Graphical abstract

Keywords: Microwave heating, l-Lysine, Wheat gluten, Structural changes, Gel properties
Highlights
-
•
The synergistic effect of l-Lysine and microwave raised WG gel properties.
-
•
Zwitterionic l-Lysine acted as buffer in electromagnetic field.
-
•
l-Lysine unfolded wheat gluten and promoted the cross-linking of protein molecules.
-
•
Intermolecular force and electromagnetic coupling effect promoted WG gel qualities.
Abstract
To improve the quality of wheat gluten (WG) gels, the effect of l-Lysine on gelatin formation of WG under microwave (MW) irradiation was studied. The strength of WG gels treated by MW heating increased significantly (P < 0.05) in the alternating electromagnetic fields with zwitterionic l-Lysine. l-Lysine enhanced the surface hydrophobicity of WG under MW irradiation indicating that the dielectric buffering of l-Lysine changed the conformation of WG. The second structure of WG by Fourier transformed infrared spectroscopy showed that the α-helix content of WG decreased, while the β-sheet content. Furthermore, compared to the non-l-Lysine addition group, the ultraviolet absorption and fluorescence intensity of the WG increased. Scanning electron microscopy presented denser porous network microstructure of WG gels by MW treatment with adding l-Lysine. These results elucidate the regulation effect of l-Lysine on WG gelation in the MW field.
1. Introduction
Wheat gluten (WG) is a kind of protein with unique characteristics in wheat flour, widely used in the field of food processing, such as noodles and bread (Hoehnel et al., 2020, Sun et al., 2021). Gel properties are important functional characteristics of WG, protein gelation is a process in which denatured protein molecules aggregate to form an ordered protein network structure. The protein molecule is induced to unfold from the native state, thus the groups originally wrapped inside the molecular chain are gradually exposed. The stretched peptide chain is partially folded in different conformation, and the denatured protein aggregates to form macromolecules. Therefore, the three-dimensional network structure is constituted, which wraps water and other substances, and finally the gelation is formed (Bhattacharya and Jena, 2007, Tanger et al., 2022, Xiao et al., 2020). All the dough-related flour products are related to the textural characteristics of WG. In the process of converting the water-wheat flour mixture into dough, WG is solvated with water. Moreover, under the action of directional shear force and heating, the disulfide bonds and secondary bonds (hydrogen bonds, hydrophobic bonds and ionic bonds) in polypeptide chains were broken and recombined to form ordered spatial network structure. WG protein built the backbone of the dough and formed three-dimensional gluten network through chemical interactions, thereby combining starch particles, fat and other ingredients stuck in the gluten network (Li, Zhu, Yadav, & Li, 2019). However, native WG consists of globular gliadin and glutenin, whose main secondary structures were β-sheet with high bond energy. The compact molecular structure and poor hydrophilicity of WG limit the improvement of its functional properties, especially the further improvement of gel properties. Moderate physical or chemical modifications are ways to develop WG protein gel potential during food processing.
In addition, the heating process is crucial to obtain WG gel with good quality, and discrepant quality of WG products might be obtained using different heating treatments. The traditional water bath (WB) heating method based on conduction, it has the problems of low energy efficiency and environmental pollution (Wang, Muhoza, Wang, Feng, Xia, & Zhang, 2019). Moreover, the thermal transmission from the outside to the inside of the traditional WB heating method reduced the gel properties of WG due to the slow conduction rate. As a green processing technology, microwave (MW) heating has the characteristics of high energy efficiency and pollution-free. Furthermore, MW directly affected the electrostatic interaction and positioning of charged groups in protein molecules, which interfered with the electric field distribution and electrostatic interaction in amino acid residues. The alternating electromagnetic field caused charge separation, thereby changing the secondary and tertiary structure of the protein (Dong, Wang, & Raghavan, 2021). MW heating has been widely used in many food processing fields, especially in changing protein gel quality. But the protein aggregation produced by the rapid heating of the MW causes the quality of the protein gel to deteriorate (Fu, Hayat, Li, Lin, Xu, & Wang, 2012). Because the WG protein molecules are in an enclosing state at the initial heated stage, it is difficult for MW to fulfill its function to unfold protein. Cao et al. found that when MW was used to replace the first stage (40 ℃ for 30 min) of the two-stage WB heating of surimi, the gel strength was significantly lower than that of the WB heating (Cao, Fan, Jiao, Huang, Zhao, Yan, et al., 2018). In addition, the action of the MW alternating electric field makes the molecular dipole moment oriented, which is inclined to cause amorphous aggregation of the protein, therefore, the formed gel appears to be harder rather than more elastic. Due to the special heating mode of MW, adding a dielectric substance into the system to control the state balance during the gelation is an effective approach to regulate and control ordered aggregation of WG under MW irradiation.
l-Lysine is a weakly alkaline amino acid, which can inhibit the disordered aggregation of protein. As a small molecule additive, l-Lysine is often used to improve the solubility and gelation characteristics of proteins in food systems. l-Lysine is the first limiting amino acid in WG, involved in the synthesis of various proteins such as skeletal muscle, enzymes, serum proteins, and peptide hormone. Additionally, l-Lysine significantly increased the solubility of myosin and the emulsifying stability of meat products (Li et al., 2019, Zhu et al., 2018). After adding l-Lysine, the electrochemical characteristics of the protein system in the MW field will change regularly with the concentration of l-Lysine, especially the conductivity of the system increased significantly (Cao, Li, Fan, Wang, Zhu, Huang, et al., 2021). However, Liao et al. studied the dielectric properties of lysine aqueous solution. The results demonstrated that the dielectric loss (ɛ“) of the solution was unchanged with the increase of l-Lysine concentration (Liao, Raghavan, Wu, & Yaylayan, 2003). Generally, the addition of dielectric will enhance the dielectric loss of the system, and the dielectric properties will change with the increase of temperature (Feng, Xue, Li, Wang, Yang, & Xue, 2015). The aforementioned research proved that owing to the directional rearrangement produced by l-Lysine during the dipole polarization, l-Lysine can serve as a dielectric buffer in the alternating electromagnetic field of MW.
Therefore, the purpose of this research was to regulate the orderly aggregation of WG under the action of the MW field through the dielectric buffer effect of l-Lysine to enhance the quality characteristics of WG protein food. This work could be meaningful for food industry to have an insight into the molecular interaction of WG under the MW irradiation and provide a novel approach for the development of new applications of WG in the agricultural food industry.
2. Materials and methods
2.1. Materials
Commercial WG flour was purchased from Anhui Ante Food Co., Ltd (Suzhou, Anhui, China) with a protein content of 77.35 ± 0.97% (The total protein contents were determined with a Kjeltec8400 (FOSS analytical instruments Co. Ltd, Denmark)) on a dry matter basis and 7.27 ± 0.04% moisture. l-Lysine was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Page Ruler Prestained Protein Ladder was purchased from Thermo Scientific (MA, USA). 5, 5-dimercapto-2, 2-dinitrobenzoic acid (DTNB) was purchased from Yuanye Biotechnology Co., Ltd (Shanghai, China). The 8-Anilino-1-naphthalenesulfonic acid hydrate ammonium salt (ANS) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). All the other chemical reagents were of analytical grade unless otherwise specified.
2.2. Preparation of WG gels
WG was dispersed in deionized water to a concentration of 16% (w/v) and l-Lysine (0%, 0.1%, 0.125%, 0.15%, 0.175% and 0.2%, w/w, referred to GB14880-2012) was added to WG dispersion. WB heating treatment: WG sample dispersion was incubated at 90 ℃ for 30 min in WB. The time–temperature history of the heating process was recorded by the thermocouple (Fig. S1). MW heating treatment: the fiber-optic probe attached to the computer MW solid–liquid phase synthesis/extraction instrument (Model XH-200A, Xianghu, Beijing, China) was inserted into the WG dispersion, the temperature curve recorded from the WB heating was programmed to ensure an equivalent heating rate. The reactions were stopped by transferring the samples to the ice water for 30 min after heating. The prepared WG gels were stored at 4 °C (Wang, Liu, Luo, Li, Mu, Zhao, et al., 2019). Part of the protein gels was used to freeze-dry for subsequent testing.
2.3. Determination of gel strength
The gel strength of WG gels was measured by using TA.TOUCH Texture Analyzer equipped with a P/0.5 diameter probe (BosinTech, Shanghai, China). The parameters were set as follows: pretest speed 2.0 mm/s; post-test speed 2.0 mm/s; test speed 1.0 mm/s; trigger force 5.0 g; the samples were pierced to depth 10 mm and the maximum force of the sustained compression was defined as the gel strength value (g). The experiment was conducted in five replicates (Xiao, Liu, Wang, Jin, Guo, Liu, et al., 2020).
2.4. Determination of water holding capacity (WHC)
Slices with the mass of 2–3 g (M1) were cut from the middle part of the prepared WG gels. The slices were then wrapped in filter paper in 50 mL centrifuge tube and centrifuged at 8000 g at 4 ℃ for 15 min in a CR22N centrifuge (Hitachi, Tokyo, Japan). The filter papers were removed and WG gels were weighted (M2). Each experiment was repeated five times in parallel (Wang, et al., 2019). The WHC was calculated as follows:
| WHC (%) = × 100 | (1) |
2.5. Determination of chemical interactions
The reagents used to break certain types of bonds were as follows: 0.05 M NaCl (PA); 0.6 M NaCl (PB); 0.6 M NaCl + 1.5 M urea (PC); 0.6 M NaCl + 8 M urea (PD). It was the difference in protein concentration that indicated the existence of ionic bonds (difference between PB and PA), hydrogen bonds (difference between PC and PB), hydrophobic interactions (difference between PD and PC). The sample (0.3 g) was homogenized in a 5 mL reagent, and reacted at 4 ℃ for 1 h and then centrifuged at 5000 g for 15 min. The soluble protein content was determined by using the bicinchoninic acid protein assay kit (Beyotime, Shanghai, China) (Cao, Li, Luo, Mu, Zhong, Jiang, et al., 2017).
2.6. Determination of free sulfhydryl (SH) groups and disulfide bonds (S—S) content
Prepared protein solution: protein samples of 75 mg were dissolved in 1 mL Tris-Gly (0.086 M Tris, 0.09 M glycine and 0.004 M EDTA, pH 8.0), mixed with 4.7 g GuHCl, and diluted to 10 mL with Tris-Gly. To determine the free SH groups, the prepared protein solution of 1 mL was mixed with 4 mL urea-guanidine hydrochloride (8 M urea and 5 M GuHCl in Tris-Gly) and 50 μL of Ellman's reagent (4 mg/mL in Tris-Gly) and incubated for 30 min in dark condition. The absorbance of the solution was measured at 412 nm with a UV-2800A spectrophotometer (UNICO, WI, USA). To determine the total SH groups, the prepared protein solution of 1 mL was mixed with 4 mL urea-guanidine hydrochloride and 50 μL of 2-mercaptoethanol, then incubated at 25 °C for 1 h. Subsequently, trichloroacetic acid (12%, w/v) of 10 mL was added to the mixture and incubated at 25 °C for 1 h. The solutions were then centrifuged at 5000 g for 10 min. The process was repeated three times. The precipitate was dissolved in 10 mL urea-guanidine hydrochloride with 40 μL Ellman's reagent, the mixed solution was incubated for 30 min in dark condition, and then recorded the absorbance at 412 nm (Zhang, Chen, Liu, Mei, Yu, & Kan, 2020). The buffer solution without proteins was used as the blank control.
| (2) |
| (3) |
Where:
73.53 = 106/(1.36 × 104), 1.36 × 104 was the extinction coefficient for Ellman's reagents/ (L/ (mol × cm));
A412 was the absorbance value of the protein sample at 412 nm;
C was the protein concentration (mg/mL);
D was the dilution factor of the sample solution, where the free sulfhydryl groups dilution factor was 5.02 and the total sulfhydryl groups dilution factor was 10.
2.7. Determination of surface hydrophobicity (H0)
H0: The protein sample was dissolved in phosphate buffer solution (0.01 M, pH 7.0) to prepare a protein solution (0.5%, w/v) and centrifuged at 4000 g for 10 min. The concentrations of the supernatants were diluted to different concentrations (0.05–0.5 mg/mL). Different diluted sample solutions (4 mL) and 20 μL ANS solution (0.008 M) were mixed evenly, and the fluorescence intensity of the sample solutions was measured within 10 min at room temperature by using an RF-5301PC fluorescence spectrophotometer (Shimadzu, Tokyo, Japan). The excitation wavelength was 390 nm, the emission wavelength was 470 nm, and the scanning slit was set at 5 nm. H0 was the initial slope of the fluorescence intensity relative to the protein concentration (Zhao, Wang, Hong, Liu, & Li, 2021).
2.8. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The concentration of protein solution was adjusted to 1 mg/mL. The protein was solubilized in 0.0625 M Tris-HCl buffer (pH 6.8) containing SDS (2.3%) and glycerol (10%) and then heated for 10 min in boiling water. The sample solution was cooled at room temperature and centrifuged for 10 min at 8000 g. A sample of 10 μL was then loaded into the polyacrylamide gel (5% stacking gel, 12% separating gel). The gel was dyed with 0.1% (w/w) coomassie brilliant blue R-250 for 40 min. The gel was subsequently decolorized until the protein bands were clear (Wang, Luo, Zhong, Cai, Jiang, & Zheng, 2017).
2.9. Fourier transformed infrared spectroscopy analysis (FTIR)
The secondary structure of WG in the gels was measured by Nicolet iS50 Fourier Infrared Spectrometer (Thermo Nicolet Inc., Waltham, MA, USA). Powder sample (1 mg) was mixed with 99 mg of KBr was ground thoroughly and pressed into pellets. The spectra were recorded in the range of 400–4000 cm−1 (He, Shi, Walid, Zhang, Ma, & Xue, 2015).
2.10. Determination of ultraviolet (UV) absorption spectrum
Protein solution (0.5%, w/v) was prepared in 0.01 M phosphate buffer (pH 7.0), the sample was centrifuged at 4000 g for 10 min, the UV spectra between 200 and 400 nm was collected (Wang, Luo, Zhong, Cai, Jiang, & Zheng, 2017).
2.11. Determination of intrinsic fluorescence spectrum
The fluorescence intensity of the sample solution (0.5%, w/v) was measured by a fluorescence spectrophotometer. The excitation wavelength and emission wavelength were set to 280 nm and 290–450 nm respectively, and the slit width of the excitation wavelength and emission wavelength were 5 nm (Wu, Wu, Wang, Fan, Chen, Ma, et al., 2019).
2.12. Scanning electron microscopy analysis (SEM)
The processed WG gel samples were cut transversally into cube and lyophilized (Du, Dang, Khalifa, Du, Xu, & Li, 2020). Freeze-dried samples were mounted on a silver specimen holder and coated with gold. The microstructure was observed with an accelerating voltage of 10 kV by HITACHI SU8010 (Hitachi, Tokyo, Japan).
2.13. Statistical analysis
The data were expressed as means ± standard deviations (SD) of three replicates at least. The ANOVA significance of data was analyzed by SPSS Statistics 22 using analysis of Tukey’s HSD, and the difference was significant (P < 0.05). All graphs were presented by Origin 9.1.
3. Results and discussion
3.1. Gel properties analysis
The gel strength of protein gels is a considerable indicator of protein aggregation and food texture, and is directly related to the microstructure of the gel. The effect of l-Lysine addition on gel strength of heat-induced WG was shown in Fig. 1A. It could be seen from the figure that the gel strength of heat-induced WG gel was significantly improved through adding l-Lysine (P < 0.05), which suggested that l-Lysine could improve the texture properties of heat-induced gels. As the addition of l-Lysine increased from 0.1 % to 0.2 %, the gel strength increased subsequently, demonstrating that the addition of l-Lysine changed the internal structure of heat-induced WG gels, resulting in changes in textural properties. It was speculated that the reason was that protein molecules were rapidly arranged to form a stronger gel network structure under the action of l-Lysine. Besides, the gel strength of WG gels prepared by MW irradiation increased significantly than WB treatment (P < 0.05), when the addition of l-Lysine to 0.2%, the gel strength reached the maximum. There are two main explanations for the enhanced gel strength of amino acid in the MW field: ① The dielectric dissociated from l-Lysine enhanced MW heating induced protein aggregation; ② l-Lysine changed the pH of the system and the acid-base variation induced protein aggregation. Regarding the first explanation, l-Lysine is an alkaline amino acid with the side chain containing the proton-able amino group (–NH2), which can dissociate charged ions and increase the conductivity in the system. The conductivity increased due to the temperature of the system increased (Fig. S2), and the addition of l-Lysine led to the weakening of steric hindrance and electrostatic repulsion between protein molecules. Therefore, under MW heating, the intense Brownian motion of protein molecules was enough to overcome the residual repulsive energy barrier between protein molecules, increasing the chance of effective collision and aggregation (Anema, 2019, Yanagida et al., 2007). Although this explanation focused on the MW thermal effect after amino acid dissociation, the MW electromagnetic effect was ignored, that could directly act on polar protein molecules. Even at the same temperature, the aggregation rate of protein heated by MW was faster. Compared with traditional conduction heating, when the energy absorption value was the same, MW can achieve higher degrees of crosslinking between protein molecules (Cao, Jiao, Fan, Huang, Zhao, Yan, et al., 2019). For the second explanation, alkaline amino acids could increase the hydration layer and net negative charge of the protein in the system, and the pH value of the mixed system gradually deviated from the isoelectric point of the protein (Fig. S3). Protein molecules were not easy to contact with each other under electrostatic interaction, and the action of the MW alternating electric field broke the balance of the system. Thus, the protein molecules were cross-linked and aggregated, so the gel strength was improved. To prove whether the change of pH is the key factor affecting the gel strength, NaOH was used to regulate the pH of the system to make it the same as the pH of adding l-Lysine. The gel strength of WG gels decreased (Fig. S4). A hydration layer was formed around the ions, which was difficult for the intervention of cations to react with proteins and the role of MW in the system was limited (Basse, Bosc, Saiter, Chan-Huot, Dupas, Maillard, et al., 2020). The influence on the aggregate formation of protein in the electromagnetic field was weakened, which proved that the change of pH value was not the key factor to improve WG gel quality. Based on the facultative ion of alkaline amino acids, we tried to explain the process of MW-induced protein aggregation regulated with l-Lysine by the dielectric buffer.
Fig. 1.
Gel strength and water holding capacity (WHC) of wheat gluten gels with different l-Lysine addition treated by water bath (WB) and microwave (MW) treatment, respectively. The different uppercase letters on the bars indicated the significant difference (P < 0.05) in the MW treatment group. The different lowercase letters on the bars indicated the significant difference (P < 0.05) in WB treatment group.
The electromagnetic field of the MW would make the charged groups of the protein directional rearrangement with l-Lysine addition. The alternating electric field of the MW altered the charge distribution of the protein, thereby destroying the electrostatic interaction between the protein-peptide chains and inducing the protein to form aggregates. Furthermore, l-Lysine could induce the unfolding of protein and hinder over-aggregation (Wang, Zhao, Zhang, Liu, Jauregi, & Huang, 2020). The positively charged side chain of l-Lysine band to WG residues through electrostatic interaction, which could promote the formation of network structure and enhance the stability of three-dimensional network structure. Then under the synergistic effect of l-Lysine and MW, the protein gel formed developed networks and improved the gel strength.
WHC is a physical parameter, indicating the proportion of total water in the gel network under certain external force. The greater the WHC, the stronger the water binding capacity of the gel. The effect of adding l-Lysine on the WHC of heat-induced WG proteins was depicted in Fig. 1B. It could be seen from the figure that the WHC of heat-induced WG gel was significantly improved through adding l-Lysine (P < 0.05), probably because l-Lysine promoted the partial unfolding of protein molecules and increased the exposure of polar amino acid side chains, which interacted more easily with water, thus increasing the WHC of WG gels. Similar results were observed by Fu et al. for l-Lysine enhanced the WHC of myosin gels (Fu, Zheng, Lei, Xu, & Zhou, 2017). Further, the addition of l-Lysine could change the pH and make WG deviate from the isoelectric point, thereby increasing the electrostatic repulsion between protein molecules and fully exposing the binding sites of water, which led to a rise in WHC. In Fig. 1B, the WHC of MW group was higher than WB group, similar to the research of Ji et al., the WHC of mixed surimi gel treated by MW was remarkably enhanced showed that MW promoted the interaction between protein molecules and made more water retention (Ji, Xue, Zhang, Li, & Xue, 2017). Adding l-Lysine in WG under alternating electric field changed the conformation of protein molecules and promoted the cross-linking of protein molecules, forming a denser and uniform gel network structure. The denser three-dimensional network structure could confine more water in the gel system and enhance the WHC of the gel.
3.2. Intermolecular interactions analysis
WG was a biomacromolecule with spatial structure composed of various amino acids connected to each other. The important intermolecular forces that maintaining the complex three-dimensional network structure and stability of gluten gels mainly included covalent bond (disulfide bond and peptide bond) and non-covalent bond (ion interaction, hydrogen bonding, hydrophobic interaction, etc.). Chemical interactions have an important impact on the structure and texture properties of WG gels.
The content of ionic bond, hydrogen bond and hydrophobic interaction in heat-induced WG protein after adding l-Lysine was shown in Fig. 2A,2B. The chemical forces in the heat-induced WG from strong to weak were hydrophobic interaction > hydrogen bond > ionic bond, and the contribution of hydrophobic interaction and hydrogen bond was much greater than that of ionic bond, suggesting that the main forces maintaining conformational stability in thermally induced WG gels were hydrophobic interactions and hydrogen bonds. Similar results were obtained by Wang et al. in their study of egg yolk gels (Wang, Zhang, Chi, & Chi, 2021). The content of ionic bonds was the lowest of the non-covalent bonds and had no significant difference in all the samples. This was mainly because l-Lysine has both positive and negative charges, so the charged environment of the whole matrix was basically in equilibrium under the action of heating. The content of hydrogen bonds and hydrophobic interactions of the gels was much higher than that of ionic bonds (Fig. 2A, 2B). The hydrogen bonding content of thermally induced WG gels was reduced and hydrophobic interactions were enhanced by the addition of l-Lysine. This might have occurred because l-Lysine acted as a dielectric buffer during the thermally induced gels and the heating resulted in the hydrogen bonds being broken, leading to a reduction in hydrogen bonding content. However, some studies had reported that l-Lysine had a strong ability to form hydrogen bonds, which might enhance the hydrogen bonding of myosin gels (Fu, Zheng, Lei, Xu, & Zhou, 2017). Hydrogen bonds mainly existed between the side amide groups of glutamine amino acids which were abundant in the repeating domains of WG and were easy to interact with through hydrogen bonds. However, the addition of l-Lysine in WG resulted in a decrease in the hydrogen bonds content, probably caused by the non-thermal effect of MW. The WG protein molecules unfolded during the heating process, and the hydrophobic parts were exposed to the hydrophilic environment. To maintain the thermodynamic stability of the system, the hydrophobic interactions between adjacent protein molecules increased. Therefore, the addition of l-Lysine combined with MW enhanced the hydrophobic interactions between protein molecules. Non-polar amino acid residues tended to accumulate inside the molecule, minimizing the area in contact with water, which led to aggregation and the formation of a three-dimensional gel.
Fig. 2.
Intermolecular interactions of wheat gluten. A: The content of non-covalent bonds (ionic bonds, hydrogen bonds and hydrophobic interaction) in wheat gluten with different l-Lysine addition treated by water bath (WB) treatment; B: The content of non-covalent bonds in wheat gluten with different l-Lysine addition treated by microwave (MW) treatment. The different uppercase letters on the bars indicated the significant difference (P < 0.05) in the content of non-covalent bonds of wheat gluten (A, ionic bonds; B, hydrogen bonds; C, hydrophobic interactions), the different lowercase letters on the bars indicated the difference between wheat gluten with different l-Lysine contents. C: The content of disulfide bonds in wheat gluten with different l-Lysine addition treated by WB and MW treatment. Different superscript letters (WB: the different lowercase letters; MW: the different uppercase letters) indicate significant differences (P < 0.05). D: Surface hydrophobicity (H0) of wheat gluten with different l-Lysine addition treated by WB and MW treatment. Different superscript letters (WB: the different lowercase letters; MW: the different uppercase letters) indicate significant differences (P < 0.05).
Disulfide bonds in proteins were one of the most functional groups. Cysteine in gluten protein formed disulfide bonds through thiol oxidation, which provided stable support for the tight structure of WG protein. The presence of disulfide bonds also made the network structure more compact. In the WG network, disulfide bonds were present both inside and outside the amino acid side chains. Intramolecular disulfide bonds were mainly found in wheat gum protein monomers or wheat gum protein polymers, and intermolecular disulfide bonds were mainly found in wheat gum protein polymers (Kondov, Verma, & Wenzel, 2011). The effect of adding l-Lysine on thermally induced disulfide bonds in WG was shown in Fig. 2C. As could be seen from the graph, the addition of l-Lysine significantly increased disulfide bond content in heat-induced WG gels (P < 0.05), which indicated that the addition of l-Lysine would promote the expansion of wheat gluten protein molecules, and more sulfhydryl groups will be exposed on the surface or inside of the protein molecules. As a result, the exposed sulfhydryl groups cross-linked and formed more disulfide bonds. Disulfide bonds played a crucial role in the thermal aggregation of protein in the formation of the gel network. Zhang et al. found that l-Lysine affected the formation of disulfide bonds in myosin gel, enhancing the gel strength (Zhang, Wu, Jamali, Guo, & Peng, 2017). Similarly, the proteins would aggregate to form larger particles, the protein gel would become denser and the gel strength would increase. Moreover, the disulfide bonds content of WG treated by MW increased than WB (Fig. 2C), during the MW heating process, the protein molecules absorbed heat and then expanded, which loosen the structure of the protein. The free SH groups embedded in the molecule were exposed and formed disulfide bonds during the gelation. MW caused the change of protein conformation and the position of free SH, so it was easier to promote the formation of disulfide bonds. The rise of disulfide bonds content caused the protein to undergo more cross-linking during the thermal gelation process and increased the gel strength. It showed that the combined action of MW and l-Lysine has the potential to orderly crosslink WG molecules.
3.3. Hydrophobicity (H0) analysis
H0 of proteins can be used to evaluate changes in protein structure. When the hydrophobic amino acid residues inside the protein molecules are exposed, some non-polar amino acid side chains of the protein molecules associated with each other form hydrophobic regions due to the repulsion of water molecules, the protein–protein aggregates maintained by hydrophobic forces. The changes in the tertiary structure of the protein are analyzed by observing the changes in H0. Additionally, ANS was used as a highly efficient fluorescent probe capable of attaching to the non-polar ends (hydrophobic groups) of protein molecules, and the amount of hydrophobic groups on the surface of the molecule is represented by the amount of fluorescence intensity. In this study, changes in wheat gluten protein H0 were characterized by detecting fluorescence intensity.
It could be seen from Fig. 2D that the addition of l-Lysine significantly increased the H0 of the heat-induced WG gel (P < 0.05), indicating that the addition of l-Lysine had led to the expansion of the molecular structure of WG protein and the exposure of hydrophobic group. Guo et al. found that l-Lysine could increase the H0 of porcine myosin. l-Lysine destroyed the protein structure, caused the exposure of aromatic acid, and led to the exposure of hydrophobic groups (Guo, Peng, Zhang, Liu, & Cui, 2015). However, with the same amount of l-Lysine added, the H0 of the MW group was lower than that of the WB group. The addition of l-Lysine might bind to the charged residues on WG due to the electrostatic effect of MW, which destroyed the ionic bonds within molecules. As the protein disassociated and stretched, some hydrophobic groups embedded in the molecule were exposed and hence the H0 increased. The H0 of WG treated by MW irradiation increased due to the polarization of alternating electromagnetic fields. MW broke the balance between hydrophilic and hydrophobic substances, which led to the stretching of protein structure and exposed the hydrophobic groups initially inside the molecules. These results enhanced the hydrophobic groups’ exposure to the surface, promoted the orderly aggregation of proteins within the MW field, and might affect the three-dimensional network structure of WG gels. However, the enhancement of H0 changed the environment around the protein molecules, causing the protein molecules to self-aggregate, resulting in a decrease in H0. It could be seen from Fig. 2D that the increases in H0 tend to balance, indicating that with the intervention of zwitterionic l-Lysine, it did act as a dielectric buffer in the MW field. The zwitterion of l-Lysine shielded the increasing H0 so that the protein molecules were in a relatively stable and stretched state, which was conducive to the further orderly aggregation of protein molecules. The combined treatments were more effective in inducing the further unfolding of WG, which favored inhibiting the flocculation between protein molecules. The increase in H0 reduced the WG gels’ water fluidity and enhanced the WHC; it was proved that the MW synergistic effect of l-Lysine on the gel quality of the WG was improved.
3.4. SDS-PAGE electrophoresis analysis
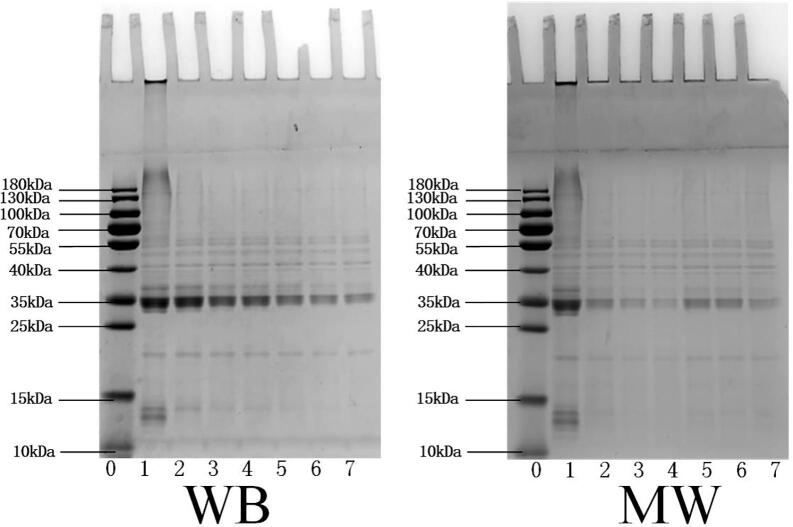
Changes in WG subunits were investigated by using SDS-PAGE, as shown in Fig. 3. Electrophoretic bands could be divided into aggregates (130–200 kDa); HMW-GS (66–130 kDa); ω-Gliadin (39–55 kDa); LMW-GS (36–44 kDa); α/β (28–40 kDa); γ-Gliadin (38–42 kDa) and soluble peptide (<25 kDa) according to molecular weight (Zhao, Hou, Tian, Zhou, Yang, Wang, et al., 2020). It could be seen from the figure the basic subunit bands were visible clearly, which mean that the addition of l-Lysine with heating treatment would not change the molecular weight of WG subunits, the energy of chemical bonds was much higher than the quantum energy of heating. Proteins will form dimers, multimers, and even insoluble protein precipitates under many physical stimuli such as heat and high pressure. Compared with the WB group, a part of the protein aggregates with the larger molecular weight accumulated at the loading port in MW group. MW is a physical stimulus that is different from heat and pressure and has its peculiarities. Under the synergistic action of l-Lysine and MW, proteins cross-linked each other through disulfide bonds to form insoluble protein aggregates. At the same time, the depth of the other subunit bands decreased obviously, indicating that the formation of the WG gel network required the cross-linking of proteins of different molecular weights. WG aggregated irregularly under the action of the alternating electric fields. The binding of these aggregates was loose, and the intermolecular interactions were weak. During the electrophoresis process, some proteins were easily dissolved from the irregular aggregates. Therefore, the intensity of the high molecular polymer band at the upper end of the electrophoresis lane was lighter, and the width of the band was also narrower. With the addition of l-Lysine, the protein molecules formed ordered aggregation. In addition to the rapid temperature rise of materials, MW may also have a non-thermal effect. The polar groups in protein molecules were affected by electromagnetic waves, which promoted the formation of new hydrophobic bonds of protein molecules and cross-linking aggregation. The proteins of some small molecules participate in the formation of gel network by embedding or chimerism, subunit bands (10–15 kDa) of soluble peptide disappearance, so the subunit bands of high polymers were gradually deepened.
Fig. 3.
SDS-PAGE of wheat gluten with different l-Lysine addition treated by water bath (WB) and microwave (MW) treatment. Lane 0: Molecular marker; Lane 1: Wheat gluten power; Lane 2–7: Wheat gluten treated with different l-Lysine addition of 0%, 0.1%, 0.125%, 0.15%, 0.175%, 0.2%, respectively.
3.5. FTIR analysis
FTIR was used to characterize the secondary structure of wheat gluten protein gel samples. The amide I band (1600 ∼ 1700 cm−1) was evaluate. Amide I band of FTIR was mainly caused by C O stretching and H—O—H bending vibration. The changes in protein secondary structure (α-helix, β-sheet, β-turn, and random coil) could be reflected in this vulnerable zone. The peaks in the amide I band corresponding to the secondary protein structures was as follows: β-sheet (1610 ∼ 1642 cm−1&1680–1700 cm−1), β-turn (1660 ∼ 1680 cm−1), random coil (1642 ∼ 1650 cm−1), α-helix (1650 ∼ 1660 cm−1)(He, Shi, Walid, Zhang, Ma, & Xue, 2015).
FTIR spectra of thermally induced WG gels treated with different concentrations of l-Lysine in the amide I region were displayed in Fig. 4. The peak patterns of all the samples remained basically unchanged, indicating that the WG molecules were able to maintain most of their helical structure under these treatment conditions. The content of α-helix decreased with the increase of l-Lysine while the content of β-sheet increased in the alternating electromagnetic field with l-Lysine. The superposition of the dipole moments of all peptide bonds that made up the α-helix, resulting in a strong directional dipole moment. The external electric field coupled with the directional dipole moment decreases the content of α-helix. Moreover, Li et al. found that l-Lysine could significantly reduce the α-helix content of myosin, while increasing the β-sheet content (Li, Li, Zhu, Ning, Cai, & Zhou, 2019). The increase in the β-sheet structure changed the protein–protein interaction and exposed the hydrophobic sites of the protein, which promoted protein network formation. Zhu et al. also reported that low α-helix and high β-sheet contents in the protein structure suggested a simultaneous increase in its flexibility and H0 (Zhu, Fu, Wu, Qi, Teng, Wang, et al., 2020). MW is an electromagnetic wave with an alternating electric field that has a “tear” effect on polar protein molecules. Under the action of a high-frequency external electric field, the polarity orientation of the water was induced to change, causing the movement of protein molecules and mutual friction. The alternating electric field of MW directly acted on the charged groups and affected the microenvironment of WG, destroying the ordered structure in the secondary structure of the protein to form a disordered structure. While the addition of l-Lysine alleviated the fierce impact on WG in the MW field and promoted the orderly aggregation. In general, l-Lysine existed in a zwitterionic form [NH3+(CH2)4CH-(NH2)COO−], which could increase the hydration layer and negative charge of protein in the system and improve the stability of the system. Therefore, under the synergistic effect of the MW heating and l-Lysine, new molecular order crosslinks appeared within proteins and prompted the secondary structure of proteins to interconvert.
Fig. 4.
Fourier transformed infrared spectra (FTIR) of wheat gluten and analysis of wheat gluten secondary structure treated by water bath (WB) and microwave (MW) treatment. Transmission rate: (A: WB; B: MW); Secondary structure content of wheat gluten (C: WB; D:MW). 0–0.2: The different l-Lysine additions (%).
3.6. UV spectra analysis
WG had a UV absorption peak at about 275 nm, which was mainly caused by the π-π* transition of aromatic heterocycles such as tryptophan (Trp) and tyrosine (Tyr) on its peptide bonds. As shown in Fig. 5A,5B, an increase in UV absorption intensity of WG after adding l-Lysine in both treatments. l-Lysine promoted the unfolding and partial expansion of polypeptide chains in WG. Some amino acid residues originally in the hydrophobic environment of protein molecules might be exposed to the molecular surface due to the changes in protein spatial structure. With the increase of l-Lysine addition, the UV absorption intensity of the MW-treated WG was raised. The protein backbone directly absorbed MW energy in the alternating electric field. Water was a polar compound, in which the atoms with high electronegativity had electron repulsion pulling effect, resulting in uneven charge distribution and dipole moment formation. Moreover, the zwitterionic of l-Lysine played the role of dielectric buffer in the MW field due to directional rearrangement of polar molecules. Therefore, the polarization effect was increased under the condition of MW heating (Hu and Cross, 1995, Liu and Ma, 2016), and the effect on the unfolding of WG protein was enhanced. Hence, molecular mobility in the polar region of the protein was increase, the tertiary structure of the protein was loosened, and the spatial conformation of the protein molecule was changed. With the increase of l-Lysine addition, the maximum UV absorption peak of WG protein had a slight blue shift, showing that the increased the polarity of the microenvironment of protein molecules, caused by the distribution of the aromatic residues (Tyr, Trp, and phenylalanine (Phe)) was transferred to the surface of protein molecules (Qin, Luo, Cai, Zhong, Jiang, Zheng, et al., 2016). The intramolecular or intermolecular interactions with the action of l-Lysine lead to the regular aggregation of protein molecules under MW field.
Fig. 5.
Ultraviolet spectroscopy of wheat gluten with different l-Lysine addition treated by water bath WB (A) and MW (B) treatment. B: Fluorescence spectra of wheat gluten with different l-Lysine addition treated by WB (C) and MW (D) treatment. 0–0.2: The different l-Lysine additions (%).
3.7. Fluorescence spectra analysis
Aromatic amino acid residues (Try, Trp and Phe) could produce characteristic spectra to reflect changes in their microenvironment. For example, the endogenous fluorescence of Trp residues was very sensitive to changes in its microenvironment. Therefore, Trp residues were usually used to characterize the variation in the tertiary structure of the protein. The maximum emission wavelength of protein λmax indicates the polarity of the environment of amino acids in protein changes. The change of fluorescence intensity is determined by the exposure degree of fluorescent amino acids. Fig. 5C,5D showed that the addition of l-Lysine had no significant effect on the peak shape of the fluorescence spectrum of WG. The maximum fluorescence emission of Trp residues corresponding to WG protein λmax was around 343 nm. Generally, Trp residues in a polar environment if λmax > 330 nm (Zhang, Chen, Liu, Mei, Yu, & Kan, 2020). With the increase of l-Lysine addition, the maximum fluorescence intensity of all the samples was around 343 nm, showing that all Trp residues were present in the polar environment. With the addition of l-Lysine increased, the fluorescence intensity of WG strengthened, suggesting the microenvironment of Trp residues has changed. The unfolding of the WG led to the exposure of the internal hydrophobic groups, which indicated the enhancement of polar environment. The charge and mass of l-Lysine molecules were non-spherical symmetrically and the positive and negative charge centers were not coincident, so l-Lysine had the permanent dipole moment. The result of fluorescence intensity was also consistent with the UV absorption intensity, indicating the MW with l-Lysine altering the tertiary structure of the protein, causing more unfolding of the protein and the exposure of internal chromophores, thus increasing the fluorescence intensity. General amino acids had an average dipole moment of about 3.8 D, which was greater than that of water molecules (1.81 D). Moreover, each protein had a certain electric dipole moment, which was the target of the electromagnetic field. MW caused the dipole to rotate and quickly rearrange with the change of the interactive electric field, resulting in the change of the tertiary structure of the protein. The stretching protein molecules orderly aggregated and gradually cross-linked under the traction of electromagnetic field, and the gel properties of WG were improved accordingly.
3.8. Microstructure of WG gels
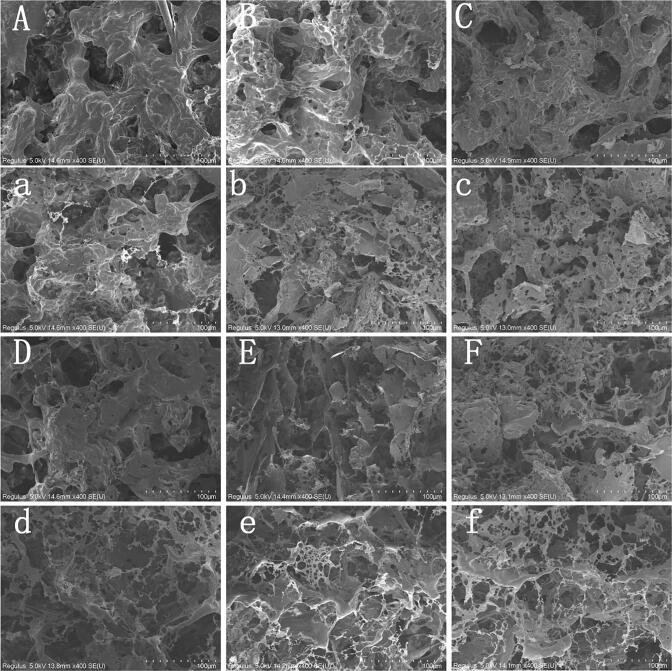
The microstructure is the basis of the gel strength and WHC of the protein gels. The SEM micrograph of WG gels without l-Lysine exhibited a discontinuous network (Fig. 6A) due to the compact structure of WG and the limited effect of MW on WG. The chambers of the gel simply by MW heating were larger and rougher, the texture structure was the irregular arrangement, and it appeared blocky. These might cause by the irregular aggregation of proteins produced by the intense electromagnetic oscillation of MW heating. The surface proteins of the MW-heated WG gels without l-Lysine addition were thick and filamentous, the chambers were small, and the texture structures were flat. Under the regulation of l-Lysine, a network structure with fine chambers was formed by the proteins orderly cross-linking, and with a more delicate and dense surface texture. MW heating could avoid forming clustered structures with the action of l-Lysine as a dielectric buffer in the alternating electric field (Wang, Jiao, Yan, Meng, Cao, Huang, et al., 2021). The addition of l-Lysine promoted the formation of disulfide bonds and the enhancement of hydrophobic interactions during gelation. With the increased intermolecular forces, the WG realized orderly cross-linking between protein molecules. Therefore, the network structure became denser and more even (Fig. 6A-F), which varied with the change of gel strength. The zwitterionic l-Lysine inhibited the disorder aggregation of WG and enhanced the solubility of the protein. The aggregation and solubility of protein could affect the formation of gelatin, thus altering the microstructure of the gels. l-Lysine influenced the charge distribution of WG and the microenvironment of the electromagnetic field. The polymerization rate of WG with charged groups was greater than the frequency of vibration and rotation, resulting in a denser WG gels network structure. These results were more favorable for the physical interception of water, thus the water in the gel network more difficult to escape, enhancing the WHC of the gel.
Fig. 6.
SEM microstructure (×400) of wheat gluten gels. Wheat gluten gels treated by water bath (A-F) and microwave (a-f) treatment with l-Lysine addition at 0%, 0.1%, 0.125%, 0.15%, 0.175% and 0.2%, respectively.
4. Conclusion
Compared to the non-l-Lysine addition in WG by MW treatment, l-Lysine improved the gel properties. The synergistic effect of the alternating electric field in MW and l-Lysine accelerated the protein molecular denaturation and unfolding. The unfolding of protein molecules caused the exposure of internal groups of protein, including hydrophobic residues. Therefore, the improvement of UV and fluorescence spectra intensity was enhanced. Moreover, zwitterionic l-Lysine reduced the damage of the secondary structure of the protein and the destruction to other intermolecular interactions due to polarization of electromagnetic coupling. The increase of β-sheet and disulfide bonds facilitated the formation of the protein gels network. Further microstructure showed the uniform network of WG proved that protein molecules can be regulated orderly in MW field with the dielectric buffering effect of l-Lysine. In conclusion, the method of MW and adding l-Lysine improved the functional properties in WG and provided a theoretical basis for further research in promoting the quality of WG based food in the agricultural industry.
CRediT authorship contribution statement
Sen Li: Conceptualization, Methodology, Writing – review & editing. Mengyao Li: Methodology, Data curation, Writing – review & editing. Hongwei Cao: Writing – original draft, Investigation, Formal analysis. Xiao Guan: Funding acquisition, Supervision. Ying Zhang: Software, Methodology. Kai Huang: Visualization. Yu Zhang: Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (32102140); Shanghai Sailing Program (20YF1433400); the Domestic Science and Technology Cooperation Projects of Shanghai (21015801100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100299.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anema S.G. Age Gelation, Sedimentation, and Creaming in UHT Milk: A Review. Comprehensive Reviews in Food Science and Food Safety. 2019;18(1):140–166. doi: 10.1111/1541-4337.12407. [DOI] [PubMed] [Google Scholar]
- Basse B., Bosc V., Saiter J.M., Chan-Huot M., Dupas J.P., Maillard M.N., Menut P. Combined effects of ionic strength and enzymatic pre-treatment in thermal gelation of peanut proteins extracts. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109362. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Jena R. Gelling behavior of defatted soybean flour dispersions due to microwave treatment: Textural, oscillatory, microstructural and sensory properties. Journal of Food Engineering. 2007;78(4):1305–1314. [Google Scholar]
- Cao F., Li X., Luo S., Mu D., Zhong X., Jiang S.…Zhao Y. Effects of organic acid coagulants on the physical properties of and chemical interactions in tofu. LWT - Food Science and Technology. 2017;85:58–65. [Google Scholar]
- Cao H., Fan D., Jiao X., Huang J., Zhao J., Yan B.…Zhang H. Effects of microwave combined with conduction heating on surimi quality and morphology. Journal of Food Engineering. 2018;228:1–11. [Google Scholar]
- Cao H., Jiao X., Fan D., Huang J., Zhao J., Yan B.…Wang M. Microwave irradiation promotes aggregation behavior of myosin through conformation changes. Food Hydrocolloids. 2019;96:11–19. [Google Scholar]
- Cao Y., Li B., Fan X., Wang J., Zhu Z., Huang J., Xiong Y.L. Synergistic recovery and enhancement of gelling properties of oxidatively damaged myofibrillar protein by l-lysine and transglutaminase. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129860. [DOI] [PubMed] [Google Scholar]
- Du J., Dang M., Khalifa I., Du X., Xu Y., Li C. Persimmon tannin changes the properties and the morphology of wheat gluten by altering the cross-linking, and the secondary structure in a dose-dependent manner. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109536. [DOI] [PubMed] [Google Scholar]
- Feng D., Xue Y., Li Z., Wang Y., Yang W., Xue C. Dielectric properties of myofibrillar protein dispersions from Alaska Pollock (Theragra chalcogramma) as a function of concentration, temperature, and NaCl concentration. Journal of Food Engineering. 2015;166:342–348. [Google Scholar]
- Fu X., Hayat K., Li Z., Lin Q., Xu S., Wang S. Effect of microwave heating on the low-salt gel from silver carp (Hypophthalmichthys molitrix) surimi. Food Hydrocolloids. 2012;27(2):301–308. [Google Scholar]
- Fu Y., Zheng Y., Lei Z., Xu P., Zhou C. Gelling properties of myosin as affected by L-lysine and L-arginine by changing the main molecular forces and microstructure. International Journal of Food Properties. 2017;20(sup1):S884–S898. [Google Scholar]
- Guo X.Y., Peng Z.Q., Zhang Y.W., Liu B., Cui Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of L-lysine and L-histidine. Food Chemistry. 2015;170:212–217. doi: 10.1016/j.foodchem.2014.08.045. [DOI] [PubMed] [Google Scholar]
- He S., Shi J., Walid E., Zhang H., Ma Y., Xue S.J. Reverse micellar extraction of lectin from black turtle bean (Phaseolus vulgaris): Optimisation of extraction conditions by response surface methodology. Food Chemistry. 2015;166:93–100. doi: 10.1016/j.foodchem.2014.05.156. [DOI] [PubMed] [Google Scholar]
- Hoehnel A., Bez J., Petersen I.L., Amarowicz R., Juskiewicz J., Arendt E.K., Zannini E. Enhancing the nutritional profile of regular wheat bread while maintaining technological quality and adequate sensory attributes. Food & Function. 2020;11(5):4732–4751. doi: 10.1039/d0fo00671h. [DOI] [PubMed] [Google Scholar]
- Hu W., Cross T.A. Tryptophan hydrogen bonding and electric dipole moments functional roles in the gramicidin channel and implications for membrane proteins. Biochemistry. 1995;34:9. doi: 10.1021/bi00043a020. [DOI] [PubMed] [Google Scholar]
- Ji L., Xue Y., Zhang T., Li Z., Xue C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocolloids. 2017;63:77–84. [Google Scholar]
- Kondov I., Verma A., Wenzel W. Performance assessment of different constraining potentials in computational structure prediction for disulfide-bridged proteins. Computational Biology and Chemistry. 2011;35(4):230–239. doi: 10.1016/j.compbiolchem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Li J., Zhu Y., Yadav M.P., Li J. Effect of various hydrocolloids on the physical and fermentation properties of dough. Food Chemistry. 2019;271:165–173. doi: 10.1016/j.foodchem.2018.07.192. [DOI] [PubMed] [Google Scholar]
- Li S., Li L., Zhu X., Ning C., Cai K., Zhou C. Conformational and charge changes induced by l-Arginine and l-lysine increase the solubility of chicken myosin. Food Hydrocolloids. 2019;89:330–336. [Google Scholar]
- Liao X., Raghavan G.S.V., Wu G., Yaylayan V.A. Dielectric properties of lysine aqueous solutions at 2450 MHz. Journal of Molecular Liquids. 2003;107(1):15–19. [Google Scholar]
- Liu C., Ma X. Study on the mechanism of microwave modified wheat protein fiber to improve its mechanical properties. Journal of Cereal Science. 2016;70:99–107. [Google Scholar]
- Qin X.S., Luo S.Z., Cai J., Zhong X.Y., Jiang S.T., Zheng Z., Zhao Y.Y. Effects of microwave pretreatment and transglutaminase crosslinking on the gelation properties of soybean protein isolate and wheat gluten mixtures. Journal of the Science of Food and Agriculture. 2016;96(10):3559–3566. doi: 10.1002/jsfa.7541. [DOI] [PubMed] [Google Scholar]
- Sun J., Chen M., Hou X.X., Li T.T., Qian H.F., Zhang H.…Wang L. Effect of phosphate salts on the gluten network structure and quality of wheat noodles. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129895. [DOI] [PubMed] [Google Scholar]
- Tanger C., Müller M., Andlinger D., Kulozik U. Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocolloids. 2022;123 [Google Scholar]
- Wang B.Z., Liu F.R., Luo S.Z., Li P.J., Mu D.D., Zhao Y.Y.…Zheng Z. Effects of High Hydrostatic Pressure on the Properties of Heat-Induced Wheat Gluten Gels. Food and Bioprocess Technology. 2019;12(2):220–227. [Google Scholar]
- Wang K.Q., Luo S.Z., Zhong X.Y., Cai J., Jiang S.T., Zheng Z. Changes in chemical interactions and protein conformation during heat-induced wheat gluten gel formation. Food Chemistry. 2017;214:393–399. doi: 10.1016/j.foodchem.2016.07.037. [DOI] [PubMed] [Google Scholar]
- Wang Q., Jiao X., Yan B., Meng L., Cao H., Huang J.…Fan D. Inhibitory effect of microwave heating on cathepsin l-induced degradation of myofibrillar protein gel. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129745. [DOI] [PubMed] [Google Scholar]
- Wang R., Zhang L., Chi Y., Chi Y. Forces involved in freeze-induced egg yolk gelation: Effects of various bond dissociation reagents on gel properties and protein structure changes. Food Chemistry. 2021;371 doi: 10.1016/j.foodchem.2021.131190. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Muhoza B., Wang X.W., Feng T.T., Xia S.Q., Zhang X.M. Comparison between microwave and traditional water bath cooking on saltiness perception, water distribution and microstructure of grass crap meat. Food Research International. 2019;125 doi: 10.1016/j.foodres.2019.108521. [DOI] [PubMed] [Google Scholar]
- Wang Y.S., Zhao J., Zhang W.W., Liu C.Q., Jauregi P., Huang M.G. Modification of heat-induced whey protein gels by basic amino acids. Food Hydrocolloids. 2020;100 [Google Scholar]
- Wu D., Wu C., Wang Z., Fan F., Chen H., Ma W., Du M. Effects of high pressure homogenize treatment on the physicochemical and emulsifying properties of proteins from scallop (Chlamys farreri) Food Hydrocolloids. 2019;94:537–545. [Google Scholar]
- Xiao Y., Liu Y., Wang Y., Jin Y., Guo X., Liu Y.…Xu H. Heat-induced whey protein isolate gels improved by cellulose nanocrystals: Gelling properties and microstructure. Carbohydrate Polymers. 2020;231 doi: 10.1016/j.carbpol.2019.115749. [DOI] [PubMed] [Google Scholar]
- Xiao Y.Q., Li J.M., Liu Y.N., Peng F., Wang X.J., Wang C.…Xu H.D. Gel properties and formation mechanism of soy protein isolate gels improved by wheat bran cellulose. Food Chemistry. 2020;324 doi: 10.1016/j.foodchem.2020.126876. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Ueda M., Murata T., Esaki S., Ishii Y. Brownian motion, fluctuation and life. Biosystems. 2007;88(3):228–242. doi: 10.1016/j.biosystems.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang H., Chen G., Liu M., Mei X., Yu Q., Kan J. Effects of multi-frequency ultrasound on physicochemical properties, structural characteristics of gluten protein and the quality of noodle. Ultrasonics Sonochemistry. 2020;67 doi: 10.1016/j.ultsonch.2020.105135. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu J., Jamali M.A., Guo X., Peng Z. Heat-induced gel properties of porcine myosin in a sodium chloride solution containing L-lysine and L-histidine. LWT - Food Science and Technology. 2017;85:16–21. [Google Scholar]
- Zhao Q., Wang L., Hong X., Liu Y., Li J. Structural and functional properties of perilla protein isolate extracted from oilseed residues and its utilization in Pickering emulsions. Food Hydrocolloids. 2021;113 [Google Scholar]
- Zhao X., Hou C., Tian M., Zhou Y., Yang R., Wang X.…Wang P. Effect of water-extractable arabinoxylan with different molecular weight on the heat-induced aggregation behavior of gluten. Food Hydrocolloids. 2020;99 doi: 10.1016/j.foodchem.2020.126477. [DOI] [PubMed] [Google Scholar]
- Zhu X., Ning C., Li S., Xu P., Zheng Y., Zhou C. Effects of L-lysine/L-arginine on the emulsion stability, textural, rheological and microstructural characteristics of chicken sausages. International Journal of Food Science & Technology. 2018;53(1):88–96. [Google Scholar]
- Zhu Y., Fu S.Y., Wu C.L., Qi B.K., Teng F., Wang Z.J.…Jiang L.Z. The investigation of protein flexibility of various soybean cultivars in relation to physicochemical and conformational properties. Food Hydrocolloids. 2020;103 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.