Abstract
Platinum derivatives are commonly used for the treatment of patients with metastatic triple-negative breast cancer (TNBC). However, resistance often develops, leading to treatment failure. This expansion cohort (part C2) of the previously reported phase 1b trial (NCT02157792) is based on the recommended phase 2 dose of the combination of the ataxia-telangiectasia and Rad3-related (ATR) inhibitor berzosertib and cisplatin observed in patients with advanced solid tumors, including TNBC. Forty-seven patients aged ≥18 years with advanced TNBC received cisplatin (75 mg/m2; day 1) and berzosertib (140 mg/m2; days 2 and 9), in 21-day cycles. Berzosertib was well tolerated, with a similar toxicity profile to that reported previously for this combination. The overall response rate (90% confidence interval) was 23.4% (13.7, 35.8). No relevant associations were observed between response and gene alterations. Further studies combining ATR inhibitors with platinum compounds may be warranted in highly selected patient populations.
Subject terms: Breast cancer, Cancer therapy
Introduction
Triple-negative breast cancer (TNBC) accounts for ~10–20% of all patients with breast cancer and is traditionally defined by the lack of expression of the estrogen and progesterone receptors, alongside no overexpression or amplification of the human epidermal growth factor receptor 2 (HER2)1. TNBC has the poorest prognosis amongst all breast cancer subtypes2; the reported 5-year survival for patients with any metastatic breast cancer is ~27%, compared with 11% in patients with metastatic TNBC3,4. At least four different subtypes of breast cancers have been identified according to their gene expression profile, namely: luminal A, luminal B, HER2-enriched, and basal-like (basal)5,6.
In the setting of advanced TNBC, carboplatin has previously demonstrated similar efficacy and a more favorable toxicity profile compared to docetaxel7–9, and therefore platinum derivates have commonly been used for the treatment of metastatic disease10. However, the treatment options for patients with advanced TNBC have expanded in the last few years as new therapeutic modalities, such as immune checkpoint inhibitors and antibody–drug conjugates, have become available11–13. Poly (ADP-Ribose) Polymerase (PARP) inhibitors have also been approved for use in patients with germline Breast Cancer Gene (BRCA) 1/2-mutated, HER2-negative metastatic breast cancer. This includes patients with TNBC who have germline BRCA1/2 mutations, with clinical benefits also extending to those with somatic BRCA1/2 mutations, although it is unclear whether similar benefits will be observed in patients with TNBC14,15 without germline or somatic BRCA1/2 mutations. Despite some progress being made, there remains a significant unmet medical need to improve treatment outcomes for patients with advanced TNBC.
Ataxia telangiectasia and Rad3-related (ATR) and ataxia-telangiectasia-mutated (ATM) protein kinases are members of the DNA damage response (DDR) family of proteins. Both ATM and ATR are key regulators of cell cycle checkpoint control and predominantly direct repair of DNA via homologous recombination16. Loss or inactivation of a single member of the DDR family of proteins has been associated with a greater reliance on other family members to respond to DNA damage, including damage induced by platinum-based chemotherapy. Preclinical experiments conducted using human ovarian and breast cancer cell lines suggest that their sensitivity to DNA-damaging agents may be increased when used in combination with ATR inhibitors17. Preclinical evidence also suggests that tumor protein 53 (TP53) mutant status correlates with the response to ATR inhibition in combination with DNA damaging agents18–20. Since basal breast cancers often present with a high frequency of TP53 mutations21,22, combining ATR inhibitors and DNA-damaging chemotherapy is a theoretically efficacious therapeutic approach in this setting.
Berzosertib (formerly M6620, VX-970) is an intravenously (i.v.) administered, highly potent and selective, first-in-class inhibitor of ATR23. Preclinical studies have demonstrated the ability of ATR inhibitors to sensitize breast cancer cell lines to platinum-based chemotherapy, namely cisplatin17. The part A and B cohorts of the first-in-human study (NCT02157792) phase 1 trial of berzosertib established the recommended phase 2 dose (RP2D) and demonstrated its tolerability, both as a single agent and in combination with certain chemotherapies; preliminary signs of efficacy were also observed in patients with a range of solid tumor types, including TNBC24,25. The tolerability, preliminary signs of efficacy, and favorable pharmacokinetics (PK) identified in this study have been confirmed in other clinical studies of berzosertib26–28. A berzosertib–topotecan combination has also been evaluated in a recent proof-in-concept phase 2 study, reporting an objective response rate of 36% (9/25) and a median duration of response of 6.4 months in patients with SCLC, including platinum-resistant patients29. Parts A and B of NCT02157792 were dose escalation studies that established the RP2D of berzosertib in combination with gemcitabine (part A; berzosertib 210 mg/m2 [days 2 and 9] in combination with gemcitabine 1000 mg/m2 [days 1 and 8])24 and cisplatin (part B; berzosertib 140 mg/m2 [days 2 and 9] in combination with cisplatin 75 mg/m2 [day 1])25 in 21-day cycles.
Here we report the results from the C2 expansion cohort of the same study, which investigated the safety and tolerability, efficacy, PK, and potential predictive biomarkers of berzosertib in combination with cisplatin in patients with advanced TNBC whose tumors were germline BRCA1/2 wild-type and basal subtype.
Results
Patient demographics and disposition
Forty-seven patients were enrolled into this study, all of whom were included in the safety analysis set (SAF) and modified full analysis set (mFAS) for efficacy (Table 1). The PK analysis set (PAS) included all enrolled patients who received at least one dose of berzosertib and provided at least one measurable post-dose concentration; 41 patients were included in the PAS, with 6 patients excluded as they did not have a measurable post-dose PK berzosertib concentration. The modified primary efficacy set (mPES) included all patients in the mFAS who were basal subtype and BRCA1/2 germline wild-type; 35 patients were included in the mPES, with 12 patients excluded due to a lack of BRCA1/2 gWT status data or were not basal subtype. Among all patients, 34 (72.3%) were TP53 mutant; 2 (4.3%) were TP53 wild-type; 31 (66%) were BRCA1/2 germline wild-type; and 36 (76.6%) were basal subtype, based on Prediction Analysis of Microarray 50 (PAM50). In the 7 patients who were not basal subtype, 4 (57.1%) had a TP53 mutation, 1 (14.3%) was TP53 wild-type and 2 (28.6%) had an unknown TP53 mutational status; in the 36 (76.6%) patients who were basal subtype, 29 (81%) had a TP53 mutation, 1 (3%) was TP53 wild-type, and 6 (17%) had an unknown TP53 mutational status. Four patients had an unknown subtype (Table 2).
Table 1.
Patient demographics and baseline characteristics.
| Characteristic, N (%) unless stated | Total N = 47 |
|---|---|
| Sex | |
| Male | 0 |
| Female | 47 (100) |
| Ethnicity | |
| Caucasian/White | 41 (87.2) |
| Non-Caucasian/non-White | 3 (6.4) |
| Age, years; median (range) | 48.0 (35–73) |
| Baseline ECOG PS | |
| 0 | 25 (53.2) |
| 1 | 22 (46.8) |
| Prior anticancer therapy | |
| Chemotherapy | 45 (95.7) |
| Immunotherapy | 1 (2.1) |
| Other | 11 (23.4) |
| Missing | 2 (4.3) |
| Number of previous anticancer therapy regimens | |
| Neoadjuvant | 16 (34.0) |
| Adjuvant | 27 (57.4) |
| 1st line, metastatic disease | 24 (51.1) |
| 2nd line, metastatic disease | 10 (21.3) |
| >2nd line, metastatic disease | 5 (10.6) |
| Missing | 2 (4.3) |
| TP53a | |
| Wild type | 2 (4.3) |
| Mutant | 34 (72.3) |
| Missing | 11 (23.4) |
| BRCA1/2b | |
| Wild type | 31 (66) |
| Mutant | 5 (10.6) |
| Missing | 11 (23.4) |
| Basal subtypec | |
| Yes | 36 (76.6) |
| No | 7 (14.9) |
| Missing | 4 (8.5) |
ATM ataxia-telangiectasia mutated, BRCA1/2 breast cancer gene 1/2, ECOG PS Eastern Cooperative Oncology Group Performance Status, TP53 tumor protein 53.
aOnly patients with biomarker status determined by FoundationOne® CDx next generation sequencing were reported.
bPatients with unknown BRCA1/2 status were prospectively tested at screening by BRCAnalysis assay (Myriad Genetics); patients that were found to be BRCA1/2 germline mutant were still enrolled in the study.
cAssessment for basal subtype was performed retrospectively via PAM50 analysis (Prosigna).
Table 2.
TP53 mutational status.
| TP53 status | Basal subtype (N = 36) | Non-basal subtype (N = 7) |
|---|---|---|
| Wild type | 1 (3.0) | 1 (14.3) |
| Mutant | 29 (81.0) | 4 (57.1) |
| Missing | 6 (17.0) | 2 (28.6) |
TP53 tumor protein 53.
In all, 24 (51.1%) patients had previously received only one line of therapy for metastatic disease, 10 (21.3%) patients had received two lines of therapy, and 5 (10.6%) received more than two lines of therapy for metastatic disease.
All 47 patients in the mFAS received at least one dose of berzosertib, 46 (97.9%) received at least one dose of cisplatin, and 12 (25.5%) received at least one dose of carboplatin; the patient who received berzosertib without cisplatin received at least one dose of carboplatin. Thirty-eight (80.9%) patients completed treatment with berzosertib, while nine (19.1%) discontinued treatment, primarily due to patient decision (four, 8.5%) or adverse events (AEs; two, 4.3%). Sixteen patients (34%) discontinued cisplatin, primarily due to AEs (12.8%) and remained on berzosertib monotherapy until PD. The median (range) duration of treatment for berzosertib in combination with cisplatin was 15.0 (5 cycles) (2.0, 137.1) weeks. No patients died during this study.
Efficacy
Forty-seven patients were included in the mFAS; however four patients were non-evaluable. The objective response rate (ORR [90% confidence interval (CI)]) was 11/47 (23.4%) patients (13.7, 35.8), the best overall response (BOR) was complete response (CR) for two (4.3%) patients, partial response (PR) for nine (19.1%) patients, and stable disease (SD) for 18 (38.3%) patients (Table 3 and Fig. 1a); all responses were confirmed. For patients with SD (N = 18), 3 patients (16.7%) progressed within 3 months, 10 (55.6%) patients progressed within 3–6 months, and 5 (27.8%) patients progressed within 6–12 months. BOR was also retrospectively stratified by prior lines of treatment for metastatic disease (Supplementary Table 1); all patients who achieved a BOR of CR were previously treated only in the neoadjuvant/adjuvant setting, while no patients who had received more than two lines of therapy for metastatic disease achieved a response.
Table 3.
Efficacy responses (modified full analysis set, N = 47).
| Efficacy outcome | Patients, n (%) unless stated |
|---|---|
| BOR | |
| CR | 2 (4.3) |
| PR | 9 (19.1) |
| SD | 18 (38.3) |
| PD | 14 (29.8) |
| Not evaluable | 4 (8.5) |
| ORR, N (%), (90% CI) | 11 (23.4) (13.7, 35.8) |
| DCR, N (%), (90% CI) | 29 (61.7) (48.7, 73.6) |
| Median PFS (months), (90% CI) | 4.0 (2.8, 6.0) |
| Median OS (months), (90% CI) | 12.4 (7.8, 14.5) |
| Median DOR (months), (90% CI) | 6.0 (5.1, nd) |
DCR was defined as the proportion of patients with disease control, defined as a BOR of CR, PR, or SD.
BOR best overall response, CI confidence interval, CR complete response, DCR disease control rate, DOR duration of response, nd not defined, ORR overall response rate, OS overall survival, PD progressive disease, PFS progression-free survival, PR partial response, SD stable disease.
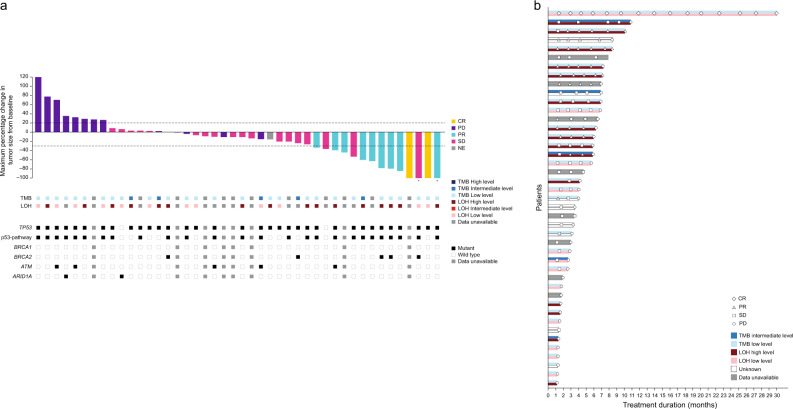
Fig. 1. Best percentage change in tumor size from baseline and tumor response by LOH and TMB scores (modified full analysis set).
a Best percentage change in tumor size from baseline, b tumor response by LOH and TMB scores. Patients without on-treatment target lesion measurements are not shown in Fig. 1a. Patients without response assessments are not shown in Fig. 1b. All response categories were determined according to RECIST 1.1 criteria. Full details of mutations can be found in Supplementary Table 5. CR complete response, LOH loss of heterozygosity, PD progressive disease, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease, TMB tumor mutational burden.
The median duration of response (DOR; [90% CI]) was 6.0 months (5.1, not defined). Median progression-free survival (PFS; [90% CI]) was 4.0 months (2.8, 6.0), and median overall survival (OS; [90% CI]) was 12.4 months (7.8, 14.5).
Similar results were observed for the mPES: the ORR (90% CI) was 9/35 (25.7%) patients (14.1, 40.6), 2 (5.7%) patients had a BOR of CR, 7 (20.0%) had a BOR of PR, and 15 (42.9%) had a BOR of SD; 10 (28.6%) patients had a BOR of PD. The ORR for patients who were germline BRCA1/2 wild-type vs mutant were 7/31 (22.6%) patients vs 2/5 (40.0%) patients, respectively, while for patients who were basal subtype vs those who were not, the ORRs were 9/36 (25.0%) patients vs 1/7 (14.3%) patients, respectively. However, no formal statistical comparison for these subgroups was made. Further information on ORR by biomarker status can be found in Table 4.
Table 4.
ORR for selected biomarker subgroups; modified full analysis set (N = 47).
| Gene | Patients, n | Responses, n | ORR, % (90% CI) |
|---|---|---|---|
| TP53a | |||
| Wild type | 2 | 0 | 0.0 (0.0, 77.6) |
| Mutant | 34 | 9 | 26.5 (14.6, 41.6) |
| ARID1A | |||
| Wild type | 34 | 9 | 26.5 (14.6, 41.6) |
| Mutant | 2 | 0 | 0.0 (0.0, 77.6) |
| ATM | |||
| Wild type | 31 | 8 | 25.8 (13.5, 41.8) |
| Mutant | 5 | 1 | 20.0 (1.0, 65.7) |
| Germline BRCA1/2 | |||
| Wild type | 31 | 7 | 22.6 (11.1, 38.3) |
| Mutant | 5 | 2 | 40.0 (7.6, 81.1) |
| Basal subtypeb | |||
| Yes | 36 | 9 | 25.0 (13.7, 39.6) |
ARID1A AT-rich interaction domain 1A, ATM ataxia-telangiectasia-mutated, BRCA1/2 breast cancer gene 1/2, CI confidence interval, ORR overall response rate, PAM50 Prediction Analysis of Microarray 50, TP53 tumor protein 53.
aOnly patients with biomarker status determined by FoundationOne® CDx next-generation sequencing were reported.
bBasal subtype was determined via PAM50 analysis (Prosigna) and was performed retrospectively.
Safety and tolerability
A summary of treatment-emergent AEs (TEAEs) is presented in Table 5. The most common TEAEs, which occurred in more than 50% of patients, were nausea (N = 39, 83.0%), fatigue (N = 32, 68.1%), neutropenia (N = 29, 61.7%), and vomiting (N = 28, 59.6%); a full list of TEAEs affecting ≥20% of patients can be found in Table 5. TEAEs of grade ≥3 occurred in 32 patients (62.1%). TEAEs of grade ≥3 occurring in more than 10% of patients were neutropenia (N = 18, 38.3%), anemia (N = 12, 25.5%), thrombocytopenia (N = 6, 12.8%), and vomiting (N = 6, 12.8%). Febrile neutropenia occurred in two (4.3%) patients. Forty-five (95.7%) patients had a berzosertib-related TEAE; of these, 27 (57.4%) had a related TEAE of grade ≥3. There were no notable adverse events relating to laboratory results, vital signs or electrocardiogram (ECG) measurements.
Table 5.
Overview of TEAEs for berzosertib and cisplatin (safety analysis set, N = 47).
| Patients, N (%) | Berzosertib + Cisplatin Any grade |
Berzosertib + Cisplatin Grade ≥3 |
|---|---|---|
| N = 47 | N = 47 | |
| TEAE | 47 (100) | 36 (76.6) |
| Berzosertib-related TEAE | 45 (95.7) | 27 (57.4) |
| Cisplatin or carboplatin-related TEAE | 47 (100) | 31 (66.0) |
| Berzosertib or cisplatin or carboplatin-related TEAE | 47 (100) | 32 (68.1) |
| TEAEs occurring in ≥20% of patients | ||
| Nausea | 39 (83.0) | 4 (8.5) |
| Fatigue | 32 (68.1) | 1 (2.1) |
| Neutropenia | 29 (61.7) | 18 (38.3) |
| Vomiting | 28 (59.6) | 6 (12.8) |
| Tinnitus | 21 (44.7) | 0 |
| Anemia | 19 (40.4) | 12 (25.5) |
| Headache | 18 (38.3) | 0 |
| Diarrhea | 16 (34.0) | 0 |
| Constipation | 14 (29.8) | 0 |
| Dizziness | 11 (23.4) | 0 |
| Decreased appetite | 10 (21.3) | 0 |
| Serious TEAE | 15 (31.9) | 13 (27.7) |
| Berzosertib-related serious TEAE | 10 (21.3) | 8 (17.0) |
| Cisplatin or carboplatin-related serious TEAE | 10 (21.3) | 8 (17.0) |
| Berzosertib or cisplatin or carboplatin-related serious TEAE | 10 (21.3) | 8 (17.0) |
| TEAEs leading to permanent discontinuation of treatment | ||
| TEAE leading to permanent discontinuation of berzosertib | 5 (10.6) | NR |
| Berzosertib-related TEAE leading to permanent discontinuation of berzosertib | 3 (6.4) | NR |
| Cisplatin-related TEAE leading to permanent discontinuation of cisplatin | 8 (17.0) | NR |
| Carboplatin-related TEAE leading to permanent discontinuation of carboplatin | 3 (6.4) | NR |
| TEAE leading to a dose reduction in at least one study drug | 11 (23.4) | NR |
| Berzosertib-related TEAE leading to dose reduction in berzosertib | 3 (6.4) | NR |
| Cisplatin-related TEAE leading to dose reduction in cisplatin | 8 (17.0) | NR |
| Carboplatin-related TEAE leading to dose reduction in carboplatin | 1 (2.1) | NR |
| TEAE leading to temporary discontinuation of at least one study drug | 24 (51.1) | NR |
| Berzosertib-related TEAE leading to temporary discontinuation of berzosertib | 21 (44.7) | NR |
| Cisplatin-related TEAE leading to temporary discontinuation of cisplatin | 15 (31.9) | NR |
| Carboplatin-related TEAE leading to temporary discontinuation of carboplatin | 5 (10.6) | NR |
| TEAE leading to death | 0 | 0 |
NR not reported, TEAE treatment-emergent adverse event.
In all, 15 (31.9%) patients had a serious TEAE, with 13 (27.7%) having a grade ≥3 serious TEAE. Of these patients, 10 (21.3%) reported a serious TEAE related to berzosertib, 8 (17%) related to cisplatin, and 2 (4.3%) related to carboplatin.
In total, 11 (23.4%) patients experienced a TEAE leading to a dose reduction in at least one study drug (3 [6.4%], 8 [17.0%], and 1 [2.1%] TEAEs leading to permanent dose reduction were related to berzosertib, cisplatin, and carboplatin, respectively).
In total, 24 (51.1%) patients experienced a TEAE leading to temporary discontinuation of at least one study drug (21 [44.7%], 15 [31.9%], and 5 [10.6%] TEAEs leading to temporary discontinuation were related to berzosertib, cisplatin, and carboplatin, respectively). Five (10.6%) patients in this expansion cohort permanently discontinued berzosertib during the study due to TEAEs, three of whom discontinued due to berzosertib-related TEAEs (one patient each experienced neutropenia [grade 2], anemia and thrombocytopenia [both grade 3], and peripheral neuropathy [grade 2]).
Biomarkers
No conclusive association was identified between clinical outcome (ORR or PFS) and the mutational status of TP53, AT-rich interaction domain 1A (ARID1A), ATM or BRCA1/2 (Table 4 and Supplementary Table 2). Post-hoc exploratory biomarker assessments of loss of heterozygosity (LOH) status and tumor mutational burden (TMB) using Foundation Medicine’s (FMI) FoundationOne® CDx assay30,31 in these patients did not identify any statistically significant association between LOH and response or TMB and response (Fig. 1b). However, 7 of the 11 patients with a confirmed response had a high LOH score and 7 of the 16 total patients with a high LOH score responded to treatment, compared with only 1 of the 12 patients with a low LOH score. Although the association was not statistically significant (p = 0.09), the results suggest an enrichment of responders in tumors with high LOH (OR = 7.96) (Supplementary Table 3). No patients had a tumor with a high TMB score. In all, 7 of the 27 patients with a low TMB score had a confirmed response, compared with only 1 of the 5 patients with an intermediate TMB score.
A 40-year-old female patient with grade 3, metastatic, BRCA1/2 negative TNBC achieved a CR with a duration of 28.6 months, remaining in CR at the end of the study. The patient achieved a BOR of CR despite disease progression on previous chemotherapeutic treatment in the (neo)adjuvant setting. Further assessment of the biomarker status of this patient revealed heterozygous germline mutations in genes including adenovirus E1A-associated cellular p300 transcriptional co-activator protein (EP300), DNA polymerase delta 1, catalytic subunit (POLD1), and Tumor Growth Factor Beta Receptor 2 (TGFBR2), all variants of unknown significance. Archival tumor demonstrated low LOH and TMB scores, somatic TP53 mutation and several gene amplification events relating to MYC (copy number 18), CCNE1 (Cyclin E1; copy number 14), and RAD21 (copy number 18).
Pharmacokinetics
The PAS included 41 patients, with six patients excluded because their post-dose berzosertib concentration was outside the limit of quantification. The administered i.v. berzosertib dose of 140 mg/m2 was within the dose range that has previously shown dose-dependent berzosertib PK as monotherapy or in combination with either carboplatin or cisplatin24–26. The observed berzosertib concentration data in this expansion cohort were generally consistent with those reported previously at the same dose level25. Cisplatin had no apparent effect on berzosertib PK (Supplementary Fig. 1). Key berzosertib PK parameters from this study, presented as geometric mean (geometric coefficient of variance), were as follows: maximum observed concentration (Cmax [ng/mL]) – 555 (42.9%); area under the curve (AUC) from start of infusion to the 4-h sampling time after start of infusion (AUC0–4 [ng.h/mL]) – 1110 (26.4%). The Cmax in this expansion cohort was slightly lower than the 652 ng/mL (25%) and 854 ng/mL (63%) observed in the lead-in period and in the berzosertib and cisplatin combination cohort, respectively, at the same dose level in part B. The partial AUC0–4 was ~23% of the reported AUC0–∞ in part B25.
Discussion
In this dose expansion cohort study, berzosertib 140 mg/m2 (days 2 and 9) administered alongside cisplatin 75 mg/m2 (day 1) in 21-day cycles was well tolerated in patients with advanced TNBC. The safety profile of this combination was broadly consistent with that of the individual agents and no new or unexpected safety signals were identified for berzosertib26. Although a high rate of treatment interruptions was observed with berzosertib, these did not translate into dose reductions or permanent treatment discontinuation. The rate of bone marrow toxicity observed in this study with a limited number of participants may indicate the potential of ATR inhibition to increase the hematologic toxicity of cisplatin and may be an on-target effect. However, these toxicities were generally manageable and did not result in significant dose reductions or treatment discontinuations.
The observed PK data were generally consistent with the PK data reported previously at the same dose levels28. A population PK model built based on pooled data from two phase 1 studies, including this expansion cohort, suggested that administration of cisplatin does not affect observed berzosertib PK parameters28. Although the effects of TNBC tumor type on clearance were estimated in the model, an association between tumor type and PK is not anticipated28.
With the implicit caveats associated with cross-trial comparisons, the response rate observed in this expansion cohort (ORR: 23.4%; DOR: 6.0 months) was broadly consistent with those observed in historical studies conducted in patients with advanced TNBC treated with platinum-based therapy (TNT trial, carboplatin ORR: 31.4%; TBCRC009 trial, ORR: 25.6%)7,32.
Although preclinical evidence suggested a potent synergistic effect of berzosertib and cisplatin on cancer cell survival, particularly in tumors with TP53 mutations, this was not observed with the berzosertib and cisplatin doses utilized in this study18–20. The lower dose of berzosertib used in this study may have been insufficient for target engagement. For instance, a Phase 2 proof-of-concept study investigating a dose of 210 mg/m2 of berzosertib in combination with topotecan demonstrated durable tumor regression in patients with small-cell neuroendocrine cancers29, whereas no benefit in PFS was shown in a Phase 2 study investigating a dose of 90 mg/m2 berzosertib in combination with gemcitabine in patients with advanced urothelial carcinoma33. Additionally, patients in the clinical setting are likely to have tumors with complex genetic aberrations; hence, solely assessing TP53 mutational status may not provide a robust assessment of p53 pathway impairment. Several co-occurring DDR alterations may be necessary to confer sensitivity to the combination of ATR inhibition and platinum-derived chemotherapeutics.
One patient with TNBC and several co-occurring DDR alterations experienced a CR lasting for 28.6 months. Further assessment of the biomarker status of this patient revealed a number of amplification events relating to MYC, CCNE1, and RAD21. This exceptional response might be explained in part by the presence of these specific gene amplifications. Overexpression of MYC and CCNE1 (Cyclin E1) have been shown to increase replication stress, thus sensitizing tumors to ATR inhibition34,35. The role of RAD21 is less clear in this context, as up-regulation of RAD21 has been shown to mitigate replication stress arising from MYC overexpression36. Future research may explain the complex interplay between co-occurring gene amplifications and ATR inhibition.
Taken together, no conclusive associations were observed for gene alterations linked to higher susceptibility to ATR inhibition or increased reliance on ATR in preclinical experiments, such as ATM and ARID1A alterations37. This may be due to the low patient numbers; for example, there were only five patients with an ATM mutation (Supplementary Table 4). However, an enrichment of responses would have been expected in patients with ATM loss, which raises questions related to the functionality of ATM, or how this is defined (i.e. based on loss-of-function mutation, mono-allelic vs bi-allelic loss, or protein expression). Other studies have pointed towards such an association between ATM protein loss and response to ATR inhibition38. The identification of biomarkers of response to ATR inhibitors continues to be a very active field of research.
As LOH can indicate the presence of historical homologous recombination deficiency39,40 and has also been reported to predict response to platinum-based neoadjuvant chemotherapy in patients with TNBC41, an exploratory biomarker analysis focused on evaluating genetic alterations and genomic signatures was conducted in this trial. Interestingly, there were a greater number of clinical responses in patients with a high LOH status (7/16) within this patient population compared with patients with a low LOH status (1/12).
In conclusion, the combination of berzosertib and cisplatin in patients with advanced, pretreated TNBC was well tolerated. Although the observed efficacy signal does not warrant further development of the present combination and dose in patients with TNBC in a phase 2/3 setting, the clinical evaluation of berzosertib in a very selective sub-population of patients with breast cancer might be of interest to confirm the role of certain genetic or molecular markers, such as LOH. Clinical trials evaluating berzosertib in combination with DNA damage-inducing chemotherapy, such as topotecan, are ongoing in a variety of solid tumors including small-cell lung cancer.
Methods
Study design
This was a multicenter, open-label, non-randomized, first-in-human, phase 1 study conducted in six parts (A, B, B2, C1, C2, C3; NCT02157792, registered 06 June 2014). Part C2 was a single-arm, dose expansion cohort evaluating the safety and preliminary efficacy of berzosertib combined with cisplatin in patients with advanced TNBC using the berzosertib RP2D identified in part B. Patients were enrolled across five sites in the UK and 12 in the USA, between 09 December 2015 (study initiation date) and 11 March 2020 (study completion date, when the final patient completed their last visit).
This study was conducted in accordance with the ethical principles of the International Council for Harmonisation guideline for Good Clinical Practice and the Declaration of Helsinki, as well as with applicable local regulations. The Clinical Study Protocol and all required associated documents were approved by the following responsible Institutional Review Boards (IRB) or Independent Ethics Committees of the individual study sites: The Washington University in St. Louis IRB, Vanderbilt University IRB, North East – Tyne & Wear South Research Ethics Committee, Northwestern University Biomedical IRB, Stanford University Administrative Panels on Human Subjects in Medical Research Board, Greenville Health System IRB, Mayo Clinic IRB, The University Hospitals IRB, US Oncology IRB, NRES Committee North East – Sunderland, Dana Farber Cancer Institute IRB, Western IRB. All patients were required to provide written informed consent prior to enrollment.
Patients
Eligible patients were: ≥18 years of age; had advanced (locally advanced incurable or metastatic), histologically confirmed estrogen receptor, progesterone receptor, and HER2-negative breast cancer; adequate available historical tumor biopsies (core biopsy or surgical specimen); 0–2 prior therapies for the treatment of advanced breast cancer; and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.142. A minimum of 30 patients who were germline BRCA1/2 wild-type and had a basal subtype, as assessed by PAM50, were to be enrolled into this study. If one or more of these characteristics was unknown before screening, BRCA1/2 status was determined by central prospective germline testing during screening, and patients found to be germline BRCA1/2 mutant were still enrolled. If possible, assessment of basal subtype was performed retrospectively.
Prior taxane and/or an anthracycline chemotherapy in the metastatic setting was allowed, alongside one other non-platinum-based chemotherapy in the first- or second-line (no restrictions were placed on prior immunotherapy or targeted treatment in the metastatic setting, unless combined with a cytotoxic agent).
Key exclusion criteria included: any prior platinum therapy in the metastatic setting (adjuvant or neoadjuvant platinum-based chemotherapy was permitted if this was completed within 6 months of screening); relapse within 3 months of completion of prior adjuvant or neoadjuvant chemotherapy; known BRCA1/2 germline mutations, either determined and documented prior to screening or determined during screening; and documented intrinsic subtype other than basal by PAM50.
Full inclusion and exclusion criteria are provided in the supplementary information.
Treatments
The patients enrolled in this cohort received the RP2D established in part B25; following enrollment, patients received berzosertib (140 mg/m2) on days 2 and 9, ~24 h after receiving cisplatin (75 mg/m2; day 1) in 21-day cycles.
A patient could receive berzosertib in combination with carboplatin if they had not progressed but were unable to tolerate treatment due to toxicities associated with cisplatin, or it was considered by the Investigator to be in their best interest. The starting doses following switching would be berzosertib (90 mg/m2) plus carboplatin (AUC 5 mg/min/mL).
Patients received treatment until PD, unacceptable toxicity, withdrawal, or non-compliance with study protocol.
Objectives
The primary objectives of this study were to evaluate the safety, tolerability, and preliminary efficacy of berzosertib when combined with cisplatin in patients with advanced, basal, germline BRCA1/2 wild-type TNBC, with or without TP53 mutations. Exploratory objectives of this study included the evaluation of biomarkers potentially associated with response to berzosertib in combination with cisplatin.
Assessments and endpoints
The primary safety endpoints were TEAEs; clinical laboratory values (chemistry, hematology, urinalysis, coagulation); ECG; and vital signs. TEAEs were defined as any AEs that were reported, or worsened, on or after study drug initiation, through the safety follow-up visit. Serious TEAEs were defined as any AEs that were a congenital or birth abnormality, resulted in persistent or significant disability or incapacity, required or prolonged in-patient hospitalization, were life-threatening or resulted in death, or were otherwise deemed medically important. Related TEAEs were defined as any AE reported by the Investigator to have a relationship to study treatment, or where the relationship was unknown.
All AEs were coded according to the Medical Dictionary for Regulatory Activities V21.043 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.044.
The primary efficacy endpoint of this study was the ORR, defined as the proportion of participants with a BOR of PR or CR (summarized as objective response [OR] according to RECIST 1.1), where both PR and CR were confirmed by repeat assessments performed no less than 4 weeks after the criteria for response was first met.
Tumor assessments were performed from baseline until either end of treatment or PD using RECIST 1.1 guidelines. Initial disease was documented using baseline imaging scans (computed tomography or magnetic resonance imaging) taken within 14 days prior to the first dose of study drug; imaging was repeated at the end of every 2 cycles for the first 12 cycles, followed by every 2 or 3 cycles, and finally 5 ± 1 weeks after completion of therapy.
The secondary efficacy endpoints of this study were PFS, DOR, OS, and clinical benefit rate (CBR). PFS was defined as the time from the date of first study drug dose to the first documentation of PD or death due to any cause, whichever occurred first. DOR was defined as the time that response criteria were first met for PR or CR until the date that recurrent or PD disease was objectively documented. OS was defined as the time from the date of the first dose of study drug to death due to any cause. CBR was defined as the proportion of patients who achieved a BOR of CR, PR, or SD of ≥6 months, measured from the date of first study drug dose.
Blood samples for PK analysis of berzosertib were collected pre-dose, 0.5 h before the end of infusion, at the end of infusion, and at 0.5, 1, 2, 3, and optionally at 7 h after the end of infusion on cycle 1, day 2. A limited berzosertib PK sampling scheme was implemented in this expansion cohort, with samples collected up to 4 h after the start of infusion for all participants and optionally at 8 h; therefore, only a partial AUC up to 4 h after the start of infusion (AUC0–4) was estimated for berzosertib. Berzosertib was administered by i.v. infusion over 60 (±10) min; if the total volume of infusion was ≥600 mL, infusion time could be extended up to 90 min, as tolerated. Tumor biopsies – either historical core or surgical specimens, or core biopsy obtained at screening (if the biopsy could be considered standard clinical practice) – were collected for potential exploratory evaluation of correlations between genetic alterations and treatment outcomes.
Biomarker analyses
Germline BRCA1/2 mutational status was determined using the BRCAnalysis assay (Myriad Genetics, Inc., Salt Lake City, UT, USA) and tumor intrinsic subtype was assessed via PAM50 molecular profiling using the Breast Cancer Prognostic Gene Signature Assay (Prosigna®; LabCorp [Covance], Burlington, NC, USA).
Exploratory biomarkers – including LOH and TMB, surrogate markers for DNA repair deficiencies – were assessed using FMI’s FoundationOne® CDx next-generation sequencing (Foundation Medicine Inc., Cambridge, MA, USA). The genomic LOH score determined by FMI is assessed based on the percent of LOH in the tumor genome and is computed by inferring LOH regions across the 22 autosomal chromosomes using the genome-wide copy number profile and minor allele frequencies of the single-nucleotide polymorphisms. Regarding LOH, patients were categorized as having high or low LOH scores (≥16 or <16, respectively), while for TMB, patients were categorized (in somatic mutations per mega base [MB]) as high (≥20), intermediate (≥6–<20), or low (<6)30,31. The single nucleotide variants provided by the CDx assay were further analyzed for their functional consequences using the Variant Effect Predictor (VEP), Ensembl release 99® (Ensembl, Cambridge, UK)45. Using VEP annotation, single nucleotide and indel variations were grouped into the following categories, irrespective of the number of affected alleles: high impact, predicted high impact, and other. Only mutations with either a high or predicted high impact were considered in the analysis.
Statistical analyses
Planned enrollment for this cohort was 50 patients, with a minimum of 30 patients who were basal subtype and BRCA1/2 germline wild type; if BRCA1/2 status was unavailable before screening it was to be determined prospectively at screening, if possible. Patients known to be non-basal subtype or BRCA1/2 germline mutant were not screened for inclusion in this study.
Based on historical response rates of ~25% for single agent carboplatin7,32, the power for a one-sided method at different treatment response rates was calculated for a minimum of 30 patients who were basal subtype and BRCA1/2 germline wild type. If at least 12 responses were observed (approximate ORR of 40%), the estimated 90% CI were calculated as 25.0% and 56.6%, respectively.
Four distinct analysis sets were established with the following definitions: SAF, all enrolled patients who received at least one dose of study drug; PAS, all enrolled patients who received at least one dose of berzosertib and provided at least one measurable post-dose concentration; mFAS, all enrolled patients who received at least one dose of study drug, had a baseline scan with a measurable target lesion; and mPES, all patients in the mFAS who were basal subtype and BRCA1/2 germline wild type.
The primary efficacy endpoint of this study was analyzed in both the mFAS and the mPES. Each efficacy endpoint was calculated using two-sided 90% CI (OR and CBR using the Clopper-Pearson method46; PFS, OS, and DOR according to Brookmeyer and Crowley47).
Bioanalysis of berzosertib concentrations for PK analysis was performed in plasma samples using validated liquid chromatograph-tandem mass spectrometry methods in compliance with standard operating procedures. All PK analyses was conducted using standard non-compartmental analyses methods.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank patients, investigators, co-investigators, and the study teams at each of the participating centers and at the healthcare business of Merck KGaA, Darmstadt, Germany. The authors thank Vertex Pharmaceuticals for their involvement in the development of berzosertib (formerly M6620, VX-970). Giuseppe Locatelli, PhD, an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, contributed to the analysis and interpretation of the biomarker results. Bart Hendriks, PhD, a former employee of the healthcare business of Merck KGaA, Darmstadt, Germany, contributed to the analysis and interpretation of PK data. Annick Seithel-Keuth, PhD, an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, contributed to the analysis and interpretation of PK data. Danyi Wang, MD, PhD, an employee of EMD Serono, Billerica, MA, USA, provided technical and operational support of biomarker sample analysis. Medical writing assistance was provided by Alexander T. Hardy of Bioscript Stirling Ltd, Macclesfield, UK and funded by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). The trial (including acquisition of data; analysis and interpretation of data; study supervision, conception, and design; development of methodology; administrative, technical or material support; and writing, review, and/or revision of the manuscript) was sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany and Vertex Pharmaceuticals Incorporated., Boston, MA, USA.
Author contributions
Acquisition of data: R.W., S.R.L., E.D., S.M.T., R.P., T.C.H., A.T., M.T., and G.I.S. Analysis and interpretation of data: I.D.-P., T.Gr., J.D., R.P., T.Go., R.W., S.R.L., E.D., S.M.T., T.C.H., A.T., M.T., and G.I.S. Study supervision: R.W., S.R.L., R.P., A.T., M.T., and G.I.S. Conception and design: R.P. Writing, review, and/or revision of the manuscript: all authors. Read and approved final manuscript: all authors.
Data availabiliity
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the healthcare business of Merck KGaA, Darmstadt, Germany Data Sharing Policy. All requests should be submitted in writing to the healthcare business of he Merck KGaA, Darmstadt, Germany data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinicaltrials/commitment-responsible-data-sharing.html). When the healthcare business of Merck KGaA, Darmstadt, Germany has a co-research, co-development, co-marketing, or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.
Competing interests
M.L.T.: advisory role: AbbVie, Aduro, Blueprint Medicines Celgene, Daiichi Sankyo, Genentech, G1 Therapeutics, Immunomedics, Lilly, Merck & Co., Natera, OncoSec Medical, Pfizer. Institutional research funding from AbbVie, AstraZeneca, Bayer, Biothera, Calithera, EMD Serono, Billerica, MA USA, Genentech, Merck & Co., OncoSec Medical, Pfizer, PharmaMar, Tesaro, Vertex. S.M.T.: Institutional research funding from AstraZeneca, Lilly, Merck & Co., Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, Bristol-Myers Squibb, Eisai, Nanostring, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck & Co., Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Bristol-Myers Squibb, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Puma, Silverback Therapeutics, G1 Therapeutics, AbbVie, Anthenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, Gilead, and Samsung Bioepsis Inc. G.I.S. has received research funding from Eli Lilly, the healthcare business of EMD Serono, Billerica, MA USA, Merck & Co., and Sierra Oncology. He has served on advisory boards for Pfizer, Eli Lilly, G1 Therapeutics, Roche, the healthcare business of EMD Serono, Billerica, MA USA, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalis and CytomX Therapeutics. In addition, he holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent, entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell. M.M.: personal fees from Amgen, grants and personal fees from Roche, grants from AstraZeneca, grants and personal fees from GSK, personal fees and other from Novartis, other from Millenium, personal fees, non-financial support and other from Immunocore, personal fees and other from BMS and Eisai, other from Pfizer, personal fees, non-financial support and other from MSD, personal fees and other from Rigontec (acquired by MSD), other from Regeneron, personal fees from BiolineRx, personal fees and other from Array Biopharma (now Pfizer), non-financial support and other from Replimune, personal fees from Kineta, and personal fees from Silicon Therapeutics, outside the submitted work. S.R.L.: personal fees from Eisai, Shionogi and Prosigna, and was previously employed by Pfizer, research funding from Pathios Therapeutics, received travel, accommodation or expenses from Pfizer, Roche, Synthon and Piqur Therapeutics, cofounder and stock holding in Mitox Therapeutics. H.-T.A. has served as an advisor/consultant for Bicycle Therapeutics, Biontech, Bayer, Beigene, Servier, Roche and Guardant Health, and honoraria from Bicycle Therapeutics, Biontech, Bayer, Beigene, Servier, Roche and Guardant Health. A.T.: AstraZeneca-Financial support to academic and hospital institutions for costs associated with academic study chair and local site costs for OlympiA trial/Travel expenses related to any trial related travel/Payments to Institution through Breast International Group for trial conduct in Olympia trial and through CRO’s for commercial PARP inhibitor trials AstraZeneca with royalties paid by Institute of Cancer Research with royalties paid for use of PARP inhibitors in DNA deficient cancers, licensee - AstraZeneca. Payments to Institute of Cancer Research and personally through ICR rewards to Inventors scheme. the healthcare business of Merck KGaA, Darmstadt, Germany: local site trial support costs associated with clinical trial. Pfizer: personal fees/Advisory Board related to targeted therapies in DNA repair deficient cancers. Vertex: personal fees from/Advisory Board related to targeted therapies in DNA repair deficient. Artios: personal fees/Advisory Board related to targeted therapies in DNA repair deficient cancers. Prime Oncology-Personal fees/Advisory Board related to targeted therapies in DNA repair deficient cancers. Medivation: financial support for research at ICR. Breast Cancer Now Charity: grant funded to study homologous recombination deficient breast and other cancers, BCN receive payments through AstraZeneca related to PARP inhibitor patents. CRUK: grant funded to study homologous recombination deficient breast and other cancers, CRUK receive payments through AstraZeneca related to PARP inhibitor patents/personal fees/honoraria associated with function as Deputy Chair and reviewer for CRUK Clinical Research committee. Inbiomotion: personal fees/Scientific Ad Board function and stock options. MD Anderson: personal fees/Moon shot Breast Cancer scientific advisory board honoraria. Medscape Education honorarium from Merck & Co. educational grant: personal fees/speaker for a video series. V.A. has received consulting fees from Daiichi Sankyo and Eisai and research grants from Genentech. E.D. was an employee and stockholder at AstraZeneca subsequent to involvement in this study. T.C.H. has received research funding from Takeda Oncology. R.W. has received research funding from Acerta Pharma and AstraZeneca; served on advisory boards for Puma Biotechnology, Pfizer; and served on speakers’ bureau for Roche Diagnostics. J.F.-P. is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. T.Go. and T.Gr. are employees of the healthcare business of Merck KGaA, Darmstadt, Germany. J.D. is an employee of EMD Serono, Billerica MA, USA. P.F.-M.: an employee of EMD Serono, Billerica MA, USA. I.D.-P. was an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany, at the time of the study and is currently an employee of GlaxoSmithKline, Zug, Switzerland. R.P. has received honoraria for attending advisory boards from Pierre Faber, Bayer, Octimet, Clovis Oncology, Novartis, Karus Therapeutics, Biosceptre, BMS, Cybrexa, Ellipses, CV6 Therapeutics, Astex Therapeutics and Sanofi Aventis. Fees for delivery of educational talks or chairing educational meetings by AstraZeneca, Novartis, Bayer, Tesaro and BMS. Funds to support attendance at conferences from BMS and MSD.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-022-00406-0.
References
- 1.Lee KJ, et al. Exploiting DNA repair defects in triple negative breast cancer to improve cell killing. Ther. Adv. Med. Oncol. 2020;12:1758835920958354. doi: 10.1177/1758835920958354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018;27:619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 3.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur. J. Cancer. 2009;45:27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Prat A, et al. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018;27:95–120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutt A, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat. Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikov WM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J. Clin. Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loibl S, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann. Oncol. 2018;29:2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann. Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 12.Schmid P, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 13.Bardia A, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. New Engl. J. Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 14.Beniey M, Haque T, Hassan S. Translating the role of PARP inhibitors in triple-negative breast cancer. Oncoscience. 2019;6:287–288. doi: 10.18632/oncoscience.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung NM, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J. Clin. Oncol. 2020;38:4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 16.Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell. 2017;66:801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Peasland A, et al. Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br. J. Cancer. 2011;105:372–381. doi: 10.1038/bjc.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reaper PM, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat. Chem. Biol. 2011;7:428–430. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 19.Sangster-Guity N, Conrad BH, Papadopoulos N, Bunz F. ATR mediates cisplatin resistance in a p53 genotype-specific manner. Oncogene. 2011;30:2526–2533. doi: 10.1038/onc.2010.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton, F. K., Pollard, J. R. & Curtin, N. J. The impact of p53 dysfunction in ATR inhibitor cytotoxicity and chemo- and radiosensitisation. Cancers (Basel)10, 275 (2018). [DOI] [PMC free article] [PubMed]
- 21.Carey LA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Bertheau P, et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22:S27–S29. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Hall AB, et al. Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget. 2014;5:5674–5685. doi: 10.18632/oncotarget.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middleton, M. R. et al. Phase 1 study of the ATR inhibitor berzosertib (formerly M6620, VX-970) combined with gemcitabine ± cisplatin in patients with advanced solid tumours. Br. J. Cancer125, 510–519 (2021). [DOI] [PMC free article] [PubMed]
- 25.Shapiro, G. I. et al. Phase 1 study of the ATR inhibitor berzosertib in combination with cisplatin in patients with advanced solid tumours. Br. J. Cancer125, 520–527 (2021). [DOI] [PMC free article] [PubMed]
- 26.Yap TA, et al. Phase I trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 2020;38:3195–3204. doi: 10.1200/JCO.19.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstantinopoulos PA, et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:957–968. doi: 10.1016/S1470-2045(20)30180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terranova, N., Jansen, M., Falk, M. & Hendriks, B. S. Population pharmacokinetics of ATR inhibitor berzosertib in phase I studies for different cancer types. Cancer Chemother. Pharmacol.87, 185–196 (2020). [DOI] [PMC free article] [PubMed]
- 29.Thomas A, et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell. 2021;39:566–579.e567. doi: 10.1016/j.ccell.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA. FoundationFocus CDxBRCA LOH - P160018/S001, https://www.fda.gov/medical-devices/recently-approved-devices/foundationfocus-cdxbrca-loh-p160018s001 (2018).
- 31.Foundation Medicine, I. FoundationOne®CDx Technical Information, https://assets.ctfassets.net/w98cd481qyp0/41rJj28gFwtxCwHQxopaEb/2725881bbc67d6f323ab893851344c4a/FoundationOne_CDx_Label_Technical_Info.pdf.
- 32.Isakoff SJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal, S. K. et al. Effect of cisplatin and gemcitabine with or without berzosertib in patients with advanced urothelial carcinoma: a phase 2 randomized clinical trial. JAMA Oncol.7, 1536–1543 (2021). [DOI] [PMC free article] [PubMed]
- 34.Toledo LI, et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murga M, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat. Struct. Mol. Biol. 2011;18:1331–1335. doi: 10.1038/nsmb.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su XA, et al. RAD21 is a driver of chromosome 8 gain in Ewing sarcoma to mitigate replication stress. Genes Dev. 2021;35:556–572. doi: 10.1101/gad.345454.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson CT, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 2016;7:13837. doi: 10.1038/ncomms13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yap, T. A. et al. First-in-human trial of the oral ataxia telangiectasia and RAD3-related (ATR) inhibitor BAY 1895344 in patients with advanced solid tumors. Cancer Discov.11, 80–91 (2020). [DOI] [PMC free article] [PubMed]
- 39.Abkevich V, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawlyn C, et al. Loss of heterozygosity as a marker of homologous repair deficiency in multiple myeloma: a role for PARP inhibition? Leukemia. 2018;32:1561–1566. doi: 10.1038/s41375-018-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telli ML, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 2016;22:3764–3773. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 43.MedDRA. Medical Dictionary for Regulatory Activities Version 21.0, https://admin.new.meddra.org/sites/default/files/guidance/file/dist_file_format_21_0_english.pdf (2018).
- 44.NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0, https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf (2009).
- 45.McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016). [DOI] [PMC free article] [PubMed]
- 46.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 47.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. doi: 10.2307/2530286. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the healthcare business of Merck KGaA, Darmstadt, Germany Data Sharing Policy. All requests should be submitted in writing to the healthcare business of he Merck KGaA, Darmstadt, Germany data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinicaltrials/commitment-responsible-data-sharing.html). When the healthcare business of Merck KGaA, Darmstadt, Germany has a co-research, co-development, co-marketing, or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.