Abstract
The diversity and complexity of sympathetic function highlight the importance of fundamental research. Little is known about the interaction of superior cervical sympathetic ganglion (SCG) and gut microbiota. In this study, the engagement of the sympathetic ganglia with gut microbiota was investigated. Bilateral superior cervical ganglionectomy (SCGx) significantly altered the microbiota composition in rats 14 days post-surgery, and these microbiotas may participate in several biological pathways in the host, suggesting the vital role of the cervical sympathetic ganglion in regulating the microbiome-brain axis, and further confirming that the sympathetic nervous system (SNS) regulates the microbiome-brain axis.
Keywords: Superior cervical sympathetic ganglion, superior cervical ganglionectomy, sympathetic nervous system, gut microbiota
Introduction
Mounting evidence supports a reciprocal relationship between intestinal bacteria and the brain [1]. The gut microbiota comprises miscellaneous microorganisms, including bacteria, viruses and fungi, which populate at the lower gastrointestinal tract [2] and regulate the host functions, such as defense, metabolism and reproduction [3]. The diversity and abundance of each individual’s specific members of microbes vary widely, with many factors such as age, host genetics, environment and diet being implicated [4]. The distinctive character of microbiome has been exclusively studied in human diseases, including obesity [5], inflammatory and functional bowel diseases [6,7] and cardiovascular diseases [8]. Recently, emerging studies have indicated that the gut microbe participates in the pathogenesis of neuropsychiatric diseases, such as autism [9], depression [10], hepatic encephalopathy [11], Parkinson’s disease [12] and Alzheimer disease [13]. Multiple tools for manipulating gut microbiota with antibiotics [14], fecal transplantation [15] and germ-free animal models [16] are broadly applied to investigate gutbrain interactions. In addition, considerable evidence demonstrates that gastric microbiota affects cerebral development and function, while the reduced brain-derived neurotropic factor (BDNF) levels [14,17], abnormal neuropeptide and neurotransmitter levels [17,18], and altered neuroreceptor expression [18-20] (primarily in the hippocampus) were found in the germ-free and antibiotic-treated animals. Some of these changes correlate with emotional behaviors and cognitive function, implying the vital role of microbial composition in the occurrence of neuropsychological diseases.
The crosstalk between the central nervous system (CNS) and the gut to maintain body homeostasis is achieved by the sympathetic and parasympathetic nervous systems [21,22], the hypothalamic-pituitary-adrenal (HPA) axis, and the enteric nervous system (ENS). Combined with the HPA axis, and neural and neuroendocrine signaling, the sympathetic and parasympathetic nervous systems regulate the essential gut duty, e.g., regional motility and permeability, maintenance of epithelial fluid, bile secretion, production of bicarbonates and mucus, and the mucosa immune response [23]. It is now well appreciated that the vagus nerve is the principal regulatory pathway in linking the gut to the brainstem and modulating social behavior [24,25]. The effect of probiotics and specific metabolites may work through the vagus neural pathway [24,26-28]. A recent study reported that the bacteria L. reuteri alleviate the social defects in a vagus nerve-dependent tone in mouse models of autism [28]. Likewise, the sympathetic nervous system (SNS) is known to mediate the intestinal mucus layer’s physiological properties, consequently regulating the mucosal immune system [29] and microbial composition and behavior alterations [30]. Despite the significance of the vagus nerve in microbiome-brain signaling thus far, the sympathetic branch of autonomic nervous system has not been explored in-depth in the underlying microbiome-gut-brain axis. In addition, the correlation between the SNS and gut microbiota remains to be seen.
The superior cervical ganglion (SCG) is one of the significant components of the SNS with the most traffic branches and special distribution positions. The SCG gives out postganglionic fibers that innervate the pineal gland, hypophysis, carotid body, the iris and eyelid, and participates in the neuroendocrine immune regulation [31]. To excavate the pivotal role of the sympathetic pathway in the microbiome-brain axis, we investigated the fecal microbiome of rats with superior cervical ganglionectomy using 16s rRNA gene sequencing analysis.
Materials and methods
Animals
Twelve male Sprague Dawley (SD) rats (300-400 g; specific pathogen-free grade) were purchased from the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, Hubei, China). Animals were individually housed in a climate-controlled room (temperate of 25±1°C, relative humidity of 50±10%, and a 12 h light/dark cycle) with ad libitum food and water access. All experimental procedures were strictly carried out following with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Institutional Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) (TJH-202102002). Animals were euthanized by overdose of anesthetics after the experiments.
Experimental design
After acclimation for one week, rats were randomly distributed to the following groups: SCGx group (n=6) received bilateral superior cervical ganglionectomy, and faeces were collected from the rats on the seventh day (SCGx-7 group) and the fourteenth day (SCGx-14 group) after surgery. CON group (n=6) received the same surgical procedure without bilateral superior cervical ganglionectomy.
All surgical procedures were conducted with sterile instruments. Rats were anaesthetized with sodium pentobarbital (40 mg/kg, i.p.) for all surgeries and body temperature was maintained with a heat regulator. The SCGx surgery was performed as previously described [32]. After anesthetic induction and disinfection, a 2 cm vertical incision was performed at the neck region and salivary glands were exposed. Then, the common carotid artery (CCA) was clearly visible after transected the cranial of the sternomastoid muscle (SMM) and omohyoid muscle (OMH) with blunt forceps. Next the CCA was dissected cranially to locate the carotid bifurcation, and the SCG was just situated behind the carotid bifurcation. Finally, the cell body of the ganglion was gently pulled until their complete avulsion from the nerves chain for collecting the SCG tissue. Absolutely superior cervical ganglionectomy was accomplished by removing the SCG on the contralateral side. After SCG extraction, the incision was closed with 4-0 sutures and compound lidocaine cream was applied topically at the incision to alleviate incision pain. Only exposure of the superior cervical ganglion was performed in the control group. After surgery, rats were put back on a heating pad for recovery and then replaced to their cages. The blepharoptosis was regarded as an indicator of the successful operation.
Fecal collections and 16s rRNA sequencing
Two pellets of faeces were collected from each subject and divided into two parts in a sterilized 1.5-ml EP tube. Fecal samples were snap-frozen on dry ice and stored at -80°C. The 16s rRNA analysis of the fecal samples was performed by OE Biotech Co., Ltd (Shanghai, China). Total genomic DNA was extracted using MagPure Soil DNA LQ kit following the manufacturer’s instructions. DNA concentration was verified with NanoDrop and agarose gel. Genomic DNA was amplified using primers targeting the 16S V3-V4 regions. Both primers were coupled with an Illumina sequencing adapter. The PCR products were purified and quantified, and then the concentration was corrected for sequencing performed on an Illumina Miseq PE300 system. The poor-quality reads were screened out and discard using QIIME (ver 1.9.1) software. The clean reads were clustered to generate operational taxonomic units (OTUs) at 97% identity using Vsearch software [33], and all the representative reads were classified with Silva database and PDR classifier via QIIME package [34,35].
Immunohistochemistry
After removal of SCG, the sections were fixed with 4% paraformaldehyde overnight at 4°C and subsequently dehydrated in 20% sucrose/PBS for 24 h and 30% sucrose/PBS for 48 h and embedded in OCT. 20 um thick serial sections of the ganglion were cut with a Leica cryostat. These sections underwent three 10-minutes washes in PBS and permeabilized with PBST for 30 minutes. After washing, the tissue slices were incubated with 5% bovine serum albumin for 1 h at 37°C. Then sections were incubated at 4°C overnight with the rabbit anti-tyrosine hydroxylase antibody (1:100, catalogue number: E2L6M, Cell Signaling Technology). After three washes in PBS for 10 min per wash, sections were incubated with secondary antibody conjugated with Alexa Fluor 488 (Goat Anti-Rabbit IgG, 1:100) for 2 h at 37°C followed by counterstaining with DAPI. The sections were examined using fluorescence microscope.
Statistical analyses
The relative abundance of gut microbiota and statistical graphs were analyzed using the GraphPad Prism v8.0 package. Data from three groups were analyzed by one-way ANOVA followed by Dunn’s post hoc test. The differences in α-diversity (Chao 1 and Shannon index) among CON, SCGx-7 and SCGx-14 groups were analyzed by the Wilcoxon rank sum test. A heat map of Binary-Jaccard distance and the principal coordinates analysis (PCoA) of Binary-Jaccard distance were utilized to visualize the β-diversity. The permutational multivariate analysis of variance analyses of PCoA were performed to observe the overall distribution among groups. The taxa summaries generated in QIIME were reformatted for inputs into LEfSE and linear discriminant analysis (LDA) was used to differentially rank the abundant taxa, followed by Kruskal-Wallis test. For KEGG functional prediction analysis, the functional differences among groups were also analyzed using the PICRUSt software [36] and the results were counted for differences at the class level according to the Kruskal-Wallis algorithm.
All the data are expressed as the mean ± SEM. P<0.05 was considered statistically significant.
Result
Verification of surgical performance
After neck dissection and retraction of the mandibular glands, sternocleidomastoid muscle was pulled to the side to expose the CCA. Then the carotid bifurcation was bluntly exposed with the CCA in the cranial direction. The SCG is localized directly underneath the carotid bifurcation. Then, the cell body of the ganglion was gently pulled with microscope forceps until complete avulsion from the sympathetic chain (Figure 1A) [37]. The same operation was performed on the contralateral side. Bilateral palpebral ptosis was regarded as an indicator to evaluate the efficacy of the surgery and the rat with bilateral blepharoptosis was apparent after surgery (Figure 1B). To demonstrate the sympathetic nature of SCG, the removed structure was stained with antibody against TH, the rate-limiting enzyme in the biosynthesis of catecholamine and a specific marker of SNS [38]. The resected structure presented TH positive immunoreactivity, whereas no distinguishable signal was detected in the absence of primary antibody (Figure 1C).
Figure 1.
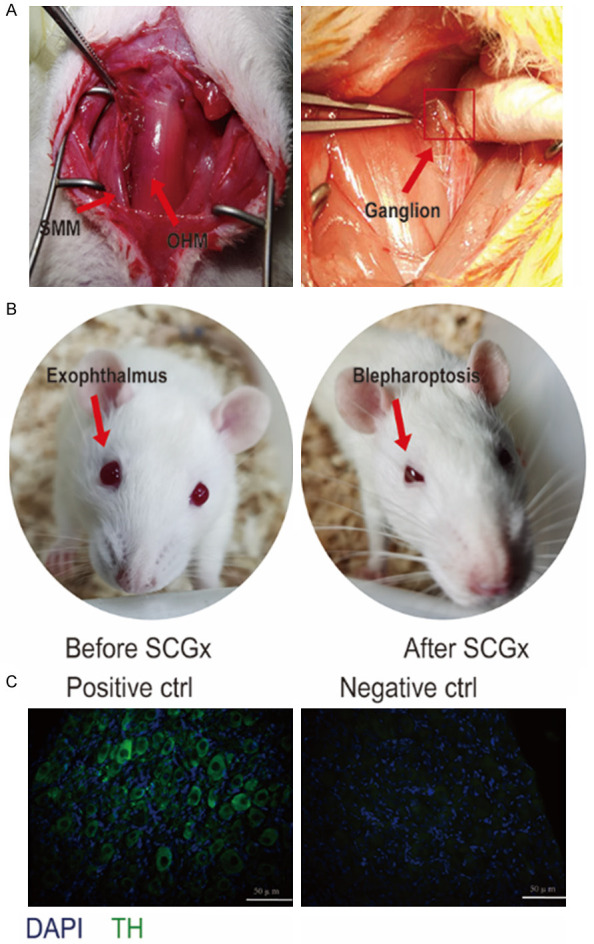
Surgical removal of the SCG in rats and verification by performance. A. The anatomy of the SCG and the neighboring tissues. The glandular tissue and sternomastoid muscle (SMM) were dissected bluntly to make clear the CCA. The SCG was situated behind the carotid bifurcation, and the cell body of the ganglion was gently pulled until their full avulsion from the sympathetic chain. B. The blephar before and after bilateral superior cervical ganglionectomy. C. Immunofluorescence staining of SCG. The same staining protocol was used as a negative control, except that the TH antibody was applied. Tyrosine hydroxylase (TH), scale bar: 50 μm.
Comparison of the gut microbiota composition among the CON, SCGx-7 and SCGx-14 groups
The 16s rRNA sequencing results showed that a total of 12 bacteria at six phylogenetic levels (phylum, class, order, family, genus, and species) were significantly altered among the three groups (Figure 2A-L). The relative abundance of 11 bacteria of the SCGx-14 group was significantly altered as compared to the CON group, in which 3 bacteria were decreased (Figure 2A, 2I, 2K) and 8 were increased (Figure 2C-H, 2J, 2L). In SCGx-7 groups, there was one significantly increased change (Figure 2B) and one significantly declined change (Figure 2K), and the remaining ten changes were not significant.
Figure 2.
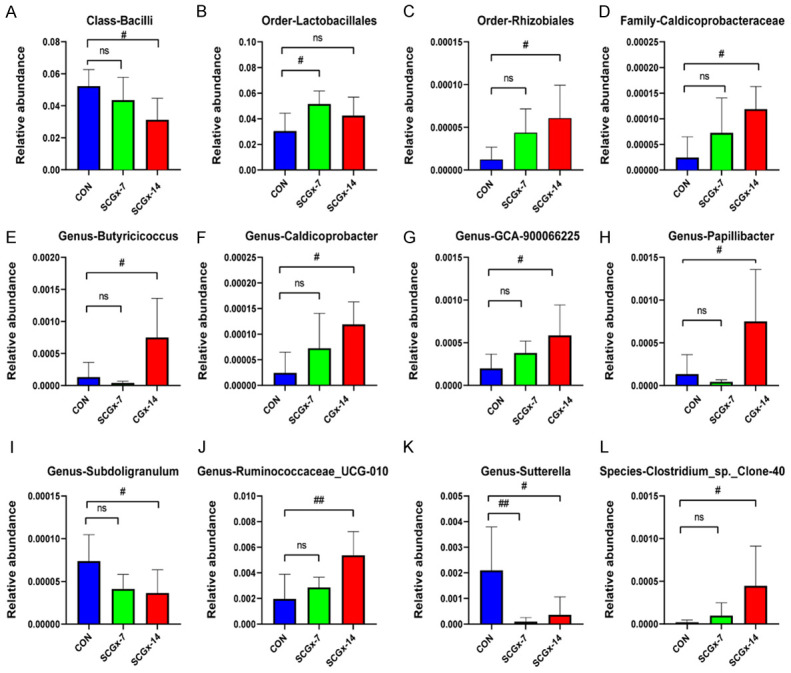
The ANOVA analysis of gut bacteria at differential levels in CON, SCGx-7 and SCGx-14 groups. A. Class-Bacilli (P=0.036714). B. Order-Lactobacillales (P=0.040188). C. Order-Rhizobiales (P=0.030745). D. Family-Caldicoprobacteraceae (P=0.023132). E. Genus-Butyricicoccus (P=0.009982). F. Genus-Caldicoprobacter (P=0.023132). G. Genus-GCA-900066225 (P=0.044258). H. Genus-Papillibacter (P=0.039208). I. Genus-Subdoligranulum (P=0.0485). J. Genus-Ruminococcaceae_UCG-010 (P=0.006185). K. Genus-Sutterella (P=0.010417). L. Species-Clostridium_sp._Clone-40 (P=0.009404). #P<0.05, ##P<0.01. All three groups were compared by one-way analysis of variance with Dunnett’s multiple comparisons test.
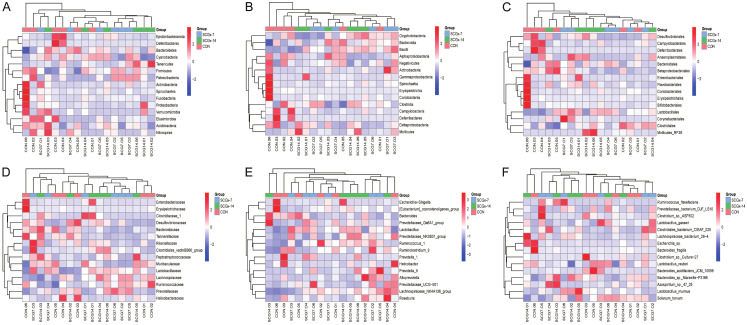
Abundance of the composition of gut microbiota at phylogenetic levels in the CON, SCGx-7 and SCGx-14 groups
Heatmaps of the top 15 gut microbiota at the phylum, class, order, family, genus and species levels in the three groups are shown (Figure 3A-F).
Figure 3.
Heatmaps of the abundance of gut bacteria (top 15) at various levels in CON, SCGx-7 and SCGx-14 groups. A. Phylum level. B. Class level. C. Order level. D. Family level. E. Genus level. F. Species level.
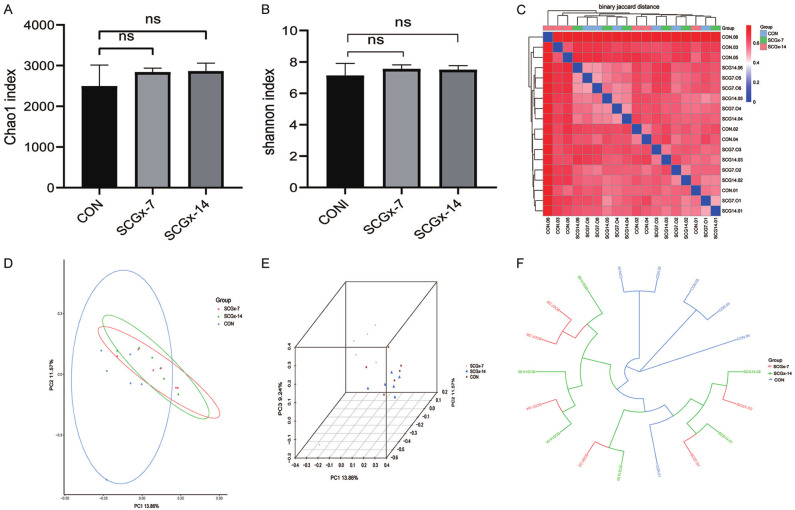
alpha-diversity and beta-diversity analysis of the CON, SCGx-7 and SCGx-14 groups
α-diversity depicts the diverseness within a sample, which is mainly relevant to the number of different species, while β-diversity represents the dissimilarity between samples [39]. The Chao1 and Shannon indices are parameters for evaluating the α-diversity. And there was no significant difference in the Chao1 index and Shannon index among the CON, SCGx-7 and SCGx-14 groups (Figure 4A, 4B). Regarding the β-diversity, the principal coordinate analysis (PCoA) plots each sample as a dot in three-dimensional spaces, and demonstrates the difference between groups. It is obvious that the gut microbiome composition profiles in the CON, SCGx-7 and SCGx-14 were clearly separated by PCoA based on Binary-Jaccard dissimilarity (Figure 4C-E). Of note, the 3D PCoA analysis depicts that the dots of the CON group were away from that of the SCGx-7 and SCGx-14 groups, but the dots of the SCGx-7 and SCGx-14 groups were adjacent. In addition, the unweighted pair-group method with arithmetic mean (UPGMA) of PCoA analysis also showed that the CON and the SCGx groups (SCGx-7 and SCGx-14) clustered clearly in their own groups (Figure 4F), which demonstrates that there are significant differences in the distribution and composition of species between the CON and SCG groups (SCGx-7 and SCGx-14).
Figure 4.
The analysis of alpha-diversity and beta-diversity. A. Chao1 index (P>0.05). B. Shannon index (P>0.05). Data are shown as mean ± SEM (n=6). P values were calculated from the Wilcoxon rank sum test. C. Binary-Jaccard distance. D, E. Binary-Jaccard based Principal component analysis (PCoA) of the three groups. (P<0.05). P was calculated from the permutational multivariate analysis of variance. F. Unweighted UniFrac-based hierarchical clustering analysis of UPGMA in the three groups.
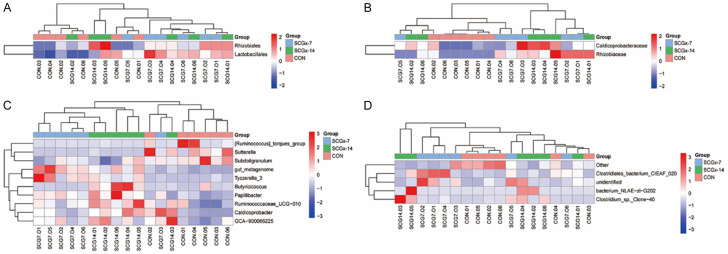
Heatmaps of the compositions of gut bacteria at differential levels in the CON, SCGx-7 and SCGx14 groups
Heatmaps of the compositions of gut bacteria at the order, family, genus, and species levels in the three groups are illustrated (Figure 5).
Figure 5.
Heatmaps of the compositions of gut bacteria at differential levels in CON, SCGx-7 and SCGx-14 groups. A. Order level. B. Family level. C. Genus level. D. Species level.
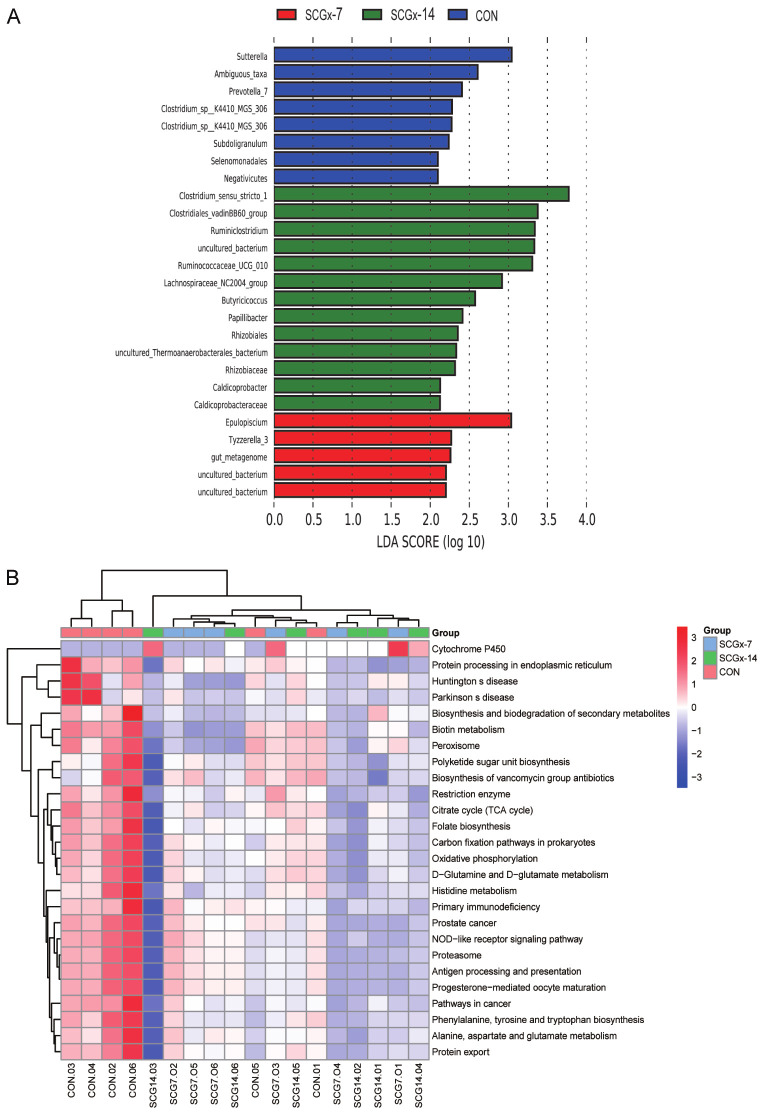
The LEfSe results and prediction of differential microbial functions
To ascertain the especial taxa variably distributed in the three groups, the LEfSe results revealed that 8 taxa were relatively over-represented in the CON group, and 5 taxa and 13 taxa were relatively over-represented respectively in the SCGx-7 and SCGx-14 groups (Figure 6A). KEGG functional prediction analysis was performed at class levels. It showed that potential pathways were associated with these differential microbes, including protein processing in the endoplasmic reticulum, neurodegenerative diseases, biosynthesis and biodegradation of secondary metabolites, oxidative phosphorylation, D-glutamine and D-glutamate metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, alanine, aspartate and glutamate metabolism, and the NOD-like receptor signaling pathway (Figure 6B).
Figure 6.
The LEfSe results and KEGG functional prediction analysis. A. The linear discriminant analysis. Red, green and blue bars respectively represent the species with relatively high abundance in the three groups. B. PICRUSt analysis of predicted functional pathway in gut microbiota.
Discussion
SCGx was introduced in rats in the last century and has been proved to be a valuable model in sympathetic nervous system research [40]. SCG contains thousands of neurons and is located uppermost in the paravertebral sympathetic chain [41]. Surgical removal of the SCG resulted in complete and irreversible sympathetic denervation to all the neuronal cell bodies [31], which disrupted the sympathetic pathway to the eye [42], resulting in blepharoptosis, a phenomenon used as an indicator after SCGx. In the present study, we successfully established SCGx models in rats and clearly found bilateral blepharoptosis post-surgery, especially in the SCGx-14 group. Furthermore, the results of immunofluorescence confirmed the sympathetic nature of the SCG.
Notably, elevated sympathetic activity contributes to the development of hypertension [43,44]. And the reciprocal communication between the gut microbiota and the brain has been shown to regulate blood pressure by modulating the interaction between the immune and sympathetic nervous systems [45]. Also, considerable evidence has shown the crucial role of microbial dysbiosis in the pathology of hypertension through bacteria metabolic products such as short chain fatty amino acids (SCFAs) [46,47]. Moreover, SCFA stimulates the paraventricular nucleus (PVN), increasing the sympathetic outflow to the gastrointestinal tract, resulting in a shift in microbial composition. Thus, there is a bidirectional regulation between the sympathetic nervous system and gut flora.
The 16s rRNA gene sequencing is a comparatively cheap and potent tool for uncovering the microbial community and the link between physiological and pathological characteristics [48-50]. In this study, we tested the altered microbiota composition induced by superior cervical ganglionectomy using 16S rRNA sequencing. And we discovered that the relative abundance of gut bacteria differed markedly between the CON group and SCGx-14 group, and a total of 11 specific gut bacteria were significantly altered between the two groups. At 5 phylogenetic levels, Order-Lactobacillales, Order-Rhizobiales, Family-Caldicoprobacteraceae, Genus-Butyricicoccus, Genus-Caldicoprobacter, Genus-GCA-900066225, Genus-Papillibacter, Genus-Ruminococcaceae_UCG-010, Species-Clostridium_sp._ and Clone-40 were significantly increased in the SCGx-14 group, compared with the CON group. On the contrary, Class-Bacilli, Genus-Subdoligranulum, and Genus-Sutterella were significantly decreased. These results suggest that superior cervical ganglionectomy caused abnormal microbiota composition, implicating the sympathetic pathway in regulating the gut microbiota.
Meanwhile, in our study, the α diversity showed no significant differences among the CON, SCGx-7 and SCGx-14 groups, which suggests that the species and quantity of microbiota did not change in rats that underwent superior cervical ganglionectomy. For the β-diversity, as represented in the PCoA pictures, the dots of the SCGx-7 group and SCGx-14 group were close together, but the dots of both two groups were clearly separated from the CON group. Besides, the circular tree data of UPGMA was in agreement with the PCA and PCoA analysis. Collectively, there was a significant discrepancy in the microbial community diversity between the SCGx and the CON groups.
As previously mentioned, gut microbial community disturbance has been reported to be associated with cardiovascular diseases [51,52], neurodegenerative disease [12] and obesity [53]. And various pathophysiological mechanisms are involved in the evolvement of these diseases, such as oxidative stress [54], endoplasmic reticulum stress [55], energy metabolism [56], neuroinflammation [57] and unbalanced neurotransmitter [58]. In the present study, we found that the differential microbes were closely related to protein processing in the endoplasmic reticulum, neurodegenerative diseases, biosynthesis and biodegradation of secondary metabolites, oxidative phosphorylation, D-glutamine and D-glutamate metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, alanine, aspartate and glutamate metabolism, and the NOD-like receptor signaling pathway.
Our current study also has several limitations, which should be addressed in the future. Our experimental results just showed the altered microbiota after SCGx, but we did not measure the circulatory and gut NE levels, and the pro-inflammatory cytokines associated with the gut microbiome. The previous study has suggested that the cervical sympathetic trunk takes part in the bidirectional communication between the immune and the sympathetic nervous system [59]. Another question we did not answer is whether cervical sympathetic denervation interferes with gut coeliac-superior mesenteric ganglion (CG-SMG), which specifically project to the gut and directly modulate gut sympathetic activity via the gut-brain circuit [60]. Likewise, the cardiac function might also be affected by SCGx that contributes to the altered microbial composition [61-63]. Hence, we could not clearly clarify the mechanism underlying the altered gut microbiome after superior cervical ganglionectomy. Our data need further studies in the characterization of sympathetic nervous system regulation of gut microbiota and relevant circuit for better understanding the regulation of intestinal motility, enteric immunity and neuropsychiatric disorders related to the gut-brain axis.
In conclusion, these findings revealed that superior cervical ganglionectomy significantly altered the microbiota composition in rats and these microbiotas may participant in several biological pathways in the host. In addition, this study confirmed the vital role of the SNS in regulating the microbiome-brain axis involved with various diseases, many of which are characterized by sympathetic dysregulation. The diversity of metabolites derived from our gut microbiota is quite astounding and also acts locally and systemically in a manner that affects the host physiology in both health and disease conditions. There is an urgent need for further research to identify the exact microbial product regulated by the sympathetic nerve system.
Acknowledgements
We thank the OEbiotech Co. Ltd. (Shanghai, China) for assistance with the data analysis of 16s rRNA sequencing. This work is funded by the National Natural Science Foundation of China (81873467, 81670240, and 82070302).
Disclosure of conflict of interest
None.
References
- 1.Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom Med. 2017;79:920–926. doi: 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 2.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nature reviews. Genetics. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. e114–515. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 7.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioessays. 2014;36:933–939. doi: 10.1002/bies.201400075. [DOI] [PubMed] [Google Scholar]
- 10.Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. 2015;21:273–282. doi: 10.5056/jnm14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini A, Campagna F, Amodio P, Tuohy KM. Gut: liver: brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct. 2018;9:1373–1388. doi: 10.1039/c7fo01528c. [DOI] [PubMed] [Google Scholar]
- 12.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167:1469–1480. e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii Y, Nguyen TTT, Fujimura Y, Kameya N, Nakamura S, Arakawa K, Morita H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci Biotechnol Biochem. 2019;83:2144–2152. doi: 10.1080/09168451.2019.1644149. [DOI] [PubMed] [Google Scholar]
- 14.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609, e1–3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 15.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 18.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 19.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Feng M, Xiang B, Fan L, Wang Q, Xu W, Xiang H. Interrogating autonomic peripheral nervous system neurons with viruses-a literature review. J Neurosci Methods. 2020;346:108958. doi: 10.1016/j.jneumeth.2020.108958. [DOI] [PubMed] [Google Scholar]
- 22.Fan L, Xiang B, Xiong J, He Z, Xiang HB. Use of viruses for interrogating viscera-specific projections in central nervous system. J Neurosci Methods. 2020;341:108757. doi: 10.1016/j.jneumeth.2020.108757. [DOI] [PubMed] [Google Scholar]
- 23.Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 24.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101:246–259. e246. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 26.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–9. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, Dauge V, Maguin E, Naudon L, Rabot S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci. 2018;12:19. doi: 10.3389/fnins.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattiolit M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101:246–259. e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 30.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 31.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savastano LE, Castro AE, Fitt MR, Rath MF, Romeo HE, Muñoz EM. A standardized surgical technique for rat superior cervical ganglionectomy. J Neurosci Methods. 2010;192:22–33. doi: 10.1016/j.jneumeth.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhang W, Sun T, Liu B, Manyande A, Xu W, Xiang HB. The role of gut microbiota in chronic itch-evoked novel object recognition-related cognitive dysfunction in mice. Front Med (Lausanne) 2021;8:616489. doi: 10.3389/fmed.2021.616489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler KA, Ahles A, Wille T, Kerler J, Ramanujam D, Engelhardt S. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc Res. 2018;114:291–299. doi: 10.1093/cvr/cvx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagatsu T, Nagatsu I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): historical overview and future prospects. J Neural Transm (Vienna) 2016;123:1255–1278. doi: 10.1007/s00702-016-1596-4. [DOI] [PubMed] [Google Scholar]
- 39.Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. [Google Scholar]
- 40.Emmelin N, Trendelenburg U. Degeneration activity after parasympathetic or sympathetic denervation. Ergeb Physiol. 1972;66:147–211. doi: 10.1007/3-540-05882-6_3. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro AA, Davis C, Gabella G. Estimate of size and total number of neurons in superior cervical ganglion of rat, capybara and horse. Anat Embryol (Berl) 2004;208:367–380. doi: 10.1007/s00429-004-0407-0. [DOI] [PubMed] [Google Scholar]
- 42.Cavallotti C, Frati A, Sagnelli P, Pescosolido N. Re-evaluation and quantification of the different sources of nerve fibres supplying the rat eye. J Anat. 2005;206:217–224. doi: 10.1111/j.1469-7580.2005.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 44.Yang T, Ahmari N, Schmidt JT, Redler T, Arocha R, Pacholec K, Magee KL, Malphurs W, Owen JL, Krane GA, Li E, Wang GP, Vickroy TW, Raizada MK, Martyniuk CJ, Zubcevic J. Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front Physiol. 2017;8:220. doi: 10.3389/fphys.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang T, Zubcevic J. Gut-brain axis in regulation of blood pressure. Front Physiol. 2017;8:845. doi: 10.3389/fphys.2017.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruning J, Chapp A, Kaurala GA, Wang R, Techtmann S, Chen QH. Gut microbiota and short chain fatty acids: influence on the autonomic nervous system. Neurosci Bull. 2020;36:91–95. doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watts GS, Youens-Clark K, Slepian MJ, Wolk DM, Oshiro MM, Metzger GS, Dhingra D, Cranmer LD, Hurwitz BL. 16S rRNA gene sequencing on a benchtop sequencer: accuracy for identification of clinically important bacteria. J Appl Microbiol. 2017;123:1584–1596. doi: 10.1111/jam.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glockner FO. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louca S, Doebeli M, Parfrey LW. Correcting for 16S rRNA gene copy numbers in microbiome surveys remains an unsolved problem. Microbiome. 2018;6:41. doi: 10.1186/s40168-018-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng G, Zhou X, Wang M, Zhou L, Wang Z, Wang M, Deng J, Wang Y, Zhou Z, Zhang Y, Lai Y, Zhang Q, Yang X, Yu L, Jiang H. Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656–664. doi: 10.1016/j.ebiom.2019.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peleli M, Flacker P, Zhuge Z, Gomez C, Wheelock CE, Persson AEG, Carlstrom M. Renal denervation attenuates hypertension and renal dysfunction in a model of cardiovascular and renal disease, which is associated with reduced NADPH and xanthine oxidase activity. Redox Biol. 2017;13:522–527. doi: 10.1016/j.redox.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi X, Zhang G, Wang X, Nguyen C, May HI, Li X, Al-Hashimi AA, Austin RC, Gillette TG, Fu G, Wang ZV, Hill JA. Endoplasmic reticulum chaperone grp78 protects heart from ischemia/reperfusion injury through akt activation. Circ Res. 2018;122:1545–1554. doi: 10.1161/CIRCRESAHA.117.312641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson VM, Breen DM, Fortin JP, Liou A, Kuzmiski JB, Loomis AK, Rives ML, Shah B, Carpino PA. Latest approaches for the treatment of obesity. Expert Opin Drug Discov. 2015;10:825–839. doi: 10.1517/17460441.2015.1044966. [DOI] [PubMed] [Google Scholar]
- 57.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramaswamy K, Mathison R, Carter L, Kirk D, Green F, Davison JS, Befus D. Marked antiinflammatory effects of decentralization of the superior cervical ganglia. J Exp Med. 1990;172:1819–1830. doi: 10.1084/jem.172.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Marmol J, Castro TBR, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu H, Zheng X, Zhang L, Yang X, Shao Y, Zhang S. Bilateral superior cervical ganglionectomy attenuates the progression of β-aminopropionitrile-induced aortic dissection in rats. Life Sci. 2018;193:200–206. doi: 10.1016/j.lfs.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 62.Madan S, Mehra MR. The heart-gut microbiome axis in advanced heart failure. J Heart Lung Transplant. 2020;39:891–893. doi: 10.1016/j.healun.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Zuo K, Li J, Li K, Hu C, Gao Y, Chen M, Hu R, Liu Y, Chi H, Wang H, Qin Y, Liu X, Li S, Cai J, Zhong J, Yang X. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8:giz058. doi: 10.1093/gigascience/giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]