Abstract
Dietary protein restriction is increasingly recognized as a unique approach to improve metabolic health, and there is increasing interest in the mechanisms underlying this beneficial effect. Recent work indicates that the hormone FGF21 mediates the metabolic effects of protein restriction in young mice. Here we demonstrate that protein restriction increases lifespan, reduces frailty, lowers body weight and adiposity, improves physical performance, improves glucose tolerance, and alters various metabolic markers within the serum, liver, and adipose tissue of wildtype male mice. Conversely, mice lacking FGF21 fail to exhibit metabolic responses to protein restriction in early life, and in later life exhibit early onset of age-related weight loss, reduced physical performance, increased frailty, and reduced lifespan. These data demonstrate that protein restriction in aging male mice exerts marked beneficial effects on lifespan and metabolic health and that a single metabolic hormone, FGF21, is essential for the anti-aging effect of this dietary intervention.
Subject terms: Ageing, Metabolic syndrome
The restriction of dietary protein or amino acid intake is well established to extend lifespan in multiple species. Here, the authors show that the endocrine hormone FGF21 is necessary for dietary protein restriction to extend lifespan and improve metabolic health in aged, male mice.
Introduction
A variety of dietary interventions (i.e., calorie restriction, intermittent fasting, fasting mimetics, and dietary restriction) improve health and lifespan1,2. Epidemiological data suggest that lowering dietary protein content supports metabolic improvements and resilience3–7, while high protein intake correlates with increased mortality8,9. Protein restriction (PR) is a form of dietary restriction in the absence of energy restriction that extends lifespan and improves general health measures in various organisms, including rodents, fruit flies, and yeast10–15. The restriction of protein but not the restriction of fat or carbohydrate increases lifespan in fruit flies16,17. In rodents, PR also extends lifespan18–21, with evidence suggesting that lowering protein consumption exerts favorable outcomes on health that are independent of energy intake18. As an alternative to total PR, the restriction of select amino acids22, including methionine restriction23,24, threonine and/or tryptophan restriction25–27, and branched-chain amino acid (BCAA) restriction28–31, also extend lifespan in various organisms. Collectively, these data suggest that protein/amino acid restriction may engage unique mechanisms that improve metabolic health and extend lifespan.
The PR-induced improvements on health naturally create an interest in the underlying cellular mechanisms. Most work has accentuated the ability of protein or amino acid restriction to engage a host intracellular nutrient-sensing pathways, including mTOR, GCN2, AMPK, autophagy, etc12,32–39. However, several years ago our lab hypothesized that an endocrine effector signal of protein restriction might exist. This focus led to the discovery that the liver-derived hormone FGF21 is robustly induced by PR and that the deletion of FGF21 blocks adaptive metabolic responses to PR in young mice40–45. FGF21 is a pleiotropic hormone, with circulating levels primarily being produced by the liver46. FGF21 signals through fibroblast growth factor receptors (FGFRs), but binds with low affinity such that proper signal transduction requires interaction with the co-receptor beta-Klotho (Klb)47. FGF21 increases energy expenditure, enhances glucose metabolism, and upregulates the thermoregulatory marker UCP148. FGF21 also crosses the blood-brain barrier49, and several studies suggest that the physiological effects of FGF21 are mediated by the brain50,51. Recent data from our lab indicate that FGF21/Klb signaling in the brain is essential for PR to increase energy expenditure, improve glucose homeostasis, and protect against diet-induced obesity in young mice45.
Factoring in FGF21’s key role in facilitating the metabolic response to PR in young mice, and that transgenic overexpression of FGF21 extends lifespan and improves insulin sensitivity52, we hypothesized that increases in FGF21 might mediate the beneficial effects of long-term PR in aging animals. Here we demonstrate that, in male mice, FGF21 is required for the effects of PR on lifespan and metabolism. Indeed, mice that are FGF21 deficient are not only resistant to the health benefits effects of PR, but they also exhibit early-onset weight loss, increased frailty, and reduced lifespan when fed a low protein diet. Collectively, these data represent a suggest that FGF21 is essential for the pro-longevity effects of PR and highlight the power of a single endocrine hormone to coordinate metabolic and behavioral responses that improve metabolism and longevity.
Results
FGF21 is essential for increasing lifespan during dietary protein restriction
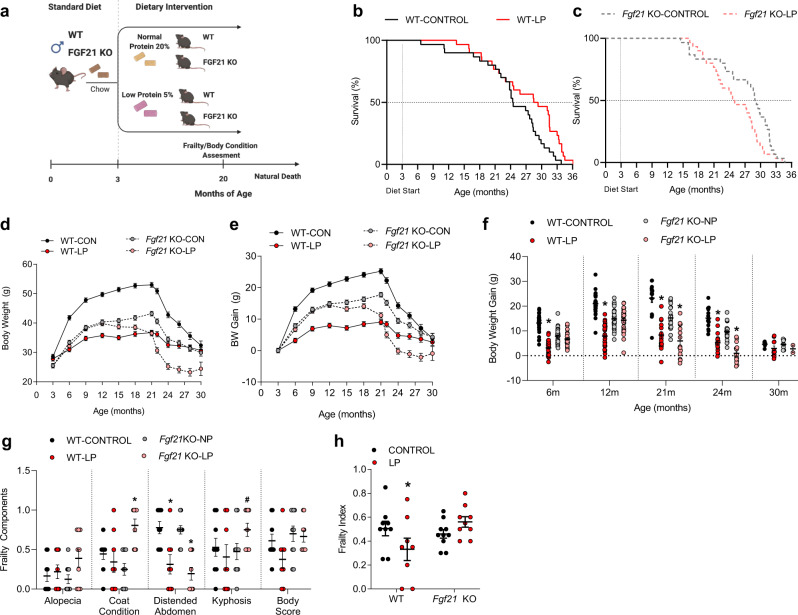
To test FGF21’s role in mediating the longevity effects of long-term dietary PR, 60 male C57BL/6 J (control wild-type) mice and 60 male Fgf21 KO mice were placed on either control (CON) or low protein (LP) diet, generating four groups of 30 mice each (Fig. 1a). Mice consumed these diets ad libitum from 3 months of age until natural death. We observed that the LP diet extended lifespan in wild type (WT) mice (Kaplan-Meier survival analysis; p = 0.01365; Fig. 1b). However, exposing Fgf21 KO mice to the LP diet reduced lifespan (p = 0.044; Fig. 1c). Parametric Survival Fit using a 2-way analysis (Diet, Genotype, G*D) resulted in a significant diet*genotype interaction (p = 0.0107). Cox Proportional Hazards analysis also results in a significant diet*genotype interaction (p = 0.002), with LP reducing the risk ratio in WT mice (RR = 0.49 for WT-LP vs WT-CON, p = 0.008) but increasing the risk ratio in Fgf21 KO mice (RR = 1.60 for Fgf21 KO-LP vs Fgf21 KO-CON; p = 0.072). Unexpectedly, a trend was observed for KO-CON mice to live longer than WT-CON mice, although the effect did not reach statistical significance (Survival analysis Log-Rank P = 0.0978; Cox Proportional Hazards Fit P = 0.0838).
Fig. 1. Deletion of FGF21 reverses the beneficial effects of dietary protein restriction on lifespan.
a Schematic representation of the longevity study. Wildtype and Fgf21-KO mice were placed on control or LP diet at 3 months of age and monitored until natural death. b Kaplan–Meier survival curve for wildtype mice on LP diet, indicating an extension of lifespan in WT mice on LP. n = 30 per group. c Kaplan–Meier survival curve for Fgf21-KO mice on LP, indicating a reduction in lifespan in Fgf21 KO mice on LP. n = 30 mice per group. d, e Bodyweight and bodyweight gain curves based on the initiation of diets, group*time p = 0.001. f Comparison of body weight change at specific time points, group*time p = 0.001. g Specific components were used from the Howlett Frailty Index assessed at 20 months of age, n = 9–10 mice per group; alopecia geno*diet p = 0.2115, coat condition geno*diet p = 0.0009, distended abdomen diet p = 0.0001, kyphosis geno*diet p = 0.09, body score geno*diet p = 0.2854. h, Frailty Index as an average from individual components in g, geno*diet p = 0.02. Statistical analyses were conducted using survival analysis for lifespan and one-way or two-way ANOVA for BW change and Frailty. Values are mean ± SEM, with significant posthoc comparison within the diet*genotype interaction noted as *p < 0.05 and #p < 0.10 compared with respective control. Source data are provided as a Source Data file.
The changes in lifespan in response to LP were reflected by distinctive changes in body weight gain over time. WT-CON mice exhibited the expected increase in BW through approximately 21 months of age, followed by a progressive age-related BW decline during the third year of life (Fig. 1d–f). WT-LP mice exhibited reduced body weight gain through the first two years of life, with a slightly delayed and less severe loss of body weight over the final year. Fgf21 KO-CON mice weigh less than WT-CON mice, yet age-related changes in body weight were similar to WT-CON mice, with weight peaking at approximately 21 months and then declining throughout the final year. Fgf21 KO-LP mice, in contrast, do not initially alter body weight in response to the LP diet. However, age-related weight loss occurred much earlier (~15–18 months vs 22 months), such that Fgf21 KO-LP mice weigh less than even WT-LP mice at ~26 months.
In both mice and humans, the prevalence and extent of frailty increase with age53. Recent data suggest that dietary interventions such as CR and BCAA restriction reduce the incidence of frailty in mice31. To examine the effects of FGF21 during LP feeding on additional geriatric measures, we adapted an analysis of frailty from the Howlett Frailty Index, including the individual factors alopecia, coat condition, distended abdomen, kyphosis, and body score (Fig. 1g, h). At 22 months of age LP diet tended to reduce frailty markers in WT mice but increased them in Fgf21 KO mice. As a result, an average across all scores (Fig. 1h) demonstrated a significant diet*genotype interaction (p = 0.02), with LP reducing frailty in the WT but not the Fgf21 KO mice.
Collectively, these data suggest that the interaction of FGF21 and PR reduces body weight gain, reduces frailty, and extends lifespan in mice. Conversely, Fgf21 KO mice do not exhibit initial changes in body weight in response to the LP diet, and then beyond ~12 months of age the LP diet begins to exert an adverse effect in Fgf21 KO mice, including earlier onset of age-related weight loss, increases in some frailty markers, and shortened lifespan.
FGF21 mediates low protein-induced changes in BW gain, food intake, and body composition during aging
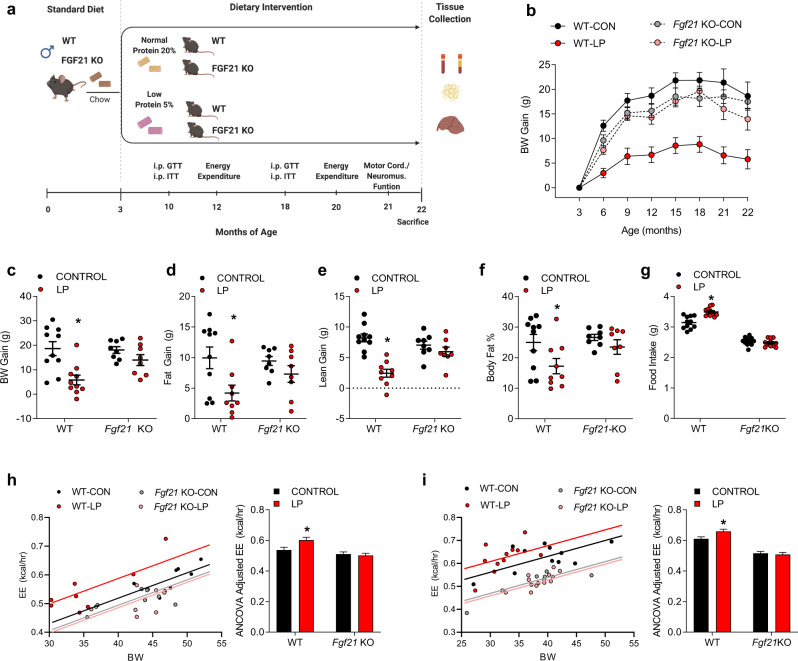
The above data demonstrate that lifespan extension due to PR requires FGF21. To test whether FGF21 contributes to the effects of long-term PR on healthspan in aged mice, a separate group of WT and Fgf21 KO mice were fed a control or LP diet from 3 months to 22 months of age (n = 12 mice per diet/genotype in 4 total groups). Metabolic and behavioral endpoints were then assessed throughout the experiment as outlined in Fig. 2a. As expected, the LP diet reduced BW gain in WT mice (Fig. 2b, c; p < 0.05), while this effect was absent in Fgf21 KO mice. Body composition analysis indicates that LP diet reduced both fat and lean gain in WT mice (Fig. 2d, e; p < 0.05), but that the reduction in fat gain was larger than that of lean gain, such that fat mass relative to body weight (percent body fat) was reduced in WT-LP mice compared to controls (Fig. 2f; p < 0.05). All of these LP-induced changes in body composition were absent in Fgf21 KO mice. Food intake was persistently elevated in WT-LP mice (p < 0.05), but again this effect was absent in Fgf21 KO mice (Fig. 2g). Lastly, energy expenditure (EE), adjusted for BW via ANCOVA, was increased by LP diet at both 12 and 20 months of age in WT mice (Fig. 2h, i, p < 0.05). Conversely, there was no increase in EE in Fgf21 KO mice on LP. These data suggest that dietary protein restriction persistently reduces body weight but increases food intake and energy expenditure in aging mice, with these effects depending on increased circulating FGF21.
Fig. 2. FGF21 mediates low protein-induced changes in BW gain, food intake, and body composition in aged mice.
a Schematic representation of the metabolic aging study. Wildtype and Fgf21-KO mice were placed on control or LP diet at 3 months of age (12 mice/group), with various metabolic endpoints assess throughout the study as indicated, and tissue collection at 22 months of age. b Body weight gain over time following initiation of experimental diets, n = 12 mice/group, group*time p = 0.001. c Final body weight gain at 22 months of age (n = 8–12 mice/group at sacrifice; geno*diet p = 0.0004). d Final body fat gain at 22 months of age as measured by NMR (n = 8–12 mice/group at sacrifice; geno*diet p = 0.03). e Final body lean gain at 22 months of age as measured by NMR (n = 8–12 mice/group at sacrifice; geno*diet p = 0.0001). f Percent body fat at 22 months of age as measured by NMR (n = 8–12 mice/group at sacrifice; geno*diet p = 0.03). g Average daily food intake throughout the study (n = 8–12 mice/group at sacrifice; geno*diet p = 0.0001). h Energy expenditure (ANCOVA adjusted for BW) as measured at 12 months of age (n = 8–12 mice/group at sacrifice; geno*diet p = 0.04). i Energy expenditure (ANCOVA adjusted for BW) as measured at 20 months of age (n = 8–12 mice/group at sacrifice; geno*diet p = 0.04). Statistical analyses were conducted using two-way ANOVA or ANCOVA. All values are mean ± SEM, with significant posthoc comparison within the diet*genotype interaction noted as *p < 0.05 compared with respective control. Source data are provided as a Source Data file.
Protein restriction protects against age-related functional decline via FGF21
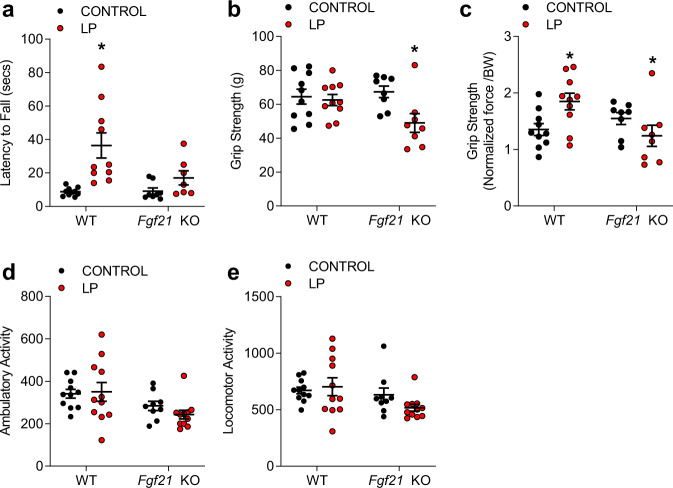
Inadequate protein intake, particularly when diagnosed as malnourishment in the elderly, may lead to adverse functional and structural events such as muscle wasting and increase the risk of bone fractures. To assess the contribution of FGF21 to functional performance at 21 months of age, mice fed either control or LP diet were placed on the rotarod to assess physical performance and coordination, which is captured as latency to fall from the rotating rod (Fig. 3a). A significant genotype*diet interaction was observed (p < 0.05), with LP diet increasing latency to fall in WT mice (p < 0.01), but this effect being largely abolished in Fgf21 KO mice (P = 0.28). Significant diet*genotype interactions (p < 0.01) were also detected for raw grip strength (Fig. 3b) and grip strength normalized to body (Fig. 3c). Raw grip strength was not influenced by the LP diet in WT mice but was significantly increased when normalized to body weight (p < 0.05). Similarly, both raw and normalized grip strength was significantly reduced by LP in Fgf21 KO mice. Finally, ambulatory and locomotor activity within the metabolic chamber at 20 months of age indicated no effect of genotype or diet (Fig. 3d, e). These data indicate that protein restriction increases functional performance, with these beneficial effects being dependent on FGF21.
Fig. 3. Protein restriction protects against age-related functional decline via FGF21.
a Latency to fall from an accelerating rod (Rotarod) at 21 months of age in WT and Fgf21 KO mice on control or LP diets (n = 8–12 mice/group; geno*diet p = 0.008). b Grip Strength at 21 months of age (n = 8–12 mice/group; geno*diet p = 0.04). c Grip Strength normalized to body mass (n = 8–12 mice/group; geno*diet p = 0.01). d Ambulatory activity as measured in the metabolic chambers at 20 months of age (n = 8–12 mice/group geno*diet p = 0.39). e Locomotor activity as measured in the metabolic chambers at 20 months of age (n = 8–12 mice/group geno*diet p = 0.19). Statistical analyses were conducted using two-way ANOVA. All values are mean ± SEM, with significant posthoc comparison within the diet*genotype interaction noted as *p < 0.05 compared with respective control. Source data are provided as a Source Data file.
FGF21 mediates low protein-induced improvements in glucose homeostasis in aged mice
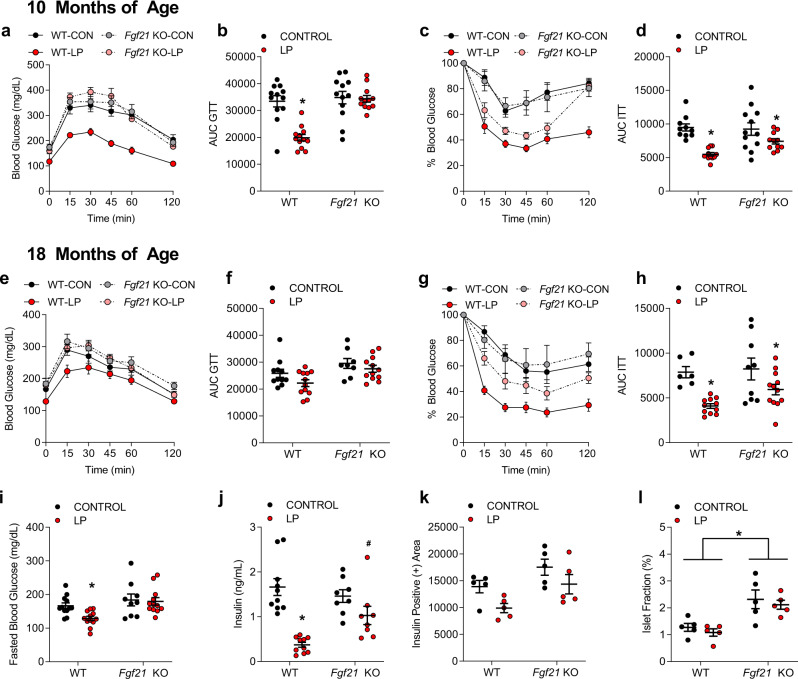
Low protein fed mice have improved glucose tolerance compared to their control-fed counterparts, furthermore these improvements are correlated with increases in circulating FGF2118. To assess the impact of PR induced-FGF21 on glucose homeostasis, i.p. glucose and insulin tolerance tests were conducted at 10 and 18 months of age. Glucose tolerance and insulin sensitivity were significantly improved in WT-LP mice (Fig. 4a–h; p < 0.05), as was fasting blood glucose (Fig. 4i; p < 0.05). Effects of LP diet on glucose tolerance were completely lost in Fgf21 KO mice (Fig. 4a, b), but insulin sensitivity was improved in both genotypes (Fig. 4c, g), although the effect was reduced in Fgf21 KO mice. Serum insulin levels at sacrifice were significantly reduced by LP in WT mice (Fig. 4j, p < 0.05), yet this reduction in insulin levels by LP was blunted in Fgf21 KO mice (p = 0.07). Finally, histological analysis of the insulin-positive area and islet fraction of the pancreas at sacrifice indicated a significant main effect of both diet and genotype, but no significant interaction, on insulin-positive area (Fig. 4k; p < 0.05), with LP tending to reduce insulin positive area and FGF21 deletion tending to increase it. There was no effect of diet on islet fraction in either group, but islet fraction was significantly higher in Fgf21 KO mice compared to controls (Fig. 4l; p < 0.05). Collectively, these data indicate that the LP diet exerts a persistent effect to protect against age-related declines in glucose homeostasis, and that these diet effects are attenuated in Fgf21 KO mice.
Fig. 4. FGF21 mediates low protein-induced improvements in glucose homeostasis in aged mice.
a Glucose tolerance test measured at 10 months of age in WT and Fgf21 KO mice control or LP diets (n = 8–12 mice/group, group*time p = 0.001). b Area under the curve glucose as measured in the GTT at 10 months of age (n = 8–12 mice/group geno*diet p = 0.001). c Insulin tolerance test at 10 months of age (n = 8–12 mice/group, group*time p = 0.001). d Area under the curve glucose as measured in the ITT at 10 months of age (n = 8–12 mice/group; diet p = 0.001). e Glucose tolerance test measured at 18 months of age in WT and Fgf21 KO mice control or LP diets (n = 8–12 mice/group group*time p = 0.236). f Area under the curve glucose as measured in the GTT at 18 months of age (n = 8–12 mice/group) geno p = 0.004, diet p = 0.062. g Insulin tolerance test at 18 months of age (n = 8–12 mice/group group*time p = 0.001). h Area under the curve glucose as measured in the ITT at 18 months of age (n = 8–12 mice/group; diet p = 0.003). i Fasting blood glucose at 18 months of age (n = 8–12 mice/group; diet p = 0.06, geno p = 0.0031, geno*diet p = 0.128). j Serum insulin levels at sacrifice at 22 months of age (n = 8–12 mice/group; geno*diet p = 0.01). k Insulin positive area as measured via insulin IHC on fixed pancreas collected at sacrifice at 22 months of age (n = 5 mice/group; geno p = 0.001, diet p = 0.02). l Islet fractional percentage as measured on fixed pancreas collected at sacrifice at 22 months of age (n = 5 mice/group; geno p = 0.01). Statistical analyses were conducted using one or two-way ANOVA. All values are mean ± SEM, with significant posthoc comparison within the diet*genotype interaction noted as *p < 0.05, #p < 0.10 compared with respective control. Source data are provided as a Source Data file.
FGF21-dependent and independent changes in molecular endpoints in the liver and adipose tissue during protein restriction
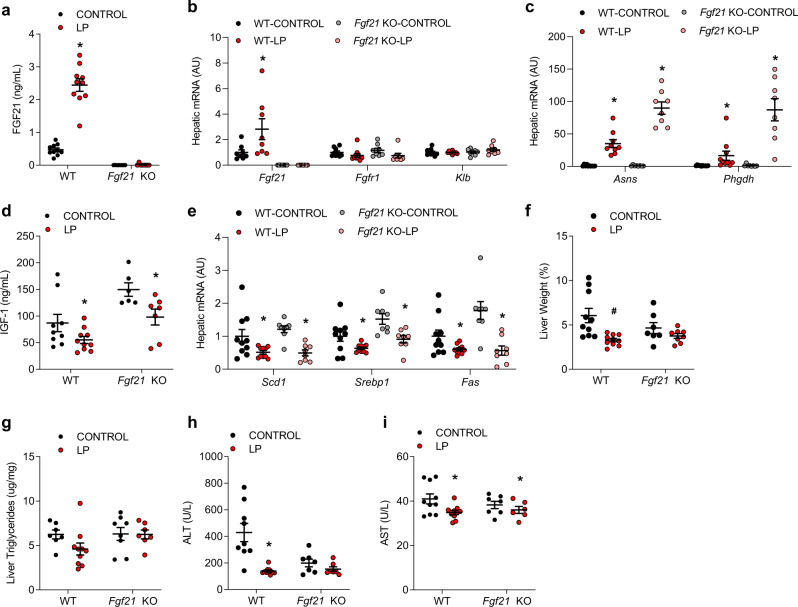
All mice within this second study were sacrificed at 22 months of age to assess the impact of PR on tissue endpoints. LP induced a robust increase in serum FGF21 in WT mice (Fig. 5a; p < 0.01), while FGF21 levels were not detectable in Fgf21 KO mice. This increase in circulating FGF21 was consistent with a significant increase in liver Fgf21 mRNA expression (Fig. 5b). The expression of the FGF21 co-receptor Klb was not altered in the liver, but Fgfr1 expression was reduced by the LP diet in both genotypes (p < 0.05; Fig. 5b). The amino acid biosynthetic genes Asns and Phgdh were also significantly increased by LP in both genotypes (Fig. 5c; p < 0.05), consistent with continuous protein restriction, and this induction was relatively larger in Fgf21 KO mice than in WT mice.
Fig. 5. FGF21-dependent and independent changes in molecular endpoints in the liver at 22 months of age.
a Serum FGF21 levels collected at sacrifice at 22 months of age in WT and Fgf21 KO mice on control or LP diets as in Fig. 2 (n = 8–10 mice/group, geno*diet p = 0.01). b Liver mRNA levels for FGF21 related markers as measured via qPCR at 22 months of age (n = 8–10 mice/group, geno*diet Fgf21 p = 0.04, Fgfr1 p = 0.556, Klb = 0.3016). c Liver markers of dietary protein restriction (amino acid biosynthetic genes) as measured via qPCR at 22 months of age (n = 8–10 mice/group; geno*diet Asns p = 0.0001, Phgdh p = 0.001. d Serum IGF1 levels at sacrifice at 22 months of age (n = 8–10 mice/group; genotype p = 0.0004, diet p = 0.003, geno*diet p = 0.4567). e Liver mRNA expression for hepatic lipogenic genes as measured via qPCR at 22 months of age (n = 8–10 mice/group; Scd1 diet p = 0.001 geno*diet p = 0.553, Srebp1 diet p = 0.001, geno*diet p = 0.89, Fas diet p = 0.001 geno*diet p = 0.2395. f Liver weight as a percentage of body weight at sacrifice at 22 months of age (n = 8–10 mice/group; diet p = 0.0026, geno*diet p = 0.10). g Liver triglyceride content at sacrifice at 22 months of age (n = 8–10 mice/group, geno*diet p = 0.2271). h Serum ALT levels at sacrifice at 22 months of age (n = 8–10 mice/group; genotype p = 0.01, diet p = 0.0003, geno*diet p = 0.0057). i Serum AST levels at sacrifice at 22 months of age (n = 8–10 mice/group; genotype p = 0.6662, diet p = 0.02, geno*diet p = 0.2852). Statistical analyses were conducted using two-way ANOVA. All values are mean ± SEM, with significant main effects of diet or posthoc comparison within the diet*genotype interaction noted as *p < 0.05, #p < 0.10 compared with respective control. Source data are provided as a Source Data file.
Serum IGF-1 levels (Fig. 5d) were independently increased by the deletion of FGF21 (genotype p = 0.004) and reduced by LP diet (diet p = 0.003); however, the diet*genotype interaction was not statistically significant. LP diet reduced the expression of liver genes associated with lipogenesis (Scd-1, Srebp1c, Fas) in both genotypes (diet p < 0.05, genotype and G*D = NS; Fig. 5e). Liver mass was significantly reduced by diet (p = 0.002; Fig. 5f), and there was a trend (p = 0.10) towards a diet*genotype interaction with WT mice exhibiting a larger reduction compared to Fgf21 KO mice. Liver triglyceride content displayed a similar pattern; however, differences were not statistically significant (Fig. 5g). Finally, changes in circulating ALT and AST levels were used as markers of liver health (Fig. 5h, i). A significant diet*genotype interaction was observed for ALT (p = 0.0057), with the LP diet markedly reducing ALT levels in WT-LP mice but not in Fgf21 KO-LP mice. However, this difference is driven largely by differences in baseline ALT between WT-CON and Fgf21 KO-CON mice. Conversely, AST levels were modestly reduced by diet in both genotypes (diet p = 0.02), but the diet*genotype interaction was not statistically significant (p = 0.285).
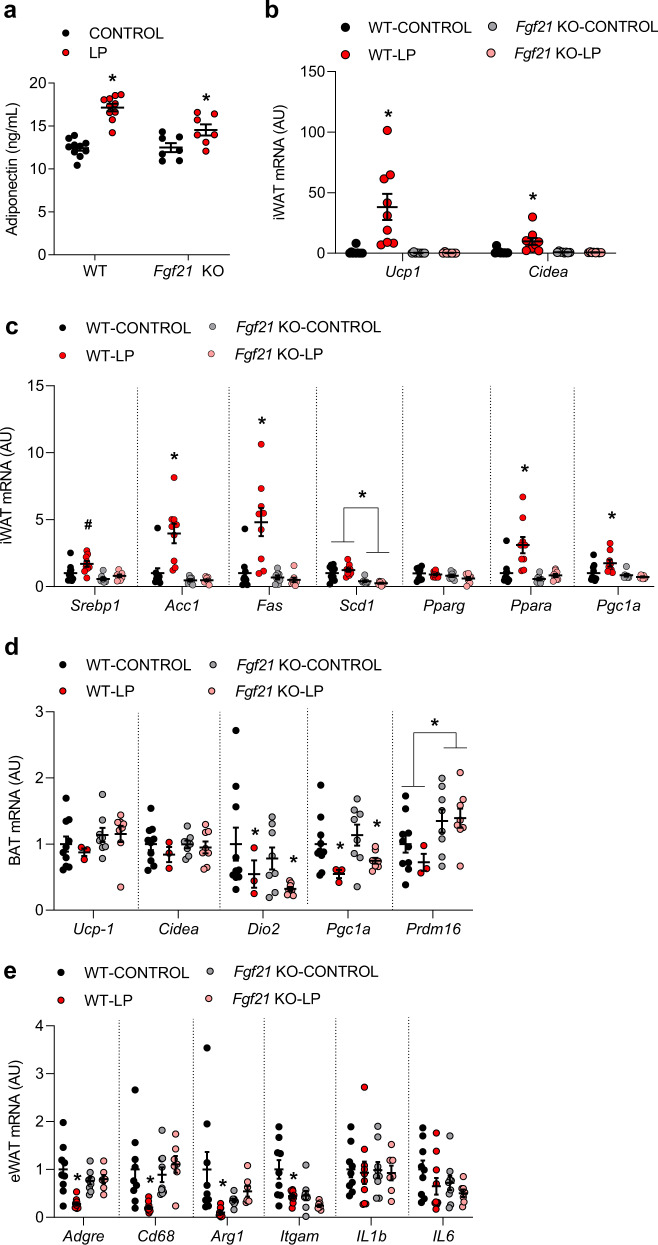
Adipose tissue-related markers were also altered by LP diet at 22 months of age. Circulating adiponectin was significantly increased by LP diet in both WT and Fgf21 KO mice (Fig. 6a). Conversely, the LP diet robustly increased thermogenic regulatory genes (Ucp1, Cidea; Fig. 6b, p < 0.01) within inguinal white adipose tissue (iWAT) in WT mice, but this effect was lost in Fgf21 KO mice. LP diet also induced genes associated with adipose tissue lipid oxidation and metabolic function, including Acc1, Fas, Ppara, and Pgc1a (Fig. 6c; p < 0.05), and again these effects were lost in Fgf21 KO mice. Interestingly, despite the increase in EE and induction of thermogenic genes in iWAT, we observed no increase in thermogenic markers within the BAT of either WT or Fgf21 KO mice on LP (Fig. 6d). Finally, LP diet reduced the expression of multiple inflammatory markers within epididymal white adipose tissue, most notably Adgre, Cd68, Arg1, and Itgam (Fig. 6e), although neither IL1b nor IL6 expression was altered. This effect of LP was largely blocked in Fgf21 KO mice, although baseline levels of Arg1 and Itgam were already low in Fgf21 KO-CON mice. Collectively, these data provide evidence that protein restriction alters multiple metabolic targets within adipose tissue, and that most but not all of these effects are dependent on FGF21.
Fig. 6. FGF21-dependent and independent changes in molecular endpoints in the adipose tissue at 22 months of age.
a Serum adiponectin collected at 22 months of age (n = 8–10 mice/group; geno*diet p = 0.009). b Thermogenic gene expression in inguinal white adipose tissue (iWAT) collected at sacrifice at 22 months of age (n = 8–10 mice/group; geno*diet Ucp1 p = 0.0023, Cidea p = 0.0065). c Energy metabolism gene expression in iWAT at 22 months of age (n = 8–10 mice/group; Srebp1 diet p = 0.01 geno*diet p = 0.1876, Acc1 geno*diet p = 0.002, Fas geno*diet p = 0.002, Scd1 geno*diet p = 0.1388, Pparg geno*diet p = 0.6344, Ppara geno*diet p = 0.01, Pgc1a geno*diet p = 0.02). d Expression of thermogenic markers within BAT (n = 3–10 mice/group; Ucp1 geno*diet p = 0.6124, Cidea geno*diet p = 0.5739, Dio2 diet p = 0.05 geno*diet p = 0.99, Pgc1a diet p = 0.007 geno*diet p = 0.37, Prdm16 geno p = 0.0079 geno*diet p = 0.37). e Expression of inflammatory markers within visceral adipose tissue (n = 8–10 mice/group; Adgre geno*diet p = 0.009, Cd68 p = 0.006, Arg1 geno*diet p = 0.01, Itgam diet p = 0.002 geno p = 0.03 geno*diet p = 0.14, IL1b geno*diet p = 0.9, IL6 geno*diet p = 0.67). Statistical analyses were conducted using two-way ANOVA. All values are mean ± SEM, with significant main effects of diet or posthoc comparison within the diet*genotype interaction noted as *p < 0.05, #p < 0.10 compared with respective control. Source data are provided as a Source Data file.
Protein restriction protects against the detriments of diet-induced obesity during middle age
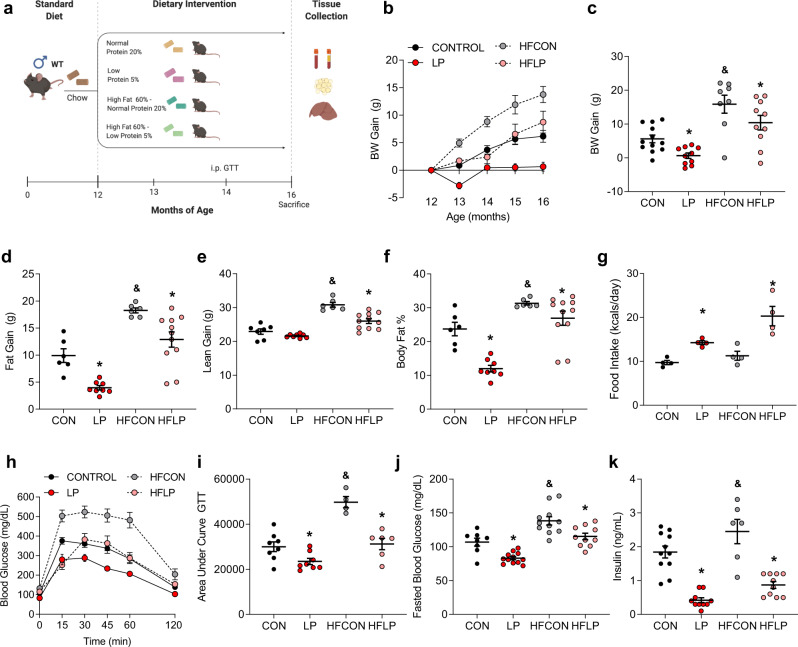
To further expound on the efficacy of PR to reduce the progression of age-related metabolic decline, forty-eight 12-month-old male C576BL/6 J mice were randomly assigned to one of 4 diets (12 mice/diet): control (CON) and low protein (LP) diets as above, as well as two additional diets, HFCON and HFLP, that varied protein on the background of a 60% high-fat diet. Mice consumed these diets ad libitum from 12 to 16 months of age (Fig. 7a) to test the impact of protein restriction in middle-aged mice with diet-induced obesity. As expected, HFCON mice gained weight more rapidly than CON mice (Fig. 7b, c; p < 0.05), and both LP and HFLP reduced weight relative to their controls. HFCON gained both fat and lean mass at a greater rate than any group, and both LP and HFLP mice exhibit reduced fat and lean gain relative to their controls (Fig. 6d, e; p < 0.05). However, the reduction in fat gain was larger than lean gain, such that LP and HFLP show a decrease in body adiposity (Fig. 7f). These changes in weight and body composition were associated with increases in food intake in both LP and HFLP relative to their controls (Fig. 7g; p < 0.05). Consistent with changes in body composition, HFCON mice exhibit impaired glucose tolerance (Fig. 7h, i; p < 0.05), increased fasting blood glucose (Fig. 7j; p < 0.05), and increased serum insulin (Fig. 7k; p < 0.05) as compared to CON mice. Both LP and HFLP improved glucose tolerance and lowered fasting glucose and insulin levels (p < 0.05). Strikingly, HFLP normalized body adiposity, glucose tolerance, and fasting glucose and insulin levels, even though these middle-aged animals remained on a 60% high-fat diet.
Fig. 7. Protein restriction promotes beneficial metabolic effects and protects against diet-induced obesity when initiated at middle age.
a Schematic representation of middle-aged study. Wildtype and Fgf21-KO mice were placed on CON, LP, HFCON, or HFLP diet at 12 months of age, with various metabolic endpoints assessed throughout the study as indicated and tissue collection at 16 months of age. b Body weight gain from initiation of diets (n = 12 mice/group). c Body weight gain from diet initiation to sacrifice at 16 months of age (n = 8–12 mice/group; fat p < 0.0001, protein p = 0.0046, fat*protein p = 0.8814). d Body fat gain from diet initiation to sacrifice at 16 months of age, as measured by NMR (n = 6–12 mice/group; fat p < 0.0001, protein p < 0.0001, fat*protein p = 0.816). e Body lean gain from diet initiation to sacrifice at 16 months of age, as measured by NMR (n = 6–12 mice/group; fat p = 0.001, protein p = 0.001, fat*protein p = 0.0144. f Percent body fat at sacrifice at 16 months of age (n = 6–12 mice/group; fat p < 0.0001, protein p < 0.0001, fat*protein p = 0.218). g Average daily food intake throughout the experiment (n = 4 cages/group fat p = 0.6676, protein p = 0.0001, fat*protein p = 0.285). h Glucose tolerance test conducted at 14 months of age (2 months on diet (n = 6–8 mice/group; group*time p = 0.001). i Area under the curve glucose for the GTT at 14 months of age (n = 6–8 mice/group fat*protein p = 0.01). j Fasting blood glucose at 14 months of age (n = 6–8 mice/group; fat p = 0.0001, protein p = 0.0001, fat*protein p = 0.94). k Serum insulin levels at sacrifice at 14 months of age (n = 6–8 mice/group; fat p = 0.0033, protein p = 0.0001, fat*protein p = 0.4746). Statistical analyses were conducted using two-way ANOVA with dietary fat content and dietary protein content as main effects. All values are mean ± SEM, with significant main effects of protein or posthoc comparisons within the fat*protein interaction noted as *p < 0.05 vs respective control, and main effects of fat noted as &p < 0.05 HFCON vs CON. Source data are provided as a Source Data file.
Protein restriction alters liver and adipose tissue endpoints in middle-aged mice
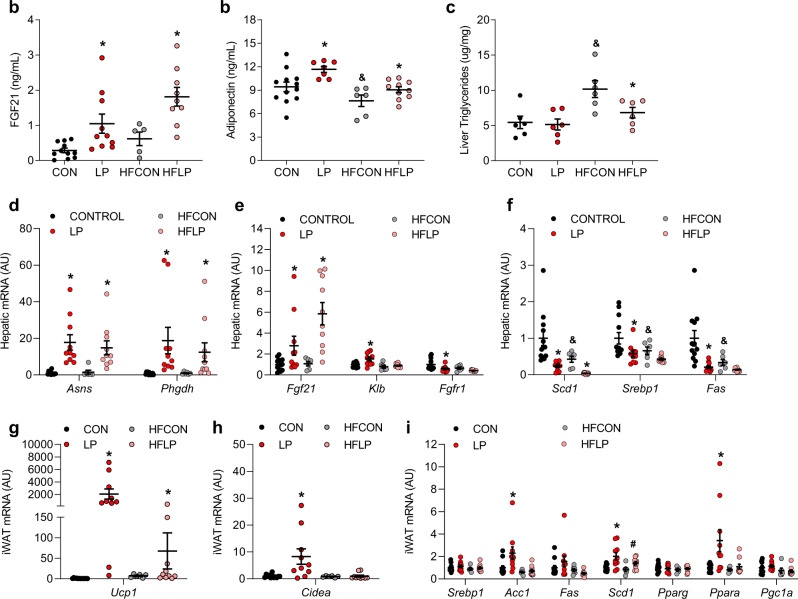
Lastly, we addressed the effect of age on a subset of metabolic genes in liver and adipose tissue during PR. As expected, LP and HFLP significantly increased circulating FGF21 (Fig. 8a; p < 0.05) and adiponectin (Fig. 8b; p < 0.05). Within the liver, HFCON increased liver triglyceride content relative to control, and this effect was prevented by HFLP (Fig. 8c). Both LP and HFLP increased liver amino acid biosynthetic genes (Asns and Phgdh; Fig. 8d), as well as hepatic Fgf21 expression (Fig. 8e). Both high fat and low protein independently reduced liver lipogenic gene expression (Fig. 7f). LP diet induced a strong increase of thermogenic gene expression (Ucp1, Cidea) in iWAT (Fig. 8g, h; p < 0.05). The increase in Ucp1 expression was also observed but smaller in magnitude in HFLP fed mice, while there was no increase in Cidea expression in HFLP. This pattern of a blunted effect on adipose tissue gene expression was also observed for related genes, with the LP-induced increase of Acc1, Fas, and Ppara being lost in HFLP (Fig. 8i).
Fig. 8. Serum, liver, and adipose tissue endpoints following the initiation of dietary protein restriction in middle age.
a Serum FGF21 concentrations in wildtype and Fgf21-KO mice consuming control or LP diet from 12 to 16 months of age as Fig. 7a (n = 6–10 mice/group; protein p = 0.0002). b Serum Adiponectin concentrations at 16 months of age (n = 6–10 mice/group; fat p = 0.0007, protein p = 0.004). c Liver triglyceride content at 16 months of age (n = 6–10 mice/group; fat p = 0.0023, protein p = 0.06, fat*protein p = 0.11). d Liver amino acid biosynthetic gene expression (markers of dietary protein restriction) as measured via qPCR at 16 months of age (n = 6–10 mice/group; Asns protein p = 0.003, Phgdh protein p = 0.02). e Liver mRNA expression of FGF21 signaling genes as measured via qPCR at 16 months of age (n = 6–10 mice/group; Fgf21 protein p = 0.01, Klb fat p = 0.001, protein p = 0.01, fat*protein p = 0.08, Fgfr1 fat p = 0.04, protein p = 0.01) f Liver mRNA expression of lipogenic genes as measured via qPCR at 16 months of age (n = 6–10 mice/group, Scd1 fat p = 0.008, protein p = 0.0002, Srebp1 fat p = 0.04, protein p = 0.009, Fas fat p = 0.01, protein p = 0.001). g iWAT expression of Ucp1 at 16 months of age (n = 6–10 mice/group; fat*protein p = 0.02). h iWAT expression of Cidea at 16 months of age (n = 6–10 mice/group; fat*protein p = 0.03). i iWAT expression of metabolic genes at 16 months of age (n = 6–10 mice/group; Srebp1 p = NS, Acc1 fat*protein p = 0.08, Fas fat p = 0.04, Scd1 fat p = 0.08, protein p = 0.008, Pparg p = NS, Ppara fat*protein p = 0.06, Pgc1a fat p = 0.03). Statistical analyses were conducted using two-way ANOVA. All values are mean ± SEM, with significant main effects of protein or posthoc comparison within the fat*protein interaction noted as *p < 0.05 vs respective control and main effects of fat noted as &p < 0.05 HFCON vs CON. Source data are provided as a Source Data file.
While obesity may occur at any age, the detrimental effects of obesity are magnified with advancing age. These data suggest that despite continued exposure to a 60% high fat diet at middle-age, the HFLP diet was sufficient to protect against the negative effects of high fat diet-induced obesity.
Discussion
The ability of PR to reduce the physical signs of aging in model organisms has been recognized for over 75 years54–56, with more recent work demonstrating the beneficial effects of dietary protein or amino acid restriction in a variety of species10–15. The specific mechanisms driving the anti-aging effects of protein restriction are not well known, and the primary research focus has been various cell-autonomous nutrient-sensing pathways, such as mTOR, AMPK, sirtuins, or GCN212,32–39. However, our prior work in young mice indicated that the endocrine hormone FGF21 was required for young mice to detect and metabolically adapt to a low protein diet42,45. Importantly, FGF21 has been previously linked to lifespan extension, as transgenic overexpression of FGF21 increases lifespan and inhibits growth in mice52,57. However, no study has previously tested whether FGF21 contributes to lifespan extension in a dietary or otherwise physiological model. To test whether FGF21 was required for the longevity effects of PR, male control and Fgf21-KO mice were placed on a low protein diet at 3 months of age and were then allowed to age naturally. Collectively, the data demonstrate that FGF21 is not only required for the longevity effects of PR, but the absence of FGF21 converts the beneficial effect of PR to a detrimental effect. This work provides clear evidence that FGF21 plays a physiological role in longevity and identifies a unique mechanism that explains PR-induced longevity in a mammalian model.
The differential effects of PR on lifespan in the WT and Fgf21 KO mice were mirrored by changes in a variety of physiological and metabolic markers, both within the lifespan group and in a second experimental cohort (termed metabolic group) of WT and Fgf21 KO mice that were fed either control or LP diets from 3 to 22 months of age. Consistent with prior work in young mice, the LP induced a persistent reduction in body weight gain over the first 2 years of life in WT-LP mice. However, we do not interpret these reductions in growth as evidence of malnutrition or poor health. Instead, the WT-LP mice in the lifespan group exhibited a reduction in clinical markers of frailty and subjectively looked healthier and more robust compared to their controls. Similarly, WT-LP mice in the metabolic group exhibited reduced total adiposity, and measures of physical performance (rotorod, grip strength) were either unchanged or improved by PR in WT mice, although we acknowledge that these effects are likely to be driven by differences in body weight in WT mice. While LP diet did reduce total BW in WT mice, there was no evidence that prolonged PR negatively impacted physical function. Instead, we suggest that the WT-LP mice were smaller but healthier, consistent with the effects on lifespan, and not unlike mice on chronic CR. However, unlike CR, these mice were consuming food ad libitum with no additional intervention on the part of the experimenter.
While long-term PR led to beneficial effects on frailty and physical performance in WT mice, the Fgf21 KO mice exhibited a much more complex response. Most notably, the Fgf21 KO mice were initially unresponsive to the diet, supporting our prior observation that FGF21 is required for mice to physiologically adapt to a state of protein restriction. While this lack of adaptation appears benign in young mice, over time the LP diet exerted increasingly negative effects in Fgf21 KO mice. For example, Fgf21 KO-LP mice exhibit age-related weight loss earlier than any other group, several clinical markers of aging and frailty (alopecia, coat condition, kyphosis) were increased by LP in the Fgf21 KO mice, the subjective appearance of Fgf21 KO-LP mice declined earlier than any other group, Fgf21 KO-LP mice showed no improvement in rotarod performance, and finally grip strength was reduced. Interestingly, separate work specifically focusing on the kidney in these same animals observed increased kidney inflammation in Fgf21 KO-LP mice, again suggesting that protein restriction induces negative outcomes in the absence of FGF2158. Collectively, these data paint a consistent picture in which protein restriction improves functional performance and reduces frailty in WT mice, but conversely leads to long-term negative outcomes for performance and frailty in Fgf21 KO mice.
The metabolic cohort also allowed us to directly assess metabolic and tissue endpoints in WT and Fgf21 KO mice following long-term protein restriction. Many of these effects are consistent with our prior work in young mice. In WT mice the LP diet persistently increased both food intake and energy expenditure, while changes in food intake and energy expenditure were absent in Fgf21 KO mice even at 22 months of age. Thus the effects of the LP diet seem to be consistent throughout the lifespan, and also to consistently require FGF21. Dietary PR has also been shown to improve measures of metabolic health in young mice3,43,45, and in WT mice the LP diet enhanced glucose tolerance and insulin sensitivity and reduced fasting blood glucose and insulin, with these effects being largely absent in Fgf21 KO mice. It seems likely that these effects are driven by LP-induced reductions in body weight and adiposity, as liver weight and triglyceride content were also reduced in WT but not Fgf21 KO-LP. However, we point out prior work indicating that PR engages unique signaling mechanisms that are not engaged by an equivalent weight loss induced by general food restriction, most notably the increase in FGF2145.
PR also altered the expression of multiple metabolic genes within the liver and adipose tissue at 22 months of age, including a consistent and robust increase in both circulating FGF21 protein and liver Fgf21 mRNA expression, which suggest that PR persistently increases FGF21 for the full duration of dietary exposure. Interestingly, the LP-induced change in the expression of several liver genes was intact in Fgf21 KO mice. Our earlier work indicated that the LP-induced reductions in the expression of liver lipogenic genes (Scd1,Srebp1,Fas) were lost in Fgf21 KO mice41, but more recent work44 and this study indicate that the reduction in lipogenic genes is intact LP-fed Fgf21 KO mice. Interestingly, the LP diet also significantly reduced circulating IGF-1 levels in both control and Fgf21 KO mice, indicating that this well-known effect of PR is not FGF21-dependent. However, interpretation of these data is complicated by the fact that the deletion of FGF21 itself had an independent effect to increase IGF-1, such that Fgf21 KO mice had increased IGF-1 levels on both diets. The role that IGF-1 might play in the metabolic or lifespan effects we observe is currently not clear, but these changes in IGF-1 are consistent with prior work linking FGF21 to hepatic GH signaling and the actions of mTOR9,57,59–61. Circulating adiponectin levels were also increased by PR, and this effect was retained in Fgf21 KO mice. Within adipose tissue, our work and that of others indicate that protein restriction promotes a remodeling (beiging) of inguinal white adipose tissue44,45, and in these aged mice we observed a similar, FGF21-dependent induction of thermogenic gene expression. LP diet also altered the expression of genes related to lipid metabolism (Fas, Acc1, Ppara), with these effects being dependent on FGF21. Interestingly, despite the increase in whole-body EE and the remodeling of iWAT, we did not observe increased thermogenic markers within BAT. These data are consistent with prior work in our lab in which BAT Ucp1 mRNA expression was increased after 2 weeks of LP exposure, but not after 27 weeks41. However, these assessments depend exclusively on measures of mRNA expression which do not necessarily reflect the total thermogenic capacity of BAT62, and it is therefore problematic to conclude that BAT may be uninvolved in the changes in whole body energy expenditure. Finally, consistent with changes in whole-body adiposity and metabolic health, the LP diet also reduced various markers of inflammation, particularly macrophage markers, within visceral adipose tissue of WT mice, and these effects were largely blunted in Fgf21 KO mice. In summary, these data indicate that changes in whole-body metabolism are also linked to specific adaptations within peripheral tissues, particularly liver and adipose tissue. While changes in adipose tissue appear to be largely downstream of and dependent on increases in FGF21, the liver’s response to PR, particularly in terms of changes in gene expression, were largely intact in Fgf21 KO mice and thus appear to be independent of FGF21. These observations are thus consistent with a model in which the liver initially detects PR and increases circulating FGF21 as an endocrine signal, with FGF21 acting in a variety of sites, most notably the brain, to coordinate adaptive metabolic responses40,45.
While the data described above clearly indicate that PR, via the induction of FGF21, protects against age-related metabolic decline, these data are based on a model in which PR is initiated in young adult mice (3 months of age). To test whether PR would also improve metabolic health in middle-aged animals, 12-month-old WT mice were placed on the previously described control and LP diets, as well as two additional diets that contained 60% fat. These latter diets were designed to test whether PR is sufficient to prevent the acceleration of age-associated metabolic dysfunction that occurs with diet-induced obesity. Collectively, these data demonstrate that the effects of protein restriction were retained in middle-aged mice, with LP reducing weight, BW gain, and adiposity, improving glucose homeostasis, and altering liver and adipose tissue gene expression when the diet was initiated at 12 months. Most notably, while HFCON diet exacerbated age-associated metabolic dysfunction as expected, these negative effects were largely prevented by HFLP. It is quite compelling to observe that mice on HFLP were generally similar to control-fed mice despite consuming a 60% fat diet ad libitum. These data are similar to a recent publication indicating that PR initiated at 18 months of age increases energy expenditure and adipose browning, although no effects on glucose homeostasis were observed63. Taken together, these data provide strong evidence that PR improves metabolic health when initiated in middle age, and importantly that PR can prevent the negative effects of high-fat diet exposure in this model.
Our data strongly suggest that PR improves healthspan and longevity via FGF21, yet it is important to comment on the limitations within our study. First, genetic variation has been shown to improve or blunt the metabolic benefits of diet on healthspan or lifespan64, and this variability in genetic response could extend to PR. Testing the effects of PR in multiple strains or mixed background strains such as HET-3 or the Diversity Outbred65–68 may provide evidence that is more translatable to humans. Although all mice in the current study are on the B6 background, it should be noted that the WT and Fgf21 KO mice are derived from separate colonies (not littermates), with potentially subtle differences in genetic background. Most notably, Fgf21 KO on the control diet exhibit reduced overall body weight and increased median lifespan compared to WT mice, and the mechanism underlying this difference is currently unclear. Second, sex also alters the response to healthy diets69. The beneficial effects of PR have been primarily illustrated in males, and only males were used in the current study. Therefore, the extent of sexual dimorphism in the improvements in healthspan and lifespan with PR remains unclear. Notably, a recent study reported that PR only improves metabolic health in males and ovariectomized female mice, suggesting that the benefits of PR are blunted in female mice70. New data indicate that neither PR nor BCAA restriction improved lifespan in females compared to males and that the benefits of BCAA restriction were reduced in middle-aged males31. On the other hand, initial findings in female rats showed that PR extended lifespan when initiated 120d of age71. Finally, this study used only a single diet to model protein restriction (5% casein) and a single genetic model to delete FGF21 (Fgf21 KO mice). It is possible that the observed effects, including the contribution of FGF21, might vary with differing levels of protein content and uniquely interact with differences in sex or genetic background.
In summary, our work collectively supports multiple overarching conclusions. First, in male C57BL/6 J mice the restriction of dietary protein intake exerts beneficial effects on body weight gain, adiposity, glucose homeostasis, physical performance, and metabolic health, with these effects ultimately reducing frailty and increasing lifespan. Second, these beneficial effects can be reproduced if the diet is initiated in middle age, where PR also protects against the harmful effects of diet-induced obesity during aging. Finally, and most importantly, nearly all these PR-induced beneficial effects depend on the liver-derived hormone FGF21. The inability to detect protein restriction in Fgf21-KO mice is initially benign but ultimately leads to negative outcomes in late life, such that Fgf21-KO mice on LP die earlier than their controls. Collectively, these data demonstrate that increases in circulating FGF21 mediate the extension of lifespan and improvement in healthspan during dietary protein restriction, and thereby identify a novel mechanism that drives the increase in longevity in response to dietary protein restriction in mammals.
Methods
Animals and diets
All procedures involving animals were approved by the PBRC Institutional Animal Care and Use Committee and were performed following the guidelines and regulations of the NIH Office of Laboratory Animal Welfare. Male C57BL/6 mice (WT, Jackson Lab) were used in all studies. Fgf21-deficient mice on the C57BL/6 background (Fgf21-KO) were originally provided by Steven Kliewer (University of Texas Southwestern, Dallas, Texas, USA) and maintained as a colony at PBRC. Diets were formulated and produced by Research Diets as previously described42,45 and were designed to be isocaloric by equally varying protein and carbohydrate while keeping fat constant. Control diets (CON) contained 20% casein (by weight) as the protein source, while the low protein diet (LP) contained 5% casein. High-fat diets (HF) also contained 20% casein (HFCON) and 5% casein (HFLP), respectively, but on a background of 60% fat. All diet compositions are provided in Table S1. At the end of the study mice were sacrificed during the mid-light cycle in the fed state (unless otherwise noted) using acute exposure to CO2 followed by rapid decapitation. Trunk blood was also collected at sacrifice, allowed to clot overnight at 4 °C, centrifuged at 3000xg, and serum collected. Tissues were collected and snap-frozen in liquid nitrogen for further analysis.
Experimental design
To link dietary protein restriction-induced FGF21 signaling to lifespan, male wildtype and Fgf21-KO mice were entered into the study at approximately 3 months of age, group-housed (4 per cage) at room temperature (23 °C), and placed on control or LP diet ad libitum (30 mice/diet/genotype). Mice were maintained on these diets until natural death or clinically determined as failure to thrive, with failure to thrive being jointly determined by laboratory staff and veterinary care staff and triggering humane euthanasia. The mice in this longevity study were solely utilized to examine the effects of dietary protein content on lifespan and determine if FGF21 is required. Therefore, this group was minimally handled except for routine body weight measurements and a one-time frailty index assessment at 18 months of age. The experimental timeline is presented in Fig. 1A (Schematics were created with BioRender).
To test if FGF21 is required for the effects of dietary protein restriction on metabolic health during aging, male wildtype and Fgf21-KO mice were entered into the study at approximately 3 months of age and group-housed (4 per cage) at room temperature (23 °C). Mice were placed on either control or LP diet ad libitum until 22 months of age (12 mice/diet/genotype). Mice that were clinically determined as being unable to thrive were removed from the study and euthanized as above. Bodyweight and food intake were recorded weekly throughout the experiment. Body composition was measured via TD-NMR (Bruker Minispec) at the start (3 months of age), middle (12 months of age), and end (22 months of age) of the study. Glucose homeostasis was assessed by glucose tolerance test and intraperitoneal insulin tolerance test at 10 and 18 months of age. Energy expenditure, ambulatory activity, and locomotor activity were measured at 12 and 20 months of age in metabolic chambers (23 °C) via TSE Systems (Phenomaster / Labmaster). Assessment of motor coordination via RotaRod treadmill (Med Associates) and grip strength (Harvard Apparatus) was measured at 21 months of age. Mice were sacrificed at 22 months of age for blood and tissue collection for further processing and analysis. The experimental timeline is presented in Fig. 2A (Schematics were created with BioRender).
To examine if age impacts the effect of dietary protein restriction to improve metabolic health, 12 month-old male mice were randomly assigned to one of 4 diets with 12 mice/diet: control (CON), LP, HFCON, or HFLP diet ad libitum. All mice were initially adapted to the control diet and then transitioned to their experimental diet while in the metabolic chambers (TSE Systems; PhenoMaster). After 14 days, mice were transitioned to standard cages, group-housed 4 per cage, and maintained on diet for four months. Bodyweight and food intake were recorded weekly throughout the experiment. Body composition was measured via TD-NMR (Bruker Minispec) at the start (12 months of age) and the end (16 months of age) of experimental diets. Glucose homeostasis was accessed by glucose tolerance test at 14 months of age. Mice were sacrificed at 16 months of age for blood and tissue collection for further processing and analysis. The experimental timeline is presented in Fig. 6A (Schematics were created with BioRender).
Frailty
Frailty was assessed in mice by using 5 clinically observed, noninvasive, health-related deficits based on the Howlett Frailty Index72,73. The deficits were scored as either absent (score 0), mild (score 0.5), or severe (score 1). Two independent, blinded individuals scored each mouse, with these numbers averaged before final analysis.
Grip strength
Forelimb grip strength (Harvard Apparatus) was evaluated at 21 months of age. The grip strength meter was positioned horizontally, and the mouse was held by the tail and lowered towards the mesh grid such that the forelimbs were allowed to grasp the metal grid. The mouse was then pulled backward in a horizontal fashion with grip strength measured as peak tension in grams. The measurement was performed three times per mouse and the scores were averaged for analysis.
Motor coordination
Rotarod (Med‐Associates, St Albans, VT) was used to assess motor function and coordination at 21 months of age. Mice were placed on a slowly accelerating rod that progresses over 300 sec, and the latency to fall was recorded for each animal. Mice were measured in a single day, with each mouse tested over 3 trials with an inter‐trial interval of 30‐40 min. Mean latency to fall was calculated per mouse and used for statistical analysis.
Glucose tolerance test (GTT)
Sixteen-hour-fasted mice underwent GTT by i.p. injection with 2 g glucose per kg of BW. Blood glucose levels were measured at 0, 15, 30, 45, 60, and 120 min via a handheld glucometer (Accu Check; Roche Diabetes Care, Inc. Indianapolis IN). The data for GTT are represented as mg/dL and as the area under the curve (AUC). Fasted glucose levels are reported at the time of GTT.
Insulin tolerance test (ITT)
Three-hour-fasted mice were injected i.p. with 0.75 IU human insulin (Eli Lilly and Company, Indianapolis, IN) per kg of BW. Blood glucose levels were measured at 0, 15, 30, 60, and 120 min with a glucometer (Accu Check; Roche Diabetes Care, Inc. Indianapolis IN). The ITT data are presented as a percentage of baseline glucose and as the area under the curve (AUC).
Real-time PCR
RNA extraction and real-time PCR were conducted as described previously36,42. Total RNA was extracted from liver and inguinal white adipose tissue (iWAT) using TRIzol reagent following the manufacturer’s protocol (Invitrogen), with the addition of an RNeasy Lipid Tissue Mini Kit (QIAGEN) for iWAT. RNA purity and quantity were determined by spectrophotometry using a NanoDrop (Thermo Scientific). cDNA synthesis was performed with iScript (BioRad) and mRNA was quantified on the ABI 7900 platform using the ABI SYBR Green PCR Master Mix in optical 384-well plates (Applied Biosystems). Primer pairs were designed using the IDT RealTime qPCR Primer Design tool to span an intro-exon boundary (Table S3). Target gene expression was normalized with cyclophilin as the endogenous control.
Immunoassay determination of FGF21, insulin, adiponectin, and IGF-1
Serum concentrations of FGF21 (no. RD291108200R, Mouse and Rat FGF-21 ELISA, BioVendor), insulin (#EZRMI-13K, Rat/Mouse Insulin ELISA, EMD Millipore Corporation), adiponectin (#EZMADP-60K, Mouse Adiponectin EMD Millipore Corporation), and IGF-1 (ab100695, Mouse IGF1 ELISA, Abcam) were determined via ELISA according to the manufacturer’s recommended protocol.
Colorimetric determination of hepatic triglycerides and serum ALT and AST
Concentrations of hepatic triglycerides were measured in hepatic homogenates with a colorimetric assay (no. 10010303, Cayman Chemical) according to the protocol recommended by the manufacturer
Concentrations of ALT (MAK052-Sigma Aldrich, Alanine Aminotransferase Activity Assay Kit) and AST (MAK055-Sigma Aldrich, Aspartate Aminotransferase Activity Assay Kit) in serum were determined with a colorimetric coupled enzymatic assay according to the procedure recommended by the manufacturer
Pancreas histology and immunohistochemistry
After fixation in 10% neutral buffered formalin (NBF) for 24–48 hr, the pancreatic tissue was embedded in paraffin. Five μm sections were cut onto positively charged slides and detection of insulin was performed on a Leica Bond-Max (Leica Biosystems, Melbourne, Australia) using the Bond Polymer Refine detection kit. The insulin primary antibody was guinea pig anti-insulin (1:800, #18-0067, Invitrogen, Grand Island, NY) with secondary detection performed using HRP-conjugated rabbit anti-guinea pig (1:800, A5545, Sigma, Saint Louis, MO). Stained sections were imaged using a Hamamatsu NanoZoomer digital slide scanner at 20x resolution. For determination of insulin positive area, insulin positive islets were detected and quantified using a custom application that detects insulin for each islet present within a section using the Visiopharm VIS software version 5.0.5.
Quantification and statistical analysis
Data were analyzed using the SAS version 9 or JMP Pro version 16 software packages (SAS Institute) using one-way, two-way, or repeated-measures ANOVA using the general linear model procedure. When experiment-wide tests were significant, post hoc comparisons were made using the LSMEANS statement with the PDIFF option, and represent least significant differences tests for pre-planned comparisons, generally within the diet*genotype or fat*protein interaction. Average daily energy expenditure was analyzed via analysis of covariance (ANCOVA) with body weight as the covariate using the general linear model procedure of SAS V9. Kaplan-Meier survival and Cox Proportional Hazards analysis were conducted using the JMP Pro 16 software program. All data are expressed as mean ± SEM, with a probability value of 0.05 considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank the leadership and staff of the PBRC Comparative Biology Core and Animal Metabolism and Behavior Core for their skillful assistance and excellent technical support. This work was supported by the National Institutes of Health (NIH) R01DK105032, R01DK121370, R01DK123083, and S10OD023703 to C.D.M. C.M.H. was supported by F32DK115137 and K99AG070273. RAS was supported by F32DK130544. H.-R.B. was supported by R01DK047348; H.M. was supported by R01DK092587; J.J.C. was supported by R21AI138136 and R01DK123183; and A.B. was supported by R21AG062985, American Diabetes Association ADA 1–19-IBS-126, and the William E. McElroy Charitable Foundation. This project used facilities within the Animal Metabolism & Behavior Core, Genomics Core, and Cell Biology and Bioimaging Core at PBRC that are supported in part by NIH center awards P30GM118430, P20GM135002, and P30DK072476, as well as an NIH equipment award S10OD023703. Schematic images in Figs. 1, 2 and 7 were created with BioRender.
Source data
Author contributions
C.M.H., A.B, and C.D.M. conceived and designed the studies. C.M.H., D.C.A., L.C., R.A.S, E.Q-C, S.J.B., J.J.C., D.H.B., and S. Y. conducted the experiments, acquired and analyzed data, and produced the figures. C.M.H., S.J.B., J.J.C., E.Q.-C., S.Y., D.H.M, H.-R.B., H.M., A.B., and C.D.M. discussed data interpretation. C.M.H. and C.D.M. wrote the original draft, whereas C.M.H., S.J.B., J.J.C., E.Q.-C., S.Y., H.-R.B., H.M., A.B., and C.D.M. edited and revised the final draft.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Data availability
All data generated in this study are provided in the Supplementary Information/Source Data file, and from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristal M. Hill, Email: Cristal.Hill@pbrc.edu
Christopher D. Morrison, Email: Christopher.Morrison@pbrc.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29499-8.
References
- 1.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch JB, et al. Advances in geroscience: impact on healthspan and chronic disease. J. Gerontol. A Biol. Sci. Med Sci. 2014;69:S1–S3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana L, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevino-Villarreal JH, et al. Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI Insight. 2018;3:e99470. doi: 10.1172/jci.insight.99470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittendorfer B, Klein S, Fontana L. A word of caution against excessive protein intake. Nat. Rev. Endocrinol. 2020;16:59–66. doi: 10.1038/s41574-019-0274-7. [DOI] [PubMed] [Google Scholar]
- 7.Orillion A, et al. Dietary protein restriction reprograms tumor-associated macrophages and enhances immunotherapy. Clin. Cancer Res. 2018;24:6383–6395. doi: 10.1158/1078-0432.CCR-18-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen AN, Kondrup J, Borsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr. Res. 2013;57:21245. doi: 10.3402/fnr.v57i0.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine ME, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soultoukis GA, Partridge L. Dietary protein, metabolism, and aging. Annu Rev. Biochem. 2016;85:5–34. doi: 10.1146/annurev-biochem-060815-014422. [DOI] [PubMed] [Google Scholar]
- 11.Le Couteur DG, et al. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol. Life Sci. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings NE, Lamming DW. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol. Cell Endocrinol. 2017;455:13–22. doi: 10.1016/j.mce.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirzaei H, Raynes R, Longo VD. The conserved role of protein restriction in aging and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:74–79. doi: 10.1097/MCO.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- 16.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl Acad. Sci. USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solon-Biet SM, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solon-Biet SM, et al. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl Acad. Sci. USA. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horakova M, Deyl Z, Hausmann J, Macek K. The effect of low protein-high dextrin diet and subsequent food restriction upon life prolongation in Fischer 344 male rats. Mech. Ageing Dev. 1988;45:1–7. doi: 10.1016/0047-6374(88)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Leto S, Kokkonen GC, Barrows CH., Jr Dietary protein, life-span, and biochemical variables in female mice. J. Gerontol. 1976;31:144–148. doi: 10.1093/geronj/31.2.144. [DOI] [PubMed] [Google Scholar]
- 22.Brown-Borg HM, Buffenstein R. Cutting back on the essentials: can manipulating intake of specific amino acids modulate health and lifespan? Ageing Res Rev. 2017;39:87–95. doi: 10.1016/j.arr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp. Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 25.Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 26.Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech. Ageing Dev. 1988;43:79–98. doi: 10.1016/0047-6374(88)90099-1. [DOI] [PubMed] [Google Scholar]
- 27.Yap YW, et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 2020;11:2894. doi: 10.1038/s41467-020-16568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solon-Biet SM, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019;1:532–545. doi: 10.1038/s42255-019-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juricic P, Gronke S, Partridge L. Branched-chain amino acids have equivalent effects to other essential amino acids on lifespan and aging-related traits in Drosophila. J. Gerontol. A Biol. Sci. Med Sci. 2020;75:24–31. doi: 10.1093/gerona/glz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings NE, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018;596:623–645. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson NE, et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging. 2021;1:73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green CL, Lamming DW. Regulation of metabolic health by essential dietary amino acids. Mech. Ageing Dev. 2019;177:186–200. doi: 10.1016/j.mad.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunpo P, et al. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J. Biol. Chem. 2009;284:32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanders D, et al. Role of GCN2-independent signaling through a noncanonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65:1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henagan TM, et al. Hepatic autophagy contributes to the metabolic response to dietary protein restriction. Metabolism. 2016;65:805–815. doi: 10.1016/j.metabol.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyo JO, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Maida A, et al. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Mol. Metab. 2017;6:873–881. doi: 10.1016/j.molmet.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill CM, Berthoud HR, Munzberg H, Morrison CD. Homeostatic sensing of dietary protein restriction: a case for FGF21. Front Neuroendocrinol. 2018;51:125–131. doi: 10.1016/j.yfrne.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laeger T, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16:707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maida A, et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Invest. 2016;126:3263–3278. doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill CM, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci. Rep. 2017;7:8209. doi: 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill CM, et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27:2934–2947 e2933. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markan KR, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, et al. Structures of beta-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature. 2018;553:501–505. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev. Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 49.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bookout AL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owen BM, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz MB, et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat. Commun. 2020;11:4618. doi: 10.1038/s41467-020-18446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 55.Slonaker JR. The effect of different per cents of protein in the diet VII. Life span and cause of death. Am. J. Physiol. 1931;2:266–275. [Google Scholar]
- 56.Iwasaki K, et al. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J. Gerontol. 1988;43:B5–B12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- 57.Inagaki T, et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang H, et al. FGF21 prevents low protein diet-induced renal inflammation in aged mice. Am. J. Physiol. Renal Physiol. 2021;321:F356–F368. doi: 10.1152/ajprenal.00107.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson AC, et al. Fibroblast growth factor 21 is not required for the reductions in circulating insulin-like growth factor-1 or global cell proliferation rates in response to moderate calorie restriction in adult mice. PLoS ONE. 2014;9:e111418. doi: 10.1371/journal.pone.0111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J, et al. Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology. 2012;153:750–758. doi: 10.1210/en.2011-1591. [DOI] [PubMed] [Google Scholar]
- 62.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim. Biophys. Acta. 2013;1831:943–949. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Dommerholt MB, et al. Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. FEBS J. 2021;288:2257–2277. doi: 10.1111/febs.15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogue MA, Churchill GA, Chesler EJ. Collaborative cross and diversity outbred data resources in the mouse phenome database. Mamm. Genome. 2015;26:511–520. doi: 10.1007/s00335-015-9595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med Sci. 2010;65:1275–1284. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melia T, Waxman DJ. Genetic factors contributing to extensive variability of sex-specific hepatic gene expression in Diversity Outbred mice. PLoS One. 2020;15:e0242665. doi: 10.1371/journal.pone.0242665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller RA, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell SJ, et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larson KR, et al. Sex differences in the hormonal and metabolic response to dietary protein dilution. Endocrinology. 2017;158:3477–3487. doi: 10.1210/en.2017-00331. [DOI] [PubMed] [Google Scholar]
- 71.Miller DS, Payne PR. Longevity and protein intake. Exp. Gerontol. 1968;3:231–234. doi: 10.1016/0531-5565(68)90006-5. [DOI] [PubMed] [Google Scholar]
- 72.Kane AE, Ayaz O, Ghimire A, Feridooni HA, Howlett SE. Implementation of the mouse frailty index. Can. J. Physiol. Pharm. 2017;95:1149–1155. doi: 10.1139/cjpp-2017-0025. [DOI] [PubMed] [Google Scholar]
- 73.Whitehead JC, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. A Biol. Sci. Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are provided in the Supplementary Information/Source Data file, and from the corresponding author upon reasonable request. Source data are provided with this paper.