Abstract
Iron and vitamin D deficiencies are some of the most common health problems in the world. Iron is essential in oxygen transport and participates in many enzymatic systems in the body, with important roles in vitamin D metabolism. Osteoporosis is one of the most prevalent chronic disease of the elderly in the world as well as in the Saudi population. The relationship between iron, vitamin D deficiency and bone health comes from clinical observations in iron overload patients who suffered bone loss. The opposite scenario, whether iron and vitamin D deficiencies affect bone metabolism, has not been fully addressed. This is of great interest, as this nutrient deficiency is a worldwide public health problem and at the same time osteoporosis and bone alterations are highly prevalent. The relationship between 25(OH)D and iron deficiencies with osteoporosis is unknown up to date. This review presents the current knowledge on nutritional iron and vitamin D deficiencies in bone remodeling, and discuss the link between iron and bone metabolism among postmenopausal women. Finally, it is hypothesized that chronic iron and vitamin D deficiencies induces bone resorption and risk of osteoporosis, thus complete recovery from anemia and its prevention should be promoted in order to improve quality of life including bone health. Several mechanisms are implicated; hence, further investigation on the possible impact of iron and vitamin D deficiencies on the development of osteoporosis is needed.
Keywords: Iron deficiency, vitamin D, bone remodeling, bone health, osteoporosis, bone formation, bone resorption
Introduction
Iron
Iron is an essential element in almost all living cells. There are characteristics of iron that make it valuable in livening up a biological system. Under physiologic conditions, iron is converted between ferric form (Fe3+) and the ferrous form (Fe2+), a thermodynamically stable oxidation state. Biological function for different enzymes depends on iron to catalyse reactions and it is incorporated with a large number of cellular proteins [3]. Iron is a trace element with a major role in energy production and metabolism. It participates as a heme in hemoglobin and myoglobin, contributes in the synthesis of varies enzymes and it is essential for energy production and respiration of mitochondrial function. Iron-sulfur cluster component is essential for many functions of enzymes in their prosthetic group such as in DNA repair, cell proliferation and heme formation [4,5]. Iron distribution in the body is about 73% in hemoglobin and myoglobin, 12% in iron storage proteins, and 15% for iron dependent enzymes [6]. In fundamental metabolic processes for cells and organisms, systemic iron is essential in homeostasis to regulate sufficient plasma iron levels. In plasma, iron is bound to transferrin, which is a glycoprotein with high affinity binding sites for Fe3+. Thus, the binding maintains the soluble form of iron in plasma and delivers it into the cell by the transferrin receptor (TfR1), therefore reducing the toxic radicals generated in the plasma [5]. In individual cells and in the body, complex regulation mechanisms for iron influx and efflux supply adequate iron and prevent excess iron under oxidative stress [3]. The homeostatic system maintains physiological levels of transferrin and depends on signals sent to cells in order to release iron into the blood. The release of iron into the circulation is from duodenal enterocytes and macrophages. In the liver, hepatocytes have a role in iron metabolism. Senescent erythrocytes can recycle and store iron (20-25 mg) as well as secrete hepcidin. Hepcidin is a regulatory hormone for systemic iron fluxes and regulates iron levels in plasma via binding to ferroportin, which is the iron exporter on the surface of iron releasing cells. Serum ferritin is a useful measurement for iron storage. Serum ferritin mainly consists of partly glycosylated, L chain subunits [5].
Iron deficiency
Iron deficiency (ID) is a widespread problem for all ethnic groups throughout the world. It influences the health of humans and socioeconomic development in all countries [6-9]. The definition of ID is the condition in which there is a decrease of iron stores even without of anemia, with maintenance of erythropoiesis. Consequently, there is an alteration of iron distribution to specific tissues or organs resulting from lack of iron. ID causes symptoms as a muscle weakness, fatigue, cognitive impairment and decreasing of physical performance [10]. There are three phases of iron status: iron depletion phase, iron deficiency phase which is non-anemic iron deficiency, and iron deficiency anemia [11,12]. The iron depletion phase can be determined by serum ferritin and the iron deficiency phase can be detected by elevated serum transferrin and sTfR, which promotes iron supply to tissues resulting in reduced transferrin saturation [12]. In plasma, the normal transferrin saturation with iron is about 30%. In iron deficiency, the saturation <16% [5]. Iron deficiency is caused by inadequate iron intake, chronic loss of blood or impaired absorption of iron by the intestines. In addition, chronic inflammatory disorders affect iron metabolism via inflammatory cytokines. Cytokines can affect hepcidin regulation by increasing its production. Thus, iron accumulates in macrophage stores, whereas transferrin saturation decreases in the circulation, resulting in an insufficient iron supply to tissue. The diagnostic tests for ID are serum ferritin, transferrin and serum iron, which indicate the total iron binding capacity, transferrin saturation and blood cell count [13].
Prevalence of iron deficiency
Iron deficiency (ID) is the greatest risk factor for death and disability, affecting about 2 billion (more than 30%) people worldwide [14]. ID is the most common cause of anemia in about 50% of cases [15,16]. ID prevalence is twice as prevalent (2 billion people) compared to iron deficiency anemia (IDA) [16]. IDA affects approximately 30% of the population and in the third world it affects about 75% of the population with all types of anemia [17]. According to a world health organization (WHO) report, on a global burden of anemia, from 1993 to 2005 about 126 million of people suffered from anima, which indicates both low health status and insufficient nutrition [18]. ID can occur with both anemia and without anemia [7]. Figures 1 and 2 show the worldwide prevalence of anemia.
Figure 1.
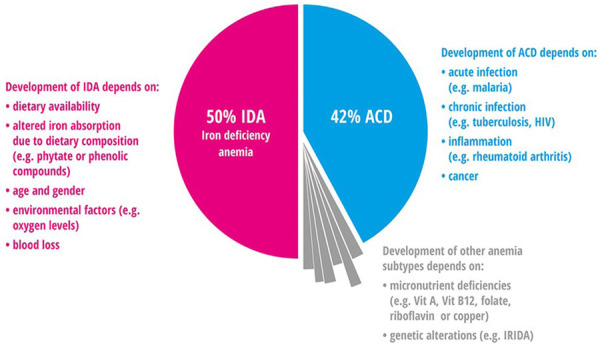
Anemia prevelence in all population in the world for both Iron deficiency anemia (IDA) and Anemia of chronic disease (ACD) [19].
Figure 2.
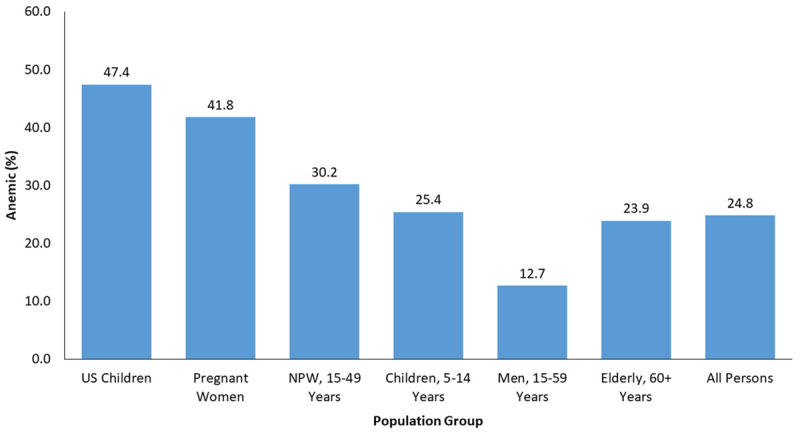
Global prevalence of anemia in different population group.
There are many causes of anemia and ID. ID can exist without decreased hemoglobin levels. There are four different types found in the population: iron anemic and iron deficient, iron deficient with anemia, which is caused without iron deficiency, and being replete of iron with normal hemoglobin [20,21]. ID caused by deficiency of nutritional iron is seen in about 50% of anemias, 42% of the time it is due to infections and inflammation and the remaining 8% is the result of nutritional deficiencies, for example, vitamin B12, vitamin A and riboflavin or other genetic causes [7]. Anemia is most common in women, from a report by the WHO in 2011, with a prevalence of 38% in pregnant women and 29% in non-pregnant women [15]. In Middle Eastern countries, iron deficiency (ID) and iron deficiency anemia (IDA) are the most common public health problems [22,23]. The highest percentage of IDA was seen in the Middle East and North Africa for preschool aged children, and also in pregnant and non-pregnant women. Most causes of IDA for women in childbearing age is due to loss of iron in blood during the menstrual cycle and pregnancy [24]. The IDA prevalence is reported at 20-67% for preschool children and 22.7-54% among pregnant women [18]. In Saudi Arabia, the prevalence of IDA was 30-56% [17]. In Riyadh city, a study showed the prevalence was 40.5% for adolescent female students aged 16-18 years old [25], and 48% to 60% for school girls aged 11-18 years [26]. The prevalence among anemic pregnant women based on hemoglobin levels was 31.9% in Asir [27], 22.9% in Taif [28] and 25.6% in Jeddah [29]. Otherwise in adults and elderly Saudi males in Riyadh it was 38% and 58% respectively [30]. One study evaluated IDA among adult healthy Saudi males and non-pregnant females in Riyadh. The prevalence in females based on hemoglobin was 21.6%, based on hematocrit was 20.6% and based on iron intake was 95.1%. Results showed that IDA among adult Saudi males was not present [31]. Table 1 shows the global prevalence of anemia according to regions while Table 2 shows the prevalence of anemia in the Greater Middle East region.
Table 1.
The prevalence of anemia across regions according to WHO [147]
| WHO Region | Pregnant Women | Non-Pregnant Women |
|---|---|---|
| Africa | 57.1% | 47.5% |
| Americas | 24.1% | 17.8% |
| SE Asia | 48.2% | 45.7% |
| Europe | 21.0% | 19.0% |
| Eastern Mediterranean | 44.2% | 32.4% |
| Western Pacific | 30.7% | 21.5% |
| GLOBAL | 41.8% | 30.2% |
Table 2.
Anemia prevalence percentage (%) in Eastern Mediterranean regions in different population groups [17]
| Member state | Women | Men | |
|---|---|---|---|
|
| |||
| Pregnant | Non-Pregnant | ||
| Bahrain | - | 40 | 20 |
| Djibouti | - | 63 | - |
| Egypt | 21-35 | - | - |
| Kuwait | 40 | 42 | 34 |
| Oman | 54 | 15-48 | 3-24 |
| Pakistan | 45 | - | - |
| Palestine | 23-44 | - | - |
| Saudi Arabia | 11-19 | - | 30-56 |
| AUE | 22-62 | - | - |
Osteoporosis
Osteoporosis is a common disease caused by impaired mass and strength of bone as well as microarchitecture that leads to an increased risk for fragility fractures [32,33]. According to WHO, the definition of osteoporosis is a reduction in bone minerals density (BMD) when assessed by dual-energy x-ray absorptiometry (DEXA) to 2.5 standard deviations or less than of mean peak BMD in young healthy adults [34]. The fracture risk of patients with osteoporosis is 40%, it can occur in the hip or wrist, spine and it affects the humerus, ribs and trochanter, thus leading to loss of mobility and is often a major reason for diminished quality of life [35]. Osteoporosis is more common in women (34%) than men (17%) [36]. It is influenced by various risk factors associated with sex and age [37]. Osteoporosis is most prevalent in postmenopausal women due to secondary estrogen deficiency that results in accelerated bone turnover, additionally, vitamin D deficiency and hyperparathyroidism are the main causes of osteoporosis in men and women [36]. A report published by WHO on the differentiation between osteoporosis and osteopenia can be related to patient’s BMD and to the mean peak bone density of young adults (T-score). In osteoporosis, the BMD is more than 2.5 SD (T-score is 2.5 or ≤2.5) means lower than that of young adults because there is a gradient of risk showing a relation between lower BMD and increased fracture risk. Whereas in osteopenia, the BMD (1 to 2.5 SD) among young adults (T-score is -1 to -2) is related to a moderately increased fracture risk [38]. The normal healthy skeleton is maintained by remodelling, a constant process of the bone throughout life. Remodelling regulates activities between osteoblast (bone formation cell) and osteoclast (bone resorbing cell), therefore it maintains the normal structure and mineral content of bone [39]. In case of osteoporosis, an uncoupling of bone resorption from bone formation occurs, thus the activity of osteoclasts far outweighs that of the osteoblasts. In early adulthood, peak bone mass is activated, after increasing age the bone mass is lost in both men and women. In osteoporotic postmenopausal women, the activity of osteoclasts increases due to loss of estrogen, which plays a significant role in maintaining cortical bone formation by supporting osteoblasts and preventing bone resorption by suppressing osteoclasts formation, hence stimulating the process of apoptosis in osteoclasts [40,41].
Prevalence of osteoporosis
Osteoporosis is considered a highly prevalent clinical and public health disease because it is related to age related fractures. According to the International Osteoporosis Foundation (IOF), osteoporosis influences 200 million women in the world; however, it also affects men significantly. One in five men compared to one in three women aged >50 years are affected [42,43]. The rate of mortalities due to hip fracture was 20% to 24%, besides that more deaths take place within six months of fracture [44]. In the United States, there are 10 million people who have osteoporosis (>50 years of age) and osteoporosis fractures occur in about 1.5 million people each year, with an economic cost of about $17 in 2005. In Europe, there were 27.6 million individuals who were reported to have osteoporosis, and osteoporosis fractures cost about >3.5 million per year with economic cost about €37 billion in 2010 [45-47]. In Sweden, it affects about 6.3% of men and about 21.2% of women (50-80 age years) [48]. There are many studies that estimated the prevalence of osteoporosis in countries of the Middle East. For example, approximately 31% of women in Lebanon are thought to be affected, with a prevalence (women >40 age years) of 13% in Jordan. Furthermore, in Kuwait, the prevalence was 18%. In Saudi Arabia, the prevalence was between the range of 35% to 48%. In females aged >31 years, the prevalence was about 7% whereas in women aged >50 years it was 28% [49]. Osteopenia and osteoporosis are highly common among postmenopausal Saudi women. The percentage of women between the age of 50-59 years who have osteopenia was 58%, and it was 89% in women between age 60-69 years who have osteoporosis, and it increased to 94% at the age of 70-80 years [50]. In Saudi Arabia, a high prevalence of osteoporosis is seen in both women and men due to different causes such as genetics, nutritional and environmental factors, such as low calcium and vitamin D intake, along with poor sun exposure, due to covering almost all of the body, combined with low physical activity [50,51]. Table 3 shows the prevalence of osteoporosis in Saudi women and men according to age groups.
Table 3.
The prevalence of osteoporosis and osteopenia in different groups of age in Saudi subject [51]
| Age | Normal N (%) | Osteopenia N (%) | Osteoporosis N (%) | Total N (%) |
|---|---|---|---|---|
| Postmenopausal Saudi women | ||||
| 50-59 | 216 (42.2) | 171 (33.4) | 124 (24.3) | 511 (100) |
| 60-69 | 28 (11) | 69 (27) | 157 (62) | 254 (100) |
| 70-80 | 3 (4.6) | 14 (21.5) | 48 (73.8) | 65 (100) |
| Saudi men (of lumbar spine) | ||||
| 30-50 | 90 (44.3) | 74 (36.5) | 39 (19.2) | 203 (100) |
| 51-90 | 94 (41.5) | 79 (35) | 53 (23.5) | 226 (100) |
Vitamin D
Vitamin D is a fat-soluble vitamin that is a hormonal precursor which includs two forms. The first form is present in plants and some fish, is called ergocalciferol or vitamin D2. The second form is cholecalciferol or vitamin D3 that is present in the skin and is synthesized by sunlight. In circulation, the normal level of vitamin D is >75 nmol/l or 30 ng/mL which is necessary for the beneficial effects of vitamin D in the physiological system for good health. The required level of vitamin D in the absence of sufficient sun exposure is about 800 to 1000 IU in both children and adults [52]. Vitamin D plays a significant role in bone metabolism, and it also has anti-inflammatory and immune-mediating properties. Vitamin D has an important role in controlling calcium absorption with parathyroid hormones in the gut, therefore, it assists in maintaining calcium homeostasis in the blood circulation and mediates skeletal mineralization [53,54]. Vitamin D deficiency is related to multiple diseases, for example, it causes rickets in children as well as osteoporosis and osteomalacia in adults. In addition, it is associated with cardiovascular disease rheumatoid arthritis, type I diabetes mellitus, increase risks for death from cancer and multiple sclerosis [55]. Vitamin D3 is synthesized in the skin from 7-dehydrocholestrol. Vitamin D3 is transported to the liver by vitamin D-binding protein. In the liver, hydroxylation occurs to inactivate it in the form of vitamin D 25(OH)D and then it is hydroxylated in the kidney to its active form 1,25(OH)D by the enzyme 1α hydroxylase, which is also found in different sites such as the skin, brain, osteoclasts and macrophages [54].
Vitamin D deficiency (VDD) and osteoporosis
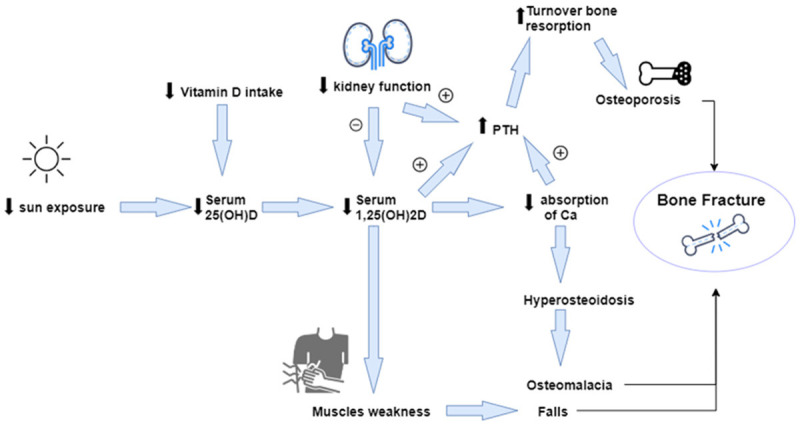
In humans, physiological calcium concentrations are required for bone remodelling process [53]. Vitamin D deficiency consequences are bone loss and secondary hyperparathyroidism which lead to osteoporosis and increased risk for bone fracture and mineralization defects which cause osteomalacia and weakness of muscles, thus leading to falls and bone fracture. Vitamin D status is associated with bone turnover, bone mineral density and risks for bone fracture. Consequently, vitamin D supplementation assists in reducing of bone turnover, increasing mineral bone and decreasing bone fractures [56] (see Figure 3). Vitamin D deficiency status in the circulation, is indicated when the concentration of 25(OH)D is <50 nmol/l or (<25 ng/ml), with an insufficient status set at 51-74 nmol/l or (21-29 ng/ml) and a sufficient status is denoted at >75 nmol/l or (>30 ng/ml) [52]. In the serum, the bone formation marker is osteocalcin and the bone resorption marker is urinary deoxypyridinoline execration, which are increased in low vitamin D levels in serum, whereas both markers are decreased when vitamin D is elevated up to 40 nmol/l [57]. A recent study by Yu et al., in 2021 found that Vitamin D deficiency is common in patients with brittle hip fractures, especially in women. With the increase of age, vitamin D continues to decrease and PTH increases. The decrease of BMD in patients with hip fractures is the result of a combination of age, gender, BMI, and vitamin D content [58].
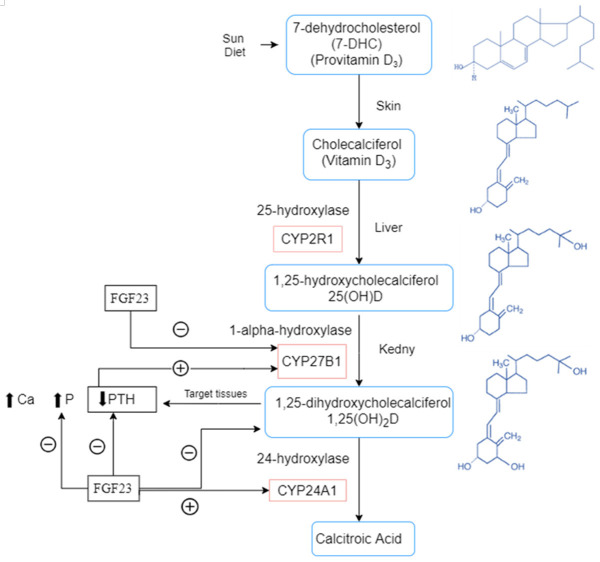
Figure 3.
Vitamin D Deficiency and its associated with osteoporosis, falls and bone fracture [56]. PTH: parathyroid hormone, Ca: calcium, 25(OH)D: 25-hydroxyvitamin D.
Prevalence of vitamin D deficiency (VDD)
Vitamin D deficiency is a common public health problem seen worldwide, in all different age groups, particularly in women and girls from Middle East countries. The prevalence of vitamin D deficiency in the world is uncertain and many of countries do not have issues; however, about 1 billion of people have vitamin D deficiency from all populations and ages [52]. The data show a high prevalence of vitamin D deficiency even in sunny countries. This high prevalence may be due to different causes such as high melanin content in dark skin individuals, or due to aging. Because, there is a low response to vitamin D synthesis from UVB, low vitamin D intake, along with high prevalence of obesity worldwide, and skin coverage causing low exposure to sunlight which occurs in people whose are from the Middle East, Africa and South America. Also, this problem is related to season, in which some countries with a long winter have a higher rate of vitamin D deficiency compared to those in sunny areas which often consume a fatty fish and have high vitamin D supplementation to replenish vitamin D levels [59]. In Europe, vitamin D deficiency is highly prevalent in older people in South Europe. The prevalence of vitamin D deficiency was 15% to 25% (<25 nmol/l from the mean serum 25(OH)D) in both young adults and adolescents, and 20% to >30% in persons aged >80 years. Also, the percentage range for all age groups was between 40% to 80% [60]. VDD is very common in Saudi Arabia as well as in most countries in the Gulf Cooperation Council (GCC) region despite yearlong sunshine (Al-Saleh et al., 2020) [61]. In the Middle East, the mean serum level of 25(OH)D was very lowest due to clothing style in most countries. For example, it was 32 nmol in Turkish women whose were wearing a hijab, and it was 56 nmol/l in western style women [62]. In Jordan, it was 28 nmol/l for women who wore a hijab and 37 nmol/l in women with a western style, while in men it was 44 nmol/l [63]. In India, it was 35 nmol/l in pregnant women and 36 nmol/l in postmenopausal women [64,65]. In Iran, the level was 20.6 nmol/l in study of 1210 men and women (20 to 69 years old) [66]. In Qatar, the level for the healthcare professionals was 29.2 nmol/l, and 25.7 nmol in females, whereas it was 34.2 nmol/l in males. The percentage of the population was 87% who had a level <50 nmol/l, and 97% with a level <75 nmol/l [67]. In Saudi Arabia, the level was 10 nmol/l in both students and older people [68]. Most studies of vitamin D in Saudi Arabia between 1982 to 1992 have revealed that the levels of vitamin D were the lower than other countries in the world [69]. One study showed that the prevalence of vitamin D deficiency was 83% of patients that have a backache [70]. Vitamin D deficiency prevalence was 30% in healthy Saudi women aged between 25 to 35 years, and 55% in postmenopausal women with an age more than ≥50 years [71].
Iron deficiency (ID) and osteoporosis
The effect of iron in bone metabolism has been given little attention, thus, the interaction is still unclear [72]. There are studies that have established that iron is an essential trace element for all cells as well as in osteoblast, which are required for bone formation. Different studies have revealed that iron deficiency and iron overload are both related to low bone mass. Some of these reports are based on a unique group of people and others are from animal models [73]. The following studies demonstrate the relationship between iron and osteoporosis.
Animal studies
Research reported by Medeiros et al., [74-76] showed that iron affects bone by influencing collagen synthesis. An important step of lysyl oxidase activity is where iron acts on two enzymes as cofactors which are lysyl and prolyl hydroxylase enzymes. This step is essential for catalyzing cross-linking of collagen fibers which are adjacent to each other, however, in case of iron deficiency, the cross-linking activity is decreased and collagen fibers become weaker if there is low iron levels available to the enzymes lysyl and prolyl hydroxylase [77] (see Figure 4). Collagen is a major component of connective tissue. In animals, collagen is considered one of the most abundant proteins [78]. In the collagen synthesis process, the principle components are proline and glycine amino acids. Assembly of the three-dimensional stranded structure to form the collagen precursor procollagen is modified by addition of hydroxyl groups to lysine and proline amino acids. This step undergoes glycosylation to form of collagen which is a triple helix structure. This reaction requires important components ferrous iron, molecular oxygen, α ketoglutarate and a reducing agent [77,79]. Reduction of the inactive state Fe+3 to active state Fe+2 occurs by ascorbate [80]. One previous animal model investigated the relationship between iron and bone resorption and formation with mineral content in rats with iron deficient anemia. In 2012, Diaz-Castro et al., observed that bone matrix formation diminished with a lower amount of procollagen type I N-terminal propeptide and bone resorption process increased with tartrate-resistant acid phosphatase, C-terminal telopeptides of type I and serum parathyroid hormone. In addition, iron deficiency affects the mineralization process by decreasing the Ca2+ and P content. This study indicates that severe iron deficiency causes an important change in bone metabolism status, decreasing bone formation and increasing bone resorption [81]. According to research by Tuderman et al., (1977) the level of C-terminal telopeptides of type I collagen increased in iron deficient rats. Iron deficiency leads to the reduction in type I collagen resulting in increased degradation of C-terminal telopeptides of type I collagen, thus weaker collagen fibers and increased bone fragility [77]. In 2006, Parelman et al., demonstrated that a severe iron deficiency caused a negative effect upon bone microarchitecture and they determined if human consumption patterns could result in a similar consequence. A cell culture model was used to study the effect of iron depletion on type I collagen deposition, and evaluated the mineralization. A study of female rats that were randomly divided into four groups and then fed different diets of adequate (A) or restricted (R) in both Fe and Ca for 10 weeks. DEXA analysis revealed that the Ca-R group had decreased bone mineral density (BMD), and the Fe-R group had decreased whole body bone mineral content (BMC) while in the group given both Fe restricted and Ca restricted diets revealed lower BMD. In cultured osteoclast cells, chelation of iron impaired mineralization but not type I collagen deposition. Similarly, in humans, results based on in vivo and in-vitro studies indicated reduced microarchitecture and bone strength. Bone abnormalities due to impaired mineralization in the case of iron depletion seem to be a possible mechanism [72]. Another study in 2006, Katsumata et al., examined the impact of dietary iron deficiency on bone metabolism. This was achieved by measuring some of the bone turnover markers in rats which were fed iron-deficient diets for 4 weeks. The results of this work suggested that dietary iron deficiency reduced bone mineral density, bone mineral content, osteocalcin concentration in serum and the mechanical strength of the femur. Thus, the results proved that dietary iron deficiency could affect bone by decreasing bone formation [82]. Additionally, in another study in 2009, the alteration in bone formation and resorption was assessed by detection of bone turnover markers and histomorphometry of bone in Wister rats with iron-deficient diet. The results suggested that the parameters of bone histomorphometry (bone formation and osteoblasts in lumber vertebra) were insignificantly lower in the iron-deficient group compared with other groups. Moreover, dietary iron deficiency reduced 1,25-dihydroxycholecalcifrol and osteocalcin. Therefore, ID can reduce both bone formation and resorption even though bone resorption is greater than bone formation resulting to overall bone loss [83]. Messer and colleagues, reported iron deficiency in growing female rats resulted from low bone mass and bone volume. Also, recent investigations showed that long term iron deficiency leads to altered bone mass and bone structure [84,85].
Figure 4.
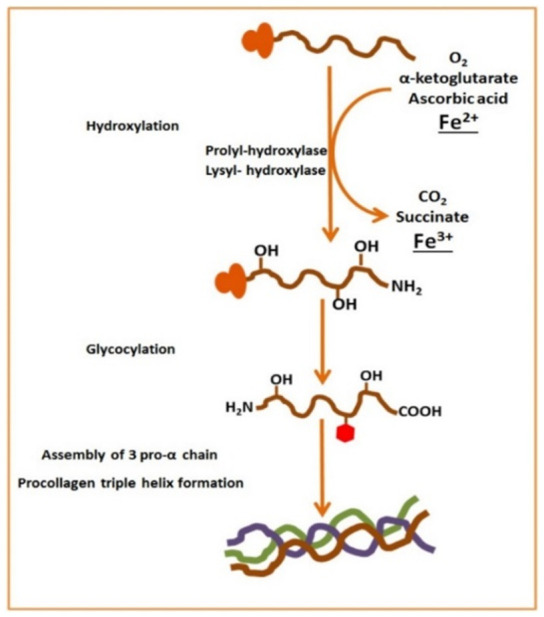
Role of iron bone metabolism and collagen synthesis [1]. CO2: carbon dioxide; O2: oxygen; Fe2+: ferrous ion.
Human studies
On the other hand, a previous study of post-menopausal women by D’Amelio et al., (2008) demonstrated a different type of haptoglobin (Hp) phenotypes could be risk factors or protective factors against fragility fracture. Iron levels in cells also depend on (Hp) phenotypes due to available of antioxidant properties which are impotent and could affect bone density and iron metabolism in osteoporotic postmenopausal women. They evaluated (Hp) phenotypes as a risk factors or protective factors against bone fragility fracture. The result showed that (Hp1.1) and (Hp2.2) phenotypes were higher in patients with fragility fractures while (Hp2.1) was lower compared with controls. In addition, (Hp2.1) acts as a protective factor against bone fragility fracture depending on its antioxidant properties and roles in iron metabolism. They also suggested an association between (Hp) and bone mass from its antioxidant properties in iron metabolism. In osteoporotic patients, serum iron levels were lower compared to those of a healthy control [86]. Previous research by Harris et al., determined whether the relationship between iron and (BMD) was clinically significant in a cross-sectional study in postmenopausal women examining many nutrients including iron. They detected a significant association (P<0.01) of iron with BMD in all bone sites in this study [73]. Similarly, in a study by Maurer et al., (2015) suggested that dietary iron in women using hormone replacement therapy was positively association with BMD [87]. In addition, in 1988, Angus et al., demonstrated a positive correlation of iron with BMC in the forearm and BMD in the hip in pre and post-menopausal women aged 23-75 years [88]. In contrast, a study in 1995 by Michaëlsson et al., determined the association between dietary iron with BMD significantly correlated (P<0.05) from univariate regression analysis at three sites in women aged 24-75 years. The results showed no associated between iron and bone site [89]. In 2013, Okyay and coworkers determined the relation between minerals found in serum, including iron, and postmenopausal osteoporosis. This study concluded that low minerals in serum including iron appear to be as risk factor and play a role in developing osteoporosis in postmenopausal women [90]. Zhao et al., (2012) performed an experiment to determine the effect of iron on osteoblast activity in humans. They found that a mild iron deficiency promoted osteoblast activity while more sever levels of iron deficiency inhibited osteogenesis [91]. In our recent study (Ghaleb et al., 2021) we found significant associations between circulating iron and 25(OH)D with select bone biomarkers in postmenopausal Arab women with osteoporosis, suggesting that these micronutrients may affect bone metabolism [92].
Relationship between iron deficiency (ID) and hypoxia in osteoporosis
Additionally, iron affects bone through hypoxia, which is a condition of reduced oxygen supply to tissues. Hypoxia causes stimulate of bone resorption by increasing of osteoclastogenesis followed by osteogenesis [93,94]. In normoxia, prolyl hydroxylase enzyme activation is required by α-ketoglutarate, iron and molecular oxygen. This enzyme participates by influencing Hypoxia Inducible Factor α (HIF-1α) through degradation and prevents its action. The impact of iron in HIF-1α seems to be similar in the collagen synthesis pathway. In contrast, HIF-1α does not undergoe degradation in case of hypoxia and translocates into nucleus resulting in transcription and regulation of more than 100 genes [79]. These genes induce PDGF, transferrin and erythropoietin (EPO) which are involved in the relationship between iron deficiency anemia and bone health. Accordingly, EPO influences several pleiotropic functions of the erythropoiesis process; furthermore, it acts in the remodelling process through direct and indirect effects [94-96]. A study in 2015 by Okito et al., reported that EPO stimulated bone resorption in mice [97]. In addition, stimulating osteoclast activity by acidosis leads to bone loss [93-98]. Hypoxia can lead to acidosis. Bone resorption is activated at low pH state. Consequently, phosphate is released and the equilibrium of acid-base restoration occurs in the extracellular fluid. However, a study by Okito et al., (2015) demonstrated that osteoblast phenotype changed from osteogenic to osteoclastogenic under acids condition, thus, the activity of both osteoblast and osteoclast are altered [97]. Furthermore, bone formation is considered to be slower than bone resorption. Thus, a long period of iron deficiency results in increased risk for osteoporosis and bone loss [12].
Relationship between iron deficiency (ID) and vitamin D deficiency (VDD) in osteoporosis
Iron deficiency and vitamin D deficiency are two of the most common nutritional disorders in the world [99]. Iron plays an important role in bone health by a cohort of enzymes as cofactors which are involved in collagen maturation, prolyl and lysyl hydroxylase. Furthermore, in vitamin D metabolism, iron participates in renal 25-hydroxyvitamin D 1-hydroxylase, which is part of a system that includes of flavoproteins which are iron-sulfur proteins along with cytochrome P450 [77,100] (See Figure 5). In case of IDA, all these iron-dependent enzymes can be affected or become inactive resulting in abnormal vitamin D and collagen metabolism [83]. Iron is involved in bone metabolism by affecting the vitamin D activation and inactivation process through participating in several heme-containing monooxygenases, a superfamily of cytochrome P450 which plays an important role in these pathways [101]. In vitamin D metabolism, iron is an essential element involved with cytochromes which catalyse a hydroxylation reaction in pathways by vitamin D substrate at specific carbons with heme binding iron [102]. In the first hydroxylation, photosynthesized vitamin D is transported by vitamin D-binding protein to the liver. After that, vitamin D is converted to its active form 1,25-hydroxvitamin D. In the kidney, a second hydroxylation occurs with key regulators that are required for production of 1,25-hydroxvitamin D. These regulators are calcium, serum phosphorus and fibroblast growth factor (FGF-23) [103]. There are observational studies that have demonstrated the relation between iron and vitamin D including of age, ethnicity, dietary calcium, fat intake, BMI, altitude, oxidative stress and inflammation. Inflammation and BMI are the most important factors because vitamin D is a fat-soluble vitamin stored in the body fat. Vitamin D concentration can be affected by the amount of fat in the tissues [104]. Ferritin is an acute phase protein and marker of increased iron in inflammation, thus resulting in IDA [105]. Increased age causes VDD [106]. Menstruation in postmenopausal women has increased risk of anemia. However, anemia developed in menopausal females may be due to inadequate nutritional intake and inflammation [107]. 1,25-hydroxvitamin D is related to bone health, more than 75 nmol/l of 1,25-hydroxvitamin D is required to prevention fracture. A previous study has suggested that chronic iron deficiency is related to increased bone resorption [83]. One of the mechanisms is through the influence of vitamin D metabolism deactivation [108]. In the condition of iron deficiency in tissues, the activity of iron-containing enzymes is reduced [109]. In the first step of the vitamin D activation pathway which involves certain types of cytochrome P450, CYP2R1, that is required for this step to form 25(OH)D3 in the liver. Another one is CYP27B1 which is required to produce 1,25(OH)2D3. CYP2R1 needs NADPH-Cytochrome P450 reductase for its biological function, whereas CYP27B1 depends on Ferredoxin reductase and Ferredoxin [110]. Heme group is a component of both of these enzymes. Consequently, iron is an important component in vitamin D metabolism and iron deficiency can be affected by vitamin D activation [108]. Vitamin D has been demonstrated to have a role in erythropoiesis. This clinical observation suggested that vitamin D repletion is associated with stimulation of reticulocytosis and reduced dose in erythrocyte stimulation agents [111,112]. Also, previous studies have shown that vitamin D affects bone function by playing a role in bone marrow [113,114]. In addition, levels of the active form of vitamin D show a higher concentration compared to plasma [115]. A study by Sim et al., (2010) evaluated whether there is an existing association between vitamin D deficiency, anemia, high intake of erythrocyte-stimulating agents and lower mean of hemoglobin (Hb). The results revealed a high prevalence and increased risk of anemia in people with vitamin D deficiency compared to normal vitamin D level in control subjects. Also, they suffered from reduced Hb levels compared to normal vitamin D level subjects. Furthermore, they elevated the level of serum iron saturation, ferritins and found lower levels of TIBC compared to normal vitamin D level subjects [116]. Vitamin D acts on erythroid precursors by direct stimulation. Vitamin D receptors are found in bone marrow and act upon non-renal target tissues [117,118]. In hematopoietic tissues, adequate amounts of vitamin D levels are a substrate provided to 1-α-hydroxylase enzymes to produce the active form of vitamin D [119]. Another study by Grindulis et at., (1986) suggested that a significant association exists between iron deficiency and lower vitamin D levels found in Asian children. Children who had a low vitamin D concentration had also a lower Hb concentration and iron concentration. Their investigations suggested that iron deficiency was due to anemia with dietary origin (Transferrin saturation, ferritin activity and low mean corpuscular volume) for both groups with and without anemia [120]. In addition, a previous study in 2007 by McGillivray et al., suggested that vitamin D deficiency is common in immigrant children with high skin pigmentation living in higher latitudes. Their findings revealed a high prevalence in this population of anemia and iron deficiency about 20% and 19%, respectively [121]. In addition to work in Korea by Jin et at., (2013) provided an association between iron status and vitamin D levels in the serum of infants. VVD was found in 67% of IDA compared to ID (53%) and normal subjects (29%). They suggested that the prevalence of VDD and iron was higher in breastfed infants (97%). Thus, they vitamin D supplementation is important particularly in IDA infants. Furthermore, the study showed a significant association between Hb and vitamin D levels which were higher in both the IDA and ID groups. Iron status was a predictor of vitamin D levels [122]. Similarly, research by Kang et al., demonstrated a correlation between iron and status of vitamin D in breastfed infants and their mothers. They also evaluated ID and VDD in breastfed infants. The results showed that there is an association of lower ferritin and vitamin D in breastfed infants and their mothers that had anemia during pregnancy [123]. Also, Yoon and Cho in 2015 indicated VDD prevalence and risk factors in anemic patients. Vitamin D prevalence was significantly higher in anemic patients compared to control group. Hence, vitamin D status measurement and its supplementation is important especially in anemic patients [124]. In 2003, Harris et al., reported that dietary iron is an important factor of bone mineralization combined with calcium on BMD in postmenopausal women. The results revealed a significant association of nutritional iron, vitamin D and calcium with BMD [73]. Another study in 2013 by Blanco-Rojo et al., observed a positive correlation between transferrin saturation and 25-hydroxyvitamin D in iron-deficient young women. Also, consuming food fortified with iron- helped recover iron status and was not affected by 25-hydroxyvitamin D. They concluded a correction between ID and VDD in iron-deficient young women [99]. All these studies showed the relationship between iron and vitamin D. Consequently, disrupted iron and vitamin D levels could lead to an increased risk of osteoporosis particularly in postmenopausal women. In recent studies from our group, it was observed that circulating iron is inversely associated with 25(OH)D levels among Saudi adolescents and was modestly decreased after vitamin D supplementation [125,126].
Figure 5.
Role of cytochromes P450 superfamily in activation and inactivation vitamin D pathway [1,127]. FGF23: Fibroblast growth factor-23, PTH: parathyroid hormones, Ca: calcium, P: phosphorous, CYP27B1: Cytochrome P450 Family 27 Subfamily B Member 1.
Effect of iron supplementation and vitamin D levels on osteoporosis
Most previous studies have suggested that iron supplementation does not influence vitamin D levels. This might be due to inappropriate design of these studies for example control group and randomization [122]. Generally, 25-hydroxyvitamin D status is measured by a normal phase high-performance liquid chromatography (HPLC), after that ultraviolet detection is used [108,128]. All studies do not use this method, thus, results may be affected. Also depletion of iron renewal is a factor. Moreover, iron supplementation does not affect iron deficiency when 25-hydroxylase enzyme is insufficient [108]. A study in 1992 by Heldenberg et al., reported that the levels of serum 24,25 dihydroxyvitamin D was lower in infants with IDA. Infants received 10 micrograms of vitamin D and they were treated with iron (Intramuscular Iron Dextran). The results relealed that 25 hydroxyvitamin D and 24,25 dihydroxyvitamin D levels were increased, likewise as were serum iron and hemoglobin concentrations. They concluded that iron might enhanced vitamin D absorption by the small intestine, thus giving rise to the recovery of vitamin D levels in the plasma [129]. Prats et al., (2013) in Spain examined the influence of ferric carboxymaltose (FCM) on metabolism of phosphate and fibro-blast growth factor 23 (FGF23) [130]. FGF23 is an osteocyte-derived hormone that is involved in the regulation hemostasis of phosphate and vitamin D [2]. Patient who suffered from chronic kidney disease (CKD) with IDA were injected with 1000 mg of FCM. The results showed that mineral metabolism markers such as 1,25-dihydroxyvitamin D, serum calcium and PTH were not changed. However, there was a significant decreasing in serum phosphate levels and FGF23 [130]. Similarly, in a study in the USA by Wolf et al., (2013) they examined the association between ID and both levels of C-terminal FGF23 and intact FGF23 in women who had a heavy uterine bleeding. Patients were injected with intravenous iron treatments FCM and dextran. The results revealed that ID lead to elevated levels of cFGF23, otherwise, iFGF23 remained normal. However, with iron supplementation intake, cFGF23 levels decreased in both group while iFGF23 was transiently increased in the group with FMC. Moreover, serum phosphate levels were decreased in the group with FMC. 25-dihydroxyvitamin D levels did not change in both groups [2]. Reducing serum phosphate levels because of acute increase in FGF23 levels results in enhanced phosphaturia and suppressed 1,25-dihydroxyvitamin D levels [131]. Normalizing iFGF23 due to reduction of 1,25-dihydroxyvitamin D and calcium levels in the case of hyperparathyroidism. Also, intravenous iron results in diminished serum phosphate levels. In ID, transcription of FGF23 was enhanced whereas with iron therapy resulted in decreased serum phosphate levels by enhanced of iFGF23 and decreased cFGF23 [2] (see Figure 6). Another study in 2013 by Wright et al., investigated the difference in bone remodelling and effect of recovery of iron status on bone turnover markers in postmenopausal women with IDA. Patients treated with iron and iron biomarkers and bone turnover markers were measured at baseline after treatment. The results showed that there was a significant increase in amino-terminal telopeptide of collagen1 (NTx) levels while procollagen type I N-terminal propeptide (PINP) levels were reduced in the IDA group compared to control group with comparable age and BMI. Also, there was no change in 25-hydroxyvitamin D levels or PTH. However, after treatment of with iron, the levels of (NTx) and (PINP) were significantly lower in the recovered group, with no difference in 25-hydroxyvitamin D levels or PTH. They concluded that recovery of iron status might give a beneficial effect to bone remodelling by reducing resorption and formation of bone in postmenopausal women with IDA [132]. Blanco-Rojo et al., (2013) studied whether iron recovery by intake of iron-fortified juice could influence bone remodeling and affected vitamin D levels in menstruating women with ID aged 18-35 years. All participants consumed of a placebo fruit juice (P) or iron-fortified juice (F). The results revealed a positive association between 25(OH)D and transferrin and no significant correlation between serum 25(OH)D and iron parameters (serum ferritin, serum transferrin and hemoglobin). Also, there was no change in ALP, NTX and PTH in either the P or F group [99]. Another study in Spain by Toxqui and colleagues (2014) determined if there was a correlation between iron status and bone metabolism. They studied the effect of iron or iron and vitamin D-fortified skim milk consumption on bone the remodeling process in menstruating women with ID aged 18 to 35 years. They measured iron biomarkers and bone turnover markers. The results revealed a significant deference in ferritin, serum transferrin, serum iron, transferrin saturation and TIBC in both the Fe and Fe+D groups. There was a significant negative association between transferrin and P1NP, and log-ferritin and log-NTX. In the Fe+D group, 25(OH)D was increased significantly specifically at week 8 and 16. 25(OH)D was decreased in the Fe group at week 8, however, it recovered its baseline levels at the end. Hemoglobin and PTH did not change. P1NB was not changed in the Fe group but in the Fe+D group there was a significant decrease. NTX significantly decreased in the Fe+D group and compared to the Fe group it was lower at week 8. NTX levels were higher in the Fe and Fe+D groups at baseline and at weeks 8 and 16 [133]. They concluded that ID was correlated with higher bone resorption in women with ID. Dairy product consumption with vitamin D3 lead to increased circulation of 25(OH)D and decreased bone turnover. A study done in Japan by Iguchi and co-works in 2015 examined whether iron supplementation could influence serum FGF23 in patients with hemodialysis (HD), ID and hyperphosphatemia, had patients who were treated with Sevelamer-HCL which is changed to ferric citrate hydrate (FCH) by phosphate binder, in order to assist in the maintenance of phosphate at constant levels. The results showed that no changes in serum phosphate and 1,25(OH)2D levels while there was an increased serum ferritin levels with FCH supplementation and intact PTH levels was elevated. Moreover, there was a significant decrease in the levels of intact FGF23 and C-term FGF23 in the serum. They suggested that with treatment of ID by oral FCH consumption it is possible to decrease the levels of serum FGF23 [134]. Secretion of FGF23 from osteoblasts is regulated by dietary intake of phosphate [135], PTH [136], serum calcium level [137] and vitamin D receptor activator (VDRA) [138,139]. In addition, iron seems to be a possible factor to regulate of FGF23 [140]. In an animal study, data suggested that ID resulted from stimulated transcription of FGF23 in osteocytes [141]. Otherwise, measurement of FGF23 in humans can be done by C-terminal immunoassay (C-Term FGF23). This assay is used in the detection of FGF23 with both its full-length and C-terminal fragments which are inversely association with iron status [142,143]. The intact FGF23 C-terminal immunoassay (intact FGF23) measures the biological active FGF23 which is not correlated with iron [144]. In hypophosphatemic rickets, an autosomal dominant disease characterized by impaired cleavage of intact FGF23 with both its full-length and C-terminal fragment are inversely associated with iron status [142]. In the case of ID, transcription of FGF23 increases while intact FGF23 levels do not increase in healthy human in ID conditions. This maybe because they have normal FGF23 cleavage functions. On the other hand, an in vitro study with osteoblastics investigated iron chelation with deferoxamine in association with stabilization of hypoxia inducible factor-1α lead to increased FGF23 mRNA expression by 20-fold [141]. All these results suggested that ID results from stimulated FGF23 transcription [134]. Recently, studies have shown that FCH administration diminished intact FGF23 levels in patient with pre-dialysis CKD [144,145]. Animal studies have suggested the relationship between restrictions of diary iron and bone health. These studies found a significant correlation between iron restriction and BMC, BMD and femur strength. In several reports, the results showed bone markers were reduced in bone formation and increased in bone resorption [76,81,83]. These parameters appears to be recovered after consumption of iron in the diet in normal or high levels [146]. Finally, it is unknown why bones respond in anemic women on iron supplementation, and there was no difference in bone remodeling in women with iron deficiency and intake of iron-fortified fruit juice. Also, it is not clear why bones improve in iron deficiency women when they are treated with vitamin D. Therefore, further studies are required in this field.
Figure 6.
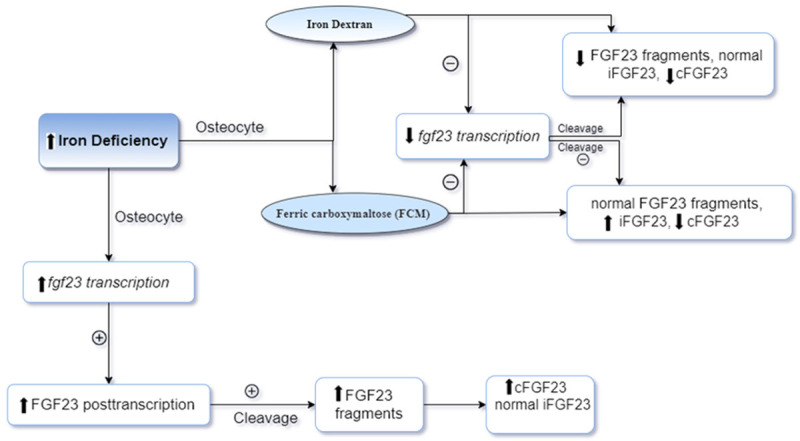
Effect of ID and corection it by using of iron dose therapy Dectran or FCM on cFGF23 and iFGF23 levels regulation in Osteosyte [2]. ID: iron deffeciency, FCM: ferric carboxymaltos, FGF23: Fibroblast growth factor-23, iFGF23: intact fibroblast growth factor-23, cFGF23: C-terminal fibroblast growth factor 23.
Literature selection method
In this narrative review article, we carried out a literature search, which identified articles describing the relationship between iron deficiency and vitamin D deficiency. The search was performed in 2020, with the electronic databases of PubMed/MEDLINE, and Web of Science. The research process was built specifically for each database and no limit was used in this research. The period of article publication was not restricted for the search. The search in the databases of electronic journals and the selection of titles, abstracts and articles were made, strictly following the pre-defined inclusion and exclusion criteria. A combination of text words was used to identify the potential articles to be included in the review. An initial analysis was carried out based on the titles of the manuscripts. When the title and the abstract were inconclusive, i.e., when there were doubts regarding the article content, the full text was sought, to not run the risk of leaving important studies out of the systematic review. Thus, after reviewing the titles and abstracts, all articles with full text were obtained and included if they met the inclusion criteria, and were subsequently read in full by the researchers. The references of all selected articles were assessed to identify other publications that could be included in the review.
Conclusion
Based on current knowledge, we hypothesize that iron deficiency inversely affects the absorption of vitamin D in the small intestine and hence bone through different mechanisms.
Interventional studies are required to determine the effect of iron and 25(OH)D supplementation over time on bone among postmenopausal women particularly those with osteoporosis.
To prove the hypothesis that iron deficiency predisposes patients to bone loss, osteoporosis, and risk of fracture, various investigation lines should be developed as there are many unsolved issues. It is not known to what extent severe or mild iron deficiency affect bone.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this research project through Vice Deanship of Scientific Research Chairs.
Disclosure of conflict of interest
None.
References
- 1.Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7:2324–2344. doi: 10.3390/nu7042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 3.Bendich A. Iron deficiency and overload. New York: Humana Press; 2010. [Google Scholar]
- 4.Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225–233. doi: 10.1016/j.blre.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Badham J, Zimmermann MB, Kraemer K. The guidebook nutritional anemia. Switzerland: Sight and Life Press Basel; 2007. [Google Scholar]
- 7.Denic S, Agarwal MM. Nutritional iron deficiency: an evolutionary perspective. Nutrition. 2007;23:603–614. doi: 10.1016/j.nut.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. 2008 [Google Scholar]
- 10.Mehmood T, Auerbach M, Earley CJ, Allen RP. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis-Ekbom disease) Sleep Med. 2014;15:1473–1476. doi: 10.1016/j.sleep.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Gibson RS. Principles of nutritional assessment. USA: Oxford University Press; 2005. [Google Scholar]
- 12.Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7:2324–2344. doi: 10.3390/nu7042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585–1594. doi: 10.3945/ajcn.114.103366. [DOI] [PubMed] [Google Scholar]
- 14.Gejyo F, Saito A, Akizawa T, Akiba T, Sakai T, Suzuki M, Nishi S, Tsubakihara Y, Hirakata H, Bessho M Japanese Society for Dialysis Therapy. 2004 Japanese Society for dialysis therapy guidelines for renal anemia in chronic hemodialysis patients. Ther Apher Dial. 2004;8:443–459. doi: 10.1111/j.1774-9987.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. The global prevalence of anaemia in 2011. 2015 [Google Scholar]
- 16.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 17.Verster A, Jolieke C, Pols V. Anaemia in the Eastern Mediterranean region. Eastern Mediterr Health J. 1995;1:64–79. [Google Scholar]
- 18.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 2009;12:444. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 19.Steinbicker AU, Muckenthaler MU. Out of balance--systemic iron homeostasis in iron-related disorders. Nutrients. 2013;5:3034–3061. doi: 10.3390/nu5083034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 21.DeMaeyer EM, Dallman P, Gurney JM, Hallberg L, Sood S, Srikantia S World Health Organization. Preventing and controlling iron deficiency anaemia through primary health care: a guide for health administrators and programme managers. 1989 [Google Scholar]
- 22.Musaiger AO. Iron deficiency anaemia among children and pregnant women in the Arab Gulf countries: the need for action. Nutr Health. 2002;16:161–171. doi: 10.1177/026010600201600302. [DOI] [PubMed] [Google Scholar]
- 23.Bagchi K. Iron deficiency anaemia--an old enemy. East Mediterr Health J. 2004;10:754–760. [PubMed] [Google Scholar]
- 24.Al Hassan NN. The prevalence of iron deficiency anemia in a Saudi University female students. J Microsc Ultrastruct. 2015;3:25–28. doi: 10.1016/j.jmau.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musaiger AO. Iron deficiency anaemia among children and pregnant women in the arab gulf countries: the need for action. Nutr Health. 2002;16:161–171. doi: 10.1177/026010600201600302. [DOI] [PubMed] [Google Scholar]
- 26.al-Othaimeen A, Osman AK, al Orf S. Prevalence of nutritional anaemia among primary school girls in Riyadh city, Saudi Arabia. Int J Food Sci Nutr. 1999;50:237–243. doi: 10.1080/096374899101111. [DOI] [PubMed] [Google Scholar]
- 27.Mahfouz AA, El-Said MM, Alakija W, Badawi IA, Al-Erian R, Moneim MA. Anemia among pregnant women in the Asir region, Saudi Arabia: an epidemiologic study. Southeast Asian J Trop Med Public Health. 1994;25:84–87. [PubMed] [Google Scholar]
- 28.Madani KA. Low birth weight in the Taif region, Saudi Arabia. 1995 [Google Scholar]
- 29.Ghaznawi HI, Hussein M. Anaemia in pregnancy in Jeddah, Saudi Arabia. An epidemiological study. Bulletin of the High Institute of Public Health. 1988;18:541–553. [Google Scholar]
- 30.Alhamdan AA. Nutritional status of Saudi males living in the Riyadh nursing home. Asia Pac J Clin Nutr. 2004;13:372–376. [PubMed] [Google Scholar]
- 31.Al-Assaf AH. Anemia and iron intake of adult Saudis in Riyadh city-Saudi Arabia. Pakistan Journal of Nutrition. 2007;6:355–358. [Google Scholar]
- 32.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis and therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 34.WHO Scientific Group. Assessment of osteoporosis at the primary health care level Geneva (Switzerland) World Health Organization. 2007 [Google Scholar]
- 35.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 36.Larijani B. Facts and statistics, osteoporosis general. Available at: https://www.iofbonehealth.org/facts-statistic#category-14. Accessed 11 March 2017.
- 37.Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B. New horizons in treatment of osteoporosis. Daru. 2017;25:23. doi: 10.1186/s40199-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 39.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 41.Khosla S, Melton LJ 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26:441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeman E, Bianchi G, Adami S, Kanis J, Khosla S, Orwoll E. Osteoporosis in men-consensus is premature. Calcif Tissue Int. 2004;75:120–122. doi: 10.1007/s00223-004-4002-4. [DOI] [PubMed] [Google Scholar]
- 43.Seeman E. Osteoporosis in men: the “silent epidemic” strikes men too. International Osteoporosis Foundation. 2004 [Google Scholar]
- 44.Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644–1650. doi: 10.1046/j.1532-5415.2002.50455.x. [DOI] [PubMed] [Google Scholar]
- 45.Office of the Surgeon General (US) Bone health and osteoporosis: a report of the surgeon general. Rockville (MD): Office of the Surgeon General (US); 2004. [PubMed] [Google Scholar]
- 46.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 48.Kanis J, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 2000;27:585–590. doi: 10.1016/s8756-3282(00)00381-1. [DOI] [PubMed] [Google Scholar]
- 49.Sadat-Ali M, Al-Habdan IM, Al-Turki HA, Azama MQ. An epidemiological analysis of the incidence of osteoporosis and osteoporosis-related fractures among the Saudi Arabian population. Ann Saudi Med. 2012;32:637–41. doi: 10.5144/0256-4947.2012.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Saleh Y, Sulimani R, Sabico S, Raef H, Fouda M, Alshahrani F, Al Shaker M, Al Wahabi B, Sadat-Ali M, Al Rayes H, Al Aidarous S, Saleh S, Al Ayoubi F, Al-Daghri NM. 2015 Guidelines for osteoporosis in Saudi Arabia: recommendations from the Saudi osteoporosis society. Ann Saudi Med. 2015;35:1–12. doi: 10.5144/0256-4947.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Daghri NM, Sabico S, Al-Saleh Y, Sulimani R, Aljohani NJ, Sheshah E, Alodhayani A, Harvey NC, Liu E, Lorentzon M, McCloskey EV, Vandenput L, Johansson H, Kanis JA. The application of FRAX in Saudi Arabia. Arch Osteoporos. 2021;16:166. doi: 10.1007/s11657-021-01024-2. [DOI] [PubMed] [Google Scholar]
- 52.Al Saleh Y, Beshyah SA, Hussein W, Almadani A, Hassoun A, Al Mamari A, Ba-Essa E, Al-Dhafiri E, Hassanein M, Fouda MA, Al Ali N, Aljohani N, Al-Sayed N, Gittoes N, Elhadd T, Al-Baker W, Sabico S, Al-Daghri N. Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: an expert consensus summary statement from the GCC vitamin D advisory board. Arch Osteoporos. 2020;15:35. doi: 10.1007/s11657-020-0709-8. [DOI] [PubMed] [Google Scholar]
- 53.Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 54.Brannon PM, Yetley EA, Bailey RL, Picciano MF. Overview of the conference “vitamin D and health in the 21st century: an update”. Am J Clin Nutr. 2008;88:483S–490S. doi: 10.1093/ajcn/88.2.483S. [DOI] [PubMed] [Google Scholar]
- 55.Al-Daghri NM, Yakout SM, Ansari MGA, Hussain SD, Wani KA, Sabico S. Vitamin D metabolites and sex steroid indices in postmenopausal women with and without low bone mass. Metabolites. 2021;11:86. doi: 10.3390/metabo11020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25:585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 58.Yu SJ, Yang Y, Zang JC, Li C, Wang YM, Wang JB. Evaluation of serum 25-hydroxyvitamin D3 and bone mineral density in 268 patients with hip fractures. Orthop Surg. 2021;13:892–899. doi: 10.1111/os.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, El-Kholie E, Yousef M, Al-Othman A, Al-Saleh Y, Sabico S, Kumar S, Chrousos GP. Increased vitamin D supplementation recommended during summer season in the gulf region: a counterintuitive seasonal effect in vitamin D levels in adult, overweight and obese Middle Eastern residents. Clin Endocrinol (Oxf) 2012;76:346–50. doi: 10.1111/j.1365-2265.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- 60.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88:500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 61.Al-Saleh Y, Al-Daghri NM, Sabico S, Alessa T, Al Emadi S, Alawadi F, Al Qasaabi S, Alfutaisi A, Al Izzi M, Mukhaimer J, Suhaili AR, Reginster JY, Sulimani R. Diagnosis and management of osteoporosis in postmenopausal women in Gulf Cooperation Council (GCC) countries: consensus statement of the GCC countries’ osteoporosis societies under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Arch Osteoporos. 2020;15:109. doi: 10.1007/s11657-020-00778-5. [DOI] [PubMed] [Google Scholar]
- 62.Atli T, Gullu S, Uysal A, Erdogan G. The prevalence of vitamin D deficiency and effects of ultraviolet light on vitamin D levels in elderly Turkish population. Arch Gerontol Geriatr. 2005;40:53–60. doi: 10.1016/j.archger.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Mishal A. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12:931–935. doi: 10.1007/s001980170021. [DOI] [PubMed] [Google Scholar]
- 64.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 65.Harinarayan C. Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int. 2005;16:397–402. doi: 10.1007/s00198-004-1703-5. [DOI] [PubMed] [Google Scholar]
- 66.Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, Pajouhi M, Soltani A, Shafaei AR, Hamidi Z, Fard ARK. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public health. 2004;4:38. doi: 10.1186/1471-2458-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahdy S, Al-Emadi SA, Khanjar IA, Hammoudeh MM, Sarakbi HA, Siam AM, Abdelrahman O. Vitamin D status in health care professionals in Qatar. Saudi Med J. 2010;31:74–77. [PubMed] [Google Scholar]
- 68.Sedrani SH, Elidrissy A, El Arabi KM. Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr. 1983;38:129–132. doi: 10.1093/ajcn/38.1.129. [DOI] [PubMed] [Google Scholar]
- 69.Sedrani S, Al-Arabi K, Abanmy A, Elidrissy A. Vitamin D status of Saudis II. Effect of regional and environmental location. Saudi Med J. 1992;13:206–213. [Google Scholar]
- 70.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine (Phila Pa 1976) 2003;28:177–179. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 71.Al-Turki HA, Sadat-Ali M, Al-Elq AH, Al-Mulhim FA, Al-Ali AK. 25-Hydoxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J. 2008;29:1765–1768. [PubMed] [Google Scholar]
- 72.Parelman M, Stoecker B, Baker A, Medeiros D. Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp Biol Med (Maywood) 2006;231:378–386. doi: 10.1177/153537020623100403. [DOI] [PubMed] [Google Scholar]
- 73.Harris MM, Houtkooper LB, Stanford VA, Parkhill C, Weber JL, Flint-Wagner H, Weiss L, Going SB, Lohman TG. Dietary iron is associated with bone mineral density in healthy postmenopausal women. J Nutr. 2003;133:3598–3602. doi: 10.1093/jn/133.11.3598. [DOI] [PubMed] [Google Scholar]
- 74.Medeiros DM, Ilich J, Ireton J, Matkovic V, Shiry L, Wildman R. Femurs from rats fed diets deficient in copper or iron have decreased mechanical strength and altered mineral composition. The Journal of Trace Elements in Experimental Medicine. 1997;10:197–203. [Google Scholar]
- 75.Medeiros DM, Plattner A, Jennings D, Stoecker B. Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J Nutr. 2002;132:3135–3141. doi: 10.1093/jn/131.10.3135. [DOI] [PubMed] [Google Scholar]
- 76.Medeiros DM, Stoecker B, Plattner A, Jennings D, Haub M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J Nutr. 2004;134:3061–3067. doi: 10.1093/jn/134.11.3061. [DOI] [PubMed] [Google Scholar]
- 77.Tuderman L, Myllyla R, Kivirikko KI. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977;80:341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- 78.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Jong L, Kemp A. Stoicheiometry and kinetics of the prolyl 4-hydroxylase partial reaction. Biochim Biophys Acta. 1984;787:105–111. doi: 10.1016/0167-4838(84)90113-4. [DOI] [PubMed] [Google Scholar]
- 81.Díaz-Castro J, López-Frías MR, Campos MS, López-Frías M, Alférez MJ, Nestares T, Ojeda ML, López-Aliaga I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur J Nutr. 2012;51:241–247. doi: 10.1007/s00394-011-0212-5. [DOI] [PubMed] [Google Scholar]
- 82.Katsumata S, Tsuboi R, Uehara M, Suzuki K. Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci Biotechnol Biochem. 2006;70:2547–2550. doi: 10.1271/bbb.60221. [DOI] [PubMed] [Google Scholar]
- 83.Katsumata S, Katsumata-Tsuboi R, Uehara M, Suzuki K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J Nutr. 2009;139:238–243. doi: 10.3945/jn.108.093757. [DOI] [PubMed] [Google Scholar]
- 84.Messer JG, Cooney PT, Kipp DE. Iron chelator deferoxamine alters iron-regulatory genes and proteins and suppresses osteoblast phenotype in fetal rat calvaria cells. Bone. 2010;46:1408–15. doi: 10.1016/j.bone.2010.01.376. [DOI] [PubMed] [Google Scholar]
- 85.Messer JG, Kilbarger AK, Erikson KM, Kipp DE. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009;45:972–9. doi: 10.1016/j.bone.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 86.D’Amelio P, Cristofaro MA, Tamone C, Morra E, Di Bella S, Isaia G, Grimaldi A, Gennero L, Gariboldi A, Ponzetto A, Pescarmona GP, Isaia GC. Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone. 2008;43:1010–1015. doi: 10.1016/j.bone.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 87.Maurer J, Harris MM, Stanford VA, Lohman TG, Cussler E, Going SB, Houtkooper LB. Dietary iron positively influences bone mineral density in postmenopausal women on hormone replacement therapy. J Nutr. 2005;135:863–869. doi: 10.1093/jn/135.4.863. [DOI] [PubMed] [Google Scholar]
- 88.Angus R, Sambrook P, Pocock N, Eisman J. Dietary intake and bone mineral density. Bone Miner. 1988;4:265–277. [PubMed] [Google Scholar]
- 89.Michaelsson K, Holmberg L, Mallmin H, Wolk A, Bergstrom R, Ljunghall S. Diet, bone mass, and osteocalcin: a cross-sectional study. Calcif Tissue Int. 1995;57:86–93. doi: 10.1007/BF00298425. [DOI] [PubMed] [Google Scholar]
- 90.Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B, Ozaksoy D. Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas. 2013;76:320–325. doi: 10.1016/j.maturitas.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 91.Zhao GY, Zhao LP, He YF, Li GF, Gao C, Li K, Xu YJ. A comparison of the biological activities of human osteoblast hFOB1. 19 between iron excess and iron deficiency. Biol Trace Elem Res. 2012;150:487–495. doi: 10.1007/s12011-012-9511-9. [DOI] [PubMed] [Google Scholar]
- 92.Ghaleb A, Abdi S, Yakout S, Danish Hussain S, Wani K, Masoud M, Alnaami A, Al-Daghri NM. Serum iron deficiency and 25-hydroxyvitamin D deficiency as an independent risk factor for osteoporosis in postmenopausal Arab women. J King Saud Univ Sci. 2021;33:101217. [Google Scholar]
- 93.Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 94.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hiram-Bab S, Liron T, Deshet-Unger N, Mittelman M, Gassmann M, Rauner M, Franke K, Wielockx B, Neumann D, Gabet Y. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015;29:1890–1900. doi: 10.1096/fj.14-259085. [DOI] [PubMed] [Google Scholar]
- 96.Ilich-Ernst JZ, McKenna AA, Badenhop NE, Clairmont AC, Andon MB, Nahhas RW, Goel P, Matkovic V. Iron status, menarche, and calcium supplementation in adolescent girls. Am J Clin Nutr. 1998;68:880–887. doi: 10.1093/ajcn/68.4.880. [DOI] [PubMed] [Google Scholar]
- 97.Okito A, Nakahama KI, Akiyama M, Ono T, Morita I. Involvement of the G-protein-coupled receptor 4 in RANKL expression by osteoblasts in an acidic environment. Biochem Biophys Res Commun. 2015;458:435–440. doi: 10.1016/j.bbrc.2015.01.142. [DOI] [PubMed] [Google Scholar]
- 98.Lee MY, Fukunaga R, Lee TJ, Lottsfeldt JL, Nagata S. Bone modulation in sustained hematopoietic stimulation in mice. Blood. 1991;77:2135–2141. [PubMed] [Google Scholar]
- 99.Blanco-Rojo R, Pérez-Granados AM, Toxqui L, Zazo P, de la Piedra C, Vaquero MP. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur J Nutr. 2013;52:695–703. doi: 10.1007/s00394-012-0375-8. [DOI] [PubMed] [Google Scholar]
- 100.DeLuca HF. Metabolism of vitamin D: current status. Am J Clin Nutr. 1976;29:1258–1270. doi: 10.1093/ajcn/29.11.1258. [DOI] [PubMed] [Google Scholar]
- 101.Pikuleva IA, Waterman MR. Cytochromes P450: roles in diseases. J Biol Chem. 2013;288:17091–17098. doi: 10.1074/jbc.R112.431916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watson RR. Handbook of vitamin D in human health: prevention, treatment and toxicity. Wageningen Academic Pub. 2013 [Google Scholar]
- 104.Zitt E, Sprenger-Mähr H, Mündle M, Lhotta K. Efficacy and safety of body weight-adapted oral cholecalciferol substitution in dialysis patients with vitamin D deficiency. BMC Nephrol. 2015;16:128. doi: 10.1186/s12882-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bárány P. Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transplant. 2001;16:224–227. doi: 10.1093/ndt/16.2.224. [DOI] [PubMed] [Google Scholar]
- 106.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 107.Han SS, Kim M, Kim H, Lee SM, Oh YJ, Lee JP, Kim S, Joo KW, Lim CS, Kim YS. Non-linear relationship between serum 25-hydroxyvitamin D and hemoglobin in Korean females: the Korean national health and nutrition examination survey 2010-2011. PLoS One. 2013;8:e72605. doi: 10.1371/journal.pone.0072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Azizi-Soleiman F, Vafa M, Abiri B, Safavi M. Effects of iron on Vitamin D metabolism: a systematic review. Int J Prev Med. 2016;7:126. doi: 10.4103/2008-7802.195212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- 110.Jones G, Prosser DE. The activating enzymes of vitamin D metabolism (25-and 1a-hydroxylases) Vitamin D. 2011;1:23–42. [Google Scholar]
- 111.Albitar S, Genin R, Fen-Chong M, Serveaux M, Schohn D, Chuet C. High-dose alfacalcidol improves anaemia in patients on haemodialysis. Nephrol Dial Transplant. 1997;12:514–518. doi: 10.1093/ndt/12.3.514. [DOI] [PubMed] [Google Scholar]
- 112.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105:c132–c138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 113.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 114.Norman AW. Vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 115.Blazsek I, Farabos C, Quittet P, Labat ML, Bringuier AF, Triana BK, Machover D, Reynes M, Misset JL. Bone marrow stromal cell defects and 1 alpha, 25-dihydroxyvitamin D3 deficiency underlying human myeloid leukemias. Cancer Detect Prev. 1995;20:31–42. [PubMed] [Google Scholar]
- 116.Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, Rasgon SA. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol. 2010;89:447–452. doi: 10.1007/s00277-009-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 118.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 119.Aucella F, Scalzulli RP, Gatta G, Vigilante M, Carella AM, Stallone C. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. Nephron Clin Pract. 2003;95:c121–c127. doi: 10.1159/000074837. [DOI] [PubMed] [Google Scholar]
- 120.Grindulis H, Scott PH, Belton NR, Wharton BA. Combined deficiency of iron and vitamin D in Asian toddlers. Arch Dis Child. 1986;61:843–848. doi: 10.1136/adc.61.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGillivray G, Skull SA, Davie G, Kofoed SE, Frydenberg A, Rice J, Cooke R, Carapetis JR. High prevalence of asymptomatic vitamin D and iron deficiency in East African immigrant children and adolescents living in a temperate climate. Arch Dis Child. 2007;92:1088–1093. doi: 10.1136/adc.2006.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin HJ, Lee JH, Kim MK. The prevalence of vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Blood Res. 2013;48:40–45. doi: 10.5045/br.2013.48.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang YS, Kim JH, Ahn EH, Yoo EG, Kim MK. Iron and vitamin D status in breastfed infants and their mothers. Korean J Pediatr. 2015;58:283–287. doi: 10.3345/kjp.2015.58.8.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoo EH, Cho HJ. Prevalence of 25-hydroxyvitamin D deficiency in Korean patients with anemia. J Clin Lab Anal. 2015;29:129–134. doi: 10.1002/jcla.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Masoud MS, Alokail MS, Yakout SM, Khattak MNK, AlRehaili MM, Wani K, Al-Daghri NM. Vitamin D supplementation modestly reduces serum iron indices of healthy Arab adolescents. Nutrients. 2018;10:1870. doi: 10.3390/nu10121870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masoud MS, Yakout SM, Al-Attas OS, Alokail MS, Al-Daghri NM. The association between iron and vitamin D status in Arab adolescents. Public Health Nutr. 2020;23:1208–1213. doi: 10.1017/S1368980019001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 128.Fraser WD, Milan AM. Vitamin D assays: past and present debates, difficulties, and developments. Calcif Tissue Int. 2013;92:118–127. doi: 10.1007/s00223-012-9693-3. [DOI] [PubMed] [Google Scholar]
- 129.Heldenberg D, Tenenbaum G, Weisman Y. Effect of iron on serum 25-hydroxyvitamin D and 24, 25-dihydroxyvitamin D concentrations. Am J Clin Nutr. 1992;56:533–536. doi: 10.1093/ajcn/56.3.533. [DOI] [PubMed] [Google Scholar]
- 130.Prats M, Font R, García C, Cabré C, Jariod M, Vea AM. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: post-hoc analysis of a prospective study. BMC Nephrol. 2013;14:167. doi: 10.1186/1471-2369-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–2337. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 132.Wright I, Blanco-Rojo R, Fernández MC, Toxqui L, Moreno G, Pérez-Granados AM, de la Piedra C, Remacha ÁF, Vaquero MP. Bone remodelling is reduced by recovery from iron-deficiency anaemia in premenopausal women. J Physiol Biochem. 2013;69:889–896. doi: 10.1007/s13105-013-0266-3. [DOI] [PubMed] [Google Scholar]
- 133.Toxqui L, Pérez-Granados AM, Blanco-Rojo R, Wright I, de la Piedra C, Vaquero MP. Low iron status as a factor of increased bone resorption and effects of an iron and vitamin D-fortified skimmed milk on bone remodelling in young Spanish women. Eur J Nutr. 2014;53:441–448. doi: 10.1007/s00394-013-0544-4. [DOI] [PubMed] [Google Scholar]
- 134.Iguchi A, Kazama JJ, Yamamoto S, Yoshita K, Watanabe Y, Iino N, Narita I. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron. 2015;131:161–166. doi: 10.1159/000440968. [DOI] [PubMed] [Google Scholar]
- 135.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 136.López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, De Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–482. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 137.David V, Dai B, Martin A, Huang J, Han X, Quarles LD. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154:4469–4482. doi: 10.1210/en.2013-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 139.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012;23:610–618. doi: 10.1016/j.tem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wolf M, White KE. Coupling FGF23 production and cleavage: iron deficiency, rickets and kidney disease. Curr Opin Nephrol Hypertens. 2014;23:411–9. doi: 10.1097/01.mnh.0000447020.74593.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108:E1146–E1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96:3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Braithwaite V, Prentice AM, Doherty C, Prentice A. FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int J Pediatr Endocrinol. 2012;2012:27. doi: 10.1186/1687-9856-2012-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Block GA. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol. 2014;9:543–52. doi: 10.2215/CJN.05170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis. 2015;65:728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 146.Díaz-Castro J, Lopez-Frias MR, Campos M, Lopez-Frias M, Alférez M, Nestares T, Ortega E, Lopez-Aliaga I. Goat milk during iron repletion improves bone turnover impaired by severe iron deficiency. J Dairy Sci. 2011;94:2752–2761. doi: 10.3168/jds.2010-4043. [DOI] [PubMed] [Google Scholar]
- 147.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]