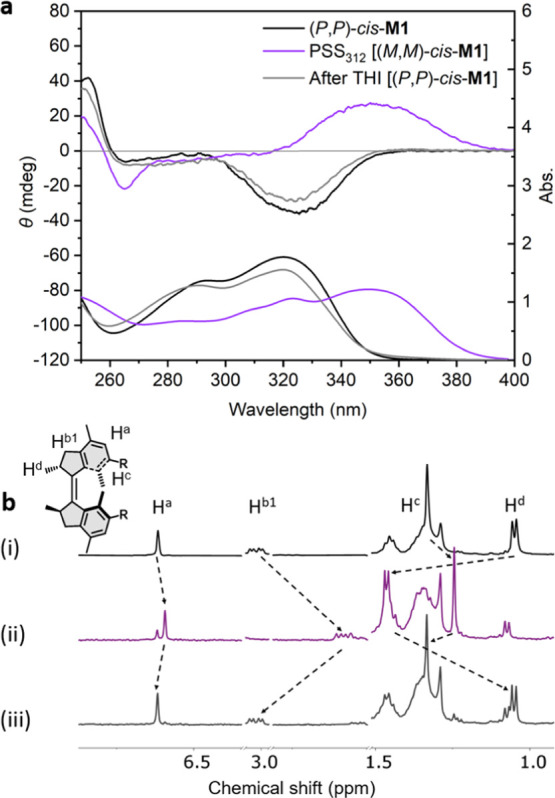
(a) Changes in the UV–vis (right axis) and CD (left axis) absorption spectra (MeOH, 293 K) of (P,P)-cis-M1 (78 μM) upon irradiation with 312 nm light for 30 min to reach the PSS and subsequently removing the light source and warming at 45 °C for 5 h to allow THI; (b) changes in the 1H NMR spectra of (i) (P,P)-cis-M1 (2 mM), (ii) upon irradiation with 312 nm light for 30 min at 293 K to the PSS, and (iii) subsequently removing the light source and warming at 55 °C for 1.5 h to induce THI.
Assembly in Water and Chirality Transfer from Molecular Motors to Supramolecular Polymers
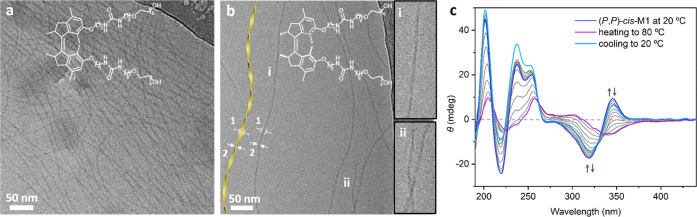
To test if the molecular motor can result in ordered assembly structures, an aqueous solution of racemic stable cis-M1 was first characterized by cryogenic electron transmission microscopy (cryo-TEM). To our delight, racemic stable cis-M1 was found to form fibers with a uniform diameter of 7.0 ± 0.8 nm and over micrometers in length (Figure 3a). As stable cis-M1 formed a well-organized 1D assembly, we envisioned that the enantiomerically pure stable cis-M1 holds promise to transfer its molecular chirality to a supramolecular polymer. Consequently, we conducted the subsequent assembly study starting from (P,P)-cis-M1.
Figure 3.
Cryo-TEM images of the assembly structure in water of (a) racemic stable cis-M1 (3 mg/mL) and (b) (P,P)-cis-M1 (1 mg/mL), inset: enlarged images at positions i and ii (additional images are shown in the Supporting Information). (c) Temperature-dependent CD spectra of (P,P)-cis-M1 (52 μM) in water.
Indeed, the cryo-TEM images revealed that (P,P)-cis-M1 formed uniform fibers with micrometer length (Figures 3b and S14). These fibers showed a regular variation in the width along the axis under cryo-TEM, indicating the helical structure of the supramolecular polymer. The 1/2 pitch was around 70 nm. The maximum width is 7.8 ± 0.7 nm (Figure 3b, label 1) and the minimum is 3.8 ± 0.4 nm (Figure 3b, label 2). The identical sample was characterized in water by small angle X-ray scattering (SAXS). The SAXS profile shows a q–1 slope of the curve at low q-values in the log–log plot, suggesting a rod (fiber)-like structure (vide infra, Figure 6, black circle). The curve was fitted with the analytical model for flexible cylindrical objects, resulting in an average diameter of 5.2 ± 0.4 nm. The total length and the Kuhn length of the structures are beyond the resolution of the measurement (>60 nm). Despite the fact that this diameter refers to a solid rod-like object, thus showing a slight deviation from the shape of twisted fiber, it is in agreement with the cryo-TEM observations. We further characterized the supramolecular polymer by Fourier transform infrared (FTIR) spectroscopy. The strong vibrational band centered at 3337 cm–1 belongs to hydrogen-bonded N–H in the urea moieties (Figure S9, black curve), confirming that the hydrogen bonds contributed to forming a supramolecular polymer of (P,P)-cis-M1.
Figure 6.
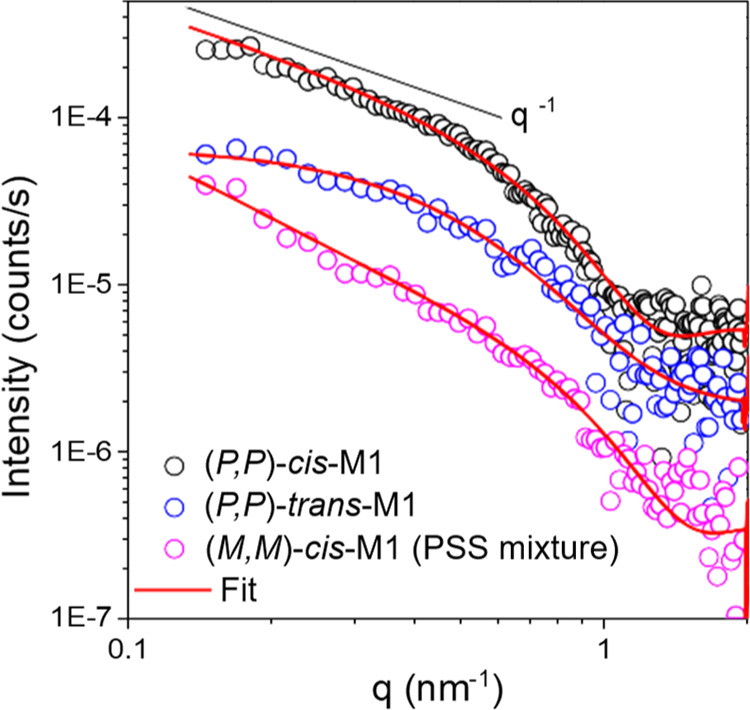
SAXS profiles for the supramolecular polymer of (P,P)-cis-M1 in water before (black circle) and after irradiation with 312 nm light for 2 min and keeping in the dark for 10 min to reach (P,P)-trans-M1 (blue circle), subsequent irradiation with 312 nm light for 10 min to get a PSS mixture of (M,M)-cis-M1 and (P,P)-trans-M1 (purple circle). The red curves are the fittings with the Guinier approximation (Supporting Information Section S11).
The presence of helical fibers encouraged us to study the chirality transfer process by temperature-dependent CD spectra. Differently from the CD spectrum in MeOH, with a characteristic negative signal at 290–350 nm, an aqueous solution of (P,P)-cis-M1 showed a positive Cotton effect at 280–360 nm at 20 °C, suggesting chiral helical packing of molecules (Figure 3c). No linear dichroism (LD) signal was measured for the sample, indicating that the CD signals are induced from molecules to supramolecular polymers (Figure S10).65 Upon heating, the positive Cotton effect diminished with an appearance of a negative band similar to that in MeOH, implying a disorder of the supramolecular polymer and the disappearance of the supramolecular chirality (Figures 3c and S11). After cooling and stabilizing at 20 °C for 2 h, the positive Cotton effect was recovered, suggesting the reformation of the chiral supramolecular polymer (Figure 3c). These features confirmed the chirality transfer from the molecular motor (P,P)-cis-M1 to the supramolecular polymer. Temperature-dependent DLS measurements revealed the changes in the size of the assemblies upon heating and cooling, confirming the disorder and reformation of the supramolecular polymers (Figure S12). In addition, a LCST-type phase separation was found in the heating process with the critical temperature at ca. 60 °C (Supporting Information, Section S9).
Rotation of Molecular Motors in the Supramolecular Polymer in Water
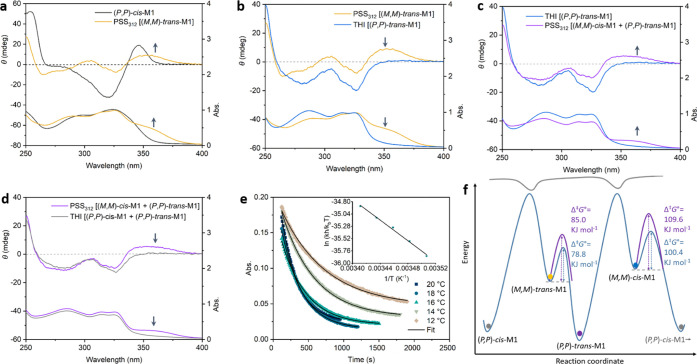
The motion of molecular motors in confined space is crucial for their applications in materials.66−68 To study the rotation of the molecular motors inside the aqueous supramolecular polymer, we monitored the rotation process by CD and UV–vis absorption spectroscopy. Interestingly, we observed the appearance of the metastable trans isomer (M,M)-trans-M1 at 20 °C (Figure 4a,b). The trans isomer (M,M)-trans-M1 was not observed in MeOH at the same temperature, in which the motors are molecularly dissolved. After exposing (P,P)-cis-M1 to 312 nm light for 2 min, the CD spectrum displayed a positive signal at 330–390 nm, and the UV–vis absorption spectrum showed a band at 330–390 nm, suggesting the presence of metastable (M,M)-trans-M1 (Figure 4a). After keeping in the dark at 20 °C for 10 min, the absorption band and positive CD signal at 330–390 nm disappeared with an increase of the absorption band and negative CD signal at 270–330 nm, indicating that the THI of metastable (M,M)-trans-M1 to stable (P,P)-trans-M1 was completed (Figure 4b). Eyring analysis of the THI in water (aggregate states) revealed a Δ⧧G° of 85.0 kJ mol–1, which was higher than 78.8 kJ mol–1 in the monomeric state (Figure 4e,f). The increased energy barrier results in a longer half-life of 2.6 min, benefitting from the fact that metastable (M,M)-trans-M1 is stabilized in the fibers.
Figure 4.
CD and UV–vis spectra of (a) (P,P)-cis-M1 (78 μM) after irradiation with 312 nm light for 2 min to get the PSS, (M,M)-trans-M1, (b) after removing the light source and keeping 20 °C for 10 min to get (P,P)-trans-M1, (c) after subsequent irradiation with 312 nm light for 10 min to get a PSS mixture of (P,P)-trans-M1 and (M,M)-cis-M1 in water, (d) after removing the light source and warming at 45 °C for 3 days, processing the THI of (M,M)-cis-M1 to get (P,P)-cis-M1 (293 K, 1 cm cuvette), and (e) time-dependent absorption changes at 365 nm during the THI of metastable (M,M)-trans-M1 in water at different temperatures, inset: Eyring plots with a liner fit. (f) Energy diagram of the rotation of molecular motor in MeOH and water, respectively.
Much to our delight, we obtained the pure stable trans isomers after irradiation and THI in water, as revealed by integrating the characteristic signals in 1H NMR (Figure S7b). Hence, the original (P,P)-cis-M1 has been fully converted to (P,P)-trans-M1. Upon subsequent irradiation with 312 nm light for 10 min, the absorption band and negative CD signal at 270–330 nm decreased with the generation of an absorption band and positive CD signal at 330–400 nm, suggesting the isomerization of stable (P,P)-trans-M1 to metastable (M,M)-cis-M1 (Figure 4c). The 1H NMR spectra of the identical sample revealed a ratio of (M,M)-cis-M1 and (P,P)-trans-M1 as 32:68 (Figure S7c), which is lower than the one in MeOH (Figure 2b). After warming at 45 °C for 3 days, the absorption band and positive CD signal at 330–400 nm disappeared, suggesting the THI of (M,M)-cis-M1 to (P,P)-cis-M1 (Figure 4d). The complete conversion of (M,M)-cis-M1 by THI to the original isomer was confirmed by 1H NMR (Figure S7d), showing the four-stage rotary cycle. Eyring analysis of the THI revealed a Δ⧧G° of 109.6 kJ mol–1 and a t1/2 of 44.5 days at 20 °C or 19.4 h at 45 °C, which were higher than those found in MeOH (Figures 4f and S5).
Multistate Chirality and Morphology Changes of Supramolecular Polymers
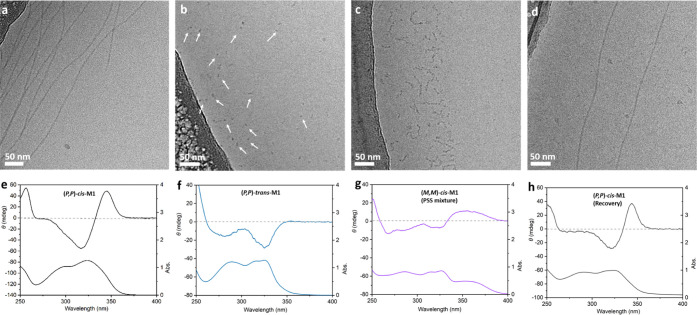
To explore the supramolecular chirality and morphology changes of the assemblies during the rotation of the molecular motor, we performed cryo-TEM, SAXS, CD, and UV–vis absorption measurements at different stages. As presented above, (P,P)-cis-M1 formed helical fibers and showed a positive Cotton effect at 280–360 nm in the CD spectra (Figures 5a,e and S14). The fibers of (P,P)-cis-M1 transformed into micelles after irradiation for 2 min and subsequently keeping in the dark for 10 min to reach (P,P)-trans-M1 (Figures 5b,f and S15). The SAXS profile of (P,P)-trans-M1 exhibits a significant decrease in intensity at low q-values, and the q–1 slope of the (P,P)-cis-M1 sample is not observed anymore, suggesting the presence of less-elongated assemblies (Figure 6, blue circle). The average diameter of the aggregates as determined using the fit of the SAXS profile is 5.0 ± 0.4 nm, which is in good agreement with the cryo-TEM study result (5.7 ± 0.7 nm). After irradiation for 10 min, cryo-TEM images showed worm-like fibers with a diameter of 6.2 ± 0.8 nm, which were formed from the mixture of (M,M)-cis and (P,P)-trans-M1 (Figures 5c,g and S16). A SAXS pattern of the same sample displays an increase of the intensity at low q-values again, indicating the existence of more extended assemblies than spherical micelles formed by (P,P)-trans-M1 (Figure 6 purple circle). Data analysis revealed a diameter of 4.8 ± 0.4 nm and a Kuhn length (LKuhn) around 20 nm, comparable to the observations from the cryo-TEM images. As described above, the ratio of (M,M)-cis and (P,P)-trans-M1 is 32:68 at the PSS in water. A considerable amount of (P,P)-trans-M1 remaining unconverted after THI might affect the recovery of the helical fibers. Indeed, the morphology of aggregates after THI of (M,M)-cis to (P,P)-cis-M1 remained as worm-like fibers, although almost all the (M,M)-cis-M1 have converted to (P,P)-cis-M1 (Figures S17 and S18). To improve the ratio of (M,M)-cis-M1 and (P,P)-trans-M1 at the PSS and speed up the following THI, we performed the irradiation and warming in a water/THF (7/3) mixture. In this way, the ratio of (M,M)-cis-M1 and (P,P)-trans-M1 at the PSS increases to 70:30, revealed by integrating 1H NMR signals (Figure S8a). Helical fibers were recovered by preparing the above sample in water, as evidenced from the cryo-TEM images and the positive Cotton effect in the CD spectrum (Figures 5d,h and S19).
Figure 5.
Cryo-TEM images of (a) (P,P)-cis-M1 (780 μM), (b) after irradiation with 312 nm light for 2 min and keeping in the dark for 10 min to reach (P,P)-trans-M1 (micelles were pointed out with arrows for clearance, and not all the micelles were pointed), (c) after subsequent irradiation with 312 nm light for 10 min to get a PSS mixture of (M,M)-cis-M1 and (P,P)-trans-M1 in water, and (d) (P,P)-trans-M1 after irradiation with 312 nm light for 10 min and keeping in the dark at 45 °C for 5 h in a water/THF (7/3) mixture to recover (P,P)-cis-M1, followed by repreparation in pure water. (e–h) CD and UV–vis absorption spectra of the identical samples in (a–d), respectively (293 K, 1 mm cuvette).
Light-Controllable Multistate AIE
The supramolecular polymer of (P,P)-cis-M1 showed a blue emission centered at 440 nm upon excitation at λex = 312 nm in water (Figure 7a). (P,P)-cis-M1 show no emission in the monomeric state, as suggested by the absence of fluorescence in MeOH (Figure S20). To identify the lowest concentration for AIE, we plotted the concentration-dependent fluorescence intensity. This value increased with the concentration after a sharp transition at a critical concentration of 6 μM (Figure S21). As the mechanism of AIE is associated with the restriction of the intramolecular rotation,69 we assumed that the emission of our supramolecular polymer was attributed to the restriction of the excited-state rotation of the molecular motor in confined space. Gratifyingly, this increased barrier is not enough to halt the photochemical isomerization of the molecular motors inside the supramolecular assembly.
Figure 7.
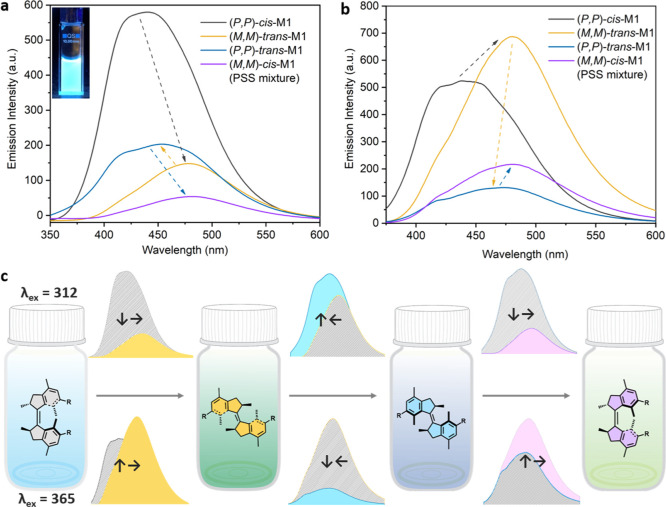
Changes in the fluorescence spectra of (P,P)-cis-M1 (78 μM) during multistate rotation in water upon the excitation at (a) λex = 312 nm, inset: an image of (P,P)-cis-M1 upon the irradiation at λex = 312 nm in water, and (b) λex = 365 nm. (c) Schematic illustration of light-tuneable multistate AIE upon excitation at λex = 312 nm and λex = 365 nm, respectively.
These results encouraged us to study the multistate photoresponsive AIE. We measured the fluorescence spectra of (P,P)-cis-M1 (78 μM) during the four-stage rotation under the excitation of 312 nm light (Figure 7a). After irradiation with 312 nm for 2 min, the emission centered at 440 nm decreased and red-shifted to 480 nm due to the presence of metastable (M,M)-trans-M1. The emission shifted in the blue region and increased at 440 nm after keeping the sample in the dark at room temperature for 10 min to reach stable (P,P)-trans-M1. After subsequent irradiation of (P,P)-trans-M1for 10 min to the PSS, the emission red-shifted to 480 nm again, which was attributed to a mixture of metastable (M,M)-cis-M1 and stable (P,P)-trans-M1. The differences in emission intensity might be attributed to the packing of molecules in the aggregates, while the emission wavelength is related to the Stokes shift of different absorption bands.70−72
Notably, the four states of M1 also showed the emission upon excitation with 365 nm light (Figure 7b). The emission wavelengths of the four states were comparable to those observed when exciting at 312 nm, while the emission intensities showed an inverse trend (Figure 7c). An increase of the emission intensity was observed in the transition of (P,P)-cis to (M,M)-trans-M1 and (P,P)-trans to (M,M)-cis-M1, and a decrease of the emission intensity was found for the THI of (M,M)-trans to (P,P)-trans-M1. In addition, (P,P)-cis and (P,P)-trans-M1 show a relatively lower emission intensity, while (M,M)-trans and (M,M)-cis-M1 show a stronger emission intensity under λex = 365 nm compared to those upon 312 nm excitation. To avoid the possible photoinduced rotation of M1 during the measurements, the fluorescence quantum yield (Φem) was measured upon the excitation of their unfavorable excitation wavelength (Supporting Information Section S14, Table S1).73−75 (P,P)-cis and (P,P)-trans-M1 were measured under λex = 365 nm; (M,M)-trans and (M,M)-cis-M1 were characterized under λex = 312 nm. Φem of (P,P)-cis reaches 9.2%, even under an unfavorable excitation wavelength. The other three states show relatively lower Φem, 3.2, 3.4, and 1.4%, under their unfavorable excitation wavelengths, respectively (Table S1). Emission lifetimes of the aggregates formed by (P,P)-cis-M1, (P,P)-trans-M1, and (M,M)-cis-M1 are shorter than 0.5 ns (Supporting Information Section S15, Figures S22–S24). Distinct from the major current two-state strategy,76 our work presented a unique and facile way toward multistate light-controllable AIE materials.
Conclusions
In conclusion, we developed a multistate photoresponsive supramolecular polymer in water based on synthetic molecular motor M1. The chirality of the molecular motor (P,P)-cis-M1 successfully transferred to the supramolecular polymer in an aqueous solution. The rotation-induced intrinsic chirality and geometric changes dramatically influenced the morphology and chirality of the supramolecular polymer, thus achieving dynamic control of the supramolecular polymer in multiple self-assembled states. Starting from the helical fibers, the morphology was transformed into micelles, followed by worm-like micelles, and finally the helical fibers were recovered. The resulting assemblies showed unique multistate AIE, which could be tuned by light. The present study showed a multistate supramolecular system in water with light-controllable properties and established the basis for developing advanced multifunctional and responsive molecular motor-based chiroptical materials.
Acknowledgments
The authors acknowledge financial support from the Netherlands Organization for Scientific Research (NWO-CW), the European Research Council (ERC; advanced grant no. 694345 to B.L.F.), the Dutch Ministry of Education, Culture and Science (Gravitation program no. 024.001.035), the China Scholarship Council (CSC; no. 201707040064 to F.X.), and the Marie Skłodowska-Curie Actions (Individual Fellowship no. 838280 to S.C.; 101025041 to Q.Z.). We thank Marco Ovalle for the 3D illustrations and helping with the TOC figure. We appreciate Vanda Dašková for helping with optical rotation [α]DT measurements. We thank prof. Wesley R. Browne for measuring emission lifetimes and helping with LD measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c01063.
Synthesis; experimental conditions for UV–vis, CD, cryo-TEM, FTIR, and SAXS; fluorescence quantum yield and emission lifetime measurements; Eyring analysis; computational study; and 1H, 13C NMR, and HRMS data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Feringa B. L.; van Delden R. A.; Delden R. Absolute Asymmetric Synthesis: The Origin, Control, and Amplification of Chirality. Angew. Chem., Int. Ed. 1999, 38, 3418–3438. . [DOI] [PubMed] [Google Scholar]
- Palmans A. R. A.; Meijer E. W. Amplification of Chirality in Dynamic Supramolecular Aggregates. Angew. Chem., Int. Ed. 2007, 46, 8948–8968. 10.1002/anie.200701285. [DOI] [PubMed] [Google Scholar]
- Yashima E.; Ousaka N.; Taura D.; Shimomura K.; Ikai T.; Maeda K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- Morrow S. M.; Bissette A. J.; Fletcher S. P. Transmission of Chirality through Space and across Length Scales. Nat. Nanotechnol. 2017, 12, 410–419. 10.1038/nnano.2017.62. [DOI] [PubMed] [Google Scholar]
- Liu M.; Zhang L.; Wang T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. 10.1021/cr500671p. [DOI] [PubMed] [Google Scholar]
- Brandt J. R.; Salerno F.; Fuchter M. J. The Added Value of Small-Molecule Chirality in Technological Applications. Nat. Rev. Chem. 2017, 1, 0045. 10.1038/s41570-017-0045. [DOI] [Google Scholar]
- Percec V.; Xiao Q. Helical Self-Organizations and Emerging Functions in Architectures, Biological and Synthetic Macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. 10.1246/bcsj.20210015. [DOI] [Google Scholar]
- Percec V.; Xiao Q. Helical Chirality of Supramolecular Columns and Spheres Self-Organizes Complex Liquid Crystals, Crystals, and Quasicrystals. Isr. J. Chem. 2021, 61, 530–556. 10.1002/ijch.202100057. [DOI] [Google Scholar]
- Ariga K.; Mori T.; Kitao T.; Uemura T. Supramolecular Chiral Nanoarchitectonics. Adv. Mater. 2020, 32, 1905657. 10.1002/adma.201905657. [DOI] [PubMed] [Google Scholar]
- de Jong J. J. D.; Lucas L. N.; Kellogg R. M.; van Esch J. H.; Feringa B. L. Reversible Optical Transcription of Supramolecular Chirality into Molecular Chirality. Science 2004, 304, 278–281. 10.1126/science.1095353. [DOI] [PubMed] [Google Scholar]
- Pijper D.; Jongejan M. G. M.; Meetsma A.; Feringa B. L. Light-Controlled Supramolecular Helicity of a Liquid Crystalline Phase Using a Helical Polymer Functionalized with a Single Chiroptical Molecular Switch. J. Am. Chem. Soc. 2008, 130, 4541–4552. 10.1021/ja711283c. [DOI] [PubMed] [Google Scholar]
- Lehn J. M.Supramolecular Chemistry; John Wiley & Sons: Strasbourg, 1995. [Google Scholar]
- Brunsveld L.; Folmer B. J. B.; Meijer E. W.; Sijbesma R. P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]
- Lehn J.-M. Supramolecular Polymer Chemistry—Scope and Perspective. Polym. Int. 2002, 51, 825–839. 10.1002/pi.852. [DOI] [Google Scholar]
- de Greef T. F. A.; Smulders M. M. J.; Wolffs M.; Schenning A. P. H. J.; Sijbesma R. P.; Meijer E. W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. 10.1021/cr900181u. [DOI] [PubMed] [Google Scholar]
- van Dijken D. J.; Beierle J. M.; Stuart M. C. A.; Szymański W.; Browne W. R.; Feringa B. L. Autoamplification of Molecular Chirality through the Induction of Supramolecular Chirality. Angew. Chem., Int. Ed. 2014, 53, 5073–5077. 10.1002/anie.201311160. [DOI] [PubMed] [Google Scholar]
- Palmans A. R. A.; Meijer E. W.; Denmark S. E. Stereochemical Language in Supramolecular Polymer Chemistry: How We Can Do Better. J. Polym. Sci. 2021, 59, 1171–1174. 10.1002/pol.20200814. [DOI] [Google Scholar]
- Xing P.; Zhao Y. Controlling Supramolecular Chirality in Multicomponent Self-Assembled Systems. Acc. Chem. Res. 2018, 51, 2324–2334. 10.1021/acs.accounts.8b00312. [DOI] [PubMed] [Google Scholar]
- Aida T.; Meijer E. W.; Stupp S. I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würthner F.; Saha-Möller C. R.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Dong R.; Zhou Y.; Huang X.; Zhu X.; Lu Y.; Shen J. Functional Supramolecular Polymers for Biomedical Applications. Adv. Mater. 2015, 27, 498–526. 10.1002/adma.201402975. [DOI] [PubMed] [Google Scholar]
- Sun P.; Qin B.; Xu J.-F.; Zhang X. Supramonomers for Controllable Supramolecular Polymerization and Renewable Supramolecular Polymeric Materials. Prog. Polym. Sci. 2022, 124, 101486. 10.1016/j.progpolymsci.2021.101486. [DOI] [Google Scholar]
- Ouchi H.; Kizaki T.; Yamato M.; Lin X.; Hoshi N.; Silly F.; Kajitani T.; Fukushima T.; Nakayama K.-i.; Yagai S. Impact of Helical Organization on the Photovoltaic Properties of Oligothiophene Supramolecular Polymers. Chem. Sci. 2018, 9, 3638–3643. 10.1039/c7sc05093c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. D.; Simmons H. T. D.; Horner K. E.; Liu K.; Thompson R. L.; Steed J. W. Braiding, Branching and Chiral Amplification of Nanofibres in Supramolecular Gels. Nat. Chem. 2019, 11, 375–381. 10.1038/s41557-019-0222-0. [DOI] [PubMed] [Google Scholar]
- Martínez M. A.; Doncel-Giménez A.; Cerdá J.; Calbo J.; Rodríguez R.; Aragó J.; Crassous J.; Ortí E.; Sánchez L. Distance Matters: Biasing Mechanism, Transfer of Asymmetry, and Stereomutation in N-Annulated Perylene Bisimide Supramolecular Polymers. J. Am. Chem. Soc. 2021, 143, 13281–13291. 10.1021/jacs.1c06125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethy R.; Kumar J.; Métivier R.; Louis M.; Nakatani K.; Mecheri N. M. T.; Subhakumari A.; Thomas K. G.; Kawai T.; Nakashima T. Enantioselective Light Harvesting with Perylenediimide Guests on Self-Assembled Chiral Naphthalenediimide Nanofibers. Angew. Chem., Int. Ed. 2017, 56, 15053–15057. 10.1002/anie.201707160. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Mondal A. K.; Das T. K.; Grinbom G.; Tassinari F.; Mabesoone M. F. J.; Meijer E. W.; Naaman R. Highly Efficient and Tunable Filtering of Electrons’ Spin by Supramolecular Chirality of Nanofiber-Based Materials. Adv. Mater. 2020, 32, 1904965. 10.1002/adma.201904965. [DOI] [PubMed] [Google Scholar]
- Mondal A. K.; Preuss M. D.; Ślęczkowski M. L.; Das T. K.; Vantomme G.; Meijer E. W.; Naaman R. Spin Filtering in Supramolecular Polymers Assembled from Achiral Monomers Mediated by Chiral Solvents. J. Am. Chem. Soc. 2021, 143, 7189–7195. 10.1021/jacs.1c02983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders M. M. J.; Filot I. A. W.; Leenders J. M. A.; van der Schoot P.; Palmans A. R. A.; Schenning A. P. H. J.; Meijer E. W. Tuning the Extent of Chiral Amplification by Temperature in a Dynamic Supramolecular Polymer. J. Am. Chem. Soc. 2010, 132, 611–619. 10.1021/ja908053d. [DOI] [PubMed] [Google Scholar]
- Gillissen M. A. J.; Koenigs M. M. E.; Spiering J. J. H.; Vekemans J. A. J. M.; Palmans A. R. A.; Voets I. K.; Meijer E. W. Triple Helix Formation in Amphiphilic Discotics: Demystifying Solvent Effects in Supramolecular Self-Assembly. J. Am. Chem. Soc. 2014, 136, 336–343. 10.1021/ja4104183. [DOI] [PubMed] [Google Scholar]
- Ślęczkowski M. L.; Mabesoone M. F. J. J.; Ślęczkowski P.; Palmans A. R. A. A.; Meijer E. W. Competition between Chiral Solvents and Chiral Monomers in the Helical Bias of Supramolecular Polymers. Nat. Chem. 2021, 13, 200–207. 10.1038/s41557-020-00583-0. [DOI] [PubMed] [Google Scholar]
- Yagai S.; Yamauchi M.; Kobayashi A.; Karatsu T.; Kitamura A.; Ohba T.; Kikkawa Y. Control over Hierarchy Levels in the Self-Assembly of Stackable Nanotoroids. J. Am. Chem. Soc. 2012, 134, 18205–18208. 10.1021/ja308519b. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Guo Z.; Chen J.; Li W.; Zhong L.; Gao Y.; Jiang L.; Chi L.; Tian H.; Zhu W.-H. Enabling Light Work in Helical Self-Assembly for Dynamic Amplification of Chirality with Photoreversibility. J. Am. Chem. Soc. 2016, 138, 2219–2224. 10.1021/jacs.5b11580. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Jiang Y.; Han J.; Zhang L.; Liu M. Helical Nanostructures: Chirality Transfer and a Photodriven Transformation from Superhelix to Nanokebab. Angew. Chem., Int. Ed. 2019, 58, 785–790. 10.1002/anie.201811060. [DOI] [PubMed] [Google Scholar]
- Feringa B. L.; Browne W. R.. Molecular Switches, 2nd ed.; Wiley-VCH: Weinheim, 2011. [Google Scholar]
- Goulet-Hanssens A.; Eisenreich F.; Hecht S. Enlightening Materials with Photoswitches. Adv. Mater. 2020, 32, 1905966. 10.1002/adma.201905966. [DOI] [PubMed] [Google Scholar]
- Wang L.; Li Q. Photochromism into Nanosystems: Towards Lighting up the Future Nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. 10.1039/c7cs00630f. [DOI] [PubMed] [Google Scholar]
- Kitamoto Y.; Aratsu K.; Yagai S.. Photoresponsive Supramolecular Polymers. In Photoactive Functional Soft Materials; Wiley Online Books, 2019; pp 45–90. [Google Scholar]
- Bisoyi H. K.; Li Q. Light-Directed Dynamic Chirality Inversion in Functional Self-Organized Helical Superstructures. Angew. Chem., Int. Ed. 2016, 55, 2994–3010. 10.1002/anie.201505520. [DOI] [PubMed] [Google Scholar]
- Liu C.; Yang D.; Jin Q.; Zhang L.; Liu M. A Chiroptical Logic Circuit Based on Self-Assembled Soft Materials Containing Amphiphilic Spiropyran. Adv. Mater. 2016, 28, 1644–1649. 10.1002/adma.201504883. [DOI] [PubMed] [Google Scholar]
- Liu G.; Sheng J.; Wu H.; Yang C.; Yang G.; Li Y.; Ganguly R.; Zhu L.; Zhao Y. Controlling Supramolecular Chirality of Two-Component Hydrogels by J- and H-Aggregation of Building Blocks. J. Am. Chem. Soc. 2018, 140, 6467–6473. 10.1021/jacs.8b03309. [DOI] [PubMed] [Google Scholar]
- Zhao D.; van Leeuwen T.; Cheng J.; Feringa B. L. Dynamic Control of Chirality and Self-Assembly of Double-Stranded Helicates with Light. Nat. Chem. 2016, 9, 250–256. 10.1038/nchem.2668. [DOI] [PubMed] [Google Scholar]
- Ryabchun A.; Lancia F.; Chen J.; Morozov D.; Feringa B. L.; Katsonis N. Helix Inversion Controlled by Molecular Motors in Multistate Liquid Crystals. Adv. Mater. 2020, 32, 2004420. 10.1002/adma.202004420. [DOI] [PubMed] [Google Scholar]
- Foy J. T.; Li Q.; Goujon A.; Colard-Itté J.-R.; Fuks G.; Moulin E.; Schiffmann O.; Dattler D.; Funeriu D. P.; Giuseppone N. Dual-Light Control of Nanomachines That Integrate Motor and Modulator Subunits. Nat. Nanotechnol. 2017, 12, 540–545. 10.1038/nnano.2017.28. [DOI] [PubMed] [Google Scholar]
- Pooler D. R. S.; Lubbe A. S.; Crespi S.; Feringa B. L. Designing Light-Driven Rotary Molecular Motors. Chem. Sci. 2021, 12, 14964–14986. 10.1039/d1sc04781g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem S.; van Leeuwen T.; Lubbe A. S.; Wilson M. R.; Feringa B. L.; Leigh D. A. Artificial Molecular Motors. Chem. Soc. Rev. 2017, 46, 2592–2621. 10.1039/c7cs00245a. [DOI] [PubMed] [Google Scholar]
- van Leeuwen T.; Lubbe A. S.; Štacko P.; Wezenberg S. J.; Feringa B. L. Dynamic Control of Function by Light-Driven Molecular Motors. Nat. Rev. Chem. 2017, 1, 0096. 10.1038/s41570-017-0096. [DOI] [Google Scholar]
- Costil R.; Holzheimer M.; Crespi S.; Simeth N. A.; Feringa B. L. Directing Coupled Motion with Light: A Key Step Toward Machine-Like Function. Chem. Rev. 2021, 121, 13213–13237. 10.1021/acs.chemrev.1c00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.; Danowski W.; Mondal A. K.; Tassinari F.; Beek C. L. F.; Heideman G. H.; Santra K.; Cohen S. R.; Feringa B. L.; Naaman R. Multistate Switching of Spin Selectivity in Electron Transport through Light-Driven Molecular Motors. Adv. Sci. 2021, 8, 2101773. 10.1002/advs.202101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Feringa B. L. Dynamic Control of Chiral Space in a Catalytic Asymmetric Reaction Using a Molecular Motor. Science 2011, 331, 1429–1432. 10.1126/science.1199844. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Neubauer T. M.; Feringa B. L. Dynamic Control of Chirality in Phosphine Ligands for Enantioselective Catalysis. Nat. Commun. 2015, 6, 6652. 10.1038/ncomms7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel R.; Feringa B. L. Stereodivergent Anion Binding Catalysis with Molecular Motors. Angew. Chem., Int. Ed. 2020, 59, 785–789. 10.1002/anie.201913054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Loos M.; van Esch J.; Stokroos I.; Kellogg R. M.; Feringa B. L. Remarkable Stabilization of Self-Assembled Organogels by Polymerization. J. Am. Chem. Soc. 1997, 119, 12675–12676. 10.1021/ja972899z. [DOI] [Google Scholar]
- de Loos M.; van Esch J.; Kellogg R. M.; Feringa B. L. Chiral Recognition in Bis-Urea-Based Aggregates and Organogels through Cooperative Interactions. Angew. Chem., Int. Ed. 2001, 40, 613–616. . [DOI] [PubMed] [Google Scholar]
- Wezenberg S. J.; Croisetu C. M.; Stuart M. C. A.; Feringa B. L. Reversible Gel–Sol Photoswitching with an Overcrowded Alkene-Based Bis-Urea Supergelator. Chem. Sci. 2016, 7, 4341–4346. 10.1039/c6sc00659k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa Y.; Nan Y.; Okuro K.; Aida T. Mechanically Robust, Readily Repairable Polymers via Tailored Noncovalent Cross-Linking. Science 2018, 359, 72–76. 10.1126/science.aam7588. [DOI] [PubMed] [Google Scholar]
- Xu F.; Pfeifer L.; Crespi S.; Leung F. K.-C.; Stuart M. C. A.; Wezenberg S. J.; Feringa B. L. From Photoinduced Supramolecular Polymerization to Responsive Organogels. J. Am. Chem. Soc. 2021, 143, 5990–5997. 10.1021/jacs.1c01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Loos M.; Feringa B. L.; van Esch J. H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 3615–3631. 10.1002/ejoc.200400723. [DOI] [Google Scholar]
- Estroff L. A.; Hamilton A. D. Effective Gelation of Water Using a Series of Bis-urea Dicarboxylic Acids. Angew. Chem., Int. Ed. 2000, 39, 3447–3450. . [DOI] [PubMed] [Google Scholar]
- Obert E.; Bellot M.; Bouteiller L.; Andrioletti F.; Lehen-Ferrenbach C.; Boué F. Both Water- and Organo-Soluble Supramolecular Polymer Stabilized by Hydrogen-Bonding and Hydrophobic Interactions. J. Am. Chem. Soc. 2007, 129, 15601–15605. 10.1021/ja074296l. [DOI] [PubMed] [Google Scholar]
- Pal A.; Karthikeyan S.; Sijbesma R. P. Coexisting Hydrophobic Compartments through Self-Sorting in Rod-like Micelles of Bisurea Bolaamphiphiles. J. Am. Chem. Soc. 2010, 132, 7842–7843. 10.1021/ja101872x. [DOI] [PubMed] [Google Scholar]
- Leenders C. M. A.; Albertazzi L.; Mes T.; Koenigs M. M. E.; Palmans A. R. A.; Meijer E. W. Supramolecular Polymerization in Water Harnessing Both Hydrophobic Effects and Hydrogen Bond Formation. Chem. Commun. 2013, 49, 1963–1965. 10.1039/c3cc38949a. [DOI] [PubMed] [Google Scholar]
- Albertazzi L.; van der Zwaag D.; Leenders C. M. A.; Fitzner R.; van der Hofstad R. W.; Meijer E. W. Probing Exchange Pathways in One-Dimensional Aggregates with Super-Resolution Microscopy. Science 2014, 344, 491–495. 10.1126/science.1250945. [DOI] [PubMed] [Google Scholar]
- Garzoni M.; Baker M. B.; Leenders C. M. A.; Voets I. K.; Albertazzi L.; Palmans A. R. A.; Meijer E. W.; Pavan G. M. Effect of H-Bonding on Order Amplification in the Growth of a Supramolecular Polymer in Water. J. Am. Chem. Soc. 2016, 138, 13985–13995. 10.1021/jacs.6b07530. [DOI] [PubMed] [Google Scholar]
- Wolffs M.; George S. J.; Tomović Ž.; Meskers S. C. J.; Schenning A. P. H. J.; Meijer E. W. Macroscopic Origin of Circular Dichroism Effects by Alignment of Self-Assembled Fibers in Solution. Angew. Chem., Int. Ed. 2007, 46, 8203–8205. 10.1002/anie.200703075. [DOI] [PubMed] [Google Scholar]
- Feringa B. L. Vision Statement: Materials in Motion. Adv. Mater. 2020, 32, 1906416. 10.1002/adma.201906416. [DOI] [PubMed] [Google Scholar]
- Krause S.; Feringa B. L. Towards Artificial Molecular Factories from Framework-Embedded Molecular Machines. Nat. Rev. Chem. 2020, 4, 550–562. 10.1038/s41570-020-0209-9. [DOI] [Google Scholar]
- Danowski W.; van Leeuwen T.; Browne W. R.; Feringa B. L. Photoresponsive Porous Materials. Nanoscale Adv. 2021, 3, 24–40. 10.1039/d0na00647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S.; Sasaki S.; Sairi A. S.; Iwai R.; Tang B. Z.; Konishi G. i. Principles of Aggregation-Induced Emission: Design of Deactivation Pathways for Advanced AIEgens and Applications. Angew. Chem., Int. Ed. 2020, 59, 9856–9867. 10.1002/anie.202000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.-W.; Li D.; Li X.; White P. B.; Mecinović J.; Ma X.; Ågren H.; Nolte R. J. M.; Tian H. Multicolor Photoluminescence Including White-Light Emission by a Single Host-Guest Complex. J. Am. Chem. Soc. 2016, 138, 13541–13550. 10.1021/jacs.6b04776. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang J.; Li H.; Song N.; Wang D.; Tang B. Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172. 10.1039/c9cs00495e. [DOI] [PubMed] [Google Scholar]
- Cao S.; Shao J.; Abdelmohsen L. K. E. A.; van Hest J. C. M. Amphiphilic AIEgen-polymer Aggregates: Design, Self-assembly and Biomedical Applications. Aggregate 2022, 3, e128 10.1002/agt2.128. [DOI] [Google Scholar]
- Xu F.; Pfeifer L.; Stuart M. C. A.; Leung F. K.-C.; Feringa B. L. Multi-Modal Control over the Assembly of a Molecular Motor Bola-Amphiphile in Water. Chem. Commun. 2020, 56, 7451–7454. 10.1039/d0cc02177f. [DOI] [PubMed] [Google Scholar]
- Vlatković M.; Feringa B. L.; Wezenberg S. J. Dynamic Inversion of Stereoselective Phosphate Binding to a Bisurea Receptor Controlled by Light and Heat. Angew. Chem., Int. Ed. 2016, 55, 1001–1004. 10.1002/anie.201509479. [DOI] [PubMed] [Google Scholar]
- Wang J.; Hou L.; Browne W. R.; Feringa B. L. Photoswitchable Intramolecular Through-Space Magnetic Interaction. J. Am. Chem. Soc. 2011, 133, 8162–8164. 10.1021/ja202882q. [DOI] [PubMed] [Google Scholar]
- Luo W.; Wang G. Photo-Responsive Fluorescent Materials with Aggregation-Induced Emission Characteristics. Adv. Opt. Mater. 2020, 8, 2001362. 10.1002/adom.202001362. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.