Abstract
Soil microbial communities are critical to plant health and productivity. Crop-associated microbial diversity may exhibit spatial specificity across regions and soil compartments. However, we lack sound evidence for the impact of variation in soil microbial diversity on plant productivity caused by regional differences. The main aims of this study are to explore the structure and functionality of the belowground (potato tuber surface and rhizosphere) microbial communities in three compartments and assess whether these communities contribute to potato productivity. Significant differences in alpha and beta diversities of belowground microbiota were detected in different compartments and regions, mainly due to differences in available soil nutrients and pH. Changes to microbial diversity between bulk soil and rhizosphere or tuber surface soil were significantly negatively correlated with potato yield and nutrient content and positively correlated with starch content. We further found some bacterial (Mucilaginibacter, Dokdonella, and Salinispora) and fungal (Solicoccozyma, Scytalidium, and Humicola) genera closely associated with potato yield and quality. Aggregated boosted tree prediction revealed that soil physicochemical properties and microbial diversity of tuber surface soil contributed more to potato yield; tuber surface soil bacterial contributed more to potato starch and nutrient content. Our findings provide experimental evidence that the significant differences in soil microbial diversity and specific microbial taxa enrichment may potentially influence crop productivity under soil physicochemical property change scenarios in the agricultural ecosystem.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03167-6.
Keywords: Belowground microbe, Niche compartment, Potato productivity, Soil physicochemical property
Introduction
Potato (Solanum tuberosum L.) is the fourth most produced food crop worldwide in yield and nutritional value. It also has strong adaptability, high yield, and high nutritional value (Waqas et al. 2021). The growth and development of potatoes are strongly affected by biological and abiotic factors in their environment (Liang et al. 2019; Lal et al. 2021). There is a major demand for potatoes in China, a country with high food safety requirements because of its large human population. Consequently, extensive planting areas are required in China; however, the viability of cultivated land is affected by geographical location and climate. As a result, distinct differences exist in the yield and quality of potato crops across regions (Hu et al. 2017). This phenomenon might be related to the soil environment of the potato root zone, including microorganisms, soil physicochemical properties, and soil extracellular enzyme activity; however, this relationship needs confirmation.
Most land crops are closely associated with a complex diversity of microorganisms (Bulgarelli et al. 2013; Wu et al. 2021b). These microbes include bacteria, fungi, archaea, and protists, which can all inhabit the rhizosphere, root endosphere, leaf phyllosphere, and other compartments of plants (Trivedi et al. 2020). These crop-related microorganisms regulate crop growth and improve crop yield and quality by direct (promoting nutrient acquisition and regulating plant hormone level) or indirect (inducing systemic resistance and biosynthesis) mechanisms (Wu et al. 2021a; Yadav et al. 2021). Microorganisms can either act alone or jointly. Nitrogen fixing bacteria, potassium dissolving bacteria, and phosphorus dissolving microorganisms can enhance plant nutrient absorption and productivity by promoting nutrient circulation and increasing soil fertility (Wang et al. 2019; Yadav et al. 2021). Rhizobium nitrogen fixation helps legumes obtain nitrogen and improve yield (Denison and Kiers 2004). To acidify biological cells and the surrounding environment, potassium and phosphorus solubilizing microorganisms release certain organic substances (such as oxalic acid, glucose, and malic acid). This causes potassium or phosphate, which are originally insoluble minerals, to be released into the soil, thereby increasing the effective nutrients of the soil, promoting plant growth, and improving the yield and quality of crops (Whitelaw 2000; Meena et al. 2014). For instance, Bona (Bona et al. 2018) showed that the addition of mycorrhizal fungi and beneficial bacteria to tomato crops resulted in plants containing higher glucose, fructose, and malic acid, with healthier and plumper fruit than the control. Pandey (Pandey et al. 2018) showed that Bacillus pumilus and Bacillus subtilis significantly improved the content of carbohydrates and essential amino acids in amaranth grains, because of their ability to decompose minerals (especially phosphorus), which enhanced crop quality. Microbes enhance crop quality by regulating plant hormones, such as indoleacetic acid, ethylene, auxin, and gibberellin. For example, de Andrade (de Andrade et al. 2019) and Pešaković (Pešaković et al. 2013) showed that microorganisms enhance strawberry biomass by producing indoleacetic acid and adjusting abscisic acid levels, which increased the total sugar concentration of fruit and improved sweetness.
In addition, microorganisms indirectly promote plant growth by inducing plant immune and resistance systems through nutritional competition, such as iron carriers and hydrogen cyanide (Zhang et al. 2020; Yadav et al. 2021). For instance, the natural microbiome of the tomato plant rhizosphere contains growth-inhibitory siderophores that often inhibit pathogens by competing for iron and protecting plants from infection (Gu et al. 2020). In addition, the composition and diversity of microbial communities in different compartments vary. Microorganisms in the plant root zone follow the three-step assembly principle (Reinhold-Hurek et al. 2015; Zhang et al. 2022), from bulk soil to rhizosphere soil and then to the endorhizosphere of roots. Microorganisms are screened across each layer of the plant root zone as they approach the roots; therefore, their diversity gradually decreases and homogenization occurs. This phenomenon is the result of the economic utilization of resources during long-term evolution (Comas and Eissenstat 2004; Fitzpatrick et al. 2018). Changes to the microbial communities from bulk soil to rhizosphere soil are closely related to crop growth and development (Lin et al. 2020). However, evidence that proves this change is related to crop yield and quality is lacking. Notably, soil abiotic factors (including soil pH, nutrients, and moisture) have a marked effect on the structure and composition of the microbial community, particularly in rhizosphere and bulk soils (Bakker et al. 2015; Naylor et al. 2017). The relationship of the microbial community with crop yield and quality is essential to support food security, especially in the developing, increasingly populated world (Lesiv et al. 2019).
Soil nutrients (especially soil dissolved organic carbon and available nitrogen, phosphorus, and potassium) are used as important indicators of soil fertility and long-term ecosystem sustainability (Maria et al. 2008; Liang et al. 2020). In potato production, soil physicochemical properties are key to obtaining high yields and efficiency. Potatoes require large quantities of available nitrogen, phosphorus, and, especially, potassium in the soil. Potassium transports carbohydrates to potato tubers and is utilized throughout the growth period. Phosphorus regulates the size and quantity of potato tubers. In parallel, nitrogen directly affects the growth of the aboveground and underground parts of the potato (Darabi et al. 2018; Jasim et al. 2020).
Acidic soil environments are optimal for the growth of potato plants. In acidic environments, potatoes absorb more cations. Potato plants in soils with pH 4.8–7 grow normally; however, soil with a pH of 5–5.5 is more suitable (Mulder et al. 1997). Extracellular enzymes are important indicators of soil fertility and function, because they contribute to the decomposition of soil organic matter and nutrient cycling (Doran et al. 1994). Soil enzymes primarily originate from microorganisms in the soil, while some originate from animals and plants (Caldwell 2005). Soil hydrolase is an intermediate substance used by microorganisms to improve the soil environment, promote nutrient cycling, and regulate crop growth and development (Yadav et al. 2021); consequently, it is closely related to crop yield and quality.
Here, we aimed to identify the relationship between different bacterial and fungal communities in different compartments with potato yield and quality. In parallel, we investigated the relationship between soil physicochemical property and soil extracellular enzyme activity and potato yield and quality, hoping to provide a new viewpoint for using the power of microorganisms to regulate crop productivity.
Materials and methods
Site description and sample collection
The three sampling sites were located in Yinzhou (Site 1: 29.67° N, 121.60° E), Xiangshan (Site 2: 29.38° N, 121.78° E), and Ninghai (Site 3: 29.32° N, 121.68° E) of Ningbo City, Zhejiang Province, China. These sites have been used for potato monoculture for many years, and have been used for breeding and cultivation experiments of new potato varieties in Ningbo in the past 3 years; they have a good research foundation and long-term stable mode of cultivation. However, there were differences in potato yield and quality among the three sites, and these sites were selected to explore the reasons underlying this difference. The mean annual temperature of the three experimental plots was 18.68, 18.70, and 17.47 °C, respectively. Precipitation levels were 1653.74, 1799.25, and 1744.1 mm, respectively. Sites 1 and 3 belonged to a humid subtropical monsoon climate, whereas Site 2 belonged to a subtropical maritime monsoon climate. According to Chinese soil taxonomy (Institute of Soil Science, CAS 2001), Xanthic Ali-Udic Cambosol was the soil type of the three sample plots. Information on the soil physicochemical properties at the three sampling sites is presented in Table S1. Most important differences among sites were in the dissolved organic carbon (DOC), nitrate nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), and available potassium (AK) levels, with Site 1 and Site 3 showing the highest and lowest DOC levels, respectively, and Site 3 showing the highest levels of NO3−-N, NH4+-N, and AK (Table S1). The seeds of the locally grown potato cultivar, NB-1, were provided by the Institute of Biotechnology, Ningbo Academy of Agricultural Sciences, China. Potatoes were planted at Sites 1, 2, and 3 on February 9, 2020. Cut tubers were placed at a 7–10 cm depth, spaced 15–20 cm apart, at the tops of ridges along a single row. Adjacent ridges were spaced 20–30 cm apart. Nitrogen, phosphorus, and potassium compound fertilizer (N: P2O5: K2O = 20:10:18) and organic fertilizer (chicken manure: Organic matter content ≥ 45%, nutrient content ≥ 5%) were applied once as basal fertilization (compound fertilizer: 600 kg·ha−1, organic fertilizer: 3750 kg·ha−1) before planting. All plant and soil samples were collected at the potato ripening stage. On May 8, 2020, all potatoes in each sample plot were harvested and weighed, and the yield was obtained.
Three square experimental districts with an area of 4 m2 were randomly selected for each location. Five sampling points were selected using the classical five-point sampling method in each experimental district. Then, five plants were randomly collected at each sample point. Finally, 25 samples from each experimental district were combined into a single sample to represent that district. Thus, for each of the three site locations, there were three composite samples (one for each of the three districts), which were further divided into bulk soil (BS), rhizosphere soil (RS), and potato tuber surface soil (PS) components. Potato tubers were removed from the soil with a shovel sterilized with alcohol. The potato plants were shaken and the dislodged soil was collected as BS. The soil still attached to the roots was defined as RS. Soil samples attached to the surfaces of potato tubers represented PS. RS and PS were obtained from potato roots and tubers, respectively, with sterile brushes. After the soil was collected, the tubers were separated from the stems with sterilized scissors. The tubers were then put into sterile bags and transported to the laboratory. All samples were placed in sterile bags. Soil samples were passed through < 2 mm sieves. One part of each soil sample was naturally air-dried or stored at 4 °C before analyzing soil physicochemical properties and extracellular enzyme activity. All other soil samples were stored at − 80 °C until DNA extraction.
Potato yield, starch, and nutrient contents
Potato yield was obtained after weighing all harvested potatoes on the day of sampling. We selected potato starch and nutrient content to represent potato quality. Tubers cleaned with sterile water were cut into square pieces of the same size. Then, one-tenth of the potato weight of each sample was dried at 105 °C for 6 h and again at 65 °C until they reached a constant weight. Dried tubers were ground to powder and mixed thoroughly. Potato starch content was measured using 0.1 g tuber dry powder using anthrone colorimetry (Haase and Plate 1996). The remaining dry tuber powder was used to analyze the content of various elements. Total nitrogen (TN) was obtained by digesting 0.1 g tuber powder with concentrated sulfuric acid. Then, 0.2 g tuber powder was extracted with 1% (v/v) HNO3, and digested with HF to obtain total phosphorus (TP) (Xing et al. 2013), calcium (Ca), magnesium (Mg), zinc (Zn), manganese (Mn), boron (B), and cobalt (Mo). These elements were measured using inductively coupled plasma optical emission spectrometry (ICP-OES-5110; Agilent Technologies, Santa Clara, CA, USA) (Xing et al. 2017).
Soil physicochemical property and extracellular enzyme activity
Soil pH was measured with a calibrated Metro-pH320 pH meter (Mettler-Toledo Instruments Ltd., Shanghai, China) at a soil/water ratio of 1:2.5. Soil organic carbon (SOC) was analyzed by the K2Cr2O7 digestion method (Wei et al. 2019). Soil NO3−-N and NH4+-N were determined with a continuous flow analytical system (AA3; SEAL Analytical GmbH, Norderstedt, Germany) after extraction with 1 mol·L−1 KCl. Available phosphorus (AP) was measured using a UV–Vis spectrophotometer (UV-2600; Shimadzu, Kyoto, Japan), and an extraction solution of 0.50 mol·L−1 NaHCO3. TN was determined with an automatic continuous flow analytical system (AA3; SEAL Analytical GmbH, Norderstedt, Germany) after digestion with concentrated H2SO4 (Zhang et al. 2022). DOC in soil was analyzed in 2 mol·L−1 K2SO4 extract (soil: solution = 1:5) for 0.3 h after passing it through a 0.45 μm filter using a total organic C analyzer (Fusion/Torch, Tekmar Dohrmann, USA) (Matlou and Haynes 2006). AK was measured after extraction with 1 mol·L−1 CH3COONH4 by atomic absorption spectrometry (AAS; NOVAA350; Analytik Jena AG, Jena, Germany) (Bao 2000).
Extracellular enzyme activity related to the carbon, nitrogen, and phosphorus cycle was analyzed. The enzymes selected for analysis included acid phosphatase (PHOS), β-xylosidase (XYL), N-acetyl-glucosaminidase (NAG), β-glucosidase (BG), α-glucosidase (AG), and cellobiohydrolase (CBH). These enzymes were detected using fluorescence microplate enzyme detection technology (Wang et al. 2021). They were measured using a multi-plate reader (Infinite M200 PRO, Tecan, Hombrechtikon, Switzerland) with the substrates 4-methylumbelliferyl phosphate, 4-methylumbelliferyl-β-d-xylopyranoside, 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide, 4-methylumbellifer-β-d-glucopyranoside, 4-methylumbelliferyl-α-d-glucopyranoside, and 4-methylumbelliferyl β-d-cellobioside for each enzyme, respectively.
Genomic DNA extraction and amplicon sequencing
Soil DNA was extracted from 0.5 g soil using the Power Soil DNA Isolation Kit (MoBio, San Diego, USA), following the manufacturer’s instructions. DNA concentration and integrality were measured using a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, USA) and 2% agarose gel electrophoresis, respectively. High-throughput sequencing was performed on the Illumina Miseq PE250 platform from Novogene Biopharm Technology Co. Ltd. (Tianjing, China) with a paired-end protocol. Primer sets 515F and 806R (5′-GTGCCAGCMGCCGCGGTAA-3′ and 5′-GGACTACHVGGGTATCTAAT-3′) (Walters et al. 2016) were used to amplify the V4 region of the bacterial 16S rRNA gene. Primer sets 1737F and 2043R (5′-GGAAGTAAAAGTCGTAACAAGG-3′ and 5′-GCTGCGTTCTTCATCGATGC-3′) (Jiao et al. 2018) were used to amplify the fungal ITS1 region. Thermal cycling consisted of initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 70 °C for 45 s. After amplification, the PCR products of each sample were detected using 1.5% agarose gel electrophoresis, and the PCR products were purified, quantified, and mixed together to construct the sequencing libraries. Finally, the library quality was determined using the Qubit® 4.0 Fluorometer (Thermo Scientific), and then, all libraries were sequenced on an Illumina NovaSeq platform. DNA sequencing data were uploaded to the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) Sequence Read Archive database under BioProject PRJNA741079.
Original data were spliced and quality controlled on QIIME 2 pipeline v.2020.8 (Caporaso et al. 2010). Original sequence data were demultiplexed and quality filtered using q2-demux plugin, and barcode and primer sequences were removed. Thereafter, reads with an average Phred score (Q-score) below 20, ambiguous bases, homopolymers greater than 6, primer mismatches, and sequence lengths less than 150 bp were removed (Bokulich et al. 2013), and the retained high-quality reads were assigned to the corresponding samples according to the specific barcode at the end of the reverse primers. Then, reads with overlap exceeding 10 bp without any mismatch were assembled into tags using FLASH (Mago and Salzberg 2011). Divisive Amplicon Denoising Algorithm 2 (DADA2) was used to assign these tags to amplicon sequence variants (ASVs) with the Quantitative Insights Into Microbial Ecology 2 (Callahan et al. 2016). The ASVs were annotated using the silva132 database (https://www.arb-silva.de/) (McDonald et al. 2012) and UNITE (v8.0, 2018.08, https://unite.ut.ee/) database in QIIME 2 with the BLAST method for bacteria and fungi, respectively. To obtain the bacteria ASV table, we removed all features with mitochondria or chloroplasts in their taxonomy using QIIME 2 with the qiime taxa filter.py script. Finally, 31,067 ASVs of bacteria and 10,277 ASVs of fungi were obtained. Next, to obtain an equivalent sequencing depth for later analyses, all samples were rarefied to 61,333 sequences in bacteria and 68,062 in fungi using the Rarefy function in the “Vegan” package in R.4.0.2 (R Core Team, Vienna, Austria) (Dixon 2003).
Statistical analyses
All figures were created in R.4.0.2 (R Core Team, Vienna, Austria). Duncan’s test was used in IBM SPSS Statistics 22 (SPSS Inc., Chicago, IL, USA) to determine differences in potato yield, starch, and nutrient content, soil physicochemical properties and enzyme activity across sites, and microbial alpha diversity and relative abundance in different compartments (Duncan’s multiple range tests, p < 0.05). Based on Pearson analysis, the R package ‘psych’ (Zhang et al. 2020) was used to draw heatmaps to show the relationships among potato properties, the relative abundance of microorganisms at the genus level, and soil environmental factors. Linear regression based on partial least squares (Xing et al. 2020) was used to determine the relationship among soil enzyme activity, beta diversity, microorganisms at the genus level, and potato properties. Zero-mean normalization (Z scores) was used to standardize microbial beta diversity and taxa before linear fitting. Principal coordinates analysis (PCoA) was performed to determine how microbial communities varied across compartments and sites based on Bray–Curtis dissimilarity (Sun et al. 2021). Analysis of similarities (ANOSIM) based on Tukey’s test was conducted using the “vegan” package in R 4.0.2 (Dixon 2003) and was used to test these differences to determine whether the groupings were meaningful. Redundancy analysis (RDA) was used to analyze the relationship between microbial communities and soil environmental factors (Wei et al. 2019). The representing indices of potato nutrients (including potato tubers TN, TP, Ca, Mg, Zn, Mn, B, and Mo contents) and soil enzyme activities (including soil PHOS, XYL, NAG, BG, AG, and CBH) were calculated using PCoA with IBM SPSS statistics 22 (Sun et al. 2021).
Aggregated boosted tree (ABT) (De’ath 2007) analysis was performed to quantify the effect of genus-level microbial taxa on potato yield and quality, as well as the relative effects of soil physicochemical properties, enzyme activity, and microbial diversity on potato yield and quality, using the “gbm” package in R 4.0.2 software. It is worth noting that soil physicochemical properties, enzyme activity, and microbial diversity in different compartments were standardized using Z scores before ABT analysis.
Results
Relationship between soil properties and the yield and quality of potatoes
The potato yield and starch content varied across the sampled plots (Duncan test, p < 0.05, Fig. 1a, b). Site 1 had the highest yield and lowest starch content, whereas Site 3 showed the opposite trend (Fig. 1a, b). Measurements of element content in potato tubers showed that TP and B were significantly higher at Site 1 than at Site 3, whereas Ca, Mg, and Mn content showed the opposite trend (Table S2). The contents of most elements were intermediate at Site 2, except TP and Mo (Table S2).
Fig. 1.
Variation in the yield (a) and starch content (b) of potatoes in the three sampled sites. Data represent the mean ± standard error (SE) (n = 3). Different lowercase letters above the bar charts represent significant differences at p < 0.05 based on Duncan’s test. Correlation of soil physicochemical properties with the yield and quality of potatoes and soil enzyme activity using a heat map based on Pearson test (c). Color intensity of the scale bar indicates the correlation. Significance is represented as: *p < 0.05; **p < 0.01; and ***p < 0.001. Regression analysis of the relationship between enzyme activity in the soil and the yield and quality of potatoes based on partial least squares (d). Soil enzyme activity indexes were obtained by PCoA of the activity of six enzymes
DOC was significantly correlated with potato yield and starch content. DOC was very significantly positively correlated with potato yield and negatively correlated with starch content (p < 0.05 Fig. 1c). NO3−-N, NH4+-N, TN, SOC, and AK were negatively correlated with potato yield (p < 0.05; Fig. 1c). There was a significant negative correlation between pH and plant nutrient content (p < 0.05; Fig. 1c). Of all other soil physicochemical properties, only pH and DOC were related to potato starch and nutrient content. Soil enzyme activity was negatively correlated with most physicochemical properties (NO3−-N, NH4+-N, TN, SOC, and AK) (Fig. 1c). Soil enzyme activity showed a significantly positive correlation with potato yield and a significantly negative correlation with starch content (p < 0.01; Fig. 2d).
Fig. 2.
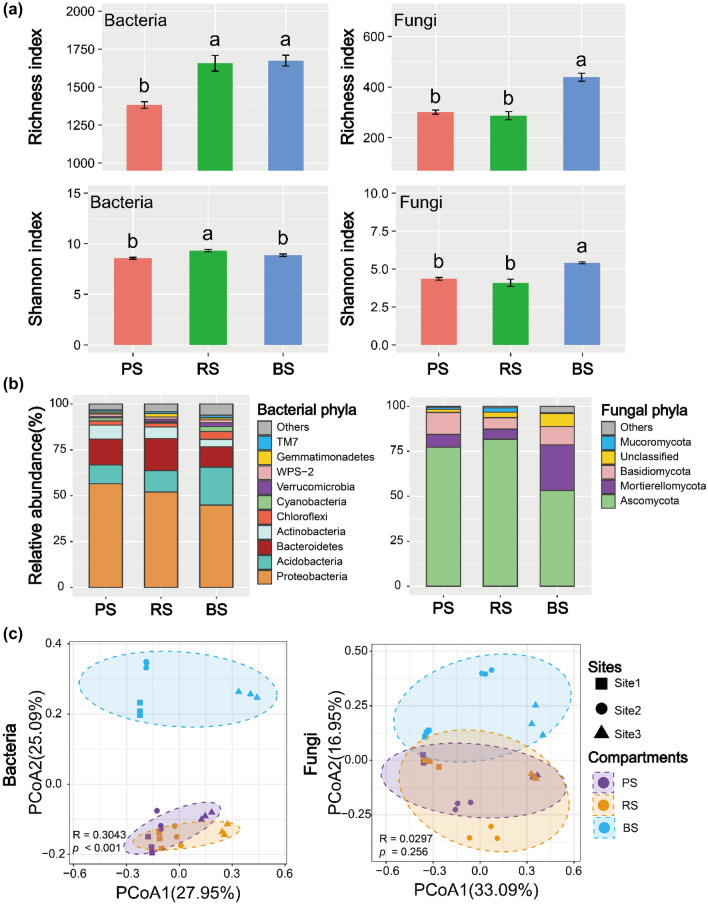
Changes in the bacterial (a) and fungal (b) ASV richness and Shannon diversity in the three potato compartments across the sites. Different lowercase letters above bar charts represent significant differences at p < 0.05 based on Duncan’s test. Principal coordinate analysis (PCoA) grouped by compartments based on Bray–Curtis distance, showing differences in the distribution of bacterial (c) and fungal communities (d). Community composition of bacteria (e) and fungi (f) across compartments at the phylum level, and classes of the total reads ranked outside the TOP10 bacteria and TOP5 fungi are grouped as “Other”
Microbial diversity and community composition across sites
Microbial community diversity of the three sites was significantly different, especially for RS (Fig. 2). RS bacterial richness and Shannon index of Site 2 and Site 3 were significantly higher than those of Site 1; RS fungal richness and Shannon index of Site 1 and Site 3 were significantly higher than those of Site 2 (Duncan test, p < 0.05; Fig. 2a, b). However, there were no significant differences in the soil microbial alpha diversity in PS and BS among the three sites. PCoA with Bray–Curtis distances showed that the structure of the microbial community in the three sample sites and three compartments differed significantly (ANOSIM, p < 0.05) (Figs. 2c, d and 3c). Sites were separated along PCoA1, whereas compartments were separated along PCoA2, especially fungi (Figs. 2c, d and 3c). It showed that site differences explained the variation to a greater degree than the compartments.
Fig. 3.
Observed microbial ASV richness and Shannon index for bacteria and fungi across the three soil compartments (a). Different lowercase letters above bar charts represent significant differences at p < 0.05 based on Duncan’s test. Community composition of bacteria and fungi across compartments at the phylum level, and classes of the total reads ranked outside the TOP10 bacteria and TOP5 fungi are grouped as “Other” (b). Principal coordinate analysis (PCoA) grouped by compartments based on Bray–Curtis distance, showing differences in the distribution of bacterial and fungal communities (c)
The bacterial and fungal community compositions in all the three potato compartments clearly shifted across sites (Fig. 2e, f). At the phylum level, the bacterial community of the three compartments was dominated by Proteobacteria (34%-60%), Acidobacteria (7–34%), Bacteroidetes (7–23%), Actinobacteria (3–11%), and Chloroflexi (1–7%) (Fig. 2e). Comparative analysis showed that the relative abundance of Acidobacteria, Chloroflexi, and WPS-2 were the highest at Site 1 and Site 2, while the relative abundance of Bacteroidetes and Gemmatimonadete were the highest at Site 3 (Duncan test, p < 0.05; Fig. S1). The fungal community of the three compartments was dominated by Ascomycota (37–92%), Mortierellomycota (3–44%), and Basidiomycota (0.8–34%) (Fig. 2f). Interestingly, the phylum Ascomycota was more abundant at Site3 than at Site 1 and Site 2, but the relative abundance of Basidiomycota and Mucoromycota were the highest at Site 1 (Duncan test, p < 0.05; Fig. S1).
The microbial community composition was also analyzed at the genus level (Fig. S3). Rhodanobacter was significantly enriched at the genus level, followed by bacteria belonging to the families Chitinophagaceae, Acidobacteriaceae, and Xanthomonadaceae (Fig. S2). In all three sites, fungal communities were dominated by the genera Mycochlamys, Humicola, Mortierella, Fusarium, and Chaetomium (Fig. S2). RDA indicated that the bacterial community was significantly affected by DOC, AK, NH4+-N, and NO3−-N. The fungal community was significantly affected by AP, pH, and TN (Fig. S3). In conclusion, the diversity and composition of bacterial and fungal communities were significantly different among sites, and this difference was affected by the soil environment and had different epithets in different compartments.
Microbial diversity and community composition across compartments
The richness index of bacteria and fungi was significantly higher in BS than in RS and PS (Duncan test, p < 0.05; Fig. 3a). The Shannon index of bacteria was higher in RS than in BS and GS. The fungal Shannon index was higher in BS than in RS and PS (Fig. 3a). PCoA with Bray–Curtis distances showed that the structure of the bacterial community in the three compartments differed significantly (ANOSIM, p < 0.05). BS was clearly separated from PS and RS, but RS and PS were not separated (Fig. 3c). From BS to RS and then to PS, the relative abundance of bacterial Proteobacteria and Bacteroidetes, and fungal Ascomycota and Basidiomycota increased gradually (Fig. 2b). In addition, the community compositions of PS and RS were similar (Fig. 3b, c).
Relationship between microbial diversity and taxa on potato yield and quality
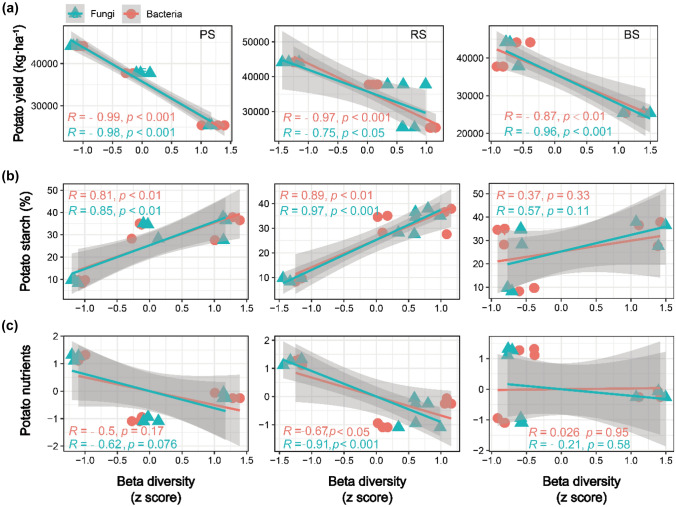
Upon exploring the relationship between microbial diversity and potato yield and quality, it was found that bacterial and fungal diversity was significantly and negatively correlated with the potato yield among compartments (p < 0.05, Fig. 4a). Bacterial and fungal diversity was significantly and positively correlated with the potato starch content in RS and PS, but not significantly correlated in BS (p < 0.05, Fig. 4b). Bacterial and fungal diversity was significantly and negatively correlated with potato nutrients in RS, but not significantly correlated in BS and PS (p < 0.05, Fig. 4c). The results showed that potato yield and quality were potentially affected by microbial community, especially the yield and starch content, but the effects of microorganisms in different compartments were not consistent. Noticeably, the microbial communities in RS and PS were more closely related to potato yield and quality.
Fig. 4.
Regression analysis based on partial least squares shows the linear relationship between microbial beta diversity (PCoA1 standardized using Z-score) and potato yield (a), starch (b), and nutrient content (c)
Using ABT, we distinguished the top 10 microorganisms that contributed to the yield and quality of potato at the genus level in the three compartments. Ranked by importance values, bacteria accounted for most microorganisms in PS (Fig. 5a). The top three contributors to PS were Solicoccozyma of the fungus genus, Betaproteobacteria of the bacteria class, and Mucilaginibacter of the bacteria genus (Fig. 5a). Most microorganisms in RS were fungi, of which most belonged to the phyla Ascomycota and Basidiomycota (Fig. 5b). The top three contributors to RS were Scytalidium of the fungus genus, Rozellomycota of the fungus phylum, and Humicola of the fungus genus (Fig. 5b). Bacteria dominated BS, most of which belonged to the phylum Proteobacteria (Fig. 5c). The top three contributors to BS were Dokdonella of the bacteria genus, Chlorobi of the bacteria phylum, and Salinispora of the bacteria genus (Fig. 5c). As shown in the bubble chart (Fig. 5), the relative abundance of these microbial taxa were different in sites, which was largely enriched in Site 1, and the relative abundance of these microorganisms was strongly correlated with pH, NO3−-N, NH4+-N, DOC, SOC, and AK (Fig. 5a–c). The microorganisms (standardized using Z score) with the top three values in all three compartments were significantly positively correlated with the potato yield and nutrient content, and negatively correlated with potato starch content, based on linear regression with partial least squares (Fig. S4). The results showed that specific underground microbial taxa had a potential impact on the potato yield and quality, and these microorganisms were regulated by the soil environment and distributed differently in different sites.
Fig. 5.
Top 10 microorganisms (both bacteria and fungi) contributing to the yield and quality of potatoes at the genus level that were identified in the surface soil of potatoes (PS) (a), rhizosphere soil (RS) (b), and bulk soil (BS) (c), respectively, using the aggregated boosted tree. The bubbles on the left show the relative abundance of these microorganisms at the genus level at the three sites; bar plots in the middle show the importance values of each microorganism in relation to the yield and quality of potatoes at the genus level, estimated by the aggregated boosted tree; heat maps based on Pearson test on the right side show the correlations between the relative abundance of microorganisms at the genus level and environmental variables. *p < 0.05; **p < 0.01; ***p < 0.001
Relative influence of microbial diversity and soil physicochemical properties and enzyme activities on potato yield and quality
The relative influence of soil physicochemical properties on potato yield was the greatest, followed by the microbial diversity of PS, BS, and RS, whereas soil enzyme activity contributed the least to potato yield (Fig. 6a). The relative influence of PS bacterial diversity on potato starch content was noticeable, followed by RS bacterial and fungal diversity; the relative influence of soil physicochemical properties and enzyme activity on potato starch content was the least (Fig. 6b). Potato tuber surface soil bacterial diversity had the greatest effect on potato nutrients, followed by BS and RS microbial diversity, but the impact values were very similar, and soil physicochemical properties and enzyme activity had the least relative influence on potato nutrients (Fig. 6c). This shows that soil physicochemical properties and microbial diversity jointly affect potato yield and quality. For potato quality, the potential impact of soil microorganisms was more significant than soil physicochemical properties and enzyme activity. When compared with BS, RS and PS had a greater impact on potato yield and quality.
Fig. 6.
Relative influence of soil physicochemical properties, enzyme activity, and microbial diversity to potato yield (a), starch content (b), and nutrients (c) predicted using the aggregated boosted tree (ABT). Higher values mean a greater influence. Soil physicochemical properties, enzyme activities, and microbial diversity (including richness index, Shannon index, and PCoA1 of beta diversity) in different compartments were standardized using Z scores
Discussion
Crop production and quality depend on soil water and mineral nutrient availability. In the present study, potato yield and starch content showed contrasting trends in the three sites, which may be associated with the soil environment and the genetic characteristics of our experimental variety “NB-1” (Schönhals et al. 2017). The soil properties at the root–soil interface could promote the increase of crop yield (Jin et al. 2017). Soil DOC is the most active part of soil organic matter, has high biological activity, contributes to the transfer and transformation of soil carbon, and strongly affects the growth and development of crops (Hansson et al. 2010). In our study, DOC was positively correlated with potato yield and negatively correlated with starch content (p < 0.05; Fig. 1c). This may be related to microorganisms and root exudates in the potato rhizosphere (Williams and Edwards 1993). Because the formation of soil DOC was mainly controlled by biological factors, microbial activity in the rhizosphere was high, root turnover was fast, and root exudates were high in the rhizosphere (Kalbitz et al. 2000). Thus, it is logical that soil DOC content is significantly correlated with potato yield and quality. As important elements of crop growth and development, nitrogen and potassium are the main limiting nutrients in potato production and strongly affect the yield and quality of tubers (Miller and Cramer 2005). Potato is highly sensitive to nitrogen and potassium fertilizers; thus, adequate nitrogen and potassium supply are necessary for the growth and development of potato. However, excessive fertilization leads to excessive vegetative growth, reducing the quality of tubers, dry matter content, and nutrient use efficiency, as well as delaying maturation (Darabi et al. 2018). In our study, nitrogen content (including NH4+-N, NO3−-N, and TN) and AK were negatively correlated with potato yield (p < 0.05; Fig. 1c). As shown in Fig. 1a and Table S1, Site 3 had the lowest yield and the highest NH4+-N, NO3−-N, TN, and AK contents. This may be related to excessive land intensification in Site 3. Previous over fertilization resulted in excess nitrogen and potassium, but not correspondingly high yields (Darabi et al. 2018). The effect of soil pH on crops is well documented. Potatoes grow better in slightly acidic soil, with soil of pH 5.0–5.5 being the most suitable. When hydrogen ion concentration is approximately 897.1–2305.2 nmol·L−1 in soil, potato vegetation causes tuber starch content to increase (Mulder et al. 1997). In the appropriate pH range, the AP also increases with increasing soil pH, but soil Fe and Al content declines (Jasim et al. 2020), which negatively affects starch content and nutrient accumulation in potato tubers.
As an important mediator of material flow and energy flow in soil ecosystems, soil enzymes generate specific biochemical reactions in soil microorganisms and plant roots, and are an important manifestation of soil function and soil fertility (Zhang et al. 1984; Qiu et al. 2004). Soil enzyme activity is the direct expression of metabolic demand and available nutrients in soil communities (Caldwell 2005). We selected extracellular hydrolases related to the carbon, nitrogen, and phosphorus cycle as the only soil enzyme activity index through IBM SPSS Statistics 22. Soil enzyme activity was significantly and positively correlated with potato yield and was negatively correlated with starch content (p < 0.01; Fig. 1d). The direct correlation between crops and soil enzyme activity has not been previously reported. However, microorganisms likely play an important role in this relationship. The microbial economic theory represents the main mechanism for maintaining soil enzyme activity (Allison et al. 2011). Microorganisms regulate enzyme activity based on the demand of potato for carbon, nitrogen, and phosphorus nutrients, as well as the content of the substrate, which feeds back to the yield and quality of potato.
Potatoes recruit microorganisms from BS through root exudates (Rolfe et al. 2019). These root exudates in the rhizosphere provide nutrients to microorganisms and promote their growth, interacting with edaphic conditions to create a soil environment different from that of BS (Lareen et al. 2016). Microbial communities in different compartments exhibit disparities (Edwards et al. 2015; Fitzpatrick et al. 2018). This could be determined by soil properties (Reinhold-Hurek et al. 2015) or microbial interactions involving competition and cooperation (Edwards et al. 2015). In the current study, BS had the highest bacterial and fungal richness index; however, the Shannon index of bacteria was lower than that of RS (Fig. 3a). This phenomenon followed the assembly principle of plant microorganisms (Reinhold-Hurek et al. 2015; Edwards et al. 2015). From BS to RS or PS, the selection of microbes by plants becomes increasingly specific. In contrast to the changes to bacterial communities, those to fungal communities from BS to RS or PS were less significant. This phenomenon might be attributed to fungi exhibiting a lower dispersal capacity than bacteria (Peay et al. 2007). Proteobacteria and Actinobacteria were gradually enriched from BS to PS of potatoes, whereas the numbers of Acidobacteria and Chloroflexi gradually decreased (Fig. 3b). This trend for Proteobacteria was also found for corn and sorghum (Peiffer et al. 2013; Sun et al. 2021). Proteobacteria might be more likely to accumulate in areas with labile carbon sources, especially r-selected microbiota (Fierer et al. 2007). In comparison, k-selected microbiota, or slow-growing microflora, with relatively stable biological populations usually aggregate in BS (Fuerst 1995; Fierer et al. 2007). In addition, the RS and BS microbiome could be subjected to low stochastic variation (Sun et al. 2021) due to the relatively high diversity of microbes (Fig. 3). In short, the assembly of microorganisms in different compartments is closely related to the consumption and turnover of plant resources. Changes in microbial diversity from BS to RS or PS are resource acquisition strategies widely used in the long-term evolution of plants (Comas and Eissenstat 2004). In turn, this might affect the yield and quality of potatoes. Relatively speaking, the microbial community variability of PS and RS was small in our study (Fig. 3b, c). This might have been caused by potato characteristics and our compartment division method. And, the degree of change in the soil microbial community from BS to RS or PS differed in the three sites (Fig. S5), indicating that the assembly of microbial communities in different compartments was common, but they are also affected by the soil environment, and may affect the growth process of potatoes (Sun et al. 2021). Finally, the ability of plants to allocate microorganisms is reflected in the yield and quality of crops (Fig. 4).
There were considerable differences in the diversity of the microbial community across sites (Fig. S1 and Fig. 2), which could be due to differences in the soil environment. Several studies have shown that soil property is an important factor that shapes plant microbial community (Berg and Smalla 2009; Edwards et al. 2015). For example, altitude, pH, soil water content, phosphorus content, and the ratio of cations in the soil impact the diversity of soil microbes (Faoro et al. 2010; Liu et al. 2021; Yu et al. 2021). Soil environment of the three selected sample plots was significantly different, i.e., pH, DOC, NO3−-N, NH4+-N, and AK (Table S1). Meanwhile, RDA showed a significant correlation between microorganisms and DOC, AK, NH4+-N, NO3−-N, AP, pH, and TN (Fig. S3). However, compared with BS, the difference in the RS in the three sites was more significant (Fig. 2a, b). This may be related to potato root exudates, because studies have shown that plant root exudates are involved in a series of biological and abiotic reactions at the root–soil interface (Mendes et al. 2013). This shows that differences among soil environments across sites resulted in differences in microbial communities; further promoting microbial community assembly from BS to RS or PS across sites (Fig. S5).
We identified the top 10 genus-level microorganisms contributing to potato yield and quality in different compartments by ABT construction. Most of the microbial taxa at the genus level were closely related to the yield, starch, and nutrient content of potatoes and belonged to the fungal phyla of Ascomycota and Basidiomycota and the bacterial phylum of Proteobacteria (Fig. 5). These microbial populations are considered the most competitive and ubiquitous in a wide range of terrestrial ecosystems (Delgado-Baquerizo et al. 2018). Thus, their abundance in potato-associated soils and their correlation with the productivity and quality of potatoes are logical. Ascomycetes have genes related to stress-tolerance and nutrition uptake. These genes enhance the response of ascomycetes to environmental pressure, allowing them to utilize more resources and strengthen their environmental competitiveness (Egidi et al. 2019). The Scytalidium genus belongs to Ascomycota, with most strains being fungal pathogens of plants that secrete plant toxins and affect crop health and production (Berger et al. 2020). Basidiomycetes are also considered to have a strong regulatory capacity to adapt to harsh environmental conditions (Peralta et al. 2017). The extracellular enzyme system of basidiomycetes effectively catalyzes cellulose and lignin and promotes the carbon cycle in ecosystems, including white-rot basidiomycetes and brown-rot fungi (Várnai et al. 2014; Peralta et al. 2017). Some strains of the genus Dokdonella, belonging to Proteobacteria, produce large quantities of diphosphatidylglycerol and promote energy flow (Ten et al. 2009). Furthermore, Lysobacter, in the phylum Proteobacteria, inhibits plant viruses via extracellular enzymes (Islam 2011). Proteobacteria, especially Alphaproteobacteria and Gammaproteobacteria, have been classified as co-trophic bacteria (Adrian et al. 2017) and exhibit strong environmental adaptability, rapidly adjusting to changes in soil moisture, pH, DOC, soil organic nitrogen, and temperature (Zhang et al. 2020). Therefore, the relative abundance of these organisms contributes to the yield and quality of potatoes. In this study, there were differences in the enrichment of these microbial taxa in different sites (Fig. 5). As a result, we suggested that differences among the soil environment in the three regions affect the composition and diversity of microbial communities, as well as the assembly process of specific microorganisms from BS to RS or PS (Bakker et al. 2015; Naylor et al. 2017), affecting the yield and quality of potato. However, this hypothesis needs to be validated by future studies.
Our ABT revealed that microbial diversity, soil physicochemical properties, and enzyme activity have potential effects on potato yield and quality in varying degrees. The relative influence of soil physicochemical properties on potato yield was the most striking, while the relative effects of microbial diversity in different compartments on potato starch and nutrient content were greater than those of physicochemical properties and enzyme activity (Fig. 6a–c). Potato tuber biomass is directly related to soil available nutrients (N/P/K), supporting the results of existing studies (Miller and Cramer 2005; Kolodziejczyk 2014; Xing et al. 2020). Potato is a crop that is highly sensitive to nitrogen, phosphorus, and potassium fertilizers, with the application of appropriate NPK being required to enhance the yield of potato tubers (Agbede 2010). In addition to soil physicochemical properties, microbial diversity also has a potential impact on potato yield. The relative influence of microbial diversity on potato yield and quality also differed across soil compartments. Potato tuber surface soil bacterial diversity contributed the most important relative influence value to potato starch and nutrient content (Fig. 6a–c). Rhizosphere (Potato tuber surface soil was also included in our study) is a unique region where plant roots interact with the soil microbial community. Rhizosphere microorganisms are important regulators of substrate and energy transformation, which affect soil fertility and plant nutrient status (Mendes et al. 2013). Plant rhizosphere bacteria stimulate plant growth directly in various ways, including in-situ production or anabolism of plant hormones (Vessey 2003; Dodd and Ruiz-Lozano 2012), enhancing plant nutrient absorption, and indirectly inhibiting plant pathogens (Kloepper et al. 1992). Fungi also play an important role in crop growth. For example, arbuscular mycorrhizal fungi facilitate the uptake of water and nutrients (particularly P and K) by plants, improve photosynthesis, and protect plants from diseases and heavy metal toxicity, promoting plant growth and enhancing crop yield (Al-Karaki 2000; Birhane et al. 2012; Nadeem et al. 2017). Fungi also act as pathogens that harm plant health. For instance, the Fusarium wilt of banana is caused by the fungus Fusarium oxysporum (Fones et al. 2017), and late blight of potato is caused by Phytophthora infestans (Fry 2008). Due to the important role of fungal and bacterial diversity in regulating crop growth and influencing the yield and quality of crops, future research should focus on developing microbial biotechnology to ameliorate the agricultural ecological environment and realize the sustainable development of agriculture by utilizing the power of microorganisms.
Conclusions
In this study, the link between potato productivity and the belowground microbial community has been described. We found that the effects of microbial diversity on potato yield and quality were mainly affected by the geographical environment and the belowground niche compartments of the plant. First, the diversity and composition of the soil microbial communities were affected by differences in geographical environment, and the responses of RS to the difference were more significant than those of BS. Secondly, the relationship between soil microbial communities in RS and PS and potato productivity was closer than between BS and potato productivity. Interestingly, some specific belowground microbial taxa were significantly correlated with potato productivity. The results underline the potential effects of biological and abiotic factors on crop growth, with important implications for the conservation of belowground microbial diversity and maintenance of crop productivity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products. We thank Haiting Wang for their assistance in bioinformatics analysis.
Author contributions
YD: conceptualization; CW: methodology; GC: software; YD, TG and JC: validation; GC: formal analysis; CW: investigation; HL and FW: resources; GC: data curation; GC: writing—original draft preparation; YL and TG: writing—review and editing; GC: visualization; JC and CY: supervision; JC: project administration; JC: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Key Project of Science and Technology Innovation 2025 in Ningbo City (2019B10017), the National Key Research and Development Program of China (2018YFD0200802), and sponsored K. C. Wong Magna Fund in Ningbo University.
Data availability
The data provided in this study can be obtained from the NCBI sequence reading file with accession number PRJNA741079.
Declarations
Conflict of interest
The authors declare that there are no competing interests with this study.
Contributor Information
Fang Wang, Email: wangfang811028@163.com.
Yangwu Deng, Email: tosang@foxmail.com.
Tida Ge, Email: getida@nbu.edu.cn.
References
- Adrian H, Di L, Bodelier P. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol Ecol. 2017 doi: 10.1093/femsec/fix006. [DOI] [PubMed] [Google Scholar]
- Agbede TM. Tillage and fertilizer effects on some soil properties, leaf nutrient concentrations, growth and sweet potato yield on an Alfisol in southwestern Nigeria. Soil Tillage Res. 2010;110:25–32. doi: 10.1016/j.still.2010.06.003. [DOI] [Google Scholar]
- Al-Karaki GN. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza. 2000;10:51–54. doi: 10.1007/s005720000055. [DOI] [Google Scholar]
- Allison SD, Weintraub MN, Gartner TB, Waldrop MP. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A, editors. Soil Enzymology. Berlin, Heidelberg: Springer; 2011. pp. 229–243. [Google Scholar]
- Bakker MG, Chaparro JM, Manter DK, Vivanco JM. Impacts of bulk soil microbial community structure on rhizosphere microbiomes of Zea mays. Plant Soil. 2015;392:115–126. doi: 10.1007/s11104-015-2446-0. [DOI] [Google Scholar]
- Bao S. Soil and agricultural chemistry analysis. Beijing: China Agriculture Press; 2000. [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere: plant species, soil type and rhizosphere communities. FEMS Microbiol Ecol. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Berger LRR, de Araújo MB, da Costa DP, et al. Agroindustrial waste as ecofriendly and low-cost alternative to production of chitosan from Mucorales fungi and antagonist effect against Fusarium solani (Mart.) Sacco and Scytalidium lignicola Pesante. Int J Biol Macromol. 2020;161:101–108. doi: 10.1016/j.ijbiomac.2020.06.024. [DOI] [PubMed] [Google Scholar]
- Birhane E, Sterck FJ, Fetene M, et al. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 2012;169:895–904. doi: 10.1007/s00442-012-2258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona E, Todeschini V, Cantamessa S, et al. Combined bacterial and mycorrhizal inocula improve tomato quality at reduced fertilization. Sci Hortic. 2018;234:160–165. doi: 10.1016/j.scienta.2018.02.026. [DOI] [Google Scholar]
- Bulgarelli D, Schlaeppi K, Spaepen S, et al. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- Caldwell BA. Enzyme activities as a component of soil biodiversity: a review. Pedobiologia. 2005;49:637–644. doi: 10.1016/j.pedobi.2005.06.003. [DOI] [Google Scholar]
- Callahan BJ, Mcmurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas LH, Eissenstat DM. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct Ecol. 2004;18:388–397. doi: 10.1111/j.0269-8463.2004.00835.x. [DOI] [Google Scholar]
- Darabi A, Omidvari S, Shafiezargar A, et al. Impact of integrated management of nitrogen fertilizers on yield and nutritional quality of potato. J Plant Nutr. 2018;41:2482–2494. doi: 10.1080/01904167.2018.1509995. [DOI] [Google Scholar]
- de Andrade FM, de Assis PT, Souza TP, et al. Beneficial effects of inoculation of growth-promoting bacteria in strawberry. Microbiol Res. 2019;223–225:120–128. doi: 10.1016/j.micres.2019.04.005. [DOI] [PubMed] [Google Scholar]
- De’ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:btfema]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Oliverio AM, Brewer TE, et al. A global atlas of the dominant bacteria found in soil. Science. 2018;359:320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- Denison RF, Kiers ET. Lifestyle alternatives for rhizobia: mutualism, parasitism, and forgoing symbiosis. FEMS Microbiol Lett. 2004;237:187–193. doi: 10.1016/j.femsle.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- Dodd IC, Ruiz-Lozano JM. Microbial enhancement of crop resource use efficiency. Curr Opin Biotechnol. 2012;23:236–242. doi: 10.1016/j.copbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Doran JW, Coleman DC, Bezdicek DF, et al. Soil enzyme activities as indicators of soil quality. Soil Sci Soc Am J. 1994;58:107–124. doi: 10.2136/sssaspecpub35.c7. [DOI] [Google Scholar]
- Edwards J, Johnson C, Santos-Medellín C, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci USA. 2015;112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egidi E, Delgado-Baquerizo M, Plett JM, et al. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat Commun. 2019;10:2369. doi: 10.1038/s41467-019-10373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro H, Alves AC, Souza EM, et al. Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic Forest. AEM. 2010;76:4744–4749. doi: 10.1128/AEM.03025-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology. 2007;88:1354–1364. doi: 10.1890/05-1839. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CR, Copeland J, Wang PW, et al. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc Natl Acad Sci USA. 2018;115:E1157–E1165. doi: 10.1073/pnas.1717617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fones HN, Fisher MC, Gurr SJ. Emerging fungal threats to plants and animals challenge agriculture and ecosystem resilience. Microbiol Spectr. 2017 doi: 10.1128/microbiolspec.FUNK-0027-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry W. Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol. 2008;9:385–402. doi: 10.1111/j.1364-3703.2007.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst JA. The planctomycetes: emerging models for microbial ecology, evolution and cell biology. Microbiology. 1995;141:1493–1506. doi: 10.1099/13500872-141-7-1493. [DOI] [PubMed] [Google Scholar]
- Gu S, Wei Z, Shao Z, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat Microbiol. 2020;5:1002–1010. doi: 10.1038/s41564-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase NU, Plate J. Properties of potato starch in relation to varieties and environmental factors. Starke. 1996;48:167–171. doi: 10.1002/star.19960480503. [DOI] [Google Scholar]
- Hansson K, Kleja DB, Kalbitz K, Larsson H. Amounts of carbon mineralised and leached as DOC during decomposition of Norway spruce needles and fine roots. Soil Biol Biochem. 2010;42:178–185. doi: 10.1016/j.soilbio.2009.10.013. [DOI] [Google Scholar]
- Hu Q, Yang N, Pan F, et al. Adjusting sowing dates improved potato adaptation to climate change in semiarid region, China. Sustainability. 2017;9:615. doi: 10.3390/su9040615. [DOI] [Google Scholar]
- Institute of Soil Science CAS . Chinese soil taxonomy. Beijing Science Press; 2001. [Google Scholar]
- Islam MdT. Potentials for biological control of plant diseases by Lysobacter spp., with special reference to strain SB-K88. In: Maheshwari DK, editor. Bacteria in agrobiology: plant growth responses. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 335–363. [Google Scholar]
- Jasim A, Sharma LK, Zaeen A, et al. Potato phosphorus response in soils with high value of phosphorus. Agriculture. 2020;10:264. doi: 10.3390/agriculture10070264. [DOI] [Google Scholar]
- Jiao S, Chen W, Wang J, et al. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome. 2018;6:146. doi: 10.1186/s40168-018-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, White PJ, Whalley WR, et al. Shaping an optimal soil by root–soil interaction. Trends Plant Sci. 2017;22:823–829. doi: 10.1016/j.tplants.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Kalbitz K, Solinger S, Park JH, et al. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci. 2000;165:277–304. doi: 10.1097/00010694-200004000-00001. [DOI] [Google Scholar]
- Kloepper JW, Tuzun S, Kuc JA. Proposed definitions related to induced disease resistance. Biocontrol Sci Technol. 1992;2:349–351. doi: 10.1080/09583159209355251. [DOI] [Google Scholar]
- Kolodziejczyk M. Effectiveness of nitrogen fertilization and application of microbial preparations in potato cultivation. Turk J Agric for. 2014;38:299–310. doi: 10.3906/tar-1305-105. [DOI] [Google Scholar]
- Lal MK, Tiwari RK, Kumar R, et al. Effect of potato apical leaf curl disease on glycemic index and resistant starch of potato (Solanum tuberosum L.) tubers. Food Chem. 2021;359:129939. doi: 10.1016/j.foodchem.2021.129939. [DOI] [PubMed] [Google Scholar]
- Lareen A, Burton F, Schafer P. Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol. 2016;90:575–587. doi: 10.1007/s11103-015-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiv M, Laso Bayas JC, See L, et al. Estimating the global distribution of field size using crowdsourcing. Glob Change Biol. 2019;25:174–186. doi: 10.1111/gcb.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Qi J, Liu EY, et al. Estimated potential impacts of soil and water conservation terraces on potato yields under different climate conditions. J Soil Water Conserv. 2019;74:225–234. doi: 10.2489/jswc.74.3.225. [DOI] [Google Scholar]
- Liang X, He J, Zhang F, et al. Healthy soils for sustainable food production and environmental quality. Front Agr Sci Eng. 2020;7:347–355. doi: 10.15302/J-FASE-2020339. [DOI] [Google Scholar]
- Lin F, Wu X, Francisco DA, et al. Changes in bulk soil affect the disease-suppressive rhizosphere microbiome against Fusarium wilt disease. Front Agr Sci Eng. 2020;7:307–316. doi: 10.15302/J-FASE-2020328. [DOI] [Google Scholar]
- Liu X, Yang T, Shi Y, et al. Strong partitioning of soil bacterial community composition and co-occurrence networks along a small-scale elevational gradient on Zijin Mountain. Soil Ecol Lett. 2021;3:290–302. doi: 10.1007/s42832-021-0122-2. [DOI] [Google Scholar]
- Mago T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria AGDS, Antonio Pavan M, Saraiva Muniz A, et al. Nutrient availability in the soil and its absorption, transport, and redistribution in vines. Commun Soil Sci Plant Anal. 2008;39:1507–1516. doi: 10.1080/00103620802006628. [DOI] [Google Scholar]
- Matlou MC, Haynes RJ. Soluble organic matter and microbial biomass C and N in soils under pasture and arable management and the leaching of organic C, N and nitrate in a lysimeter study. Appl Soil Ecol. 2006;34:160–167. doi: 10.1016/j.apsoil.2006.02.005. [DOI] [Google Scholar]
- McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena VS, Maurya BR, Verma JP. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res. 2014;169:337–347. doi: 10.1016/j.micres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Cramer M. Root nitrogen acquisition and assimilation. Plant Soil. 2005;274:1–36. doi: 10.1007/1-4020-4099-7_1. [DOI] [Google Scholar]
- Mulder A, Van der Wal AF, Velema RAJ, Roosjen JS. Effects of soil characteristics on the relationship between potato cyst nematodes and yield. II. Acidity (soil pH) Potato Res. 1997;40:375–381. doi: 10.1007/BF02357996. [DOI] [Google Scholar]
- Nadeem SM, Khan MY, Waqas MR, et al. Arbuscular mycorrhizas and stress tolerance of plants. Singapore: Springer; 2017. pp. 1–24. [Google Scholar]
- Naylor D, DeGraaf S, Purdom E, Coleman-Derr D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017;11:2691–2704. doi: 10.1038/ismej.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey C, Bajpai VK, Negi YK, et al. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J Biol Sci. 2018;25:1066–1071. doi: 10.1016/j.sjbs.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peay KG, Bruns TD, Kennedy PG, et al. A strong species-area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol Lett. 2007;10:470–480. doi: 10.1111/j.1461-0248.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Peiffer JA, Spor A, Koren O, et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci. 2013;110:6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta RM, da Silva BP, Gomes Côrrea RC, et al. Chapter 5 - enzymes from basidiomycetes—peculiar and efficient tools for biotechnology. In: Brahmachari G, et al., editors. Biotechnology of microbial enzymes. Academic Press; 2017. pp. 119–149. [Google Scholar]
- Pešaković M, Karaklajić-Stajić Ž, Milenković S, Mitrović O. Biofertilizer affecting yield related characteristics of strawberry (Fragaria×ananassa Duch.) and soil micro-organisms. Sci Hortic. 2013;150:238–243. doi: 10.1016/j.scienta.2012.11.016. [DOI] [Google Scholar]
- Qiu L, Liu J, Wang Y. Research on relationship between soil enzyme activities and soil fertility. Plant Nutr Fert Sci. 2004;10:277–280. [Google Scholar]
- Reinhold-Hurek B, Bünger W, Burbano CS, et al. Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol. 2015;53:403–424. doi: 10.1146/annurev-phyto-082712-102342. [DOI] [PubMed] [Google Scholar]
- Rolfe S, Griffiths J, Ton J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr Opin Microbiol. 2019;49:73–82. doi: 10.1016/j.mib.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Schönhals EM, Ding J, Ritter E, et al. Physical mapping of QTL for tuber yield, starch content and starch yield in tetraploid potato (Solanum tuberosum L.) by means of genome wide genotyping by sequencing and the 8.3 K SolCAP SNP array. BMC Genomics. 2017;18:642. doi: 10.1186/s12864-017-3979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Jiao X-Y, Chen Q, et al. Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil Biol Biochem. 2021;153:108113. doi: 10.1016/j.soilbio.2020.108113. [DOI] [Google Scholar]
- Ten LN, Jung H-M, Im W-T, et al. Dokdonella ginsengisoli sp. nov., isolated from soil from a ginseng field, and emended description of the genus Dokdonella. Int J Syst Evol Microbiol. 2009;59:1947–1952. doi: 10.1099/ijs.0.004945-0. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Leach JE, Tringe SG, et al. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18:607–621. doi: 10.1038/s41579-020-0412-1. [DOI] [PubMed] [Google Scholar]
- Várnai A, Mäkelä MR, Djajadi DT, et al. Chapter four - carbohydrate-binding modules of fungal cellulases: occurrence in nature, function, and relevance in industrial biomass conversion. In: Sariaslani S, Gadd GM, et al., editors. Advances in applied microbiology. Academic Press; 2014. pp. 103–165. [DOI] [PubMed] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Walters W, Hyde ER, Berg-Lyons D, et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2016 doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Han Y, Xu Z, et al. Hummock-hollow microtopography affects soil enzyme activity by creating environmental heterogeneity in the sedge-dominated peatlands of the Changbai Mountains, China. Ecol Indic. 2021;121:107187. doi: 10.1016/j.ecolind.2020.107187. [DOI] [Google Scholar]
- Wang Z, Li Y, Zhuang L, et al. A rhizosphere-derived consortium of bacillus subtilis and trichoderma harzianum suppresses common scab of potato and increases yield. Comput Struct Biotechnol J. 2019;17:645–653. doi: 10.1016/j.csbj.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas MS, Cheema MJM, Hussain S, et al. Delayed irrigation: An approach to enhance crop water productivity and to investigate its effects on potato yield and growth parameters. Agric Water Manag. 2021;245:106576. doi: 10.1016/j.agwat.2020.106576. [DOI] [Google Scholar]
- Wei X, Hu Y, Razavi BS, et al. Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol Biochem. 2019;131:62–70. doi: 10.1016/j.soilbio.2018.12.025. [DOI] [Google Scholar]
- Whitelaw MA. Growth promotion of plants inoculated with phosphate-solubilizing fungi. In: Sparks DL, editor. Advances in agronomy. San Diego: Elsevier Academic Press Inc; 2000. pp. 99–151. [Google Scholar]
- Williams BL, Edwards AC. Processes influencing dissolved organic nitrogen, phosphorus and sulphur in soils. Chem Ecol. 1993;8:203–215. doi: 10.1080/02757549308035309. [DOI] [Google Scholar]
- Wu C, Wang F, Ge A, et al. Enrichment of microbial taxa after the onset of wheat yellow mosaic disease. Agric Ecosyst Environ. 2021;322:107651. doi: 10.1016/j.agee.2021.107651. [DOI] [Google Scholar]
- Wu C, Wang F, Zhang H, et al. Enrichment of beneficial rhizosphere microbes in Chinese wheat yellow mosaic virus-resistant cultivars. Appl Microbiol Biotechnol. 2021;105:9371–9383. doi: 10.1007/s00253-021-11666-4. [DOI] [PubMed] [Google Scholar]
- Xing W, Wu H, Hao B, et al. Bioaccumulation of heavy metals by submerged macrophytes: looking for hyperaccumulators in eutrophic lakes. Environ Sci Technol. 2013;47:4695–4703. doi: 10.1021/es303923w. [DOI] [PubMed] [Google Scholar]
- Xing W, Wu H, Hao B, et al. Trace element stoichiometry of submerged macrophytes in Yangtze floodplain lakes and Yunnan plateau lakes (China) Aquat Sci. 2017;79:89–98. doi: 10.1007/s00027-016-0481-4. [DOI] [Google Scholar]
- Xing Y, Niu X, Wang N, et al. The Correlation between soil nutrient and potato quality in loess plateau of China based on PLSR. Sustainability. 2020;12:1588. doi: 10.3390/su12041588. [DOI] [Google Scholar]
- Yadav AN, Kour D, Kaur T, et al. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal Agric Biotechnol. 2021;33:102009. doi: 10.1016/j.bcab.2021.102009. [DOI] [Google Scholar]
- Yu J, Xia J, Ma Q, et al. Soil particle and moisture-related factors determine landward distribution of bacterial communities in a lateral riverside continuum of the Xilin River Basin. Soil Ecol Lett. 2021;3:303–312. doi: 10.1007/s42832-021-0106-2. [DOI] [Google Scholar]
- Zhang H, Wu C, Wang F, et al. Wheat yellow mosaic enhances bacterial deterministic processes in a plant-soil system. Sci Total Environ. 2022;812:151430. doi: 10.1016/j.scitotenv.2021.151430. [DOI] [PubMed] [Google Scholar]
- Zhang K, Delgado-Baquerizo M, Zhu Y-G, Chu H. Space is more important than season when shaping soil microbial communities at a large spatial scale. mSystems. 2020 doi: 10.1128/mSystems.00783-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cao C, Zhou L. Study on enzymatic activities of farming brown soils. Acta Pedol Sin. 1984;21:281–285. [Google Scholar]
- Zhang J, Marcel GA, Zhang F, et al. Soil biodiversity and crop diversification are vital components of healthy soils and agricultural sustainability. Front Agr Sci Eng. 2020;7:236–242. doi: 10.15302/J-FASE-2020336. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data provided in this study can be obtained from the NCBI sequence reading file with accession number PRJNA741079.