Abstract
The ambulatory glucose profile (AGP) is now established as the standardised, practical one-page report for graphically presenting a summary of glycaemic control status in patients with diabetes who use continuous glucose monitoring (CGM) systems as part of their daily diabetes care. The AGP report provides both a visual and a statistical summary of the glucose metrics that, as agreed in the 2019 international consensus for assessing glycaemic control, should be analysed in all people with diabetes who are using CGM systems. The AGP report can be analysed in a systematic fashion to understand current glycaemic control and to monitor, in real time, the impact of adjustments to therapy in both type 1 diabetes and type 2 diabetes. Here we provide a practical guide to the glycaemic measures that are summarised in the AGP Report and illustrate the essential components of an AGP review in a series of hypothetical, real-world, patient-centred case studies (see Supplementary Materials).
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01229-9.
Keywords: Ambulatory glucose profile (AGP), Continuous glucose monitoring (CGM), Type 1 diabetes, Type 2 diabetes
Key Summary Points
| This practical guideline demonstrates how the metrics and visual components of the standardised ambulatory glucose profile (AGP) report should be applied in the management of real-world patients. |
| A guide to understanding and investigating issues in glycaemic control is provided and illustrated with a series of case studies, each of which reflects a patient profile that is likely to be encountered in daily practice. |
| A step-by-step clinical algorithm is provided that can be used to guide the decision path through an effective, patient-centred review of an AGP Report. |
| Different components of the AGP Report derived from continuous glucose monitoring (CGM) glucose data are represented, including daily patterns, daily log and weekly summary charts. |
Introduction
The combination of high glucose measurement accuracy and ease and convenience of use has contributed in recent years to a significant uptake in the use of continuous glucose monitoring (CGM) systems, which provide a significant advantage over self-monitored blood glucose (SMBG) testing [1]. CGM is particularly useful in situations where the periodic measurement of HbA1c and infrequent SMBG testing may be inadequate to either predict or explain potentially harmful changes in glucose levels [1, 2]. These include impaired awareness of hypoglycaemia, nocturnal hypoglycaemia, recurrent unexplained hypoglycaemia, and unexplained fasting hyperglycaemia or glucose variability, including the dawn phenomenon. In such cases, CGM can provide immediate feedback to alert the user to impending low glucose or to allow a retrospective analysis of glucose fluctuation patterns that require investigation. Importantly, the use of CGM systems is proven to reduce HbA1c in people with type 1 diabetes or type 2 diabetes [3] on intensive insulin therapy, but they are equally effective for people with type 2 diabetes on basal insulin therapy only [4, 5] or those on non-insulin therapy [4]. Ultimately, CGM systems can be used in the same way to reduce long-term high glucose exposure for people with either type 1 or type 2 diabetes.
The full value of CGM systems is realised through the extensive insight into glycaemic data that they can provide. An international consensus published in 2019, which summarises expert discussions at the Advanced Technology & Treatment for Diabetes Congress, identified 10 metrics for assessing glycaemic control that should be analysed by healthcare professionals (HCPs) in all people with diabetes using CGM systems [6]. At the same time, the consensus proposed a standardised, practical one-page report for graphically presenting a summary of glycaemic control status in patients with diabetes—the ambulatory glucose profile (AGP) report [6].
A systematic analysis of the AGP report is a valuable and practical way to obtain a comprehensive assessment of glycaemic control and the effectiveness of any treatment changes in real time. At the same time, a comprehensive AGP analysis facilitates the identification of crucial problems in achieving optimal glycaemic control, which helps to identify the appropriate therapeutic management adjustment.
The aims of this paper are:
To identify and discuss the importance of the individual glycaemic data presented in the AGP report
To show an effective and systematic method for interpreting the data in the report
To provide guidance for HCPs on how to use the AGP Report in the daily care of patients with diabetes.
Glycaemic Control Data Provided in the AGP Report
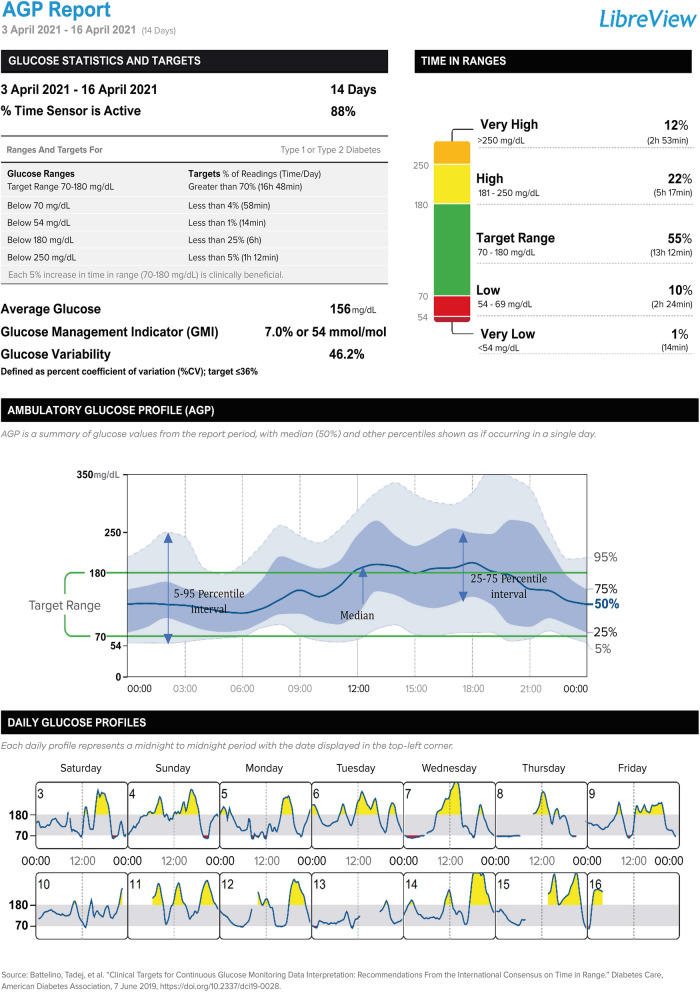
When presenting patient glycaemic data (Fig. 1), the AGP report consists of three sections: (1) glucose statistics and targets; (2) the ambulatory glucose profile; and (3) daily glucose profiles [6]. The recommended standard reports that provide summaries of glycaemic control have been incorporated into dedicated data-management applications for the analysis and archiving of patient glycaemic data provided by manufacturers of CGM systems. For example, for the FreeStyle Libre system (Abbott Diabetes Care, Witney, UK), one of the most widely used glucose monitoring systems globally, a consensus-recommended AGP report for a given patient can be generated in LibreView, a cloud-based platform available at http://www.libreview.com. In LibreView, the period covered by the AGP report is set by default to 14 days, but it can be generated for a longer period—up to a maximum of 90 days.
Fig. 1.
Ambulatory glucose profile (AGP) report
Section 1: Standardised Parameters for Glycaemia Assessment
Section 1 of the AGP report presents the 10 CGM metrics that are key to a comprehensive assessment of glycaemic control in people using CGM systems [6, 7]. Their meaning, target values and ranges are presented in Table 1.
Table 1.
| No. | Metric | Definition and target value and range |
|---|---|---|
| 1 | Number of days the sensor is active | The recommended minimum duration of use of the CGM system to assess glycaemic parameters is 14 consecutive days |
| 2 | % of sensor data captured |
This parameter specifies the percentage of time that the CGM system has recorded glucose readings For reliable analysis of glycaemic indices, the recommended minimum system activity is 70% of the time |
| 3 | Mean glucose concentration | The mean value of the glucose measurements recorded by the system during the period analysed |
| 4 | Glucose management indicator (GMI) |
A new parameter assessing glycaemic control based on mean glucose levels GMI replaces the previously used estimated HbA1c value (eA1c)—see also Table 3 Mathematical calculation: GMI (%) = 3.31 + 0.02392 × mean glucose concentration (mg/dL) [14] |
| 5 | Glucose variability |
Assessed as the coefficient of variation (CV) of glucose measurements during the analysis period The recommended CV value is ≤ 36% (higher variability is associated with a high risk of hypoglycaemia) Mathematical calculation: CV (%) = [standard deviation/mean glucose] × 100 |
| 6 | Time above target glucose range (TAR)—level 2 |
Percentage of time of the analysed period when glycaemia is > 250 mg/dL (> 13.9 mmol/L) Recommended targets: < 5% (i.e. < 1 h 12 min per day)a < 10% (i.e. < 2 h 24 min per day)b |
| 7 | Time above target glucose range (TAR)—level 1 |
Percentage of time during the analysed period when glucose is > 180 mg/dL (> 10 mmol/L)a,b or > 140 mg/dL (> 7.8 mmol/l)c Recommended targets: < 25% (i.e. < 6 h per day)a,c < 50% (< 12 h per day)b |
| 8 | Time in range (TIR) |
Percentage of the time of the analysed period when glycaemia is within the range 70–180 mg/dL (3.9–10 mmol/l)a,b or 63–140 mg/dL (3.5–7.8 mmol/l)c Recommended targets: > 70% (i.e. > 16 h 48 min per day)a,c > 50% (> 12 h)b |
| 9 | Time below target glucose range (TBR)—level 1 |
Percentage of time during the analysed period when glycaemia is < 70 mg/dL (< 3.9 mmol/l)a,b or < 63 mg/dL (3.5 mmol/l)c Recommended targets: < 4% (i.e. < 58 min per day)a,c < 1% (< 15 min per day)b |
| 10 | Time below target glucose range (TBR)—level 2 |
Percentage of time during the analysed period when glycaemia is < 54 mg/dL (< 3 mmol/l)a,c Recommended objectives: < 1% (i.e. < 14 min per day)a,c 0%b |
aRanges and targets for the general population of patients with type 1 or 2 diabetes
bRanges and targets for older patients and/or those at a high risk of hypoglycaemia
cRanges and targets for women with type 1 diabetes in pregnancy
The TIR/TAR/TBR ranges for women with type 2 diabetes in pregnancy or gestational diabetes are the same as those for women with type 1 diabetes in pregnancy. The specific targets in this patient group have not yet been defined because of limited evidence
Particular weight is given to assessing the percentage of time spent by the patient with glucose readings in the target glycaemic range 70–180 mg/dL. This metric is called time in range (TIR). A recommended target of > 70% time in the 70–180 mg/dL range corresponds approximately to a glycated haemoglobin A1c (HbA1c) level of close to 7.0% (53 mmol/mol), which has been confirmed using data from studies that have reported both %TIR and HbA1c in different patient populations using a variety of CGM devices [8]. Each 10 percentage point increase in %TIR correlates with a reduction in HbA1c percentage of approximately 0.8% [8]. It has been shown that a reduction in %TIR is associated with an increased risk of developing microvascular complications of diabetes. A 10% reduction in TIR is associated with a 64% increase in the risk of retinopathy progression and a 40% increase in the development of albuminuria [9]. In addition, a lower %TIR has been shown to be associated with a higher risk of macrovascular diabetes complications, including cardiovascular mortality [10]. Assessment of changes in %TIR in the AGP report allows for faster and more-accurate adjustments of therapy and treatment recommendations than the evaluation of HbA1c levels. In case of AGP, the analysis can be carried out on an ongoing basis, while HbA1c is usually measured every 3–4 months.
Section 2: Ambulatory Glucose Profile
The AGP graph itself is an internationally agreed standard for summarising and interpreting CGM data in a visually impactful format that allows diabetes healthcare professionals and people with diabetes to identify patterns and trends in daily glucose control [11, 12]. This form of presentation allows easy identification of patterns of glycaemic variation, especially periods with an increased risk of hypo- and hyperglycaemia.
The assessment of consistent glycaemic patterns is based on the analysis of distribution curves determined from the combined glucose readings visualised in the AGP. These are the median line, the 25th to 75th percentile range also called the interquartile range, (IQR), and the 5th to 95th percentile range. In the AGP, the IQR and the 5th to 95th percentile ranges appear as a darker-blue inner band and a lighter outer band, respectively. These ranges will widen (or narrow) with increasing (or decreasing) variability in the glucose values obtained by the patient at particular hours of the day (Table 2). The IQR band between the 25th to 75th percentile boundaries contains the 50% of glucose readings that are closest to the median line. The band between the 5th and 95th percentiles contains 90% of the glucose readings. The remaining 10% of readings fall outside of this area, with 5% being lower and 5% higher glucose readings. The variability and consistency of glucose readings should be identified separately for each period of the day as well as for the complete profile. The interpretation of the picture must take into account key information from the patient on insulin dosages, timing of insulin administration, timing and quantities of meals and snacks, physical and occupational activity, as well as sleep.
Table 2.
The main elements for the assessment of glycaemic variability in the ambulatory glucose profile section
| Description | Definition |
|---|---|
| Median line (50th percentile) |
The dark-blue median line traces the 50th percentile value across the 24 h period. 50% of all glucose readings recorded will be above this line and 50% will be below Recommended image: the median line should be as flat as possible over the entire 24 h and within the target glucose range |
| The interquartile range (IQR); the 25th–75th percentile band) |
This is the range within which 50% of all glucose readings fall—25% above and 25% below the median. A wide IQR band is the result of high glucose variability. When the 25th percentile curve falls below the target range, this indicates frequent hypoglycaemia. When the 75th percentile curve extends above the target range, this indicates frequent hyperglycaemia Recommended image: a narrow IQR blue band that is within the target glucose range |
| The 5th–95th percentile band |
This is the range within which 90% of all glucose readings fall. A wide band is the result of high variability in glucose. When the 5th percentile curve falls below the target range, this indicates a higher incidence of hypoglycaemia. When the 95th percentile curve rises above the target range, this indicates a higher incidence of hyperglycaemia Recommended image: a narrow, lighter-coloured 5th–95th percentile band that is as close as possible to the target glucose range |
Section 3: Daily Glucose Profiles
An analysis of glucose changes across each of the last 14 days before the generation of the AGP report is available in this section, allowing the assessment of the impact of patient behaviour on individual days in shaping the summary picture of daily glucose variability observed in the AGP. For instance, comparing glucose changes on days with notably higher physical activity vs days with sedentary behaviour, and work days vs weekend days, can lead to better adjustment of insulin supplementation depending on the circumstances. This part can help us to recognise specific causes that, when repeated on individual days, can modify the glycaemic area shape summarised in the 14-day AGP.
Interpretation of the AGP Report
Therapeutic decision-making requires a holistic view of the glycaemic data available in the AGP report and the information obtained during the interview with the patient. The observed glycaemic variability is the result of many factors, the most important of which is the type of treatment used, including insulin therapy and diet, physical activity, and a range of other factors that should be taken into consideration when reviewing patterns of glucose variability. These can include irregular meal timings, fasting (such as during religious festivals), incorrect calculation of the insulin correction factor (ICF) or insulin-to-carbohydrate ratio (ICR), insulin injection-site lipohypertrophy, acute intercurrent illness (such as gastroenteritis), malabsorption syndromes (such as celiac disease), and renal or hepatic dysfunction.
In this context, CGM systems and interpretation of the AGP work best when users are educated and motivated to maintain a record of changes in diet, physical activity, illness and medication adherence that can aid understanding of the patterns revealed in the AGP report. This can most readily be done using the smartphone apps through which CGM data are recorded and stored, and from which the AGP reports are generated using a cloud-based data platform. This is exemplified by the FreeStyle LibreLink app, which stores glucose data from the FreeStyle Libre system along with patient-entered details on insulin doses, diet and exercise, all of which can then be output in reports in the LibreView diabetes management system. The level of knowledge and experience of the patient is also an important factor, particularly with regard to the interpretation of glucose trend arrows. All of these factors help to better define and understand the risk profiles for individual patients, such that decisions about their diabetes care are fully informed.
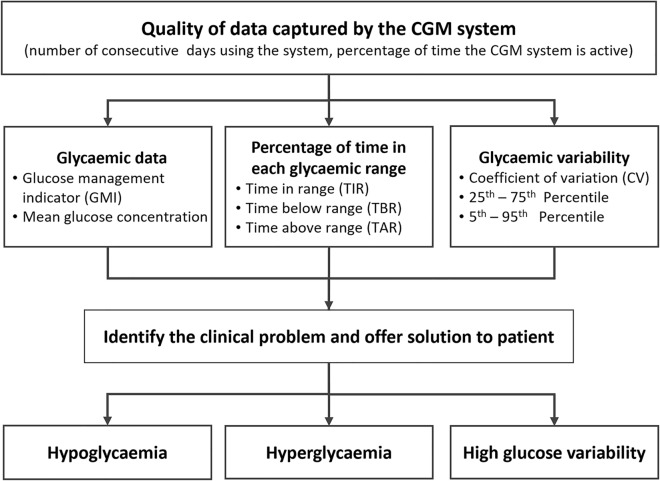
German diabetes experts have proposed a scheme for conducting the AGP report analysis in a strictly determined order [13]. However, in the opinion of the authors of this set of practical recommendations, when interpreting the AGP report, it seems necessary to maintain an individualised scheme of analysis adjusted to the preferences of the clinician and/or the individual problems presented by a given patient. However, it is important that such analysis should include each of the elements described in Fig. 2 and Table 3. The clinical experience and routines of the authors indicate that it is reasonable to define the main glycaemic control problem in each case and to modify the therapy accordingly, introducing gradual changes to the patient’s management to achieve their individualised goals. The principles of this approach are illustrated by four practical case studies that accompany these recommendations (see the “Supplementary Information”). These case studies are hypothetical but reflect practical, real-world scenarios that emphasise the value of the structured approach. Each case complies with the ethical guidelines applicable for publication. We are convinced that this knowledge will be useful not only for diabetologists but also for primary care physicians.
Fig. 2.
Algorithm for the assessment and interpretation of an AGP report. This simple algorithm illustrates the flow of activities that should be followed when interpreting an AGP report, starting at the top with an assessment of the data quality. AGP ambulatory glucose profile, CGM continuous glucose monitoring
Table 3.
Practical guidance on the basic elements of AGP report interpretation
| Elements of assessment | Parameters in report | Additional guidance |
|---|---|---|
| Issue | ||
| Quality of data captured by the CGM system | Section 1: number of days using the system, percentage of time that the CGM system is active |
The recommended analysis period is 14 days For patients with high glycaemic variability, a longer period of observation is recommended Recommended percentage of CGM system activity time is > 70% Evaluate the frequency of scans (for FreeStyle Libre system) |
| Percentages of time in each target range | Section 1: TIR, TAR, TBR |
The priority of the procedure is to increase the TIR while reducing TBR % of time in each range is also presented to the patient on a smartphone (e.g. FreeStyle Libre Link app)/reader |
| Average glucose control | Section 1: glucose management indicator (GMI) [8] |
Even when the target GMI value is the same as for HbA1c, the GMI value can be different to the laboratory-tested HbA1c value (it does not take into account several non-glycaemic factors that affect the HbA1c value, such as changes in the survival time of erythrocytes, haemoglobin, chemical modifications, anaemias, renal disease) Shorter periods are indicated for comparative assessment of progressive improvement or deterioration of glycaemic control in each patient |
| Glycaemic variability |
Section 1: CV Section 2: 25th–75th percentile band (IQR) 5th–95th percentile band |
A wide IQR band indicates the need for the correction of therapy-dependent factors (e.g. inadequate insulin dose, incorrect insulin correction factor or insulin/carbohydrate ratio, incorrectly predicted effects of exercise) A wide 5th–95th percentile band indicates the need to verify/reinforce education regarding insulin therapy, diet, physical activity or other behavioural factors, as well as the motivation of the patient to comply with treatment recommendations The most common causes of high glycaemic variability are a mismatch of insulin dosing or timing with meals, physical activity, inability to accurately carbohydrate count, or chasing glucose in response to hypoglycaemia The %CV describes the intensity of fluctuations between successive high and low glucose levels during the day, which may be outside the target range but also within the target range; patients meeting the TIR target may not meet the target for glycaemic variability (e.g. TIR = 75% and CV = 40%) |
| Identify clinical problem and offer solution to patient | ||
| Hypoglycaemia |
Section 2: ambulatory glucose profile (AGP) Section 3: daily glucose profiles |
Identify: Consistent patterns of hypoglycaemia during the day Patterns and trends in patient behaviour and activity Recurrent causes of hypoglycaemia Individualise assessment and targets, especially for pregnant women, the elderly and/or others with a high risk of hypoglycaemia Pay attention to low-glucose events with periods of hypoglycaemia lasting > 15 min Assess the glucose values at which the symptoms of hypoglycaemia occur A more detailed assessment of the frequency, severity and duration of hypoglycaemia can be performed using additional detailed online reports (e.g. LibreView) |
| High glycaemic variability |
Section 2: ambulatory glucose profile (AGP) Section 3: daily glucose profiles |
Identify: Patterns of glycaemic variability within and between days Patterns and trends in patient behaviour and activity Consistent causes of high glucose variability |
| Hyperglycaemia |
Section 2: ambulatory glucose profile (AGP) Section 3: daily glycaemic profiles |
Identify: Recurrences of hyperglycaemic episodes during the day Patterns and trends in patient behaviour and activity Consistent causes of hyperglycaemia |
Periodic AGP analysis by the physician should not replace the need for the diabetes healthcare team to educate the patient or their caregivers in this area as well. The role of the physician and the diabetes educators is to teach the patient how to independently interpret the AGP and make, based on the report data, the right therapeutic decisions for themselves. It should be stressed that the structure of the information contained in the AGP has added educational value. As part of their training, patients should be encouraged not only to independently evaluate the whole 14-day period, but also to examine the glucose logs of individual days to identify the results both of good and bad decisions. Patients should understand and be able to interpret the information provided by the AGP format. This gives them the opportunity to adjust their diabetes therapy not only after a visit to the diabetic clinic, but above all on their own, in the periods between medical advice.
Summary
The AGP report format is a practical tool that provides a quick yet comprehensive assessment of the patient’s glycaemic control based on the currently recommended metrics. It provides information about the time spent in each glucose range as well as highlighting patterns of glycaemic variation to provide a clearer picture of glycaemic control compared to HbA1c and traditional self-monitoring with blood glucose meters. The AGP report is an easy-to-use, easy-to-read summary of glycaemic data that helps to identify the main clinical difficulties in achieving optimal diabetes control. Correct analysis and identification of the causes of a lack of good glycaemic control allows the appropriate adjustment of therapeutic management and the appropriate emphasis in patient education. The AGP reports enable tried and trusted individualised care for patients with diabetes to be put into everyday practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Funding for this article was provided by Abbott, who also funded the Rapid Service Fee.
Editorial Assistance
Editorial assistance in the preparation of this manuscript was provided by Dr Robert Brines of Bite Medical Consulting. Funding for writing support was provided by Abbott Diabetes Care.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors equally contributed to the concept and design of the manuscript and worked collaboratively to review and prepare the final manuscript.
Disclosures
Leszek Czupryniak has received honoraria from Abbott, Abbott, Ascencia, Medtronic, Roche. Grzegorz Dzida has received honoraria from Abbott, Roche. Piotr Fichna has received honoraria from Abbott. Przemyslawa Jarosz-Chobot has received honoraria from Abbott, Ascencia, Dexcom, Medtronic, Roche and research funding from Medtronic. Janusz Gumprecht has received honoraria from Abbott, Ascencia. Tomasz Klupa has received honoraria from Abbott, Ascencia, Medtronic, Roche, and research support from Medtronic. Malgorzata Mysliwiec has received honoraria from Abbott, Ascencia, Dexcom, Medtronic. Agnieszka Szadkowska has received honoraria from Abbott, Ascencia, Dexcom, Medtronic, Roche. Dorota Bomba-Opon has received honoraria from Abbott. Krzysztof Czajkowski has received honoraria from Abbott. Maciej T. Malecki has received honoraria from Abbott, Ascensia, Dexcom, Medtronic, Roche. Dorota A. Zozulinska-Ziolkiewicz has received honoraria from Abbott, Ascensia, Dexcom, Medtronic, Roche.
Compliance with Ethics Guidelines
This article is based on expert interpretation and illustration of real-world scenarios. All cases are hypothetical and no actual patient information is presented and human subjects have not been involved.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Ajjan R, Slattery D, Wright E. Continuous glucose monitoring: a brief review for primary care practitioners. Adv Ther. 2019;36:579–596. doi: 10.1007/s12325-019-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubihal S, Goyal A, Gupta Y, Khadgawat R. Glucose measurement in body fluids: a ready reckoner for clinicians. Diabetes Metab Syndr Clin Res Rev. 2020;15:45–53. doi: 10.1016/j.dsx.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2019;11:83–95. doi: 10.1007/s13300-019-00720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EE, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184–189. doi: 10.2337/ds20-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin. JAMA. 2021;325:2262–2272. doi: 10.1001/jama.2021.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 9.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549–555. doi: 10.2337/dc20-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10:111–117. doi: 10.2337/diacare.10.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. Diabetes Technol Ther. 2013;15:198–211. doi: 10.1089/dia.2013.0051. [DOI] [PubMed] [Google Scholar]
- 13.Kröger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol. 2020;14(3):586–594. doi: 10.1177/1932296819883032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.