Abstract
The idea of functional non-coding RNAs is taking precedence over the previous notion which believed that they only comprise the auxiliary and junk material of the genome. Newer technologies and studies have proven their importance in regulating and affecting several cellular processes. One such area of research wherein their importance has started to take light is in cancer research, particularly leukemia. Myeloid leukemia is a blood malignancy birthed from mutations in hematopoiesis that disable myeloid progenitor cells from proper differentiation. This review will compile the most recent findings regarding the effects of these regulatory non-coding RNAs on the two types of myeloid leukemia. In particular, the effects of circular RNAs, micro RNAs and long non-coding RNAs, on the pathogenesis and proliferation of Acute and Chronic myeloid leukemia will be revealed in a molecular, cellular and prognostic light. The mechanisms of proliferation, gene-to-gene interactions and possible therapeutic effects will also be discussed. Finally, an understanding of the overall “goodness” and “badness” of these non-coding RNAs will be summarised. This review hopes to provide a platform for easy access to data regarding the current non-coding RNAs in myeloid leukemia, for faster and easier research. Finally, the review will summarize a few key players that have protagonistic and antagonistic functions, and those that regulate multiple pathways in leukemia simultaneously.
Keywords: MiRNAs, lncRNAs, circRNAs, AML, CML
The act
As science progresses the notion that non-coding RNA (ncRNAs) comprises the “junk” of the genetic material is slowly fading away. There is now increasing evidence pointing to the regulatory effects that these non-coding RNAs may have in the cell. Non-coding RNAs such as micro RNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) especially, have started to emerge as key players in epigenetic gene regulation. More research in these fields has piqued the interest among researchers to find if these non-coding RNAs have any effect in causing leukemia.
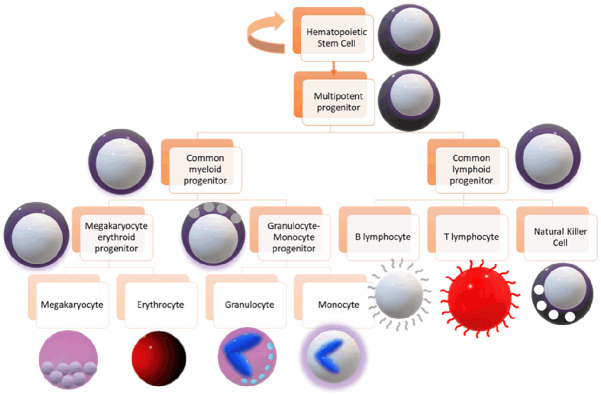
Leukemia is a disorder of the blood cells (also known as hematologic malignancies) that causes uncontrolled cell division in cells formed from hematopoietic stem cells (HSCs) [1]. Leukemia has affected around 474,519 people world-wide as of 2020 [2]. The four major types of leukemia are acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML) and chronic lymphocytic leukemia (CLL). The acute form of leukemia usually affects younger individuals and is more severe due to its sudden onset and rapid proliferation. The chronic form usually affects an older age group and may take time to be diagnosed. During HSC differentiation, mutations in the blast stage can give rise to mutated myeloid and lymphoid cell progenies, which can harbor oncogenes and turn leukemic [3]. ‘Blasts’ are unmatured and undifferentiated cells, and can differentiate to form erythrocytes, megakaryocytes, granulocytes, monocytes, T-lymphocytes, B-lymphocytes and natural killer cells (Figure 1). These differentiated cells can become cancerous and form specialized leukemias that can be a subset of the original four types of leukemias, such as acute megakaryoblastic leukemia, acute biphenotypic leukemia, Burkitt’s leukemia, precursor acute T-lymphoblastic leukemia, among many other types. Many approaches have been taken to understand its cause, such as the effects of external disturbances like radiation, smoke and alcohol, along with a more molecular approach including the effects of oncogenes, tumor suppressor genes, Philadelphia translocations, mutations, along with studies on cell cycle regulators like p53.
Figure 1.
A schematic of the differentiation of hematopoietic stem cells. This figure gives an overview of the pathway of differentiation of HSC’s that gives rise to the different types of HSC progenies in the body. Different cell types are depicted with different shapes and colors, and are described inside the boxes next to them.
The non-coding RNAs focused on in this review are the miRNAs, lncRNAs and cirRNAs. These have distinct physical characteristics as well as distinct modes of action in the cell. It has been observed that many of these ncRNAs do not act independently, instead they sponge other ncRNAs, regulate the expression levels of other proteins by different pathways, or inhibit other non-coding RNAs from being expressed. Complex relationships among them give rise to a wide variety of epigenetic modifications that eventually lead to tumorigenesis. Up-regulation of certain ncRNAs promotes leukemogenesis, while some others inhibit it. Some lncRNAs and miRNAs are found to have dual roles in AML and CML pathogenesis. Many serve prognostic roles and are a means of identifying the stage of leukemia while targeting others can give rise to therapeutic biomarkers. There are already several commercial drugs in the market for curing CML, however, a wide population of patients have drug resistance against these verified drugs. Several ncRNAs are seen to be overexpressed in such patients who suffer from drug resistance. These are gaining the spotlight for being potential therapeutic targets in the attempt to reverse multi drug-resistance. This review will bring forth all of the recent ncRNAs that have been studied in an attempt to increase the understanding of their roles in AML and CML. Finally, a general overview summarizing the positive and negative effects of the RNAs will be made to find potential therapeutic targets among the wide and varied RNA pool.
The backstory of hematopoietic stem cell differentiation
Everything has an origin. True to this statement, all blood cells originate from a common precursor known as hematopoietic stem cells. Hematopoietic stem cells, referred to as HSCs have the properties of self-renewal, differentiation, quiescence and apoptosis. Careful regulation of these properties can give rise to all the different types of the blood cells in the body [4]. HSCs differentiate in the bone marrow of the adult vertebrate [5], though initially they replicate in the embryonic yolk sac in the first week of development. Studies on hematopoiesis have revealed the different paths they undergo for differentiation. This is pictorially shown in Figure 1 [6].
It is at this stage, when the myeloid progenitor cell and lymphoid progenitor cell differentiates, that any genetic or epigenetic changes in the blast cell differentiation can give rise to myeloid leukemia or lymphoid leukemia, respectively [7]. Affecting the chromatin accessibility and structure is one of the ways by which the HSC self-renewal and differentiation properties can be controlled. This is done by DNA modifying enzymes like the DNA methyltransferases (DNMTs) and ten-eleven translocation methylcytosine dioxygenases (TETs). Histone modifiers like histone acetyl transferase (HATs), histone methyltransferases (HMTs), histone demethylases (HDMS) and histone deacetylases (HDACs) are enzymes that change histone structures and thereby modify chromatin accessibility [8,9]. It has also been observed that signals from the microenvironment also determine the differentiation patterns of the HSCs [10]. Recent research has delved into the effects that ncRNAs have over HSC differentiation. It is now known that ncRNAs have major roles to play in the post-transcriptional regulation of HSC differentiation [11,12].
The key players: non-coding RNAs
miRNAs
MicroRNAs (miRNAs) are a type of regulatory ncRNA that take part in post-transcriptional modifications, by regulating target mRNAs that have been transcribed from genetic material. miRNAs are short RNA sequences which typically consist of 19-25 nucleotides. The seed region of miRNAs is a nucleotide stretch from 2-7 nucleotides upstream of the 3’UTR. This is a unique sequence which binds complementary to the mRNA of interest [13]. The miRNA, which in conjugation with the RISC complex [14], binds to the 5’ end of the mRNA and can regulate the mRNA by either cleaving it, through endonucleases, or by changing its conformation, thereby preventing mRNA binding proteins from accessing the mRNA and translating it. RNA Pol II enzyme forms the primary transcript of the miRNA (100 s of nucleotides long) in the nucleus, which is then further processed by the Drosha enzyme to form a pre-miRNA which is 70-100 nucleotides long and has a stem-loop structure. The HASTY enzyme transports this pre-miRNA to the cytoplasm after which the Dicer enzyme converts the pre-miRNA to a double stranded miRNA. When miRNA combines with RISC it forms miRNP, which binds to the mRNAs [15-19]. In case of a double stranded miRNA, the guide (mature strand) miRNA is used for binding the mRNA, while the passenger strand is cleaved off [20]. Though miRNAs make up only 1% of the entire human genome, it is responsible for regulating around 30% of all human protein synthesis [21,22]. This is due to the fact that miRNAs do not have 100% sequence complementarity with the target mRNA of humans, giving rise to flexibility of the miRNA to bind to different mRNAs that have some sequence complementarity. Hence, miRNAs are important post-transcriptional modifiers, and as will be discussed, they have important roles in the occurrence of AML and CML.
lncRNAs
Long non-coding RNAs (lncRNAs) are another type of regulatory ncRNA which are usually classified as having more than 200 nucleotides. The GENECODE project lists that there are 17,957 lncRNAs in our entire genome as of 2019 [23,24]. lncRNAs are very similar to mRNAs in that they too can be polyadenylated by RNA Pol II, spliced, capped, and transcribed [25-30]. Essentially, they are just like mRNAs, but the main difference is that they do not contain reading frames necessary for encoding proteins. Their functions include regulating transcription by cis- and trans-acting pathways, proteins and RNA molecules in the organisation of nuclear domains. They also participate in regulating cell division, stability and translation of mRNAs, along with maintaining the cytoplasmic factors and protein scaffolding pathways required for the cell [31-33]. They are usually localised in the nucleus, but they have also been seen to have important functions in the cytoplasm. Some lncRNAs also have specific localised functions, like the lncRNA RMRP, which is transported out of the nucleus via the exportin CRM1, which is then localised to the mitochondria and regulates mitochondrial DNA replication, mitochondrial function and structure maintenance [34].
circRNAs
These are covalently closed, circular RNA molecules (circRNA) that are formed via a back-splicing method. They are formed when the 3’ end of an exon of a gene, covalently links to its own 5’ upstream exon, thereby forming a 3’-5’ phosphodiester bond. They are formed by the spliceosome machinery and are transcribed by RNA Pol II. Two models have been proposed to explain circRNA synthesis. These are the intron-interaction driven circularization model and the lariat driven circularization model [35]. Due to this closed structure, they do not have 3’ poly A tails or 5’ 7-methylguanosine caps and they are resistant to the action of RNase R present in the cytoplasm [36-40]. circRNAs such as the exonic circRNAs (ecircRNAs) are usually located in the cytoplasm, though certain circRNAs such as the circular intronic RNAs (ciRNAs), and exon-intron circRNAs (EIcircRNAs) are localised in the nucleus [41]. They mainly serve as regulatory roles. It has been found that many circRNAs have miRNA binding sites on their surfaces, and they can act as miRNA sponges. This prevents the miRNA from binding to the mRNA, thereby preventing silencing of that target mRNA by that miRNA [42]. circRNAs can also act as transcriptional regulators, by binding to their parent genes in the nucleus followed by up-regulating or down-regulating the gene of interest [43,44]. Similarly, they can also act as spicing regulators, they are involved in protein-protein interactions, in ribosomal RNA processing and they are also involved in recruiting proteins to certain locations [35].
Setting the stage for leukemia
Leukemia can be broadly classified into two types: Myeloid leukemia and lymphoid leukemia. Myeloid leukemia is a clonal expansion of myeloid progenitor cells, which if left untreated can cause bone marrow deformations, and eventually death [45]. Acute myeloid leukemia occurs mainly in the younger age groups, while chronic myeloid leukemia is more prevalent among older patients. The acute myeloid leukemia is considered to be more vigorous due to its aggressively proliferating nature, while the chronic form is considered to be long lasting and requires years of treatment [46].
Acute myeloid leukemia
Acute myeloid leukemia has become a central research focus because it is prevalent in high numbers across the world. When more than 20% of the cells in the bone marrow or blood become myeloblasts then it is diagnosed as AML [47]. Many years of research has now concluded some key factors that are the causes for this leukemia. Some of the primary reasons are: point mutations in the genes responsible for differentiation of myeloid progenitor cells, translocations, inversions, non-specific mutations, gene regulators and more recently, non-coding RNAs, which are the main focus of this review [48,49].
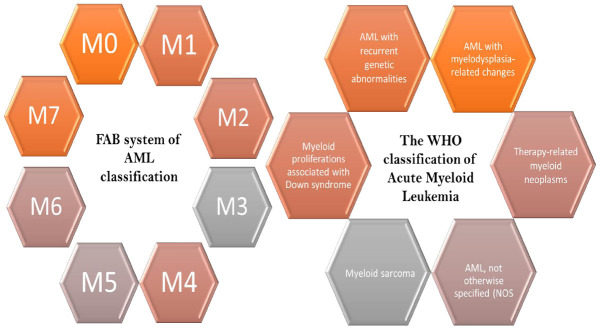
Learning about the classification of AML is necessary to learn about the methods by which AML is clinically studied. This can help researchers to find a cure for certain causative agents in a very systematic and globally accepted way. AML can be broadly sub-divided into three types of leukemias: Acute promyelocytic leukemia, acute myeloblastic leukemia and acute megakaryoblastic leukemia [50]. However, to classify AML there have been two broad systems of classification, which are based on structural and genetic factors. The earlier method of classifying AML was through the French-American-British classification method which took into account the different cell shapes, sizes, immuno-phenotyping and pathologic mechanisms used to identify them. In 2016, WHO came up with a new system of classifying AML based on a more molecular and genetic approach. This approach focused on classifying AML based on the genetic abnormalities that come in hand with the disease and molecular changes that are associated with it and has given researchers an easier way to study particular aspects of the disease. An overview of both classifications is shown in Figure 2.
Figure 2.
Classification of AML based on the FAB and WHO system of classification. This figure shows the FAB and WHO systems of classifying AML. FAB system classifies AML into eight different subsections (M0-M7). This method of classification was based on cell shapes, sizes, immuno-phenotyping and pathologic mechanisms. WHO classifies AML into six different subsections based on genetic abnormalities and the associated molecular changes. They have been listed here.
AML studies now-a-days focus on identifying any biomarkers that can be used to find out the different stages that the patient is in. These can also serve as a prognostic tool and help to determine life expectancy and possible routes to save the individual.
Effects of non-coding RNAs in acute myeloid leukemia
As discussed earlier, it has been found that several regulatory ncRNAs are being found to have significant effects on the pathogenesis of AML. This review will primarily focus on circRNAs, miRNAs and lncRNAs. These have been shown in Tables 1, 2 and 3.
Table 1.
Effects of circRNAs in Acute Myeloid Leukemia
| circRNA name and ID | Expression | Gene | Effects | miRNA that the circRNA sponges or targets | Does the circRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References |
|
| |||||||
| circ-PVT1; hsa_circ_001821 | Upregulated | PVT1 | Suppresses tumor suppressor miRNA, thereby causing oncogenic protein formation. | let-7 or miR-125. | Promotes AML formation. | Could be used as a therapeutic target. | [51] |
| circNPM1 75001; (hsa_circ_0075001) | Upregulated | NPM1 | Involved with myeloid differentiation and NPM1 mutation leads to increased AML pathogenesis. | miR-181 family/TLR signaling pathway. | Reduces AML formation. | Could be used as a biomarker. | [54] |
| circ-ANAPC7; (hsa_circ_101141) | Upregulated | ANAPC7 | Involved in pathogenesis of AML, can be used as a potential biomarker for AML. | miR-181 family. | Proliferates AML formation. | Could be used as a biomarker. | [55] |
| circRNA-DLEU2; (hsa_circ_0000488) | Upregulated | DLEU2 | Promotes cell proliferation and reproduction. | miR-496/PRKACB. | Proliferates AML formation. | Could be used as a biomarker. | [56] |
| hsa_circ_100290 | Upregulated | SLC30A7 | Causes AML and oral squamous cell cancer while promoting cell proliferation and inhibiting apoptosis. | miR-293/Rab10. | Proliferates AML formation. | Could be used as a biomarker. | [57] |
| circ_0009910; (hsa_circ_100053) | Upregulated | MFN2 | Used as a biomarker. Highly abundant in AML patients. Promotes cell cycle arrest and proliferation. | miR-20a-5p. | Proliferates AML formation. | Could be used as a biomarker and prognostic tool. High levels indicate poor prognosis. | [58] |
| circ-PAN3; (hsa_circ_0100181) | Upregulated | PAN3 | Induces drug resistance and removes the effects of miRNAs. | miR-153-5p, miR-183-5p, miR-338-3p, miR-346, miR-545-3p, miR-574-5p, miR-599, miR-653-5p, miR-766-3p, miR-767-3p. | Upregulated stage induces drug resistance in AML patients, thereby making them more susceptible to AML pathogenesis. | Could be used as an indicator of chemotherapy efficacy and understanding drug resistance. | [59] |
| circ-VIM | Upregulated | VIM | Involved in lymphocyte adhesion and transcellular migration. It can also serve as a potential biomarker because of its upregulation in AML patients. | Does not bind to any miRNA. | High level proliferates AML. | Used as a prognostic tool. High levels indicate poor prognosis. Could be used as a biomarker. | [60,61] |
| circ-HIPK2; (hsa_circ_0001756) | Downregulated | HIPK2 | Involved in regulation of ATRA-induced differentiation. | miR-124-3p. | Regulates APL differentiation by the miR-124/CEBP axis. | Could be used as a biomarker. | [62] |
| hsa_circ_0004277 | Downregulated | WDR37 | Can be used as a biomarker. Less abundant in AML patients, but abundancy increases after treatment. | miR-138-5p, miR-30c-1-3p, miR-892b, miR-571, miR-328-3p/SH3GL2, PPARGC1A, PIP4K2C, SH2B3, ZNF275, and ATP1B4. | Increasing levels of this circRNA correlates with efficient and proper treatment, which implies a decrease in AML pathogenesis. | Used as a biomarker. | [63] |
| circKHLH8 | Downregulated | KHLH8 | Overexpression leads to better survival of AML patients. | No data found. | Reduces AML pathogenesis in overexpressed condition. | Does not show any prognostic significance in younger AML patients. | [64] |
| circFBXW7 | Upregulated | FBXW7 | Tumor suppressor. | No data found. | Reduces AML pathogenesis. | Could be used as a prognostic tool. | [64] |
|
| |||||||
| Fusion circRNAs; f-circRNAs | |||||||
|
| |||||||
| circRNA name and ID | Expression | Fusion genes | Effects | Does the circRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References | |
|
| |||||||
| f-CircM9_1 | Upregulated | MLL/AF9 fusion | Increased resistance to leukemia treatments in vitro, contributes to leukemogenesis in vivo, gives resistance to leukemia treatment agents like arsenic trioxide and cytarabine. | Promotes AML formation. | Targeting their downstream effectors can give therapeutic targets. | [65,66] | |
| f-circPR | Upregulated | PML/RARα | Increases clonogenicity and they are pro-proliferative and proto-oncogenic, they also maintain the viability of the leukemic cells by inhibiting apoptosis. | Their expression leads to AML proliferation. | Targeting their downstream effectors can give therapeutic targets. | [66] | |
| f-circM9 | Upregulated | MLL/AF9 fusion | Increases clonogenicity and they are pro-proliferative and proto-oncogenic, they also maintain the viability of the leukemic cells by inhibiting apoptosis. | Their expression leads to AML proliferation. | Targeting their downstream effectors can give therapeutic targets. | [66] | |
|
| |||||||
| CircRNAs that bind to RNA binding Proteins | |||||||
|
| |||||||
| circRNA name and ID | Expression | RBP or gene to which the circ-RNA binds | Effects | Does the circRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References | |
|
| |||||||
| hsa_circ_0004870 | Downregulated | RBM39 | Causes enzalutamide resistance. Depletion of RBM39 causes decreased AML. | RBM39 expression causes AML cell migration and invasion but it is generally not expressed in healthy cells. | Could be a potential therapeutic tool. | [67,68] | |
| circMYBL2 | Upregulated | FLT3-ITD | Upregulates AML proliferation and inhibits differentiation of AML cells. | Increases AML proliferation. | It is a potential therapeutic target of FLT3-ITD AML. | [69] | |
| circFoxo3 | Downregulated | CDK2 and cyclin dependent kinase inhibitor p21 | Forms circfoxo3-p21-CDK2 which results in differentiation of AML cell lines. | Increases AML proliferation. | Could be potential prognostic tool. | [70] | |
Table 2.
Effects of miRNAs in acute myeloid leukemia
| miRNA | Expression | Gene targets | Effects | Does the miRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References |
|---|---|---|---|---|---|---|
| miR-126* | Upregulated | ADAT2, FOF1, LMO7. | Induces inhibition of apoptosis. Results in chromosomal translocations, results in AML cell viability and proliferation. | Promotes AML formation. | More research required. | [71,74] |
| miR-223 | Downregulated | ANKH, SCN1A, SCN3A, CBFB, CDH11, NEBL, RILPL1, CENPN. | miR-223 inhibits cell cycle regulator protein E2F1, but in AML condition, E2F1 acts as a transcriptional regulator of miR-223, thereby downregulating it. This results in cell proliferation. | Promotes AML. | More research required. | [75] |
| miR-126 | Upregulated | TOM1, CKMT2, ZNF131, RGS3. | This targets the polo-like kinase2 protein (PLK2) which is a tumor suppressor protein, thereby aiding in tumor proliferation. | Aids in AML formation. | More research required. | [71] |
| miR-124-1 | Downregulated | Inhibits cell growth, promotes cell apoptosis and promotes cell differentiation [76]. | Suppresses AML formation. | Serves as a prognostic marker. Those with miR-124-1 under expression have good prognosis. | [76,77] | |
| However, another study has noted that it targets the C/EBPα pathway, and it’s silenced and inhibited differentiation gives rise to leukemia phenotype [77]. | ||||||
| miR-221 | Upregulated | HIPK1, RAB18, DNM3, ZNF547. | Inhibits p27 which in turn inhibits CDK, thereby it enhances cell proliferation in AML. | Aids in AML formation. | Could be used as biomarker, as it’s amount increasing a lot in AML condition. | [78] |
| miR-20a and miR-17 | Downregulated | POLQ, KLF12, STK38, CENTD1, NUP35, GNB5, CTSK. | HIF-1α represses miR-20a and miR-17 expression. miR-17 and miR-20a removes HIF-1α mediated AML differentiation. Hence, their downregulation results in increase of AML differentiation. They also inhibit p21 and STAT3 expression. | In HIF-1α expressed condition, these miRNAs increase AML differentiation, but they inhibit AML cell proliferation. | More research required. | [72] |
| miR-29b | Upregulated | CD93, HBP1, SNX21, GNS, HMGCR, HNF4G, DNMT3B. | Reduces the expression of DNA methyltransferases. | Decreases AML pathogenesis. | Therapeutic role of synthetic miR-29b is being identified. | [79] |
| miR-29b | Upregulated | SCML2, C1orf96, COL3A1, COL7A1, COL11A1. | Reduces tumor proliferation. | Reduces AML tumor formation. | More research required. | [79] |
| miR-193b | Downregulated | MMP19, ARMC1, ARPC5. | c-Kit proto-oncogene results in abnormal cell proliferation. In AML cases miR-193b serves to post-transcriptionally modify c-Kit proto-oncogene, but it is downregulated. Artificially increasing miR-193b can lead to decrease in AML. | Increasing miR-193b can lead to decrease in AML pathogenesis. | miR-193b is used as a therapeutic agent, as increasing its amount in AML cells leads to better prognosis. | [73] |
| miR-196a and miR-196b | Upregulated in early hematopoiesis | FOS, GATA6, HOXB6, HOXC8, ZNF24, CCDC47. | Induces downregulation of ERG, which has adverse prognostic effects on elder AML patients. | Decreases the adverse effects of AML. | Could be used as a prognostic tool and biomarker. | [80] |
indicates that miR-126* is the complementary analog of miR-126. A common precursor from the egfl7 gene gives rise to both these miRNAs.
Table 3.
Effects of lncRNAs in acute myeloid leukemia
| lncRNA | Expression | Effects | Does the lncRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References |
|---|---|---|---|---|---|
| H19 | Upregulated | H19 upregulation shows that it behaves as a proto-oncogene in several AML cases, however it has also been observed to be downregulated and causes apoptosis in several other AML/CML cases through the H19/IGF2 axis. This differing characteristic can be attributed to the small sample size of observations. | It has oncogenic properties in several AML cases when upregulated, but can also have tumor suppressing roles in certain AML cases. Its effect differs with the sex and age of the patient as well. | Unmethylated H19 can be used as a prognostic biomarker. H19/ID2 has a therapeutic target value. | [81,82] |
| HOTAIR | Upregulated | It regulates LSC self-renewal by sponging miR-193a and by modulating c-KIT expression. | Its expression increases the pathogenicity of AML. | High HOTAIR expression is correlated with poor prognosis in AML patients. | [89-93] |
| UCA1 | Upregulated | It results in increased AML cell viability, growth, metastasis and cell invasion. This lncRNA binds with miR-126 and downregulates its action. However, overexpressed miR-126 binds to 3’UTR of RAC1 and inhibits its action. The PI3/AKT and JAK/STAT pathway is also blocked by miR-126 overexpression. Hence the PI3/AKT pathways are activated by lncRNA UCA1. UCA1 also sponges miR-125a and miR-16. | Its expression aids in AML formation. | Could be used as a therapeutic target as it is abundant in patients with CEBPA mutation. | [94-96] |
| CRNDE | Upregulated | It is found to be overexpressed in AML cells and it results in inhibition of apoptosis, increased proliferation, and increased cloning. | It increases AML pathogenicity. | Its presence is a measure of poor prognosis. Can be used as a therapeutic target. It is seen to be overexpressed in M4 and M5 type of FAB cells, as compared to M1, M2 and M3 cells. | [97] |
| PVT1 | Upregulated | It is elevated in t(8;21) AML and APL cases. It sponges miR-1204. It regulates MYC activation which is necessary for maintaining oncogenic state. PVT1 knockdown results in reduced cell migration and proliferation. | It increases AML pathogenicity. | Its expression is a measure of poor prognosis. | [84-87] |
| PANDAR | Upregulated | It results in AML pathogenesis by inhibiting the proapoptotic gene expression. This is done by interacting with the NF-YA transcription factor. It also results in higher BM (bone marrow) blast count and lower CR (complete remission) rate. The overall survival of the patients is also found to be shortened. | It results in AML pathogenesis. | It is used as a biomarker and as a prognostic marker. High expression levels result in poor prognosis especially in older individuals. | [98,99] |
| RUNXOR | Upregulated | It is involved in chromosomal translocation by interacting with the RUNX1 promoter via its 3’-terminal fragment. This results in the formation of an intrachromosomal loop. Mostly seen in t(8;21) AML cases. It also interacts with EZH2 which is a H3K27 methylase. | It results in AML proliferation. | Could play therapeutic and prognostic role. | [88] |
| CCAT1 | Upregulated | Promotes HL-60 cell growth by inhibiting miR-155 which has tumor suppressive properties. Hence, AML patients have less amounts of miR-155. This results in repressed monocytic differentiation, and promotes cell growth. CCAT1 upregulation is found in mainly M4 and M5 AML patients according to the FAB system. | Promotes AML cell growth by sequestering miR-155. | Its expression is a measure of poor prognosis. Downregulating CCAT1 can serve as a therapeutic target. | [84,100] |
| TUG1 | Upregulated | TUG1 suppresses miR-34a by recruiting EZH2, this results in ADR resistance. It also induces cell proliferation and inhibits AML cell apoptosis by targeting the aurora kinase A (AURKA). | Promotes AML proliferation. | High expression of TUG1 results in poor prognosis. | [101,102] |
| CCDC26 | Upregulated | It is also known as retinoid modification (RAM) and is associated with pediatric AML. It is involved with the regulation of the differentiation and apoptosis of acute monocytic leukemia cell lines via regulating c-KIT expression. | Increases AML proliferation. | High expression is a measure of poor prognosis. | [103,104] |
| MONC | Upregulated | Enhances the proliferation of immature erythroid progenitor cells. | Increases AML proliferation. | Could play therapeutic role. | [105] |
| MIR100HG | Upregulated | Involved in AML cell proliferation, viability, and colony formation. | Increases AML proliferation. | Could play therapeutic role. | [105] |
| HOXA-AS2 | Upregulated | Through a TRAIL mediated pathway, HOXA-AS2 suppresses apoptosis that is induced by ATRA. However, this HAOX-AS2 is also induced by ATRA. Net result is increase in the number of viable cells, and decrease in apoptosis. | Increases AML proliferation. | HOXA-AS2 is therapeutic targets need to be identified. | [106,107] |
| MALAT | Upregulated | Sponges and downregulates miR-96. It increases AML cell proliferation, and decreases apoptosis. It’s knockdown results in increased cytarabine (Ara-C) chemosensitivity by upregulating miR-96. | Increases AML proliferation. | Results in poor prognosis. | [108,109] |
| MEG3 | Downregulated | In a p53 dependent manner, MEG3 is seen to inhibit tumorigenesis. However, its methylation is seen to increase tumor formation and results in poor prognosis. MEG3 can also inhibit tumorigenesis in a p53 independent manner as well. Ten-eleven translocation 2 (TET2) is seen to upregulate MEG3 expression via a TET2-WT1-MEG3 axis, and this results in increased AML proliferation. | Decreases AML proliferation. | Methylation of MEG3 results in poor prognosis and reduced overall survival rates. Can be used as a biomarker. | [110,111] |
| CASC15 | Upregulated | CASC15 is a tumor suppressor gene and it results in reduced colony formation. It also limits AML cell proliferation. It regulates the SOX4 gene by modulating the transcription factor YY1. | It suppresses AML proliferation. | Studies on CASC15 have given insights into using them as potential therapeutic agents. | [112] |
| IRAIN | Downregulated | It is involved in inhibition of tumor cell migration. It is found to be more expressed in low-risk AML patients, but less expressed in high-risk AML patients. It is closely linked with the IGF1 promoter sequence. Exposing the AML cells to cytarabine (AraC), which is a chemotherapeutic agent, results in enhancing IRAIN expression. | It suppresses AML proliferation. | Results in shorter overall survival and results in a refractory response to chemotherapy. | [113,114] |
| NEAT1 | Downregulated in AML pathogenesis condition. | Results in suppressing AML cell proliferation and induces apoptosis. This is done via the NEAT1/miR-23a-3p/SMC1A axis. NEAT1 expression downregulates miR-23a-3p expression which leads to SMC1A upregulation, which then reverses AML proliferation. | It is generally downregulated in AML state, but inducing its upregulation results in decreasing AML proliferation. | Could be used as a therapeutic target by upregulating NEAT1 expression in AML affected cells. | [115] |
| LOC285758 | Upregulated | LOC285758 increases AML pathogenesis by regulating HDAC2 expression. Knockdown of LOC285758 results in increased cell apoptosis and inhibits cell proliferation. | Increases AML proliferation. | Indicates poor prognosis in AML patients. | [116-118] |
circRNAs and AML
Table 1 summarizes an overview of the different types of circRNAs that have been found to affect AML. Some of these act by sponging miRNAs, which then in turn leads to either over-expression or down-regulation of genes necessary for AML. Others may bind to RNA binding proteins (RBPs) and similarly affect the translation of necessary AML pathogenesis proteins. Some fusion circRNAs are showing prevalence, wherein, they are formed by the translocation of certain genes and their transcripts to form the circRNAs. These are important because they can be of many types and can have different functions based on the translocations. Few circRNAs will be discussed in brief here.
Circ-PVT1 (hsa_circ_001821)
The 8q24 amplicons usually generate chimeric genes and can generate oncogenic lncRNAs that result in leukemia (Figure 4). One such chimeric gene product formed from the exon 2 of PVT1 present on chromosome 8 gives rise to circ-PVT1. This circular RNA sponges the miRNAs of let-7 family and miRNA let-125 family. These miRNAs are tumor suppressors, hence sponging those results in AML proliferation. These circRNAs are abundant in those AML patients wherein the 8q24 amplicons are more. Circ-PVT1 can be used as therapeutic targets against AML [51-53].
Figure 4.
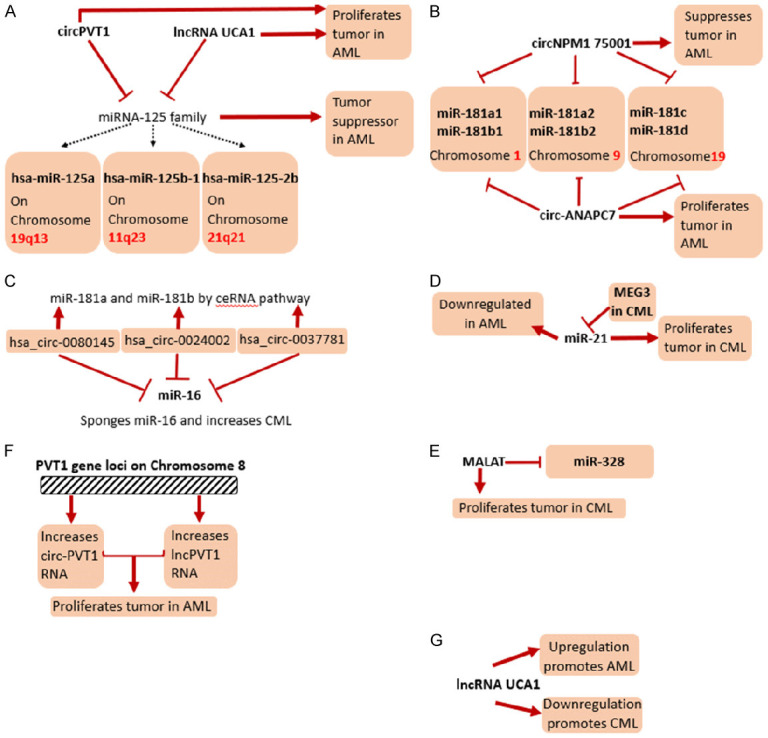
A summary on few of the mechanistic roles of miRNAs, lncRNAs and circRNAs on the pathogenesis of AML and CML. A. Effects of circPVT1 and lncRNA UCA1 on the miR-125 family have been shown. Their mechanistic effects on the pathogenesis of AML have been presented. B. Effects of circNPM1 75001 and circ-ANAPC7 on the miR-181 family has been shown in the pathogenesis of AML. C. Effects of the hsa_circ family on the miR-181 family and miR-16 family in the pathogenesis of CML have been shown. D. Effect of miR-21 in both AML and CML has been shown, along with special focus on the lncRNA MEG3 in CML. E. Effect of the lncRNA MALAT on miR-328 and in the pathogenesis of CML has been shown. F. The role of the PVT1 gene loci in formation of circ-PVT1 and lncPVT1 in proliferation of AML has been shown. G. Role of lncRNA UCA1 in proliferation of both AML and CML has been shown.
CircNPM1 75001 (hsa_circ_0075001)
The expression of circNPM1 75001 is seen to lower the TLR (Toll-Like Receptor) gene expression, thereby, affecting the TLR signaling cascade. The TLR signaling cascade has been observed to aid leukemic stem cell differentiation and survival. Hence, circNPM1 75001 aids in reducing AML pathogenicity. These circRNAs are over-expressed in NPM1 mutated AML patients. NPM1 mutation results in both tumor suppression and proto-oncogenic product formation. These circRNAs can also be used as a biomarker to check prevalence of AML in patients [54].
Circ-ANAPC7 (hsa_circ_101141)
This circRNA is seen to sponge the miRNA-181 family. It results in proliferation of pathogenesis of AML. It is observed to be up-regulated in AML patients and can be used as a potential biomarker for AML studies [55].
Circ-DLEU2 (hsa_circ_0000488)
Circ-DLEU2 is observed to be over-expressed in AML patients, thus it can be used as a potential biomarker. It acts by sponging miR-496 and thereby increases PRKACB expression by the competitive endogenous RNA mechanism. This leads to inhibition of apoptosis and increased proliferation of AML cells [56].
hsa_circ_100290
This circRNA is formed from the SLC30A7 gene. Similar to circ-DLEU2 it is also over-expressed in AML patients and can be used as a potential biomarker. It regulates the miR-203 and Rab10 axis. This promotes cell proliferation and inhibits apoptosis in AML cell lines, thereby increasing the pathogenicity of AML [57].
Circ_0009910 (hsa_circ_100053)
This originates from the gene MFN2 and it sponges miR-20a-5p. This sponging results in increased cell proliferation and its knockdown induces apoptosis. It is usually up-regulated in AML bone marrow samples and can be used as a potential biomarker and as a prognostic marker too, as high levels result in poor prognosis [58].
circ-PAN3 (hsa_circ_0100181)
These circRNAs are involved primarily in drug resistance. They are up-regulated in AML cells and cause resistance to THP-1/ADM cells by the circ-PAN3-miR-153-5p/miR-183-5p-XIAP axis [59].
Circ-vimentin
This circular RNA is seen to be up-regulated in AML patients and can be used as a measure of prognosis. Up-regulation of circ-VIM results in poor prognosis in elder patients. The vimentin protein is a part of intermediate filament protein III and is used for lymphocyte adhesion and trans cellular migration [60,61].
circ-HIPK2 (hsa_circ_0001756)
These circRNAs have an effect on acute promyelocytic leukemia, which is a subdivision of acute myelocytic leukemia. APL is caused by the blockage of cell differentiation at the promyelocytic stage by the PML/RARα fusion protein. They are down-regulated in APL cases and sponge miR-124. This causes astrocyte activation. miR-124, on the other hand, inhibits CEBPA which is a downstream protein. Hence, circ-HIPK2 regulates APL proliferation by the miR-124-CEBP axis [62].
hsa_circ_0004277
This is used as a biomarker in treatment of AML. It is de-regulated in AML pathogenesis conditions and up-regulated after treatment. It has many miRNA down-stream targets such as miR-138-5p, miR-30c-1-3p, miR-892b, miR-571 and miR-328-3p [63].
Fusion circRNAs
Some chromosomal translocations give rise to chimeric cancerous gene products. These can give rise to circRNAs which are then called fusion circRNAs or f-circRNAs.
miRNAs and AML
Table 2 summarizes the effect of different miRNAs in the pathogenesis of AML. Several of them have been seen to increase AML proliferation, while others are down-regulated and decrease tumorigenesis. Few miRNAs and their pathways of action are described below.
miR-126
miR-126 is found on intron7 of the epidermal growth factor-like 7 (EGFL7). Its expression is dependent on methylation regulation of a 287 bp region on EGFL7. Over-expression of miR-126 results in reduced apoptosis, and increased cell viability of AML cells. The Polo-like kinase family is involved in regulating the cell cycle and DNA damage checkpoints. One such polo-like kinase is PLK2, which is a tumor suppressor and it is a target of miR-126. Up-regulation of miR-126 negatively regulates PLK2, thereby causing proliferation of AML cell lines. The other gene targets of miR-126 are TOM1, CKMT2, ZNF131 and RGS3. The therapeutic effects of miR-126 are being researched currently [71].
miR-20a and miR-17
miR-20a and miR-17 are involved in reducing AML cell differentiation by affecting HIF-1α expression. HIF-1α is a transcription factor necessary for inducing differentiation of AML cells. HIF-1α can also repress miR-20a and miR-17 expression by down-regulating the c-Myc transcription factor. These miRNAs also inhibit p21 and STAT3 expression. This results in up-regulation of the miRNAs, as the p21 and STAT3 can inhibit the miRNAs from affecting HIF-1α. In summary, the miRNAs 20a and miR-17 are down-regulated in AML pathogenesis condition. This happens due to HIF-1α targeting their expression. If the miRNAs can be up-regulated, then they not only inhibit HIF-1α from acting as an oncogene, but they also inhibit p21 and STAT3 from acting as a miRNA inhibitor. It is being researched if these miRNAs can be up-regulated in cancer cells so that they can act as therapeutic targets. POLQ, KLF12, STK38, CENTD1, NUP35, GNB5 and CTSK are its gene targets [72].
miR-193b
miR-193b is found to be inversely related to the expression levels of c-Kit proto-oncogene. In AML cases, miR-193b is down regulated; hence c-Kit proto-oncogene is expressed. c-Kit proto-oncogene over-expression leads to abnormal cell proliferation and is a measure of poor prognosis. Hence, to inhibit AML pathogenesis, miR-193b must be over-expressed, as this can then post-transcriptionally modify c-Kit proto-oncogene expression levels. The gene targets of miR-193b are MMP19, ARMC1 and ARPC5. miR-193b expression levels are used as a prognostic biomarker as, it’s under expression is an indication of poor prognosis, while its over-expression is a measure of good prognosis [73].
LncRNAs and AML
Table 3 summarizes the effects different lncRNAs have on the pathogenesis of AML. A few of these lncRNAs are also seen to be involved in the pathogenesis of CML as well. The pathways of action of a few of these are explained below.
H19
H19 is located on chromosome 11p15. By regulating IGF2-IGFR1 activity it maintains quiescence of hematopoietic stem cells at the transcriptional and post-transcriptional level. H19 is usually up-regulated in AML and increases tumorigenesis along with cell proliferation. It exhibits pro-proliferative and anti-apoptotic characteristics. In AML, H19 is associated with lower Complete Remission (CR) rate and shorter Overall Survivability (OS). H19 overexpression is also correlated with the sex, age, WBC count, FLT3-ITD and DNMT3 mutation levels of the patients. H19 regulates ID2 expression (the downstream gene of H19), by regulating hsa-miR-19a/b, and this results in increased leukemogenesis [81-83].
PVT1
Acute pro-myelocytic leukemia is a subtype of AML which is formed by blockages in granulocytic differentiation during the pro-myelocytic stages. PVT1 stands for Plasmacytoma Variant Translocation 1 and it is located on chromosome 8q24 along with c-myc. c-Myc knockdown results in down-regulation of PVT1 and reverses the oncogenic effects of up-regulated PVT1. In up-regulated stage it is involved in increasing cell migration and proliferation. It mainly occurs in t(8;21) translocations. It is also known to sponge miR-1204 which is another factor to increase oncogenes in AML cells. It can be used as a prognostic tool [84-87].
RUNXOR
RUNXOR is an intragenic lncRNA that is present upstream of the RUNX1 promoter. It gets its name because of overlapping with the promoter region (RUNX1 overlapping RNA). RUNXOR is up-regulated in AML cells, especially those cells that occur with t(8;21) translocations. Its expression was also increased when treating the cells with Ara-C. It interacts with EZH2 which is a H3-K27 methylase and the RUNX1 proteins via intra-chromosomal loops, and it thereby regulates RUNX1 gene. This leads to regulation of AML pathogenesis and its over-expression increases proliferation of AML cells [88].
Chronic myeloid leukemia
Similar to AML, chronic myeloid leukemia (CML) is a leukemia characterized by the uncontrolled cell division of the myeloid cells that gives rise to a large population of blast cells in the blood. It is a myeloproliferative neoplasm and patients with more than 20% of blast cells are said to be under the blast crisis (BC) stage at which point the leukemia becomes painful, and bone pain and bleeding can occur [119,120]. It mainly affects an older age group, with the median age of 56-57 years, and 20% of cases are specific to 70-year-old individuals [46,121-123]. An abnormal reciprocal chromosomal translocation between chromosomes 9 and 22 (t(9;22)) causes the formation of a minute chromosome known as Philadelphia (Ph) chromosome. This translocation is considered to be one of the causative reasons of CML [124]. The breakpoint cluster region, BCR, is a 5.8kb region on chromosome 22, which when fuses with the ABL1 region on chromosome 9, causes the fusion of different exons on BCR with different exons on ABL1. This results in a chimeric mRNA which then translates to form oncogenic proteins [125-127]. Recently a fusion of BCR-ABL1 has given rise to a different fusion protein which has tyrosine kinase activity, but only for those patients who are positive for Ph+ [128-131]. Research is now focused on targeting this tyrosine kinase in attempts to reduce the pathogenicity of the disease. More information has been given in Tables 4, 5 and 6.
Table 4.
Effects of circRNAs in chronic myeloid leukemia
| circRNA name and ID | Expression | Gene | Effects | miRNA that the circRNA sponges or targets | Does the circRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References |
|---|---|---|---|---|---|---|---|
| circBA9.3 (fusion circRNA) | Upregulated | BCR-ABL1 | Promote cell proliferation, causes TKI resistance due to increased ABL1 expression, causes drug resistance and inhibits apoptosis. | None | Promotes CML formation. | It can be targeted to be used as a therapeutic target. | [45,131] |
| hsa_circ_0080145; hsa_circ_0024002; hsa_circ_0037781 | Upregulated | Sponges the miRNAs involved in inhibiting vascular inflammation, thereby promoting CML pathogenesis. | miR-16, miR-181a, miR-29b | Promotes CML proliferation. | Since hsa_circ_008014 sponges miR-29b it has potential for being a potential prognostic and therapeutic target for curing CML cell lines. | [137] |
Table 5.
Effects of miRNAs in chronic myeloid leukemia
| miRNA name | Expression | Gene targets | Effects | Does the miRNA promote or demote AML formation? | Can it be used as a biomarker or prognostic tool? | References |
|---|---|---|---|---|---|---|
| miR-17-92 | Downregulated | IRF9, RAB10, TXNIP, TET2 | A BCR/ABL-c-MYC-miR-17-92 pathway takes place in CML cell lines. | Upregulation causes increase in CML. | It is being checked as a possible therapeutic agent. | [74,141] |
| miR-21 | Upregulated | TXPAN2, LUM, SUZ12, MSH2, PDZD2 | Antisense inhibition causes induction of apoptosis and inhibits cell growth and migration in CML cells. It also upregulates PDCD4 which is a tumor suppressor gene. | Antisense inhibition of miR-21 decreases CML growth, but in normal cell conditions, miR-21 is oncogenic. | Antisense inhibited miR-21 is being used as a therapeutic agent. | [138] |
| miR-29b | Downregulated | HAS3, SNX24, CD93, SCML2, COL7A1, ZNF396, HMGCR, ICOS | Inhibits cell growth, colony formation, and induces apoptosis of CML cells by inhibiting ABL1 and BCR/ABL1 in overexpressed condition. | It is downregulated in CML cells, but inducing its overexpression leads to decreased CML formation. | Could be used as therapeutic agent. | [140] |
| miR-138 | Downregulated | KLF12, H3F3B, MYO5C, NXN, NEBL, PDPN, STK38 | In overexpressed condition it inhibits cell growth and it is a tumor suppressor of CML cells by inhibiting BCR/ABL1 and CCND3 by binding to their 3’UTR regions. It is downregulated in normal conditions but GATA1 activation and imatinib treatment restores its amount. | Decreases CML formation. | Could be used as a therapeutic target to control CML. | [139] |
| miR-203 | Can become hypermethylated in CML cells | RTKN2, AAK1, MYST4, CD109, IL21, PLD2 | In normal cells miR-203 has tumor suppressor roles. Sometimes it gets hypermethylated in CML cells which leads to increase of BCR/ABL fusion protein thereby causing cellular proliferation of CML cells. | Hypermethylation causes increased CML pathogenesis. | Un hypermethylated miR-203 is used as a therapeutic agent to control CML. | [142] |
| miR-451 | Downregulated | TSC1, ACADSB, GRSF1, MAML1, GDI1, NAMPT | It is associated with BCR/ABL1 activity. | Has a complex relationship in CML pathogenesis. | Further research is required to check its therapeutic effects. | [143] |
| miR-212 | Downregulated | APAF1, EP300, EDNRA, CFL2, NOS1, SOX4, SOX11 | Downregulated miR-212 causes upregulation of ABCG2 protein. ABCG2 is observed in imatinib resistant CML cells. | Downregulated miR-212 causes CML cells to be resistant to imatinib, thereby increasing susceptibility to CML. | Anti-miR-212 can cause downregulation of ABCG2 and can cause imatinib resistant cells to be susceptible to imatinib. | [144] |
Table 6.
Effects of lncRNAs in chronic myeloid leukemia
| lncRNA name | Expression | Effects | Does the lncRNA promote or demote CML formation? | Can it be used as a biomarker or prognostic tool? | References |
|---|---|---|---|---|---|
| HOTAIR | Upregulated | It results in imatinib resistance in CML patients. Its knockdown results in lowering the resistance and better sensitivity to imatinib via a PI3/Akt pathway. | Upregulated condition results in resistance against imatinib, thereby causing CML. | It is used as a therapeutic target to check sensitivity to CML drugs. | [149] |
| H19 | Upregulated | H19 is upregulated in human cancers by the c-Myc transcription factor. This results in cellular transformations. In CML it is upregulated by the Bcr-Abl kinase, resulting in efficient tumor growth and inhibits apoptosis. | Causes CML proliferation. | Further research is being conducted to check the role of hypomethylated H19 as a potential biomarker. | [147,148] |
| HAND2-AS1 | Downregulated | In CML cells, HAND2-AS1 is downregulated. This results in increased miR-1275 which results in cell proliferation. However, upregulation of HAND1-AS2 results in sponging of miR-1275 which inhibits cell proliferation and promotes apoptosis. | It is downregulated in CML cells but its upregulation causes sponging of miR-1275 which results in inhibition of CML proliferation. | Upregulation of HAND2-AS2 is being checked and it can be a potential therapeutic target for combatting CML. | [150] |
| SNHG5 | Upregulated | Overexpression of SNHG5 acts as a CeRNA against miR-205-5p and suppresses it, while overexpression of miR-205-5p suppresses the expression of ABCC2. SNHG5 results in imatinib resistance in CML by regulating ABCC2 via miR-205-5p. It also regulates CML cell proliferation, apoptosis and cell differentiation. | Results in imatinib resistance and aids in CML proliferation. | The role of SNHG5 as a drug inhibitor is being researched and more research is being done to see if it can be targeted as a therapeutic target. | [151,152] |
| NEAT1 | Upregulated | It is essential for forming nuclear body paraspeckles. It is regulated by c-Myc transcription factor. Knockdown of NEAT1 results in imatinib induced apoptosis. Additionally, SFPQ is a NEAT1-binding paraspeckle protein which induces NEAT1 to mediate apoptosis in K562 cells. | Results in proliferation of CML. | Its role as a therapeutic agent is being researched. | [153] |
| MALAT1 | Upregulated | Targets miR-328 and via the lncRNA MALAT1/miR-328 axis it promotes CML cell proliferation and induces imatinib resistance. Silencing of MALAT1 arrests cell cycle of CML cells. | Results in CML proliferation. | Its role as a therapeutic target is being researched. | [146] |
| HULC | Upregulated | Knockdown of HULC inhibits cell proliferation and induces apoptosis by repression of c-Myc and Bcl-2. It’s silencing also results in apoptosis by imatinib treatment, and it suppresses the phosphorylation of PI3K/Akt pathway. It also acts as a endogenous sponge of miR-200. | Results in CML proliferation. | Its role as a potential therapeutic target is being researched. | [154] |
| PLIN2 | Upregulated | CEBPA upregulates PLIN2 which then increases CML tumor growth via the GSK3 and Wnt/β-catenin signaling pathway. PLIN2 increases cell proliferation and inhibits apoptosis. | Increases CML proliferation. | A new CML treatment based on targeting the CEBPA/PLIN2 axis is being researched. | [155] |
| UCA1 | Downregulated | UCA1 acts as a ceRNA of MDR1 and sponges miR-16, which results in increased imatinib resistance, and increases CML proliferation. | Increases CML proliferation. | The UCA1/MDR1 axis is being studied as a potential therapeutic target. | [156] |
| MEG3 | Downregulated | It is downregulated in CML, however its overexpression results in sponging of miR-21 which results in inhibition of CML cell proliferation and induces apoptosis. Additionally, it also regulates miR-147 and the JAK/STAT pathway. | It is downregulated in CML but its overexpression reduces CML proliferation. | Used as a prognostic tool and is observed to be lower in AP and BP CML cases compared to CP stage. | [157-159] |
| FENDRR | Downregulated | FENDRR sponges the RNA binding protein HuR as well as miR-184 which results in attenuation of Adriamycin resistance in CML cells, and results in suppression of tumor growth and increased cell apoptosis rates. | Decreases CML pathogenicity. | FENDRR is a potential target for reversing the roles of Adriamycin resistance and it is being researched as a potential therapeutic target. | [145] |
| BGL3 | Downregulated | BGL3 acts as a ceRNA for binding miR-17, miR-93, miR-20a, miR-20b, miR-106a and miR-106b so that it can cross regulate PTEN expression. BGL3 acts as a tumor suppressor. | It is a tumor suppressor and reduces CML proliferation. | More research is being conducted on its therapeutic and prognostic effects. | [160] |
Circular RNAs and CML
Table 4 summarizes the two types of circRNAs seen to be involved in CML pathogenesis. Both types are up-regulated and result in increased proliferation of CML cells. They have been explained below.
circBA9.3 (fusion circRNA)
A major reason for CML pathogenesis is the unregulated tyrosine kinase activity of BCR-ABL1. circBA9.3 is a fusion circRNA derived from BCR-ABL1. In its up-regulated state it aids in increasing the tyrosine kinase activity of BCR-ABL1. Such patients are resistant to tyrosine kinase inhibitors (TKIs). Up-regulation of circBA9.3 increases the BCR-ABL1 and c-ABL1 expression which increases the proliferation of CML and inhibits cell apoptosis. Targeting the expression levels of this circular RNA and down-regulating it can aid in eliminating CML in TKI resistant patients [132].
hsa_circ_0080145; hsa_circ_0024002; hsa_circ_0037781
hsa_circ_0080145, hsa_circ_0024002 and hsa_circ_0037781 target the leukemia associated miRNAs (miR-16, miR-181a and miR-29b) by a ceRNA pathway. miR-16 is seen to be up-regulated in the peripheral lymphoid. miR-29b is involved in down-regulating the expression of BCR-ABL1, hence it is concerned with malignant hematopoiesis. miR-181b is involved in inhibiting vascular inflammation in human macrophages. Their sponging by these circRNAs results in proliferation of CML and increased pathogenesis. These circRNAs are usually up-regulated in CML and they can be used as potential biomarkers and in CML prognosis [53,133-136].
miRNAs and CML
Table 5 summarizes the effects of different miRNAs on the pathogenesis of CML. Several of these miRNAs are involved in reversing imatinib resistance in cells, while others result in imatinib resistance. The functional pathways of a few miRNAs are discussed below.
miR-21
miR-21 is involved in increased CML cell migration, it inhibits apoptosis and it increases cell growth. It’s inhibition by antisense oligonucleotides (ASOs) are used as therapeutic tools in order to suppress the effects of miR-21. miR-21 is itself up-regulated in tumor cells and they down-regulate the expression of the tumor suppressor gene PDCD4. The gene targets of miR-21 are TXPAN2, LUM, SUZ12, MSH2 and PDZD2 [138].
miR-138
miR-138 is a tumor suppressor, but it is down-regulated in CML pathogenesis conditions. It is up-regulated when the CML cells are treated with imatinib. This results in inhibited cell proliferation, increased apoptosis leading to inhibited colony formation by granulocyte-macrophages. One of the reasons for this is that miR-138 down-regulates BCR-ABL by binding to its coding region, another is it binds to the 3’UTR of CCND3, which also results in its tumor suppression role. Additionally, miR-138 up-regulation results in up-regulation of GATA1 activity which then binds to miR-138 promoter, and in turn up-regulates its activity. All these point to the therapeutic roles miR-138 has in stopping the proliferation of CML [139].
miR-29b
In CML cells it is down-regulated which leads to CML proliferation. It binds to the 3’UTR region of ABL1. By up-regulating it artificially and over-expressing it, CML cells undergo apoptosis by cleavage of procaspase3 and PARP. This happens because the miR-29b binds to the 3’UTR of ABL1 and suppresses its oncogene formation. Hence, up-regulation of miR-29b acts as a tumor suppressor and is used as a therapeutic target for combatting CML proliferation. The gene targets are HAS3, SNX24, CD93, SCML2, COL7A1, ZNF396, HMGCR, and ICOS [140].
LncRNAs and CML
Table 6 summarizes the effects of different lncRNAs on the pathogenesis of CML. They all have different modes of action and they affect different pathways. A few of them have been discussed below.
FENDRR
Adriamycin is a drug used in chemotherapy to combat cancer. However, many patients are resistant to Adriamycin due to the expression of multi drug resistance 1 (MDR1) gene. FENDRR is another gene in the genome which is usually down-regulated in CML cases. However, it has been noted that over-expression of FENDRR results in silencing of MDR1, thereby, increasing Adriamycin sensitivity. This results in increased apoptosis and decreased tumor growth. The mechanism of FENDRR action is discussed here. The 3’UTR of FENDRR and MDR1 contain multiple HuR binding sites. HuR is an RNA binding protein. miR-184 also has biding sites on the 3’UTR of FENDRR. It is observed that miR-184 competes with HuR to bind FENDRR. It was observed that miR-184 positively regulates MDR1 expression via the miR-184/FENDRR/HuR/MDR1 pathway. Hence, up-regulation of FENDRR and downregulation of miR-184 can serve as a therapeutic target by decreasing Adriamycin resistance in CML cells [145].
MALAT1
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is an lncRNA, which is known as being a proto-oncogene. It is up-regulated in CML cells and increases cell proliferation. It is also involved in increased imatinib resistance, which is why targeting and down-regulating it can serve therapeutic effects. Silencing MALAT1 targets the miR-328, which results in arresting the cell cycle of CML cells, thereby preventing CML proliferation (Figure 4). This proves that the MALAT1/miR-328 axis is a crucial pathway that can be targeted to stop CML and increase imatinib sensitivity [146].
H19
H19 is an oncogene and tumor suppressor whose transcription is positively regulated by the c-Myc transcription factor. Knockdown of H19 promotes apoptosis, makes CML cells more susceptible to imatinib and reduces the oncogenic tumor proliferation of BCR-ABL1. One reason for its over-expression in CML is accounted for the hypomethylation of certain differentially methylated regions or imprinting control regions in the H19 locus [147,148].
Analysis of the effects of ncRNAs in AML and CML
Among all of the non-coding RNAs mentioned in this review, several of them are connected in one way or the other. This section will provide a summary and will tie up several connections among the circular, micro and long non-coding RNAs discussed thus far. This can help us to study certain gene loci or ncRNAs which have multi-faceted roles in more detail.
Connections between circRNAs and lncRNAs
The circ-PVT1 and lncPVT1 RNAs, as the name suggests, are derived from the same gene loci, the PVT1 gene present on chromosome 8. Though they are from the same gene loci, their transcription occurs by different promoters [161]. Both are up-regulated and result in the proliferation of AML.
Connections between miRNAs, circRNAs and lncRNAs
The miRNA-125 family consists of three homologs: hsa-miR-125a located on 19q13, hsa-miR-125b-1 located on 11q23 and hsa-miR-125-2b located on 21q21 [162]. This miRNA family is known to be involved in tumour suppression in several leukaemias. The miR-125b in AML acts as a tumour suppressor; the circ-PVT1 RNA and lncRNA UCA1 both increases AML by sponging miR-125 family and miR-125a respectively. The miRNA-181 family is another set of conserved miRNAs that has different subtypes. They are mainly separated along three different genomic clusters, the miR-181a1 and miR-181b1 are located on chromosome 1, miR-181a2 and miR-181b2 are on chromosome 9, and miR-181c and miR-181d are on chromosome 19 [163]. circNPM1 75001 sponges the miR-181 family, however it reduces AML. circ-ANAPC7 also sponges miR-181 family but leads to the proliferation of AML. hsa_circ_0080145, hsa_circ_0024002 and hsa_circ_0037781 circRNAs target miR-181a and miR-181b by ceRNA pathway and result in increased CML pathogenesis. The miR-124 family is also involved in AML formation. miR-124-1 is down-regulated and inhibits AML formation, while the circ-HIPK2 sponges miR-124-3p in AML. The miR-20 family which is encoded from miR-17-92 [164] is present as miR-20a in AML and is down-regulated, which results in decreased AML proliferation leading to increased differentiation. lncRNA BGL3 is down-regulated in CML, but it sponges miR-20a along with miR-20b and decreases CML. circ_0009910 is up-regulated, it sponges miR-20a-5p and increases AML proliferation. miR-138 is down-regulated in CML and over-expression inhibits CML growth. miR-138-5p is sponged by hsa_circ_0004277 and it decreases AML pathogenesis. hsa_circ_0004277 sponges miR-328-3p and regulates miR-328-3p/SH3GL2 axis resulting in decreased AML. MALAT targets miR-328 and results in increased CML proliferation. miR-17 increases AML cell differentiation thereby decreases AML cell proliferation and miR-17-92 family increases CML proliferation, while BGL3 sponges miR-17 and decreases CML proliferation.
LncRNAs involved in pathogenesis of both AML and CML
lncRNA UCA1 is up-regulated in AML leading to increased proliferation, while, lncRNA UCA1 is down-regulated in CML causing higher proliferation. Interestingly, this lncRNA is involved in both AML and CML (Figure 4). All the three, hsa_circ_0080145, hsa_circ_0024002 and hsa_circ_0037781 in CML could sponge miR-16 and result in increased tumorigenesis. lncRNA H19 is up-regulated in both CML and AML, causing increased tumorigenesis in both diseases. However newer research is pointing to H19 tumor suppressive role in AML as well. lncRNA HOTAIR is also up-regulated in both AML and CML leading to increased tumor proliferation in both cases. lncRNA MALAT is up-regulated and causes increased proliferation in both AML and CML. MEG3 is down-regulated in both AML and CML causing reduction in AML proliferation in down-regulated state. However, in CML, its over-expression causes decreased CML proliferation. MEG3 in CML sponges miR-21, which is oncogenic in nature. miR-21 is known to be up-regulated in CML cases and could be used as a prognostic tool. lncRNA NEAT1 is up-regulated in CML and causes increased proliferation of CML, however in AML, it is down-regulated. Up-regulation of NEAT1 in AML causes decrease of AML pathogenesis.
Conclusion
This review has tried to give an overview of the different non-coding RNAs and their effects on the two types of myeloid leukemia. It has focused on the pathway of action of several of them, and has also delved into their prognostic value. Their use as biomarkers and possible therapeutic targets have also been listed and discussed. Figure 3 gives summary of circRNAs, miRNAs, lncRNAs in AML and CML. A statistical overview of the different types of ncRNAs are discussed and the tendency of them to cause the disease. Our interpretation shows that around 67% of the circRNAs mentioned here cause AML, while 100% of the circRNAs mentioned here cause CML. We could conclude that circRNAs in general have a more antagonistic function in myeloid leukemias. In the case of miRNAs, 60% of them result in lowering of AML in patients, while 67% of them cause CML pathogenesis. This can be interpreted as: miRNAs have a positive protagonistic role in AML, but it has an antagonistic role in CML. Finally, when considering lncRNAs, 84% of lncRNAs result in increased AML pathogenesis, while 83% of them cause increased CML pathogenesis. We could assume that lncRNAs have an overall antagonistic effect while causing myeloid tumorigenesis. Several of the circRNAs, miRNAs and lncRNAs are yet to be discovered. We await the day when myeloid tumors can be cured by targeting these ncRNAs.
Figure 3.
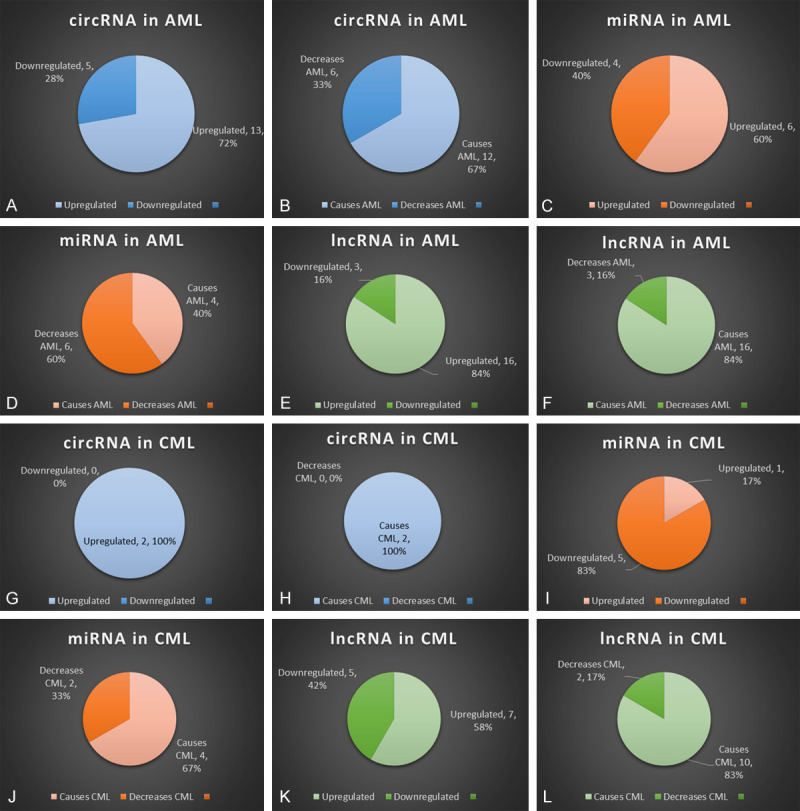
Summary of circRNAs, miRNAs, lncRNAs in AML and CML. The figure shows percentage of downregulated verses upregulated ncRNAs for all three types in AML and CML, of all the different types listed in this review. Panels (A-F) show the analyses pertaining to AML, and panels (G-L) represent analysis for CML. The percentage of downregulated versus upregulated ncRNAs are depicted as pi-charts: (A and G) circRNA; (C and I) miRNA; (E and K) lncRNA. The effects of each ncRNA in terms of whether they cause or decrease AML (B, D, F) or CML (H, J, L) are also shown. Figure also shows percentage of causing the disease versus percentage of not causing the disease in AML and CML.
Acknowledgements
This work was supported by University of Hyderabad-Institution of Eminence Grant (No. UOH-IOE-RC3-21-006), Science and Engineering Research Board (SERB, EEQ/2018/00853), Indian Council of Medical Research (ICMR, 56/5/2019-Nano/BMS), DBT BUILDER and Council of Scientific and Industrial Research (CSIR, No. 27(0343)/19/EMR-II) grants of Government of India. We appreciate the funding in form of the Council of Scientific and Industrial Research (CSIR) and UGC Fellowships from the Government of India.
Disclosure of conflict of interest
None.
References
- 1.Chennamadhavuni A, Lyengar V, Shimanovsky A. StatPearls. Treasure Island (FL): StatPearls Publishing; Leukemia. [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Raghuwanshi S, Dahariya S, Kandi R, Gutti U, Undi RB, Sharma DS, Sahu I, Kovuru N, Yarla NS, Saladi RGV, Gutti RK. Epigenetic mechanisms: role in hematopoietic stem cell lineage commitment and differentiation. Curr Drug Targets. 2018;19:1683–1695. doi: 10.2174/1389450118666171122141821. [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, Link DC. Cellular complexity of the bone marrow hematopoietic stem cell niche. Calcif Tissue Int. 2014;94:112–124. doi: 10.1007/s00223-013-9805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartenstein V. Blood cells and blood cell development in the animal kingdom. Annu Rev Cell Dev Biol. 2006;22:677–712. doi: 10.1146/annurev.cellbio.22.010605.093317. [DOI] [PubMed] [Google Scholar]
- 6.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiskopf K, Schnorr PJ, Pang WW, Chao MP, Chhabra A, Seita J, Feng M, Weissman IL. Myeloid cell origins, differentiation, and clinical implications. Microbiol Spectr. 2016;4:10. doi: 10.1128/microbiolspec.MCHD-0031-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams K, Christensen J, Helin K. DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 2012;13:28–35. doi: 10.1038/embor.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 11.Sanchez-Elsner T, Gou D, Kremmer E, Sauer F. Noncoding RNAs of trithorax response elements recruit drosophila Ash1 to Ultrabithorax. Science. 2006;311:1118–1123. doi: 10.1126/science.1117705. [DOI] [PubMed] [Google Scholar]
- 12.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Wu Y, Zhang J, Sun H, Wang X. Role of miRNA-424 in cancers. Onco Targets Ther. 2020;13:9611–9622. doi: 10.2147/OTT.S266541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 16.Carmell MA, Hannon GJ. RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol. 2004;11:214–218. doi: 10.1038/nsmb729. [DOI] [PubMed] [Google Scholar]
- 17.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 19.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 20.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, Barnes I, Berry A, Bignell A, Carbonell Sala S, Chrast J, Cunningham F, Di Domenico T, Donaldson S, Fiddes IT, García Girón C, Gonzalez JM, Grego T, Hardy M, Hourlier T, Hunt T, Izuogu OG, Lagarde J, Martin FJ, Martínez L, Mohanan S, Muir P, Navarro FCP, Parker A, Pei B, Pozo F, Ruffier M, Schmitt BM, Stapleton E, Suner MM, Sycheva I, Uszczynska-Ratajczak B, Xu J, Yates A, Zerbino D, Zhang Y, Aken B, Choudhary JS, Gerstein M, Guigó R, Hubbard TJP, Kellis M, Paten B, Reymond A, Tress ML, Flicek P. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali T, Grote P. Beyond the RNA-dependent function of LncRNA genes. Elife. 2020;9:e60583. doi: 10.7554/eLife.60583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carninci P, Kasukawa T, Katayama S, Gough J, Frith M, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schönbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y FANTOM Consortium; RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 26.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heesch S, van Iterson M, Jacobi J, Boymans S, Essers PB, de Bruijn E, Hao W, MacInnes AW, Cuppen E, Simonis M. Extensive localization of long noncoding RNAs to the cytosol and mono-and polyribosomal complexes. Genome Biol. 2014;15:R6. doi: 10.1186/gb-2014-15-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, Zeng Z, Xiong W. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. doi: 10.1186/s12943-020-1147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noh JH, Kim KM, McClusky WG, Abdelmohsen K, Gorospe M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip Rev RNA. 2018;9:e1471. doi: 10.1002/wrna.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noh JH, Kim KM, Abdelmohsen K, Yoon JH, Panda AC, Munk R, Kim J, Curtis J, Moad CA, Wohler CM, Indig FE, de Paula W, Dudekula DB, De S, Piao Y, Yang X, Martindale JL, de Cabo R, Gorospe M. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016;30:1224–1239. doi: 10.1101/gad.276022.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao KY, Sun HS, Tsai SJ. Circular RNA-new member of noncoding RNA with novel functions. Exp Biol Med (Maywood) 2017;242:1136–1141. doi: 10.1177/1535370217708978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63. doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503. doi: 10.7150/thno.42174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, Xu RH. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen LS, Andersen MS, Stagsted LV, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 41.Zaiou M. Circular RNAs as potential biomarkers and therapeutic targets for metabolic diseases. Adv Exp Med Biol. 2019;1134:177–191. doi: 10.1007/978-3-030-12668-1_10. [DOI] [PubMed] [Google Scholar]
- 42.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 43.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Böhmdorfer G, Wierzbicki AT. Control of chromatin structure by long noncoding RNA. Trends Cell Biol. 2015;25:623–632. doi: 10.1016/j.tcb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93:1267–1291. doi: 10.1002/ajh.25214. [DOI] [PubMed] [Google Scholar]
- 46.Hehlmann R. Chronic myeloid leukemia in 2020. Hemasphere. 2020;4:e468. doi: 10.1097/HS9.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020;55:S1–S4. doi: 10.5045/br.2020.S001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelcovits A, Niroula R. Acute myeloid leukemia: a review. R I Med J (2013) 2020;103:38–40. [PubMed] [Google Scholar]
- 49.Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2016;127:29–41. doi: 10.1182/blood-2015-07-604496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn AW, Li B, Prouet P, Giri S, Pathak R, Martin MG. Acute megakaryocytic leukemia: what have we learned. Blood Rev. 2016;30:49–53. doi: 10.1016/j.blre.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 51.L’Abbate A, Tolomeo D, Cifola I, Severgnini M, Turchiano A, Augello B, Squeo G, D’Addabbo P, Traversa D, Daniele G, Lonoce A, Pafundi M, Carella M, Palumbo O, Dolnik A, Muehlematter D, Schoumans J, Van Roy N, De Bellis G, Martinelli G, Merla G, Bullinger L, Haferlach C, Storlazzi CT. Correction: MYC-containing amplicons in acute myeloid leukemia: genomic structures, evolution, and transcriptional consequences. Leukemia. 2018;32:2304. doi: 10.1038/s41375-018-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Z, Sun H, Li J, Jin H. Circular RNAs in leukemia. Aging (Albany NY) 2019;11:4757–4771. doi: 10.18632/aging.102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mei M, Wang Y, Li Z, Zhang M. Role of circular RNA in hematological malignancies. Oncol Lett. 2019;18:4385–4392. doi: 10.3892/ol.2019.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirsch S, Blätte TJ, Grasedieck S, Cocciardi S, Rouhi A, Jongen-Lavrencic M, Paschka P, Krönke J, Gaidzik VI, Döhner H, Schlenk RF, Kuchenbauer F, Döhner K, Dolnik A, Bullinger L. Circular RNAs of the nucleophosmin (NPM1) gene in acute myeloid leukemia. Haematologica. 2017;102:2039. doi: 10.3324/haematol.2017.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H, Liu T, Liu J, Feng Y, Wang B, Wang J, Bai J, Zhao W, Shen Y, Wang X, Yang J, Ji Y, He A, Yang Y. Circ-ANAPC7 is upregulated in acute myeloid leukemia and appears to target the MiR-181 family. Cell Physiol Biochem. 2018;47:1998–2007. doi: 10.1159/000491468. [DOI] [PubMed] [Google Scholar]
- 56.Wu DM, Wen X, Han XR, Wang S, Wang YJ, Shen M, Fan SH, Zhang ZF, Shan Q, Li MQ, Hu B, Chen GQ, Lu J, Zheng YL. Role of circular RNA DLEU2 in human acute myeloid leukemia. Mol Cell Biol. 2018;38:e00259–18. doi: 10.1128/MCB.00259-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan H, Li Y, Liu C, Liu Y, Bai J, Li W. Circular RNA-100290 promotes cell proliferation and inhibits apoptosis in acute myeloid leukemia cells via sponging miR-203. Biochem Biophys Res Commun. 2018;507:178–184. doi: 10.1016/j.bbrc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Ping L, Jian-Jun C, Chu-Shu L, Guang-Hua L, Ming Z. Silencing of circ_0009910 inhibits acute myeloid leukemia cell growth through increasing miR-20a-5p. Blood Cells Mol Dis. 2019;75:41–47. doi: 10.1016/j.bcmd.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Shang J, Chen WM, Wang ZH, Wei TN, Chen ZZ, Wu WB. CircPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol. 2019;70:42–54. e43. doi: 10.1016/j.exphem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Wu S, Du Y, Beckford J, Alachkar H. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J Transl Med. 2018;16:1–9. doi: 10.1186/s12967-018-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi YY, Yi J, Zhu X, Zhang J, Zhou J, Tang X, Lin J, Wang P, Deng ZQ. Circular RNA of vimentin expression as a valuable predictor for acute myeloid leukemia development and prognosis. J Cell Physiol. 2019;234:3711–3719. doi: 10.1002/jcp.27145. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Ma Y, Tan Y, Ma X, Zhao M, Chen B, Zhang R, Chen Z, Wang K. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018;9:651. doi: 10.1038/s41419-018-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Zhong C, Jiao J, Li P, Cui B, Ji C, Ma D. Characterization of hsa_circ_0004277 as a new biomarker for acute myeloid leukemia via circular RNA profile and bioinformatics analysis. Int J Mol Sci. 2017;18:597. doi: 10.3390/ijms18030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papaioannou D, Nicolet D, Volinia S, Mrózek K, Yan P, Bundschuh R, Carroll AJ, Kohlschmidt J, Blum W, Powell BL, Uy GL, Kolitz JE, Wang ES, Eisfeld AK, Orwick SJ, Lucas DM, Caligiuri MA, Stone RM, Byrd JC, Garzon R, Bloomfield CD. Prognostic and biologic significance of long non-coding RNA profiling in younger adults with cytogenetically normal acute myeloid leukemia. Haematologica. 2017;102:1391–1400. doi: 10.3324/haematol.2017.166215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH, Pandolfi PP. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 66.Bonizzato A, Gaffo E, Te Kronnie G, Bortoluzzi S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. 2016;6:e483. doi: 10.1038/bcj.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamal M, Song T, Chen B, Faisal M, Hong Z, Xie T, Wu Y, Pan S, Yin Q, Shao L, Zhang Q. Recent progress on circular RNA research in acute myeloid leukemia. Front Oncol. 2019;9:1108. doi: 10.3389/fonc.2019.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greene J, Baird AM, Casey O, Brady L, Blackshields G, Lim M, O’Brien O, Gray SG, McDermott R, Finn SP. Circular RNAs are differentially expressed in prostate cancer and are potentially associated with resistance to enzalutamide. Sci Rep. 2019;9:10739. doi: 10.1038/s41598-019-47189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun YM, Wang WT, Zeng ZC, Chen TQ, Han C, Pan Q, Huang W, Fang K, Sun LY, Zhou YF, Luo XQ, Luo C, Du X, Chen YQ. circMYBL2, a circRNA from MYBL2, regulates FLT3 translation by recruiting PTBP1 to promote FLT3-ITD AML progression. Blood. 2019;134:1533–1546. doi: 10.1182/blood.2019000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Lu J, Sun M, Mi S, Zhang H, Luo RT, Chen P, Wang Y, Yan M, Qian Z, Neilly MB, Jin J, Zhang Y, Bohlander SK, Zhang DE, Larson RA, Le Beau MM, Thirman MJ, Golub TR, Rowley JD, Chen J. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc Natl Acad Sci U S A. 2008;105:15535–15540. doi: 10.1073/pnas.0808266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He M, Wang QY, Yin QQ, Tang J, Lu Y, Zhou CX, Duan CW, Hong DL, Tanaka T, Chen GQ, Zhao Q. HIF-1α downregulates miR-17/20a directly targeting p21 and STAT3: a role in myeloid leukemic cell differentiation. Cell Death Differ. 2013;20:408–418. doi: 10.1038/cdd.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao XN, Lin J, Gao L, Li YH, Wang LL, Yu L. MicroRNA-193b regulates c-Kit proto-oncogene and represses cell proliferation in acute myeloid leukemia. Leuk Res. 2011;35:1226–1232. doi: 10.1016/j.leukres.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Karnati HK, Raghuwanshi S, Sarvothaman S, Gutti U, Saladi RGV, Komati JK, Tummala PR, Gutti RK. microRNAs: key players in hematopoiesis. microRNA: basic science. Springer; 2015. pp. 171–211. [DOI] [PubMed] [Google Scholar]
- 75.Eyholzer M, Schmid S, Schardt JA, Haefliger S, Mueller BU, Pabst T. Complexity of miR-223 regulation by CEBPA in human AML. Leuk Res. 2010;34:672–676. doi: 10.1016/j.leukres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Chen XX, Lin J, Qian J, Qian W, Yang J, Ma JC, Deng ZQ, Xie D, An C, Tang CY. Dysregulation of miR-124-1 predicts favorable prognosis in acute myeloid leukemia. Clin Biochem. 2014;47:63–66. doi: 10.1016/j.clinbiochem.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 77.Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, Chen SS, Blagitko-Dorfs N, Maharry K, Whitman SP, Schmittgen TD, Lübbert M, Marcucci G, Bloomfield CD, Plass C. Epigenetic modification of CCAAT/enhancer binding protein α expression in acute myeloid leukemia. Cancer Res. 2008;68:3142–3151. doi: 10.1158/0008-5472.CAN-08-0483. [DOI] [PubMed] [Google Scholar]
- 78.Cammarata G, Augugliaro L, Salemi D, Agueli C, Rosa ML, Dagnino L, Civiletto G, Messana F, Marfia A, Bica MG, Cascio L, Floridia PM, Mineo AM, Russo M, Fabbiano F, Santoro A. Differential expression of specific microRNA and their targets in acute myeloid leukemia. Am J Hematol. 2010;85:331–339. doi: 10.1002/ajh.21667. [DOI] [PubMed] [Google Scholar]
- 79.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, Havelange V, Volinia S, Blum W, Rush LJ, Perrotti D, Andreeff M, Bloomfield CD, Byrd JC, Chan K, Wu LC, Croce CM, Marcucci G. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coskun E, von der Heide EK, Schlee C, Kühnl A, Gökbuget N, Hoelzer D, Hofmann WK, Thiel E, Baldus CD. The role of microRNA-196a and microRNA-196b as ERG regulators in acute myeloid leukemia and acute T-lymphoblastic leukemia. Leuk Res. 2011;35:208–213. doi: 10.1016/j.leukres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Gao J, Wang F, Wu P, Chen Y, Jia Y. Aberrant LncRNA expression in leukemia. J Cancer. 2020;11:4284–4296. doi: 10.7150/jca.42093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang TJ, Zhou JD, Zhang W, Lin J, Ma JC, Wen XM, Yuan Q, Li XX, Xu ZJ, Qian J. H19 overexpression promotes leukemogenesis and predicts unfavorable prognosis in acute myeloid leukemia. Clin Epigenetics. 2018;10:47. doi: 10.1186/s13148-018-0486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao TF, Jia HZ, Zhang ZZ, Zhao XS, Zou YF, Zhang W, Wan J, Chen XF. LncRNA H19 regulates ID2 expression through competitive binding to hsa-miR-19a/b in acute myelocytic leukemia. Mol Med Rep. 2017;16:3687–3693. doi: 10.3892/mmr.2017.7029. [DOI] [PubMed] [Google Scholar]
- 84.El-Khazragy N, Elayat W, Matbouly S, Seliman S, Sami A, Safwat G, Diab A. The prognostic significance of the long non-coding RNAs “CCAT1, PVT1” in t(8; 21) associated acute myeloid leukemia. Gene. 2019;707:172–177. doi: 10.1016/j.gene.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 85.Salehi M, Sharifi M, Bagheri M. Knockdown of long noncoding RNA plasmacytoma variant translocation 1 with antisense locked nucleic acid GapmeRs exerts tumor-suppressive functions in human acute erythroleukemia cells through downregulation of C-MYC expression. Cancer Biother Radiopharm. 2019;34:371–379. doi: 10.1089/cbr.2018.2510. [DOI] [PubMed] [Google Scholar]
- 86.Salehi M, Sharifi M. Induction of apoptosis and necrosis in human acute erythroleukemia cells by inhibition of long non-coding RNA PVT1. Mol Biol Res Commun. 2018;7:89–96. doi: 10.22099/mbrc.2018.29081.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng C, Yu X, Lai J, Yang L, Chen S, Li Y. Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J Hematol Oncol. 2015;8:126. doi: 10.1186/s13045-015-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Li W, Guo R, Sun J, Cui J, Wang G, Hoffman AR, Hu JF. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int J Cancer. 2014;135:2783–2794. doi: 10.1002/ijc.28922. [DOI] [PubMed] [Google Scholar]
- 89.Hao S, Shao Z. HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int J Clin Exp Pathol. 2015;8:7223–8. [PMC free article] [PubMed] [Google Scholar]
- 90.Wu S, Zheng C, Chen S, Cai X, Shi Y, Lin B, Chen Y. Overexpression of long non‑coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemia. Oncol Lett. 2015;10:2410–2414. doi: 10.3892/ol.2015.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang YY, Huang SH, Zhou HR, Chen CJ, Tian LH, Shen JZ. Role of HOTAIR in the diagnosis and prognosis of acute leukemia. Oncol Rep. 2016;36:3113–3122. doi: 10.3892/or.2016.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY, Bin-Zhou , Wu JB, Tang LY, Gao SM. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–1987. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 93.Gao S, Zhou B, Li H, Huang X, Wu Y, Xing C, Yu X, Ji Y. Long noncoding RNA HOTAIR promotes the self-renewal of leukemia stem cells through epigenetic silencing of p15. Exp Hematol. 2018;67:32–40. e33. doi: 10.1016/j.exphem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Sun M, Zheng Y, Wang L, Zhao H, Yang S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur Rev Med Pharmacol Sci. 2018;22:2233–2245. doi: 10.26355/eurrev_201804_14809. [DOI] [PubMed] [Google Scholar]
- 95.Hughes JM, Legnini I, Salvatori B, Masciarelli S, Marchioni M, Fazi F, Morlando M, Bozzoni I, Fatica A. C/EBPα-p30 protein induces expression of the oncogenic long non-coding RNA UCA1 in acute myeloid leukemia. Oncotarget. 2015;6:18534–44. doi: 10.18632/oncotarget.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Liu Y, Xu X. Knockdown of LncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J Cell Biochem. 2018;119:6296–6308. doi: 10.1002/jcb.26899. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Zhou Q, Ma JJ. High expression of lnc-CRNDE presents as a biomarker for acute myeloid leukemia and promotes the malignant progression in acute myeloid leukemia cell line U937. Eur Rev Med Pharmacol Sci. 2018;22:763–770. doi: 10.26355/eurrev_201802_14310. [DOI] [PubMed] [Google Scholar]
- 98.Yang L, Zhou JD, Zhang TJ, Ma JC, Xiao GF, Chen Q, Deng ZQ, Lin J, Qian J, Yao DM. Overexpression of lncRNA PANDAR predicts adverse prognosis in acute myeloid leukemia. Cancer Manag Res. 2018;10:4999. doi: 10.2147/CMAR.S180150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Wang Y, Kong B, Langerød A, Børresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L, Wang W, Cao L, Li Z, Wang X. Long non-coding RNA CCAT1 acts as a competing endogenous RNA to regulate cell growth and differentiation in acute myeloid leukemia. Mol Cells. 2016;39:330–6. doi: 10.14348/molcells.2016.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q, Song W, Wang J. TUG1 confers Adriamycin resistance in acute myeloid leukemia by epigenetically suppressing miR-34a expression via EZH2. Biomed Pharmacother. 2019;109:1793–1801. doi: 10.1016/j.biopha.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Zhang L, Zhao F, Xu R, Jiang J, Zhang C, Liu H, Huang H. Long non-coding RNA taurine-upregulated gene 1 correlates with poor prognosis, induces cell proliferation, and represses cell apoptosis via targeting aurora kinase A in adult acute myeloid leukemia. Ann Hematol. 2018;97:1375–1389. doi: 10.1007/s00277-018-3315-8. [DOI] [PubMed] [Google Scholar]
- 103.Chen C, Wang P, Mo W, Zhang Y, Zhou W, Deng T, Zhou M, Chen X, Wang S, Wang C. lncRNA‑CCDC26, as a novel biomarker, predicts prognosis in acute myeloid leukemia. Oncol Lett. 2019;18:2203–2211. doi: 10.3892/ol.2019.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirano T, Yoshikawa R, Harada H, Harada Y, Ishida A, Yamazaki T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer. 2015;14:90. doi: 10.1186/s12943-015-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Emmrich S, Streltsov A, Schmidt F, Thangapandi VR, Reinhardt D, Klusmann JH. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol Cancer. 2014;13:171. doi: 10.1186/1476-4598-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao H, Zhang X, Frazão JB, Condino-Neto A, Newburger PE. HOX antisense lincRNA HOXA-AS2 is an apoptosis repressor in all trans retinoic acid treated NB4 promyelocytic leukemia cells. J Cell Biochem. 2013;114:2375–2383. doi: 10.1002/jcb.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dong X, Fang Z, Yu M, Zhang L, Xiao R, Li X, Pan G, Liu J. Knockdown of long noncoding RNA HOXA-AS2 suppresses chemoresistance of acute myeloid leukemia via the miR-520c-3p/S100A4 axis. Cell Physiol Biochem. 2018;51:886–896. doi: 10.1159/000495387. [DOI] [PubMed] [Google Scholar]
- 108.Hu N, Chen L, Wang C, Zhao H. MALAT1 knockdown inhibits proliferation and enhances cytarabine chemosensitivity by upregulating miR-96 in acute myeloid leukemia cells. Biomed Pharmacother. 2019;112:108720. doi: 10.1016/j.biopha.2019.108720. [DOI] [PubMed] [Google Scholar]
- 109.Huang JL, Liu W, Tian LH, Chai TT, Liu Y, Zhang F, Fu HY, Zhou HR, Shen JZ. Upregulation of long non-coding RNA MALAT-1 confers poor prognosis and influences cell proliferation and apoptosis in acute monocytic leukemia. Oncol Rep. 2017;38:1353–1362. doi: 10.3892/or.2017.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lyu Y, Lou J, Yang Y, Feng J, Hao Y, Huang S, Yin L, Xu J, Huang D, Ma B, Zou D, Wang Y, Zhang Y, Zhang B, Chen P, Yu K, Lam EW, Wang X, Liu Q, Yan J, Jin B. Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and-independent pathways. Leukemia. 2017;31:2543–2551. doi: 10.1038/leu.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benetatos L, Hatzimichael E, Dasoula A, Dranitsaris G, Tsiara S, Syrrou M, Georgiou I, Bourantas KL. CpG methylation analysis of the MEG3 and SNRPN imprinted genes in acute myeloid leukemia and myelodysplastic syndromes. Leuk Res. 2010;34:148–153. doi: 10.1016/j.leukres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 112.Fernando TR, Contreras JR, Zampini M, Rodriguez-Malave NI, Alberti MO, Anguiano J, Tran TM, Palanichamy JK, Gajeton J, Ung NM, Aros CJ, Waters EV, Casero D, Basso G, Pigazzi M, Rao DS. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol Cancer. 2017;16:1–15. doi: 10.1186/s12943-017-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun J, Li W, Sun Y, Yu D, Wen X, Wang H, Cui J, Wang G, Hoffman AR, Hu JF. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42:9588–9601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pashaiefar H, Izadifard M, Yaghmaie M, Montazeri M, Gheisari E, Ahmadvand M, Momeny M, Ghaffari SH, Kasaeian A, Alimoghaddam K, Ghavamzadeh A. Low expression of long noncoding RNA IRAIN is associated with poor prognosis in non-M3 acute myeloid leukemia patients. Genet Test Mol Biomarkers. 2018;22:288–294. doi: 10.1089/gtmb.2017.0281. [DOI] [PubMed] [Google Scholar]
- 115.Zhao C, Wang S, Zhao Y, Du F, Wang W, Lv P, Qi L. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J Cell Physiol. 2019;234:6161–6172. doi: 10.1002/jcp.27393. [DOI] [PubMed] [Google Scholar]
- 116.Bug G, Ritter M, Wassmann B, Schoch C, Heinzel T, Schwarz K, Romanski A, Kramer OH, Kampfmann M, Hoelzer D, Neubauer A, Ruthardt M, Ottmann OG. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer. 2005;104:2717–2725. doi: 10.1002/cncr.21589. [DOI] [PubMed] [Google Scholar]
- 117.Lei L, Xia S, Liu D, Li X, Feng J, Zhu Y, Hu J, Xia L, Guo L, Chen F. Genome-wide characterization of lncRNAs in acute myeloid leukemia. Brief Bioinform. 2018;19:627–635. doi: 10.1093/bib/bbx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang FY, Gu ZY, Gao CJ. Emerging role of long non-coding RNAs in normal and malignant hematopoiesis. Chin Med J (Engl) 2020;133:462. doi: 10.1097/CM9.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Minciacchi VR, Kumar R, Krause DS. Chronic myeloid leukemia: a model disease of the past, present and future. Cells. 2021;10:117. doi: 10.3390/cells10010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 121.Hoffmann V, Baccarani M, Hasford J, Lindörfer D, Burgstaller S, Sertic D, Costeas P, Mayer J, Indrak K, Everaus H, Koskenvesa P, Guilhot J, Schubert-Fritschle G, Castagnetti F, Di Raimondo F, Lejniece S, Griskevicius L, Thielen N, Sacha T, Hellmann A, Turkina AG, Zaritskey A, Bogdanovic A, Sninska Z, Zupan I, Steegmann JL, Simonsson B, Clark RE, Covelli A, Guidi G, Hehlmann R. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European countries. Leukemia. 2015;29:1336–1343. doi: 10.1038/leu.2015.73. [DOI] [PubMed] [Google Scholar]
- 122.Hehlmann R, Cortes JE, Zyczynski T, Gambacorti-Passerini C, Goldberg SL, Mauro MJ, Michallet M, Simonsson B, Williams LA, Gajavelli S, DeGutis I, Sen GP, Paquette RL. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronic-phase chronic myeloid leukemia in routine clinical practice: simplicity. Am J Hematol. 2019;94:46–54. doi: 10.1002/ajh.25306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malhotra H, Radich J, Garcia-Gonzalez P. Meeting the needs of CML patients in resource-poor countries. Hematology Am Soc Hematol Educ Program. 2019;2019:433–442. doi: 10.1182/hematology.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 125.Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, BCR, on chromosome 22. Cell. 1984;36:93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- 126.Heisterkamp N, Stephenson JR, Groffen J, Hansen PF, de Klein A, Bartram CR, Grosveld G. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983;306:239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- 127.Bartram CR, de Klein A, Hagemeijer A, Grosveld G, Heisterkamp N, Groffen J. Localization of the human c-sis oncogene in Ph1-positive and Ph1-negative chronic myelocytic leukemia by in situ hybridization. Blood. 1984;63:223–5. [PubMed] [Google Scholar]
- 128.Jain P, Kantarjian H, Patel KP, Gonzalez GN, Luthra R, Shamanna RK, Sasaki K, Jabbour E, Romo CG, Kadia TM, Pemmaraju N, Daver N, Borthakur G, Estrov Z, Ravandi F, O’Brien S, Cortes J. Impact of BCR-ABL transcript type on outcome in patients with chronic-phase CML treated with tyrosine kinase inhibitors. Blood. 2016;127:1269–1275. doi: 10.1182/blood-2015-10-674242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 130.Stam K, Heisterkamp N, Grosveld G, de Klein A, Verma RS, Coleman M, Dosik H, Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N Engl J Med. 1985;313:1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- 131.Konopka JB, Watanabe SM, Witte ON. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984;37:1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 132.Pan Y, Lou J, Wang H, An N, Chen H, Zhang Q, Du X. CircBA9. 3 supports the survival of leukaemic cells by up-regulating c-ABL1 or BCR-ABL1 protein levels. Blood Cells Mol Dis. 2018;73:38–44. doi: 10.1016/j.bcmd.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 133.Liu J, Kong F, Lou S, Yang D, Gu L. Global identification of circular RNAs in chronic myeloid leukemia reveals hsa_circ_0080145 regulates cell proliferation by sponging miR-29b. Biochem Biophys Res Commun. 2018;504:660–665. doi: 10.1016/j.bbrc.2018.08.154. [DOI] [PubMed] [Google Scholar]
- 134.Musolino C, Oteri G, Allegra A, Mania M, D’Ascola A, Avenoso A, Innao V, Allegra AG, Campo S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann Hematol. 2018;97:1259–1269. doi: 10.1007/s00277-018-3296-7. [DOI] [PubMed] [Google Scholar]
- 135.Du XJ, Lu JM, Sha Y. MiR-181a inhibits vascular inflammation induced by ox-LDL via targeting TLR4 in human macrophages. J Cell Physiol. 2018;233:6996–7003. doi: 10.1002/jcp.26622. [DOI] [PubMed] [Google Scholar]
- 136.Litwińska Z, Machaliński B. miRNAs in chronic myeloid leukemia: small molecules, essential function. Leuk Lymphoma. 2017;58:1297–1305. doi: 10.1080/10428194.2016.1243676. [DOI] [PubMed] [Google Scholar]
- 137.Liu J, Kong F, Lou S, Yang D, Gu L. Global identification of circular RNAs in chronic myeloid leukemia reveals hsa_circ_0080145 regulates cell proliferation by sponging miR-29b. Biochem Biophys Res Commun. 2018;504:660–665. doi: 10.1016/j.bbrc.2018.08.154. [DOI] [PubMed] [Google Scholar]
- 138.Hu H, Li Y, Gu J, Zhu X, Dong D, Yao J, Lin C, Fei J. Antisense oligonucleotide against miR-21 inhibits migration and induces apoptosis in leukemic K562 cells. Leuk Lymphoma. 2010;51:694–701. doi: 10.3109/10428191003596835. [DOI] [PubMed] [Google Scholar]
- 139.Xu C, Fu H, Gao L, Wang L, Wang W, Li J, Li Y, Dou L, Gao X, Luo X, Jing Y, Chim CS, Zheng X, Yu L. BCR-ABL/GATA1/miR-138 mini circuitry contributes to the leukemogenesis of chronic myeloid leukemia. Oncogene. 2014;33:44–54. doi: 10.1038/onc.2012.557. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Wang H, Tao K, Xiao Q, Huang Z, Zhong L, Cao W, Wen J, Feng W. miR-29b suppresses CML cell proliferation and induces apoptosis via regulation of BCR/ABL1 protein. Exp Cell Res. 2013;319:1094–1101. doi: 10.1016/j.yexcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU, Ganser A, Eder M, Scherr M. Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood. 2007;109:4399–4405. doi: 10.1182/blood-2006-09-045104. [DOI] [PubMed] [Google Scholar]
- 142.Chim CS, Wong KY, Leung CY, Chung LP, Hui PK, Chan SY, Yu L. Epigenetic inactivation of the hsa-miR-203 in haematological malignancies. J Cell Mol Med. 2011;15:2760–2767. doi: 10.1111/j.1582-4934.2011.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lopotová T, Žáčková M, Klamová H, Moravcová J. MicroRNA-451 in chronic myeloid leukemia: miR-451-BCR-ABL regulatory loop? Leuk Res. 2011;35:974–977. doi: 10.1016/j.leukres.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 144.Turrini E, Haenisch S, Laechelt S, Diewock T, Bruhn O, Cascorbi I. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG2 expression. Pharmacogenet Genomics. 2012;22:198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- 145.Zhang F, Ni H, Li X, Liu H, Xi T, Zheng L. Lnc RNA FENDRR attenuates adriamycin resistance via suppressing MDR 1 expression through sponging HuR and miR-184 in chronic myelogenous leukaemia cells. FEBS Lett. 2019;593:1993–2007. doi: 10.1002/1873-3468.13480. [DOI] [PubMed] [Google Scholar]
- 146.Wen F, Cao YX, Luo ZY, Liao P, Lu ZW. LncRNA MALAT1 promotes cell proliferation and imatinib resistance by sponging miR-328 in chronic myelogenous leukemia. Biochem Biophys Res Commun. 2018;507:1–8. doi: 10.1016/j.bbrc.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 147.Guo G, Kang Q, Chen Q, Chen Z, Wang J, Tan L, Chen JL. High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett. 2014;588:1780–1786. doi: 10.1016/j.febslet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 148.Zhou JD, Lin J, Zhang TJ, Ma JC, Li XX, Wen XM, Guo H, Xu ZJ, Deng ZQ, Zhang W, Qian J. Hypomethylation-mediated H19 overexpression increases the risk of disease evolution through the association with BCR-ABL transcript in chronic myeloid leukemia. J Cell Physiol. 2018;233:2444–2450. doi: 10.1002/jcp.26119. [DOI] [PubMed] [Google Scholar]
- 149.Wang H, Li Q, Tang S, Li M, Feng A, Qin L, Liu Z, Wang X. The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematology. 2017;22:208–216. doi: 10.1080/10245332.2016.1258152. [DOI] [PubMed] [Google Scholar]
- 150.Yang J, Shi M, Zeng Y. LncRNA HAND2-AS1 inhibits proliferation and promotes apoptosis of chronic myeloid leukemia cells by sponging with micRNA-1275. Eur Rev Med Pharmacol Sci. 2019;23:2103–2111. doi: 10.26355/eurrev_201903_17254. [DOI] [PubMed] [Google Scholar]
- 151.He B, Bai Y, Kang W, Zhang X, Jiang X. LncRNA SNHG5 regulates imatinib resistance in chronic myeloid leukemia via acting as a CeRNA against MiR-205-5p. Am J Cancer Res. 2017;7:1704–1713. [PMC free article] [PubMed] [Google Scholar]
- 152.Gao B, Li S, Li G. Long noncoding RNA (lncRNA) small nucleolar RNA host gene 5 (SNHG5) regulates proliferation, differentiation, and apoptosis of K562 cells in chronic myeliod leukemia. Med Sci Monit. 2019;25:6812. doi: 10.12659/MSM.916661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng C, Liu S, Lu S, Yu X, Lai J, Wu Y, Chen S, Wang L, Yu Z, Luo G. The c-Myc-regulated lncRNA NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML cells. Mol Cancer. 2018;17:130. doi: 10.1186/s12943-018-0884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu Y, Li Y, Chai X, Kang Q, Zhao P, Xiong J, Wang J. Long noncoding RNA HULC promotes cell proliferation by regulating PI3K/AKT signaling pathway in chronic myeloid leukemia. Gene. 2017;607:41–46. doi: 10.1016/j.gene.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 155.Sun C, Luan S, Zhang G, Wang N, Shao H, Luan C. CEBPA-mediated upregulation of the lncRNA PLIN2 promotes the development of chronic myelogenous leukemia via the GSK3 and Wnt/β-catenin signaling pathways. Am J Cancer Res. 2017;7:1054–1067. [PMC free article] [PubMed] [Google Scholar]
- 156.Xiao Y, Jiao C, Lin Y, Chen M, Zhang J, Wang J, Zhang Z. lncRNA UCA1 contributes to imatinib resistance by acting as a ceRNA against miR-16 in chronic myeloid leukemia cells. DNA Cell Biol. 2017;36:18–25. doi: 10.1089/dna.2016.3533. [DOI] [PubMed] [Google Scholar]
- 157.Li Z, Yang L, Liu X, Nie Z, Luo J. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed Pharmacother. 2018;104:181–192. doi: 10.1016/j.biopha.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 158.Li ZY, Yang L, Liu XJ, Wang XZ, Pan YX, Luo JM. The long noncoding RNA MEG3 and its target miR-147 regulate JAK/STAT pathway in advanced chronic myeloid leukemia. EBioMedicine. 2018;34:61–75. doi: 10.1016/j.ebiom.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou X, Yuan P, Liu Q, Liu Z. LncRNA MEG3 regulates imatinib resistance in chronic myeloid leukemia via suppressing microRNA-21. Biomol Ther (Seoul) 2017;25:490. doi: 10.4062/biomolther.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guo G, Kang Q, Zhu X, Chen Q, Wang X, Chen Y, Ouyang J, Zhang L, Tan H, Chen R, Huang S, Chen JL. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene. 2015;34:1768–1779. doi: 10.1038/onc.2014.131. [DOI] [PubMed] [Google Scholar]
- 161.Adhikary J, Chakraborty S, Dalal S, Basu S, Dey A, Ghosh A. Circular PVT1: an oncogenic non-coding RNA with emerging clinical importance. J Clin Pathol. 2019;72:513–519. doi: 10.1136/jclinpath-2019-205891. [DOI] [PubMed] [Google Scholar]
- 162.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Concepcion CP, Bonetti C, Ventura A. The miR-17-92 family of microRNA clusters in development and disease. Cancer J. 2012;18:262. doi: 10.1097/PPO.0b013e318258b60a. [DOI] [PMC free article] [PubMed] [Google Scholar]