Graphical abstract

Keywords: Agriculture, Microplastics, Plastisphere, Rhizosphere, Soil functions, Soil microbiome
Abbreviations: AMF, arbuscular mycorrhizal fungi; DOC, dissolved organic carbon; FDA, fluorescein diacetate hydrolase; HDPE, high density polyethylene; LDPE, low density polyethylene; MP, microplastic; NP, nanoplastic; PBAT, polybutylene adipate-co-terephthalate; PES, polyethersulfone; PET, polyethylene terephthalate; PGPF, plant growth promoting fungi; PGPR, plant growth promoting rhizobacteria; PLA, polyactic acid; PP, polypropylene; PS, polystyrene; PVC, polyvinylchloride; SOC, soil organic carbon; SOM, soil organic matter
Abstract
Soil organisms and specifically microorganisms are indispensable to life on Earth. They regulate essential ecosystem functions from carbon sequestration to primary production. These organisms often experience stress when the balance of the soil system is disrupted by agricultural practices and environmental disturbances. A new stressor is plastic, which can be found in soils, in and around soil-dwelling organisms, and close to plants. The presence of plastic can affect soil chemistry, plant growth and the survival of higher-order organisms. Microbial organisms respond sensitively to these changes in their surroundings and will thus be (in)directly affected by plastic. Eventually, this results in a different microbial activity, composition and reduced diversity. Plastic might even serve as a specific habitat for microorganisms, generally referred to as the plastisphere. In this review, we make predictions based on the observed effects of (micro)plastics and the potential impact on the plant-soil-microbiome system. We use prior knowledge of other disturbances (e.g. tillage and pesticides) which have been studied for many years in relation to the soil microbial community. Further research is needed to develop standardized methods to study smaller plastic particles (micro- and nanoplastics) as these play the most dominant role in terrestrial ecosystems.
1. Introduction
Soils are a heterogeneous habitat formed by a complex mixture of minerals, organic matter and a network of water- and air-filled pore spaces [1] (Fig. 1). This habitat is the home of a wide abundance and diversity of soil organisms performing key soil processes and functions. The most dominant players in all of these processes are the microorganisms residing in the bulk soil and/or rhizosphere (the soil surrounding plant roots) [2]. Microorganisms belong to the most abundant and diverse groups of soil organisms, representing an estimated total biomass of 3–4% on earth (up to 20 Gt carbon (C)) [3]. They contribute to key biological processes by interacting with their surroundings, other organisms living in the soil, and plants. Specifically, 80–90% of the soil processes are mediated by the soil microbiome including bacteria, fungi, archaea, viruses and protista, for which they form the main focus of this review [4].
Fig. 1.

Illustration of the vital roles of organisms in soil. Numbers indicate the main soil processes to which microorganisms contribute. (1) carbon (C) sequestration; C enters the soil mainly via plants uptake and organic material. It is the second largest C sink, sequestering around 80% of the global terrestrial C, of which 58% is contained in the soil organic matter. Soil microorganisms contribute to this C cycle through respiration and decomposition from root deposits and plant litter (2) nutrient cycling; Macrofauna, such as earthworms, breaks down the larger organic material into smaller pieces, making it available for microorganisms which can either consume or degrade these smaller pieces. This breaks down the complex chemical compounds into more simple compounds that can again be taken up by plants. (3) soil structure; the soil structure consists of air and water-filled pore spaces created by organisms such as earthworms. Roots can reach these water-filled spaces, and these spaces are also inhibited by hydrophilic organisms. Fungi are able to bridge air-filled pore spaces with their hyphae. Microorganisms themselves contribute to the soil structure by converting organic material. The rhizosphere is magnified in black square and highlights the role of plant-growth promoting rhizobacteria and -fungi (PGPR and PGPF) for the growth, productivity and health of crops. These microorganisms can also suppress pathogens by their biological control activity. PGPR plant-growth promoting rhizobacteria; PGPF plant-growth promoting fungi; AM arbuscular mycorrhizal fungi; SOC soil organic carbon; C carbon; N nitrogen; P phosphorus; CO2 carbon dioxide; CH4 methane; N2 dinitrogen; N2O nitrous oxide; NO3– nitrate; NH4 ammonium; SOM soil organic matter.
Microorganisms mediate a multitude of soil processes and thus contribute directly to key ecosystem processes such as: (1) C sequestration, (2) nutrient cycling, and (3) the formation of soil structure [5] (Fig. 1).
After the oceans, soil is the second largest C sink, sequestering around 80% of the global terrestrial C underground. It is estimated that 58% of the soil organic C is contained in soil organic matter (SOM) [6], [7], [8], [9]. Soil bacteria and fungi contribute substantially to the flux of the below- and aboveground C through respiration and decomposition from the root deposits and plant litter.
Nutrient (C, nitrogen (N) and phosphorus (P)) cycling directly affects soil dynamics and ecosystem services such as plant growth [5]. The decomposition of organic material as well as the input of root exudates contributes to nutrient cycling [10]. Macrofauna, especially earthworms, break down the organic material into smaller pieces, thereby increasing the surface area available for colonization by microorganisms or by redistributing the organic material [11]. Especially bacteria and fungi transform the complex chemical compounds using enzymatic activity into simpler compounds and molecules that can be absorbed by plants, thus providing indirect feedback for plant productivity [12]. Positive direct effects of microbially-mediated nutrient inputs for plants are associated with symbiotic relationships. Well-studied examples are the N-fixing bacteria and archaea that convert atmospheric N into ammonium-N [13], [14] and the increased P uptake by plants via arbuscular mycorrhizal fungi (AMF) [15]. Overall, the rate of organic matter decomposition and subsequent nutrient cycling depends on the composition of the microbial community and environmental conditions (e.g. temperature, pH, soil moisture, etc.).
Soil structure refers to the size and arrangement of soil particles and associated pore spaces that create room for water, nutrients, gases and soil organisms [16]. The soil particles and pore spaces are arranged into aggregates of different sizes; their stability is an important aspect of the soil structure [17]. Several studies revealed not only that soil structure is a major driver for the adaptation of soil organisms, but also that microorganisms contribute to the soil structure, e.g. by adding organic matter through decomposition and conversion of organic material [18], [19], [20], [21]. The role of earthworms is also unmistakable for the soil structure, as they grind and remold ingested particles into new aggregates and pores, thereby both compacting and loosening the soil [21], [22].
In addition to their prominent role in key ecosystem processes, microorganisms play a vital role in the overall growth, productivity and health of crops. Plants actively attract microorganisms towards the root, by which microbial species will reside in the rhizosphere [23], [24] (Fig. 1). Plant species can shape the microbial composition and activity in their rhizosphere via differences in root architecture [25] and rhizodeposition [26], [27]. Although rhizodepositions cost C for the plant, the C exuded by the roots serves as a major energy source that nurtures a diverse and abundant microbial population originating from the bulk soil [28]. Beneficial microbial organisms can colonize the root surface and the inner root tissues [29]. Some of these colonizers have the potential to enhance plant development, biological control activity and stress tolerance; these are referred to as plant-growth promoting rhizobacteria and -fungi (PGPR and PGPF, respectively) [30] (Fig. 1).
The diversity and composition of the soil microorganisms in the bulk soil are primarily driven by (1) soil characteristics such as pH, organic matter content [31], [32] or moisture content [33], [34], (2) soil structure and (3) the soil food web (Fig. 1). Bacteria (specifically Gram-negative bacteria) are more sensitive to moisture fluctuations due to their thinner cell wall and lost capacity to sporulate, than Gram-positive bacteria, archaea and fungi [35]. Within the root microbiome, specific increases of Gram-positive Actinobacteria and Firmicutes were observed after an extended drought period, while Gram-negative bacteria declined [35]. Fungi can more easily reach water-rich environments using their hyphae [36], [37]. In addition, soil structure can influence the ability of soil organisms to sense food sources or prey [38]. The appearance of other soil organisms (micro-, meso- and macrofauna) and the interaction of all organisms in the soil food web mediates both the abundance and activity of microorganisms in soil. The soil food web represents possible feeding connections in a soil ecosystem by clustering organisms in trophic levels [39]. The biomass of each level is controlled by other trophic levels, either bottom-up or top-down. The interactions between all these trophic levels are major determinants of soil processes, notably in litter decomposition [40], [41] and nutrient turnover [42], [43].
Soil microorganisms are also influenced by the soil composition and global and local patterns of the soil. Multiple studies have contributed to the general understanding of the biogeography of the abundance and biomass of different organisms, including bacteria [44], [45], [46], [47], [48], [49], [50], fungi [34], [44], [50], [51], earthworms [52], nematodes [53], [54], [55], [56], protists [50], [57], [58] and microbial biomass [33], [59], [60]. From these studies, patterns of distribution are related to climatic conditions such as annual moisture availability (protists, fungi and nematodes) and pH (bacteria).
Because some soil processes are carried out by a variety of microorganisms, the loss of a few species is assumed to have limited impact on the soil system. This phenomenon is called functional redundancy and reflects the potential robustness of terrestrial ecosystems [61]. The buffering capacity of soil has its limits however, which should not be explored too much as it can lead to degraded soils [62]. Human-induced changes, particularly those brought by intensive agriculture and global warming, have led to the modification of soil structure and physicochemical properties [62], [63]. This has resulted in tremendous changes in the microbial community composition and diversity, which in turn alters the dynamics of the soil food web [64].
Soil disturbances linked to agricultural intensification include mechanical impacts (tillage and compaction), monoculture, and fertilizer and pesticide application [1]. These frequently applied practices, used to maximize crop yield, cause a loss of microbial diversity and alter the microbial composition, resulting in unbalanced ecosystem functions and services and worsening soil health [65], [66], [67], [68], [69]. For instance, tillage practices can affect the soil organisms by making significant modifications to the soil’s physical and chemical properties [70]. Conventional tillage disrupts soil aggregates, exposing soil microorganisms to an increased risk of desiccation and restricted access to food sources [71]. This type of tillage typically results in a bacterial-dominated community [72], [73], with a higher abundance of protists and bacterivores thriving under the increase of the bacteria [74], [75], [76], [77]. In contrast, less disturbed soils often have a higher fungal and predatory nematode biomass [76], [78], [79] and are correlated with lower N leaching and higher C sequestration [80], [81]. Soil compaction caused by passage of agricultural machinery affects the physical structure of the soil by reducing the porosity and increasing soil bulk density [82]. This leads to a slower soil water infiltration rate and negatively affects air diffusion [83]. Compaction prevents roots from penetrating the deeper soil layers, resulting in shallow root growth [84]. Additionally, lower biomass of bacteria, fungi, nematodes and microbial activity and smaller earthworm populations are found in more compacted soils [85], [86].
Soil microorganisms are not only sensitive to agricultural practices; they also respond to environmental disturbances (e.g. moisture availability and temperature). Most microorganisms are dependent on the water films surrounding soil particles as a resource for microbial cellular function and transport medium [87]. Changes in moisture availability affect the soil microbiome through osmotic stress and fluctuating accessibility to nutrients via the water film [88]. Next to bacteria, nematodes are sensitive to moisture fluctuations as they are restricted to live in water matrixes. They can survive desiccation however by going dormant [89], [90].
Seasons are correlated with ambient temperature fluctuations and therefore also soil temperature. The higher temperatures during summer are correlated with an increased microbial respiration and activity of the plant roots, resulting in a higher loss of CO2, CH4 and N2O from the soils [91], [92]. Higher temperatures as a result of climate change will only increase these respiration rates. The soil microbiome will be affected by more extreme weather events such as more frequent, longer and more intense heat waves. It is therefore thought that by 2050 soils will no longer serve as a Csink, but will become a Csource [93]. In contrast, cold stress during winter periods affect the soil microbiome and rhizobiome, with increased abundances of PGPR in the plant root that can protect the plants from chilling temperatures [94].
The soil and the soil microbiome are therefore under continuous pressure by the changing climate and pollution due to human activities. A more recent stressor on the soil ecosystem is the presence of (micro)plastic pollution. In this review, we will focus on how plastic pollution, with specific attention to microplastics (MPs), i.e. plastics with a diameter smaller than 5 mm, disrupts the reciprocal interactions between the plant, the soil and the microbial community. In the first part, we will focus on the accumulation and distribution of (micro)plastic in soil and its effect on the soil structure and plant development. Second, we elaborate on the disruptions in community activity, complexity and composition MP pollution causes on the microbial community. In the last part of this review, we highlight the role of plastic as a microbial hotspot within the soil ecosystem.
2. A new stressor in the field: plastic pollution
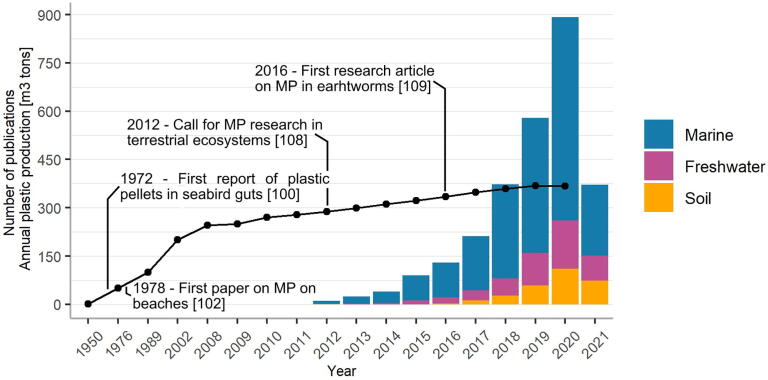
Mass production of plastic dates from the early 1950s [95]. Since then, the production rate of plastic has grown to over 300 million tons per year (Fig. 2). The popularity of plastics is easy to explain: they are cheap, lightweight, strong and durable [96]. In an ecological context, plastics can be distinguished based on either form (filaments, beads, sheets, foams, etc.) or polymer (the most common are polyethylene terephthalate (PET), high density polyethylene (HDPE) and polypropylene (PP)) [97]. The widespread use of plastics has led to plastic pollution in nearly every conceivable environment, even in the deepest marine trenches [98]. We focus here on the impact of plastic pollution in terrestrial (agricultural) environments.
Fig. 2.
Annual plastic production and overview of the number of research papers on Web of Knowledge (on 21/5/2021) regarding microplastic pollution in terrestrial, freshwater or marine environments. Annual plastic production is displayed by a black line starting from 1862. The search included the following keywords: terrestrial (soil + MP OR terrestrial + MP), freshwater (river + MP, freshwater + MP), marine (marine + MP, aquatic + MP, sea + MP). All reviews were excluded from the literature search.
2.1. Plastic pollution in the terrestrial ecosystem
The first appearances of plastic were made in seabird guts and alongside plankton in oceanographic sampling nets in the 1960’s and early 1970’s, respectively [99], [100], [101], whereas the appearance of MPs was reported for the first time on New-Zealand beaches in 1978 [102] (Fig. 2). It has been estimated that up to 5.25 trillion plastic particles are now scattered in the marine environment [103].
Most (up to 80%) of the plastic particles found in aquatic environments were produced, used, and disposed of on land. Terrestrial plastic pollution is therefore expected to be 4- to 23-fold larger than that of marine environments [104]. Agricultural soils alone may contain more (micro)plastics than oceanic basins [105]. Despite these expectations of intense plastic pollution on land, only limited research has been performed on plastic contamination in soils. Especially the smaller particles, nanoplastics (NPs; ≤1 µm) and MPs (≤5 mm), are not currently being studied, probably because advanced techniques and methodologies for sampling, extraction and detection have not yet been developed [106], [107]. The extraction of MP filaments from water is comparatively easy compared to extraction from a complex organo-mineral soil matrix [108]. Nevertheless, it is expected that the smaller particles can cause the most damage to biodiversity and the food web. Despite the call for research on MP contamination in terrestrial ecosystems dates back from 2012, it took over four years before the first research article was published, studying the effects of MP contamination on the growth rate and mortality of earthworms [108], [109]. To date, only very limited data on the occurrence of MP particles in terrestrial systems are available. A search on Web of Knowledge shows that from 2012 until May 2021, only 10% of all research articles on MP pollution focuses on terrestrial ecosystems (Fig. 2; used keywords: terrestrial (soil + MP OR terrestrial + MP), freshwater (river + MP, freshwater + MP), marine (marine + MP, aquatic + MP, sea + MP)). The current focus on MPs in marine environments is probably due to researchers’ tendency to build on previous studies, but this leaves an enormous knowledge gap regarding MP pollution in other ecosystems.
2.2. Sources and accumulation of terrestrial (micro)plastic
Plastic contamination in soil is both intentional and unintentional [110] (Fig. 3). Examples of intentional plastic addition to soil are plastic mulching and addition of plastic to fertilizers [104], [105], while unintentional contamination can occur via composts and fermented organic waste products [111], sewage sludge [112] or irrigation with water from contaminated lakes or rivers [111]. For example, Nizzetto et al. (2016) estimated that between 125 and 850 tons MPs per million inhabitants are added annually to European agricultural soils through sewage sludge or processed biosolids [105]. An annual sludge application on croplands can lead to 2- or 3-fold increases in MP concentration in soil [113], [114], [115]. In addition, MPs enter the soil ecosystem through atmospheric deposition such as tire abrasion [116] and disposal of industrial and consumer plastic waste [117].
Fig. 3.

The known and potential effects of micro- and nanoplastics on the soil physicochemical and microbial characteristics. Proven effects of microplastics are indicated in black boxes: first it has been shown that the presence of MP changes the soil bulk density and is able to increase the dissolved organic carbon (DOC) in the soil. This is related to changes in the water availability (increase) and evaporation (increase). Effects on plant development have also been noted. These effects are still uncertain either (increase or a decrease) and are therefore indicated with a crossed-out tilde. MPs also affect the soil food web. Decreases in the survival and reproduction of earthworms and nematodes have been noted and also an active uptake of MPs by these organisms has been shown. The combination of these effects can explain at least in part the effect on the microbial community, with changes in the microbial activity, composition and a decrease in the microbial diversity. In addition, we added some hypothetical effects (in orange) plastics might cause. The degradation of plastic might result in an increase in the carbon:nitrogen (C:N) ratio. Also, differences in the rhizosphere microbiome are expected as the root architecture of the plant is different in plastic-polluted soil. The presence of plastic might also increase the abundance of plastic degrading organisms, either in the soil or residing on the plastic. Plastic can thus serve as a vector; however, it remains unclear if this will be more for pathogens or beneficial microorganisms as indicated by exclamation point and check mark, respectively. DOC dissolved organic carbon; MP microplastics; C carbon; N nitrogen; H2O water. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The high level of plastic input results in high accumulation levels in the soil. In Europe, concentrations range from 0.3 and 3.4 mg MPs per kg soil near Malmö, Sweden [118] up to 55.5 mg MPs per kg soil on floodplain sites in Switzerland [119]. Industrial soils in Sydney, Australia were documented with a MP contamination in excess of 500 mg per kg soil [106]. In southwestern China, the abundance of plastic particles was found to range from 7,100 to 42,960 items per kg soil [120]. All of these studies were conducted on soils with a history of sewage sludge application, plastic mulching or a geographic location near an industrial site. The only study that reportedly studied uncontaminated farmlands (located in Southern Germany) found concentrations of only 0.31 items per kg soil [111], [121]; however, this study did not account for particles smaller than 1 mm, which were the majority of MPs in other studies [111].
2.3. Disturbance of the soil and plant system due to microplastic pollution
To protect the current status of the soil, models indicate that the number of MPs should not exceed 2,128 mg per kg soil or 14,435 mg per kg soil to maintain 50% of the currently present soil biota or soil properties, respectively. For a protection of 95%, values should stay even below 520 and 655 mg per kg soil [122]. As MP contamination of soils will only increase in the near future, presumable effects on the soil chemistry [123], plant performance [124], [125] and soil biota are to be expected. So far, it is known that MPs interferes with the soil physicochemical composition by: (1) decreasing soil bulk density, (2) changing the water availability, (3) increasing soil pH, and (4) increasing dissolved organic matter (Fig. 3).
First, it has been shown that the incorporation of MP particles such as polystyrene (PS), PP, PET, polyethersulfone (PES) or HDPE at concentrations up to 2% (w:w) can decrease soil bulk density [126], [127]. In addition, Lehmann et al. (2019) showed that the incorporation of generally large (5 mm) microfibers at concentrations over 0.2% (w:w) reduces the stability of soil aggregates [128]. Microfibers thus have the potential to alter the soil structure [128], [129], which is at least in part mediated by the presence of soil microorganisms [128]. For other polymer shapes like foams and particles, the polymer type is an important factor that co-modulates the soil physicochemical properties [130].
Second, for water availability, contradictory results have been noticed. Soil contamination with polyester fibers can lead to increasing water availability in the soil [126], [127], which might affect soil moisture and evapotranspiration. On the other hand, the study of Wan et al. (2019) showed that the addition of plastic films (fragment sizes 2, 5, 10 mm and an average concentration of 0.5% or 1%) leads to increased water evaporation and desiccation cracking [131]. This can lead to higher water losses and soil drying with negative consequences for both the soil microbiome [132] and plant performance [133].
Third, tire debris application in soil led to an increase in pH in several soils [134].
Fourth, addition of PP in extremely high concentrations (7% w:w to 28% w:w; particle size < 180 µm) to Chinese loess soil increased soil enzymatic activity, with an accumulation of dissolved organic C, N and P as a result [123]. In contrast, studies making use of MP concentrations as found in industrialized soils (5% to 7% w:w) did not have a significant effect on dissolved organic C (DOC) in the short term [106], [123], [135]. On the other hand, detrimental effects on Potassium (K), Magnesium (Mg) and Sulfur (S) were noted in the presence of MP fibers (0.4% w/w), while the bioavailability of Zinc (Zn) increased [136].
The number of studies focusing on the effect of MPs on plant growth and plant development are limited and show contradictory results (Fig. 3; Table 1). The growth of wheat was retarded by the presence of MPs (concentration 1% w:w) [137], but in spring onion, both positive and negative effects were noted depending on the polymer type (concentration 2% w:w) [127]. Exclusively positive effects were noted for Daucus carota, when MPs in different shapes and concentrations were added, ranging from shoot mass increases of 27% up to 60% [129]. In roots, the presence of MPs increased the total root length and root area in spring onion, whereas it decreased root growth of cress seeds and the average root diameter of spring onion [127], [138]. MPs are also able to accumulate in seed capsules of cress seeds, which can cause delayed germination [138]. Recently, the smallest plastic particles (NP) were even found to accumulate in edible fruits and vegetables. On average 223,000 and 97,800 NP particles per gram were found in fruits and vegetables, respectively, with the highest concentrations found in apples and carrots [139].
Table 1.
Information of the effect of specific polymers on the shoot and root of certain plant species. Data represents the plant species (+reference), the type of polymer (PA Polyamide, PEHD polyethylene high-density, PP Polypropylene, PS polystyrene, PET polyethylene terephthalate, PE Polyethylene, PU Polyurethane, PC polycarbonate), the concentration in plastic weight over soil weight, the polymer size (med. = median size of the fragments) and the effect on shoot and root development.
| Plant species Reference | Polymer | Conc. (w/w) | Polymer size | Effect shoot | Effect root |
|---|---|---|---|---|---|
| Allium fistulosum (spring onion) [127] | PA | 2.0% | 15–20 µm | Decrease dry biomass onion Increase total biomass |
No reported effects |
| PES fibers | 0.2% | Length: 5000 µm Diameter: 8 µm |
Increase total biomass | Increase root biomass | |
| PEHD | 2.0% | med. 643 µm | No reported effects | Trendwise increase root biomass | |
| PP | 2.0% | med. 624 µm | No reported effects | Trendwise increase root biomass | |
| PS | 2.0% | med. 492 µm | Increase total biomass | Increase root biomass | |
| PET | 2.0% | med. 187 µm | Increase total biomass | Trendwise increase root biomass | |
| Daucus carota (carrot) [129] | PP (fibers, film, foam or fragments) | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | Increase shoot biomass of 53.1% (fibers), 64.2% (films) and 56.3% (fragments) | Increase of root biomass of 71.5% (fragments) |
| Polyester fibers | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | No reported effects | No reported effects | |
| PA fibers | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | No reported effects | No reported effects | |
| PE films or foams | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | Increase shoot biomass of 43.7% (films) and 64.6% (foams) | Increase of root biomass of 79.9% (films) and 40.4% (foams) | |
| PET films or fragments | 0.1% 0.2% 0.3% 0.4% |
max 5 mm | Increase shoot biomass of 72.4% (films) and 51.1% (fragments) | Increase of root biomass of 70.0% (films) and 38.0% (fragments) | |
| PS foams | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | No reported effects | No reported effects | |
| PU foams | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm | Increase of shoot biomass of 50.6% | Increase of root biomass of 160.3% | |
| PC fragments | 0.1% 0.2% 0.3% 0.4% |
max. 5 mm |
Increase of shoot biomass of 54.6% | Increase of root biomass of 42.6% | |
| Lepidium sativum (cress) [138] | Not defined | 50 nm 500 nm 4800 nm |
No reported effects | Increase (50 nm), decrease (500 nm) or no effects (4800 nm) on relative root growth | |
| Triticum aestivum (wheat) [137] | LDPE | 1% | 12.5% [501 µm–1 mm] 62.5% [251 µm–500 µm] 25% [50 µm–250 µm] |
Decrease shoot biomass | No reported effects |
| Starch-based biodegradable plastic (37.1% Pullulan, 44.6% PET, 18.3% PBT) |
1% | 12.5% [501 µm–1 mm] 62.5% [251 µm–500 µm] 25% [50 µm–250 µm] |
Decrease shoot biomass | Decrease root biomass at 2 months harvest, |
3. Can plastic disrupt the soil and plant microbiome?
The disturbance of soil chemistry and plant development by MP pollution will disturb the interactions between the soil, the plant and the microbial community. Here we summarize studies that describe the disruption on the soil food web, with a focus on the soil microbiome, due to MP contamination.
3.1. Plastic decreases growth rates and increases mortality of invertebrates
Invertebrates, specifically earthworms, have been the major focus for researchers in terms of MP pollution in soil. Whereas they cannot be classified as microorganisms, earthworms play an important role in the soil food web [11]. The effect of MPs has been studied on several species, such as Lumbricus terrestris [109], [140], [141], Eisenia fetida [142], [143], [144], [145] and Eisenia andrei [146], [147]. Earthworms can ingest and digest MPs [109], [148] and can also transport and incorporate MPs into the soil matrix [140], [148] (Fig. 3). Decreases in growth rate and higher mortality (28 to 60%) of L. terrestris when MPs were introduced in the soil have been noticed [109]. In contrast, no adverse effects on survival, reproduction or body weight of the earthworm E. andrei was observed [147]. In addition, MPs can cause skin lesions and reduce reproductive rates in earthworms [149], the latter potentially caused by damage to male reproductive organs [146]. The uptake of MPs by earthworms can lead to the production of NPs through activity of the earthworm’s gut microbiome, which will release smaller plastics in the environment [146], [148]. Upon excretion, these particles become available to other soil organisms, e.g., smaller decomposers, such as microarthropods. Indeed, springtails can experience critical damage (e.g. reduced mobility, mortality) by MP exposure [150], [151] and transport the MPs to deeper soil layers [152]. The isopod Porcellia scaber was however not affected at all [153]. In conclusion, we can say that the presence of MPs in soil will have an effect on the invertebrate community, but that the sensitivity of each of the species will be different.
3.2. Negative effects on the survival and reproduction of nematodes by soil MP pollution
The species Caenorhabditis elegans is the most commonly studied nematode in terms of MP toxic effects. It has been shown that MPs will not only be ingested by C. elegans, but can also affect reproduction, survival and behavior [154], [155], [156], [157], [158], [159], [160]. Particle ingestion is limited to the particle-to-mouth size ratio, and therefore will be species dependent. For the species C. elegans, the maximum particle size to be ingested is estimated to be 4.4 ± 0.5 µm [156]. The negative effects on the survival and reproduction of MPs on nematodes are dependent on the polymer type, particle size and MP concentration [154], [155]. The exposure of micro-sized low density polyethylene (LDPE), polylactide polymers (PLA) and polybutylene adipate-co-terephthalate (PBAT) will lead to fewer offspring (up to 22.9%) of C. elegans [154], [155], [158], [159]. The behavior of C. elegans changes as well: the frequency of body bending and head thrashing accelerated and crawling speed increased, indicating that MPs can induce size-dependent excitatory toxicity on locomotor behavior [157]. The mechanism behind these effects is currently unknown, but might be related to (1) MP uptake, as smaller and higher concentration of MPs have higher toxic effects [154], [155] and (2) chemical additives on the plastic particles [159]. We hypothesize that the uptake of MP particles can interrupt the digestive tract of nematode species, leading to growth reductions. In addition, the observed relation between extractable additives and the MP toxicity on nematodes indicates toxic effects of chemical additives bound to MP particles [159]. In addition, it has been shown that bulk density, cation exchange capacity, and clay and sand content are dominant factors influencing the toxicity of PS particles on nematodes [159].
MPs thus seem to have a major effect on nematodes. Three remarks should be made, however. First, a recent study of Mueller et al. (2020) indicated that the nematodes Acrobeloides nanus and Plectus acuminatus responded differently after long term exposure to MPs [156]. Although P. acuminatus was not affected by the MP treatment, A. nanus populations developed significantly faster 31 days after MP treatment. More studies should thus be undertaken to verify the effects of MPs on nematode species other than C. elegans, particularly because nematodes play a pivotal role in many trophic levels [161]. Second, most of these studies were performed using non-soil media or spherical beads [159]. To elucidate the effect in terrestrial ecosystems, more research should be undertaken in a soil matrix and with more representative concentrations of MP. Last, all studies conducted focused on one to maximum three nematode species. We suggest that effects on the total soil nematode community should also be evaluated to elucidate the effect on the soil food web and soil health.
3.3. Interruption of the soil microbial activity and composition by MP soil pollution
After an initial focus on invertebrates and nematodes, studies looking into the effects of MPs on the soil microbiome are now emerging. Most of these studies focus on microbial activity rather than composition. So far, effects on fluorescein diacetate hydrolase (FDA), phosphatase activity, dehydrogenase activity, soil microbial respiration, and enzymes involved in the C, N or P cycles have been observed (Table 2).
Table 2.
Information of the effect of specific polymers on the microbial activity. Data represents the microbial activity (+reference), the soil type and composition, the type of polymer (PA Polyamide, PVC Polyvinyl chloride, PP Polypropylene, PS polystyrene, PET polyethylene terephthalate and PE Polyethylene), the polymer size and increase or decrease of the activity (% decrease or increase added when available in the original manuscript).
| Microbial activity Reference | Soil type | Soil composition | Polymer type | Polymer size | Increase/Decrease activity |
|---|---|---|---|---|---|
| FDA [123], [126], [162] | Unpolluted shrub field, China | Not defined | PVC | 20 mm × 20 mm | Decrease (−1.6% up to −30.7%) |
| Top loess soil, China | 18.4% clay 25.0% silt 55.9% sand |
PP | 180 µm | Increase | |
| Experimental site, Freie Universität Berlin | Sandy loam | PA, Polyester, PE | Beads: 8–20 µm Fragments: 643 µm |
Decrease for PA and PES | |
| Dehydrogenase [163], [164] | Unpolluted shrub field, China | Not defined | PVC | 20 mm × 20 mm | Decrease (−14.9 up to −59.0%) |
| Not defined | Silt loam | PS | 69.5 ± 0.5 nm | Increase until day 14 Decrease at day 28 | |
| N-(leucineaminopeptidase) cycle [164] | Not defined | Silt loam | PS | 69.5 ± 0.5 nm | Decrease |
| C-(β-glucosidase and cellobiohydrolase) cycle [163], [164] | Not defined | Silt loam | PS | 69.5 ± 0.5 nm | Decrease |
| Top loess soil, China | 18.4% clay 25.0% silt 55.9% sand |
PP | 20 mm × 20 mm | No consistent effect | |
| P-(alkaline-phosphatase) cycle [164] | Not defined | Silt loam | PS | 69.5 ± 0.5 nm | Decrease |
| Urease activity [163], [165] | Cinnamon soil, China | 35.7% clay 46.8% silt 17.5% sand |
PE | 2 mm × 2 mm | Increase (+175% up to +234%) |
| Top loess soil China | 18.4% clay 25.0% silt 55.9% sand |
PP | 20 mm × 20 mm | No consistent effect | |
| Catalase activity [165] | Cinnamon soil, China | 35.7% clay 46.8% silt sand 17.5% sand |
PE | 2 mm × 2 mm | Increase (+139% up to +149%) |
| Invertase activity [165] | Cinnamon soil, China | 35.7% clay 46.8% silt 17.5% sand |
PE | 2 mm × 2 mm | No consistent effect |
| Phosphatase activity [163] | Top loess soil China | 18.4% clay 25.0% silt 55.9% sand |
PP | 20 mm × 20 mm | Increase |
For FDA, a measure for total microbial activity, both positive and negative effects have been observed by the presence of plastic. In the presence of plastic mulch residues, FDA in soil decreases [162], whereas relatively high concentrations of PP (7% to 28% w:w) in Loess soil increases FDA activity [123]. A significant relation between MP concentration (0.05% to 2%) and FDAse activity was observed for various polymer types including polyamide beads, PE fragments, polyactic acid (PLA) and polyester fibers [126]. Likewise, phosphatase activity increases in the presence of MPs (7% to 28% w:w), whereas it decreases in the presence of low amounts of NPs (10 to 1000 µg per kg soil) [163], [164]. Both the incorporation of NPs (10 to 1000 µg per kg soil) and MPs (7% to 28%) have shown to decrease the dehydrogenase activity [162], [164] and temporarily (less than 60 days) enhance the soil microbial respiration [163], [164]. In addition, decreases in most enzyme activities involved in C (β-glucosidase and cellobiohydrolase), N (leucine-aminopeptidase) and P (alkaline phosphatase) cycles have been noted 28 days after exposure of low concentrations (0.1–1 mg per kg dry matter of soil) of NPs [164]. Also LDPE (2 mm × 2 mm fragments, 0.076 g per kg soil) altered the soil microbial activity, with increases of urease and catalase activities, and no effect on soil invertase activity [165].
It can be suggested that the altered microbial activities may reflect an altered microbial community composition or diversity, which eventually will impact the soil food web (Fig. 3). An initial study using PCR-denaturing gradient gel electrophoresis showed that the incorporation of a PBAT film in soil resulted in enrichments of the fungal phylum Ascomycota after seven months of exposure, whereas the bacterial community stayed relatively stable [166]. More recent studies using rRNA gene metabarcoding did show differences in bacterial and fungal community composition upon MP addition, however [127], [135]. The incorporation of PE led to a significantly higher abundance of OTUs assigned to the orders Flavobacteriales and Longimicrobiales and a family belonging to the Betaproteobacteria. In addition, PES treatment significantly enhanced the colonization of spring onion roots by soil bacteria and fungi and increased the abundance of AMF hyphae [127].
Several studies indicated a decrease in soil microbial biodiversity or biomass when MPs or NPs were present [135], [162], [164], [166] (Fig. 3). In contrast, it has also been shown by serial dilution that the biennial plastic mulching can significantly increase the population of the soil microbial physiological groups [167]. Null effects of MP incorporation (PE, 2 mm × 2 mm fragments, 0.076 g per kg soil) on the microbial biodiversity and richness have also been shown after 90 days of exposure [165]. The observed differences in effect are probably related to the size of the plastic: Ren et al. (2020) showed that larger MPs decreased the fungal richness and diversity, whereas smaller MPs appeared to increase richness and diversity of both bacterial and fungal communities [135]. The mechanism behind these effects is so far unknown, however could be related to the relation of soil particle size fractions and microbial diversity: small size fractions generally yield higher microbial diversity [168].
Whereas the mechanisms of MPs on the changes of the soil microbial activities and community composition have not been elucidated so far, we can hypothesize the following (Fig. 3).
First, the small size and high surface area-to-volume ratio of MPs and NPs allow it to closely interact with the microbial cell and sub-cellular structure, which might lead to antimicrobial activities and an overall decrease in enzyme activities (FDA, phosphatase, dehydrogenase and C, N and P cycles) [169]. Second, in relation to the nematode community, the presence of toxic compounds on MPs could have contributed to a decline in microbial activity and diversity [170]. Recently, it has been shown that the changes induced in N cycling by MP addition (polyvinylchloride (PVC)) was mainly a function of the phthalate residing on the plastic, whereas the pure PVC was relatively inert under the studied conditions [171]. This is an important message that special attention should be given to chemical additives on the plastic surface in terms of toxicity. In aquatic environments it has already been shown that (micro)plastics can serve as a vector of chemical contaminants [172]. This raises the question whether a similar process occurs in soil ecosystems. For example, pesticides and mineral fertilizers residing in the soil, might attach to plastic particles, resulting in local, high concentrations of the chemical substances. Pesticides can kill or inhibit soil microorganisms as well as pests and pathogens, resulting in a lower microbial diversity, biomass and/or soil respiration [173]. It was observed that the number of viable Rhizobium sp. decreased after fungicide treatment [174] and a reduction in N-fixation was noted after application of herbicides [175].
Third, the presence of MPs can change the soil bulk density and porosity [126], [127], [128], [129], [130]. The change in soil porosity can result and explain the increase of the soil microbial respiration. Furthermore, we hypothesize that the observed decrease in soil bulk density by MP pollution can eventually lead to lower soil compaction as described in the introductory section of this review. An increased soil aggregate stability and therefore an increase of pore spaces in which the microbial community can move is to be expected [176]. This increase in microbial habitat, will result in the increase in microbial activity and degradation of organic matter, releasing more plant- and microbial-available nutrients and thus potentially leads to an increase in microbial biomass, which should be the focus of future studies.
Based on the observed effects on soil chemistry and plant performance, we predict that the addition of MP particles can also lead to an increase in the C:N ratio, a higher fungal biomass in the soil, and a change in the rhizosphere community of the plant.
Plastic particles have a very high C content [177]. It has already been suggested that plastic material can be degraded (slowly) in soil ecosystems, and therefore might interfere with the C:N ratio. This might lead to microbial immobilization [124], but might also shift the microbial community towards a higher abundance of plastic-degrading microorganisms.
In addition, it can be expected that the fungal biomass will increase for three main reasons. First, for tillage it has been shown that less disturbed soil with higher C:N ratios contain a higher fungal biomass (see introduction). Second, plastic interfere the soil moisture fluctuations, potentially influencing the composition of the microbial community [88], [132]. With the observed increase in soil water availability and evaporation, we expect higher abundances of drought-tolerant organisms, such as fungi, but potentially also sporulating bacteria, in plastic polluted soils. Third, an elevated soil pH is noted in the presence of tire particles. As described earlier, bacteria are sensitive to pH fluctuations and thus the bacterial community is expected to change drastically. Fungi will be less affected by this change. However, as most fungi favor slightly acidic environments, an increase in pH might lead to a decrease in fungal biomass. The elevated pH will also indirectly disturb plant and microbial growth. Increases in pH to levels above 7 will lead to a drastic decrease in the bioavailability of many essential metal ions. For example, it has been shown that the solubility of Cd is reduced 8.8-fold by an increase in pH from 6 to 7 [178].
Furthermore, the observed change in root structure by the presence of MPs will lead to other microorganisms being attracted to the plant’s rhizosphere (see introduction), either directly through the change of the root architecture [25] or by the release of root exudates [26], [27], which will attract mycorrhizae, N-fixers or PGPR (Fig. 3). It is unclear whether this will have a negative result for the plant.
Last, in aquatic environments, it has been shown that especially smaller organisms such as microalgae, Bryozoa, insects, macrobenthos, bacteria and fungi might benefit from the presence of plastic particles, as they can use them as a water vehicle to travel to new foreign habitats, among others [179], [180], [181], [182], [183]. In comparison to soil ecosystems, aquatic environments contain however a low amount of particles. Nonetheless, plastic might still play a role as transport vehicle as well in soil ecosystems, as other organisms might be attracted towards the plastic compared to the soil particles [165]. This may enable these microorganisms to travel into deeper soil layers or even into groundwater. This is hard to verify for microbes; the first step will be to determine whether plastic carries any of these organisms.
Most of this information is still preliminary; more research regarding the effects of MPs on the soil–plant-microbiome interplay is needed. The results described in this review give a first impression on how the soil–plant-microbiome interactions can be interrupted. Results are often contradictory, however. We suggest that this can be attributed to: (1) the lack of standardized methods, and (2) the focus on macro- and larger MPs in soil systems. The first concern is that different polymer types, soils and plants are used, as well as different concentrations and sizes of MPs. Several publications have shown that at least the type of polymer, particle size, shape and probably concentration will result in different effects on the soil microbiome and in relation the soil physicochemistry and plant growth. In addition, soil type might play a role. For instance, Wan et al. (2019) showed that the reduction of soil water evaporation by plastic addition is not only related to smaller plastic sizes, but was much more pronounced if the soil matrix consisted of clay minerals compared to silt or sand [131]. The lack of information on MP contamination in soil makes it, however, difficult to set up good standardized experiments. In-depth studies are urgently needed throughout all different soil ecosystems to determine target concentrations of MPs. In addition, these target concentrations of MPs should be based not only on the current, but presumably higher future levels of contamination as well [184]. Second, most studies still focus on bigger (micro)plastics as these are “easier” to use in studies. We expect major consequences for the tiniest fractions (NPs and MPs) however for three main reasons: (1), it has been shown that these can change the soil structure and the soil permeability; (2) these can be ingested by larger soil biota such as earthworms and nematodes, thereby affecting the soil food web and soil compaction; (3) they can be taken up by the plant [185], [186], potentially harming plant development.
4. The plastisphere: a microbial environment on its own
The presence of microorganisms on plastic was reported for the first time in a marine environment. In the early 1970s, Carpenter and Smith reported the presence of diatoms, bacteria and hydroids on the surfaces of plastic debris in the Sargasso Sea [99], [100]. By using Scanning Electron Microscopy, Sieburth (1975) was able to make images of pennate diatoms, filamentous cyanobacteria, coccoid bacteria and bryozoans on HDPE plastic bottles [187]. These were the first glimpses of what we now refer to as the “plastisphere” [188], a name originally assigned to the diverse assemblage of taxa that inhabit the thin layer of life on the outer surface of plastic debris, but which now more broadly refers to life on the surface of plastic litter in general.
Previous studies in aquatic environments have shown that microbial biofilms on MP surfaces are formed within one to two weeks and that the plastisphere selects for particular microbial communities that are distinct from those of the surrounding environment, e.g. water and sediment [179], [189], [190], [191], [192] and other inert surfaces, e.g., glass [192]. Data on the plastisphere microbiome in terrestrial ecosystems are scarce. In relation to aquatic environments, the microbiome of MPs contains a significantly smaller number of bacteria and fungi and microbial diversity compared to its environment, the bulk soil [165], [193]. Whereas the complex microbial assemblage of the plastisphere differs significantly from those of the bulk soil [193], [194], [195], this effect seems to diminish over time. Puglisi et al. (2019) showed that the microbial communities on more degraded plastics present for a longer time in landfills become more similar to the surrounding soil [196]. Analysis of the bacterial communities indicated that MPs might serve as a “special microbial accumulator” in farmland soil, enriching taxa that might degrade the plastic, such as Pseudomonas sp., Streptomyces sp. and Leptothrix sp. [194]. These effects are influenced by the type of soil [193], but also the polymer type [193], [196] used in the study. In addition, it has been hypothesized that biodegradable plastics like PLA show larger changes in microbial community structure in the plastisphere compared to non-degradable fragments like PE [193]. We believe this is related to higher metabolic activity (due to biodegradation), which will release metabolites and attract other species towards the plastisphere community. Recently, it has been shown that the presence of biodegradable polyhydroxyalkanoates did not only alter the soil microbial community, but increase the activity of the microbial biomass in the plastisphere with higher activities of β-glucosidase and leucine aminopeptidase and lower enzyme affinity. The authors claimed that this was the driving factor of higher C and nutrient turnover in the soil [197].
These first studies reveal results similar to the studies that show how plastic serves as a new microbial habitat [165], [193], [194], [195], [196]. It remains unknown whether this community will differ between environments, will influence the surrounding environment, or will interfere with soil–plant-microbiome interactions; these subjects should be the focus of future studies.
5. Summary and future perspectives
In this review we have shown the importance of the microbial community in the soil ecosystem. Microbes are largely responsible for the soil physicochemistry dynamics, as they mediate C sequestration and play a role in the formation of the soil structure. In addition, the growth and defense response of plants is influenced by the microbial community as well, as microbes play a dominant role in nutrient cycling and can be actively attracted to the plant root. Human interference is the main reason the interaction of soil microbial communities, soil processes and plant development are disrupted. Soil research has primarily focused on agricultural processes such as tillage, compaction, monoculture and the addition of soil amendments, fertilizers and pesticides. Human-caused pollution, of which plastic is the most common and well-known, will disrupt the microbial community as well. We indicated that the decrease in soil bulk density, the fluctuations in soil moisture content and the change of the soil pH might form the basis of the decreased microbial activity and microbial biomass and the increase in soil microbial respiration by the presence of MP particles. In addition, we addressed that the presence of chemical additives compounds (e.g. pesticides and mineral fertilizers) on the surface of plastic particles might be even more important to explain the toxic effects of MP particles on the soil microbiome. Furthermore we present the hypothesis that MP incorporation will lead to an increase in fungal biomass by the decrease of the soil pH and C:N ratio, a larger abundance of plastic degraders, and a change in the rhizosphere microbiome due to the changed root architecture of MP-exposed plants. Due to the importance of microorganisms in soil ecosystem functioning, we therefore urge the research community to focus on terrestrial and not exclusively marine MP pollution, and to focus on the effect of MP pollution on the microbial community in future experiments. In addition, we urge the research community to develop standardized methods to study the smaller plastics (MP and NP) environmentally realistic MP concentrations, as the concentration of these small plastic particles will strongly influence the observed effects.
CRediT authorship contribution statement
L. Joos: Conceptualization, Writing – original draft. C. De Tender: Conceptualization, Supervision, Writing – review & editing.
Acknowledgments
Acknowledgements
We thank Bart Vandecasteele, Jane Debode and Adriaan Vanderhasselt for their insights regarding soil ecosystem functioning and the microbial community, Sofie Vandendriessche for her expertise in plastic pollution, and Miriam Levenson for English-language editing. Caroline De Tender received a grant of the Research Foundation Flanders (FWO) with application number 12S9418N. This research was supported by SoilCom, an Interreg project funded by the North Sea Region programme of the European Regional Development Fund of the European Union with project number 38-2-25-18. We acknowledge Flanders Research Institue of Agriculture, Fisheries and Food (ILVO) for the strategic fellowship granted to Lisa Joos.
Declarations of interest
None.
References
- 1.Orgiazzi A, Bardgett RD, Barrios E, Behan-Pelletier V, Briones MJI, Chotte J-L, et al. Global soil biodiversity atlas. European Commission; 2016. https://doi.org/10.2788/799182.
- 2.Philippot L., Raaijmakers J.M., Lemanceau P., van der Putten W.H. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 3.Bar-On Y.M., Phillips R., Milo R. The biomass distribution on Earth. Proc Natl Acad Sci. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nannipieri P., Ascher J., Ceccherini M.T., Landi L., Pietramellara G., Renella G. Microbial diversity and soil functions. Eur J Soil Sci. 2003;54:655–670. doi: 10.1046/j.1365-2389.2003.00556.x. [DOI] [Google Scholar]
- 5.Kibblewhite M.G., Ritz K., Swift M.J. Soil health in agricultural systems. Philos Trans R Soc B Biol Sci. 2008;363:685–701. doi: 10.1098/rstb.2007.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirschbaum M.U.F. Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry. 2000;48:21–51. doi: 10.1023/A:1006238902976. [DOI] [Google Scholar]
- 7.Lal R. Sequestration of atmospheric CO2 in global carbon pools. Energy Environ Sci. 2008;1:86–100. doi: 10.1039/b809492f. [DOI] [Google Scholar]
- 8.Lal R. Soil carbon sequestration to mitigate climate change. Geoderma. 2004;123:1–22. doi: 10.1016/j.geoderma.2004.01.032. [DOI] [Google Scholar]
- 9.Lal R. Global potential of soil carbon sequestration to mitigate the grechristophe verheyenenhouse effect. CRC Crit Rev Plant Sci. 2003;22:151–184. doi: 10.1080/713610854. [DOI] [Google Scholar]
- 10.Barrios E. Soil biota, ecosystem services and land productivity. Ecol Econ. 2007;64:269–285. doi: 10.1016/j.ecolecon.2007.03.004. [DOI] [Google Scholar]
- 11.Jouquet P., Dauber J., Lagerlöf J., Lavelle P., Lepage M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl Soil Ecol. 2006;32:153–164. doi: 10.1016/j.apsoil.2005.07.004. [DOI] [Google Scholar]
- 12.Scow K, Bardgett RD, Pennock D, Vargas Rojas R, Singh BK, Eisenhauer N, et al. State of knowledge of soil biodiversity: Status, challenges and potentialities 2020. https://doi.org/10.1016/b978-0-12-348536-6.50017-4.
- 13.Franche C., Lindström K., Elmerich C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil. 2009;321:35–59. doi: 10.1007/s11104-008-9833-8. [DOI] [Google Scholar]
- 14.Leininger S., Urich T., Schloter M., Schwark L., Qi J., Nicol G.W., et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 15.Bolan N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil. 1991;134:189–207. doi: 10.1007/BF00012037. [DOI] [Google Scholar]
- 16.Bronick C.J., Lal R. Soil structure and management: a review. Geoderma. 2005;124:3–22. doi: 10.1016/j.geoderma.2004.03.005. [DOI] [Google Scholar]
- 17.Merino-Martín L., Stokes A., Gweon H.S., Moragues-Saitua L., Staunton S., Plassard C., et al. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol Biochem. 2021;154 doi: 10.1016/j.soilbio.2020.108072. [DOI] [Google Scholar]
- 18.Degens B.P. Macro-aggregation of soils by biological bonding and binding mechanisms and the factors affecting these: a review. Aust J Soil Res. 1997;35:431–459. doi: 10.1071/S96016. [DOI] [Google Scholar]
- 19.Edgerton D.L., Harris J.A., Birch P., Bullock P. Linear relationship between aggregate stability and microbial biomass in three restored soils. Soil Biol Biochem. 1995;27:1499–1501. doi: 10.1016/0038-0717(95)00076-Q. [DOI] [Google Scholar]
- 20.Gupta V.V.S.R., Germida J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol Biochem. 2015;80:A3–A9. doi: 10.1016/j.soilbio.2014.09.002. [DOI] [Google Scholar]
- 21.Lehmann A., Zheng W., Rillig M.C. Soil biota contributions to soil aggregation. Nat Ecol Evol. 2017;1:1828–1835. doi: 10.1038/s41559-017-0344-y.Soil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blouin M., Hodson M.E., Delgado E.A., Baker G., Brussaard L., Butt K.R., et al. A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci. 2013;64:161–182. doi: 10.1111/ejss.12025. [DOI] [Google Scholar]
- 23.Berendsen R.L., Pieterse C.M.J., Bakker P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Olanrewaju O.S., Ayangbenro A.S., Glick B.R., Babalola O.O. Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biotechnol. 2019;103:1155–1166. doi: 10.1007/s00253-018-9556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleem M., Law A.D., Sahib M.R., Pervaiz Z.H., Zhang Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere. 2018;6:47–51. doi: 10.1016/j.rhisph.2018.02.003. [DOI] [Google Scholar]
- 26.Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 27.Mendes R., Garbeva P., Raaijmakers J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 28.Ahkami A.H., Allen White R., Handakumbura P.P., Jansson C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere. 2017;3:233–243. doi: 10.1016/j.rhisph.2017.04.012. [DOI] [Google Scholar]
- 29.Vacheron J., Desbrosses G., Bouffaud M.L., Touraine B., Moënne-Loccoz Y., Muller D., et al. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lugtenberg B., Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Micr. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 31.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017 doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 32.Kivlin S.N., Winston G.C., Goulden M.L., Treseder K.K. Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol. 2014;12:14–25. doi: 10.1016/j.funeco.2014.04.004. [DOI] [Google Scholar]
- 33.Serna-Chavez H.M., Fierer N., Van Bodegom P.M. Global drivers and patterns of microbial abundance in soil. Glob Ecol Biogeogr. 2013;22:1162–1172. doi: 10.1111/geb.12070. [DOI] [Google Scholar]
- 34.Tedersoo L., Bahram M., Põlme S., Kõljalg U., Yorou N.S., Wijesundera R., et al. Global diversity and geography of soil fungi. Science. 2014;346 doi: 10.1126/science.aaa1185. [DOI] [PubMed] [Google Scholar]
- 35.Naylor D., Coleman-Derr D. Drought stress and root-associated bacterial communities. Front Plant Sci. 2018;8:2223. doi: 10.3389/fpls.2017.02223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vries F.T., Griffiths R.I., Bailey M., Craig H., Girlanda M., Gweon H.S., et al. Soil bacterial networks are less stable under drought than fungal networks. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S., Li S., Avera B.N., Strahm B.D., Badgley B.D. Soil bacterial and fungal communities show distinct recovery patterns during forest ecosystem restoration. Appl Environ Microbiol. 2017;83:e00966–e1017. doi: 10.1128/AEM.00966-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erktan A., Or D., Scheu S. The physical structure of soil: determinant and consequence of trophic interactions. Soil Biol Biochem. 2020;148 doi: 10.1016/j.soilbio.2020.107876. [DOI] [Google Scholar]
- 39.Scheu S. The soil food web: structure and perspectives. Eur J Soil Biol. 2002;38:11–20. doi: 10.1016/S1164-5563(01)01117-7. [DOI] [Google Scholar]
- 40.Handa I.T., Aerts R., Berendse F., Berg M.P., Bruder A., Butenschoen O., et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature. 2014;509:218–221. doi: 10.1038/nature13247. [DOI] [PubMed] [Google Scholar]
- 41.Hättenschwiler S., Tiunov A.V., Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst. 2005;36:191–218. doi: 10.1146/annurev.ecolsys.36.112904.151932. [DOI] [Google Scholar]
- 42.Morriën E., Hannula S.E., Snoek L.B., Helmsing N.R., Zweers H., De Hollander M., et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat Commun. 2017;8:1–10. doi: 10.1038/ncomms14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vries F.T., Thébault E., Liiri M., Birkhofer K., Tsiafouli M.A., Bjørnlund L., et al. Soil food web properties explain ecosystem services across European land use systems. Proc Natl Acad Sci. 2013;110:14296–14301. doi: 10.1073/pnas.1305198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahram M., Hildebrand F., Forslund S.K., Anderson J.L., Soudzilovskaia N.A., Bodegom P.M., et al. Structure and function of the global topsoil microbiome. Nature. 2018;560:233–237. doi: 10.1038/s41586-018-0386-6. [DOI] [PubMed] [Google Scholar]
- 45.Crowther T.W., van den Hoogen J., Wan J., Mayes M.A., Keiser A.D., Mo L., et al. The global soil community and its influence on biogeochemistry. Science. 2019;365 doi: 10.1126/science.aav0550. [DOI] [PubMed] [Google Scholar]
- 46.Delgado-baquerizo M., Oliverio A.M., Brewer T.E., Benavent-gonzález A., Eldridge D.J., Bardgett R.D., et al. A global atlas of the dominant bacteria found in soil. Science. 2018;359:320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- 47.Fierer N., Jackson R.B. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson L.R., Sanders J.G., McDonald D., Amir A., Ladau J., Locey K.J., et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozupone C.A., Knight R. Global patterns in bacterial diversity. Proc Natl Acad Sci. 2007;104:11436–11440. doi: 10.1073/pnas.0611525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Gruyter J., Weedon J.T., Bazot S., Dauwe S., Fernandez-Garberí P.-R., Geisen S., et al. Patterns of local, intercontinental and interseasonal variation of soil bacterial and eukaryotic microbial communities. FEMS Microbiol Ecol. 2020;96 doi: 10.1093/femsec/fiaa018. [DOI] [PubMed] [Google Scholar]
- 51.Davison J., Moora M., Öpik M., Adholeya A., Ainsaar L., Bâ A., et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science. 2015;349:970–973. doi: 10.1126/science.aab1161. [DOI] [PubMed] [Google Scholar]
- 52.Phillips H.R.P., Guerra C.A., Bartz M.L.C., Briones M.J.I., Brown G., Ferlian O., et al. Global distribution of earthworm diversity. Science. 2019;366:480–485. doi: 10.1101/587394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Hoogen J., Geisen S., Routh D., Ferris H., Traunspurger W., Wardle D.A., et al. Soil nematode abundance and functional group composition at a global scale. Nature. 2019;572:194–198. doi: 10.1038/s41586-019-1418-6. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen U.N., Ayres E., Wall D.H., Li G., Bardgett R.D., Wu T., et al. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob Ecol Biogeogr. 2014;23:968–978. doi: 10.1111/geb.12177. [DOI] [Google Scholar]
- 55.Song D., Pan K., Tariq A., Sun F., Li Z., Sun X., et al. Large-scale patterns of distribution and diversity of terrestrial nematodes. Appl Soil Ecol. 2017;114:161–169. doi: 10.1016/j.apsoil.2017.02.013. [DOI] [Google Scholar]
- 56.Wu T., Ayres E., Bardgett R.D., Wall D.H., Garey J.R. Molecular study of worldwide distribution and diversity of soil animals. Proc Natl Acad Sci. 2011;108:17720–17725. doi: 10.1073/pnas.1103824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates S.T., Clemente J.C., Flores G.E., Walters W.A., Parfrey L.W., Knight R., et al. Global biogeography of highly diverse protistan communities in soil. ISME J. 2013;7:652–659. doi: 10.1038/ismej.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliverio A.M., Geisen S., Delgado-Baquerizo M., Maestre F.T., Turner B.L., Fierer N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fierer N., Strickland M.S., Liptzin D., Bradford M.A., Cleveland C.C. Global patterns in belowground communities. Ecol Lett. 2009;12:1238–1249. doi: 10.1111/j.1461-0248.2009.01360.x. [DOI] [PubMed] [Google Scholar]
- 60.Xu X., Thornton P.E., Post W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr. 2013;22:737–749. doi: 10.1111/geb.12029. [DOI] [Google Scholar]
- 61.Jurburg S.D., Salles J.F. In: Biodiversity in ecosystems - Linking structure and function. Blanco J.A., Lo Y.-H., Roy S., editors. Intech; Rijeka: 2015. Functional redundancy and ecosystem function - the soil microbiota as a case study; pp. 29–42. [Google Scholar]
- 62.Kopittke P.M., Menzies N.W., Wang P., McKenna B.A., Lombi E. Soil and the intensification of agriculture for global food security. Environ Int. 2019;132 doi: 10.1016/j.envint.2019.105078. [DOI] [PubMed] [Google Scholar]
- 63.Prasad M.N.V., Pietrzykowski M. Elsevier; 2020. Climate change and soil interactions. https://doi.org/https://doi.org/10.1016/C2018-0-03008-X. [Google Scholar]
- 64.Tsiafouli M.A., Thébault E., Sgardelis S.P., de Ruiter P.C., van der Putten W.H., Birkhofer K., et al. Intensive agriculture reduces soil biodiversity across Europe. Glob Chang Biol. 2015;21:973–985. doi: 10.1111/gcb.12752. [DOI] [PubMed] [Google Scholar]
- 65.Cadotte M.W., Dinnage R., Tilman D. Phylogenetic diversity promotes ecosystem stability. Ecology. 2012;93:S223–S233. doi: 10.1890/11-0426.1. [DOI] [Google Scholar]
- 66.Foley J.A., Ramankutty N., Brauman K.A., Cassidy E.S., Gerber J.S., Johnston M., et al. Solutions for a cultivated planet. Nature. 2011;478:337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 67.Loreau M. Linking biodiversity and ecosystems: towards a unifying ecological theory. Philos Trans R Soc B Biol Sci. 2010;365:49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBratney A., Field D.J., Koch A. The dimensions of soil security. Geoderma. 2014;213:203–213. doi: 10.1016/j.geoderma.2013.08.013. [DOI] [Google Scholar]
- 69.Wagg C., Bender S.F., Widmer F., van der Heijden M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci. 2014;111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kladivko E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001;61:61–76. doi: 10.1016/S0167-1987(01)00179-9. [DOI] [Google Scholar]
- 71.Holland J.M. The environmental consequences of adopting conservation tillage in Europe: reviewing the evidence. Agric Ecosyst Environ. 2004;103:1–25. doi: 10.1016/j.agee.2003.12.018. [DOI] [Google Scholar]
- 72.Govaerts B., Mezzalama M., Sayre K.D., Crossa J., Nicol J.M., Deckers J. Long-term consequences of tillage, residue management, and crop rotation on maize/wheat root rot and nematode populations in subtropical highlands. Appl Soil Ecol. 2006;32:305–315. doi: 10.1016/j.apsoil.2005.07.010. [DOI] [Google Scholar]
- 73.Kuntz M., Berner A., Gattinger A., Scholberg J.M., Mäder P., Pfiffner L. Influence of reduced tillage on earthworm and microbial communities under organic arable farming. Pedobiologia. 2013;56:251–260. doi: 10.1016/j.pedobi.2013.08.005. [DOI] [Google Scholar]
- 74.Briar S.S., Grewal P.S., Somasekhar N., Stinner D., Miller S.A. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Appl Soil Ecol. 2007;37:256–266. doi: 10.1016/j.apsoil.2007.08.004. [DOI] [Google Scholar]
- 75.Fu S., Coleman D.C., Hendrix P.F., Crossley D.A. Responses of trophic groups of soil nematodes to residue application under conventional tillage and no-till regimes. Soil Biol Biochem. 2000;32:1731–1741. doi: 10.1016/S0038-0717(00)00091-2. [DOI] [Google Scholar]
- 76.Zhang S., Li Q., Lü Y., Sun X., Jia S., Zhang X. Conservation tillage positively influences the micro flora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015;149:46–52. doi: 10.1016/j.still.2015.01.001. [DOI] [Google Scholar]
- 77.Rønn R., Vestergård M., Ekelund F. Interactions between bacteria, protozoa and nematodes in soil. Acta Protozool. 2012;51:223–235. doi: 10.4467/16890027AP.12.018.0764. [DOI] [Google Scholar]
- 78.Minoshima H., Jackson L.E., Cavagnaro T.R., Sánchez-Moreno S., Ferris H., Temple S.R., et al. Soil food webs and carbon dynamics in response to conservation tillage in California. Soil Sci Soc Am J. 2007;71:952–963. doi: 10.2136/sssaj2006.0174. [DOI] [Google Scholar]
- 79.van Capelle C., Schrader S., Brunotte J. Tillage-induced changes in the functional diversity of soil biota - a review with a focus on German data. Eur J Soil Biol. 2012;50:165–181. doi: 10.1016/j.ejsobi.2012.02.005. [DOI] [Google Scholar]
- 80.De Vries F.T., van Groenigen J.W., Hoffland E., Bloem J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol Biochem. 2011;43:997–1005. doi: 10.1016/j.soilbio.2011.01.016. [DOI] [Google Scholar]
- 81.Six J., Frey S.D., Thiet R.K., Batten K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J. 2006;70:555–569. doi: 10.2136/sssaj2004.0347. [DOI] [Google Scholar]
- 82.Raghavan GSV, Alvo P, McKyes E. Soil compaction in agriculture: a view toward managing the problem. Springer, New York, NY; 1990. https://doi.org/10.1007/978-1-4612-3322-0_1.
- 83.Jung K.Y., Kitchen N.R., Sudduth K.A., Lee K.S., Chung S.O. Soil compaction varies by crop management system over a claypan soil landscape. Soil Tillage Res. 2010;107:1–10. doi: 10.1016/j.still.2009.12.007. [DOI] [Google Scholar]
- 84.Colombi T., Torres L.C., Walter A., Keller T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth – a vicious circle. Sci Total Environ. 2018;626:1026–1035. doi: 10.1016/j.scitotenv.2018.01.129. [DOI] [PubMed] [Google Scholar]
- 85.Pupin B., Freddi O.da.S., Nahas E. Microbial alterations of the soil influenced by induced compaction. Rev Bras Cienc Do Solo. 2009;33:1207–1213. doi: 10.1590/s0100-06832009000500014. [DOI] [Google Scholar]
- 86.Smeltzer D.L.K., Bergdahl D.R., Donnelly J.R. Forest ecosystem responses to artificially induced soil compaction. II. Selected soil microorganism populations. Can J For Res. 1986;16:870–872. doi: 10.1139/x86-154. [DOI] [Google Scholar]
- 87.Schimel J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst. 2018;49:409–432. doi: 10.1146/annurev-ecolsys-110617-062614. [DOI] [Google Scholar]
- 88.Barnard R.L., Osborne C.A., Firestone M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013;7:2229–2241. doi: 10.1038/ismej.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alpert P. The limits and frontiers of desiccation-tolerant life. Integr Comput Biol. 2005;45:685–695. doi: 10.1093/icb/45.5.685. [DOI] [PubMed] [Google Scholar]
- 90.Møbjerg N., Halberg K.A., Jørgensen A., Persson D., Bjørn M., Ramløv H., et al. Survival in extreme environments - on the current knowledge of adaptations in tardigrades. Acta Physiol. 2011;202:409–420. doi: 10.1111/j.1748-1716.2011.02252.x. [DOI] [PubMed] [Google Scholar]
- 91.Pendall E., Bridgham S., Hanson P.J., Hungate B., Kicklighter D.W., Johnson D.W., et al. Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phytol. 2004;162:311–322. doi: 10.1111/j.1469-8137.2004.01053.x. [DOI] [Google Scholar]
- 92.Lai T.V., Farquharson R., Denton M.D. High soil temperatures alter the rates of nitrification, denitrification and associated N2O emissions. J Soils Sediments. 2019;19:2176–2189. doi: 10.1007/s11368-018-02238-7. [DOI] [Google Scholar]
- 93.Cox P.M., Betts R.A., Jones C.D., Spall S.A., Totterdell I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408:184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 94.Beirinckx S., Viaene T., Haegeman A., Debode J., Amery F., Vandenabeele S., et al. Tapping into the maize root microbiome to identify bacteria that promote growth under chilling conditions. Microbiome. 2020;8:1–13. doi: 10.1186/s40168-020-00833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.PlasticEurope. Plastics - the facts 2014/2015: an analysis of European plastics production, demand and waste data. 2015. https://doi.org/10.1016/j.marpolbul.2013.01.015.
- 96.Derraik J.G.B. The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull. 2002;44:842–852. doi: 10.1016/S0025-326X(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 97.Tod H. The basics on 7 common types of plastic 2021. https://plasticoceans.org/7-types-of-plastic/.
- 98.Chiba S., Saito H., Fletcher R., Yogi T., Kayo M., Miyagi S., et al. Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar Policy. 2018;96:204–212. doi: 10.1016/j.marpol.2018.03.022. [DOI] [Google Scholar]
- 99.Carpenter E.J., Anderson S.J., Harvey G.R., Miklas H.P., Peck B.B. Polystyrene spherules in coastal waters. Science. 1972;178:749–750. doi: 10.1126/science.178.4062.749. [DOI] [PubMed] [Google Scholar]
- 100.Carpenter E.J., Smith K.L. Plastics on the Sargasso Sea surface. Science. 1972;175:1240–1241. doi: 10.1126/science.175.4027.1240. [DOI] [PubMed] [Google Scholar]
- 101.Thompson RC, Swan SH, Moore CJ, Vom Saal FS. Our plastic age 2009. https://doi.org/10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed]
- 102.Gregory M.R. Accumulation and distribution of virgin plastic granules on New Zealand beaches. New Zeal J Mar Freshw Res. 1978;12:399–414. doi: 10.1080/00288330.1978.9515768. [DOI] [Google Scholar]
- 103.Eriksen M., Lebreton L.C.M., Carson H.S., Thiel M., Moore C.J., Borerro J.C., et al. Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 105.Nizzetto L., Futter M., Langaas S. Are agricultural soils dumps for microplastics of urban origin? Environ Sci Technol. 2016;50:10777–10779. doi: 10.1021/acs.est.6b04140. [DOI] [PubMed] [Google Scholar]
- 106.Fuller S., Gautam A. A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol. 2016;50:5774–5780. doi: 10.1021/acs.est.6b00816. [DOI] [PubMed] [Google Scholar]
- 107.Bläsing M., Amelung W. Plastics in soil: analytical methods and possible sources. Sci Total Environ. 2018;612:422–435. doi: 10.1016/j.scitotenv.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 108.Rillig M.C. Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol. 2012;46:6453–6454. doi: 10.1021/es302011r. [DOI] [PubMed] [Google Scholar]
- 109.Huerta E.L., Gertsen H., Gooren H., Peters P., Salánki T., Van Der Ploeg M., et al. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae) Environ Sci Technol. 2016;50:2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- 110.Meixner K., Kubiczek M., Fritz I. Microplastic in soil–current status in Europe with special focus on method tests with Austrian samples. AIMS Environ Sci. 2020;7:174–191. doi: 10.3934/environsci.2020011. [DOI] [Google Scholar]
- 111.Büks F., Kaupenjohann M. Global concentrations of microplastics in soils - a review. Soil. 2020;6:649–662. doi: 10.5194/soil-6-649-2020. [DOI] [Google Scholar]
- 112.de Souza Machado A.A., Kloas W., Zarfl C., Hempel S., Rillig M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol. 2018;24:1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vollertsen J., Hansen A.A. The Danish Environmental Protection Agency; 2017. Microplastic in Danish wastewater: sources, occurrences and fate. [Google Scholar]
- 114.Huang Y., Liu Q., Jia W., Yan C., Wang J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ Pollut. 2020;260 doi: 10.1016/j.envpol.2020.114096. [DOI] [PubMed] [Google Scholar]
- 115.van den Berg P., Huerta E.L., Corradini F., Geissen V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114198. [DOI] [PubMed] [Google Scholar]
- 116.Chae Y., An Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: a review. Environ Pollut. 2018;240:387–395. doi: 10.1016/j.envpol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Gionfra S. Plastic pollution in soil. 2018.
- 118.Ljung E, Olesen KB, Andersson P-G, Fälström E, Vollertsen J, Wittgren HB, et al. Microplastics in the water and nutrient-cycle (Mikroplaster i kretsloppet). 2018.
- 119.Scheurer M., Bigalke M. Microplastics in Swiss floodplain soils. Environ Sci Technol. 2018;52:3591–3598. doi: 10.1021/acs.est.7b06003. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G.S., Liu Y.F. The distribution of microplastics in soil aggregate fractions in Southwestern China. Sci Total Environ. 2018;642:12–20. doi: 10.1016/j.scitotenv.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Piehl S., Leibner A., Löder M.G.J., Dris R., Bogner C., Laforsch C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-36172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S.W., Rillig M.C. Research trends of microplastics in the soil environment: comprehensive screening of effects. Soil Ecol Lett. 2021 doi: 10.1007/s42832-021-0077-3. [DOI] [Google Scholar]
- 123.Liu H., Yang X., Liu G., Liang C., Xue S., Chen H., et al. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017;185:907–917. doi: 10.1016/j.chemosphere.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 124.Rillig M.C., Lehmann A., de Souza Machado A.A., Yang G. Microplastic effects on plants. New Phytol. 2019;223:1066–1070. doi: 10.1111/nph.15794. [DOI] [PubMed] [Google Scholar]
- 125.Rillig M.C. Plastic and plants. Nat Sustain. 2020;3:887–888. doi: 10.1038/s41893-020-0583-9. [DOI] [Google Scholar]
- 126.de Souza Machado A.A., Lau C.W., Till J., Kloas W., Lehmann A., Becker R., et al. Impacts of microplastics on the soil biophysical environment. Environ Sci Technol. 2018;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Souza Machado A.A., Lau C.W., Kloas W., Bergmann J., Bachelier J.B., Faltin E., et al. Microplastics can change soil properties and affect plant performance. Environ Sci Technol. 2019;53:6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- 128.Lehmann A., Fitschen K., Rillig M.C. Abiotic and biotic factors influencing the effect of microplastic on soil aggregation. Soil Syst. 2019;3:21. doi: 10.3390/soilsystems3010021. [DOI] [Google Scholar]
- 129.Lozano Y.M., Lehnert T., Linck L.T., Lehmann A., Rillig M.C. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front Plant Sci. 2021;12:169. doi: 10.3389/fpls.2021.616645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehmann A., Leifheit E.F., Gerdawischke M., Rillig M.C. Microplastics have shape- and polymer-dependent effects on soil aggregation and organic matter loss – an experimental and meta-analytical approach. Microplastics Nanoplastics. 2021;1:1–14. doi: 10.1186/s43591-021-00007-x. [DOI] [Google Scholar]
- 131.Wan Y., Wu C., Xue Q., Hui X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci Total Environ. 2019;654:576–582. doi: 10.1016/j.scitotenv.2018.11.123. [DOI] [PubMed] [Google Scholar]
- 132.Joos L, De Tender C, Holderbeke A, Clement L, Vandecasteele B, Debode J. The soil microbiome after drought-rewetting and chitin disturbances: the magnitude of the change matters! Under Rev 2021.
- 133.Lipiec J., Doussan C., Nosalewicz A., Kondracka K. Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophysics. 2013;27:463–477. doi: 10.2478/intag-2013-0017. [DOI] [Google Scholar]
- 134.Smolders E., Degryse F. Fate and effect of zinc from tire debris in soil. Environ Sci Technol. 2002;36:3706–3710. doi: 10.1021/es025567p. [DOI] [PubMed] [Google Scholar]
- 135.Ren X., Tang J., Liu X., Liu Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113347. [DOI] [PubMed] [Google Scholar]
- 136.Moreno-Jiménez E, Leifheit EF, Plaza C, Feng L, Bergmann J, Wulf A, et al. Effects of microplastics on crop nutrition in fertile soils and interaction with arbuscular mycorrhizal fungi. J Sustain Agric Environ 2021:1–7. https://doi.org/10.1002/sae2.12006.
- 137.Qi Y., Yang X., Pelaez A.M., Huerta Lwanga E., Beriot N., Gertsen H., et al. Macro- and micro- plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci Total Environ. 2018;645:1048–1056. doi: 10.1016/j.scitotenv.2018.07.229. [DOI] [PubMed] [Google Scholar]
- 138.Bosker T., Bouwman L.J., Brun N.R., Behrens P., Vijver M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere. 2019;226:774–781. doi: 10.1016/j.chemosphere.2019.03.163. [DOI] [PubMed] [Google Scholar]
- 139.Oliveri Conti G, Ferrante M, Banni M, Favara C, Nicolosi I, Cristaldi A, et al. Micro- and nano-plastics in edible fruit and vegetables. the first diet risks assessment for the general population. Environ Res 2020;187:109677. https://doi.org/10.1016/j.envres.2020.109677. [DOI] [PubMed]
- 140.Rillig M.C., Ziersch L., Hempel S. Microplastic transport in soil by earthworms. Sci Rep. 2017;7:1–6. doi: 10.1038/s41598-017-01594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prendergast-Miller M.T., Katsiamides A., Abbass M., Sturzenbaum S.R., Thorpe K.L., Hodson M.E. Polyester-derived microfibre impacts on the soil-dwelling earthworm Lumbricus terrestris. Environ Pollut. 2019;251:453–459. doi: 10.1016/j.envpol.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 142.Cao D., Wang X., Luo X., Liu G., Zheng H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf Ser Earth Environ Sci. 2017;61:12148. doi: 10.1088/1755-1315/61/1/012148. [DOI] [Google Scholar]
- 143.Wang J., Coffin S., Sun C., Schlenk D., Gan J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ Pollut. 2019;249:776–784. doi: 10.1016/j.envpol.2019.03.102. [DOI] [PubMed] [Google Scholar]
- 144.Chen Y., Liu X., Leng Y., Wang J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol Environ Saf. 2020;187 doi: 10.1016/j.ecoenv.2019.109788. [DOI] [PubMed] [Google Scholar]
- 145.Rodríguez-Seijo A., da Costa J.P., Rocha-Santos T., Duarte A.C., Pereira R. Oxidative stress, energy metabolism and molecular responses of earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics. Environ Sci Pollut Res Int. 2018;25:33599–33610. doi: 10.1007/s11356-018-3317-z. [DOI] [PubMed] [Google Scholar]
- 146.Kwak J Il, An Y-J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J Hazard Mater 2021;402:124034. https://doi.org/10.1016/j.jhazmat.2020.124034. [DOI] [PubMed]
- 147.Rodriguez-Seijo A., Lourenço J., Rocha-Santos T.A.P., da Costa J., Duarte A.C., Vala H., et al. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ Pollut. 2017;220:495–503. doi: 10.1016/j.envpol.2016.09.092. [DOI] [PubMed] [Google Scholar]
- 148.Huerta E.L., Thapa B., Yang X., Gertsen H., Salánki T., Geissen V., et al. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci Total Environ. 2018;624:753–757. doi: 10.1016/j.scitotenv.2017.12.144. [DOI] [PubMed] [Google Scholar]
- 149.Büks F., van Schaik N., Kaupenjohann M. What do we know about how the terrestrial multicellular soil fauna reacts to microplastic? Soil. 2020;6:245–267. doi: 10.5194/soil-6-245-2020. [DOI] [Google Scholar]
- 150.Kim S.W., An Y.-J. Soil microplastics inhibit the movement of springtail species. Environ Int. 2019;126:699–706. doi: 10.1016/j.envint.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 151.Ju H., Zhu D., Qiao M. Effects of polyethylene microplastics on the gut microbial community, reproduction and avoidance behaviors of the soil springtail, Folsomia candida. Environ Pollut. 2019;247:890–897. doi: 10.1016/j.envpol.2019.01.097. [DOI] [PubMed] [Google Scholar]
- 152.Maaß S., Daphi D., Lehmann A., Rillig M.C. Transport of microplastics by two collembolan species. Environ Pollut. 2017;225:456–459. doi: 10.1016/j.envpol.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Kokalj A.J., Horvat P., Skalar T., Kržan A. Plastic bag and facial cleanser derived microplastic do not affect feeding behaviour and energy reserves of terrestrial isopods. Sci Total Environ. 2018;615:761–766. doi: 10.1016/j.scitotenv.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 154.Lei L., Wu S., Lu S., Liu M., Song Y., Fu Z., et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ. 2018;619–620:1–8. doi: 10.1016/j.scitotenv.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 155.Schöpfer L., Menzel R., Schnepf U., Ruess L., Marhan S., Brümmer F., et al. Microplastics effects on reproduction and body length of the soil-dwelling nematode Caenorhabditis elegans. Front Environ Sci. 2020;8:41. doi: 10.3389/fenvs.2020.00041. [DOI] [Google Scholar]
- 156.Mueller M-TT, Fueser H, Höss S, Traunspurger W. Species-specific effects of long-term microplastic exposure on the population growth of nematodes, with a focus on microplastic ingestion. Ecol Indic 2020;118:106698. https://doi.org/10.1016/j.ecolind.2020.106698.
- 157.Lei L., Liu M., Song Y., Lu S., Hu J., Cao C., et al. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ Sci Nano. 2018;5:2009–2020. doi: 10.1039/C8EN00412A. [DOI] [Google Scholar]
- 158.Kim S.W., Waldman W.R., Kim T.-Y., Rillig M.C. Effects of different microplastics on nematodes in the soil environment: Tracking the extractable additives using an ecotoxicological approach. Environ Sci Technol. 2020;54:13868–13878. doi: 10.1021/acs.est.0c04641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim S.W., Kim D., Jeong S.-W., An Y.-J. Size-dependent effects of polystyrene plastic particles on the nematode Caenorhabditis elegans as related to soil physicochemical properties. Environ Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113740. [DOI] [PubMed] [Google Scholar]
- 160.Zhao L., Qu M., Wong G., Wang D. Transgenerational toxicity of nanopolystyrene particles in the range of μg L−1 in the nematode Caenorhabditis elegans. Environ Sci Nano. 2017;4:2356–2366. doi: 10.1039/C7EN00707H. [DOI] [Google Scholar]
- 161.Boag B., Yeates G.W. Soil nematode biodiversity in terrestrial ecosystems. Biodivers Conserv. 1998;7:617–630. doi: 10.1023/A:1008852301349. [DOI] [Google Scholar]
- 162.Wang J., Lv S., Zhang M., Chen G., Zhu T., Zhang S., et al. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere. 2016;151:171–177. doi: 10.1016/j.chemosphere.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 163.Yang X., Bento C.P.M., Chen H., Zhang H., Xue S., Lwanga E.H., et al. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ Pollut. 2018;242:338–347. doi: 10.1016/j.envpol.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 164.Awet T.T., Kohl Y., Meier F., Straskraba S., Grün A.-L., Ruf T., et al. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ Sci Eur. 2018;30:1–10. doi: 10.1186/s12302-018-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang Y., Zhao Y., Wang J., Zhang M., Jia W., Qin X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut. 2019;254 doi: 10.1016/j.envpol.2019.112983. [DOI] [PubMed] [Google Scholar]
- 166.Muroi F., Tachibana Y., Kobayashi Y., Sakurai T., Kasuya K. Influences of poly(butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym Degrad Stab. 2016;129:338–346. [Google Scholar]
- 167.Wang S.L., Liang T.W., Yen Y.H. Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr Polym. 2011;84:732–742. doi: 10.1016/j.carbpol.2010.06.022. [DOI] [Google Scholar]
- 168.Sessitsch A., Weilharter A., Gerzabek M.H., Kirchmann H., Kandeler E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol. 2001;67:4215–4224. doi: 10.1128/AEM.67.9.4215-4224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown D.M., Wilson M.R., MacNee W., Stone V., Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 170.Velzeboer I., Kwadijk C.J.A.F., Koelmans A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol. 2014;48:4869–4876. doi: 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- 171.Zhu F., Yan Y., Doyle E., Zhu C., Jin X., Chen Z., et al. Microplastics altered soil microbiome and nitrogen cycling: the role of phthalate plasticizer. J Hazard Mater. 2021;127944 doi: 10.1016/j.jhazmat.2021.127944. [DOI] [PubMed] [Google Scholar]
- 172.Caruso G. Microplastics as vectors of contaminants. Mar Pollut Bull. 2019;146:921–924. doi: 10.1016/j.marpolbul.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 173.Wang M.-C., Gong M., Zang H.-B., Hua X.-M., Yao J., Pang Y.-J., et al. Effect of methamidophos and urea application on microbial communities in soils as determined by microbial biomass and community level physiological profiles. J Environ Sci Heal Part B, Pestic Food Contam Agric Wastes. 2006;41:399–413. doi: 10.1080/03601230600616155. [DOI] [PubMed] [Google Scholar]
- 174.Kyei-Boahen S., Slinkard A.E., Walley F.L. Rhizobial survival and nodulation of chickpea as influenced by fungicide seed treatment. Can J Microbiol. 2001;47:585–589. doi: 10.1139/w01-038. [DOI] [PubMed] [Google Scholar]
- 175.Kucey R.M.N., Chaiwanakupt P., Arayangkool T., Snitwongse P., Siripaibool C., Wadisirisuk P., et al. Nitrogen fixation (15N dilution) with soybeans under Thai field conditions - II. Effect of herbicides and water application schedule. Plant Soil. 1988;108:87–92. doi: 10.1007/BF02370103. [DOI] [Google Scholar]
- 176.Shah A.N., Tanveer M., Shahzad B., Yang G., Fahad S., Ali S., et al. Soil compaction effects on soil health and crop productivity: an overview. Environ Sci Pollut Res. 2017;24:10056–10067. doi: 10.1007/s11356-017-8421-y. [DOI] [PubMed] [Google Scholar]
- 177.Rillig M.C., Leifheit E., Lehmann J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021;19 doi: 10.1371/JOURNAL.PBIO.3001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Olaniran A.O., Balgobind A., Pillay B. Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci. 2013;14:10197–10228. doi: 10.3390/ijms140510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.De Tender C.A., Devriese L.I., Haegeman A., Maes S., Ruttink T., Dawyndt P. Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ Sci Technol. 2015;49:9629–9638. doi: 10.1021/acs.est.5b01093. [DOI] [PubMed] [Google Scholar]
- 180.Barnes D.K.A. Invasions by marine life on plastic debris. Nature. 2002;416:808–809. doi: 10.1038/416808a. [DOI] [PubMed] [Google Scholar]
- 181.Gregory M.R. Environmental implications of plastic debris in marine settings-entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2013–2025. doi: 10.1098/rstb.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Keswani A., Oliver D.M., Gutierrez T., Quilliam R.S. Microbial hitchhikers on marine plastic debris: human exposure risks at bathing waters and beach environments. Mar Environ Res. 2016;118:10–19. doi: 10.1016/j.marenvres.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 183.De Tender C., Schlundt C., Devriese L.I., Mincer T.J., Zettler E.R., Amaral-Zettler L.A. A review of microscopy and comparative molecular-based methods to characterize “Plastisphere” communities. Anal Methods. 2017;9:2132–2143. doi: 10.1039/C7AY00260B. [DOI] [Google Scholar]
- 184.Rillig M.C., Ryo M., Lehmann A. Classifying human influences on terrestrial ecosystems. Glob Chang Biol. 2021;27:2273–2278. doi: 10.1111/gcb.15577. [DOI] [PubMed] [Google Scholar]
- 185.Lian J, Wu J, Xiong H, Zeb A, Yang T, Su X, et al. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J Hazard Mater 2020;385:121620. https://doi.org/10.1016/j.jhazmat.2019.121620. [DOI] [PubMed]
- 186.Li L., Luo Y., Li R., Zhou Q., Peijnenburg W.J.G.M., Yin N., et al. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat Sustain. 2020;3:929–937. doi: 10.1038/s41893-020-0567-9. [DOI] [Google Scholar]
- 187.John McNeill S., Harold L.P. University Park Press; 1975. Microbial seascapes. 10.4319/lo.1975.20.6.1059. [Google Scholar]
- 188.Zettler E.R., Mincer T.J., Amaral-Zettler L.A. Life in the “Plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- 189.McCormick A., Hoellein T.J., Mason S.A., Schluep J., Kelly J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol. 2014;48:11863–11871. doi: 10.1021/es503610r. [DOI] [PubMed] [Google Scholar]
- 190.Oberbeckmann S., Löder M.G.J., Labrenz M. Marine microplastic-associated biofilms – a review. Environ Chem. 2015;12:551–562. [Google Scholar]
- 191.Ogonowski M., Motiei A., Ininbergs K., Hell E., Gerdes Z., Udekwu K.I., et al. Evidence for selective bacterial community structuring on microplastics. Environ Microbiol. 2018;20:2796–2808. doi: 10.1111/1462-2920.14120. [DOI] [PubMed] [Google Scholar]
- 192.Kirstein I.V., Wichels A., Gullans E., Krohne G., Gerdts G. The Plastisphere – Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0215859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rüthi J., Bölsterli D., Pardi-Comensoli L., Brunner I., Frey B. The, “plastisphere” of biodegradable plastics is characterized by specific microbial taxa of Alpine and Arctic soils. Front Environ Sci. 2020;8:173. [Google Scholar]
- 194.Zhang M., Zhao Y., Qin X., Jia W., Chai L., Huang M., et al. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci Total Environ. 2019;688:470–478. doi: 10.1016/j.scitotenv.2019.06.108. [DOI] [PubMed] [Google Scholar]
- 195.Bandopadhyay S, Liquet y González JE, Henderson KB, Anunciado MB, Hayes DG, DeBruyn JM. Soil microbial communities associated with biodegradable plastic mulch films. Front Microbiol 2020;11:2840. [DOI] [PMC free article] [PubMed]
- 196.Puglisi E., Romaniello F., Galletti S., Boccaleri E., Frache A., Cocconcelli P.S. Selective bacterial colonization processes on polyethylene waste samples in an abandoned landfill site. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-50740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou J., Gui H., Banfield C.C., Wen Y., Zang H., Dippold M.A., et al. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol Biochem. 2021;156 doi: 10.1016/j.soilbio.2021.108211. [DOI] [Google Scholar]