Abstract
Objective: This study was designed to evaluate the efficacy of adaptive support ventilation (ASV) and lung recruitment maneuvering (LRM) on the hemodynamics and respiratory mechanics of patients with acute respiratory distress syndrome (ARDS). Methods: A total of 100 patients with ARDS admitted to the intensive care unit (ICU) of our hospital from July 2016 to October 2019 were randomly divided into the control group (n=50) receiving synchronized intermittent mandatory ventilation (SIMV) and the study group (n=50) receiving ASV + LRM. The hemodynamics, respiratory mechanics, oxygen metabolism parameters, pulmonary index of microcirculatory resistance and prognosis were compared between the two groups. Results: No significant difference was observed between the two groups in terms of baseline data (P > 0.05). Positive end-expiratory pressure (PEEP), mean arterial pressure (MAP), central venous pressure (CVP), heart rate (HR), systemic vascular resistance index (SVRI), pulmonary arterial pressure (PAP), and cardiac output index (CI) were not significantly different between the two groups (P > 0.05). PEEP, peak inspiratory pressure (PIP), pulmonary vascular resistance index (PVRI), and extravascular lung water (EVLW) were lower, and arterial oxygen pressure (PaO2), global oxygen delivery (DO2), oxygen-uptake (VO2), and dynamic compliance (Cdyn) were higher in the study group than in the control group (P < 0.05). Time to withdrawal, APACHE II score, and length of stay in ICU were lower in the study group than in the control group (P < 0.05). Conclusion: ASV + LRM can improve respiratory mechanics, oxygen metabolism, reduce microcirculatory resistance, shorten ICU stay and alleviate the conditions of ARDS patients, but has no significant effect on hemodynamics.
Keywords: Acute respiratory distress syndrome, adaptive support ventilation, lung recruitment maneuvering, hemodynamics, respiratory mechanics
Introduction
Acute respiratory distress syndrome (ARDS) is a type of inflammatory, diffuse acute lung injury characterized by intractable hypoxemia and respiratory distress, and patients are often accompanied by hypoxia and increased respiration rate and cyanosis [1,2]. The specific pathogenesis of ARDS is not yet fully understood, the treatment methods are limited, and the morbidity and mortality rates are still as high as 40%-50%, posing a great threat to patients [3,4]. Mechanical ventilation is a common management option. In recent years, low tidal volume ventilation and positive end-expiratory pressure (PEEP) have gained clinical attention, which can effectively regulate microcirculation and inhibit disease progression. Due to decreased lung compliance, low tidal volume ventilation causes collapsed alveoli in ARDS patients, affecting gas exchange in the lungs and reducing global oxygen delivery (DO2) [5]. Clinically, PEEP levels are often increased to keep DO2 within the normal range. However, PEEP can trigger or aggravate lung injury associated with mechanical ventilation, causing many complications, affecting prognosis [6,7]. Therefore, exploring an efficient and safe ventilation protocol is the key to improve the efficacy of ARDS, ensure patient safety and improve prognosis.
Adaptive support ventilation (ASV) is a positive pressure mode of mechanical ventilation with closed-loop control and automatic adjustment according to the needs of patients. It is designed to ensure optimization of breathing work for the patients [8]. Multiple methods have been described for lung recruitment maneuvering (LRM), including 3 consecutive sighs/min with a plateau pressure of 45 cmH2O, which improves lung compliance and oxygenation function by reopening the alveoli, reduces the degree of lung injury, and decreases the overall morbidity and mortality [9,10]. Currently, there are few clinical reports on the effectiveness of ASV combined with LRM modality in ARDS. In this study, 100 patients with ARDS were enrolled and grouped based on synchronized intermittent mandatory ventilation (SIMV) and ASV + LRM modes to investigate the effects of ASV + LRM mode on hemodynamic, respiratory mechanics, and microcirculatory resistance.
Materials and methods
Clinical data
A total of 100 patients with ARDS admitted to the intensive care unit (ICU) of The First Affiliated Hospital of Wenzhou Medical University from July 2016 to October 2019 were included in the study, and were randomly divided into the control group (n=50) receiving synchronized intermittent mandatory ventilation (SIMV) and the study group (n=50) receiving ASV + LRM. This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. All subjects or their families signed the informed consent prior to participating in the study.
Inclusion criteria: patients who met the diagnostic criteria of ARDS [11] and received mechanical ventilation; acute onset within 1 week; oxygenation index ≤ 200 and PEEP ≥ 5 cmH2O; and chest X-ray radiographs showing reduced translucency in both lungs and no pleural effusion. Exclusion criteria: death within 24 h of admission; oxygen saturation (SaO2) ≤ 90% following ventilator use and invasive mean arterial pressure (MAP) ≤ 65 mmHg in the brachial artery 2 h after resuscitation; pneumothorax and alveolar disease.
Methods
All patients were mechanically ventilated using a Galerio Gold ventilator (Hamilton, Switzerland), and a Swan-Ganz floating catheter was placed via the patient’s internal jugular or subclavian vein, and mechanical ventilation was performed using the ventilator. SIMV mode was set with a PEEP of 0, a tidal volume of 8 mL/kg, and an oxygen concentration of 60% for 8 h. When the condition was stable, the control group continued to receive the SIMV mode with sequential increases of 0, 5, and 10 cmH2O and each PEEP was maintained for 60 min. The study group received ASV + LRM mode. The control group underwent ASV with the same parameters.
Outcome measurements
Pulmonary arterial pressure (PAP) and central venous pressure (CVP) were measured using a Swan-Ganz floating catheter, and systemic vascular resistance index (SVRI), pulmonary vascular resistance index (PVRI) and cardiac output index (CI), and extravascular lung water (EVLW) were measured using a PiCCO monitor; peak inspiratory pressure (PIP) and dynamic compliance (Cdyn) were measured using a ventilator; 3 mL of mixed venous and femoral blood was obtained from the pulmonary artery end of a Swan-Ganz float catheter, and arterial oxygen pressure (PaO2), global oxygen delivery (DO2), and oxygen-uptake (VO2) were measured with an ABL90 blood gas analyzer.
The time to withdrawal, APACHE II score, and ICU stay were recorded in both groups. The APACHE II scores were determined in terms of age, acute physiology and chronic health on a 4-point Likert scale, with high scores indicating severe disease and poor prognosis.
Statistical analysis
SPSS 23.0 statistical analysis software was used. The graphics were prepared using GraphPad Prism 8. The measurement data were expressed as x̅ ± s. Comparisons between groups were made by the independent samples t test. Count data [n (%)] were examined by χ2 test, with P < 0.05 regarding as a significant difference.
Results
Baseline data
The control group included 27 males and 23 females aged (45.2±8.2) years, while the study group included 26 males and 24 females aging (45.9±7.6) years. The differences in baseline data such as gender, age, body mass index, oxygenation index, APACHE II score and primary disease were not significant between the two groups (P > 0.05) (Table 1).
Table 1.
Comparison of baseline data (n/χ ± S)
| Group | Male/female | Age (years) | Body mass index (kg/m2) | Oxygenation index | APACHE II score | Primary disease |
|---|---|---|---|---|---|---|
|
| ||||||
| Acute pancreatitis/pulmonary infection/severe infection/trauma/other | ||||||
| Control group (n=50) | 27/23 | 45.2±8.2 | 22.95±2.68 | 145.26±10.29 | 26.13±2.25 | 12/10/14/12/2 |
| Study group (n=50) | 26/24 | 45.9±7.6 | 23.15±3.02 | 146.35±9.95 | 26.38±2.37 | 14/9/13/10/4 |
| χ2/t | 0.040 | 0.443 | 0.350 | 0.341 | 0.541 | 0.244 |
| P | 0.841 | 0.659 | 0.727 | 0.734 | 0.590 | 0.970 |
Hemodynamics
At a PEEP of 0 cmH2O, 5 cmH2O, and 10 cmH2O, no significant difference was found in MAP, CVP, and HR levels between the two group (P > 0.05), indicating that SIMV and ASV + LRM modes did not affect patient hemodynamics (Table 2).
Table 2.
Comparison of hemodynamic indices (χ ± S)
| Group | MAP (mmHg) | CVP (cmH2O) | HR (beats/min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| PEEP=0 cmH2O | PEEP=5 cmH2O | PEEP=10 cmH2O | PEEP=0 cmH2O | PEEP=5 cmH2O | PEEP=10 cmH2O | PEEP=0 cmH2O | PEEP=5 cmH2O | PEEP=10 cmH2O | |
| Control group (n=50) | 99.62±9.52 | 98.86±10.02 | 99.76±9.68 | 10.26±3.21 | 9.64±3.12 | 10.65±3.57 | 113.25±15.45 | 112.57±14.26 | 109.32±13.02 |
| Study group (n=50) | 98.68±9.57 | 99.94±10.34 | 98.86±9.76 | 9.97±4.02 | 10.25±4.12 | 10.98±5.11 | 112.37±14.25 | 110.34±13.25 | 109.57±14.75 |
| t | 0.492 | 0.530 | 0.463 | 0.399 | 0.835 | 0.374 | 0.296 | 0.810 | 0.090 |
| P | 0.624 | 0.597 | 0.644 | 0.691 | 0.406 | 0.709 | 0.768 | 0.420 | 0.928 |
Respiratory mechanics
At a PEEP of 0 cmH2O, 5 cmH2O, and 10 cmH2O, the PIP level was significantly lower and the Cdyn level in the study group was significantly higher than that in the control group (P < 0.05), suggesting that compared with SIMV mode, ASV + LRM could improve the respiratory function of the patients with ARDS (Table 3).
Table 3.
Comparison of respiratory mechanics (χ ± S)
| Group | PIP (cmH2O) | Cdyn (mL/cmH2O) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PEEP=0 cmH2O | PEEP=5 cmH2O | PEEP=10 cmH2O | PEEP=0 cmH2O | PEEP=5 cmH2O | PEEP=10 cmH2O | |
| Control group (n=50) | 34.26±8.26 | 37.12±9.26 | 43.26±8.26 | 20.16±5.12 | 24.49±6.32 | 29.62±7.71 |
| Study group (n=50) | 26.31±6.25 | 29.62±6.94 | 33.35±7.25 | 24.49±5.28 | 32.26±6.58 | 37.26±8.02 |
| T | 5.427 | 4.583 | 6.376 | 4.163 | 6.022 | 4.856 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Oxygen metabolism parameters
At a PEEP of 0 cmH2O, 5 cmH2O, and 10 cmH2O, PaO2, DO2, and VO2 levels in the study group were significantly higher than those in the control group (P < 0.05), showing that compared with SIMV mode, ASV + LRM could improve oxygen metabolism parameters of the patients with ARDS (Figure 1).
Figure 1.
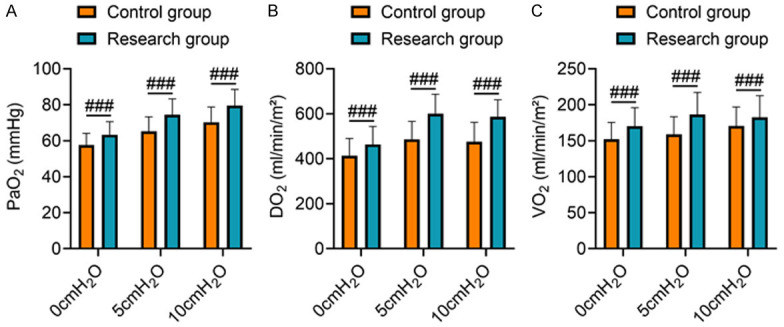
Comparison of oxygen metabolism parameters. Note: (A) PaO2; (B) DO2; (C) VO2. Compared to the control group under the same PEEP, ###P < 0.001.
Pulmonary index of microcirculatory resistance
At a PEEP of 0 cmH2O, 5 cmH2O, and 10 cmH2O, SVRI, PAP, and CI levels in the study group were not significantly different from the control group (P > 0.05). At the PEEP of 0 cmH2O, 5 cmH2O, and 10 cmH2O, PVRI and EVLW levels in the study group were significantly lower than those in the control group (P < 0.05), showing that compared with SIMV mode, the ASV + LRM could improve pulmonary microcirculatory resistance of the patients with ARDS and prevent pulmonary microcirculation disturbance (Figure 2).
Figure 2.
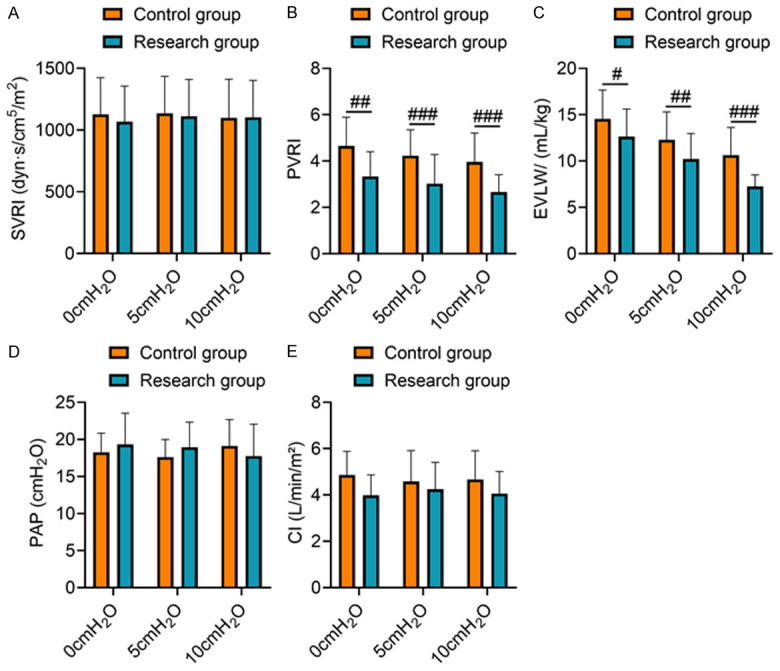
Comparison of microcirculatory resistance. Note: (A) SVRI; (B) PVRI; (C) EVLW; (D) PAP; (E) CI. Compared to the control group under the same PEEP, ##P < 0.01 and ###P < 0.001.
Prognosis
The time to withdrawal, APACHE II score, and ICU length of stay in the study group were significantly lower than those in the control group (P < 0.05), indicating that compared with SIMV mode, the ASV + LRM mode could shorten the length of stay, alleviate the condition of the patients with ARDS, and facilitate rehabilitation (Table 4).
Table 4.
Comparison of prognostic indicators (χ ± S)
| Group | Withdrawal time (h) | APACHE II score | Length of ICU stay (d) |
|---|---|---|---|
| Control group (n=50) | 302.16±29.58 | 16.35±5.57 | 15.26±3.02 |
| Study group (n=50) | 258.86±25.57 | 12.16±3.75 | 11.19±3.15 |
| T | 7.831 | 4.412 | 6.595 |
| P | < 0.001 | < 0.001 | < 0.001 |
Discussion
Lung-protective ventilator settings (LPS) were initially thought to be beneficial for patients with ARDS, but recent studies have shown that lower tidal volume is beneficial for non-ARDS patients as well [12-14]. LPS works by limiting the concentration of oxygen and tidal volume, while applying PEEP to increase alveolar pressure and alveolar volume, prevent damage caused by repeated closure and opening of the atrophied lung, improve gas exchange at the alveoli and lung compliance, and prevent infection and pulmonary edema [15]. However, due to the presence of heterogeneous lesions in the lungs of ARDS patients, high PEEP fails to expand the partially collapsed alveoli [16]. Therefore, the key to ARDS treatment is to reduce PEEP and PIP as much as possible while effectively promoting alveolar collapse and providing adequate oxygen supply.
ASV is a closed-loop pressure-controlled ventilation mode, which is an intelligent automatic ventilation mode for optimal respiratory mode while ensuring an adequate air supply [17]. ASV is based on individual patient parameters such as respiratory resistance and lung compliance, with significant advantages. It automatically adjusts to the most appropriate support pressure, respiratory frequency, and tidal volume by analyzing the parameters of each breath, so as to meet the body’s requirements for DO2 with the minimum work of breathing. The ventilator provides air in a constant deceleration mode, so there is no excessive peak airway pressure [18,19]. However, ASV alone cannot adequately dilate collapsed alveoli, and other measures need to be taken to enhance oxygenation capacity, promote alveolar collapse, and correct hypercapnia [20]. LRM reopens the collapsed alveoli and increases functional residual capacity. A study found that the implementation of LRM contributed to a reduction in collapsed alveoli by 11.7% of whole lung tissue [21]. Animal tests have shown that LRM can prevent ventilator-related exacerbation of lung injury and reduce the levels of inflammatory factors in plasma and bronchoalveolar lavage fluid, thereby reducing the risk of developing multiple organ dysfunction syndrome [22]. In this study, no significant difference was observed in MAP, CVP, and heart rate (HR) between the two groups under the same PEEP, indicating that the two modalities did not have a significant effect on the hemodynamics of the patients. Inspiratory pressure in the study group varied with pulmonary compliance, and ventilation was consistently with lower PIP. The study group showed lower PIP, PVRI, and EVLW, and higher PaO2, DO2, VO2, and Cdyn than the control group, suggesting that the ASV + LRM mode helped improve inspiratory mechanics, oxygen metabolism, and reduce pulmonary microcirculatory resistance in patients with ARDS. Moreover, the time to withdrawal, APACHE II score, and ICU length of stay in the study group were lower than those in the control group, indicating that the ASV + LRM could accelerate the recovery process from ARDS, shorten the length of stay, and alleviate the patient’s condition. The reason for this may be that the ASV is a volume-targeted pressure support mode that automatically adjusts the pressure support according to the spontaneous respiratory rate, thus promoting a more even distribution of airflow in the alveoli, which allows fully oxygenated blood, thereby maintaining the balance of oxygen supply and demand. Meanwhile, ASV could provide ideal ventilation at a low airway pressure. With small tidal volume and respiratory frequency, the patients can breathe independently and comfortably, which can stabilize hemodynamics, reduce the work of respiration as well as oxygen consumption, and increase gas exchange in the lungs, thereby avoiding respiratory muscle atrophy, human-machine confrontation, reducing the consumption of sedation and analgesia, and shortening the use time of ventilator and ICU hospitalization time [23,24]. LRM increases lung volume, reopens non-ventilated alveoli, keeps reventilated alveoli and ventilated alveoli open, and connected airways open, increases the patient’s functional residual capacity, reduces the shear forces formed by repeated alveolar closure and opening, and avoids the occurrence of mismatched ventilation perfusion [25,26]. In ASV + LRM mode, the deceleration wave during the ventilation can improve the ratio of pulmonary gas and blood flow and promote alveolar gas exchange. Combined with LRM, it can reopen the alveoli and keep them in a state of dilation, thereby increasing lung volume and improving oxygenation, lung compliance, oxygen supply and consumption.
This was a single-center study with a small number of cases and short period of followed-up, leading to a possible certain bias in the conclusions of the study. In the next step, a multi-center study will be carried out with increased number of cases and extended follow-up period to further explore the reliability of the conclusions of this study.
In conclusion, ASV + LRM mode can improve respiratory mechanics and oxygen metabolism, reduce microcirculatory resistance, shorten ICU stay and alleviate the condition of ARDS patients, but has no significant effect on hemodynamics.
Disclosure of conflict of interest
None.
References
- 1.van der Zee P, Gommers D. Recruitment maneuvers and higher PEEP, the so-called open lung concept, in patients with ARDS. Crit Care. 2019;23:73. doi: 10.1186/s13054-019-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherian SV, Kumar A, Akasapu K, Ashton RW, Aparnath M, Malhotra A. Salvage therapies for refractory hypoxemia in ARDS. Respir Med. 2018;141:150–158. doi: 10.1016/j.rmed.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321:846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85:1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 5.Guo L, Xie J, Huang Y, Pan C, Yang Y, Qiu H, Liu L. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18:172. doi: 10.1186/s12871-018-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tusman G, Gogniat E, Madorno M, Otero P, Dianti J, Ceballos IF, Ceballos M, Verdier N, Böhm SH, Rodriguez PO, San Roman E. Effect of PEEP on dead space in an experimental model of ARDS. Respir Care. 2020;65:11–20. doi: 10.4187/respcare.06843. [DOI] [PubMed] [Google Scholar]
- 7.McNicholas BA, Rooney GM, Laffey JG. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care. 2018;24:41–48. doi: 10.1097/MCC.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology. 2013;18:1108–1115. doi: 10.1111/resp.12126. [DOI] [PubMed] [Google Scholar]
- 9.Arnal JM, Wysocki M, Novotni D, Demory D, Lopez R, Donati S, Granier I, Corno G, Durand-Gasselin J. Safety and efficacy of a fully closed-loop control ventilation (IntelliVent-ASV®) in sedated ICU patients with acute respiratory failure: a prospective randomized crossover study. Intensive Care Med. 2012;38:781–787. doi: 10.1007/s00134-012-2548-6. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Cao R, Wang Y, Li G. Lung recruitment maneuvers for ARDS patients: a systematic review and meta-analysis. Respiration. 2020;99:264–276. doi: 10.1159/000501045. [DOI] [PubMed] [Google Scholar]
- 11.Chinese Society of Critical Care Medicine. Guidelines for the diagnosis and treatment of acute lung injury/acute respiratory distress syndrome (2006) Chinese Journal of Internal Medicine. 2007;46:430–435. [Google Scholar]
- 12.Bergez M, Fritsch N, Tran-Van D, Saghi T, Bounkim T, Gentile A, Labadie P, Fontaine B, Ouattara A, Rozé H. PEEP titration in moderate to severe ARDS: plateau versus transpulmonary pressure. Ann Intensive Care. 2019;9:81. doi: 10.1186/s13613-019-0554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanchal RS, Truwit JD. Recent advances in understanding and treating acute respiratory distress syndrome. F1000Res. 2018;7:F1000 Faculty Rev-1322. doi: 10.12688/f1000research.15493.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karbing DS, Panigada M, Bottino N, Spinelli E, Protti A, Rees SE, Gattinoni L. Changes in shunt, ventilation/perfusion mismatch, and lung aeration with PEEP in patients with ARDS: a prospective single-arm interventional study. Crit Care. 2020;24:111. doi: 10.1186/s13054-020-2834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, Wu B, Ran YN. A clinical study on mechanical ventilation PEEP setting for traumatic ARDS patients guided by esophageal pressure. Technol Health Care. 2019;27:37–47. doi: 10.3233/THC-181380. [DOI] [PubMed] [Google Scholar]
- 16.Arnal JM, Garnero A, Novonti D, Demory D, Ducros L, Berric A, Donati S, Corno G, Jaber S, Durand-Gasselin J. Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study. Crit Care. 2013;17:R196. doi: 10.1186/cc12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khemani RG, Parvathaneni K, Yehya N, Bhalla AK, Thomas NJ, Newth CJL. Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med. 2018;198:77–89. doi: 10.1164/rccm.201707-1404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnal JM, Saoli M, Garnero A. Airway and transpulmonary driving pressures and mechanical powers selected by INTELLiVENT-ASV in passive, mechanically ventilated ICU patients. Heart Lung. 2020;49:427–434. doi: 10.1016/j.hrtlng.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Bialais E, Wittebole X, Vignaux L, Roeseler J, Wysocki M, Meyer J, Reychler G, Novotni D, Sottiaux T, Laterre PF, Hantson P. Closed-loop ventilation mode (IntelliVent®-ASV) in intensive care unit: a randomized trial. Minerva Anestesiol. 2016;82:657–668. [PubMed] [Google Scholar]
- 20.Dai YL, Wu CP, Yang GG, Chang H, Peng CK, Huang KL. Adaptive support ventilation attenuates ventilator induced lung injury: human and animal study. Int J Mol Sci. 2019;20:5848. doi: 10.3390/ijms20235848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 22.Badet M, Bayle F, Richard JC, Guérin C. Comparison of optimal positive end-expiratory pressure and recruitment maneuvers during lung-protective mechanical ventilation in patients with acute lung injury/acute respiratory distress syndrome. Respir Care. 2009;54:847–854. doi: 10.4187/002013209793800448. [DOI] [PubMed] [Google Scholar]
- 23.Goligher EC, Hodgson CL, Adhikari NKJ, Meade MO, Wunsch H, Uleryk E, Gajic O, Amato MPB, Ferguson ND, Rubenfeld GD, Fan E. Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S304–S311. doi: 10.1513/AnnalsATS.201704-340OT. [DOI] [PubMed] [Google Scholar]
- 24.Lovisari F, Fodor GH, Peták F, Habre W, Bayat S. Effect of PEEP and I:E ratio on cerebral oxygenation in ARDS: an experimental study in anesthetized rabbit. BMC Anesthesiol. 2019;19:110. doi: 10.1186/s12871-019-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pensier J, de Jong A, Hajjej Z, Molinari N, Carr J, Belafia F, Chanques G, Futier E, Azoulay E, Jaber S. Effect of lung recruitment maneuver on oxygenation, physiological parameters and mortality in acute respiratory distress syndrome patients: a systematic review and meta-analysis. Intensive Care Med. 2019;45:1691–1702. doi: 10.1007/s00134-019-05821-9. [DOI] [PubMed] [Google Scholar]
- 26.Kung SC, Hung YL, Chen WL, Wang CM, Chang HC, Liu WL. Effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. J Clin Med. 2019;8:231. doi: 10.3390/jcm8020231. [DOI] [PMC free article] [PubMed] [Google Scholar]


