Abstract
Aim: The purpose of the present research was to investigate the effect and mechanism of Astragaloside IV (AS-IV) on liver cancer progression in vivo and in vitro. Since M1 macrophages play an essential role in suppressing tumors, while M2 macrophages can accelerate the incidence and progression of tumors by promoting angiogenesis, increasing tumor cell invasion and inhibiting tumor immune response, the effect and mechanism of AS-IV on macrophage polarization and their role in the development of HCC was explored. Methods: The effects of different concentrations of AS-IV (0, 50, 80, 100, 120, and 150 μM) on the capacity of hepatocellular carcinoma (HCC) cells to proliferate, migrate, and invade were detected. THP-1 cells were subjected to incubation in PMA for the purpose of stimulating differentiation into M0 macrophages. These macrophages were treated using LPS, IFN-γ, and PMA to produce M1 macrophages or treated using PMA, IL-13, and IL-4 to produce M2 macrophages. HCC cells and M1 or M2 macrophages were co-cultured for 48 hours, then the cell proliferation and migration were measured. The MTT assay was employed to determine cell viability. The capability of the cells to migrate and invade was investigated utilizing the Transwell assay and the wound healing assay. The expression of the M2 macrophage CD206 in macrophages treated with AS-IV was evaluated by flow cytometry. The expression of p-signal transducer and activator of transcription 3 (STAT3), phosphorylated (p)-NF-κB, and toll-like receptor 4 (TLR4) in macrophages was measured after treatment with AS-IV and M2 induction. To verify the function of the TLR4/NF-κB/STAT3 signaling pathway, TLR4 expression was knocked down in M2 macrophages, then the proliferation and migration and the M2 macrophage markers of HCC cells were measured. The effect of AS-IV on HCC in vivo was confirmed by a subcutaneous tumor mouse model. AS-IV was 2 was administered by gavage (0, 40, 80, and 100 mg/kg) for every 3 days. The tumor volume and weight were recorded. Results: AS-IV suppressed the capacities of HCC cells to proliferate, migrate, and invade in a dose-dependent way. M2 macrophages could promote the proliferative, migratory, and invasive ability of Huh-7 cells, which were suppressed by AS-IV. AS-IV directly attenuated the expression of M2 macrophage markers, indicating that AS-IV can inhibit macrophage M2 polarization. M2 macrophages stimulated the expression of p-STAT3, p-NF-κB, and TLR4, while AS-IV decreased the expression compared to the M2 group, indicating that AS-IV can regulate the TLR4/NF-κB/STAT3 signaling pathway. TLR4 small interfering RNA (siRNA/si) inhibited the proliferation of Huh-7 cells. The tumor volume, as well as weight of mice, was significantly reduced by AS-IV, indicating the antitumor impact of AS-IV in vivo. Conclusion: AS-IV can inhibit the proliferative, invasive, and migratory ability of liver cancer through the suppression of the M2 polarization of macrophages, and the mechanism may involve the TLR4/NF-κB/STAT3 signaling pathway. The present study indicates that AS-IV could be an alternative drug to treat liver cancer, and the polarization of macrophages may be a novel treatment target for HCC.
Keywords: Astragaloside IV, macrophages, hepatocellular carcinoma, M1 macrophages, M2 macrophages, TLR4, NF-κB
Introduction
Primary liver cancer is one of the most prevalent cancers. It has an elevated incidence rate and is ranked as the third major contributor to cancer-associated mortality [1]. According to the 2017 report of the American Cancer Society, the incidence and mortality rates of hepatocellular carcinoma (HCC) are increasing, and the five-year survival is as low as 17.7% [2]. Primary liver cancer includes HCC, cholangiocarcinoma, and mixed-type liver cancer, of which HCC is the most frequent form, are responsible for 85-90% of cases [3]. The main characteristics of HCC are its aggressive invasive and metastatic potential and poor prognosis [4]. Even after surgical resection, patients with HCC have a remarkably low five-year overall survival [5]. The main reason is that the recurrence and metastasis rates of HCC are high, and the specific pathogenesis of HCC remains unclear [6]. Thus, it is critical to further comprehend the molecular mechanisms of HCC progression as well as to explore innovative treatment approaches so as to manage the progression and metastasis of HCC.
Astragaloside IV (AS-IV) is among the most representative effective constituent of Astragalus membranaceus. It is a type of AS monomer, which performs diverse biological functions, such as anti-infection, anti-inflammatory, and antioxidant properties [7]. Previous studies have shown that AS-IV can effectively improve the immunity of mice, promote the production of immune factors, and improve the antitumor effect of immune cells; thus, it can be used as a natural immune regulator [8-12]. Certain studies suggested that AS-IV can suppress tumor growth by suppressing the expression of VEGF and cyclooxygenase 2 in tumor cells [13,14]. The mechanism of AS in the treatment of malignant tumors is similar to that of a variety of clinical chemotherapy drugs, which lead to apoptosis through cytotoxicity, thereby inhibiting tumor proliferation [15]. Tin et al. applied AS-IV to human colon cancer cells and discovered that it can effectively stimulate the apoptosis of tumor cells by DNA fragmentation and chromatin condensation [16]. AS-IV can inhibit the invasive and migratory abilities of cervical cancer cells in vivo and in vitro by TGF-β1-mediated PI3K and MAPK signaling pathways [17]. However, the effects and mechanism of AS-IV on HCC have not been well-investigated to date.
Under different stimulating conditions, macrophages can polarize into two cell subtypes with different phenotypes, namely M1 (classically activated macrophages) and M2 (selectively activated macrophages) [18]. The induction of M1 macrophages can be achieved with the aid of lipopolysaccharide (LPS) and interferon (IFN)-γ. M1 macrophages have been shown to express nitric oxide synthase (iNOS), which is inducible and secretes numerous pro-inflammatory factors (TNF-α, IL-12, and IL-6) and produce reactive oxygen species, which result in an inflammatory response and tissue damage [19]. M2 macrophages may be stimulated by IL-4 and IL-13, and their characteristics include an elevated expression of macrophage mannose receptor (MMR) CD206 and arginase-1 (Arg-1). M2 macrophages secrete large quantities of the anti-inflammatory factor IL-10 and produce TGF-β [20]. Therefore, M2 macrophages have important anti-inflammatory properties and participate in tissue repair and angiogenesis [21-24]. In the early stage of tumorigenesis, tumor-associated macrophages (TAMs) are mainly produced from normal tissues near the cancerous area. Macrophages in normal tissues are recruited into the cancerous area by the factors C-C motif chemokine ligand 2, granulocyte-macrophage CSF, and macrophage colony-stimulating factor (CSF), which are secreted by tumor cells and promote tumorigenesis [25]. During tumor development, TAMs derive from monocytes in peripheral blood circulation, and a number of them will undergo differentiation and proliferation to finally form mature TAMs [26]. Different tumor microenvironments determine whether TAMs enter the classical activation pathway and polarize into M1 or M2 types [27,28]. M1-type TAMs can release TNF and play a role in suppressing tumors, while M2-type TAMs can accelerate the occurrence and development of tumors by promoting angiogenesis, increasing tumor cell invasion, and inhibiting tumor immune response [29]. However, the association between M1 or M2-type TAMs and the development of HCC has not been well explored thus far.
Therefore, to identify novel treatments for HCC, the present research focused on investigating the effect and mechanism of AS-IV on the progression of HCC in vivo and in vitro. Since certain studies suggested that AS-IV may affect macrophage polarization [11,30,31], and macrophages exert a crucial function in the tumor immune response, we examined the involvement of macrophages in the effects of AS-IV on HCC.
Materials and methods
Cell culture and AS-IV treatment
THP-1 and human hepatoma Huh-7 cells were procured from the Shanghai Institute of Biochemistry and Cellular Biology of the Chinese Academy of Sciences. Subsequently, culturing of these cells was performed in DMEM that contained 10 percent fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100 U/ml penicillin (Beyotime, Shanghai, China). Cells were cultured at a temperature of 37°C in a humid environment containing 5% CO2. AS-IV (Shanghai Ronghe, Shanghai, China) was dissolved in DMSO to treat macrophages. DMSO was used at a final concentration of <0.1 percent (v/v). The cells were subjected to incubation for 48 hours in the presence or absence of IL-4/IL-13, followed by treatment with AS-IV. Macrophages that had adhered to the walls were subjected to treatment using 0.1 percent DMSO for 48 hours for the purpose of removing the influence of DMSO. The concentration of AS-IV was set as 0, 50, 80, 100, 120, and 150 μM, and the incubation time was 24 or 48 h.
Macrophage polarization
For the purpose of differentiating into M0 macrophages, THP-1 cells were subjected to incubation in 320 nmol/L PMA (Sigma-Aldrich, St Louis, MO, USA) for 18 hours. The M0 macrophages were treated using 320 nmol/l PMA for 12 hours and subjected to further incubation in 100 nmol/l PMA, 100 ng/ml LPS (Sigma-Aldrich, St Louis, MO, USA), and 20 ng/ml IFN-(Sigma-Aldrich, St Louis, MO, USA) for 48 hours at a temperature of 37°C to yield M1 polarized macrophages. M0 macrophages were subjected to treatment using 320 nmol/l PMA for 12 hours. Subsequently, they were cultured in 100 nmol/l PMA, 20 ng/ml IL-4, and 20 ng/ml IL-13 for 48 hours at a temperature of 37°C for the purpose of generating M2 polarized macrophages. Following that, macrophages together with HCC cells were co-cultured in the co-culture chamber for 48 h at 37°C, then used in subsequent experiments.
MTT assay
The MTT test was performed for the purpose of determining cell viability. At a density of 3,000-5,000 cells per well, cells were plated in 96-well plates to obtain the desired results. After being cultured all night at 37°C, the cells were exposed to a range of increasing AS-IV dosages (0, 50, 80, 100, 120, and 150 M) for 48 hours. A 100-microliter volume of MTT (Sigma-Aldrich, St Louis, MO, USA; final concentration: 500 g/ml) was introduced into each well following the removal of the media. Then, the cells were incubated in an incubator for 4 hours at 37°C. Subsequently, 100 μl 20% SDS solution (Bio-Bad Laboratories) was added for 20 h. Finally, the microplate reader (BioTek Instruments, Inc.) was utilized to measure absorbance at 570 nm. Cell viability was presented as the proportion of the experimental group in contrast with that of the controls.
Wound healing assay
For culturing the cells, they were placed into 6-well plates (1105 cells/well) and subjected to culture till they reached 90-95 percent confluence. After 24 h, a straight line was drawn perpendicularly to the horizontal line at the bottom of the plate using a sterile pipette tip. To eliminate the unattached cells, rinsing was performed 3 times using PBS, followed by drying. DMEM was subsequently introduced, and the cells were subjected to culturing at a temperature of 37°C in a 5% CO2 incubator. Wound healing was determined by photographing the cells at 0 and 48 h after scratching using a Nikon Coolpix 990 camera (Nikon Corporation) and was examined with the aid of ImageJ software (version 1.43, National Institutes of Health, Bethesda, Maryland, USA).
Transwell assay
Similar to Shao et al. [32], the cells were digested and centrifuged before resuspension in a serum-free medium that contained 0.2 percent bovine serum albumin (Gibco Invitrogen, Shanghai, China). The concentration was increased to 5×105 cells per milliliter of solution. Subsequently, 100 µl of cell suspension was introduced into the Transwell chamber (BD Biosciences, San Jose, CA, USA), and 600 µl of media comprising 20% FBS was introduced to the bottom chamber of a 24-well plate. The cells were then grown for 48 hours. Then, the Transwell chamber was withdrawn, and the culture media contained within the well was disposed of. The cells were rinsed twice with the aid of PBS, followed by the fixing in methanol for 30 minutes. Moreover, the chamber was stained using 0.1 percent crystal violet for 20 minutes. The cells on the top layer that had not migrated were carefully brushed off utilizing a cotton swab and subjected to rinse thrice with PBS to remove any remaining residue. Finally, the cells were observed in five fields of view and counted using a microscope (BX-40, Olympus, Tokyo, Japan) at 400× magnification.
Flow cytometry
In total, 2 ml cells in the logarithmic growth phase (1×106 cells/ml) were inoculated in a 6-well plate before being subjected to the corresponding treatment. The cells were harvested 24 hours after they were placed in culture. The cell precipitate was filtered after being centrifuged for 5 minutes at a rate of 1200× g at ambient temperature, and the supernatant was removed. Afterward, rinsing of the cells was performed twice using pre-cooled PBS, incubated with pre-cooled 75% ethanol, followed by fixing for 4 hours at 4°C. After being centrifuged for 5 minutes at a rate of 3000× g, the supernatant was extracted and washed once with 3 ml PBS. Subsequently, incubation of the cells was conducted in the fluorescent antibody (Sigma-Aldrich, St Louis, MO, USA) at 4 degrees Celsius in the darkness for 30 minutes. Flow cytometry was subsequently employed for the purpose of identifying the number of apoptotic cells. A computer program called ModFit (Verity Software House, Top-sham, ME, USA) was used to examine the results.
Immunofluorescence staining
First, the adherent cells were rinsed using 0.01 M PBS for 5 min each and then subjected to incubation in 10 percent normal goat serum (Sigma-Aldrich, St Louis, MO, USA) at a temperature of 37°C for 45 minutes. Next, the excess liquid was removed, and a mouse anti-CD68 primary antibody (1:500, Sigma-Aldrich, St Louis, MO, USA) was introduced, followed by incubation throughout the night at 4°C. After washing the cells thrice for 5 minutes using 0.01 M PBS, they were subjected to incubation in darkness at 4°C in a sheep anti-rabbit Fluorescein Isothiocyanate (FITC)-conjugated IgG (1:500, Sigma-Aldrich, St Louis, MO, USA). Subsequently, the cells were rinsed thrice in the darkness using 0.01 M PBS for 5 minutes each time, followed by six washes with 0.01 M PBS (Sigma-Aldrich, St Louis, MO, USA) for 5 minutes each time. After that, the results were examined with the aid of a fluorescence microscope (400×, Axiovert 135TV, Carl Zeiss) and photographed.
Western blotting
After 48 hours of culture with AS-IV, the cells were digested for 20 minutes using 0.25% trypsin (Sigma-Aldrich, St Louis, MO, USA) and placed into a 1.5 ml Eppendorf tube, followed by washing twice using PBS. A total of 50 μl protein lysate was added, and the lysed protein solution was centrifuged for 10 minutes at a rate of 12,000× g and a temperature of 4°C. Then, the supernatant was obtained. A BCA kit (Sigma-Aldrich, St Louis, MO, USA) was utilized for the purpose of measuring the abundance of proteins. According to the quantitative results, the concentration of proteins in all samples was adjusted to the same level, followed by denaturation at 100°C for 10 minutes. The samples were treated with 10 percent SDS-PAGE (40 μg/lane). Electrophoresis was performed at 90 V for 90 min. Subsequently, the proteins were loaded onto a nitrocellulose membrane for 2 h. In total, 5 ml blocking solution (P0023B, Beyotime, Shanghai, China) was introduced into the membrane, followed by incubation at 25°C for 1 hour. Next, 3 ml primary antibodies (Sigma-Aldrich, St Louis, MO, USA) were introduced and subjected to incubation throughout the night at 4°C. Keeping the membrane at ambient temperature, it was rinsed thrice (5 min each time) using PBS-Tween 20 buffer (PBST, 0.05 percent Tween 20) before being used. Next, we added a 3 ml secondary antibody (Sigma-Aldrich, St Louis, MO, USA) for the purpose of incubating the membranes for 2 hours at ambient temperature. The membrane was rinsed in PBST buffer three times (5 min each). In accordance with the instructions of the SuperSignal West Pico chemiluminescence detection kit (Pierce; Thermo Fisher Scientific, Inc.), the substrate and enhancer were mixed in equal proportions and added to the membrane for 3-5 min. Eventually, the bands were displayed by means of an ECL technique. The quantification of the protein levels was performed with ImageJ software (version 1.43, National Institutes of Health, Bethesda, Maryland, USA), followed by normalization to those of α-tubulin.
Reverse transcription-quantitative PCR (RT-qPCR)
First, TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was employed for the purpose of extracting total RNA, and cDNA was generated as per the protocols stipulated by the manufacturer of the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.). RNA was collected and thawed at 4°C, and then the RT reaction system was prepared in a 0.2-ml PCR tube with the following components: Total RNA (0.5-1 μg), 1 μl iScript reverse transcriptase, 4 μl 5X reaction mixture, 14 μl H2O and 1 μl RNA. The PCR tube was subsequently placed for 5 minutes at 25°C, incubated at 42°C for 30 min, denatured at 85°C for 5 minutes, and sustained at 4°C. The reaction system was as follows: A total of 10 μl 2× SYBR Green PCR buffer (Sigma-Aldrich, St Louis, MO, USA), 3 μl ddH2O, 1 μl 1 μl 5 μl template DNA, reverse primer (10 μM), and, forward primer (10 μM). Table 1 lists the primers that were utilized in the RT-qPCR experiment. The reaction was as follows: Incubation at 50°C for 2 minutes, followed by incubation at 95°C for 10 minutes, and then 40 cycles of 95°C for 15 minutes and 60°C for 1 minute. The quantification cycle (Cq) values were obtained using ABI 7500 instrument (v2.0.6, Applied Biosystems; Thermo Fisher Scientific, Inc.). The final gene expression was presented as relative expression based on the following calculation formula: Ratio (reference/target) =2Cq (reference)-Cq (target).
Table 1.
Primers used in real-time PCR
| Gene | Primer (5’→3’) |
|---|---|
| CD206 | Forward: GCAGAAGGAGTAACCCACCC |
| Reverse: TGGCAAATGAAGGCGTTTGG | |
| TGF-β | Forward: CCAAGCTTATGCCGCCCTCCGGGC |
| Reverse: GCGTCGACCAGCTGCACTTGCAGGAG | |
| CD209 | Forward: GCAGAAGGAGTAACCCACCC |
| Reverse: TGGCAAATGAAGGCGTTTGG | |
| IL-10 | Forward: ACCTGGTAGAAGTGATGCC |
| Reverse: CAAGGAGTTGTTTCCGTTA | |
| GAPDH | Forward: CTGCACCACCAACTGCTTAG |
| Reverse: GTCTGGGATGCAAATTGTGA |
Cell transfection
The experiment included the following groups: Control, induction (M2), AS-IV, AS-IV + induction (M2 + AS-IV), induction + si-TLR4 (M2 + si-TLR4) and induction + si-TLR4 + AS-IV (M2 + si-TLR4 + AS-IV). Macrophages were classified into six groups: Control, induction (M2), AS-IV, AS-IV + M2, M2 + si-TLR4 and M2 + AS-IV + si-TLR4. Shanghai GeneChem Co., Ltd synthesized the human TLR4 siRNA. The sequences for si-TLR4 were forward (F), 5’-UUCGAG-ACUGGACAAGCCATT-3’ and reverse (R), 5’-UGGCUUGUCCAGUCUCGAATT-3’, while the sequences for siRNA-negative control (NC) were F, 5’-UUCUCCGAACGUGUCACGUTT-3’ and R, 5’-ACGUGACACGUUCGGAGAATT-3’. Briefly, cells were inoculated into 12-well plates (5×105 cells/well). Then, 100 nM siRNA was dissolved in 50 μl serum-free Opti-MEM (Gibco, Grand Island, NY, USA), while 1 μl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was dissolved in 50 μl serum-free Opti-MEM and agitated well. The solutions were placed at ambient temperature for 5 minutes, followed by mixing and incubation for another 20 minutes. For transfection, the normal DMEM medium with serum in the 12-well plates containing the cells was replaced with 400 µl serum-free Opti-MEM medium/well. The transfection solution containing Lipofectamine and siRNAs was introduced to the corresponding wells of the 12-well plates. After 6 hours, the serum-free media was replenished using a normal DMEM medium (Gibco, Grand Island, NY, USA). The cells were then treated with the corresponding reagents (AS-IV, macrophage polarization reagents).
Animal experiments
Fifty male BALB/c-nude mice (aged between 4-5 weeks and weighing 20±1.2 g) were procured from Shanghai Bikai Biotechnology Co., Ltd (Shanghai, China). They were housed at 24°C in the animal center of Shanghai Jiaotong University School of Medicine (humidity of 41%; 12 hours dark/light cycle). The mice were classified into 5 groups in a random manner: 0, 20, 40, 80, and 100 mg/kg AS-IV (10 mice/group). Huh-7 cells (5×107 cells/0.2 ml) and M2-type macrophages (1×107 cells/0.2 ml) were administered by subcutaneous injection into nude mice, similarly to Fu et al. [33]. Approximately 2 weeks later, when the tumor diameter increased to 5 mm, AS-IV was administered every 3 days. There were four different doses of the medication, each of which was administrated by gavage at 20, 40, 80, and 100 mg/kg. Tumor size was measured every 5 days. After 40 days, we performed cervical dislocation for the purpose of sacrificing the mice, harvested, and photographed the tumor. The volume of the tumor was calculated. We measured the tumor’s long diameter (a) and short diameters (b), both of which were subsequently utilized to derive the volume of the tumor (V) according to the following equation: V = π×a×b2/6. The present research had a limit of 2.0 cm3 for the greatest possible tumor volume allowed. The humane outcomes comprised a swift increase in body weight by more than 15%; the mice were incapable of selffeeding and drinking; the tumor growth exceeded >10% of the animal original body weight, or the average tumor diameter became >20 mm; and sustained infection of body organs. Approval of the present animal experimentation was granted by the Ethics Committee of Shanghai Jiaotong University School of Medicine (approval no. RJH-8956).
Data analysis
SPSS 17.0 software (SPSS, Inc.) was employed for performing all analyses of statistical data. Analysis of variance (one-way analysis of variance (ANOVA) and Tukey’s post-hoc test) was utilized to examine the differences existing among various groups. We conducted all tests a minimum of three times in total. Additionally, all the data were presented as the mean ± standard deviation. P<0.05 was considered a significant difference.
Results
Impacts of varying dosages of AS-IV on the capability of HCC cells to proliferate, migrate, and invade
The MTT test was used for detecting the viability of HCC cells in each of the groups. The results shown in Figure 1A revealed that the cell viability began to decrease upon reaching the AS-IV dosage of 100 μM, and it decreased to the lowest level once the dosage was elevated to 150 μM AS-IV. These results showed that AS-IV had an effect on the proliferative capacity of HCC cells in a dose-dependent way. For subsequent experiments, 120 μM AS-IV was selected. To verify the impact of AS-IV on cell migration, changes in the width of the wound were observed at 0 and 48 h after treatment of HCC cells with AS-IV. The findings illustrated that the width of the wound of cells treated with 100, 120, and 150 μM AS-IV was considerably wider in contrast with the width in the control group, and the healing rate in these groups was lower as opposed to the control (Figure 1B and 1D). To explore the impact of AS-IV on the invasiveness of HCC cells, a Transwell assay was conducted following 48 h of the invasion experiment. The findings demonstrated that as opposed to the control group, the proportion of cells invading the lower compartment following the treatment with AS-IV was significantly reduced (P<0.05; Figure 1C and 1E), indicating that the invasiveness of cells following AS-IV treatment was substantially attenuated.
Figure 1.

Impact of varying doses of AS-IV on the proliferation, migration, and invasion of HCC cells. Values are presented as mean ± S.E.M. A. Cell viability of HCC cells; B. Healing rates of HCC cells; C. Migration cell counts of HCC cells; D. Representative images obtained from wound closure assay; E. Representative images obtained from Transwell assay. Scale bar =50 μm. ***P<0.01 compared to control using ANOVA and Tukey’s post-hoc test.
AS-IV inhibits tumor proliferation and migration by inhibiting macrophage polarization
To delve into whether the influence of AS-IV on the capacity of HCC cells to proliferate was achieved by inhibiting the polarization of macrophages, HCC cells and macrophages were first co-cultured for 48 h, and divided into M1 induction (M1), M2 induction (M2), M1 induction + AS-IV treatment (M1 + AS-IV) and M2 induction + AS-IV treatment (M2 + AS-IV) groups. The MTT test was used to determine the viability of the cells in each group. The findings (Figure 2A) illustrated an elevation in the cell viability in the M2 group, but a decrease in the AS-IV group. The cell viability was decreased in the M2 + AS-IV group, indicating that AS-IV has an impact on the proliferative capacity of HCC cells and can reverse the effect of M2 induction. The findings from the wound healing assay demonstrated that, as opposed to the control group, the width of the wound in the M2 group was substantially reduced, whereas the widths of the wounds in the AS-IV and M2 + AS-IV groups were wider in contrast with the M2 group (Figure 2B and 2D). Similarly, the number of cells that passed across the chamber was substantially elevated following the treatment with M2 macrophages compared with the control, but it was substantially lowered by AS-IV treatment (Figure 2C and 2E).
Figure 2.

Impacts of macrophage polarization on the proliferation, migration, and invasion of HCC cells. Values are expressed as mean ± S.E.M. A. Cell viability of HCC cells; B. Healing rates of HCC cells; C. Migration cell counts of HCC cells; D. Representative images obtained from wound closure assay; E. Representative images obtained from Transwell assay. Scale bar =50 μm. **P<0.01 versus Control; ***P<0.01 versus Control; ##P<0.01 versus M2; ###P<0.01 versus M2. The statistical analysis was performed using ANOVA and Tukey’s post-hoc test.
Distribution of macrophage subpopulations and M2 macrophage markers
The expression of the M2 macrophage CD206 in macrophages treated with 120 M AS-IV was assessed with the aid of flow cytometry for the purpose of determining if AS-IV suppressed IL-4 and IL-13-stimulated macrophage M2 polarization. As illustrated in Figure 3A and 3B, upon treating THP-1 monocytes for 72 hours using IL-4 and IL-13, a remarkable up-modulation of CD206 was detected, which was greatly diminished by AS-IV therapy. These findings revealed that AS-IV blocked the polarization of macrophage M2 cells. The levels of mRNA expression in macrophage surface markers were detected utilizing RT-qPCR to further verify the influence of AS-IV on M2 polarization. As depicted by Figure 3C and 3D, 120 μM AS-IV lowered the CD209 and TGF-β in M2 macrophage expression. Thus, these findings show that AS-IV can suppress the polarization of M2 macrophages in vitro in an effective manner.
Figure 3.

Distribution of macrophage subpopulations and the proportions of M2 macrophage markers. Values are presented as mean ± S.E.M. A. Representative images of flow cytometry results of CD206; B. Percentages of CD206-positive cells (***P<0.01 in contrast to M2); C. Relative mRNA levels of CD209; D. Relative mRNA levels of TGF-β. ***P<0.01 in contrast to Control; ###P<0.01 in contrastto M2. Statistical analyses were performed using ANOVA and Tukey’s post-hoc test.
Expression of the TLR4/NF-κB/STAT3 signaling pathway
In order to thoroughly examine the possible molecular mechanism of AS-IV on the M2 polarization of macrophages, the total p65, p-STAT3, p-p65, TLR4, and total STAT3 expression levels in macrophages were measured after treatment with AS-IV and IL-4/IL-13. The findings (Figure 4) demonstrated that the M2 polarization of macrophages upregulated the expression of TLR4, p-STAT3, and p-p65 as opposed to the control group, while AS-IV treatment reduced the expression of TLR4, p-STAT3, and p-p65 as opposed to the M2 group.
Figure 4.

Expression of the TLR4/NF-κB/STAT3 signaling pathway. A. Representative images of western blots; B. Results of the expression of TLR4; C. Results of the ratios of p-NF-κB/NF-κB; D. Results of the ratios of p-STAT3/ STAT3. ***P<0.01 in contrast to Control; ###P<0.01 in contrast to M2. The statistical analyses were performed using ANOVA and Tukey’s post-hoc test.
Effect of treatment of macrophages with TLR4 siRNA on the proliferative and migratory capabilities of HCC cells
In order to verify whether AS-IV affects the growth of HCC cells by the TLR4/NF-κB/STAT3 signaling pathway, TLR4 expression was knocked down in M2 macrophages. The expression of TLR4 mRNA was determined with the aid of RT-qPCR. As depicted by Figure 5A and 5B, the TLR4 mRNA expression was considerably reduced by si-TLR4, which demonstrated that the siRNA transfection was successful. To verify that the influence of AS-IV on HCC cells is facilitated by the TLR4/NF-κB/STAT3 signaling pathway, HCC cells were treated with AS-IV and co-cultured with macrophages for 24 h. The results showed that AS-IV inhibited cell proliferation (Figure 5C). After transfecting the HCC cells with TLR4 siRNA, their proliferation was also inhibited, which indicates that AS-IV inhibits the HCC cells’ capacity of proliferating through the TLR4 signaling pathway. As demonstrated by Figure 5D and 5F, the proportion of cells that invade the lower compartment in the Transwell assay was considerably reduced by AS-IV, and the effect was more pronounced after treating the cells with TLR4 siRNA (P<0.05), indicating that the impact of AS-IV on the migratory and invasive capabilities of Huh-7 cells was associated with TLR4.
Figure 5.

Impact of treating macrophages with TLR4 siRNA on the capacity of HCC cells to proliferate and migrate. A. The mRNA levels of TLR4 in M2 macrophages; B. The expression of TLR4 in M2 macrophages; C. The cell viability of HCC cells; D. The healing rates of HCC cells; E. The migration cell counts of HCC cells; F. Illustrative pictures of wound closure assay; G. Illustrative pictures of Transwell assay. Scale bar =50 μm. ***P<0.01 in contrast to Control; ###P<0.01 in contrast to M2. Statistical analyses were performed using ANOVA and Tukey’s post-hoc test.
Changes in mRNA expression of M2 macrophage markers after TLR4 siRNA transfection
In order to confirm whether the influence of AS-IV on the polarization of M2 was correlated with the TLR4, the mRNA levels of macrophage surface markers were evaluated by RT-qPCR after treating the macrophages with TLR4 siRNA. As shown in Figure 6A and 6B, compared to normal macrophages treated with AS-IV, the expression of TGF-β, CD209, and M2 macrophage markers was substantially attenuated by AS-IV treatment in the si-TLR4 group (P<0.05). The above findings show that AS-IV can inhibit the polarization of the M2 macrophages in vitro through the TLR4 signaling pathway.
Figure 6.

Alterations in mRNA expression of M2 macrophage markers after TLR4 siRNA transfection. A. mRNA levels of CD209; B. mRNA levels of TGF-β. **P<0.05 in contrast to Control; ***P<0.01 in contrast to Control; ###P<0.01 in contrast to M2. The statistical analyses were performed using ANOVA and Tukey’s post-hoc test.
Differences in the expression of proteins correlate with the TLR4/NF-κB/STAT3 signaling pathway after transfection with TLR4 siRNA
The total p65, p-STAT3, p-p65, TLR4, and total STAT3 expressions in macrophages were detected by means of western blotting. The findings were displayed in Figure 7. It was found that AS-IV attenuated the expression levels of p-p65, TLR4, and p-STAT3 in macrophages, which was further decreased by transfection with TLR4 siRNA. Therefore, AS-IV, together with si-TLR4, exacerbated the decrease in the p-p65, TLR4, and p-STAT3 expression in macrophages.
Figure 7.

Expression of the TLR4/NF-κB/STAT3 signaling pathway after transfection with TLR4 siRNA. A. Representative images of western blots; B. Results of the expression of TLR4; C. Results of the ratios of p-NF-κB/NF-κB; D. Results of the ratios of p-STAT3/STAT3. **P<0.05 in contrast to Control; ***P<0.01 in contrast to Control; ###P<0.01 compared to M2. The statistical analyses were performed using ANOVA and Tukey’s post-hoc test.
Inhibitory influence of AS-IV on hepatoma in vivo
To confirm the aforementioned in vitro results, the present research subsequently examined if AS-IV exhibited an in vivo impact on the growth of liver cancer. A Huh-7 hepatoma mouse model was created through the injection of Huh-7 cells into the mice’s subcutaneous tissue. In contrast with the control group, AS-IV suppressed the growth of tumors in the Huh-7 hepatoma model (Figure 8). In contrast with the control group, therapy with 40, 80, and 100 mg/kg AS-IV resulted in substantial reductions in both tumor weight and volume.
Figure 8.
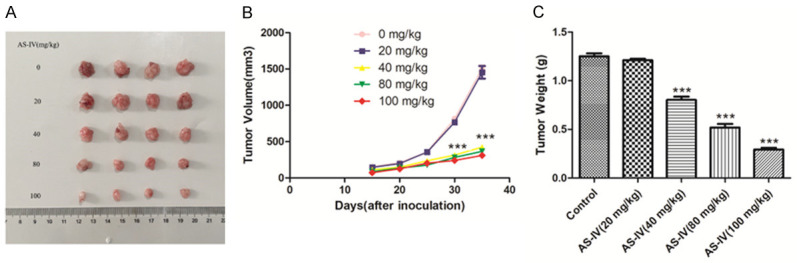
The inhibitory impact of AS-IV on hepatoma in vivo. A. Illustrated pictures of tumors; B. Changes in the tumor volumes; C. Changes in the tumor weights. ***P<0.01 in contrast to Control using the method of ANOVA and Tukey’s post-hoc test.
Discussion
Primary liver cancer is the third major contributor to cancer-associated mortality worldwide. Theincidence has been increasing in recent years [34]. The characteristics of primary liver cancer include rapid advancement, a lower resection rate, a higher incidence of postsurgical recurrence, and a negative reaction to conservative treatment [35]. Although improvements in surgical procedures have boosted the resection rates of liver cancer, this rate remains unsatisfactory (25-40%) [36]. Since most of the liver cancer patients are in Barcelona Clinic Liver Cancer stage B or C when they are first diagnosed, they cannot undergo surgical resection, percutaneous ablation, or liver transplantation [3]. As a consequence, it is critical to investigate novel therapeutic approaches for the purpose of treating liver cancer. The findings of the present research illustrate that AS-IV influenced the capability of HCC cells to proliferate in a dosage-dependent way, thus suppressing the migratory and invasive capacities of HCC cells.
In the past few years, AS-IV has been demonstrated to have antitumor activity in a number of studies. AS-IV can significantly improve the chemosensitivity of non-small-cell lung cancer (NSCLC) cells to cisplatin [37]. Zheng et al. discovered that AS-IV can enhance the taxol chemosensitivity of breast cancer and inhibit their proliferation [38]. In addition, AS-IV can inhibit the proliferative, invasive, and migratory capabilities of hepatoma cells by suppressing the Akt/GSK-3β/β-catenin signaling pathway [39]. It may also promote Bax expression, inhibit Bcl-2 expression, and activate the caspase-3-dependent apoptosis process [40]. AS-IV also assumes an integral function in tumor-infiltrating lymphocytes in the cancer microenvironment. It was reported that AS-IV may suppress the protein and mRNA expression of indoleamine 2,3-dioxygenase in the microenvironment of lung cancer, downregulate the proportion of regulatory T cells, as well as increase the proportion of cytotoxic T cells and the antitumor immune responsiveness [41]. AS-IV can also partially block the AMP-activated protein kinase signaling pathway and suppress the polarization of the M2 macrophages in the microenvironment, thus inhibiting the proliferative, invasive, migratory, and angiogenesis of lung cancer [31]. To explore the impact of AS-IV on M2 macrophages in liver cancer cells, HCC cells and control macrophages (M2 macrophages) were co-cultured in the present study and then treated with AS-IV for 48 h before measuring their proliferative, migratory, and invasive capacities. The results indicated that M2 macrophages can promote the proliferative, migratory, and invasive capacities of Huh-7 cells, which were inhibited by AS-IV. The differential expression of specific genes and cell surface marker proteins is the most common method to distinguish different polarization states of macrophages [42]. M1 macrophages stimulated by IFN-γ and LPS showed high expression of CD86 on their cell surfaces, and upregulated expression of CD197, TNF-α, IL-6, IL-1β, and iNOS. M2 macrophages induced by IL-13 and IL-4 showed high expression of the mannose receptor CD206 on their cell surface, and upregulated expression of CD209, TGF-β, Arg-l, peroxisome proliferator-activated receptor-γ, macrophage galactose N-acetyl-galactosamine-specific lectin 2, and other genes [43,44]. Flow cytometry illustrated that AS-VI reduced the expression of the M2 macrophage markers CD206, CD209, and TGF-β, indicating that AS-IV can inhibit macrophage M2 polarization.
Macrophages are widely distributed in various organs of the body, such as the spleen, liver, and brain [45-49]. Macrophages are a type of immune cell with variable phenotype and diverse functions. They are easily affected by the microenvironment of different physiologic and pathologic conditions and can be polarized into several functional subtypes. In the tumor microenvironment, there are two types of TAM subgroups with different molecular characteristics and biologic functions, namely M1 type TAMs with classical polarization, and M2 type TAMs with alternative polarization [50]. M1 type TAMs are mainly induced by IFN-γ and LPS, thus presenting a type I immune response, secreting a variety of pro-inflammatory cytokines, and playing a role in killing bacteria and tumors [51]. By contrast, M2-type TAMs are mainly induced by IL-4 and IL-13 from tumor cells or T cells, which inhibit the cellular immune response and secrete factors to promote angiogenesis [52]. They also release metalloproteinases to promote matrix protein degradation and secrete growth factors so as to enhance the proliferative capacities of tumor and endothelial cells [53]. As a result, M2-type TAMs can accelerate the occurrence and progression of tumors [54]. The current results suggest, for the first time, that AS-IV can inhibit macrophage M2 polarization in liver cancer, which may be one of the important mechanisms of its antitumor effect in HCC.
To further explore the mechanism by which AS-IV inhibits macrophage M2 polarization, the function performed by the TLR4/NF-κB/STAT3 signaling pathway was investigated. TLR4 is a crucial molecule belonging to the TLR family, since it can mediate innate immunity, and acts as an endotoxin recognition receptor and a link between acquired and innate immunity [55]. TLR4 mainly works through the TLR4/NF-κB/STAT3 signaling pathway. As illustrated by the findings of the present study, the M2 polarization of macrophages stimulated the expression of TLR4, p-STAT3, and p-p65 as opposed to the control group, while AS-IV treatment attenuated the expression of these molecules as opposed to the M2 group. Such findings indicate that AS-IV can regulate the TLR4/NF-κB/STAT3 signaling pathway. Without any stimulation signal, NF-κB is positioned in the cytoplasm and binds to the inhibitor of NF-κB (Iκb) as a complex, thus inhibiting its activity [56]. LPS or free fatty acids from gut-derived LPS can activate TLR4 and bind to myeloid differentiation factor 88 to form a complex, which might trigger the NF-κB inhibitor kinase and promote the dissociation of NF-κB into the nucleus [57]. This action can activate target gene transcription and cause a series of cytokine synthesis and release. A previous study has shown that AS-IV can suppress the triggering of the NF-κB signaling pathway and reduce the phosphorylation of Iκb, thereby reducing the nuclear translocation of NF-κB, and downregulating the expression of IL-1β, TNF-α, and other inflammatory cytokines [58]. Research indicates that AS-IV might suppress the LPS-induced acute inflammatory response across many organs of rats by the mechanisms of modulating TLR4/NF-κB and decreasing the production of TNF-α and IL-6 [59]. AS-IV could also inhibit TLR4 and the NF-кB signaling pathway in vitro and in vivo by treating LPS-induced epithelial cells and unilateral ureteral obstruction model mice with astragaloside IV [60]. Upon transfection of cells with TLR4 siRNA, their proliferation was also inhibited, which indicates that AS-IV inhibits the proliferation of HCC cells by the TLR4/NF-κB/STAT3 signaling pathway. The invasive and migratory capacities of Huh-7 cells were also significantly suppressed by transfection with TLR4 siRNA, indicating that the effect of AS-IV on the invasion and metastasis abilities of Huh-7 cells may be associated with the inhibition of TLR4.
To confirm the role of TLR4 in macrophage M2 polarization, the markers of M2 macrophages were determined after TLR4 siRNA intervention. Since TLR4 siRNA considerably attenuated the expression of the TGF-β and M2 macrophage markers CD209, these findings suggested that AS-IV may successfully suppress the polarization of the M2 macrophages in vitro by suppressing the TLR4 signaling pathway. Similar results were obtained in other animal models. For example, Zhou et al. highlighted that AS-IV might suppress the inflammatory response through the TLR4/NF-κB signaling pathway, thus impeding the progression of renal fibrosis [60]. Zhang et al. demonstrated the effectiveness of AS-IV in suppressing LPS-induced acute inflammatory response in rats through the modulation of TLR4/NF-κB [61]. AS-IV could also reduce the levels of TNF-α and IL-6 by modulating the TLR4/NF-κB signaling pathway, as well as improving vascular endothelial dysfunction caused by hyperglycemia [62]. It was also reported that AS-IV could protect rat cardiac hypertrophy by suppressing the TLR4/NF-κB signaling pathway as well as its downstream inflammatory cytokines [63]. In a myocardial ischemia/reperfusion rat model, AS-IV can downmodulate the TLR4/NF-κB signaling pathway and suppresses apoptosis, thereby reducing myocardial damage [64]. Finally, to confirm that AS-IV exhibited an antitumor impact on liver cancer in vivo, a mouse model was created by injecting the mice subcutaneously with Huh-7 cells and M2-type macrophages. The findings confirmed that treatment with 40, 80, and 100 mg/kg AS-IV substantially reduced tumor volume and weight in contrast to the control group, showing that AS-IV has an anticancer impact in vivo.
In conclusion, the present findings illustrate that AS-IV can suppress the proliferative, invasive, and migratory ability of liver cancer by suppressing the polarization of M2 macrophages. The mechanism may involve the TLR4/NF-κB/STAT3 signaling pathway. The present research suggested that AS-IV might be an alternative drug to treat liver cancer, and the polarization of macrophages may be a new target for HCC treatment.
Acknowledgements
Shanghai Jiaotong University Medical College’s Natural Science Foundation Project provided funding for the present research (No. 12xj10051).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Zhao YR, Wang JL, Xu C, Li YM, Sun B, Yang LY. HEG1 indicates poor prognosis and promotes hepatocellular carcinoma invasion, metastasis, and EMT by activating Wnt/β-catenin signaling. Clin Sci. 2019;133:1645–1662. doi: 10.1042/CS20190225. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- 7.Wang SG, Xu Y, Chen JD, Yang CH, Chen XH. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules. 2013;18:12809–12819. doi: 10.3390/molecules181012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112. doi: 10.1016/bs.apha.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Deng S. Effects of astragaloside IV on inflammation and immunity in rats with experimental periodontitis. Braz Oral Res. 2019;25:e032. doi: 10.1590/1807-3107bor-2019.vol33.0032. [DOI] [PubMed] [Google Scholar]
- 10.Tan YQ, Chen HW, Li J. Astragaloside IV: an effective drug for the treatment of cardiovascular diseases. Drug Des Devel Ther. 2020;14:3731–3746. doi: 10.2147/DDDT.S272355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Ran F, He H, Chen L. Astragaloside IV exerts anti-tumor effect on murine colorectal cancer by re-educating tumor-associated macrophage. Arch Immunol Ther Exp (Warsz) 2020;68:33. doi: 10.1007/s00005-020-00598-y. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Xing B, Zhao J, Zhang X, Zhou L, Yang S, Wang Y, Yang F. Astragaloside IV relieves gestational diabetes mellitus in genetic mice through reducing hepatic gluconeogenesis. Can J Physiol Pharmacol. 2020;98:466–472. doi: 10.1139/cjpp-2019-0548. [DOI] [PubMed] [Google Scholar]
- 13.Mehmood K, Zhang H, Yao W, Jiang X, Waqas M, Li A, Wang Y, Lei L, Zhang L, Qamar H, Li J. Protective effect of Astragaloside IV to inhibit thiram-induced tibial dyschondroplasia. Environ Sci Pollut Res Int. 2019;26:16210–16219. doi: 10.1007/s11356-019-05032-1. [DOI] [PubMed] [Google Scholar]
- 14.Liang C, Ni GX, Shi XL, Jia L, Wang YL. Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis after ischemic stroke. Restor Neurol Neurosci. 2020;38:271–282. doi: 10.3233/RNN-201001. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Lu XJ, Zhang J, Diao H, Li G, Xu L, Wang T, Wei J, Meng W, Ma JL, Yu H, Wang YG. Oridonin, a novel lysine acetyltransferases inhibitor, inhibits proliferation and induces apoptosis in gastric cancer cells through p53- and caspase-3-mediated mechanisms. Oncotarget. 2016;7:22623–22631. doi: 10.18632/oncotarget.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28:1347–55. doi: 10.1093/carcin/bgl238. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Zhou J, Qin X, Huang H, Nie C. Astragaloside IV inhibits the invasion and metastasis of SiHa cervical cancer cells via the TGF-β1-mediated PI3K and MAPK pathways. Oncol Rep. 2019;41:2975–2986. doi: 10.3892/or.2019.7062. [DOI] [PubMed] [Google Scholar]
- 18.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feito MJ, Diez-Orejas R, Cicuéndez M, Casarrubios L, Rojo JM, Portolés MT. Characterization of M1 and M2 polarization phenotypes in peritoneal macrophages after treatment with graphene oxide nanosheets. Colloids Surf B Biointerfaces. 2019;176:96–105. doi: 10.1016/j.colsurfb.2018.12.063. [DOI] [PubMed] [Google Scholar]
- 20.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 21.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol Aspects Med. 2011;32:123–145. doi: 10.1016/j.mam.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Gao S, Song L, Liu M, Sun Z, Liu J. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Mol Immunol. 2021;130:113–121. doi: 10.1016/j.molimm.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, Gong WY, Dong JC, Liu BJ. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37:207. doi: 10.1186/s13046-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao J, Fan W, Ma B, Wu Y. Breast cancer stem cells expressing different stem cell markers exhibit distinct biological characteristics. Mol Med Rep. 2016;14:4991–4998. doi: 10.3892/mmr.2016.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu XT, Song K, Zhou J, Shi YH, Liu WR, Shi GM, Gao Q, Wang XY, Ding ZB, Fan J. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019;19:71. doi: 10.1186/s12935-019-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 35.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He CS, Liu YC, Xu ZP, Dai PC, Chen XW, Jin DH. Astragaloside IV enhances cisplatin chemosensitivity in non-small cell lung cancer cells through inhibition of B7-H3. Cell Physiol Biochem. 2016;40:1221–1229. doi: 10.1159/000453175. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Dai Y, Liu W, Wang N, Cai Y, Wang S, Zhang F, Liu P, Chen Q, Wang Z. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin-1-targeting oxidant damage. J Cell Physiol. 2019;234:4277–4290. doi: 10.1002/jcp.27196. [DOI] [PubMed] [Google Scholar]
- 39.Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH, Wang YC, Ye BG, Cao MQ, Gao DM, Tang ZY. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol Rep. 2017;37:1725–1735. doi: 10.3892/or.2017.5389. [DOI] [PubMed] [Google Scholar]
- 40.Jia L, Lv D, Zhang S, Wang Z, Zhou B. Astragaloside IV inhibits the progression of non-small cell lung cancer through the Akt/GSK-3β/β-catenin pathway. Oncol Res. 2019;27:503–508. doi: 10.3727/096504018X15344989701565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang A, Zheng Y, Que Z, Zhang L, Lin S, Le V, Liu J, Tian J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140:1883–1890. doi: 10.1007/s00432-014-1744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Paolicelli RC, Ferretti MT. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schafer DP, Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol. 2015;7:a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borst K, Frenz T, Spanier J, Tegtmeyer PK, Chhatbar C, Skerra J, Ghita L, Namineni S, Lienenklaus S, Köster M, Heikenwaelder M, Sutter G, Kalinke U. Type I interferon receptor signaling delays Kupffer cell replenishment during acute fulminant viral hepatitis. J Hepatol. 2018;68:682–690. doi: 10.1016/j.jhep.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446–453. doi: 10.1159/000336423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U, Müller-Brüsselbach S, Müller R. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller E, Christopoulos PF, Halder S, Lunde A, Beraki K, Speth M, Øynebråten I, Corthay A. Toll-like receptor ligands and interferon-γ synergize for induction of antitumor M1 macrophages. Front Immunol. 2017;8:1383. doi: 10.3389/fimmu.2017.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binnemars-Postma K, Bansal R, Storm G, Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018;32:969–978. doi: 10.1096/fj.201700629R. [DOI] [PubMed] [Google Scholar]
- 53.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, Cui XB, Yang L, Zhao J, Shen XH, Jiang JF, Liang WH, Yuan XL, Li F. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8:21526–21538. doi: 10.18632/oncotarget.15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:14.12.1–14.12.10. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- 56.Wei J, Shi M, Wu WQ, Xu H, Wang T, Wang N, Ma JL, Wang YG. IκB kinase-beta inhibitor attenuates hepatic fibrosis in mice. World J Gastroenterol. 2011;17:5203–5213. doi: 10.3748/wjg.v17.i47.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J, Petzelbauer P, Assinger A, Schmid JA. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31:17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- 59.Ren S, Zhang H, Mu Y, Sun M, Liu P. Pharmacological effects of Astragaloside IV: a literature review. J Tradit Chin Med. 2013;33:413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Sun X, Gong X, Yang Y, Chen C, Shan G, Yao Q. Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol. 2017;42:18–24. doi: 10.1016/j.intimp.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Zhang WJ, Frei B. Astragaloside IV inhibits NF-κB activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314. doi: 10.1155/2015/274314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leng B, Tang F, Lu M, Zhang Z, Wang H, Zhang Y. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sci. 2018;209:111–121. doi: 10.1016/j.lfs.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, Wang HX, Zhang YJ, Yang YH, Lu ML, Zhang J, Li ST, Zhang SP, Li G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol. 2013;150:1062–1070. [PubMed] [Google Scholar]
- 64.Lu M, Tang F, Zhang J, Luan A, Mei M, Xu C, Zhang S, Wang H, Maslov LN. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-κB signaling pathway. Phytother Res. 2015;29:599–606. doi: 10.1002/ptr.5297. [DOI] [PubMed] [Google Scholar]


