Abstract
Background.
Data on norovirus epidemiology among all ages in community settings are scarce, especially from tropical settings.
Methods.
We implemented active surveillance in 297 households in Peru from October 2012 to August 2015 to assess the burden of diarrhea and acute gastroenteritis (AGE) due to norovirus in a lower-middle-income community. During period 1 (October 2012–May 2013), we used a “traditional” diarrhea case definition (≥3 loose/liquid stools within 24 hours). During period 2 (June 2013–August 2015), we used an expanded case definition of AGE (by adding ≥2 vomiting episodes without diarrhea or 1–2 vomiting episodes plus 1–2 loose/liquid stools within 24 hours). Stool samples were tested for norovirus by reverse-transcription polymerase chain reaction.
Results.
During period 1, overall diarrhea and norovirus-associated diarrhea incidence was 37.2/100 person-years (PY) (95% confidence interval [CI], 33.2–41.7) and 5.7/100 PY (95% CI, 3.9–8.1), respectively. During period 2, overall AGE and norovirus-associated AGE incidence was 51.8/100 PY (95% CI, 48.8–54.9) and 6.5/100 PY (95% CI, 5.4–7.8), respectively. In both periods, children aged <2 years had the highest incidence of norovirus. Vomiting without diarrhea occurred among norovirus cases in participants <15 years old, but with a higher proportion among children <2 years, accounting for 35% (7/20) of all cases in this age group. Noroviruses were identified in 7% (23/335) of controls free of gastroenteric symptoms.
Conclusions.
Norovirus was a significant cause of AGE in this community, especially among children <2 years of age. Inclusion of vomiting in the case definition resulted in a 20% improvement for detection of norovirus cases.
Keywords: incidence, norovirus, acute gastroenteritis, Peru
Diarrhea remains an important cause of morbidity and mortality with approximately 2 billion illnesses across the age range and 752 000 million deaths occurring among children <5 years of age annually [1, 2]. Noroviruses are one of the leading causes of diarrheal disease in both developing [1, 3] and industrialized countries [4]. Although the highest incidence of norovirus infection is among children <5 years of age, infection and disease occur throughout life, with norovirus associated with an estimated 18% of acute gastroenteritis (AGE) cases in both <5 and ≥5 years age groups [5].
A recent comprehensive study on the causes of enteric infection in children in developing countries found norovirus genogroup (G) II to have the first and second highest attributable incidences of all pathogens as a cause of diarrhea in the first (5.2%) and second (5.4%) years of life, respectively, and was also frequently associated with vomiting [3]. Norovirus-associated diarrhea incidence peaks between 6 and 23 months of age and declines thereafter [6].
Understanding of the epidemiology and natural history of norovirus has increased in recent years, but important gaps remain. For example, there are few data on the norovirus burden among adults at the community level, especially from developing countries or tropical settings. Additionally, case definitions have generally excluded vomiting-only syndromes, which may be a common presentation of norovirus infection [7]. In this article, we report findings from a community-based cohort study in Puerto Maldonado, Peru, that included surveillance of both children and adults, comparing alternative case definitions of AGE with diarrhea only vs diarrhea and/or vomiting to better characterize norovirus disease across age groups and yield the best estimates of norovirus incidence in this community.
METHODS
Study Population and Design
Data were collected from periurban communities in the city of Puerto Maldonado, which is located in the department of Madre de Dios in the southeastern tropical rainforest basin region of Peru. Households were randomly selected from a community georeferenced census that was previously enrolled in an influenza community cohort study [8]. Households already participating in the influenza cohort were invited to participate in the prospective AGE cohort study and were enrolled when at least 60% of household members agreed to participate, resulting in a total of 297 households with 1556 people among all age groups (Table 1). Detailed household characteristics and demographic data were collected at the time of enrollment. We identified 1 or more representative adult household member(s) to be trained in the identification of AGE symptoms and stool sample collection. They serve as household informants and were relied on to provide information about gastroenteritis symptoms for all household members. The cohort was open: Participants could leave or enter the cohort based on their residence and willingness to participate, which was assessed weekly. Each household was provided a standard thermometer for monitoring and recording of oral temperatures during ill periods for household members and also clean plastic containers for collection of stools during diarrhea episodes.
Table 1.
Characteristics of Study Population, Puerto Maldonado, Peru
| First Period | Second Period | |
|---|---|---|
|
|
||
| Study Population | (October 2012–May 2013) | (June 2013–August 2015) |
| Total population, No. | 1556 | 2062 |
| Mean No. of personsa | 1359 | 1052 |
| Total households, No. | 297 | 408 |
| Mean No. of householdsa | 278 | 240 |
| Mean No. of persons per household | 4.9 | 4.4 |
| Age, y, mean (standard deviation) | 25 (19) | 25 (20) |
| Male sex, No. (%) | 734 (47) | 933 (45) |
| Age group, y, No. (%)b | ||
| <2 | 99 (6) | 145 (7) |
| 2–4 | 121 (8) | 170 (8) |
| 5–14 | 384 (25) | 513 (25) |
| 15–44 | 680 (44) | 874 (42) |
| ≥45 | 272 (18) | 360 (18) |
Monthly mean.
Fisher exact test was negative for age group.
Household Surveillance and Case and Control Definitions
Active surveillance for diarrhea among the community began in October 2012, leveraging the participants and infrastructure of our ongoing Influenza Cohort Study [8]. During the first 8 months of follow-up (until May 2013), we used a traditional case definition of diarrheal disease, defined as ≥3 loose or liquid stools in any 24-hour period. From June 2013 until August 2015 (26 months), we switched to an expanded case definition for AGE to detect episodes where vomiting was present without diarrhea, which may occur in norovirus infections [7]. AGE was defined as a participant with any of the following: (1) ≥3 loose or liquid stools in a 24-hour period; (2) ≥2 vomiting episodes in a 24-hour period; or (3) ≥1 vomiting episodes plus ≥1 loose or liquid stools in any 24-hour period. Trained study field workers performed follow-up visits 2 or 3 times a week to ask if any of the household members had experienced any symptoms either of diarrhea or AGE. When a participant reported an event meeting the diarrhea or AGE case definition, the participant or primary caregiver of the children in the household collected the stool specimens in a provided plastic container during the acute phase of illness, as near as possible to the onset of symptoms and within a maximum of 10 days following the onset of symptoms. For the purposes of this analysis, we considered an individual at risk of a (subsequent) diarrhea or AGE episode after they had been free of diarrhea or vomiting for 10 days.
During the second period of active surveillance (June 2013 to August 2015), we also enrolled a control household for every fifth index case of AGE identified in a household. Control households were matched based on the total number of household members and children aged <5 years in the AGE index case household. To be included as a control household, each member of the household had to be free of gastroenteritis symptoms (vomiting and/or loose or liquid stools) for 3 weeks (2 weeks before and 1 week after day of symptom onset of the matched AGE index case). Once a control household was identified, we requested each household member to collect 1 stool specimen. The specimen had to be collected in a period of 10 days, starting from day 7 of symptom onset of the index-matched AGE case.
Laboratory Methods
Stool specimens were transported in portable coolers on ice packs to the nearby field laboratory, where they were aliquoted and stored at −80°C until they were shipped in batches on dry ice to our central laboratory in Lima for testing, where specimens were treated as described in the protocol of Apaza et al [9] with some modifications. In brief, fecal samples were diluted 10% in 1× phosphate-buffered saline, vortexed, and centrifuged at 5943g for 10 minutes at 4°C [10]. Total RNA was extracted from stool diluted samples using the Qiagen QIAmp Viral RNA mini kit (Valencia, California) as described by the manufacturer. RNA was eluted with 60 μL of 0.01% of RNAse inhibitor (Qiagen) in DEPC Treated Water (Invitrogen). RNA samples were stored at −80°C until use. Norovirus detection was performed using a Duplex genogroup-specific real-time reverse-transcription polymerase chain reaction (qRT-PCR) developed and described by the National Calicivirus Laboratory at the Centers for Disease Control and Prevention in a 7500 FAST real-time platform (Applied Biosystems) [11, 12].
RT-PCR was performed at 45°C for 10 minutes and 95°C for 10 minutes followed by 45 cycles of 15 seconds at 95°C and 1 minute at 60°C using primers and probes from previous assays [13, 14]. A sample was considered positive for norovirus GI when the calculated cycle threshold (Ct) was ≤37 cycles, and considered positive for GII when the Ct was ≤39 cycles. Test results were only considered valid when all quality control worked properly; positive reactions were below Ct cutoff for all positive controls, and did not exhibit fluorescence above the threshold for the negative template control reactions.
Statistical Analysis
Incidence of diarrheal disease (period 1), AGE (period 2), and norovirus-associated illness (period 1 and 2) was calculated and expressed per 100 person-years (PY) at risk. Because samples were not collected from participants for all episodes meeting the case definition, we corrected incidence rates to account for undersampling for both diarrhea and AGE cases by multiplying the crude incidence rate by 1 / (proportion of cases with samples collected). Confidence intervals (CIs) were based on quadratic approximation of the Poisson function. Incidence rates were stratified by age.
To characterize the clinical presentations of norovirus and the value of the vomiting-based case definitions, we report the percentage of norovirus positives meeting each component of the expanded case definition of AGE. The overall proportions of norovirus cases detected were reported using each part of the expanded case definition for AGE by age group. Stata software version 13.1 (StataCorp, College Station, Texas) was used for all statistical analysis.
Ethical Considerations
This study was reviewed and approved by the Institutional Review Board of US Naval Medical Research Unit No. 6 (NAMRU6 2012.0013) in compliance with all applicable federal regulations governing the protection of human subjects. Host country approval was obtained from the Peruvian Ministry of Health. Informed written consent was obtained from each adult participant or parent or guardian of a child at the time of enrollment.
RESULTS
From October 2012 to May 2013, a total of 1556 participants in 297 households were enrolled. Monthly averages of 1359 participants from 278 households were under active surveillance (Table 1). During this period using the diarrhea case definition, 294 cases were identified and 188 (64%) provided a stool sample. Fifteen percent (29/188) of specimens were positive for norovirus, 76% (22/29) of which were GII. The incidence of diarrhea and norovirus-associated diarrhea was 37.2/100 PY (95% CI, 33.2–41.7) and 5.7/100 PY (95% CI, 3.9–8.1), respectively (Table 2).
Table 2.
Incidence of All and Norovirus-Specific Diarrhea, Overall and by Age Group, October 2012–May 2013, Puerto Maldonado, Peru
| Age Group, y | Person-years | First Period (October 2012–May 2013) |
||||
|---|---|---|---|---|---|---|
| Diarrhea Incidence |
Sampled, No. (%) | Norovirus Diarrhea Incidenceb |
||||
| Events | Rate (95% CI)a | Events | Rate (95% CI)a | |||
| Overall | 789 | 294 | 372 (33.2–41.7) | 188 (64) | 45 | 5.7 (3.9–8.1) |
| <2 | 37 | 74 | 199 (1573–248.3) | 56 (76) | 19 | 49.7 (28.3–81.5) |
| 2–4 | 64 | 63 | 98.7 (76.5–125.5) | 40 (63) | 3 | 4.9 (.8–16.4) |
| 5–14 | 199 | 52 | 26.2 (19.7–34) | 37 (71) | 6 | 2.8 (.8–6.9) |
| 15–44 | 344 | 54 | 15.7 (11.9–20.4) | 26 (48) | 10 | 3 (1–6.6) |
| ≥45 | 146 | 51 | 34.9 (26.3–45.6) | 29 (57) | 7 | 4.8 (1.6–11.6) |
Abbreviation: CI, confidence interval.
Per 100 person-years.
Adjusted by undersampling.
From June 2013 to August 2015, a total of 2062 participants in 408 households were enrolled, with a monthly average of 1052 participants from 240 households under active surveillance (Table 1). During this period using the expanded AGE case definition, 1123 cases were identified and 896 (80%) provided a stool sample. Thirteen percent (113/896) of specimens were positive for norovirus, 84% (95/113) of which were GII, and 1 GI/GII coinfection. The incidence of AGE and norovirus-associated AGE was 51.8/100 PY (95% CI, 48.8–54.9) and 6.5/100 PY (95% CI, 5.4–7.8), respectively (Table 3). When applying the diarrhea case definition (as used in the first surveillance period) to the second surveillance period, diarrhea and norovirus-associated diarrhea incidence decreased to 42.7/100 PY (95% CI, 40.1–45.6) and 5.2/100 PY (95% CI, 4.2–6.4), respectively (Table 3), highlighting the increased sensitivity of the second case definition.
Table 3.
Incidence of All and Norovirus-Specific Diarrhea and Acute Gastroenteritis, Overall and by Age Group, June 2013–August 2015, Puerto Maldonado, Peru
| Age Group, y | Person-years | Second Period (June 2013–August 2015) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea Incidence |
Norovirus Diarrhea Incidenceb |
AGE Incidence |
Norovirus AGE Incidenceb |
||||||||
| Events | Rate (95% CI)a | Sampled, No. (%) | Events | Rate (95% CI)a | Events | Rate (95% CI)a | Sampled, No. (%) | Events | Rate (95% CI)a | ||
| Overall | 2169 | 927 | 42.7 (40.1–45.6) | 754 (81) | 115 | 5.2 (4.2–6.4) | 1123 | 51.8 (48.8–54.9) | 896 (80) | 142 | 6.5 (5.4–78) |
| <2 | 90 | 205 | 227.7 (198.2–260.6) | 169 (82) | 44 | 48.5 (34.4–66.5) | 248 | 275.6 (242.8–311.5) | 203 (82) | 56 | 62.4 (46.3–82.6) |
| 2–4 | 179 | 189 | 105.8 (91.5–121.7) | 158 (84) | 17 | 9.4 (5.4–15.3) | 222 | 124.3 (108.7–141.5) | 185 (83) | 20 | 11.4 (6.8–17.9) |
| 5–14 | 577 | 185 | 32.1 (27.7–36.9) | 181 (98) | 15 | 1.9 (1–3.4) | 265 | 45.9 (40.6–51.7) | 194 (73) | 23 | 4 (2.5–6.3) |
| 15–44 | 891 | 202 | 22.7 (19.7–26) | 156 (77) | 23 | 2.5 (1.5–3.9) | 231 | 25.9 (22.7–29.4) | 173 (75) | 25 | 2.8 (1.7–4.4) |
| ≥45 | 432 | 146 | 33.8 (28.6–39.6) | 130 (89) | 17 | 3.9 (2.3–6.5) | 157 | 36.3 (30.9–42.3) | 141 (90) | 17 | 3.9 (2.2–6.2) |
Abbreviations: AGE, acute gastroenteritis; CI, confidence interval.
Per 100 person-years.
Adjusted by undersampling.
With the AGE case definition, detection of norovirus-associated AGE incidence was 20% higher than norovirus-associated diarrhea-only incidence (Table 3). Importantly, all of the norovirus AGE cases presenting with vomiting only, without any other symptoms, were found exclusively among participants <15 years of age and primarily in children <2 years of age (Figure 1). The proportion of norovirus-positive cases with vomiting alone was 35% (7/20) among children aged <2 years, 13% (1/8) among 2–4 years, and 8% (3/37) among 5–14 years (Table 4). The incidence of norovirus–associated AGE in the absence of diarrhea was 9.7/100 PY (95% CI, 4.3–19.3), 0.8/100 PY (95% CI, 0–4.2), and 0.8/100 PY (95% CI, 0.2–2.1) among children aged <2 years, 2–4 years, and 5–14 years, respectively (Figure 1).The highest incidence of norovirus-associated diarrhea and norovirus-associated AGE occurred among children <2 years of age in both periods with an overall incidence of 49.7/100 PY (95% CI, 28.3–81.5) and 62.4/100 PY (95% CI, 46.3–82.6), respectively (Tables 2 and 3). Norovirus-positive cases were also present among very young children, with 6% of cases among children <6 months of age and 28% among those 6–11 months of age (Figure 2). Norovirus infections circulated year round without a noticeable seasonal pattern (Figure 3).
Figure 1.
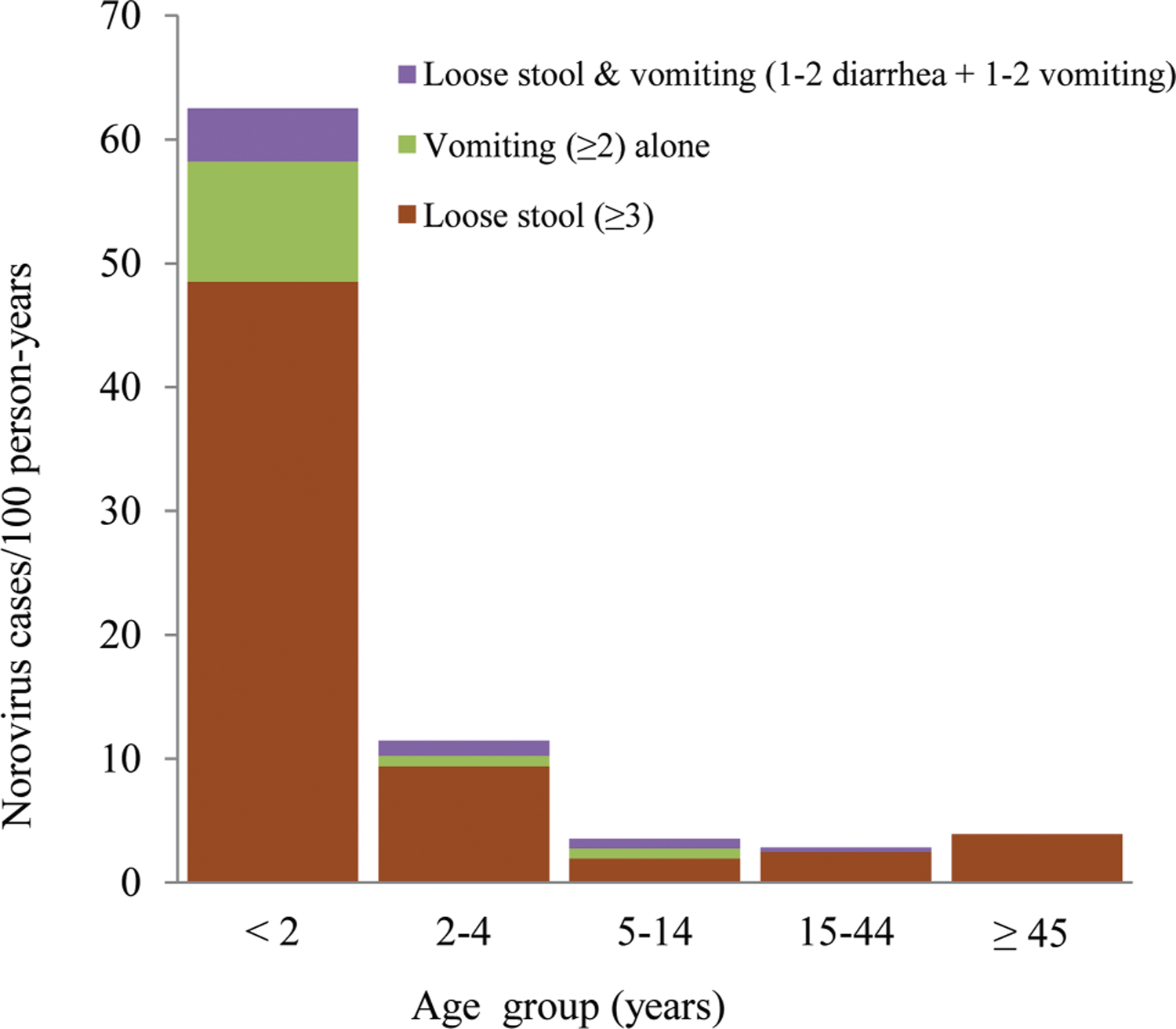
Norovirus incidence by age group and case definition, June 2013–August 2015.
Table 4.
Proportion of Norovirus-Positive Cases by Age Group and Case Definition
| Age Group, y | Total AGE Sampled | ≥3 Loose or Liquid Stoolsa | ≥2 Vomiting Episodesa | 1–2 Vomiting Episodes + 1–2 Loose Stoolsa |
|---|---|---|---|---|
| <2 | 203 | 36/169 (21) | 7/20 (35) | 3/14 (24) |
| 2–4 | 185 | 14/158 (9) | 1/8 (13) | 2/19 (11) |
| 5–14 | 194 | 11/141 (8) | 3/37 (8) | 3/16 (19) |
| 15–44 | 173 | 17/156 (11) | 0/9 (0) | 2/8 (25) |
| ≥45 | 141 | 15/130 (12) | 0/4 (0) | 0/7 (0) |
| Total | 896 | 93/754 (12) | 11/78 (14) | 10/64 (16) |
Data are presented as No. (%).
Abbreviation: AGE, acute gastroenteritis.
Fisher exact test according to each component of AGE case definition was not significant.
Figure 2.
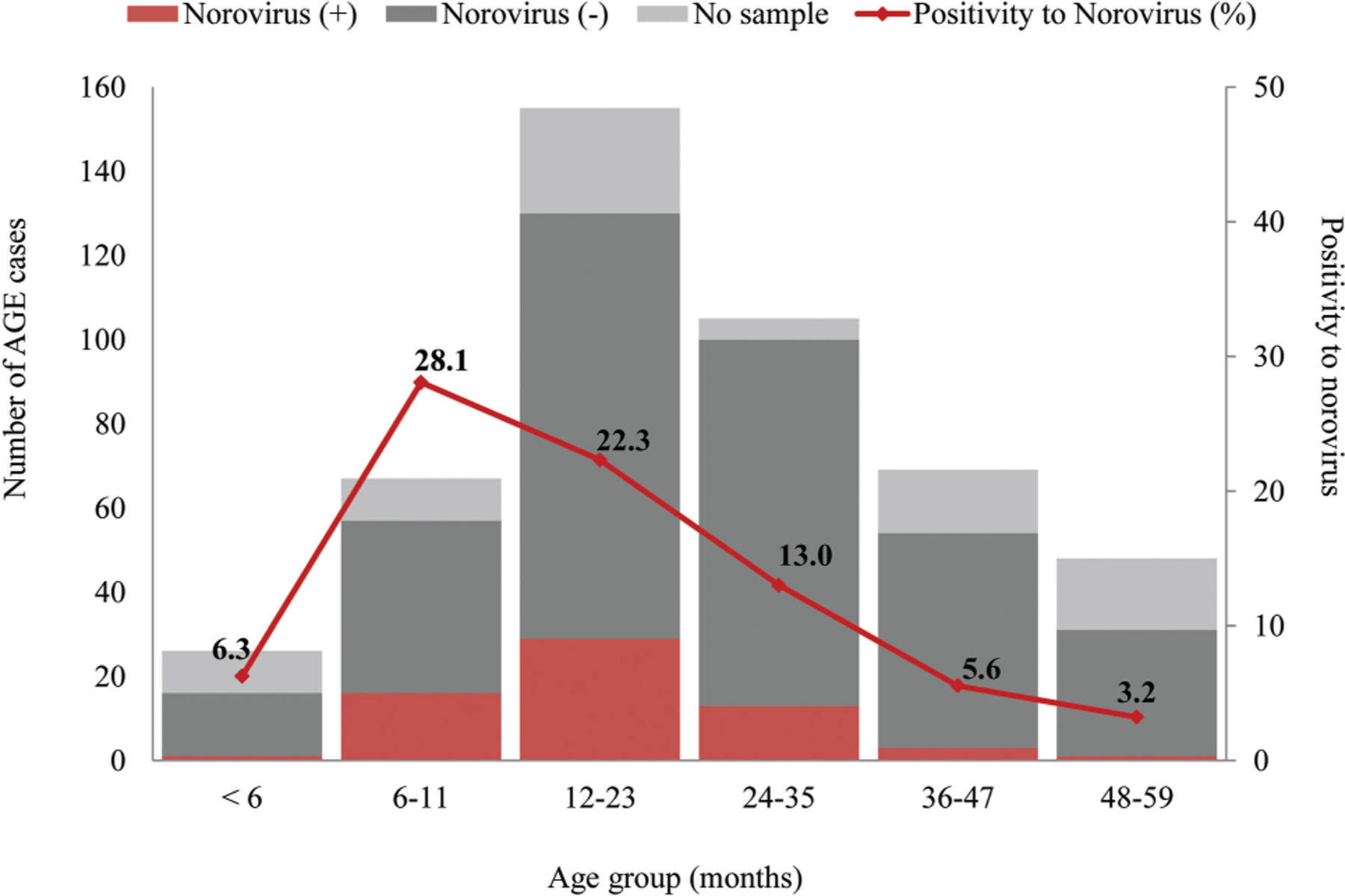
Number of acute gastroenteritis (AGE) cases by norovirus testing result and percentage positive for norovirus among children <5 years of age, June 2013–August 2015.
Figure 3.
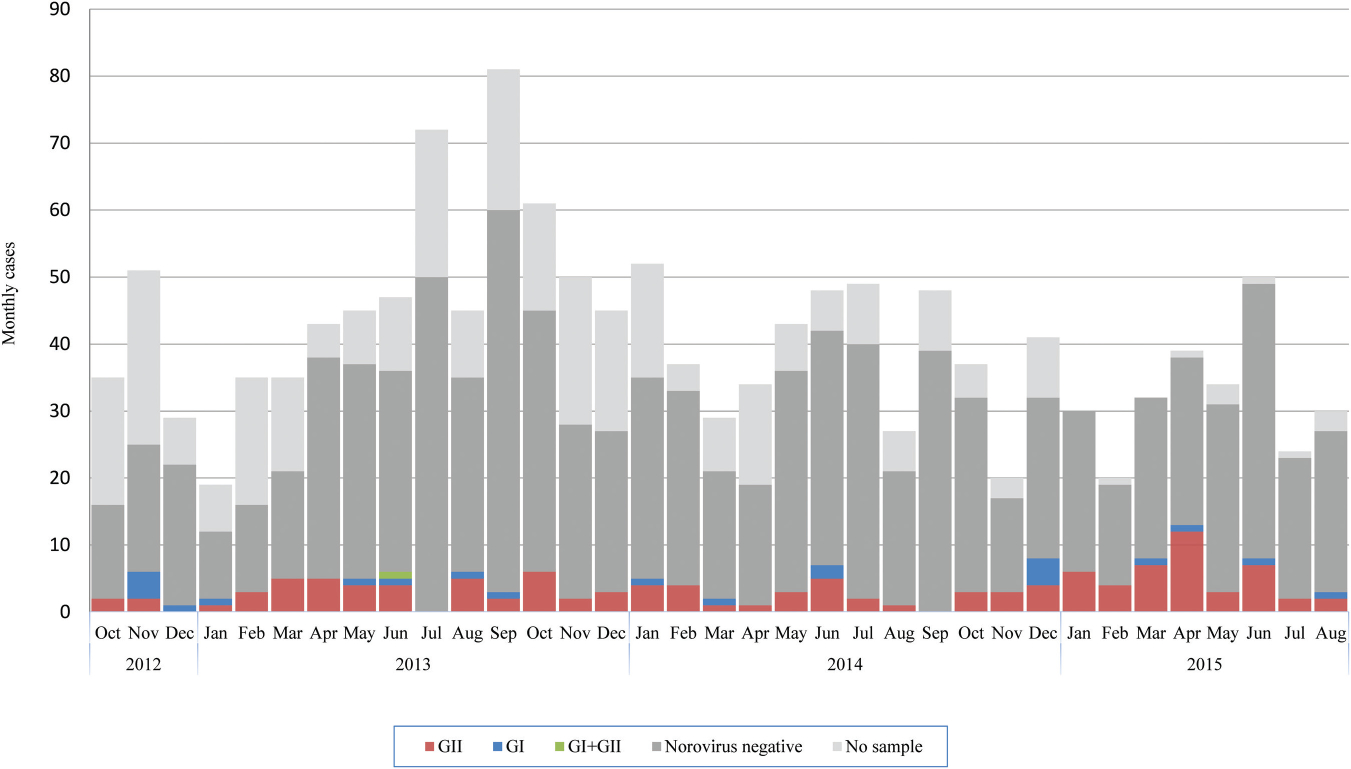
Total cases and norovirus testing results per month. October 2012–May 2013 includes only diarrhea cases and June 2013–August 2015 includes acute gastroenteritis (AGE) cases. Abbreviations: GI, genogroup i; GII, genogroup ii.
During the first surveillance period, 6 persons were hospitalized due to diarrheal disease, 4 of whom were children <5 years old, and 1 child of 3 who provided stool specimens was positive for norovirus GII. During the second period, 8 persons were hospitalized with diarrheal disease, 6 of whom were children <5 years old, and 1 child of 3 who provided stool specimens was positive for norovirus GII.
During the second period, 105 households were selected as controls including a total of 578 household members. Stool samples were collected from 58% (335/578) of the control household members, 7% (23/335) of which were positive for norovirus (16 GII and 7 GI), with median age of 9 years (interquartile range, 3–16). The 23 norovirus-positive controls came from 11 different households, 6 of which had >1 household member positive for norovirus and 3 of which had co-circulation of norovirus GI and GII. The greatest proportion positive for norovirus among controls was in children <2 years old (24%) followed by those 5–14 years old (9%).
Norovirus was significantly associated with disease in only the ≥45 years age group (P = .026), and the overall detection rates were significantly higher in AGE cases than controls (Table 5).
Table 5.
Acute Gastroenteritis and Controls Positive for Norovirus, June 2013–August 2015, Puerto Maldonado, Peru
| Age Group, y | Controls Positive to Norovirus | AGE Positive to Norovirus | P Value (AGE vs Controls Positive to Norovirus) |
|---|---|---|---|
| <2 | 5/21 (24) | 46/203 (23) | 1.000 |
| 2–4 | 2/50 (4) | 17/185 (9) | .372 |
| 5–14 | 9/105 (9) | 17/194 (9) | 1.000 |
| 15–44 | 7/114 (6) | 19/173 (11) | .232 |
| ≥45 | 0/45 (0) | 15/141 (11) | .026 |
| Total | 23/335 (7) | 114/896 (13) | .004 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: AGE, acute gastroenteritis.
DISCUSSION
Owing to its community cohort study design with intensive follow-up, this study provides a number of novel observations about norovirus epidemiology for which there are few other data, especially from tropical settings. First, we quantified the incidence of norovirus-associated AGE across the age range at 6.5% per year. Second, we noted substantially higher rates in young children, especially those aged <2 years at 62% per year. Third, we quantified the percentage of norovirus cases missed with a traditional diarrhea-based case definition at 20% by including presentations of vomiting only and vomiting plus 1 or 2 diarrhea episodes. Fourth, we showed that when using a strict symptom-free exclusion period, norovirus was detected in 7% of healthy controls across the age range and 24% among <2-year-olds.
Community-based studies that describe norovirus incidence in children and adults have rarely been conducted. Our estimate of 5.7%–6.5% per year is similar to estimates from community-based studies in Kenya at 4.1%–9.6% [15], England at 4.5%–4.7% [16, 17], and the United States at 6.8% [18]. Among children <2 years old, we found a much higher incidence (49.7%–62.4% per year) than in any other age group. This incidence of norovirus-associated AGE, while exceedingly high, is similar to estimates from birth cohort studies in Peru for <2-year-olds (approximately 50% per year), higher than Ecuador for <3-year-olds (17% per year), and consistent with estimates for a broader <5-year-old in England (21% per year) and Nicaragua (23% per year) [16, 19–21].
A cardinal symptom of norovirus-associated AGE is vomiting. However, surveillance and etiological studies typically require diarrhea as the key component of the case definition. The inclusion of vomiting only and vomiting with 1 or 2 loose stool syndromes increased norovirus case detection in the second period of our study by 20%. This broader case definition increased incidence similarly for norovirus as all-cause AGE, either because (1) norovirus is as frequently a cause of vomiting as diarrhea or (2) nonpathogenic “background” infections are equally common in both syndromes. Interestingly, all of the vomiting-only cases were among the <15 years age group, and 35% of those aged <2 years with vomiting only were positive for norovirus. This observation somewhat contradicts observations from experimental challenge studies in US adults where vomiting only was as frequent as vomiting with diarrhea [22]. Regardless, vomiting-only cases are not included in some burden of disease studies [23], and our study suggests that this may lead to considerable underestimation.
Postsymptomatic shedding of norovirus can be prolonged and persist following the resolution of AGE symptoms for a median period of >1 month [5, 20]. Similar to our findings, in high-exposure environments, norovirus has been detected in healthy controls at or near levels similar to AGE cases [24]. The Global Enteric Multicenter Study (GEMS), for example, used an exclusion period of only 7 days and attributed only a small fraction of diarrheal disease to norovirus [25]. Regardless, using our strict exclusion criteria that controls were asymptomatic for 3 weeks, the detection rate was 7%, which is similar to 7% reported globally [5] and 9.7% (range, 7%–31%) reported in Africa [26], but higher than a study among US children (4%) that used a similarly strict exclusion period for controls [27].
A number of limitations of this study should be noted. First, we only tested stool for norovirus GI and GII. Due to the limited capacity of the field-based laboratory, we were unable to perform on-site stool culture for detection of bacterial pathogens. Second, we were not able to describe the norovirus epidemiology of the elderly population (≥60 years) given that this age group represents only 4% of total population in this city [28], which was reflected in the age distribution of our study population. Finally, our first period of surveillance was only 8 months in length before we switched to the second case definition. In many settings, norovirus is highly seasonal [18]; however, in our study we did not observe a consistent seasonal pattern during the 34-month study period that would have resulted in a systematic bias in our incidence estimates. Despite these limitations, this study is unique and robust in that it (1) provides some of the first population-based estimates of norovirus incidence from a rapidly developing tropical region; (2) covered the entire age range; (3) included intensive twice-/thrice-weekly home visits to detect episodes meeting the case definition; (4) included both a traditional diarrheal disease case definition and a more sensitive AGE case definition; and (5) included sampling of healthy control households with a strict 3-week symptom-free exclusion period.
Our study and others clearly identify young children as an important age group for interventions aimed at controlling norovirus. They have highest incidence and also appear to be particularly important in the transmission of virus to other family members within the household [29–31]. Accordingly, this group likely stands to benefit most from prevention and control strategies, including vaccination. Norovirus vaccines are progressing steadily through the development pipeline, with encouraging results in phase 1/2 vaccination and challenge studies [32, 33]. Our results support continued efforts in vaccine development and other control strategies for populations in Latin America, especially young children. Our study, though relatively small, also has implications for design of future field studies of norovirus. Such studies should include vomiting-based case definitions and have strict exclusion periods for controls. Proper estimates of norovirus incidence among communities and especially in children will be an important foundation to justify implementation of norovirus vaccine programs and for evaluation of vaccine efficacy among targeted populations.
Financial support.
This work was supported by Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US government.
References
- 1.Kirk MD, Pires SM, Black RE, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 2015; 12:e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization-Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62(suppl 2):S96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shioda K, Kambhampati A, Hall AJ, Lopman BA. Global age distribution of pediatric norovirus cases. Vaccine 2015; 33:4065–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirby AE, Streby A, Moe CL. Vomiting as a symptom and transmission risk in norovirus illness: evidence from human challenge studies. PLoS One 2016; 11:e0143759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razuri H, Romero C, Tinoco Y, et al. Population-based active surveillance cohort studies for influenza: lessons from Peru. Bull World Health Organ 2012; 90:318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apaza S, Espetia S, Gilman RH, et al. Detection and genogrouping of noroviruses from children’s stools by TaqMan One-step RT-PCR. J Vis Exp 2012. doi: 10.3791/3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trujillo AA, McCaustland KA, Zheng DP, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol 2006; 44:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinje J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis 2011; 17:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debbink K, Costantini V, Swanstrom J, et al. Human norovirus detection and production, quantification, and storage of virus-like particles. Curr Protoc Microbiol 2013; 31:15K.1.1–.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kageyama T, Kojima S, Shinohara M, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 2003; 41:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent R, Hill BM, Narayanan J, Karen F, Vinje J. Detection of GI and GII noroviruses in ground water using ultrafiltration and TaqMan real-time RT-PCR. Food Environ Virol 2010; 2:218–24. [Google Scholar]
- 15.Shioda K, Cosmas L, Audi A, et al. Population-based incidence rates of diarrheal disease associated with norovirus, sapovirus, and astrovirus in Kenya. PLoS One 2016; 11:e0145943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol 2010; 171:1014–22. [DOI] [PubMed] [Google Scholar]
- 17.Tam CC, Rodrigues LC, Viviani L, et al. ; IID2 Study Executive Committee. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012; 61:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grytdal SP, DeBess E, Lee LE, et al. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012–2013. PLoS One 2016; 11:e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker-Dreps S, Bucardo F, Vilchez S, et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J 2014; 33:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito M, Goel-Apaza S, Espetia S, et al. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis 2014; 58:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopman BA, Trivedi T, Vicuña Y, et al. Norovirus infection and disease in an Ecuadorian birth cohort: association of certain norovirus genotypes with host FUT2 secretor status. J Infect Dis 2015; 211:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14:1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE; Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopman B, Simmons K, Gambhir M, Vinjé J, Parashar U. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol 2014; 179:507–12. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 26.Mans J, Armah GE, Steele AD, Taylor MB. Norovirus epidemiology in Africa: a review. PLoS One 2016; 11:e0146280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Instituto Nacional de Estadísticañ e Informática. Peru: analisis etnodemografico de las Comunidades Nativas de la Amazonia Peruana, 1993 y 2007. Lima, Peru: INEI, 2007. [Google Scholar]
- 29.Phillips G, Tam CC, Rodrigues LC, Lopman B. Risk factors for symptomatic and asymptomatic norovirus infection in the community. Epidemiol Infect 2011; 139:1676–86. [DOI] [PubMed] [Google Scholar]
- 30.de Wit MA, Koopmans MP, van Duynhoven YT. Risk factors for norovirus, Sapporo-like virus, and group A rotavirus gastroenteritis. Emerg Infect Dis 2003; 9:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsten C, Baumgarte S, Friedrich AW, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis 2009; 28:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein DI, Atmar RL, Lyon GM, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


