Abstract
Myocardial ischemia-reperfusion injury (MIRI) is a complicated pathologic process that involves multiple factors including oxidative stress (free radical damage), inflammatory response, calcium overloading, and apoptosis in cardiomyocytes. According to Traditional Chinese Medicine (TCM), MIRI belongs to the categories of “chest numbness”, “palpitations” and “angina pectoris”. Present data indicate that the application of TCM in myocardial ischemia-reperfusion injury is promising and continues to attract research attention. While the efficacy of Chinese herbal medicine has been well-proven, the underlying molecular mechanisms remain elusive. The common proven mechanisms of Chinese herbal medicine in the treatment of MIRI include regulating lipid metabolism, protecting mitochondria, and improving energy metabolism, attenuating calcium (Ca2+) overload, scavenging oxygen free radicals, inhibiting apoptosis, and reducing autophagy. Others are the regulation of inflammatory cytokine expressions and healing of inflammatory lesions, modulation of cell signaling pathways, improvement of endothelial cell function, and protection of myocardial cells. In this review, we highlight recent studies that focus on elucidating these molecular mechanisms and the therapeutic effects of natural compounds deriving from TCM in MIRI, to ascertain the research progress made and the prospects in this field.
Keywords: Myocardial ischemia-reperfusion injury, traditional Chinese medicine therapy, ingredients of traditional Chinese medicine, compound Chinese medicine
Introduction
In the last 10 years, the improvement of people’s living standards and the acceleration of population aging have also increased the prevalence of cardiovascular disease in China, where rural cardiovascular disease mortality has been more than urban mortality [1,2]. With the occurrence of Percutaneous Transluminal Coronary Angioplasty (PTCA), Percutaneous Transluminal Coronary Intervention (PCI) interventional therapy, intravenous thrombolysis, and coronary artery bypass graft, a series of phenomena, such as increased myocardial infarction area, arrhythmia, and persistent low ventricular systolic function may occur while rapidly restoring coronary blood flow and saving dying heart muscle at the same time [3]. Therefore, reperfusion therapy is also a “double-edged sword”, which has become the main problem of preventing the ischemic myocardium from obtaining the best efficacy from reperfusion therapy. In this phenomenon, cellular metabolic dysfunction, tissue structural injury, and even irreversible injury occur after the restoration of blood supply on the basis of myocardial ischemia is called myocardial ischemia-reperfusion injury (MIRI). This kind of injury can induce myocardial cell apoptosis, myocardial damage, no re-flow phenomenon, and microvascular endothelial injury, which directly affect the prognosis of patients and greatly increase the follow-up cost of patients.
MIRI mechanisms are complicated and mainly related to inflammation, intracellular Ca2+ overload, oxygen-free radicals (OFR), neutrophil infiltration, energy metabolic disorders, vascular endothelial cell dysfunction, and myocardial cell apoptosis. These events cause damage to the myocardial intima, and induce abnormal mitochondrial metabolism, formation of intima plaque, and ventricular remodeling, resulting in the increased myocardial ischemic infarct area and reduced cardiac function [4]. Clinical treatment is mainly focused on inhibiting oxidative stress, inflammatory response, calcium overload, and apoptosis damage to improve body metabolism [5]. Due to the holistic treatment concept and advantages of multi-target, multi-link, and multi-approach, TCM has been widely valued in recent years. Several animal experiments and clinical studies have demonstrated that TCM can inhibit myocardial injury caused by ischemia-reperfusion.
Based on the latest research progress, this paper discusses the intervention of TCM and its main components on MIRI from the perspectives of single Chinese herbal extracts effects on apoptosis, necroptosis, autophagy, and pyroptosis, as well as treatment with Chinese herbal compound, providing documented evidence and prospects for the prevention and treatment of MIRI.
Etiology, pathogenesis, and dialectical type of MIRI
Although there is no direct classification of MIRI in TCM, considering that its lesion is located in the heart and its clinical manifestations are chest pain, chest tightness, breath shortness, fatigue, and sense of weakness, the disease therefore belongs to the category of “Chest Obstruction”, “Palpitation” and “True Heart Pain” in TCM. Associated knowledge has been recorded in the Yellow Emperor’s Canon of Medicine written two thousand years ago. For example, in Chapter 22 of Plain Conversation: Discussion on the Association of the Zang-Qi with the Four Seasons, Qibo replied that heart disease is characterized by pain in the chest, distending hypochondriac fullness, hypochondriac pain, pain involving the chest, back, and scapula as well as the pain of the medial sides of the arms [6]. Chapter 20 of Spiritual Pivot: Five Pathogen recorded that if the pathogen is in the heart, then the disease would be manifested by heart pain.
Etiology and pathogenesis of MIRI
The TCM discussions on the etiology and pathogenesis of MIRI are widely recorded. Zhang Zhongjing, known as the medical sage, has recorded in his work Synopsis of Prescriptions of the Golden Chamber: Chapter 9-On Pulse, Symptom Complex and Treatment of Chest Obstruction, Heart Pain and Shortness of Breath that Chest Obstruction syndrome has the symptoms and signs of panting, coughing, spitting, shortness of breath, and pain in the chest and back. The pulse is deep and slow in the Inch and slender-tense-speedy in the Bar [7]. Here, Zhang illustrated the pathogenesis according to the change of pulse condition. The Plain Conversation: Chapter 43-Discussion on Obstructive Syndrome states that Obstructive Syndrome of the heart leads to obstruction of the channels, dysphoria, throb of the heart, sudden panting, dry throat, eructation, and fear due to adverse flow of Qi [6]. The Comprehensive Recording of Divine Assistance: Chapter 55-Heart Pain indicates that Heart pain is caused by the pathogen retention in the pericardium channel of Hand-Jueyin and it is either caused by improper diet or wind attack, with the pathogen retained in the deficient Zang viscera and the channel blocked, thus resulting in the pain. In the Prescriptions for Succouring the Sick, it is recorded that Palpitation indicates heart blood insufficiency. Danxi’s experiential therapy has also proposed that Asthenia and phlegm are the cause of palpitation. Moreover, Correction on errors in medical classics: Palpitation states that internal retention of blood stasis is related to the occurrence of MIRI.
In summary, the pathogenesis of MIRI falls into the category of heart vessel obstruction, where the pain is either caused by obstruction or malnourishment. The characteristics of the disease follow the pattern of deficiency in origin and enrichment in symptom, with the Yang-Qi deficiency in the heart, spleen, and kidney as the original cause, the cold stagnation, blood stasis, and turbid phlegm as the superficial cause and the mixture of phlegm and stasis as the direct cause. After a patient experiences myocardial infarction, Yang-Qi suffers great loss, causing the generation of Yin cold and blood stasis inside the body, eventually resulting in the mixture of phlegm and stasis. After the reperfusion injury, massive amounts of blood flow into the ischemic area in a very short time, Yang-Qi suddenly gets recovered, causing the syndrome of predominant yin rejecting Yang. The stagnation and obstruction of phlegm and stasis impair the cardiac muscle and result in a no-reflow phenomenon.
Dialectical type of MIRI
In Subtle Meaning of the Jade Swivel: Heart Pain, it is indicated that for patients who suffered enduring illness and has deficiency on Qi and blood, or those who are weak and fragile due to hard labor, their heart pain is deficient pain and the Treatment of Different Kinds of Diseases: Chest Obstruction also states that Where Yang Qi in the chest is weak. Yin Qi will dominate the chest and cause chest obstruction. In addition, an Essay on medical reading records that Blood stasis is generated by the fact that deficient Qi fails to promote blood circulation. According to these written records and clinical manifestations of MIRI, the dialectical type of MIRI can be categorized as Qi and blood deficiency syndrome, Qi stagnation and blood stasis syndrome, turbid phlegm obstruction syndrome, and Qi-Yin deficiency syndrome.
The TCM treatment principle of MIRI
During an acute attack period of MIRI, the patient is dominated by enrichment of symptoms, and during the remission period, the patient is dominated by deficiency. Therefore, when treating MIRI patients, the primary principle is to first cure the symptom, then to treat the deficiency; or to treat the two causes at the same time according to the priority of deficiency, excess, symptom, and origin (of the disease). Excess requires purgation, while deficiency requires tonification, a basic treatment principle in TCM. Excess requiring purgation refers to the regulation of Qi movement, promotion of blood circulation, and the removal of blood stasis, as well as the elimination of turbid phlegm. Deficiency requiring tonification refers to the balance of Qi, Blood, Yin, and Yang, and the correction on zang-fu viscera. Specific treatment methods include Qi nourishment and Yang-warming, Yin nourishment and kidney tonification, and complement on heart Qi. TCM has many treatment target points that could provide new clinical exploratory grounds for preventing and treating MIRI. Therefore, using the modern medical understanding of MIRI to select the corresponding TCM therapy for the patient will significantly improve the patient’s recovery rate and living quality.
Advances in the treatment with single Chinese herbal extracts
Exploration of prevention and treatment of MIRI by apoptosis
Under certain pathologic conditions, apoptosis refers to orderly cell death induced by certain genes to balance cell development and homeostasis [8]. Apoptosis pathways mainly include exogenous apoptosis pathway, endogenous mitochondrial pathway, and endogenous endoplasmic reticulum pathway regulated by death receptors [9]. Apoptosis is controlled by a variety of genes, such as the caspase family, B-lymphocytoma-2 gene (Bcl-2) family, and tumor suppressor gene P53 [10]. Various gene families and signal pathways cross each other and lead to apoptosis directly or indirectly. At the present, many studies have shown that apoptosis plays a very important role in MIRI. Meanwhile, the severity of MIRI is mainly determined by the number of apoptotic cells. Therefore, inhibition of apoptosis-related genes or interruption of the apoptotic pathway can reduce MIRI, as shown in Figure 1A.
Figure 1.
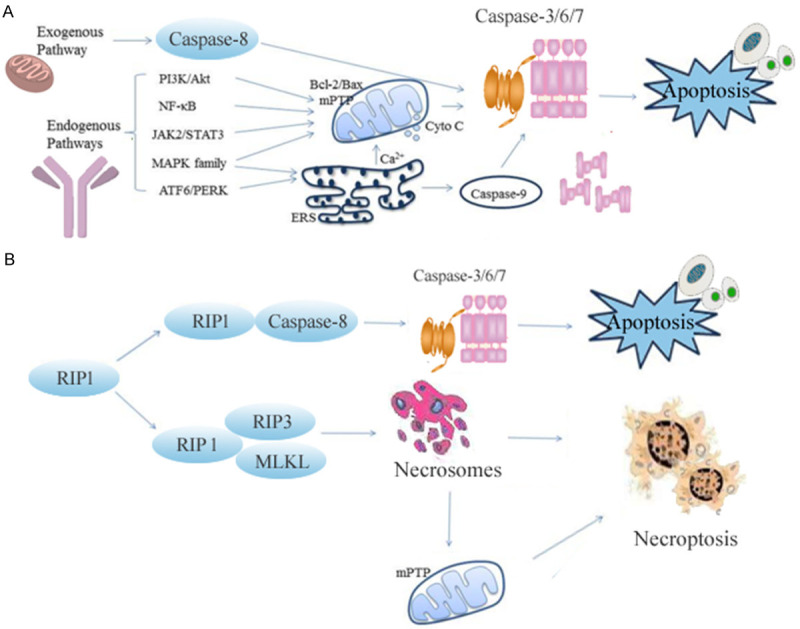
Main regulatory mechanisms of apoptosis and necroptosis in MIRI. The exogenous and endogenous pathways of apoptosis are regulated by death receptors as shown in (A). The link of RIP1 to apoptosis and necroptosis is illustrated in (B). PI3K: phosphoinositide-3-kinase; Akt: protein kinase B; NF-κB: nuclear factor kappa B; JAK2: Janus Kinase 2; STAT3: signal transducer and activator of transcription 3; ATF6: activating transcription factor 6; MAPK: mitogen-activated protein kinase; PERK: (PKR)-like ER kinase; Bcl-2: B-lymphocytoma-2 gene; Bax: Bcl2 associated X protein; MPTP: mitochondrial permeability transition pore; ERS: ER Stress; RIP1: receptor-interacting protein kinase 1; RIP3: receptor-interacting protein kinase 3; MLKL: mixed lineage kinase domain-like protein.
Luteolin (3’,4’,5’,7’-tetrahydroxyflavone, Lu) is a natural, soluble flavone that is found in high levels in many herbs. A study found that Lu enhanced Sarco/endoplasmic reticulum Ca2+-ATPase-a (SERCA2a) activity to improve systolic/diastolic function during MIRI in rat hearts and cardiomyocytes by attenuating the inhibition effects of the p38 pathway on phosphoprotein phosphorylation (p-PLB) [11]. Another study found that Lu regulated SERCA2a through SUMOylation at lysine 585 to attenuate MIRI [12]. Moreover, recent research showed that Lu pretreatment improved SERCA2a expression by the upregulation of Sp1 to exert myocardial protective effects [13].
Danshen, the dried root of Salvia miltiorrhiza Bunge, is a Chinese medicinal herb. Salvianolic acid B (Sal B) is an active water-soluble compound that can be isolated from S. miltiorrhiza Bunge. A study found that Sal B ameliorated MIRI in a dose-dependent manner, ameliorated cardiac function, reduced myocardial infarction size, decreased myocardial injury marker expression, decreased inflammatory responses, reduced apoptosis, activated the phosphoinositide-3-kinase (PI3K)/Akt signaling pathway, and inhibited the release of high mobility group box 1 (HMGB1) in rats [14]. In addition, Tanshinone IIA (TSA) is the major active compound present in the root extracts of Salvia miltiorrhiza Bunge. A study found that TSA exerted the protective role against MIRI-induced apoptosis, oxidation, and mitochondrial membrane potential (MMP) loss of cardiomyocytes by regulating AK003290 and miR-124-5p signaling [15]. In another study, TSA activated silent information regulator 1 (SIRT1)-PGC1α signaling pathway to rescue mitochondrial function, block cardiac microvascular endothelial cell (CMEC) apoptosis, and preserve microvascular structure and function. This happens to be the first study to show the beneficial role of TSA in microvascular injury [16].
Astragalus membranaceus is a widely used Chinese herbal medicine. Astragaloside IV (As-IV) is one of the important effective chemical constituents of this herb. Studies show that As-IV can alleviate MIRI in rats. The possible mechanism is related to its increased phosphorylation of PI3K/AKT and glycogen synthase kinase 3 beta (GSK-3β) protein, and activation of the PI3K/AKT/GSK-3β signaling pathway [17]. A study demonstrated for the first time that the protective effect of As-IV against MIRI was related to inhibition of the calcium-sensing receptor (CaSR)/ERK1/2 (extracellular signal-regulated kinase 1/2) axis and related apoptotic signaling pathways [18]. A similar study found that AS-IV could attenuate MIRI in human cardiomyocytes by the miR-101a/TGFBR1 (TGFβ type 1 receptor)/TLR2 (toll-like receptor 2)/MAPK (mitogen-activated protein kinase) signaling pathway [19].
Baicalin is a flavonoid active substance derived from the roots of Scutellaria baicalensis Georgi (Lamiaceae). It is reported that the protective role of baicalin on MIRI, was shown to be mediated through the Janus kinases/signal transducer and activator of transcription proteins (JAK/STAT) pathway. In addition, baicalin alleviated myocardial injury, inhibited apoptosis following MIRI, and dampened myocardial inflammation response through switching the polarization of macrophages from M1 to M2 [20]. A similar study showed that baicalin downregulates protein expression of CaSR, but upregulates the protein expression of ERK1/2. Therefore, baicalin probably attenuates MIRI through the modulation of the CaSR/ERK1/2 signaling pathway [21].
Ginsenosides are isolated from Asian ginseng (Panax ginseng), a traditional herbal medicine frequently used in eastern countries. Ginsenoside Rb1, Rb2, Rb3 are the main active compounds in the stem, leaves, and root of ginseng. A study showed that Rb1 binds directly to RhoA, inactivating the RhoA/ROCK1 signaling pathway to reduce apoptosis and prevent MIRI [22]. Similarly, Rb2 alleviated MIRI in rats by inhibiting oxidative stress and inflammatory response through SIRT1 activation [23].
In addition, several other researchers have reported the promising therapeutic effects of other TCM and their main components, where they significantly reduce the degree of MIRI by inhibiting myocardial cell apoptosis, as shown in Table 1 [24-33].
Table 1.
Summary of TCM treatment of MIRI by effects on apoptosis in the past five years
| Type | TCM name | Active constituent | Experimental model | Related pathways | Efficacies and mechanisms | References |
|---|---|---|---|---|---|---|
| Apoptosis | Luteolin (Lu) | Flavonoid compound | Ischemia/reperfusion (I/R) | p38 MAPK pathway | Promoted p-PLB, enhanced the activity and stability of SERCA2a via lysine 585 and Sp1, and relieved calcium overload to promote the recovery of the Δψm. | [11-13] |
| S. miltiorrhiza Bunge, Danshen | Salvianolic acid B (Sal B) | Myocardial ischaemic-reperfusion (I/R) | PI3K/Akt/HMGB1 signaling pathway | Reduced TNF-α, IL-18, IL-1β, HMGB1, and TLR4, and increased Bcl-2 while decreasing Bax. | [14] | |
| Salvia miltiorrhiza Bunge | Tanshinone IIA (TSA) | I/R model | AK003290 and miR-124-5p signaling | Decreased the amount of LDH, MDA, ROS, elevated MMP, and miR-124-5p directly targeted AK003290, and up-regulated the expression of AK00329. | [15] | |
| Danshen | Tanshinone IIA (Tan IIA) | Cardiac microvascular endothelial cells isolation and hypoxia/reoxygenation (HR) model in vitro | SIRT1/PGC1α pathway | Activated SIRT1 to sustain the mitochondrial potential, reduced the mPTP opening, benefited the CMEC survival, and preserved microvascular structure and function. | [16] | |
| Astragalus membranaceus | astragaloside IV (As-IV) | MIRI model of SD rats | PI3K/AKT/GSK-3β signaling pathways | Increased the left ventricular systolic pressure, fractional shortening, and ejection fraction, decreased the left ventricular end-diastolic pressure, decreased the serum LDH, CK levels, the HW/BW ratio, and myocardial infarct size, increased the p-Akt/Akt ratio and pGSK-3β/GSK-3β ratio. | [17] | |
| A hypoxia/reoxygenation (H/R) model in vitro; an SD rat MI/R model in vivo | CaSR/ERK1/2 signaling pathways | Decreased LDH, Ca2+ and CaSR expression, and increased the ERK1/2 phosphorylation levels in vitro. Decreased the myocardium infarct size, CK-MB, and cTnI levels in vivo. | [18] | |||
| H9c2 cardiomyocytes hypoxia/reoxygenation (H/R) cell model | miR-101a/TGFBR1/TLR2/MAPK signaling pathway axis | Promoted SOD activity and the expression level of miR-101a, decreased MDA, LDH, TGFBR1, and TLR2, suppressed p-ERK and p-p38, decreased Bax/Bcl-2 ratio, and cleaved caspase-3/caspase-3 ratio. | [19] | |||
| Scutellaria baicalensis Georgi (Lamiaceae) | Baicalin, a flavonoid active substance | an ischemia/reperfusion (I/R) model | JAK/STAT pathway | Decreased iNOS, IL-1β, and IL-6, up-regulated Arg-1, IL-10, and TGF-β, and inhibited the phosphorylation levels of JAK2 and STAT3. | [20] | |
| SD rats’ heart and myocardial cells I/R model | CaSR/ERK1/2 signaling pathway | Improved LV hemodynamic parameters, down-regulated the protein expression of CaSR, and up-regulated the protein expression of ERK1/2. | [21] | |||
| Ginsenosides | Ginsenoside Rb1 (Rb1) | I/R model | RhoA/ROCK1 signaling pathway | Reduced damaged myocardial structure, increased myocardial blood flow, improved heart function, and microcirculation, reduced cTnI, inhibited the activation of RhoA, and restored the production of ATP. | [22] | |
| Ginsenosides | Rb2 | Myocardial ischemia/reperfusion (MI/R) model | SIRT1 signaling pathway | Reduced myocardial superoxide generation, downregulated gp91phox expression, decreased the mRNA of IL-1β, IL-6, and TNF-α, upregulated SIRT1 expression and downregulated Ac-p53 expression. | [23] | |
| Ginkgo biloba leaves | Ginkgolide B, a flavonoid monomer | Myocardial I/R model | PI3K/AKT/mTOR signaling pathway | Suppressed TNI, TNT, LDH, and Mb and ameliorated the damaged and irregularly arranged myocardial cells, suppressed the expression levels of p-PERK, p-IRE1α, and ATF6, and upregulated p-AKT and p-mTOR expressions. | [24] | |
| Dihydroquercetin (DHQ) | A dihydroxyflavone | Isolated rat hearts and H9c2 cardiomyocytes model | PI3K/Akt pathway | Alleviated cardiac dysfunction, scavenged free radicals, reduced lipid peroxidation, increased the activity of antioxidant enzymes, inhibited the expression of CHOP, Caspase-12, and p-JNK, reduced GRP78, p-PERK, and p-eif2α expression levels, and increased HO-1 expression and Nrf2 binding to antioxidant response elements. | [25] | |
| Curculigo orchioides Gaertn | Curculigoside, a phenolic glycoside antioxidant | H9c2 cells hypoxia/reoxygenation (H/R) model | MPTP opening | Improved cell viability, reduced the infarct size, inhibited MPTP opening and preserved ΔΨm, decreased LDH activity, the expression of cytochrome c, apoptotic protease activating factor-1, and cleaved caspase-9 and cleaved caspase-3. | [26] | |
| Ganoderma lucidum | Ganoderic acid A (GA) | MIR rat model | JAK2/STAT3/NF-κB pathway | Reduced the myocardial infarction extension, decreased LDH, CK, the phosphorylation of JAK2, STAT3, and NF-κB. | [27] | |
| Epimedium brevicornum | Icariin (ICA), a flavonoid | Isolated rat hearts and neonatal rat cardiomyocytes and H9c2 cells model | SIRT1/FoxO1 signaling | Improved heart contraction and limited the infarct size and CK-MB and LDH leakage, decreased MDA, increased SOD activity, MnSOD expression, mitochondrial membrane potential and cytochrome C stabilization, up-regulated SIRT1 and down-regulated Ac-FoxO1. | [28] | |
| The roots of Sophora flavescens (Kushen), Sophora tonkinensis, and Sophora alopecuroides (Kudouzi) | Matrine, a quinolizidine alkaloid | Hypoxia/reoxygenation (H/R) of CMECs in rats’ model | JAK2/STAT3 signaling pathway | Increased cell viability, cell ratio at the S phase, expression levels of p-JAK2 and p-STAT3, increased tube formation ability and decreased the ratio of cells at the G1 phase and Bax/Bcl-2 ratio. | [29] | |
| Platycodon grandiflorum | Platycodin D (PD) | Cardiomyocyte H9c2 cells hypoxia/reoxygenation (H/R) model | Akt/Nrf2/HO-1 pathway | Decreased ROS and MDA, increased SOD and CAT, reduced Bax cleaved caspase-3, and induced Bcl-2. | [30] | |
| Quercetin (QU) | Flavonoids | Ischemia/reperfusion (I/R) model | SIRT1/PGC-1α signaling | Improved myocardial pathological morphology, upregulated SIRT1, PGC-1α, and Bcl-2 proteins expression, and downregulated Bax protein expression. | [31] | |
| Schisandra chinensis | Schisandrin B, Sch B | Ischemia/reperfusion (I/R) injury model | ATF6 and PERK pathway | Decreased CK, LDH, MDA, and the mRNA levels of ATF6, PERK, and CHOP; Downregulated the levels of caspase-9, caspase-3, and Bax, and upregulated the expression of Bcl-2. | [32] | |
| Radix Paeoniae Rubra | Total paeony glycoside (TPG), a monoterpene compound | Ischemia/reperfusion (I/R) injury model | PI3K/Akt signaling pathway | TPG decreased ROS, MDA, and LDH, increased SOD, and GPX activities upregulated the expression levels of pro-caspase-3 and Bcl-2, downregulated cleaved-caspase-3, poly (ADP-ribose) polymerase 1, Bcl-2-associated X protein, and phosphorylated PI3K and Akt expression. | [33] | |
| Apoptosis and Autophagy | Hongjingtian injection (HJT) | Extracts of Rhodiola wallichiana var. cholaensis | Cardiac ischemia-reperfusion (I/R) model | AMPK/mTOR pathway | Decreased the infarct area, the levels of cleaved caspase 3, LC3-II, and p-AMPK expression, increased the Bcl-2/Bax ratio and p-mTOR, increased the mitochondrial membrane potential, intracellular ATP contents, and oxygen consumption. | [64] |
MAPK: mitogen-activated protein kinase, p-PLB: phosphoprotein phosphorylation, SERCA2a: sarco/endoplasmic reticulum Ca2+-ATPase-a, ΔΨm: mitochondrial membrane potential, PI3K: phosphoinositide-3-kinase, HMGB1: high mobility group box 1, TNF-α: tumour necrosis factor-α, IL-18: interleukin-18, IL-1β: interleukin-1β, TLR4: toll-like receptor 4, Bax: bcl-2 associated x, Bcl-2: beclin-2, LDH: lactate dehydrogenase, MDA: malonaldehyde, ROS: reactive-oxygen species, MMP: mitochondrial membrane potential, SIRT1: silent information regulator 1, CMEC: cardiac microvascular endothelial cell, GSK-3β: glycogen synthase kinase 3 beta, CK: creatine kinase, HW/BW: heart weight/body weight, CaSR: calcium-sensing receptor, ERK1/2: extracellular signal-regulated kinase 1/2, TGFBR1: TGFβ type 1 receptor, TLR2: toll-like receptor 2, JAK: janus kinases, STAT: signal transducer and activator of transcription proteins, ROCK-1: RhoA/Rho-associated coiled-coil containing protein kinase-1, mTOR: mammalian target of rapamycin, TnI: troponin I, TnT: troponin T, Mb: myoglobin, PERK: type I transmembrane ER-resident protein kinase, ATF6: activating transcription factor 6, JNK: Jun N-terminal kinase, Nrf2: nuclear factor erythroid 2 related factors 2, MPTP: mitochondrial permeability transition pore, NF-κB: nuclear factor kappa B, FoxO1: forkhead box O 1, SOD: superoxide dismutase, CAT: catalase, GPX: glutathione peroxidase, ATP: adenosine triphosphate.
Exploration of prevention and treatment of MIRI by necroptosis
Alexei Degterev, an associate professor of developmental, molecular, and chemical biology, discovered a new form of cell death with a similar signal mechanism to apoptotic cells and morphologic characteristics of necrotic cells in 2005 and named it necroptosis [34]. The activation of necroptosis depends on the activity of receptor-interacting protein 3 (RIP3). RIP3 interacts with receptor-interacting protein 1 (RIP1) to produce necrosomes which phosphorylate mixed lineage kinase domain-like protein (MLKL) and translocate it to cellular membranes, leading to the plasma membrane damage, cell content overflow, and consequently causing inflammation and triggering the immune system [35]. Studies have shown that necroptosis plays an important role in MIRI [36], as shown in Figure 1B.
Tanshinone I (TI), a fat-soluble compound, is a major ingredient in Danshen. A study indicated that TI exerts cardiovascular protective activities in vitro and in vivo through suppressing the RIP1/RIP3/MLKL axis and activating the Akt/Nrf2 (nuclear factor erythroid 2 related factors 2) signaling pathways [37].
Baicalin is one of the predominant flavonoids isolated from the dry roots of Scutellaria baicalensis Georgi. It is reported that Baicalin mitigates necroptosis by inhibiting the protein expression of RIP1, RIP3, and p-MLKL and protects CMECs in MIRI rats by promoting the release of nitric oxide (NO) through the PI3K-AKT-eNOS pathway [38].
Exploration of prevention and treatment of MIRI by autophagy
Autophagy refers to the process in which cells actively degrade their over-accumulated and misfolded proteins, undergo functional decline, senescence, and damage of organelles, causing recycling of the generated materials and energy by the lysosomal pathway [39]. Autophagy is mainly divided into molecular chaperone-mediated autophagy, small autophagy, and large autophagy. Large autophagy is commonly referred to as autophagy and the autophagy process can be divided into four stages: induction of autophagy, formation of autophagosomes, formation of autophagolysosomes, and degradation and reuse of autophagolysosome [40]. Currently, studies have shown that autophagy has a two-way adjustment function. At the initial stage of MIRI, cardiomyocytes are subjected to a state of ischemia and hypoxia. Autophagy induces adenosine triphosphate (ATP) production, as an energy recovery process [41]. At the same time, it makes up for the damage of ubiquitin-protein so as to maintain the protein steady-state [42]. It is the key to cell survival and protects cells against various types of stress. However, in the reperfusion stage, autophagy is over-activated. Because of the increase of lysosomal enzymes, necessary proteins and organelles are excessively cleared which can lead to cellular dysfunction, further enhancing cell death and expanding the area of myocardial infarction [43-46]. Another harmful cause of autophagy may be the crosstalk between autophagy and apoptosis in the Bcl-2 family [47]. Therefore, the dual role of autophagy in MIRI is considered as a potential intervention target for the reperfusion therapy of ischemic heart disease, as shown in Figure 2A.
Figure 2.
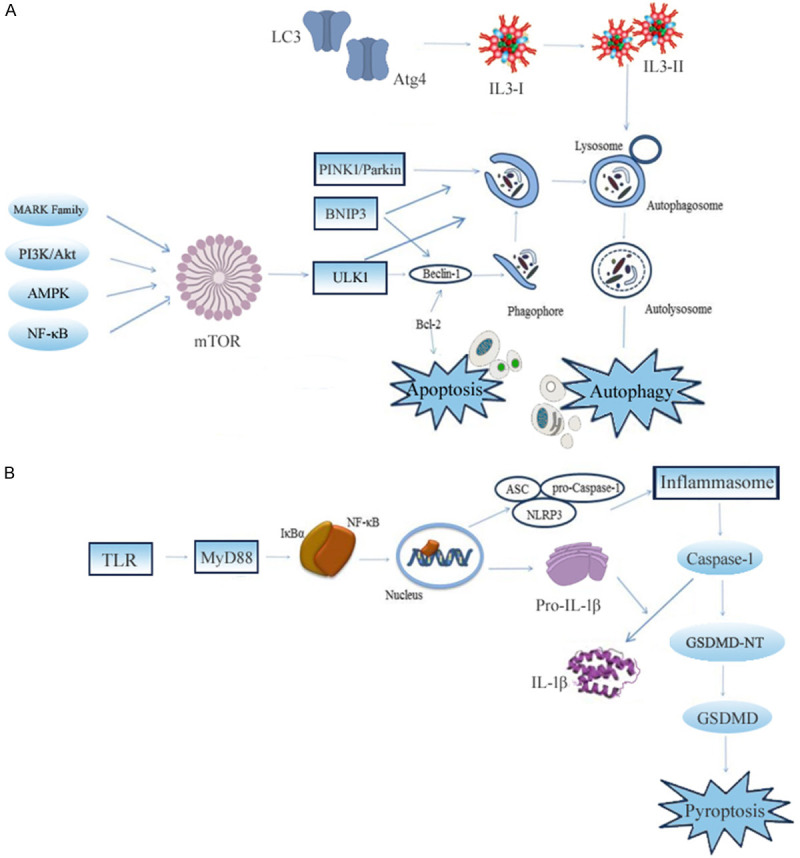
The main regulatory mechanisms of autophagy and pyroptosis in MIRI. The chain of molecular reactions associated with autophagy and apoptosis in MIRI is presented in (A). The inflammasome-associated inflammatory caspases activation of pyroptosis is illustrated in (B). AMPK: AMP-activated protein kinase; mTOR: mammalian target of Rapamycin; ULK1: unc-51-like kinase 1; BNIP3: BCL2 interacting protein 3; PINK1: PTEN-induced kinase 1; Parkin: Parkinson juvenile disease protein 2; LC3: microtubule-associated protein light chain 3; TLR: toll-like receptor; MyD88: myeloid differentiation factor 88; IκBα: recombinant inhibitory subunit of NF-κB alpha; NLRP3: NOD-like receptor protein 3; ASC: apoptosis-associated speck-like protein containing CARD; IL-1β: interleukin-1β; GSDMD: pro-pyroptotic factor gasdermin D; GSDMD-NT: GSDMD N-terminal.
An important active ingredient in TCM is Scutellarin (Scu), a flavonoid purified from Erigeron breviscapus. SCU improves diastolic dysfunction, ameliorates myocardium structure abnormality, inhibits myocyte apoptosis and inflammatory response, and promotes autophagy. These protective effects are attributed to its regulation of the Akt/(mechanistic target of rapamycin complex 1) mTORC1/NLRP3 (NLR family pyrin domain containing 3) signaling pathway [48].
Another active ingredient is Artemisinin (ARS), a sesquiterpene lactone compound with a peroxisome bridging group structure, mainly found in Artemisia annua, a traditional medicinal plant in China. A study confirmed that artemisinin preconditioning could effectively protect against MIRI through suppression of the NLRP3 inflammasome activation [49].
Furthermore, Panax notoginseng saponins (PNS) are active ingredients extracted from Panax notoginsen (Burk) F. H. Chen (Sanqi in Chinese). Researchers have established the protective effect of PNS in MIRI, mainly due to its ability to enhance the mitochondrial autophagy of myocardial tissue through the hypoxia-inducible factor (HIF)-1a/BCL2 interacting protein 3 (BNIP3) pathway [50].
In addition to the studies indicated above, several other TCM and their main components have been documented to mitigate MIRI by inhibiting myocardial cell autophagy, as summarized in Table 2 [51-56].
Table 2.
Summary of TCM treatment of MIRI by regulating necroptosis, autophagy, and pyroptosis in the past five years
| Type | TCM name | Active constituent | Experimental model | Related pathways | Efficacies and mechanisms | References |
|---|---|---|---|---|---|---|
| Necroptosis | Salvia milorrhiza Bunge | Tanshinone I (TI) | Ttert-butyl hydroperoxide (t-BHP)-stimulated H9c2 cells model; myocardial ischemia-reperfusion (MI/R) model | RIP1/RIP3/MLKL and Akt/Nrf2 signaling pathways | Inhibited the expression of p-RIP1, p-RIP3, and p-MLKL, promoted the phosphorylation of Akt, Nrf2, NQO-1, and HO-1, mitigated ROS generation, and reversed MMP loss. Recovered ECG, reversed the counts of WBC, Neu, Lym, and the release of TNF-α and IL-6, and increased SOD level while decreasing MDA level. | [37] |
| Scutellaria baicalensis Georgi (Lamiaceae) | Baicalin, a flavonoid active substance | myocardial ischemia-reperfusion (IR) model | PI3K-AKT-eNOS pathway | Improved cardiac function, decreased the myocardial infarction area, promoted the production of NO and the level of cGMP, suppressed the protein expression of RIP1, RIP3, and p-MLKL to interrupt CMEC necroptosis. | [38] | |
| Autophagy | Scutellarin (SCU) | A flavonoid purified | Acute myocardial I/R injury and anoxia/reoxygenation (A/R)-induced H9c2 injuries model | Akt/mTORC1/NLRP3 signaling pathway | Reduced NLRP3 inflammasome activation, inhibited mTORC1 activity, and increased Akt phosphorylation. | [48] |
| Artemisinin (ARS) | A sesquiterpene lactone compound | The rats I/R injury model | NLRP3 inflammasome pathway | Reduced infarct size, increased p62, decreased CK-MB, LDH, LC3II/I, NLRP3, ASC, cleaved caspase-1, and IL-1β. | [49] | |
| Panax notoginseng | Panax Notoginseng Saponins (PNS) | A rat model of myocardial injury | HIF-1α/BNIP3 pathway | Decreased CK, MDA, LDH, SOD, and ROS, increased mitochondria autophagosome in myocardial cells, increased the expression of LC3 and the ratio of LC3II/LC3I, increased the expression of HIF-1α, BNIP3, Atg5, and Beclin-1. | [50] | |
| Coptidis Rhizoma | Coptisine, a naturally occurring isoquinoline alkaloid | A hypoxia/reoxygenation (H/R)-treated H9c2 cardiomyocyte model | Autophagosome formation | Reduced the protein level of LC3-II, cleaved Caspase-3, Beclin1, and Sirt1. | [51] | |
| Gastrodian, GAS | Model of Hypoxia/Reoxygenation (H/R) | Autophagic flux | Reduced heart infarct size inhibited expression of p62 and increased LC3II, increased the mitochondrial membrane potential of NRCs, activated AMPK phosphorylation, and reduced mTOR phosphorylation. | [52] | ||
| The root of Pueraria lobata (Willd.) | Pueraria lobata (Pur) | Myocardial hypoxia/reoxygenation (H/R) | Akt signaling pathway | Decreased the ratio of LC3-II/LC3-I and the degradation of p62 and increased the level of Akt. | [53] | |
| Puerarin (Pue) | 8-C-β-D-glucopyranosyl-7,4-hydroxy-isoflavone | Myocardial ischemia/reperfusion (MI/R) injury | SIRT1/NF-κB pathway | Reduced myocardial infarct size and CK-MB, decreased the mRNA and protein levels of TNF-α, IL-6, and IL-1β, decreased the protein levels of Ac-NF-κB, NLRP3, cleaved caspase-1, cleaved IL-1β, and cleaved IL-18, and increased the protein level of SIRT1. | [54] | |
| The rhizome of curcuma | Curcumin, curcumin-peptide hydrogel, a polyphenolic compound | Rat | JAK2/STAT3 pathway | Reduced ROS formation and mitochondrial damage, improved cardiac function, inhibited left ventricular dilatation, ventricular remodeling, and collagen synthesis. | [55] | |
| ischemia-reperfusion model | ||||||
| Turmeric plant | Curcumin (Cur), an alcohol-based molecule that exists in an organic solvent | H9c2 cardiomyocytes model | ER stress and the MAPK pathway | Decreased the levels of LDH and MDA and increased the activity of SOD, inhibited the phosphorylation of ERK1/2 and JNK. | [56] | |
| Pyroptosis | The rhizome of Rheum palmatum | Emodin, an anthraquinone derivative | I/R model | TLR4/MyD88/NF-κB/NLRP3 inflammasome pathway | Increased the rate of cell survival and decreased the myocardial infarct size, suppressed the expression of TLR4, MyD88, phospho-IκBα, phospho-NF-κB, and the NLRP3 inflammasome. | [60] |
| Gastrodian, GAS | Cardiac microvascular endothelial cells and myocardial I/R animal model | NLRP3/caspase-1 pathway | Attenuated infarct size and inflammatory cells infiltration and increased capillary formation, reduced IL-1β production, blocked NLRP3-dependent pyroptotic cell death. | [61] | ||
| Luteolin (Lu) | A flavonoid compound | I/R model | Sirt1/TLR4/NLRP3/NF-κB pathway | Decreased LDH, AST and CK-MB, increased the level of Sirt1, and decreased the levels of NLRP3, ASC, Caspase-1, IL-1β, IL-18, TNF-α, TLR4, MyD88, p-NF-κB p65, and p-IκBα. | [62,63] |
RIP3: receptor-interacting protein 3, RIP1: receptor-interacting protein 1, MLKL: mixed lineage kinase domain-like protein, Akt: protein kinase B, HO-1: Heme oxygenase-1, NQO-1: quinone oxidoreduct-ase-1, ECG: electrocardiograph, WBC: white blood cell, Neu: neutrophil, Lym: lymphocyte, IL-6: interleukin-6, NO: nitric oxide, mTORC1: mechanistic target of rapamycin complex 1, P3: NLR family pyrin domain containing 3, CK-MB: creatine kinase-MB, LC3: microtubule-associated protein light chain 3, ASC: apoptosis-associated speck-like protein, HIF-1α: hypoxia-inducible factor 1α, BNIP3: BCL2 interacting protein 3, Atg5: autophagy-related gene-5, NRCs: neonatal rat cardiomyocytes, AMPK: AMP-activated protein kinase, ER: endoplasmic reticulum, MyD88: myeloid differentiation factor 88, AST: aspartate transaminase.
Exploration of prevention and treatment of MIRI by pyroptosis
Cookson and Brennan proposed a proinflammatory programmed cell death which has come to be known as pyroptosis [57]. The inflammasome-associated inflammatory caspases are activated to drive cleavage of the pro-pyroptotic factor gasdermin D (GSDMD), generating an N-terminal fragment that is oligomerized to form pores on the cell membrane and finally causes cell death [58,59], as shown in Figure 2B.
Emodin is an anthraquinone derivative from the rhizome of Rheum palmatum, which is widely used as a laxative in TCM. A study provided strong evidence that emodin treatment is able to alleviate MIRI and inhibit pyroptosis in vivo and in vitro by suppressing the toll-like receptor 4 (TLR4)/MyD88/NF-κB/NLRP3 inflammasome pathway [60].
Gastrodian (GAS), a TCM monomeric component, has been traditionally used for the treatment of cardiovascular and cerebrovascular diseases for centuries. In a study, it was demonstrated that GAS reversed CMECs and heart tissue pyroptosis, and decreased myocardial infarct size and inflammatory cell infiltration by inhibiting the NLRP3/caspase-1 signaling pathway [61].
Luteolin (Lu) is a flavonoid compound, which has been isolated from a variety of natural medicinal materials, fruits, and vegetables. Some researchers established the I/R model in rats to undertake cardiac hemodynamic measurement, assess myocardial infarction and damage, analyze antioxidant enzymes activities, and estimate various biochemical indexes of myocardial tissue. They found that Lu decreased aspartate transaminase (AST), creatine phosphokinase-isoenzyme (CK-MB), and lactate dehydrogenase (LDH), increased the level of Sirt1, and decreased the levels of NOD-like receptor 3 (NLRP3), apoptosis-associated speck-like protein containing CARD (ASC), Caspase-1, interleukin-1β (IL-1β), interleukin-18 (IL-18), tumor necrosis factor-α (TNF-α), toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), nuclear factor kappa B (NF-κB). The above results indicated that Lu may reduce MIRI by regulation of the Sirt1/TLR4/NLRP3/NF-κB pathway in vivo and in vitro [62,63].
It is not surprising to find that there exists mutual restriction and dynamic balance among apoptosis, necroptosis, autophagy, and pyroptosis. For instance, Hongjingtian injection (HJT) improved mitochondrial function and regulated autophagy to inhibit cell apoptosis through the AMPK/mTOR pathway [64]. Moreover, Lu protected the myocardium by controlling apoptosis and pyroptosis [11,63], while GAS inhibited MIRI through autophagy and pyroptosis [52,61]. The different active components of Danshen play important roles in combating MIRI through apoptosis and necroptosis [14,15,37]. Therefore, to make full use of the therapeutic potential of TCM and its main components in inhibiting cardiac myocyte death during MIRI, it is necessary to study the similarities between these cell death pathways and intervene in their key targets, to obtain effective MIRI treatment.
Progress in the treatment with Chinese herbal compound
The application of TCM compounds or compound extracts to inhibit MIRI can reflect the guiding role of TCM theory in the clinic. In literature reports, the selection of the compounds is based on which of them has the most effects of replenishing Qi, activating blood circulation, regulating Qi, resolving phlegm, nourishing Yin, and restoring Yang to save the heart from collapse.
The method of replenishing qi and activating blood
Researchers indicate that the administration of QiShenYiQi Pills (QSYQ) can improve the synergistic effect of their main components, improving myocardial energy metabolism, protecting from oxidative stress, decreasing the production of pro-inflammatory cytokines and infiltration of inflammatory cells, enabling QSYQ to attenuate the impairment of microcirculation dysfunction, myocardial injury and cardiac fibrosis [65]. Similarly, treatment with Yi Qi decoction (MYQ) consisting of five herbs-Milkvetch roots, Semen pharbitidis, CinnamoN twig, Fructus amomi, and White peony root, significantly improved cardiac function in cardiac injury, accompanied by an increase of Bcl-2, NCX1, and Serca2a expressions, and decrease of Bax and Beclin-1 expressions. This shows that MYQ has potential therapeutic effects on IR-induced cardiac injury [66]. It has also been noted that Tongxinluo (TXL) treatment increased ejection fraction, promoted angiogenesis in the peri-infarct region, and decreased fibrosis and the size of the infarcted area. Moreover, TXL could promote autophagy, inhibit apoptosis, and preserve the myocardium through activation of the AMPK signaling pathway [67].
The method of nourishing Qi and Yin
A new drug combination GRS comprising of ginsenoside Rb1 (G-Rb1), ruscogenin (R-Rus), and schisandrin (S-SA) were screened based on ShengMai preparations and was found to ameliorate MIRI and H/R-induced cardiomyocytes apoptosis by energy modulation and anti-inflammatory and anti-oxidative effects, by individually activating AMPKα phosphorylation, inhibiting the of NF-κB pathway, and regulating of ERK1/2, Akt-Bad/14-3-3 pathway [68].
The method of moving Qi and activating blood circulation
Guanxin Shutong capsules (GXSTC) is a widely used Chinese medicinal formula, which is clinically administered for palpitations, short breath, restlessness, fatigue, dizziness, and chest pain, enhancing blood circulation, removing blood stasis, and protecting against cardiovascular diseases [69]. Researchers have found that four main active ingredients (FMAI), protocatechuic acid, cryptotanshinone, borneol, and eugenol, found in GXSTC suppressed calcium overload and exerted their protective effect via its antioxidant, anti-inflammatory, and anti-apoptosis activities in vitro and in vivo [70]. Tongmai formula (TM) has also been demonstrated to exert a cardiac protective effect on ischemic myocardium of rats after reperfusion and improves mitochondrial quality control through mitochondrial dynamics in neonatal rat ventricular myocytes (NRVMs) after MIRI [71]. Another study found that the cardioprotective effect of Xuefu Zhuyu Decoction (XFZY) during MIRI is mediated by the inhibition of autophagy through regulating the AMPK-MTOR signaling pathway [72].
The method of activating Yang and dissipating cold
Shenxian-Shengmai Oral Liquid (SXSM), a compound formula for commonly treating bradyarrhythmias been found to elevate heart rate, improve cardiac dysfunction, and correct cardiac morphological and structural changes during MIRI, and as well protect cardiomyocytes from oxidative stress by upregulating the expressions of superoxide dismutase (SOD)1 and glutamate-cysteine ligase catalytic subunit (GCLC) [73]. In other studies, the herbal formula Shexiang Tongxin dripping pills (STDP), used for treating angina pectoris in Chinese clinics and hospitals, could reduce peripheral inflammation of blood vessels and the degree of atrophy of myocardial muscle fiber, through the down-regulation of Sirt1, PGC-1α, and PPARα, STDP again induced the up-regulation of ERK1/2, TLR4, and UCP2 in the myocardial tissues of MIRI to improve coronary microcirculation disorder and cardiac dysfunction after myocardial ischemia-reperfusion [74]. One study demonstrated that Suxiao Jiuxin Pills (SXJ) protected against myocardial I/R injury by activating AKT/GSK3β and GATA4 signaling pathways [75].
The method of clearing the heart and reducing fire
Sanwei-Tanxiang powder (SWTX) has been validated concerning its efficacy and mechanism in improving MIRI. In that study, pretreatment with SWTX reduced ST-segment elevation, pathological changes, and myocardial infarct size. Meanwhile, some monomers of SWTX showed antioxidant capacity and inhibited cardiomyocytic apoptosis which correlated with the activation of the PI3K/Akt/FoxO3a signaling pathway [76].
Progress of acupuncture in the treatment of MIRI
Acupuncture and moxibustion is a medical technique of “treating internal diseases and external diseases”. Acupuncture and moxibustion on corresponding meridians and acupoints can connect meridians and regulate qi and blood, so that Yin and Yang are relatively balanced and, zang-fu functions tend to harmonize, to achieve the purpose of disease prevention and treatment.
Some researchers employed rat models as research subjects to prove that electroacupuncture and moxibustion pretreatment at Neiguan point (an acupoint of the skin stimulated with various techniques in acupuncture) could reduce apoptosis index, LC3-II expression level, and LC3-II/I ratio of MIRI rats, moderately enhancing the occurrence of cardiac autophagy, reducing cell apoptosis, and improving cell damage caused by myocardial ischemia [77]. Other researchers found that electroacupuncture pretreatment at Neiguan point could reduce infarct size and tissue weight of MIRI rats, decrease left ventricular end-diastolic pressure (LVEDP) value and the expression of CK-MB and iNOS in serum, slightly expand myocardial fiber space, reduce myocardial cell swelling, and maintain the integrity of nuclear and mitochondrial structures. The above-mentioned effect could promote autophagy flux to alleviate MIRI damage through the PI3K-Akt-mTOR pathway [78]. Other studies also found that after electroacupuncture pretreatment at the Neiguan, Guanyuan, and Zusanli points, the level of TNF-α and IL-6 in serum and the expressions of farnesoid X receptor (FXR) and small heterodimer partner (SHP) in myocardial tissue were significantly reduced [79]. Another study reported that the infarct area of an electroacupuncture group was smaller than that of the model control group. Moreover, the serum LDH, CK, and cTnI levels, as well as FXR and SHP gene expression levels of the electroacupuncture group were lower than that of the model group [80].
In conclusion, electroacupuncture pretreatment could significantly improve cardiac function after MIRI in rats, reduce infarct size, reduce inflammatory factors, regulate FXP/SHP pathway to inhibit myocardial apoptosis, and play a preventive and protective role in MIRI. Other supportive studies of this observation include a study that found that the ST segment displacement of the electroacupuncture cardiac meridian group was lower than that of the control group after pretreatment at Shenmen and Tongli points. The serum IL-1β content was decreased and IL-10 content was increased in rats. The protein expression of NF-κB P65 and IKKβ in myocardial tissue were also decreased, while the protein expression of IκBα was increased. The mechanism may be mediated by regulating the IKK/IκB/NF-κB signaling pathway [81]. Other researchers developed a new method of acupoint gel embedding at bilateral Neiguan points in rats and found that it could significantly reduce the size of myocardial infarction, repair pathological changes, relieve oxidative stress injury and inflammatory response, and inhibit cell apoptosis through the notch-1/JagGED-1 signaling pathway. Acupoint gel catgut embedding method has the advantages of continuous acupuncture point stimulation, dose control, no side effects in the treatment process, and relief of pain caused by frequent acupuncture, which can become an alternative therapy of acupuncture in the treatment of diseases in the future [82].
Prospects
With the continuous development of TCM, the application of Chinese medicine has been given attention in the treatment of MIRI. Through continuous pharmacological exploration, both single TCM extracts and compounds from TCM have been confirmed to possess microscopic pharmacological effects of TCM. The concepts of traditional medicine have been adopted and the clinical effects are more prominent when combined with TCM acupuncture and moxibustion. The existing research on MIRI and therapeutic mechanism of TCM are insufficient and mostly less in-depth. The following challenges require further explorations in future studies. Firstly, although several animal model experiments have been conducted, those studies have not closely been integrated with clinical practice. Considering the increasing number of basic diseases and pathological conditions such as diabetes, hyperlipidemia, hypertension, etc., the effectiveness of TCM and associated mechanisms need further research. Secondly, the purification technology of TCM is not yet perfect and the equipment of professional and technical personnel needs to be strengthened. Furthermore, research on the mechanism of TCM mostly draws ideas from the research model of western medicine, which could lead to the ignorance of certain guiding principles of TCM theory. The failure to recognize the “syndrome” in each case is inconsistent with the theory of “treatment based on syndrome differentiation”, and results in certain blindness in clinical practice. All these challenges require studies for a future solution. Therefore, based on the TCM’s theory of “Organic wholeness” and “Treatment based on syndrome differentiation”, researchers should give more attention to TCM advantages and characteristics regarding “multi-link, multi-target, multi-access, and cost-effectiveness”. Moreover, there should be an emphasis on the combination of western medicine and TCM in terms of the disease diagnosis and organic combined research of “disease, syndrome, and prescription”.
In the meantime, there is the need to explore the relevant regulatory mechanisms and molecular changes to clarify the material basis and main action mechanism of TCM and its related components in the prevention and treatment of MIRI, which will help promote the objectification of TCM theory. On the other hand, the results of clinical studies should be used to further optimize the compatibility of effective ingredients, dosage, administration time, and administration method of TCM, to carry out more scientific design, large-sample studies, multi-center clinical observation, and classification system studies which will improve the research on the mechanism of MIRI and provide therapeutic strategies and a theoretical basis for clinical treatment of MIRI. It is expected that with the continuous development and progress of science and technology, researchers will be able to solve the problem of patients’ pain and improve their quality of life through continuous development and exploration.
Acknowledgements
Supported by WJ2019H411 from Hubei Provincial Population and Family Planning Commission, WZ19A04 from Health and Family Planning Commission of Wuhan Municipality and TC2020JCYL17 from Tai-cang Science and Technology Planning Project. The authors report no conflict of interest.
Disclosure of conflict of interest
None.
References
- 1.Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML, Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, Zheng Z, Hu SS. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept. 2000;9:160–171. doi: 10.1159/000014636. [DOI] [PubMed] [Google Scholar]
- 4.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Zhaoguo, Liu Xiru., translators. Yellow emperor’s canon of medicine·plain conversation. Xi’an: World Publishing Corporation; 2005. pp. 304–515. [Google Scholar]
- 7.Zhang ZJ. In: Synopsis of prescriptions of the golden chamber. Luo Xiwen., translator. Beijing: New World Press; 2007. p. 108. [Google Scholar]
- 8.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Obeng E. Apoptosis (programmed cell death) and its signals-a review. Braz J Biol. 2021;81:1133–1143. doi: 10.1590/1519-6984.228437. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu S, Xu T, Luo Y, Zhang Y, Xuan H, Ma Y, Pan D, Li D, Zhu H. Luteolin enhances sarcoplasmic reticulum Ca2+-ATPase activity through p38 MAPK signaling thus improving rat cardiac function after ischemia/reperfusion. Cell Physiol Biochem. 2017;41:999–1010. doi: 10.1159/000460837. [DOI] [PubMed] [Google Scholar]
- 12.Du Y, Liu P, Xu T, Pan D, Zhu H, Zhai N, Zhang Y, Li D. Luteolin modulates SERCA2a leading to attenuation of myocardial ischemia/reperfusion injury via sumoylation at lysine 585 in mice. Cell Physiol Biochem. 2018;45:883–898. doi: 10.1159/000487283. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Zhang C, Zhu H, Wang S, Zhou Y, Zhao J, Xia Y, Li D. Luteolin modulates SERCA2a via Sp1 upregulation to attenuate myocardial ischemia/reperfusion injury in mice. Sci Rep. 2020;10:15407. doi: 10.1038/s41598-020-72325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Liu W, Qiu H, Zou D, Cai H, Chen Q, Zheng C, Xu D. Salvianolic acid B protects against myocardial ischaemia-reperfusion injury in rats via inhibiting high mobility group box 1 protein expression through the PI3K/Akt signalling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1527–1539. doi: 10.1007/s00210-019-01755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Wei L, Yu Q, Shi H, Liu G. Tanshinone IIA alleviates hypoxia/reoxygenation induced cardiomyocyte injury via lncRNA AK003290/miR-124-5p signaling. BMC Mol Cell Biol. 2020;21:20. doi: 10.1186/s12860-020-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong J, Ouyang H, Sun M, Lu J, Zhong Y, Tan Y, Hu Y. Tanshinone IIA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1alpha-mitochondrial apoptosis pathway. Cell Stress Chaperones. 2019;24:991–1003. doi: 10.1007/s12192-019-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei D, Xu H, Gai X, Jiang Y. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3beta signaling pathways. Acta Cir Bras. 2019;34:e201900708. doi: 10.1590/s0102-865020190070000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. 2019;40:599–607. doi: 10.1038/s41401-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Fan Z, Chen Z, Hu J, Cui J, Liu Y, Wang Y, Guo B, Shen J, Xie L. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470:41–51. doi: 10.1007/s11010-020-03743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M, Li X, Song L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm Biol. 2020;58:655–663. doi: 10.1080/13880209.2020.1779318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Zhang S, Xu C, Sun Y, Sui S, Zhang Z, Luan Y. The protective of baicalin on myocardial ischemia-reperfusion injury. Curr Pharm Biotechnol. 2020;21:1386–1393. doi: 10.2174/1389201021666200605104540. [DOI] [PubMed] [Google Scholar]
- 22.Cui YC, Pan CS, Yan L, Li L, Hu BH, Chang X, Liu YY, Fan JY, Sun K, Li Q, Han JY. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci Rep. 2017;7:44579. doi: 10.1038/srep44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue Y, Fu W, Liu Y, Yu P, Sun M, Li X, Yu X, Sui D. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J Food Sci. 2020;85:4039–4049. doi: 10.1111/1750-3841.15505. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Zhang J, Zhang P, Si A, Zhang Z, Zhao L, Lv F, Zhao G. Ginkgolide B ameliorates myocardial ischemia reperfusion injury in rats via inhibiting endoplasmic reticulum stress. Drug Des Devel Ther. 2019;13:767–774. doi: 10.2147/DDDT.S179101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Shu Z, Yang Y, Yang L, Jiang H, Yu X, Wang Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019;10:203–215. doi: 10.1039/c8fo01256c. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Guo Y, Chen Y, Liu S, Wu N, Jia D. Curculigoside attenuates myocardial ischemiareperfusion injury by inhibiting the opening of the mitochondrial permeability transition pore. Int J Mol Med. 2020;45:1514–1524. doi: 10.3892/ijmm.2020.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Shi K, Lin T, Xia F, Cai Y, Ye Y, Liu L, Liu F. Ganoderic acid A alleviates myocardial ischemia-reperfusion injury in rats by regulating JAK2/STAT3/NF-kappaB pathway. Int Immunopharmacol. 2020;84:106543. doi: 10.1016/j.intimp.2020.106543. [DOI] [PubMed] [Google Scholar]
- 28.Wu B, Feng JY, Yu LM, Wang YC, Chen YQ, Wei Y, Han JS, Feng X, Zhang Y, Di SY, Ma ZQ, Fan CX, Ha XQ. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br J Pharmacol. 2018;175:4137–4153. doi: 10.1111/bph.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao XB, Qin Y, Niu YL, Yang J. Matrine inhibits hypoxia/reoxygenation-induced apoptosis of cardiac microvascular endothelial cells in rats via the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2018;106:117–124. doi: 10.1016/j.biopha.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Che J, Zhao H, Tang J, Shi G. Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem Biophys Res Commun. 2018;503:3219–3224. doi: 10.1016/j.bbrc.2018.08.129. [DOI] [PubMed] [Google Scholar]
- 31.Tang J, Lu L, Liu Y, Ma J, Yang L, Li L, Guo H, Yu S, Ren J, Bai H, Yang J. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1alpha signaling. J Cell Biochem. 2019;120:9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Sun Z, Meng F. Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation. 2017;40:1903–1911. doi: 10.1007/s10753-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 33.Shen P, Chen J, Pan M. The protective effects of total paeony glycoside on ischemia/reperfusion injury in H9C2 cells via inhibition of the PI3K/Akt signaling pathway. Mol Med Rep. 2018;18:3332–3340. doi: 10.3892/mmr.2018.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Adameova A, Goncalvesova E, Szobi A, Dhalla NS. Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev. 2016;21:213–221. doi: 10.1007/s10741-016-9537-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhuo Y, Yuan R, Chen X, He J, Chen Y, Zhang C, Sun K, Yang S, Liu Z, Gao H. Tanshinone I exerts cardiovascular protective effects in vivo and in vitro through inhibiting necroptosis via Akt/Nrf2 signaling pathway. Chin Med. 2021;16:48. doi: 10.1186/s13020-021-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X, Ren Y, Yang F. Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine. 2019;63:153035. doi: 10.1016/j.phymed.2019.153035. [DOI] [PubMed] [Google Scholar]
- 39.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 42.Calise J, Powell SR. The ubiquitin proteasome system and myocardial ischemia. Am J Physiol Heart Circ Physiol. 2013;304:H337–349. doi: 10.1152/ajpheart.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 44.Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond) 2018;132:1669–1683. doi: 10.1042/CS20180510. [DOI] [PubMed] [Google Scholar]
- 45.Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol. 2017;174:4329–4344. doi: 10.1111/bph.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Y, Undyala VV, Gottlieb RA, Mentzer RM Jr, Przyklenk K. Autophagy: definition, molecular machinery, and potential role in myocardial ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2010;15:220–230. doi: 10.1177/1074248410370327. [DOI] [PubMed] [Google Scholar]
- 47.Pedro JM, Wei Y, Sica V, Maiuri MC, Zou Z, Kroemer G, Levine B. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy. 2015;11:452–459. doi: 10.1080/15548627.2015.1017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu LJ, Chen RC, Ma XY, Zhu Y, Sun GB, Sun XB. Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine. 2020;68:153169. doi: 10.1016/j.phymed.2020.153169. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Gao Q, Yang J, Wang C, Cao J, Sun J, Fan Z, Fu L. Artemisinin suppresses myocardial ischemia-reperfusion injury via NLRP3 inflammasome mechanism. Mol Cell Biochem. 2020;474:171–180. doi: 10.1007/s11010-020-03842-3. [DOI] [PubMed] [Google Scholar]
- 50.Liu XW, Lu MK, Zhong HT, Wang LH, Fu YP. Panax notoginseng saponins attenuate myocardial ischemia-reperfusion injury through the HIF-1alpha/BNIP3 pathway of autophagy. J Cardiovasc Pharmacol. 2019;73:92–99. doi: 10.1097/FJC.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Wang Q, Zhang L, Ke Z, Zhao Y, Wang D, Chen H, Jiang X, Gu M, Fan S, Huang C. Coptisine protects cardiomyocyte against hypoxia/reoxygenation-induced damage via inhibition of autophagy. Biochem Biophys Res Commun. 2017;490:231–238. doi: 10.1016/j.bbrc.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Fu S, Chen L, Wu Y, Tang Y, Tang L, Zhong Y, Wang S, Liu H, Wang X, Chen A. Gastrodin pretreatment alleviates myocardial ischemia/reperfusion injury through promoting autophagic flux. Biochem Biophys Res Commun. 2018;503:2421–2428. doi: 10.1016/j.bbrc.2018.06.171. [DOI] [PubMed] [Google Scholar]
- 53.Tang H, Song X, Ling Y, Wang X, Yang P, Luo T, Chen A. Puerarin attenuates myocardial hypoxia/reoxygenation injury by inhibiting autophagy via the Akt signaling pathway. Mol Med Rep. 2017;15:3747–3754. doi: 10.3892/mmr.2017.6424. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZK, Chen RR, Li JH, Chen JY, Li W, Niu XL, Wang FF, Wang J, Yang JX. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: the role of the SIRT1/NF-kappaB pathway. Int Immunopharmacol. 2020;89:107086. doi: 10.1016/j.intimp.2020.107086. [DOI] [PubMed] [Google Scholar]
- 55.Liao CL, Liu Y, Huang MZ, Liu HY, Ye ZL, Su Q. Myocardial ischemia reperfusion injury is alleviated by curcumin-peptide hydrogel via upregulating autophagy and protecting mitochondrial function. Stem Cell Res Ther. 2021;12:89. doi: 10.1186/s13287-020-02101-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Wei W, Peng J, Li J. Curcumin attenuates hypoxia/reoxygenation induced myocardial injury. Mol Med Rep. 2019;20:4821–4830. doi: 10.3892/mmr.2019.10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 58.McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, Lu JQ, Branton WG, Power C. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A. 2018;115:E6065–E6074. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553–H1568. doi: 10.1152/ajpheart.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye B, Chen X, Dai S, Han J, Liang X, Lin S, Cai X, Huang Z, Huang W. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019;13:975–990. doi: 10.2147/DDDT.S195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun W, Lu H, Lyu L, Yang P, Lin Z, Li L, Sun L, Lu D. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J Physiol Biochem. 2019;75:531–547. doi: 10.1007/s13105-019-00702-7. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L, Zhou Z, Zhu C, Fu Z, Yu D. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-kappaB pathway. Int Immunopharmacol. 2020;85:106680. doi: 10.1016/j.intimp.2020.106680. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-kappaB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042–1052. doi: 10.1016/j.biopha.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 64.Zhao J, Zhang J, Liu Q, Wang Y, Jin Y, Yang Y, Ni C, Zhang L. Hongjingtian injection protects against myocardial ischemia reperfusion-induced apoptosis by blocking ROS induced autophagic-flux. Biomed Pharmacother. 2021;135:111205. doi: 10.1016/j.biopha.2020.111205. [DOI] [PubMed] [Google Scholar]
- 65.Han JY, Li Q, Pan CS, Sun K, Fan JY. Effects and mechanisms of QiShenYiQi pills and major ingredients on myocardial microcirculatory disturbance, cardiac injury and fibrosis induced by ischemia-reperfusion. Pharmacol Res. 2019;147:104386. doi: 10.1016/j.phrs.2019.104386. [DOI] [PubMed] [Google Scholar]
- 66.Yu X, Zhao XD, Bao RQ, Yu JY, Zhang GX, Chen JW. The modified Yi qi decoction protects cardiac ischemia-reperfusion induced injury in rats. BMC Complement Altern Med. 2017;17:330. doi: 10.1186/s12906-017-1829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q, Li N, Cui HH, Tian XQ, Jin C, Chen GH, Yang YJ. Tongxinluo exerts protective effects via anti-apoptotic and pro-autophagic mechanisms by activating AMPK pathway in infarcted rat hearts. Exp Physiol. 2017;102:422–435. doi: 10.1113/EP086192. [DOI] [PubMed] [Google Scholar]
- 68.Yang W, Lai Q, Zhang L, Zhang Y, Zhang Y, Yu B, Li F, Kou J. Mechanisms dissection of the combination GRS derived from ShengMai preparations for the treatment of myocardial ischemia/reperfusion injury. J Ethnopharmacol. 2021;264:113381. doi: 10.1016/j.jep.2020.113381. [DOI] [PubMed] [Google Scholar]
- 69.Cao Y, He X, Lui F, Huang Z, Zhang Y. Chinese medicinal formula Guanxin Shutong capsule protects the heart against oxidative stress and apoptosis induced by ischemic myocardial injury in rats. Exp Ther Med. 2014;7:1033–1039. doi: 10.3892/etm.2014.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F, Huang ZZ, Sun YH, Li T, Yang DH, Xu G, Su YY, Zhang T. Four main active ingredients derived from a traditional Chinese medicine Guanxin Shutong capsule cause cardioprotection during myocardial ischemia injury calcium overload suppression. Phytother Res. 2017;31:507–515. doi: 10.1002/ptr.5787. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Guo R, Li L, Li S, Fan G, Zhao X, Wang Y. Tongmai formula improves cardiac function via regulating mitochondrial quality control in the myocardium with ischemia/reperfusion injury. Biomed Pharmacother. 2020;132:110897. doi: 10.1016/j.biopha.2020.110897. [DOI] [PubMed] [Google Scholar]
- 72.Shi X, Zhu H, Zhang Y, Zhou M, Tang D, Zhang H. XuefuZhuyu decoction protected cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. BMC Complement Altern Med. 2017;17:325. doi: 10.1186/s12906-017-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y, Zhang X, Luan J, Zhao B, An N, Sun N, Wang X, Zhao T, Sun Y, Fu ZD, Zhang Y, Zhang Y. Shenxian-Shengmai oral liquid reduces myocardial oxidative stress and protects myocardium from ischemia-reperfusion injury. Cell Physiol Biochem. 2018;48:2503–2516. doi: 10.1159/000492688. [DOI] [PubMed] [Google Scholar]
- 74.Zhang S, Liu H, Fang Q, He H, Lu X, Wang Y, Fan X. Shexiang Tongxin dropping pill protects against chronic heart failure in mice via inhibiting the ERK/MAPK and TGF-beta signaling pathways. Front Pharmacol. 2021;12:796354. doi: 10.3389/fphar.2021.796354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan YF, Yu J, Pan WJ, Qi JY, Zhang MZ. Protective mechanisms of Suxiao Jiuxin pills () on myocardial ischemia-reperfusion injury in vivo and in vitro. Chin J Integr Med. 2020;26:583–590. doi: 10.1007/s11655-020-2726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun YH, Bu R, Wang YW, Hu YC, Wang XM, Dong X, Zu W, Niu Y, Zhao PW, Sun P, Ru SH, Lu JK, Na SS. Validation of efficacy and mechanism of Sanwei-Tanxiang powder in improving myocardial ischemia reperfusion injuries. Sci Rep. 2021;11:664. doi: 10.1038/s41598-020-80861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Y, Chen S, Wang H, Peng XM. Electroacupuncture pretreatment regulates apoptosis of myocardial ischemia-reperfusion injury in rats through RhoA/p38MAPK pathway mediated by miR-133a-5p. Evid Based Complement Alternat Med. 2021;2021:8827891. doi: 10.1155/2021/8827891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han YL, Chen S, Peng X. Electroacupuncture Pretreatment at Neiguan (PC6) attenuates autophagy in rats with myocardial ischemia reperfusion through the phosphatidylinositol 3-kinase-Akt-mammalian target of rapamycin pathway. J Tradit Chin Med. 2021;41:455–462. doi: 10.19852/j.cnki.jtcm.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 79.Li N, Li L, Wu H, Zhou H. Antioxidative property and molecular mechanisms underlying geniposide-mediated therapeutic effects in diabetes mellitus and cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7480512. doi: 10.1155/2019/7480512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Zhu L, Li H, Tang Q. Electroacupuncture pretreatment as a novel avenue to protect heart against ischemia and reperfusion injury. Evid Based Complement Alternat Med. 2020;2020:9786482. doi: 10.1155/2020/9786482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lou Y, Yu Q, Xu K, Tu Y, Balelang MF, Lu G, Zhu C, Dai Q, Geng W, Mo Y, Wang J. Electroacupuncture preconditioning protects from lung injury induced by limb ischemia/reperfusion through TLR4 and NFkappaB in rats. Mol Med Rep. 2020;22:3225–3232. doi: 10.3892/mmr.2020.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji C, Song F, Huang G, Wang S, Liu H, Liu S, Huang L, Liu S, Zhao J, Lu TJ, Xu F. The protective effects of acupoint gel embedding on rats with myocardial ischemia-reperfusion injury. Life Sci. 2018;211:51–62. doi: 10.1016/j.lfs.2018.09.010. [DOI] [PubMed] [Google Scholar]


