Abstract
This work investigated the influence of process variables of extraction temperature (35–55 °C), solid to liquid ratio (1:20–1:50 g/mL) and time (100–200 min) on the total phenolic content (TPC) and yield (EY) of Carica papaya leaves (CPL) extracts using Box-Behnken experimental design available in Design Expert software. Bi-objective process optimization was also carried out using the desirability function algorithm. The optimum process variables were later used to design an integrated process for the production of CPL extracts with the assistance of SuperPro Designer software. Scale-up studies and economic analysis for CPL extracts production were investigated in the range of 0.638–20.431 × 103 kg CPL extracts/y to determine the most economically feasible production capacity based on the minimum unit production cost (UPC) of CPL extracts. The risk and sensitivity analyses of the most economically feasible production scale were carried out using the Monte Carlo simulation in Oracle Crystal Ball software. Process variables had notable influences on the TPC and EY of CPL extracts. The extraction temperature of 35 °C, solid to liquid ratio of 40.25 g/mL and time of 100 min gave the optimum TPC of 74.65 mg GAE/g d.b and EY of 18.76 % (w/w). HPLC results indicated that CPL extracts were rich in gallic, betulinic, chlorogenic, ellagic, ferulic and caffeic acids. The designed integrated process showed similar behavior with the laboratory scale of 0.18758 g CPL extracts/batch. The preliminary techno-economic analysis indicated that plant capacity has a strong dependence on the material & energy demands and process economics. Plant capacity of 19.857 × 103 kg CPL extracts/y possessed the least UPC and was selected as the most economically feasible scale. The certainty of obtaining base case UPC value of 525.21 US$/kg CPL extracts was 75.20%. Sensitivity analysis showed that extracts recovery, CPL/water, centrifuge purchase cost, extraction time, extractor purchase cost and extraction temperature contributed -5.3 %, +42.8%, +4.0%, +47.1%, +0.1%, and +0.5%, respectively to the variance in UPC of CPL extracts.
Keywords: Heat-assisted extraction technology, Integrated process design, Techno-economic analysis, Carica papaya leaves, Process optimization, Unit production cost
Graphical abstract

Highlights
-
•
Carica papaya leaves (CPL) extracts are rich in polyphenols.
-
•
Heat assisted extraction technology was capable of recovering CPL extracts.
-
•
Industrial extracts scale-ups were achievable using SuperPro Designer software.
-
•
Plant capacity of 19.857 × 103 kg extracts/y was selected most economical scale.
-
•
The 525.21 US$/kg extracts unit production cost base case certainty was 75.20%.
Heat assisted extraction technology; Integrated process design; Techno-economic analysis; Carica papaya leaves; Process optimization; Unit production cost.
1. Introduction
Bioactive compounds from medicinal plants have a long history of prevention and treatment of diseases (Sitarek et al., 2020; Ahmed et al., 2021). These bioactive organic compounds (often refers to as phytochemicals) include alkaloids, phenolics, terpenoids and tannins (Koche et al., 2016) and can be found in the root, leaves, flower and stem bark of plants (Ugboko et al., 2020). There are scientific evidences of the potencies of phenolic bioactive compounds of medicinal plants in the treatment of cancer (Majolo et al., 2019; Desai et al., 2008), diabetes (Shanmugam et al., 2021), malaria (Titanji et al., 2008), immune disorder (Venkatesha et al., 2016) and heart problems (Mashour et al., 1998). Hence, these phytochemical compounds can be extracted from plant matrices, optimized and processed into new therapeutic drugs (Ugboko et al., 2020). Plant phytochemicals also find applications in food and cosmetics industries. Characterization methods such as high performance liquid chromatography (Obafemi et al., 2017; Maria et al., 2018), Fourier-transform infrared spectroscopy (Meenakshi et al., 2011) and liquid chromatography quadrupole time of flight mass spectrometry (Alara et al., 2021) have been used to elucidate bioactive compounds from natural sources.
One of the many established and potent ethnomedicinal plant sources in Nigeria and Africa is Carica papaya L. leaves (CPL). The Carica papaya L trees are mainly cultivated for its fruits while the leaves are regarded as a waste (Vuong et al., 2013). Presently, Nigeria is ranked 6th in the production of pawpaw fruits with around 8,36,702 million tonnes/year (Alara et al., 2020). The Carica papaya tree being a deciduous shrub sheds its leaves and the dried shed leaves are used either as a sole or part of herbal concussions by traditional herbal practitioners in Nigeria to treat various types of human diseases. However, there have been documented scientific reports on the use of aqueous CPL extracts to treat dengue fever, amoebic dysentery, gastric digestion problems and intestinal worms. It also reduces the risk of cardiovascular diseases, serves as an immune-adjuvant for vaccine therapy, alleviates allergic disorders, possesses antitumor activities and reduces symptoms of asthma (Vuong et al., 2013). Hence, the need for detailed protocol to achieve optimal extraction of the bioactive compounds (phytochemicals) responsible for the numerous activities of the CPL extracts from the plant matrix.
Technologies for the extraction of bioactive compounds from natural sources can be classified as conventional and emerging technologies. Prominent emerging extraction technologies for bioactive recovery from plant matrices include supercritical and subcritical fluid extractions (Cardenas-Toro et al., 2014), microwave-assisted extraction (Alara et al., 2021), high-pressure processing (Yuan et al., 2018), pulsed electric field processing (Nowacka et al., 2019), infrared-assisted extraction (Raafat et al., 2019), high-voltage electrical discharge extraction (El Kantar et al., 2019) and ultrasound-assisted extraction (Sharayei et al., 2019). Although emerging technologies have been reported to achieve higher bioactive yields, consume less solvent and use less time and energy when compared with the conventional technologies such as heat-assisted extraction, many of these technologies have scale-up issues (e.g. microwave generator of more than 100 kW is not presently available) and require high investment cost. They also require huge instrumentation, automation and maintenance costs (Adeyi et al., 2021). Moreover, unlike the conventional technologies, the emerging technologies could only be adequate for a particular matrix and target the recovery of a specific class of bioactive compounds (Maroun et al., 2018). The environmental impacts and thorough energy evaluation of these emerging technologies still need to be investigated.
The conventional vessel heating solid-liquid extraction is a widely studied technology for the recovery of bioactive compounds from plant matrices (Kamarudin et al., 2020). The procedure involves using a solvent (with high affinity) to remove the solutes (bioactive compounds) which are dispersed in the solid (plant) matrix at elevated temperature and for a specific time. The conventional heat-assisted extraction (HAE) still remains popular in the bioactive extraction industry because of its relative advantages such as ease of operation and scalability, availability of different extractor sizes, relatively low equipment purchase cost, and low maintenance and instrumentation costs. While solvent selection plays a key role in the extraction of bioactive compounds using HAE technology, other important variables such as solid to liquid ratio, extraction temperature, solid particle size and time (Adeyi et al., 2021) do affect the integrity and quantity of bioactive compounds removal from plant matrix. However, to achieve bioactive compounds of high quality and quantity from a natural source such as CPL, optimization of these process variables is a must. Response surface methodology (RSM) is an optimization technique that uses a reduced number of experiments to evaluate the effects of multiple parameters and their interactions on a response (Kadam et al., 2015). Also, when the response of interest in a process (such as extraction) is being influenced by many process parameters, RSM can be useful in optimizing such a process to obtain the best process variables that optimize the extraction of bioactive compounds. The global optimum data therein obtained can form the basis for process design, scale up studies and preliminary techno-economic analysis of industrial production (Adeyi et al., 2020, 2021; Oke et al., 2021) of the bioactive compounds.
Although the extraction of plant bioactive compounds has been reported feasible at laboratory scale, many of the documented reports lack detailed engineering endeavors and failed to assess the economic viability of these processes industrially. The literature survey revealed a few works on laboratory extraction of bioactive compounds from CPL (Vuong et al., 2013; Jagtap et al., 2019; Alara et al., 2021), however, the type of CPL (either freshly harvested green leaves or already shed leaves) used was not clearly stated in their methodology. Also, none of these authors focused on process design, scale up investigations and economic analysis of the extraction of bioactive compounds from CPL. Detailed techno-economic analysis of the extraction of bioactive compounds from CPL will assess its commercialization potential and also guides further process optimization towards achieving an economically viable process. Presently, computer aided process simulation (CAPS) software is available for the design, scale up and economic analysis of a production plant. CAPS uses computer packages to carry out steady state energy and material balances, do sizing of process equipment and costing analysis of a process. It is also useful in overall process optimization by pin pointing processing steps with high operating and capital cost with low yields or throughputs (Adeyi et al., 2021). However, among the presently available simulation software, SuperPro Designer (Adeyi et al., 2020) Aspen Plus (Adeniyi et al., 2019), Aspen Hysys (Sunny et al., 2016) and Aspen Batch (Oke et al., 2021) have been widely engaged. Moreover, in the process of deterministic techno-economic analysis of production processes, some of the technical and cost variables used in the analysis may vary widely and therefore might introduce some uncertainties in the analysis. Hence, to evaluate the effects of parameter and uncertainty level in techno-economic analysis, the uncertainty and sensitivity analyses have been widely conducted.
Therefore, this work investigated laboratory optimization protocols for the HAE of bioactive compounds from shed CPL and thereafter used the optimized extraction conditions for the design and analysis of industrial CPL extracts production with the aim of assessing the commercial viability of this venture due to rapidly growing global bioactive ingredients market. This endeavor is necessary to analyze the commercial feasibility of bioactive extracts production in a country like Nigeria where the Carica papaya L cultivation is presently high and there is an abundance of the shed CPL as cheap raw materials in Carica papaya L plantations. The objectives of this work were to (i) investigate the effect of process variables on the extractability of bioactive compounds extraction from CPL and optimize the extraction process conditions using the desirability function in Design expert software (ii) characterize the CPL extracts using high performance liquid chromatography (HPLC) (iii) carry out process design and techno-economic analysis using the optimum extraction conditions to determine the most economically viable scale of commercial production of CPL bioactive extracts based on minimum unit production cost with the assistance of SuperPro Designer software as process simulator (iv) conduct uncertainty and sensitivity analyses using the Monte Carlo simulation in Oracle Crystal Ball software to quantify the associated risks and identify technical and cost variables of high significance to the perturbation in unit production cost of the selected most economically feasible CPL extracts production capacity.
2. Materials and methods
2.1. Plant materials and reagents
Already shed Carica papaya leaves (CPL) were collected from the commercial farm of Michael Okpara University of Agriculture Umudike, Abia state, Nigeria. The CPL were rinsed in running water to remove all the adhering dirt. The rinsed CPL were spread on a tray and air dried in Chemical Engineering laboratory for two weeks at room temperature of approximately 25 °C to attain a stable weight. After the sample drying, the CPL were pulverized, using an electric blender, screened with sieve aperture of 0.105 mm and kept in an airtight black polythene bag at 4 °C before the extraction experiment commenced. The moisture content of the air dried CPL sample was determined using the oven drying method and was found to be approximately 9% (w/w). The chemicals used in the course of experimentation such as sodium carbonate (Na2CO3) and Folin Ciocateu phenol reagent were purchased from Sigma Aldrich, Poole, England. Other HPLC grade phenolic standards were gallic acid, betulinic acid, chlorogenic acid, ellagic acid, ferulic acid, caffeic acid, quercetin and rutin and were procured from GFS Chemicals, Inc. USA (Sigma Aldrich, Germany). The distilled water was obtained from the Chemical Analysis Laboratory of Chemical Engineering Department, Michael Okpara University of Agriculture Umudike. All chemicals used were of analytical grades.
2.2. Laboratory heat-assisted extraction of CPL
The laboratory heat-assisted extraction (HAE) of CPL was conducted in a thermostated water bath equipped with electromagnetic stirrer and was according to the procedure of Pinela et al. (2019). The HAE experiment commenced by carefully weighing out one gram of dried CPL using a digital scale (with precision of ±0.01 g) into extracting vessel and processed under continuous stirring at desired solid/liquid, extracting temperature and time according to the experimental design. The solvent used for the CPL extraction was distilled water and this was because of the reported high CPL bioactive compounds solubility in water (Vuong et al., 2013) and high immunological potencies and no adverse effects of the aqueous extracts of CPL (Nwiloh et al., 2009). All extracting vessels loaded in the thermostated water bath were sealed to avoid solvent evaporation. The resulting extracts-water mixture was afterwards centrifuged at room temperature (600 rpm for 10 min) and the supernatant (aqueous extracts) was carefully separated from the fiber sediments.
2.3. Box-Behnken experimental design and statistical analysis for investigating aqueous HAE of CPL
Box-Behnken Experimental Design (BBED) involving three levels and three-variables (Table 1) designed using Design Expert software (Design Expert version 7.0 Stat-Ease Inc., Minneapolis, MN) was used for this investigation. The process variables considered on the extractability of bioactive compounds (in terms of extracts yield (EY) and total phenolic content (TPC)) for this study were extraction temperature (A, 35–55 °C), CPL/water (B, 1: 20–1:55 g/mL) and time (C, 100–200 min). The BBED for the three (3) investigated process variable produced 17 experimental runs. All the ranges of process variables investigated were carefully selected in accordance with the preliminary studies and previously reported variables ranges for the extraction of bioactive compounds from plant materials in the literature.
Table 1.
Heat-assisted extraction experimental design.
| Process variables | Level |
||
|---|---|---|---|
| -1 | 0 | +1 | |
| A, Temperature (oC) | 35 | 45 | 55 |
| B, Solid to liquid ratio (g/mL) | 20 | 35 | 50 |
| C, Time (min) | 100 | 150 | 200 |
In order to establish the needed relationships between the process variables and desired responses, the extracts yield and TPC data obtained from the laboratory experiments were independently fitted to a quadratic model of the form in Eq. (1).
| (1) |
Where Y is the predicted response parameter (EY or TPC), , , and are constant, linear, quadratic and interaction coefficients and were estimated by the Design Expert software. The ∑ indicates the summation of terms. and represent the coded independent variables. The statistical significance of the fitted quadratic model and all models' (regression) coefficients was determined by applying the analysis of variance (ANOVA) at 95% confidence level. The adequacy of the fitted quadratic model was determined by lack of fit analysis, coefficient of determination (R2) (Eq. (2)), adjusted coefficient of determination (Eq. (3)) and predicted coefficient of determination (Pred. R2) (Eq. (4).
| (2) |
| (3) |
| (4) |
Here, , and are the sum of square of variation for the residual, model and total and are defined in Eq. (5) – Eq. (7). The “model” and “residual” here, refers to the constructed quadratic predictive model and the error between the model predicted and experimental value. is the addition of and .
| (5) |
| (6) |
| (7) |
Where, are the observed value, mean value of sample and value estimated by the regression model and n is the observed values. The degree of freedom, which indicate the number of values that are free to vary, for the model and residuals are denoted as and respectively.
Also, PRESS, a measure of predictive power, defined as sums of squares of the prediction residual errors for sample observations not used to estimate the model, is defined in Eq. (8) (Raissi, 2009)
| (8) |
Where is known as PRESS residuals and is defined in Eq. (9)
| (9) |
is the observed value and is the fitted value of the ith response based on all the observed values except the ith one.
2.4. Determination of optimum process conditions and validation
Optimum process conditions for the extraction of CPL were determined according to the procedure of Oke et al. (2020). The numerical optimizer available in the Design Expert software version 7 was used to achieve the optimization of process variables (extraction temperature, CPL/water and time) for the simultaneous maximization of CPL bi-responses of EY and TPC. Table 2 shows the desired goal, weight and importance selected for the optimization of extraction variables on the investigated responses. Here, the optimization goal was set to maximize both the EY and TPC simultaneously in the observed experimental range while keeping all process variables within the designed ranges. All the settings were done manually in the numerical optimization environment of the software. Hence the optimum process variables (based on extracts with high EY and TPC) were determined by using the desirability profiling function available in the Design Expert version 7.0 software.
Table 2.
Optimization goal for HAE of CPL.
| Name | Goal | Lower limit | Upper limit | Lower weight | Upper weight | Importance |
|---|---|---|---|---|---|---|
| Temperature (oC) | is in range | 35 | 55 | 1 | 1 | 3 |
| Solid: liquid (g/mL) | is in range | 20 | 50 | 1 | 1 | 3 |
| Time (min) | is in range | 100 | 200 | 1 | 1 | 3 |
| TPC (mg GAE/g) | maximize | 12.56 | 75.22 | 1 | 1 | 3 |
| EY (w/w %) | maximize | 10.53 | 26.31 | 1 | 1 | 3 |
The validation experiment was conducted at the predicted optimum process variables and the obtained experimental EY and TPC were thereafter compared with the model predicted values. The disparity between the predicted and experimental response value was expressed in terms of the relative standard deviation (RSD). The predicted and experimental value with RSD of <10 was considered as similar. The RSD is expressed in Eq. (10) according to Domínguez et al. (2020).
| (10) |
Where and are the standard deviations and mean values between the predicted and experimental values respectively.
2.5. Determination of the CPL extracts yield
The obtained aqueous extracts of CPL from Section 2.2 were collected and concentrated in the laboratory. The concentrated extracts were further dried in an oven till constant weight was obtained. The yield of the recovered extracts from CPL was calculated according to Alara et al. (2021) by using Eq. (11).
| (11) |
Where, EY is the yield of CPL extracts, is the weight (g) of CPL extracts and is the weight (g) of CPL used for the extraction.
2.6. Determination of TPC in the extracts of CPL
The Folin-Ciocalteu method was used for the determination of TPC in the aqueous CPL extracts and was according to the procedure of Gan and Latiff (2011). Briefly, the aqueous CPL extracts (I mL) which has been pre diluted with distilled water to a ratio of 1:10 was mixed with 1.8 mL of Folin-Ciocalteu reagent and allowed to stand for 5 min. 1.2 mL of 7.5% (w/v) sodium bicarbonate was added to the mixture, allowed to stand for 60 min at room temperature and the absorbance measured at 765 nm using a spectrophotometer. The amount of TPC (gallic acid equivalence (GAE)) was interpreted from the gallic acid calibration curve (y = 0.024x +0.0014, R2 = 0.998) which has been earlier constructed and was calculated and expressed in mg GAE/g d.w using Eq. (12).
| (12) |
Where C (mg/mL) represents the concentration interpreted from the calibration curve, V (mL) is the volume of the extracting solvent and m (g) is the weight of dried CPL used.
2.7. High performance liquid chromatography (HPLC) profiling of CPL extracts
The CPL extracts obtained at the optimum extraction conditions was used for this analysis and was according to the procedure of Krishna Murthy and Manohar (2014). 10 μL of the filtered (using 0.45 μm membrane filter) CPL water extracts was injected into the reverse VP-ODS column (150 × 4.6 mm, 5μm particle size), of HPLC (operating at 40 °C) that consisted of Ultra-Fast LC-20AB prominence and equipped with SIL-20AC autosampler, DGU-20A3 degasser, SPD-M20A UV diode array detector (UV-DAD, wavelength of 190–800 nm), column oven CTO-20AC, system controller CBM-20A lite and Windows LC solution software (Shimadzu Corporation, Kyoto Japan). The chromatographic conditions included mobile phase solvent A: 0.2% v/v formic acid and solvent B: acetonitrile; mode: isocratic elution (mobile phase solvent A and B in the ratio 80:20). The constant flow rate of 0.6 mL/min was used, total run time was 15 min and the wavelength of detection was at 220 nm. Reference standards of phenolic compounds were independently analyzed under similar condition with the CPL extracts. Bioactive compounds in the CPL extracts were identified by comparing the retention times of the standard phenolic acids with extracts.
2.8. Computer aided design, scale ups, economics and risk analysis of CPL extracts production
2.8.1. Process design, description and scale-up studies
The base case flowsheet for the production of CPL extracts was designed with the optimum extraction conditions obtained in Section 2.4 by using Superpro Designer software (Intelligen, 2014) and plant configuration similar to Adeyi et al. (2021) was adopted. The Superpro Designer software contains equipment models that housed unit procedures, unit operations and has various chemicals in its library. The software was capable of carrying out material and energy balances as well as equipment sizing and costing. It was also responsible for the estimation of the amount of heating and cooling agents and other utility demands needed in the CPL extracts production plant. The major equipment models typical of an aqueous extracts production plant were featured in the process plant and included grinder, extractor, centrifuge, evaporator, dryer, pump and storage tanks. The CPL extracts production was modeled as a 100 % batch process and annual operating time (AOT) of 7920 h was assumed (Adeyi et al., 2020). CPL was registered as user defined component and its measured density of 1065 g/L was used in the course of process simulation.
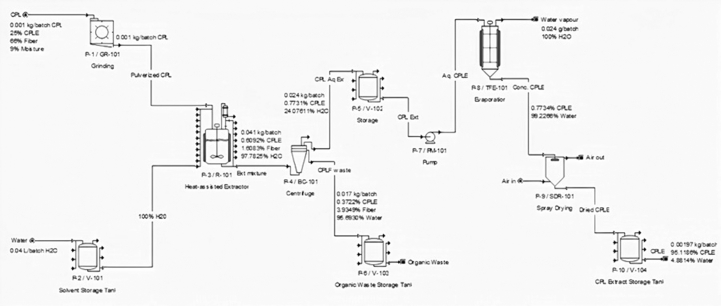
Figure 1 is the designed flowsheet for the production of phenolic rich extracts from CPL using HAE-T based process. The plant was designed to utilize already dried CPL and the CPL was modeled to contain moisture, extracts and fiber. The process of CPL extracts production started by pulverizing the dried CPL in the grinder (P-1/GR-101) to particle size of 0.105 mm for 5 min. Distilled water from the storage tank (P-2/V-101) and pulverized CPL were transferred into conventional heat assisted extractor (P-3/R-101) to actualize the extraction of bioactive compounds from CPL. Here (P-3/R-101), the optimal extraction conditions obtained in Section 2.4 were used for the operation of the extractor. Therefore, 1 g of CPL and 40.25 mL of water were processed at an extraction temperature of 35 °C for 100 min with continuous stirring to achieve bioactive yield of 18.76%. The content of P-3/R-101 was afterwards sent to a bowl centrifuge (P-4/BC-101) where aqueous bioactive extracts and CPL fibers were separated (100% CPL fiber removal and 40% solvent loss were assumed (Athimulam et al., 2006)) and temporarily stored in storage tanks P-5/V-102 and P-6/V-103 respectively. The centrifugal pump (P-7/PM-101) was used to transfer the aqueous bioactive extracts from P-5/V-102 to evaporator (P-8/TFE-101) where extracts concentration took place. The operating temperature was fixed at 65 °C while the operating pressure of P-8/TFE-101 (170.70 mm Hg) was simulated using the Peng-Ronbison cubic equation of state similar to the published work of Vieira et al. (2013). The concentration of the extracts in P-8/TFE-101 took approximately 60 min before high extracts content was obtained in solution. The concentrated CPL extracts stream was then sent to spray dryer (P-9/SDR-101) (5 w drying air/w water evaporated was assumed) where water was further removed and CPL phenolic rich extracts (<5% moisture content) was stored in P-10/V-104. Process scheduling was well detailed and set-up time of 5 min was assumed for each operation.
Figure 1.
Flowsheet and mass balance for base case production of phenolic rich CPL extracts using HAE technology (Adeyi et al., 2021).
The scale-up algorithm available in SuperPro Designer software was employed to achieve up scaling of laboratory CPL extracts production to industrial setups. The scale up was anticipated to determine/identify the optimum economically viable industrial scale to operate the CPL extracts production plant and this was investigated between 0.638 and 20.431 × 103 kg/y. The simulated range was carefully selected from preliminary simulation experiments to benefit from economies of scale effect. Also, the high upper plant capacity limit of 20.431 × 103 kg/y was anticipated considering the average daily polyphenol intake per person of 1 g/day (approximately 365 g/y) (Shahidi and Ambipaipalan, 2015). SuperPro Designer software was responsible for the calculation of material and energy balances, equipment sizing and utility demands estimations of each simulated scale.
2.8.2. Economic analysis of CPL extracts production
SuperPro Designer software was used for the assessment of the economic implications of the CPL extracts production plant and was according to the method of Baral and Shah (2016). The economic analysis of CPL extracts production entailed the determination of total capital investment cost (TCC), total operating cost (TOC) and unit production cost (UPC) of CPL extracts produced. The equipment purchase cost (EPC) of all the production scales investigated was obtained from the cost database of SuperPro Designer software and all equipment was assumed to be constructed with stainless steel. SuperPro Designer software has constantly maintained and updated databases and hence highly reliable for economic analysis of processes. Other authors have utilized SuperPro Designer databases for the design and economic analysis of their processes (Adeyi et al., 2020, 2021; Vieira et al., 2013; Sayar et al., 2019). The TCC was the addition of direct fixed capital cost (DFC), working capital and start up and validation costs. The DFC was computed as the summation of total plant direct cost (TPDC), total plant indirect cost (TPIC) and miscellaneous cost (MC). The TPDC comprised of process piping (35% EPC), instrumentations (40% EPC), insulation (3% EPC), electrical (10% EPC), buildings (45% EPC), yard improvement (15% EPC), and auxiliary facilities (40% EPC). The TPIC consisted of the engineering (25% TPDC) and construction (35% TPDC) costs. The MC cost parameters were contractor's fees (5% (TPDC + TPIC)) and contingency (10% (TPDC + TPIC)). The working capital was estimated to cover 30 days of raw material procurement, labor and waste treatment costs. However, CPL fiber wastes are organic in nature and can be used as fertilizer in farms and therefore no treatment cost was allocated to its treatment. The start-up cost was calculated as 5% DFC.
TOPC is the addition of raw materials, facility dependent, labor dependent, laboratory QC/QA, and utility costs. All the raw material costs and labor cost were obtained in the Nigerian context. Therefore, the electricity cost was 0.05 US$/kWh, cost of distilled water was 0.1 US$/kg, cooling and chilled water was 0.05 and 0.4 US$/MT respectively and steam was 12 US$/MT. The cost of CPL was obtained at a low price of 0.5 US$/kg at a market in Umudike Nigeria but was however, considered in sensitivity and uncertainty analysis. It was proposed that the CPL extracts production plant is sited very close to the source of material (proximity to Cararica papaya plantation). The wastes generated in the CPL extracts production plant were mainly organic and therefore can be used as fertilizer on farms to improve soil fertility, hence no cost was attached to CPL transportation and waste treatment. Three (3) operators were proposed to work in CPL extracts production plant. Information regarding the labor cost was obtained from the 2020 minimum wage of national salaries, income and wage commission, Nigeria and was obtained as 1.2 US$/h (Oke et al., 2021) which included, the basic rate, benefits, administrative and supervision costs. The facility dependent cost parameters were maintenance and repair (6% DFC), insurance (1% DFC), local taxes (1% DFC), factory expenses (5% DFC). The lab/QC/QA cost was estimated as 15 % of total labor cost. Furthermore, project life time of 15 years, construction period of 30 months, inflation rate of 4%, equity of 40%, income tax rate of 40% and the straight line depreciation method were assumed (Baral and Shah 2016). The UPC of CPL extracts was used as the profitability index and was calculated as the sum of variable and fixed costs divided by the total amount of CPL extracts produced (Adeyi et al., 2021).
2.8.3. Risk analysis of CPL extracts production process
The uncertainty and sensitivity analyses were conducted on the most economical scale of CPL extracts production to identify both technical and cost variables of profound perturbation effect on the UPC of CPL extracts. Therefore, the plant capacity of 19.857 × 103 kg CPL extracts/y was used for this investigation being the capacity with the least UPC and hence the most economically viable scale of production. The Monte Carlo simulation available in the Oracle Crystal Ball (OCB) software was employed for this analysis and was according to the procedure of Adeyi et al. (2020). The technical variables with tendencies of high fluctuations due to human and instrument errors during laboratory investigations such as extraction temperature (oC), time (min), CPL/water (g/mL) and optimized bioactive CPL extracts yield (%) were considered for this analysis. Also, major equipment purchase costs such as extractor, grinder, centrifuge, evaporator and dryer costs and CPL purchase cost which have possibilities of varying widely in the market were also considered. All the investigated variables were varied between + and - 20% of their original values. In the course of Monte Carlo simulation in OCB, the technical and cost variables were inserted in the assumption cells while the UPC of CPL extracts was made the forecast cell. Uniform distribution was assumed for all forecast variables and 30,000 trials were run to achieve a low mean standard error in UPC of CPL extracts.
3. Results and discussion
3.1. Quadratic model fitting and statistical analysis for TPC and EY of CPL
The Box-Behnken experimental design was adopted for the study of extraction, model construction and HAE process variables optimization of bioactive compounds from CPL. Table 3 shows the analysis of variance (ANOVA) results for the quadratic models developed (for TPC and EY), respective model coefficients and model performance evaluation parameters. The TPC and EY models had significant (p < 0.05) model F-values with non-significant lack of fit. The model F-value is a value on F-distribution used to determine the statistical significance of a fitted model. A large F-value indicates that the variance among group means cannot occur by chance. In the same vain, the lack of fit parameter informs about the adequacy of the constructed model to perfectly describe the functional relationship between the process variables and response parameter. A non-significant lack of fit implied that the model fitted well (Oke et al., 2021).
Table 3.
Analysis of variance for TPC and crude extraction yield quadratic models∗.
| Source | TPC (mg GAE/g d.w) |
EY (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of squares | df | Mean square | F-value | P-value P > F |
Sum of square | df | Mean squares | F-value | P-value P > F | |
| Model | 4994.74 | 9 | 554.97 | 41.16 | <0.0001 | 203.44 | 9 | 22.60 | 72.95 | <0.0001 |
| A | 1677.70 | 1 | 1697.70 | 125.90 | <0.0001 | 26.79 | 1 | 26.79 | 86.47 | <0.0001 |
| B | 1029.67 | 1 | 1029.67 | 76.36 | <0.0001 | 51.92 | 1 | 51.92 | 167.56 | <0.0001 |
| C | 112.95 | 1 | 112.95 | 8.38 | 0.0232 | 16.59 | 1 | 16.59 | 53.54 | 0.0002 |
| A2 | 472.94 | 1 | 472.94 | 35.07 | 0.0006 | 48.57 | 1 | 48.57 | 156.75 | <0.0001 |
| B2 | 0.92 | 1 | 0.92 | 0.068 | 0.8011 | 40.17 | 1 | 40.17 | 129.65 | <0.0001 |
| C2 | 304.44 | 1 | 304.44 | 22.58 | 0.0021 | 12.69 | 1 | 12.69 | 40.97 | 0.0004 |
| AB | 1148.87 | 1 | 1148.87 | 85.20 | <0.0001 | 1.53 | 1 | 1.53 | 4.92 | 0.0620 |
| AC | 70.48 | 1 | 70.48 | 5.23 | 0.0561 | 8.73 | 1 | 8.73 | 28.18 | 0.0011 |
| BC | 105.58 | 1 | 105.58 | 7.83 | 0.0266 | 0.54 | 1 | 0.54 | 1.74 | 0.2282 |
| Residual | 94.39 | 7 | 13.48 | 2.17 | 7 | 0.31 | ||||
| Lack of fit | 75.30 | 3 | 25.10 | 5.26 | 0.0713 | 1.39 | 3 | 0.46 | 2.39 | 0.2092 |
| Pure error | 19.09 | 4 | 4.77 | 0.78 | 4 | 0.19 | ||||
| Cor. Total | 5089.13 | 18 | 205.61 | 16 | ||||||
| CV% | 12.05 | 3.17 | ||||||||
| PRESS | 1234.62 | 23.50 | ||||||||
| Adeq Precision | 22.380 | 36.512 | ||||||||
| R2 | 0.9815 | 0.9895 | ||||||||
| Adj R2 | 0.9576 | 0.9759 | ||||||||
| Pred R2 | 0.7574 | 0.8857 | ||||||||
A is extraction temperature; B is S/L ratio; C is extraction time.
All model evaluation statistical parameters (such as R2, Adj R2, Pred R2, adequate precision and coefficient of variation (CV)) showed good performance and indicated that both developed models were able to predict the experimental TPC and EY of CPL satisfactorily. The linear and quadratic effects of some of the process variables investigated such as extraction temperature and time were significant within their investigated ranges for TPC and EY models. However, although the linear effect of S/L of extraction was significant for both TPC and EY, its quadratic effect was only significant for EY and had significant interactions with other variables in TPC and EY models.
3.2. Effect of HAE process variables on TPC and EY of CPL
3.2.1. Effect of HAE process variables on TPC
The influence of the investigated process variables on TPC is shown in Table 4. The experimental TPC obtained in the range of the investigated process variables (extraction temperature (35–55 °C); S/L (1:20–1:50 g/mL) and time (100–200 min)) was between 12.18 and 75.22 mg GAE/g d.w. This range of TPC was in close agreement with earlier reported results of Jagtap et al. (2019).
Table 4.
Box-Benkhen experimental design and observed responses.
| Run | HAE conditions |
Experimental values |
|||
|---|---|---|---|---|---|
| Temperature (oC) | Solid to liquid ratio (g/mL) | Time (min) | EY (w/w %) | TPC (mg GAE/g d.w) | |
| 1 | 35.00 | 1:35 | 100.00 | 20.11 | 60.02 |
| 2 | 55.00 | 1:35 | 100.00 | 20.45 | 22.12 |
| 3 | 55.00 | 1:35 | 200.00 | 26.31 | 29.11 |
| 4 | 55.00 | 1:20 | 150.00 | 21.71 | 23.34 |
| 5 | 45.00 | 1:35 | 150.00 | 17.02 | 21.10 |
| 6 | 35.00 | 1:20 | 150.00 | 16.45 | 18.21 |
| 7 | 45.00 | 1:35 | 150.00 | 16.08 | 24.23 |
| 8 | 45.00 | 1:50 | 200.00 | 14.12 | 29.42 |
| 9 | 45.00 | 1:50 | 100.00 | 10.53 | 53.32 |
| 10 | 45.00 | 1:35 | 150.00 | 16.17 | 20.54 |
| 11 | 35.00 | 1:50 | 150.00 | 13.34 | 75.22 |
| 12 | 55.00 | 1:50 | 150.00 | 16.13 | 12.18 |
| 13 | 45.00 | 1:35 | 150.00 | 16.82 | 22.18 |
| 14 | 35.00 | 1:35 | 200.00 | 20.06 | 50.22 |
| 15 | 45.00 | 1:20 | 200.00 | 19.23 | 17.43 |
| 16 | 45.00 | 1:35 | 150.00 | 16.91 | 18.28 |
| 17 | 45.00 | 1:20 | 100.00 | 17.11 | 20.78 |
Process variables combination with temperature of 35 °C, S/L of 1:50 and extraction time of 150 min gave the maximum TPC of 75.22 mg GAE/g d.w while temperature of 55 °C, S/L of 1:50 and time of 150 min gave the least TPC of 12.18 mg GAE/g d.w. The quadratic model developed for TPC as a function of the investigated process variables (A, temperature; B. S/L and C, time) in coded terms is presented in Eq. (13).
| (13) |
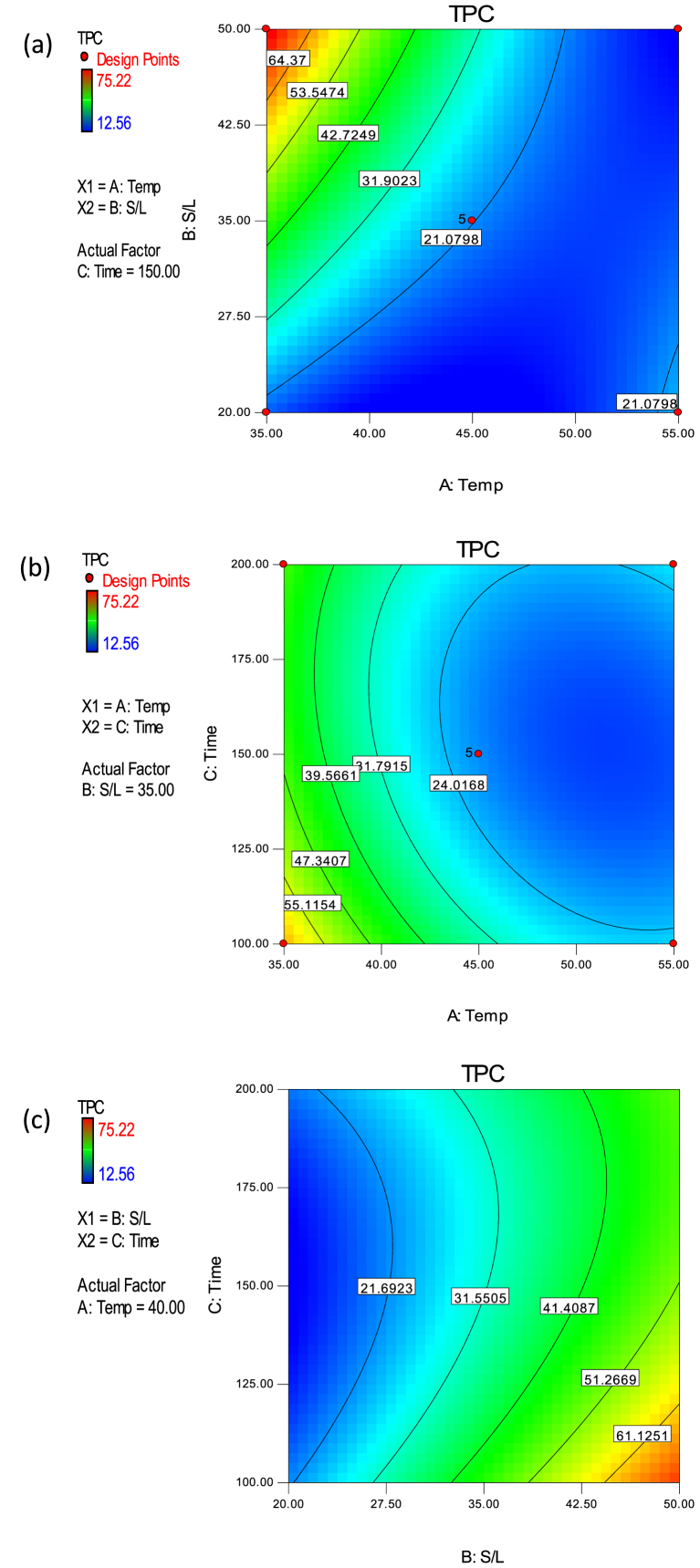
The TPC model is significant (p < 0.05) with F-value of 41.16 and high R2 (0.9815) and Adj R2 (0.9576) values. It also has a non-significant lack of fit F-value and high adequate precision value of 5.26 and 22.38 respectively. Therefore, as evident from ANOVA results, the developed TPC model was able to predict the experimental TPC data adequately. The linear effects of temperature, S/L and time; quadratic effects of temperature and time; and interactive effects of temperature and S/L, and S/L and time were significant (p < 0.05) on TPC. However, the quadratic effect of S/L and interactive effect of temperature and time were not significant (p > 0.05) on TPC within the ranges of their investigation (Table 3). TPC increased with a decrease in extraction temperature (A) and time (C) and an increase in S/L (B) as implied in Eq. (13). The quadratic effects of temperature and time were also positive (increased with increased TPC) while the simultaneous increase in S/L and time (BC) resulted in a reduction in TPC (Eq. (13)). The combined effect of increased temperature and time (AC) however, caused a significant increase in TPC (positive interactive effect indicated in the TPC model). The relationship between the investigated process variables and TPC can be visualized on the contour plots in Figure 2.
Figure 2.
Contour plots for the effects of (a) S/L and temperature (b) time and temperature (c) time and S/L on the TPC of CPL.
3.2.2. Effect of HAE process variables on EY
The experimental results of EY within the ranges of the investigated process variable are indicated In Table 4. EY of CPL ranged from 10.53 to 26.31 % (w/w). The range of EY is comparable with the reported experimental range of Alara et al. (2021). The process variables combination with extraction temperature of 55 °C, S/L of 1:35 and time of 200 min gave the highest EY of 26.31 % (w/w) while the experiment performed at 45 °C, S/L of 1:50 for 100 min gave the least EY of 10.53 % (w/w). The developed quadratic regression equation for EY as a function of the investigated process variables is shown in Eq. (14).
| (14) |
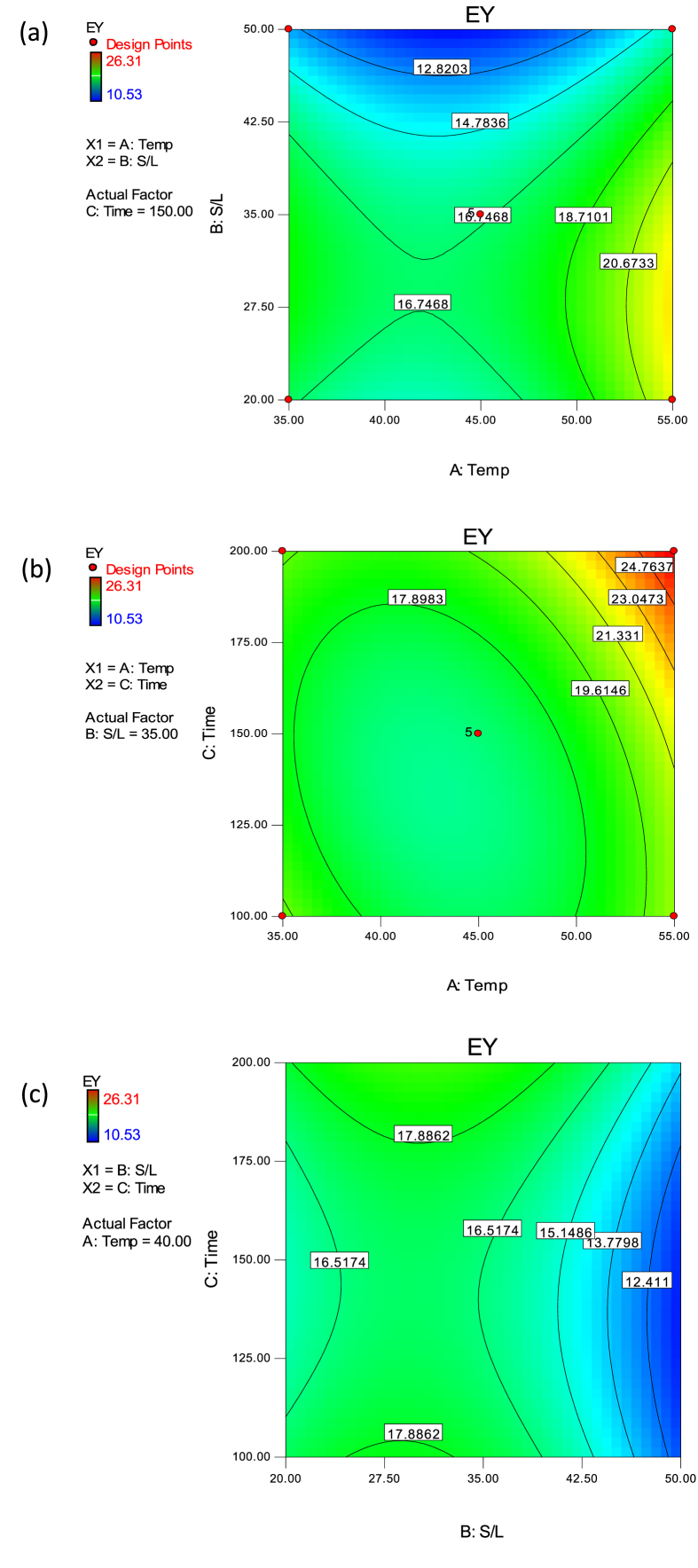
The developed EY model has a significant (p < 0.05) F-value of 72.95, R2 of 0.9895 and Adj R2 of 0.9759. The pred R2 of 0.8857 was in reasonable agreement with the Adj R2 value. The EY model also possessed a non-significant lack of fit with adequate precision of 36.512 and hence was capable of making satisfactory predictions of observed experimental EY as a function of process variables. The Prob > F less than 0.05 in Table 3 indicated that model terms were significant, therefore, linear and quadratic effects of extraction temperature, S/L and time and interactive effect of temperature and time were significant model terms. The interactive effect of temperature and S/L and S/L and time were, however, not significant model terms. The relationship existing between the investigated process variables and EY of CPL is shown in Figure 3. The EY increased with increased temperature (A) and time (C), while it reduced with increase in S/L (B). The increase in the quadratic effects of temperature (A2) and time (C2) significantly produced a positive increase in EY of CPL. However, a quadratic increase in S/L resulted into a decrease in EY. When the extraction temperature and time were increased together (i.e., AC), the EY of CPL showed a significant increase (Table 3 and Eq. (14)). The elliptical contour curves in Figure 3(b) have been attributed to the very high interactive relationship (Alara et al., 2021).
Figure 3.
Contour plots for the effects of (a) S/L and temperature (b) time and temperature (c) time and S/L on the EY of CPL.
3.3. Heat-assisted extraction process optimization and validation
Table 2 presents the criteria used for the optimization of process variables that simultaneously maximized EY and TPC of CPL. Here, the desirability algorithm in the Design Expert software (Design Expert version 7.0 Stat-Ease Inc., Minneapolis, MN) was used and weighted coefficient values of 1 was assigned for all process variable, TPC and EY. The desirability function approach is a powerful tool for process variables optimization in a multi response system. This approach transforms all obtained responses into a scale-free value (which stands between 0 and 1) known as desirability. The desirability value of 0 and 1 are attributed when the process variables give undesirable and optimal response respectively (Amdoun et al., 2018). A combined desirability is afterwards obtained as the geometric mean of the individual desirability values of each response. The main purpose of maximizing both responses was to obtain high extracts yield while still keeping the TPC as high as possible.
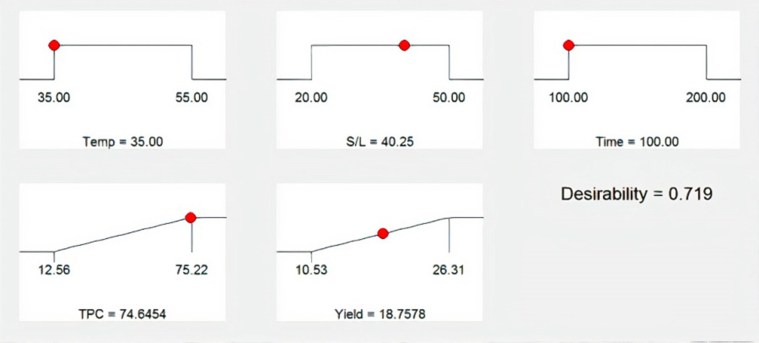
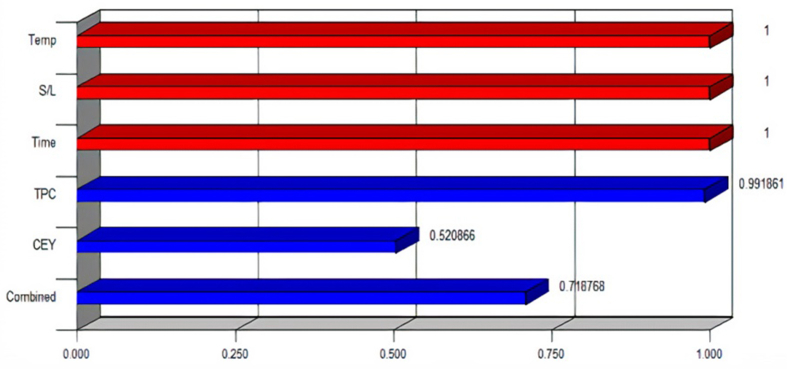
The information obtained from the optimization study was used for the preliminary process design and deterministic techno-economic analysis of the production of phenolic rich extracts from CPL. The software presented 20 solutions ranked by combined desirability value. The solution with the highest combined desirability value (desirability value closest to 1) is adjudged the best optimal solution (Oke et al., 2020). Figure 4 is the optimal ramp results obtained for the simultaneous maximization of TPC and EY. Figure 4 showed that the extraction of 1 g of CPL using 40.25 mL of water at 35 °C and for 100 min achieved optimum TPC of 74.65 mg GAE/g d.b and EY of 18.76% (w/w) with a combined desirability of 0.719. Figure 5 shows the desirability bar graph of the individual investigated process variables (extraction temperature, S/L ratio and time), responses (TPC and EY) and combined for the optimum solution. It is clear from Figure 5 that extraction temperature, S/L and time had desirability of 1, TPC and EY had desirability of 0.9919 and 0.5209 respectively, while combined desirability was 0.719.
Figure 4.
Numerical optimization ramps for HAE of CPL crude extracts.
Figure 5.
Desirability values of investigated process variables and responses.
The suggested optimum was further experimented in the laboratory for validation purposes. Hence, the validation experiment was performed at S/L of 40.25 g/mL, temperature of 35 °C and time of 100 min using water as solvent. The validation experiment obtained TPC of 74.65 GAE/g d.b and EY of 19.22 %. The obtained experimental values were in close agreement with the predicted values with RSD values of 1.38 and 1.71 for TPC and EY respectively. These low RSD values for TPC and EY indicated that the experimental and predicted TPC and EY are very similar (Domínguez et al., 2020) and therefore the developed models (TPC and EY) were well fitted for polyphenol extraction from CPL and can be used for the prediction of optimal extraction conditions. The little disparity observed between the predicted and experimental TPC and EY values during validation experiments could be as a result of uncontrolled human, procedural, environmental or machine errors in the course of experimentation.
3.4. HPLC elucidation of bioactive contents of the CPL extracts
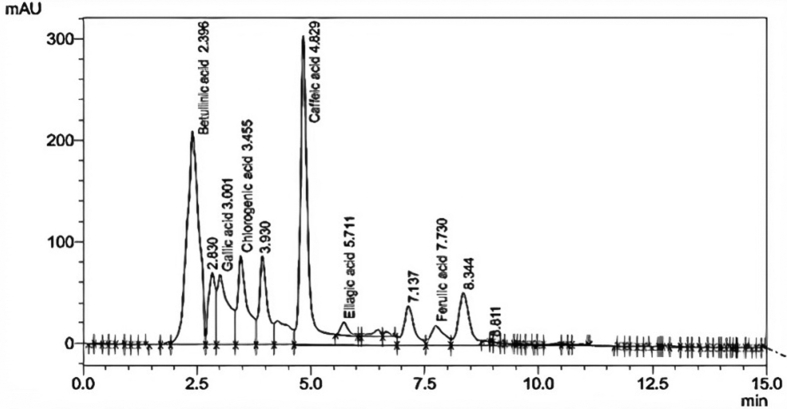
HPLC characterization of the CPL extracts was carried out to determine the nature of bioactive compounds present in the extracts considering its significant TPC. The CPL extracts were analyzed for eight (8) phenolic compounds of high biological functionalities which include gallic acid, betulinic acid, chlorogenic acid, ellagic acid, ferulic acid, caffeic acid, quercetin and rutin. The phenolic compounds present in CPL extracts showed peaks with different retention times (RT) and absorption areas. Figure 6 is the HPLC chromatogram of CPL extracts at optimum extraction conditions. Based on the standards used for HPLC analysis, the phenolic compounds detected in CPL extracts included betulinic acid (RT = 2.396 min), gallic acid (RT = 3.001 min), chlorogenic acid (RT = 3.455), caffeic acid (RT = 4.829 min), ellagic acid (RT = 5.711 min) and ferulic acid (RT = 7.730 min).
Figure 6.
HPLC chromatogram of CPL extracts.
Phenolic compounds identified in CPL extracts have well documented bioactivities. For instance, betulinic acid exhibits anticancer, anti-HIV, antimalarial and antibacterial properties (Yogeeswari and Sriram, 2005; Cichewicz and Kouzi, 2004) and gallic acid possesses anticancer, antimelanogenic and antioxidant properties (Kim, 2007; Verma et al., 2013). Also, caffeic acid has antioxidant, anti-inflammatory and anticarcinogenic activities (Espíndola et al., 2019; Gülçin, 2006). Furthermore, chlorogenic acid has antibacterial and antioxidant activity (Lou et al., 2011; Olthof et al., 2001) and ellagic acid and ferulic acid has antioxidant (Graf, 1992) and antiproliferative activities (Han et al., 2006; Daniel et al., 1989).
3.5. Techno-economic analysis of CPL bioactive extracts production using HAE technology
3.5.1. Base case and economic analysis
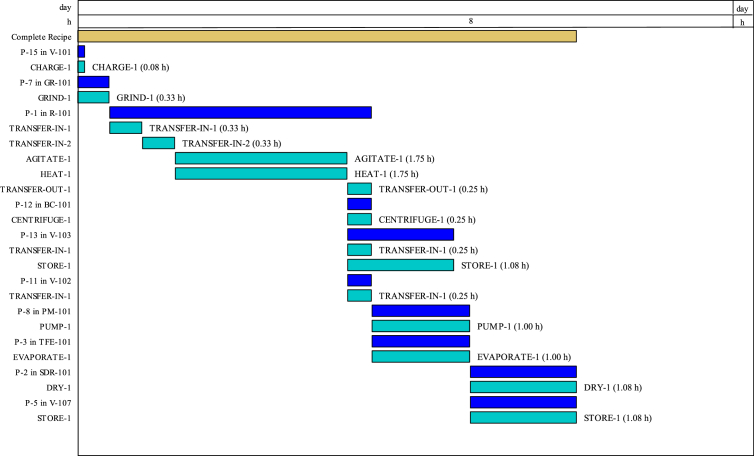
The base case model (Figure 1) for the production of dried phenolic rich bioactive extracts from CPL in this investigation was the laboratory proof of concept and was designed using the global optimum values of CPL extraction conditions. CPL extracts drying was also considered to achieve long term bioactive stability and therefore unit operations such as evaporator and dryer were also integrated. This process was designed with the assumption that industrial capacity will have similar characteristics with the laboratory scale if the same operating conditions were used (Lee et al., 2018). Figure 7 is the operational Gantt chart of the base case for the production of dried CPL bioactive extracts. The batch processing of dried CPL extracts took 5.08 h while the cycle time was 2.67 h. 2969 batch/y was achieved with the reference AOT of 7920 h.
Figure 7.
Operation Gantt chart for dried CPL extracts production base case model.
Figure 1 presents flowsheet and material balances of the important stream in the batch production of CPL bioactive extracts. The plant utilized 3 kg/y (0.01 kg/batch) CPL, 119 kg/y (0.04 kg/batch) water and 357 kg/y (0.12 kg/batch) drying air to produce 0.585 kg/y (0.197 g/batch) dried CPL extracts. The 0.585 kg/y (0.197 g/batch) dried CPL extracts comprised of 0.556 kg/y (0.18758 g/batch) CPL bioactive and 0.0286 kg/y (0.00963 g/batch) water and this is in agreement with the optimize extracts yield value. The product was designed to contain relatively low moisture content of 4.88% in order to reduce the microbial activities to the barest minimum. The electricity demand of 462 kW-h, steam of 0.4464 MT and cooling agent of 32 MT were required to meet the target base case production rate of 0.585 kg/y. As expected, the base case production cost of dried CPL extracts was outrageously high (US$ 3789795.04/kg) as a result of high TCC (US$ 9,733,009) and TOPC ($ 2,218,888) incurred largely due to relatively low production capacity. Hence, there is a need to determine an economically viable production scale of CPL extracts that gives reduced UPC. The developed base case model therefore showed similar characteristics with the laboratory proof of concept and therefore can be used for the scale up investigations.
3.5.2. Scale-up studies and economic analysis
3.5.2.1. Effect of plant capacity on material and energy demands
Preliminary simulation experiments were conducted to identify a range of CPL extracts production capacity with strong dependence on plant economics using different scaling factors. Therefore, the plant capacities in the range of 0.638–20.431 × 103 kg/y CPL extracts were analyzed and reported for the scale up of CPL extracts production. The major raw materials needed in the CPL extracts production plant were CPL (substrate from which extracts were obtained), water (solvent for bioactive extraction) and air (ingredient for extracts drying). Table 5 shows the annual material and energy demands for each investigated scale. The CPL ranged from 3,238 to 103,607 kg/y; water from 389,406 to 4,148,087 kg/y and drying air from 389,406 to 12,461,679 kg/y for a plant capacity range of 0.638–20.431 × 103 kg/y CPL extracts. The CPL, water and drying air demands were observed to increase with increased plant capacity.
Table 5.
Annual mass and energy demands for the selected CPL extracts plant capacities.
| Items |
Units | Plant capacity (x 103 kg/y) |
|||||
|---|---|---|---|---|---|---|---|
| Raw Material | 0.638 | 6.384 | 12.770 | 19.155 | 19.857 | 20.431 | |
| Water | Kg/y | 129,621 | 1,296,265 | 2,592,569 | 3,888,854 | 4,031,575 | 4,148,087 |
| Air | Kg/y | 389,406 | 3,894,297 | 7,788,593 | 11,682,890 | 12,111,652 | 12,461,679 |
| CPL | Kg/y | 3,238 | 32,377 | 64,755 | 97.132 | 100,697 | 103,607 |
| Utilities | |||||||
| Electricity demand | kW-h/y | 12,044 | 88,673 | 177,346 | 266,019 | 272,897 | 285,048 |
| Heating demand | MT/y | 163 | 1,627 | 3,254 | 4,881 | 5,060 | 5,206 |
| Cooling demand | MT/y | 778 | 5,601 | 11,202 | 16,803 | 17,222 | 18,012 |
The utility category comprised of electricity, heating and cooling demands. The electricity was needed in P-1, P-3, P-4, P-7 and P-9, steam was required in P-8 and P-9 and chilled water was demanded in P-4. The electricity demand was in the range 12,044–285,048 kW-h/y, heating demand was in the range 163–5,206 MT/y and cooling demand was in the range 778–18,012 MT/y for plant capacity in the range of 0.638–20.431 × 103 kg/y CPL extracts. All utility demands increased with plant capacity. It is also clear from Table 5 that only material demands were linearly scaled with plant capacity scaling factor, energy demands had been scaled up disproportionately.
3.5.2.2. Effect of plant capacity on production costs and UPC of CPL extracts
The economic analysis was based on the determination of TCC, TOPC and UPC of CPL extracts. Table 6 presents the summary of TOPC and TCC and cost components as a function of the investigated plant capacity range. The EPC of each scale of CPL extracts production, which served as a seed for the estimation of the DFC are also presented alongside. The TCC ranged from US$ 10,273,226 to US$ 46,078,175 for the investigated plant capacity range of 0.638–20.431 × 103 kg CPL extracts/y with plant capacity of 0.638 × 103 kg CPL extracts/y and 20.431 × 103 kg CPL extracts/y having the least and highest TCC respectively. It is interesting to note from Table 6 that although TCC increased with plant capacity, it did not scale proportionately with scaling factor. For example, when CPL plant capacity was increased 10 folds (0.638 × 103 kg/y to 6.384 × 103 kg/y), 2 folds (6.384 × 103 kg/y to 12.770 × 103 kg/y), 1.5 folds (12.770 × 103 kg/y to 19.155 × 103 kg/y), 1.03 folds (19.155 × 103 kg/y to 19.857 × 103 kg/y) and 1.03 folds (19.857 × 103 kg/y to 20.431 × 103 kg/y) TCC correspondingly increased 2.01 folds (US$ 20,675,340 - US$ 10,273,226), 1.56 folds (US$ 32,257,801 - US$ 20,675,340), 1.35 folds (US$ 43,587,940 - US$ 32,257,801), 1.01 folds (US$ 44,224,673 -US$ 43,587,940), and 1.04 folds (US$ 46,078,175 - US$ 44,224,673) respectively. This observation is not surprising because all TCC cost components such as DFC (TPIC + TPDC), working capital and start up and validation costs, though also increased but disproportionately with increased CPL extracts production capacity. This is expected since the EPC was the basis for the DFC which also increased disproportionately with increased plant capacity. The disproportionate increase in EPC as a function of plant capacity was partly due to multiple equipment units required by some higher plant capacities. It should also be noted that the respective equipment sizes for each production capacity was calculated based on the material balance information in Table 5.
Table 6.
Cost Summary for the Selected Plant Capacities of CPL extracts∗.
| Cost Items | Plant capacity (X 103 kg/y) |
|||||
|---|---|---|---|---|---|---|
| 0.638 | 6.384 | 12.770 | 19.155 | 19.857 | 20.431 | |
| EPC (US$) | 1,627,500 | 3,223,750 | 4,998,750 | 6,733,750 | 6,831,000 | 7,115,000 |
| DFC (US$) | 9,779,508 | 19,673,786 | 30,690,786 | 41,467,482 | 42,072,382 | 43,836,344 |
| TPIC (US$) | 3,189,000 | 6,415,000 | 10,008,000 | 13,522,000 | 13,719,000 | 14,294,000 |
| TPDC (US$) | 5,315,000 | 10,692,000 | 16,680,000 | 22,537,000 | 22,865,000 | 23,824,000 |
| CFC (US$) | 1,276,000 | 2,566,000 | 4,003,000 | 5,409,000 | 5,488,000 | 5,718,000 |
| Working Capital (US$) | 4,742 | 17,867 | 32,476 | 47,084 | 48,672 | 50,014 |
| Start-up Cost (US$) | 488,975 | 983,689 | 1,534,539 | 2,073,374 | 2,103,619 | 2,191,817 |
| TOPC (US$) | 2,355,776 | 4,623,340 | 7,575,926 | 10,269,632 | 10,429,306 | 10,888,648 |
| TCC (US$) | 10,273,226 | 20,675,340 | 32,257,801 | 43,587,940 | 44,224,673 | 46,078,175 |
| UPC (US$/kg) | 3689.21 | 756.36 | 593.11 | 536.01 | 525.21 | 531.32 |
EPC - Equipment Purchase Cost; DFC - Direct Fixed Capital; TIC - Total Plant Indirect Cost; TDC - Total Plant Direct Cost; CFC - Contractor's Fees and Contingencies cost; TOPC - Total Operating Cost; TCC – Total Capital Cost.
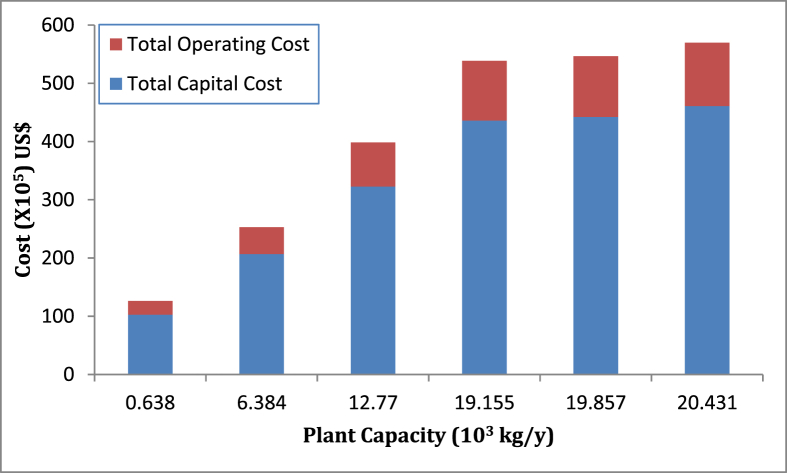
The TOPC of the investigated CPL extracts production capacities was also calculated and detailed in Table 6. The TOPC ranged from US$ 2,355,776 to US$ 10,888,648 for plant capacity in the range 0.638–20.431 × 103 kg CPL extracts/y. The plant capacity with 0.638 × 103 kg CPL extracts/y and 20.431 × 103 kg CPL extracts/y possessed the least and highest TOPC respectively. The TOPC also increased disproportionately with increased plant capacity. This implied that one or more cost components of TOPC were not scaled linearly. The disproportionate increase observed in TOPC as a function of plant capacity was as a result of contributions from utility, labour and facility dependent costs. This is possible because energy demands did not linearly increase with plant capacity (Table 5), the same number of operators was assigned to the production plant irrespective of capacity and facility dependent cost was calculated as percentage of DFC. In summary, therefore, both TCC and TOPC increased non-linearly with increased plant capacity. A similar observation was reported by Adeyi et al. (2020) and Mahmud and Rosentrater (2019). The stack graph of production costs for all investigated plant capacity is presented in Figure 8. It can be observed that the TCC far outweighed corresponding TOPC of CPL extracts production for all investigated capacities. The ratio of TCC to TOPC for plant capacity 0.638 × 103, 6.384 × 103, 12.770 × 103, 19.155 × 103, 19.857 × 103 and 20.431 × 103 kg/y is 4.36, 4.47, 4.26, 4.24, 4.24 and 4.23 respectively.
Figure 8.
CPL extracts production cost of selected plant capacity.
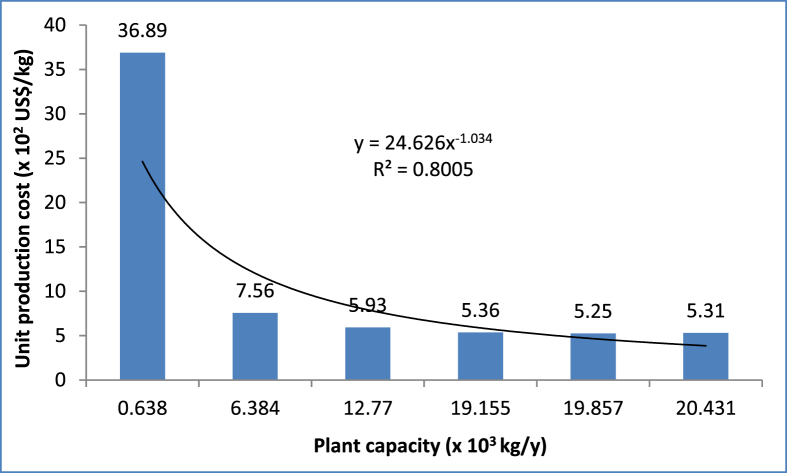
The unit production cost of CPL extracts production was used as the economic parameter to assess the cost effectiveness of the investigated scales. Many researchers have used similar parameter for the economic analysis of different production plants (Adeyi et al., 2021; Mahmud and Rosentrater, 2019). UPC of CPL extracts is the cost of production incurred to produce one kilogram of the extracts. Plant capacity with the minimum CPL extracts UPC is adjudged the most profitable scale of CPL extracts production. The UPC of CPL extracts ranged from 5.25 to 36.89 × 102 US$/kg for the plant capacity in the range of 0.638–20.431 × 103 kg CPL extracts/y. The UPC as a function of the investigated CPL extracts production capacity is presented in Figure 9. The UPC was observed to decrease with increased CPL extracts plant capacity up till the production scale of 19.857 × 103 kg CPL extracts/y above which an increase in UPC was observed. This observation showed that diseconomies of scale effect has set in at above plant capacity of 19.857 × 103 kg CPL extracts/y because further increase in production capacity did not result in a significant decrease in UPC (Mahmud and Rosentrater, 2019). Therefore, the plant capacity of 19.857 × 103 kg CPL extracts/y possessed the least UPC of 5.25 × 102 US$/kg, while plant capacity of 0.638 × 103 kg CPL extracts/y possessed the highest UPC of 36.89 × 102 US$/kg CPL extracts. The UPC of 525 US$/kg obtained for HAE (T = 35 °C, S: L = 1:40.25, time = 100 min, production scale = 19.857 × 103 kg CPL extracts/y) of bioactive extracts from CPL was comparable with the cost of manufacturing of 475 US$/kg reported by Canabarro et al. (2020) for supercritical extraction of bioactive extracts (T = 80 °C, P = 25 MPa, extractor capacity = 100 L) from Eugenia uniflora leaves. Although Canabarro et al. (2020) did not quantify the associated risk of their designed extraction process. Hence, plant capacity of 19.857 × 103 kg CPL extracts/y was therefore selected (based on the minimum UPC) as the most economical CPL extracts production scale among the plant production capacities investigated. Therefore, further engineering works that may improve and optimize the CPL extracts production process can be done using this identified scale of production.
Figure 9.
Unit production cost of selected CPL extracts plant capacity.
3.6. Uncertainty and sensitivity analyses of process and cost parameters on UPC
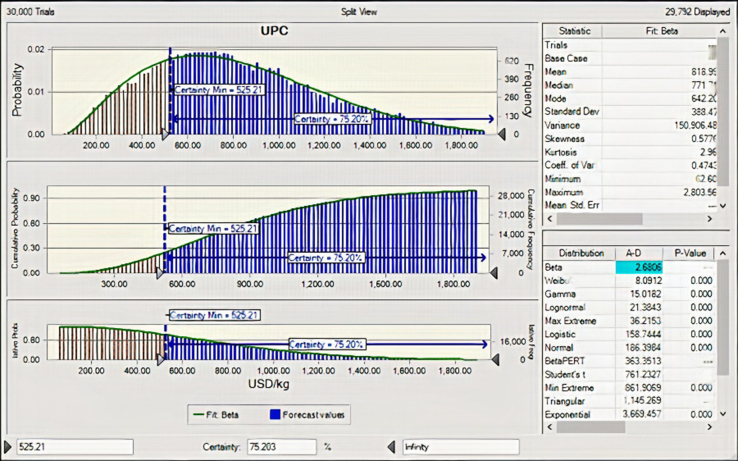
The uncertainty and sensitivity analyses were done to assess the risk involved and to achieve technical and cost variables ranking (in terms of relative importance) for further process optimization purposes in the production of phenolic rich bioactive extracts from CPL. These analyses are very important because the deterministic techno-economic assessment of the CPL extracts production used technical (process parameters) and cost (CPL and equipment costs) variables that have tendencies to vary widely in reality. Hence, process variables such as extraction temperature, CPL/water, extraction time and extracts recovery (yield) that have tendencies to vary due to human and machine errors (during weighing, measurements and operation) were selected as technical variables. Cost variables used for these analyses were costs of major process equipment (grinder, heat-assisted extractor, centrifuge, evaporator and spray dryer) and CPL purchase cost. Major equipment costs were selected because EPC form the basis of DFC which indirectly determines TCC of production plant and these costs vary widely in the market. Cost of CPL may as well be very volatile and was a key parameter in TOPC. All these selected variables may have notable influences on the UPC of CPL extracts. The identified most economical production scale of 19.857 × 103 kg CPL extracts/y was used for these analyses. The ensemble of 30000 Monte Carlo simulated UPC outcomes obtained from different combinations of the investigated technical and economic variables ranges (−20 to +20% of original values) is presented in Figure 10. Asides the UPC probability distribution, cumulative frequency and reversed cumulative frequency curves, Figure 10 also presents the UPC distribution data statistics and model fittings at the top and bottom right split views of the graph respectively. The UPC distribution of CPL extracts was assessed in terms of mean, median and standard deviation. The beta model was found most suitable with Anderson-Darling value of 2.6806 for the description of the UPC distribution of CPL extracts. The UPC distribution ranged between 62.60 and 2803.56 US$/kg CPL extracts.
Figure 10.
Probability distribution of UPC (30000 trials). Mean = 818.99, median = 771.71, S.D = 388.47, range = 62.60–2803.56.
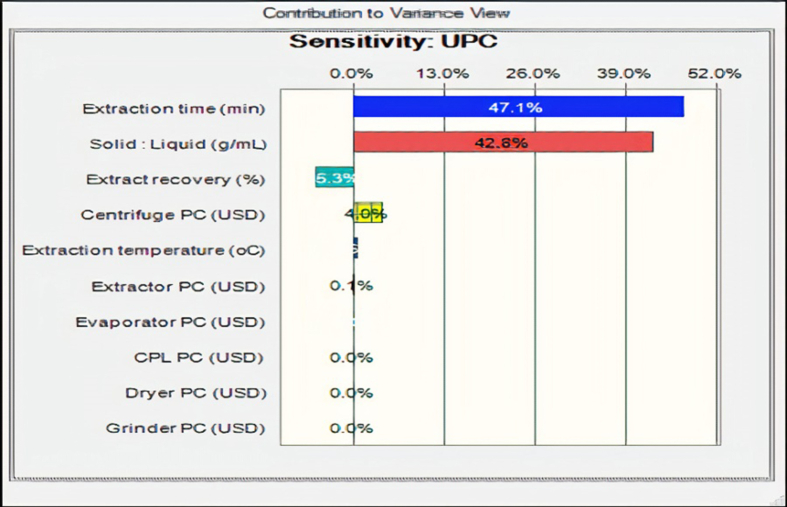
The UPC distribution is normal with skewness and kurtosis of 0.5776 and 2.96 respectively. The distribution has a mean value of 818.99, median value of 771.71 and a mode value of 642.20 US$/kg CPL extracts. The certainty of obtaining the UPC base case value of 525.21 US$/kg CPL extracts was 75.20%. The dynamic sensitivity graph which quantified the cumulative effects of each investigated factor on the UPC of CPL extracts is presented in Figure 11. It is obvious from Figure 11 that extracts recovery, CPL/water, extraction time, extraction temperature, centrifuge purchase cost and heat-assisted extractor purchase cost had notable influences on UPC of CPL extracts. However, changes in evaporator purchase cost, grinder purchase cost, dryer purchase cost and CPL purchase cost did not seem to perturb the UPC of CPL extracts. Hence the positive and negative contributions observed in the chart indicated an increase and decrease in UPC of CPL extracts as these factors increased. Therefore, increased extraction recovery yield reduced the UPC, while increased CPL/water, extraction time, extraction temperature, centrifuge purchase cost and heat-assisted extractor increased UPC of CPL extracts in US$. In summary, the quantification of the contribution of all investigated technical and cost variables to the variance in UPC is - 5.3% extracts recovery, +42.8% CPL/water, +4.0% centrifuge purchase cost, +47.1% extraction time, +0.1% extractor purchase cost, +0.5% extraction temperature, 0% CPL purchase cost, 0% evaporator purchase cost, 0% dryer purchase cost and 0% grinder purchase cost.
Figure 11.
Contribution of uncertain parameters to the variance in UPC.
4. Conclusion
The following investigations were carried out in sequence: laboratory process variables’ optimization for the optimum extraction of bioactive extracts from CPL; CPL extracts characterization to elucidate the phenolic profiling; scale up and economic studies to identify the most feasible economic scale for industrial production of CPL extracts; and risk quantification and sensitivity analysis of the selected technical and cost variables conducted on the most economically feasible production scale to determine their respective contributions to the variance in UPC of CPL extracts. It can be concluded that:
-
(a)
All investigated process variables (temperature, S/L and time) were significant on the total phenolic content extraction and yield of CPL extracts. The extraction temperature of 35 °C, S/L of 1:40.25 g/mL and time of 100 min gave the optimum TPC of 74.65 mg GAE/g d.b and EY of 18.76 % (w/w).
-
(b)
CPL extracts were rich in gallic acid, betulinic acid, chlorogenic acid, ellagic acid, ferulic acid and caffeic acid.
-
(c)
Laboratory scale up was feasible and all the production capacities investigated possessed high dependence on the material & energy demands and process economics. The plant capacity of 19.857 × 103 kg CPL extracts/y possessed the least UPC and therefore was selected as the most economically feasible scale among the investigated range.
-
(d)
The certainty of obtaining the base case UPC value of 525.21 US$/kg CPL extracts was 75.20%. Only the extracts recovery yield (%) had a negative contribution to the variance in UPC of the selected most economical scale. Changes in CPL purchase cost, evaporator purchase cost, dryer purchase cost and grinder purchase cost did not result in notable perturbation in UPC. However, increased CPL/water, extraction time, extraction temperature, centrifuge purchase cost and heat-assisted extractor had positive contributions to the variance in UPC of CPL extracts.
Declarations
Author contribution statement
Oladayo Adeyi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Emmanuel O. Oke: Conceived and designed the experiments; Wrote the paper.
Bernard I. Okolo, Samuel Okhale: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Abiola J. Adeyi: Analyzed and interpreted the data; Wrote the paper.
John A. Otolorin: Conceived and designed the experiments; Analyzed and interpreted the data.
Kenechi Nwosu-Obieogu, James A. Adeyanju, Goziya William Dzarma: Contributed reagents, materials, analysis tools or data.
Denilson Ogu, Precious N. Onu: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adeniyi A.G., Ighalo J.O., Amosa M.K. Modelling and simulation of banana (Musa spp.) waste pyrolysis for bio-oil production. Biofuels. 2019:1–5. [Google Scholar]
- Adeyi Oladayo, OKe O. Emmanuel., Adeyi J. Abiola, Bernard Okolo, Kenechi N.O. Techno-economic analysis of catechin mix manufacture from camellia sinensis leaves using green extraction technology. Chem. Ind. Chem. Eng. Q. 2020:44. [Google Scholar]
- Adeyi O., Adeyi A.J., Oke E.O., Okolo B.I., Olalere A.O., Otolorin J.A., Taiwo A.E. Techno-economic and uncertainty analyses of heat-and ultrasound-assisted extraction technologies for the production of crude anthocyanins powder from Hibiscus sabdariffa calyx. Cogent Eng. 2021;8(1):1947015. [Google Scholar]
- Ahmed S.R., Rabbee M.F., Roy A., Chowdhury R., Banik A., Kubra K., et al. Therapeutic promises of medicinal plants in Bangladesh and their bioactive compounds against ulcers and inflammatory diseases. Plants. 2021;10(7):1348. doi: 10.3390/plants10071348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alara O.R., Abdurahman N.H., Alara J.A. Carica papaya: comprehensive overview of the nutritional values, phytochemicals and pharmacological activities. Adv. Trad. Med. 2020:1–31. [Google Scholar]
- Alara O.R., Abdurahman N.H., Ali H.A., Zain N.M. Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: an optimization study and LC-QTOF-MS analysis. Future Foods. 2021;3:100035. [Google Scholar]
- Amdoun R., Khelifi L., Khelifi-Slaoui M., Amroune S., Asch M., Assaf-Ducrocq C., Gontier E. The desirability optimization methodology; a tool to predict two antagonist responses in biotechnological systems: case of biomass growth and hyoscyamine content in elicited Datura starmonium hairy roots. Iran. J. Biotechnol. 2018;16(1):1339. doi: 10.21859/ijb.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athimulam A., Kumaresan S., Foo D.C.Y., Sarmidi M.R., Aziz R.A. Modelling and optimization of Eurycoma longifolia water extract production. Food Bioprod. Process. 2006;84(2):139–149. [Google Scholar]
- Baral N.R., Shah A. Techno-economic analysis of cellulosic butanol production from corn stover through acetone–butanol–ethanol fermentation. Energy Fuels. 2016;30(7):5779–5790. [Google Scholar]
- Canabarro N.I., Veggi P.C., Vardanega R., Mazutti M.A., do Carmo Ferreira M. Techno-economic evaluation and mathematical modeling of supercritical CO2 extraction from Eugenia uniflora L. leaves. J. Appl. Res. Med. Aromat. Plants. 2020;18:100261. [Google Scholar]
- Cardenas-Toro F.P., Forster-Carneiro T., Rostagno M.A., Petenate A.J., Maugeri Filho F., Meireles M.A.A. Integrated supercritical fluid extraction and subcritical water hydrolysis for the recovery of bioactive compounds from pressed palm fiber. J. Supercrit. Fluids. 2014;93:42–48. [Google Scholar]
- Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24(1):90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- Daniel E.M., Krupnick A.S., Heur Y.H., Blinzler J.A., Nims R.W., Stoner G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989;2(4):338–349. [Google Scholar]
- Desai A.G., Qazi G.N., Ganju R.K., El-Tamer M., Singh J., Saxena A.K., et al. Medicinal plants and cancer chemoprevention. Curr. Drug Metabol. 2008;9(7):581–591. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R., Zhang L., Rocchetti G., Lucini L., Pateiro M., Munekata P.E., Lorenzo J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020;330:127266. doi: 10.1016/j.foodchem.2020.127266. [DOI] [PubMed] [Google Scholar]
- El Kantar S., Rajha H.N., Boussetta N., Vorobiev E., Maroun R.G., Louka N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019;295:165–171. doi: 10.1016/j.foodchem.2019.05.111. [DOI] [PubMed] [Google Scholar]
- Espíndola K.M.M., Ferreira R.G., Narvaez L.E.M., Silva Rosario A.C.R., da Silva A.H.M., Silva A.G.B., et al. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019;9:541. doi: 10.3389/fonc.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan C.Y., Latiff A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011;124(3):1277–1283. [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992;13(4):435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- Gülçin İ. Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid) Toxicology. 2006;217(2-3):213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Han D.H., Lee M.J., Kim J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26(5A):3601–3606. [PubMed] [Google Scholar]
- Intelligen I. 2014. SuperPro Designer-User's Guide. [Google Scholar]
- Jagtap N.S., Wagh R.V., Chatli M.K., Kumar P., Malav O.P., Mehta N. Optimisation of extraction protocol for Carica papaya L. to obtain phenolic rich phyto-extract with prospective application in chevon emulsion system. J. Food Sci. Technol. 2019;56(1):71–82. doi: 10.1007/s13197-018-3456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S.U., Tiwari B.K., Smyth T.J., O’Donnell C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015;23:308–316. doi: 10.1016/j.ultsonch.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Koche D., Shirsat R., Kawale M. An overerview of major classes of phytochemicals: their types and role in disease prevention. Hislopia J. 2016;9:1–11. [Google Scholar]
- Kamarudin A.A., Esa N.M., Saad N., Sayuti N.H., Razak N.A.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind. Crop. Prod. 2020;144:112064. [Google Scholar]
- Kim Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007;30(6):1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- Krishna Murthy T.P., Manohar B. Optimization of supercritical carbon dioxide extraction of phenolic compounds from mango ginger rhizome (Curcuma amada Roxb.) using response surface methodology. Biomed. Biotechnol. 2014;2(1):14–19. [Google Scholar]
- Lee C.S., Chong M.F., Binner E., Gomes R., Robinson J. Techno-economic assessment of scale-up of bio-flocculant extraction and production by using okra as biomass feedstock. Chem. Eng. Res. Des. 2018;132:358–369. [Google Scholar]
- Lou Z., Wang H., Zhu S., Ma C., Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011;76(6):M398–M403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Majolo F., Delwing L.K.D.O.B., Marmitt D.J., Bustamante-Filho I.C., Goettert M.I. Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochem. Lett. 2019;31:196–207. [Google Scholar]
- Mahmud N., Rosentrater K.A. Techno-economic analysis of low moisture anhydrous ammonia (LMAA) pretreatment for butanol production from oil palm frond. Biomass Convers. Biorefin. 2019:1–15. [Google Scholar]
- Maria M.F.F., Ikhmal W.M.K.W.M., Sabri M.G.M., Adnan A. IOP Conference Series: Materials Science and Engineering. Vol. 440. IOP Publishing; 2018. Identification of functional group present in Andrographis paniculata (kalmegh) leaves by FTIR analysis; p. 12035. No. 1. [Google Scholar]
- Maroun R.G., Rajha H.N., El Darra N., El Kantar S., Chacar S., Debs E., et al. Polyphenols: Properties, Recovery, and Applications. Woodhead Publishing; 2018. Emerging technologies for the extraction of polyphenols from natural sources; pp. 265–293. [Google Scholar]
- Mashour N.H., Lin G.I., Frishman W.H. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch. Intern. Med. 1998;158(20):2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- Meenakshi S., Umayaparvathi S., Arumugam M., Balasubramanian T. In vitro antioxidant properties and FTIR analysis of two seaweeds of Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2011;1(1):S66–S70. [Google Scholar]
- Nowacka M., Tappi S., Wiktor A., Rybak K., Miszczykowska A., Czyzewski J., et al. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods. 2019;8(7):244. doi: 10.3390/foods8070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwiloh B.I., Nwinuka N.M., Monanu M.O. The effect of aqueous extract of Carica papaya leaves on liver enzymes and blood cell counts of normal albino rats. Int. J. Brain Cognit. Sci. 2009;3(3) [Google Scholar]
- Obafemi T.O., Akinmoladun A.C., Olaleye M.T., Onasanya A., Komolafe K.C., Falode J.A., Athayde M.L. High performance liquid chromatography (HPLC) fingerprinting, mineral composition and in vitro antioxidant activity of methanol leaf extract of Synsepalum dulcificum (sapotaceae) J. Appl. Pharmaceut. Sci. 2017 [Google Scholar]
- Oke E.O., Adeyi O., Okolo B.I., Ude C.J., Adeyi J.A., Salam K.K., et al. Heterogeneously catalyzed biodiesel production from Azadiricha Indica oil: predictive modelling with uncertainty quantification, experimental optimization and techno-economic analysis. Bioresour. Technol. 2021;332:125141. doi: 10.1016/j.biortech.2021.125141. [DOI] [PubMed] [Google Scholar]
- Oke E.O., Adeyi O., Okolo B.I., Adeyi J.A., Ayanyemi J., Osoh K.A., Adegoke T.S. Phenolic compound extraction from Nigerian Azadirachta indica leaves: response surface and neuro-fuzzy modelling performance evaluation with Cuckoo search multi-objective optimization. Result. Eng. 2020;8:100160. [Google Scholar]
- Olthof M.R., Hollman P.C., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131(1):66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- Pinela J., Prieto M.A., Pereira E., Jabeur I., Barreiro M.F., Barros L., Ferreira I.C. Optimization of heat-and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019;275:309–321. doi: 10.1016/j.foodchem.2018.09.118. [DOI] [PubMed] [Google Scholar]
- Raafat K., El-Darra N., Saleh F.A., Rajha H.N., Louka N. Optimization of infrared-assisted extraction of bioactive lactones from Saussurea lappa L. and their effects against gestational diabetes. Phcog. Mag. 2019;15(61):208. [Google Scholar]
- Raissi S. Developing new processes and optimizing performance using response surface methodology. World academy of science. Eng. Technol. 2009;25:1039–1042. [Google Scholar]
- Sayar N.A., Şam S.D., Pinar O., Serper D., Akbulut B.S., Kazan D., Sayar A.A. Techno-economic analysis of caffeine and catechins production from black tea waste. Food Bioprod. Process. 2019;118:1–12. [Google Scholar]
- Shahidi F., Ambipaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects- A review. J. Funct.Foods. 2015;18:820–897. [Google Scholar]
- Shanmugam K.R., Shanmugam B., Subbaiah G.V., Ravi S., Reddy K.S. Medicinal plants and bioactive compounds for diabetes management: important advances in drug discovery. Curr. Pharmaceut. Des. 2021;27(6):763–774. doi: 10.2174/1381612826666200928160357. [DOI] [PubMed] [Google Scholar]
- Sharayei P., Azarpazhooh E., Zomorodi S., Ramaswamy H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT (Lebensm.-Wiss. & Technol.) 2019;101:342–350. [Google Scholar]
- Sitarek P., Kowalczyk T., Wieczfinska J., Merecz-Sadowska A., Górski K., Śliwiński T., Skała E. Plant extracts as a natural source of bioactive compounds and potential remedy for the treatment of certain skin diseases. Curr. Pharmaceut. Des. 2020;26(24):2859–2875. doi: 10.2174/1381612826666200417160049. [DOI] [PubMed] [Google Scholar]
- Sunny A., Solomon P.A., Aparna K. Syngas production from regasified liquefied natural gas and its simulation using Aspen HYSYS. J. Nat. Gas Sci. Eng. 2016;30:176–181. [Google Scholar]
- Titanji V.P., Zofou D., Ngemenya M.N. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. Afr. J. Tradit., Complementary Altern. Med. 2008;5(3):302. [PMC free article] [PubMed] [Google Scholar]
- Ugboko H.U., Nwinyi O.C., Oranusi S.U., Fatoki T.H., Omonhinmin C.A. Antimicrobial importance of medicinal plants in Nigeria. Sci. World J. 2020;2020 doi: 10.1155/2020/7059323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha S.H., Astry B., Nanjundaiah S.M., Kim H.R., Rajaiah R., Yang Y., et al. Control of autoimmune arthritis by herbal extracts and their bioactive components. Asian J. Pharm. Sci. 2016;11(2):301–307. [Google Scholar]
- Verma S., Singh A., Mishra A. Gallic acid: molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013;35(3):473–485. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Vieira G.S., Cavalcanti R.N., Meireles M.A.A., Hubinger M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis) J. Food Eng. 2013;119(2):196–204. [Google Scholar]
- Vuong Q.V., Hirun S., Roach P.D., Bowyer M.C., Phillips P.A., Scarlett C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013;3(3):104–111. [Google Scholar]
- Yogeeswari P., Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr. Med. Chem. 2005;12(6):657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- Yuan B., Danao M.G.C., Stratton J.E., Weier S.A., Weller C.L., Lu M. High pressure processing (HPP) of aronia berry purée: effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innovat. Food Sci. Emerg. Technol. 2018;47:249–255. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.