Abstract
Background: Postoperative cognitive dysfunction (POCD), also known as delayed neurocognitive recovery (up to 30 days) and postoperative neurocognitive disorder (up to 12 months), is a frequent complication of the neurological system associated with poor outcome. This randomized controlled trial aimed to determine whether bispectral (BIS) monitoring is correlated with delayed neurocognitive recovery, postoperative neurocognitive disorder, or postoperative delirium (POD). Methods: Among 197 patients included in the study, 100 were assigned to the BIS group and 97 to the control group. The BIS index was kept at 40-60 in the BIS group, and the depth of anesthesia in the control group was maintained according to anesthetists’ clinical experience. Cognitive function was evaluated from the 1st-7th day after the operation and the time of discharge, and at 1st month, 6th months, and 1 year after the operation. Results: The incidence of delayed neurocognitive recovery (3% vs. 21.6%, P<0.001, at 7th day) (3% vs. 21.1%, P<0.001, at 1st month) and postoperative neurocognitive disorder (6.2% vs. 21.3%, P=0.002, at 6th month) (4.4% vs. 16.3%, P=0.009, at 1 year) were lower in the BIS group, while there was no significant difference in POD between the two groups (12% vs. 19.6%, P=0.144). The average value of intraoperative BIS was lower in the BIS group (43.75 vs. 50.69, P<0.001). The postoperative hospitalization time (9.99 vs. 12.41, P<0.001) and the mortality (5.4% vs. 14.4%, P=0.042) were significantly decreased, while satisfaction was higher in the BIS group (39% vs. 24.7%, P=0.009). Conclusion: BIS decreases delayed neurocognitive recovery and postoperative neurocognitive disorder; however, it is not associated with POD. BIS monitoring could effectively lessen postoperative hospitalization and mortality and increase patient satisfaction.
Keywords: Delayed neurocognitive recovery, postoperative neurocognitive disorder, postoperative delirium, BIS monitoring
Introduction
Postoperative cognitive dysfunction (POCD), also known as neurocognitive recovery (up to 30 days) and postoperative neurocognitive disorder (up to 12 months), is a complication of the neurological system secondary to surgery and anesthesia, that mainly manifests as impairment in memory, cognition, and computing, disability to combine activities of daily living, and psychomotor dexterity [1,2]. The disorder has been associated with dementia [3], which is probably due to the similar mechanism of amyloid β peptide oligomerization and deposition [4]. The onset of postoperative delirium (POD), however, is always acute and fluctuating and is mainly characterized by inattention, impairments in thinking, perception, memory, psychomotor behavior, sleep-wake schedule, and change of consciousness level [5-7]. According to previous studies, the morbidity of POCD in non-cardiac surgery patients at discharge from hospital was 36.6% in those aged 18-39 years, 30.4% in those aged 40-59 years, and 41.15% in those older than 60 years. 12.7% of patients over 60 years were diagnosed with POCD three months after the operation [8]. According to the type of surgery, the incidence of POD ranged from 10% to 55%. In elderly patients, the incidence of POD was reported to reach 50% [9]. The incidence of the two diseases was high, particularly in elderly patients, which is consistent with a number of studies suggesting age as an important risk factor for both diseases [10-12]. The developments of society and improved medical care have resulted in an increasingly aging population. Elderly patients increasingly undergo surgery and anesthesia, which inevitably results in a substantial increase in the incidence of delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD. Consequently, the social health and medical problems caused by these diseases have attracted the attention of many scholars [9].
Some studies have summarized and classified cognitive dysfunction. For example, cognitive impairment during the preoperative and postoperative period, which includes cognitive decline diagnosed before operation (neurocognitive disorder), POD and delayed neurocognitive recovery (up to postoperative 30 days), and postoperative neurocognitive disorder (up to postoperative 12 months), have been classified as perioperative neurocognitive disorders (PND) [13].
Although the mechanism of neuroinflammation is well known, the pathogenesis of postoperative cognition impairment remains unclear [14]. There are still many studies attempting to investigate effective ways to prevent its occurrence. Intra-operative neuromonitoring has been increasingly used to monitor the effectiveness of anesthesia which primarily inhibits the experience of surgery and then disconnects consciousness from the environment [15]. For example, the BIS monitor is mainly used to indicate the depth of anesthesia by analyzing and integrating several different descriptors of the electroencephalogram (EEG) to form a single value [12,16]. Numerous studies have indicated that monitoring anesthesia with BIS can effectively reduce anesthesia exposure and reduce the occurrence of POD [17] and POCD, as well as accelerate the recovery from anesthesia [18,19]. While Radtke et al. [12] reported that monitoring depth of anesthesia did not alter the morbidity of POCD after the operation on the 7th day and 90th day, the available research on the relationship between the use of BIS and delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD is controversial and lacking [20].
Therefore, this study aimed to explore the correlation between BIS-monitoring and the incidence of delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD in laparoscopic gastrointestinal surgery.
Material and methods
This single-center prospective randomized controlled trial included 220 patients scheduled to undergo laparoscopic gastrointestinal surgery at the Second People’s Hospital of Yibin, Sichuan, China. This trial was approved by the Medical Ethics Committee of the Second People’s Hospital of Yibin (referral number 2020-055-01, ChiCTR2000032463), and every patient provided informed consent. The study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Patients were equally enrolled in the BIS and control groups according to computer-generated random sequences. First, member staff A designed two anesthesia plans and put them in envelopes. Second, member staff B screened the patients and extracted the envelopes. Third, member staff C implemented the anesthesia plan according to the envelope and recorded the data. Fourth, member staff D evaluated and recorded cognitive function. Finally, member staff E analyzed all the data and obtained the results. The staff participating in the study were blinded to the other data; they all received training lasting for a week before the trial started.
The inclusion criteria were as follows: the anesthesia time >2 hours; age >18 years; American Society of Anesthesiologists’ physical status (ASA) of I-III.
The exclusion criteria included patients with a history of mental and neurological disorders, excessive drinking and drug abuse, addiction to opioids or tranquilizers; severe organ functional diseases, stroke; mini-mental state examination (MMSE) score <20 (as there are many rural people with no educational background in China, we chose the lower score as the standard); those that could not complete the questionnaire, and who failed to complete the operation or anesthesia.
This trial’s main outcome was the incidence of delayed neurocognitive recovery on 7th day and 1 month, postoperative neurocognitive disorder at 6th month and 1 year, POD from 1st day to 7th day. Only two surgeons participated in the present trial, which should effectively reduce the difference caused by having more surgeons. Most of the patients were diagnosed with gastrointestinal cancer in the trial. They needed to be re-hospitalized for chemotherapy after the operation; therefore, their long-term cognitive function could be easily acquired. A series of scales could lead to adverse effects and affect patients’ completion and accuracy [21]. The MMSE scale was easy to complete and reliable (in the present study, most patients had a low education level, so the acceptance of this scale was higher). We selected this scale as the only screening tool before surgery and evaluated the cognitive function, including the memory, recollection, attention, computational ability, and writing and painting abilities postoperatively [1,22,23]. All patients were tested with the MMSE one day before the surgery. We used the MMSE and CAM (The Confusion Assessment Method) scale, which was derived from the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) [5,12], to diagnose postoperative cognitive function. A patient was considered to have delayed neurocognitive recovery (at 7th day and 1 month), or postoperative neurocognitive disorder (at 6th month and 1 year) when postoperative MMSE score was ≥2 points compared to preoperative scores [24,25]; or was deemed as having POD if CAM scores were >22. If there were any doubts about the scale score, a specialist neurologist was invited to re-diagnose it.
None of the patients were premedicated. Before induction, we had already placed the electrode sensor of the BIS index (Covidien 186-0106, YZB/USA 0793-2013) on the patient’s forehead and recorded the number every 5 minutes. In the BIS group, the BIS index was maintained between 40 and 60 during anesthesia, while in the control group, it was kept at the appropriate depth for anesthesia that was decided by the anesthesiologist based on his experience. The BIS monitor was covered and anesthesiologists were blinded to it. Next, propofol 2 mg/kg, midazolam 0.05 mg/kg, sufentanil 0.4 ug/kg, and cisatracurium 0.1 mg/kg were used to complete the induction, while an appropriate depth of anesthesia was maintained with propofol 4-12 mg/kg/h and remifentanil 0.1-0.3 ug/kg/min. The right invasive radial artery pressure was monitored, and the artery blood gas was analyzed.
During the anesthesia, mean arterial blood pressure was maintained at about 20% of its baseline. Otherwise, ephedrine, metaraminol, or nitroglycerin was used. Heart rate was kept at 50 to 100 beats per minute. Atropine and esmolol were needed if they exceeded the range. PetCO2 was held at 35 to 45 mmHg by adjusting the ventilation parameters. The patients were excluded from the study if they refused the trial, or developed serious complications. EEG, PR, SPO2, NIBP, IBP, PetCO2, and BIS index were monitored and recorded in all patients for data collection.
Finally, the patient’s characteristic data, such as age, sex, weight, height, body mass index (BMI), education status (years), ASA, and preoperative complications, were collected. The surgery data included infusion volume, blood transfusion volume, bleeding volume, urine volume, the dosage of anesthetics, the dose of vasoactive drugs, recovery time after the operation, concentration of CRP (C-reactive protein), and BIS value. The postoperative data included the incidence of delayed neurocognitive recovery, postoperative neurocognitive disorder and POD, anesthesia satisfaction, length of postoperative hospitalization, complications, and mortality.
Statistical analysis
Data were assessed using the Statistical Product and Service Solutions Group version 22.0. Data that conformed to normal distribution were described by mean ± standard deviation, while those that did not conform to normal distribution were shown as median and range interquartile. Comparisons were made using T-tests or Rank sum test for continuous variables and Chi-square test or Fisher’s exact test for dichotomous variables. A P<0.05 was considered a significant difference.
Results
Five patients in each group were excluded because their MMSE score was <20. Surgeries were delayed for 2 patients in the BIS group and 5 patients in the control group. 1 patient in the BIS group and 3 patients in the control group were excluded as their anesthesia was <120 min. Two patients from the BIS group refused to participate in the experiment after signing informed consent. Eventually, 100 patients from the BIS group and 97 from the control group were included in the analysis (Figure 1).
Figure 1.
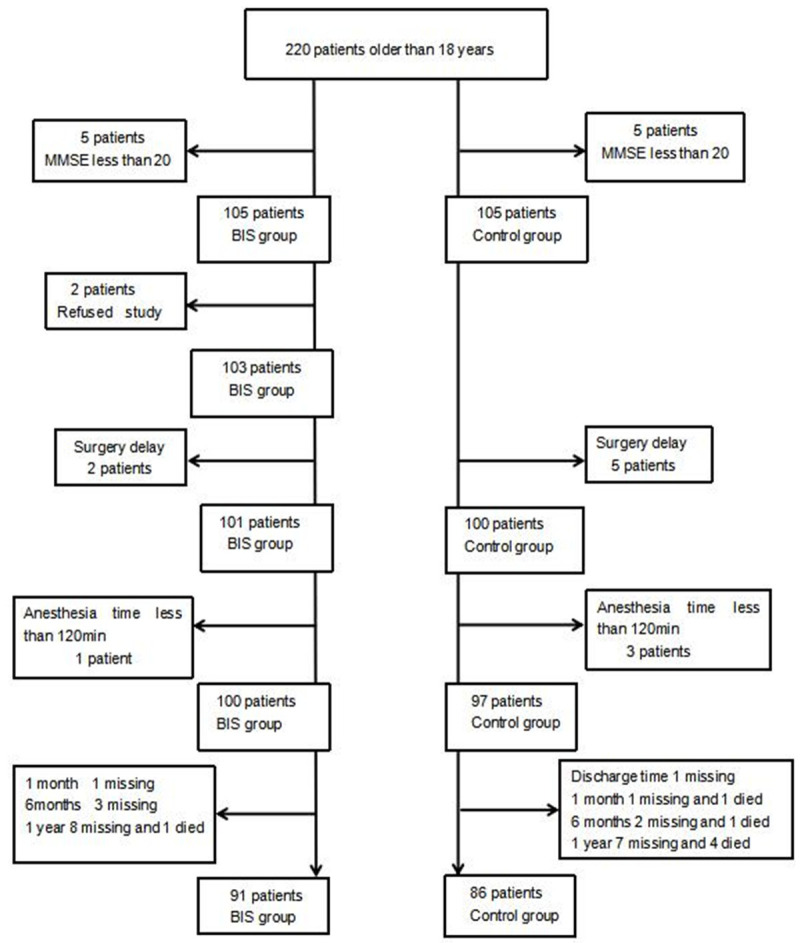
Flowchart of trial enrollment. MMSE, mini-mental state examination; BIS indicates bispectral index.
There was no significant difference in educational status, ASA, age, height, weight, BMI, and preoperative complications (coronary heart disease, hypertension, and diabetes) between the two groups (Table 1).
Table 1.
Preoperative information of patients’ characteristics
| Characteristic | BIS group (n=100) | Control group (n=97) | P |
|---|---|---|---|
| Height (cm) | 162.64±7.69 | 161.72±6.02 | 0.351 |
| Weight (kg) | 59.45±7.64 | 59.78±10.64 | 0.805 |
| BMI | 22.61±3.49 | 22.95±3.70 | 0.512 |
| Age (yr) | 62.98±10.77 | 61.69±10.27 | 0.391 |
| Education status (yr) | 0.252 | ||
| Illiteracy | 1 (1.0%) | 6 (6.2%) | |
| Primary school | 31 (31.0%) | 36 (37.1%) | |
| Junior school | 38 (38.0%) | 32 (33.0%) | |
| High school | 27 (27.0%) | 21 (21.6%) | |
| Higher degree (University) | 3 (3.0%) | 2 (2.1%) | |
| ASA | 0.943 | ||
| I | 25 (25.0%) | 13 (13.4%) | |
| II | 53 (53.0%) | 75 (77.3%) | |
| III | 22 (22.0%) | 9 (9.3%) | |
| Gender | 0.232 | ||
| Male | 66 (66.0%) | 56 (57.7%) | |
| Female | 34 (34.0%) | 41 (42.3%) | |
| Preoperative complications | |||
| Hypertension | 23 (23.0%) | 16 (16.5%) | 0.252 |
| Diabetes | 4 (4.0%) | 2 (2.1%) | 0.429 |
| Coronary heart disease | 8 (8.0%) | 5 (5.2%) | 0.421 |
Data are shown as mean ± standard deviation, median (range interquartile), and number (%). P<0.05 indicates significant difference.
There was no significant difference in transfusion volume, anesthesia time, operation time, bleeding volume, mean arterial pressure (MAP), and mean concentration of C-reaction protein (mg/L) between the two groups (Table 2). However, the dosage of propofol (1810.00±533.33 vs. 1336.08±461.75, P<0.001), cisatracurium (37.61±13.59 vs. 30.57±8.08, P<0.001), and remifentanil (2207.00±766.25 vs. 1970.62±870.23, P=0.044) were higher in the BIS group, while the dose of sufentanil (43.86±7.60 vs. 46.90±10.73, P=0.023) was lower than that in the control group. The MAP was similar in the two groups, while the use of vasoactive drugs (5.0 vs. 18.6%, P=0.003) was higher in the control group (Table 2).
Table 2.
Perioperative details
| BIS group (n=100) | Control group (n=97) | P | |
|---|---|---|---|
| Operation time (min) | 235.65±62.42 | 229.59±75.87 | 0.542 |
| Anesthesia time (min) | 261.74±63.09 | 253.28±76.76 | 0.400 |
| Liquid infusion (ml) | 2021.10±583.09 | 2037.11±687.59 | 0.860 |
| Volume of bleeding (ml) | 277.30±105.14 | 236.70±206.11 | 0.082 |
| Dosage of anesthesia | |||
| Propofol (mg) | 1810.00±533.33 | 1336.08±461.75 | <0.001 |
| Remifentanil (ug) | 2207.00±766.25 | 1970.62±870.23 | 0.044 |
| Cisatracurium (mg) | 37.61±13.59 | 30.57±8.08 | <0.001 |
| Sufentanil (ug) | 43.86±7.60 | 46.90±10.73 | 0.023 |
| Dose of vasoactive drugs | 5 (5.0%) | 18 (18.6%) | 0.003 |
| Intraoperative MABP (mmHg) | 89.22±4.92 | 90.73±7.31 | 0.093 |
| Intraoperative mean BIS | 43.75±6.78 | 50.69±6.33 | <0.001 |
| Concentration CRP (mg/L) | 32.97±22.80 | 36.86±28.43 | 0.292 |
Data are shown as mean ± standard deviation, median (range interquartile), and number (%). P<0.05 is a statistically significant difference.
There was no significant difference in postoperative ICU admission between the two groups (6% vs. 5.2%, P=0.796). In the BIS group, the time of postoperative recovery (24.76±9.36 vs. 29.49±9.72, P=0.001) and the length of postoperative hospitalization (9.99±3.94 vs. 12.41±4.61, P<0.001) were shorter than that in the control group. Also, the mortality (5.4% vs. 14.4%, P=0.042) in the BIS group was lower than that in the control group. Patients’ satisfaction (P=0.009) in the BIS group was better compared to the control group (Table 3).
Table 3.
Patients’ information after the operation
| BIS group (n=100) | Control group (n=97) | P | |
|---|---|---|---|
| Stop medication-discharged (min) | 24.76±9.36 | 29.49±9.72 | 0.001 |
| ICU admission | 6 (6.0%) | 5 (5.2%) | 0.796 |
| Postoperative hospital stay (d) | 9.99±3.94 | 12.41±4.61 | <0.001 |
| Satisfaction | 0.009 | ||
| 0 | 0 | 0 | |
| 1 | 25 (25.0%) | 44 (45.4%) | |
| 2 | 36 (36.0%) | 29 (29.9%) | |
| 3 | 39 (39.0%) | 24 (24.7%) | |
| Death | 5 (5.4%) | 13 (14.4%) | 0.042 |
Data are shown as mean ± standard deviation, median (range interquartile), and number (%). P<0.05 indicates a significant difference. Satisfaction: 0 means dissatisfied, 1 means average, 2 means slightly satisfied, and 3 means satisfied.
There was a significant difference between the two groups when comparing BIS regarding delayed neurocognitive recovery and postoperative neurocognitive disorder (Table 4).
Table 4.
Main outcomes of delayed neurocognitive recovery, postoperative neurocognitive disorder, and postoperative delirium
| BIS group | Control group | P | |
|---|---|---|---|
| Delayed neurocognitive recovery | |||
| 7th day (B=100, C=97) | 3 (3.0%) | 21 (21.6%) | <0.001 |
| 1 month (B=100, C=95) | 3 (3.0%) | 20 (21.1%) | <0.001 |
| Discharge (B=100, C=96) | 1 (1.0%) | 8 (8.3%) | 0.035 |
| Postoperative neurocognitive disorder | |||
| 6th month (B=97, C=94) | 6 (6.2%) | 20 (21.3%) | 0.002 |
| 1 year (B=91, C=86) | 4 (4.4%) | 14 (16.3%) | 0.009 |
| POD (B=100, C=97) | 12 (12.0%) | 19 (19.6%) | 0.144 |
Data are shown as mean ± standard deviation, median (range interquartile), and number (%). B is the number of patients in the BIS group, and C is the number of patients in the control group.
The incidence of delayed neurocognitive recovery and postoperative neurocognitive disorder generally showed a downward trend. The lowest morbidity of delayed neurocognitive recovery in the two groups was at discharge time (1% vs. 8.3%). The highest morbidity of postoperative cognitive dysfunction was 21.6% in the control group. The highest incidence of 6.2% at postoperative 6th month in the BIS group was still lower than that at each time point in the control group (Table 4).
Figure 2 shows the mean score of MMSE in the BIS group that was moderately increased until discharge time point and then decreased. At the same time, there was a decrease in the control group except for the discharge time point. The results showed that the BIS value in the BIS group was lower than in the control group (43.75 vs. 50.69, P<0.001).
Figure 2.

MMSE scores of the two groups at different time points.
In order to further explore the relationship of BIS values and the incidence of cognitive dysfunction, patients were divided in the control group into a postoperative cognitive impairment group (those who experienced the BIS value beyond 40-60, including delayed neurocognitive recovery and postoperative neurocognitive disorder) and a non-postoperative cognitive impairment group. There was no significant difference between the two groups in the time distribution when the BIS number was out of the standard range (Table 5).
Table 5.
Comparison of the time distribution of BIS beyond the range in the control group
| No postoperative cognitive impairment (min) | Postoperative cognitive impairment (min) | P | |
|---|---|---|---|
| POCD 7th day (Np=21, Nn=76) | |||
| BIS<40 | 20.0 (0.00, 55.0) | 40.0 (10.0, 67.5) | 0.144 |
| BIS>60 | 40.0 (11.25, 82.5) | 15.0 (7.5, 60.0) | 0.155 |
| POCD 1 month (Np=20, Nn=75) | |||
| BIS<40 | 20.0 (0.0, 60.0) | 32.5 (6.25, 57.5) | 0.523 |
| BIS>60 | 30.0 (10.0, 75.0) | 40.0 (11.25, 68.75) | 0.745 |
| POCD discharge (Np=8, Nn=88) | |||
| BIS<40 | 22.5 (0.0, 55.0) | 42.5 (2.5, 73.75) | 0.502 |
| BIS>60 | 40.0 (10.0, 75.0) | 25.0 (6.25, 62.5) | 0.562 |
| POCD 6th month (Np=20, Nn=74) | |||
| BIS<40 | 20.0 (0.0, 56.25) | 32.5 (11.25, 63.75) | 0.231 |
| BIS>60 | 40.0 (10.0, 71.25) | 22.5 (6.25, 66.25) | 0.433 |
| POCD 1 year (Np=14, Nn=72) | |||
| BIS<40 | 20.0 (0.0, 58.75) | 32.5 (3.75, 65.0) | 0.657 |
| BIS>60 | 35.0 (10.0, 68.75) | 27.5 (5.0, 57.5) | 0.441 |
Data are shown as the median (range interquartile). (Np means the number in the postoperative cognitive impairment group; Nn means the number in the non-postoperative cognitive impairment group).
The occurrence of POD in the BIS group and control group was 12% and 19.6% in the first three days, without statistical difference between the two groups.
Discussion
The main finding of the present study was that BIS-monitoring during anesthesia reduced the incidence of delayed neurocognitive recovery and postoperative neurocognitive disorder. This may be because the BIS value within the normal range reduced the effects of neuroinflammation [26] associated with lighter anesthesia and the toxic effects of drugs associated with deeper anesthesia. Also, patients in the BIS group who were continuously under deeper anesthesia (mean BIS=43.75) had decreased postoperative cognitive impairment. Moreover, the time under deep anesthesia (BIS <40) and light anesthesia (BIS>60) among patients with POCD and patients without POCD were similar in the control group. Thus, the BIS value and the value combined with the duration could be construed as a risk factor.
The curve of the score with MMSE (Figure 1) revealed that the mean score of MMSE in the two groups had increasing and decreasing trends. This illustrated that the BIS monitoring effectively reduced postoperative cognitive impairment or even improved postoperative cognitive function [27]. It is possible that besides the learning effect, most patients performed well at answering the questionnaire since they were in a better mood and were already familiar with the hospital environment at discharge.
Our study demonstrated that the postoperative cognitive disorder at different time points had different fluctuations and downtrends. According to our data, the postoperative cognitive disorder was a common complication after surgery, whose onset time may be very late. Although most of the patients’ symptoms were usually reversible, some people still suffered from a decline in cognitive function. Moreover, the highest morbidity was 21.6%, which was similar to the results reported by Chan et al. [18]. In general, the occurrence of delayed neurocognitive recovery and postoperative neurocognitive disorder was higher in the control group at all time points, which showed that the use of BIS could effectively decrease the occurrence of postoperative cognitive impairment [17,28]. In our study, the BIS group who received high doses of anesthesia had a more stable cardiovascular response, better postoperative recovery, and overall higher satisfaction, which may lead to a lower cognitive dysfunction in the BIS group.
Our results were in line with Chan et al. [19], who reported that BIS-guided anesthesia could decrease the risk of POCD and POD three months after surgery in 921 elderly patients who underwent non-cardiac surgery. Also, their median BIS value was 53 vs. 38.6 in the BIS group, with a lower dose of anesthetic in the control group, which was opposite to our BIS value in the BIS and control groups (43.75 vs. 50.69). This may be due to the larger doses of narcotic drugs used in our study.
At the same time, Farag et al. [29] discovered that a deeper (median BIS 39 vs. 51) anesthesia was associated with better cognitive function 4-6 weeks postoperatively in 74 patients, which may be due to lower BIS. Deeper anesthesia means a lower metabolic rate of the brain, which can increase the tolerance to ischemia and hypoxia, reducing the body’s stress response [18]. These results were similar to the findings of Tasbihgou and colleagues [30], arguing that deepening anesthesia attenuated the brain changes associated with hypoxia in rats. In addition, these studies illustrated that deep anesthesia, which was associated with inhibition of inflammation [31] and burst suppression, may be protective factors against POCD [32]. This is consistent with our findings, where patients with a relatively lower value (43.75 vs. 50.69) had a lower incidence of delayed neurocognitive recovery and postoperative neurocognitive disorder.
Radtke et al. [12] evaluated BIS with POCD and POD in 1155 patients, revealing that BIS did not change the incidence of POCD on 7th and 90th day postoperatively. This may be because the BIS values in their two groups of patients were similar, while in our study, there were substantial differences (43.75 vs. 50.69, P<0.001). Also, the observation period in our study was longer, and the results from 3 months after operation were not included. Cao et al. [1] also pointed out that there were no significant differences in the BIS group compared to the control group (15.15% vs. 33.33%) after 7 days, which was inconsistent with our study as our sample size was small. Moreover, their postoperative cognition impairment incidence was higher compared to both groups in our study, which is probably due to substantial trauma induced by liver transplantation. Compared to the stress response to surgical trauma, anesthesia may have a more negligible effect on cognitive impairment.
Several studies showed that lower BIS implied a larger dose of anesthetic, which in turn contributed to the risk of POCD. For example, intra-operative hypotension [5] and increased toxicity of drugs [17] were found to be detrimental to postoperative cognitive function [18,27,33].
In our study, a relatively lower mean BIS value was normal. Although the anesthetic dose was increased and the active vascular drug dosage was lower in the BIS group, there was no noticeable difference in the mean ABP between the two groups. Therefore, our study’s relatively lower BIS value was appropriate, and using BIS-monitoring could decrease the incidence of postoperative cognitive disorder. The morbidity of POD was 19.6% in the control group, which was consistent with Evered’s results [13] (15-53%). Also, the morbidity mainly occurred during the first three days [34] due to the effects of anxiety, pain [35], and residual anesthetic. Yet, the difference was not statistically significant between the groups in the present study, which may be due to the following reasons: first, postoperative cognitive disorder occurred more frequently in elderly patients with poor outcomes and increased mortality [11]. However, some patients were younger than 60 years old, although the two groups’ mean age was >60 years old (62.98 vs. 61.69, P=0.391). Second, the sample size was too small to reveal the obvious differences. Third, the patients’ satisfaction in the two groups was high (no dissatisfaction was reported) due to the good postoperative analgesia, consultation, and comfort provided from 1 to 7 days after the operation, which may be the protective factors for POD [36]. Finally, stable circulation in the perioperative period and the absence of serious complications in patients may reduce the occurrence of POD. In addition, the low activity of POD [5] may be omitted by researchers, which can also decrease the POD rates and may result in no significant difference between the two groups.
When BIS was maintained in a fixed range of 40-60, it could reduce the inflammatory reaction and intraoperative awareness induced by light anesthesia. At the same time, it decreased the harm of hypotension and burst suppression caused by deep anesthesia. The BIS monitoring reduced the time of post-operation recovery (24.76±9.36 vs. 29.49±9.72, P=0.001), and the length of postoperative hospitalization (9.99±3.94 vs. 12.41±4.61, P<0.001), as well as increased the patients’ satisfaction (P=0.009).
The lower average value of BIS group patients led to a higher intraoperative dose of anesthetic drugs. In contrast, the control group had a lower dose of anesthetic drugs and a higher dosage of vasoactive drugs due to an unstable cardiovascular system, which led to increased use of sufentanil for postoperative analgesia that in turn may lead to a longer recovery from anesthesia and lower satisfaction.
Furthermore, the significantly shorter postoperative recovery time and the shorter length of postoperative hospital stays in the BIS group suggested that patients had better recovery from surgery and disease. Also, a lower mortality rate after surgery was observed in the BIS group (5.4% vs. 14.4%, P=0.042).
Finally, CRP concentration in the two groups associated with inflammation showed no significant difference, probably due to the mean BIS value in the standard range of 40-60. Delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD were frequent complications after the operation, whose detailed pathogenesis remains unclear [37]. The risk factors were increasing age [24], poor education [5], preoperative complications, pre-operation cognitive impairment, poor functional status, depression, alcohol abuse, the duration and type of anesthesia, homeostasis, hypotension, infection, hypoxia, and pain [6,8,27,36,38,39]. Besides, a more significant risk factor may be surgery, as it has been previously reported that surgery is more likely to lead to cognitive decline than anesthetics [40].
At present, there is no golden standard [41,42] for diagnosis or effective treatment [36] of delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD. Furthermore, although BIS has been widely used, there are few controversial studies on the relationship between BIS and postoperative cognitive impairment. Therefore, this prospective randomized clinical trial aimed to investigate whether there was a correlation between BIS and delayed neurocognitive recovery, postoperative neurocognitive disorder, and POD in order to provide a new method to reduce postoperative cognitive impairment.
In general, the operation type was single, there were two surgeons who performed all the operations, and there were no ASA IV patients in the two groups, so the results were reliable. Consultation and comfort were provided timely. The most important aspect is that our study involved both long-term and short-term cognitive outcomes.
There are several limitations to the present study. First, MMSE could not accurately judge the damage of specific brain functional areas or the false-negative results of delayed neurocognitive recovery and postoperative neurocognitive disorder caused by mutual compensation of functional brain areas. Second, there was no other control group in the general population that could be used to exclude normal cognitive changes. Third, our study included a small proportion of diabetic patients, and diabetes was previously associated with POCD [43].
In summary, delayed neurocognitive recovery and postoperative neurocognitive disorders are common, reversible, and long-term complications after an operation. Using BIS can decrease delayed neurocognitive recovery and postoperative neurocognitive disorder regardless of the patient’s age (>18 years old), shorten the postoperative hospitalization stay and the mortality, and increase the patients’ satisfaction.
Disclosure of conflict of interest
None.
References
- 1.Cao YH, Chi P, Zhao YX, Dong XC. Effect of bispectral index-guided anesthesia on consumption of anesthetics and early postoperative cognitive dysfunction after liver transplantation: an observational study. Medicine (Baltimore) 2017;96:e7966. doi: 10.1097/MD.0000000000007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Z, Fu P, Chen M, Bi Q. Association of CT perfusion and postoperative cognitive dysfunction after off-pump coronary artery bypass grafting. Neurol Res. 2016;38:533–537. doi: 10.1080/01616412.2016.1187830. [DOI] [PubMed] [Google Scholar]
- 3.Cottrell JE, Hartung J. Anesthesia and cognitive outcome in elderly patients: a narrative viewpoint. J Neurosurg Anesthesiol. 2020;32:9–17. doi: 10.1097/ANA.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmetz J, Siersma V, Kessing LV, Rasmussen LS. Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. Br J Anaesth. 2013;110(Suppl 1):i92–97. doi: 10.1093/bja/aes466. [DOI] [PubMed] [Google Scholar]
- 5.Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz J, Rasmussen LS. Peri-operative cognitive dysfunction and protection. Anaesthesia. 2016;71(Suppl 1):58–63. doi: 10.1111/anae.13308. [DOI] [PubMed] [Google Scholar]
- 7.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111:119–125. doi: 10.3238/arztebl.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappa M, Theodosiadis N, Tsounis A, Sarafis P. Pathogenesis and treatment of post-operative cognitive dysfunction. Electron Physician. 2017;9:3768–3775. doi: 10.19082/3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Z, Tanzi RE. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol. 2006;41:346–359. doi: 10.1016/j.exger.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Callaway JK, Jones NC, Royse AG, Royse CF. Memory impairment in rats after desflurane anesthesia is age and dose dependent. J Alzheimers Dis. 2015;44:995–1005. doi: 10.3233/JAD-132444. [DOI] [PubMed] [Google Scholar]
- 12.Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 13.Evered L, Silbert B, Scott DA, Eckenhoff RG. Recommendations for a new perioperative cognitive impairment nomenclature. Alzheimers Dement. 2019;15:1115–1116. doi: 10.1016/j.jalz.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Jildenstål PK, Hallén JL, Rawal N, Berggren L, Jakobsson JG. AAI-guided anaesthesia is associated with lower incidence of 24-h MMSE <25 and may impact the IL-6 response. Int J Surg. 2014;12:290–295. doi: 10.1016/j.ijsu.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Sanders RD, Tononi G, Laureys S, Sleigh JW. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiefenthaler W, Colvin J, Steger B, Pfeiffer KP, Moser PL, Walde J, Lorenz IH, Kolbitsch C. How bispectral index compares to spectral entropy of the EEG and A-line ARX index in the same patient. Open Med (Wars) 2018;13:583–596. doi: 10.1515/med-2018-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo C, Zou W. Cerebral monitoring of anaesthesia on reducing cognitive dysfunction and postoperative delirium: a systematic review. J Int Med Res. 2018;46:4100–4110. doi: 10.1177/0300060518786406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MT, Cheng BC, Lee TM, Gin T CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 19.Strøm C, Rasmussen LS, Sieber FE. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69(Suppl 1):35–44. doi: 10.1111/anae.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Jin X, Yang S, Xia Y. The correlation of the depth of anesthesia and postoperative cognitive impairment: a meta-analysis based on randomized controlled trials. J Clin Anesth. 2018;45:55–59. doi: 10.1016/j.jclinane.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Shu AH, Wang Q, Chen XB. Effect of different depths of anesthesia on postoperative cognitive function in laparoscopic patients: a randomized clinical trial. Curr Med Res Opin. 2015;31:1883–1887. doi: 10.1185/03007995.2015.1075968. [DOI] [PubMed] [Google Scholar]
- 22.Meineke M, Applegate RL 2nd, Rasmussen T, Anderson D, Azer S, Mehdizadeh A, Kim A, Allard M. Cognitive dysfunction following desflurane versus sevoflurane general anesthesia in elderly patients: a randomized controlled trial. Med Gas Res. 2014;4:6. doi: 10.1186/2045-9912-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu T, Bo L, Wang J, Zhao Z, Xu Z, Deng X, Zhu W. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg. 2013;8:204. doi: 10.1186/1749-8090-8-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng LQ, Hou LN, Song FX, Zhu HY, Zhao HY, Chen G, Li JJ. Effect of pre-emptive analgesia by continuous femoral nerve block on early postoperative cognitive function following total knee arthroplasty in elderly patients. Exp Ther Med. 2017;13:1592–1597. doi: 10.3892/etm.2017.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127:496–505. doi: 10.1213/ANE.0000000000003514. [DOI] [PubMed] [Google Scholar]
- 27.Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119:i115–i125. doi: 10.1093/bja/aex354. [DOI] [PubMed] [Google Scholar]
- 28.Bocskai T, Kovács M, Szakács Z, Gede N, Hegyi P, Varga G, Pap I, Tóth I, Révész P, Szanyi I, Németh A, Gerlinger I, Karádi K, Lujber L. Is the bispectral index monitoring protective against postoperative cognitive decline? A systematic review with meta-analysis. PLoS One. 2020;15:e0229018. doi: 10.1371/journal.pone.0229018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farag E, Chelune GJ, Schubert A, Mascha EJ. Is depth of anesthesia, as assessed by the bispectral Index, related to postoperative cognitive dysfunction and recovery? Anesth Analg. 2006;103:633–640. doi: 10.1213/01.ane.0000228870.48028.b5. [DOI] [PubMed] [Google Scholar]
- 30.Tasbihgou SR, Netkova M, Kalmar AF, Doorduin J, Struys M, Schoemaker RG, Absalom AR. Brain changes due to hypoxia during light anaesthesia can be prevented by deepening anaesthesia; a study in rats. PLoS One. 2018;13:e0193062. doi: 10.1371/journal.pone.0193062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan C, Chen J, Luo Y, Zhou L, He X, Liao Y, Chou J, Guo Q, Chen AF, Wen O. BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 2019;9:e01238. doi: 10.1002/brb3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deiner S, Luo X, Silverstein JH, Sano M. Can intraoperative processed EEG predict postoperative cognitive dysfunction in the elderly? Clin Ther. 2015;37:2700–2705. doi: 10.1016/j.clinthera.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou R, Wang H, Chen L, Qiu Y, Li S. POCD in patients receiving total knee replacement under deep vs light anesthesia: a randomized controlled trial. Brain Behav. 2018;8:e00910. doi: 10.1002/brb3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franck M, Nerlich K, Neuner B, Schlattmann P, Brockhaus WR, Spies CD, Radtke FM. No convincing association between post-operative delirium and post-operative cognitive dysfunction: a secondary analysis. Acta Anaesthesiol Scand. 2016;60:1404–1414. doi: 10.1111/aas.12779. [DOI] [PubMed] [Google Scholar]
- 35.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 36.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi YL, Li ZS, Lin CS, Wang Q, Zhou YK. Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:1346–1354. [PubMed] [Google Scholar]
- 38.Gong GL, Liu B, Wu JX, Li JY, Shu BQ, You ZJ. Postoperative cognitive dysfunction induced by different surgical methods and its risk factors. Am Surg. 2018;84:1531–1537. [PubMed] [Google Scholar]
- 39.Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Silbert BS, Evered LA, Scott DA. Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J Anaesth. 2014;113:784–791. doi: 10.1093/bja/aeu163. [DOI] [PubMed] [Google Scholar]
- 41.Bilotta F, Stazi E, Zlotnik A, Gruenbaum SE, Rosa G. Neuroprotective effects of intravenous anesthetics: a new critical perspective. Curr Pharm Des. 2014;20:5469–5475. doi: 10.2174/1381612820666140325110113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jildenstål PK, Hallén JL, Rawal N, Gupta A, Berggren L. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. Eur J Anaesthesiol. 2011;28:213–219. doi: 10.1097/EJA.0b013e328340dbb9. [DOI] [PubMed] [Google Scholar]
- 43.Lachmann G, Feinkohl I, Borchers F, Ottens TH, Nathoe HM, Sauer AM, Dieleman JM, Radtke FM, van Dijk D, Spies C, Pischon T. Diabetes, but not hypertension and obesity, is associated with postoperative cognitive dysfunction. Dement Geriatr Cogn Disord. 2018;46:193–206. doi: 10.1159/000492962. [DOI] [PubMed] [Google Scholar]


