ABSTRACT.
Zika virus (ZIKV) infection during pregnancy may cause severe fetal abnormalities and therefore it is important to diagnose and distinguish between recent and past Zika infection. Serological diagnosis assays detect antibodies against the envelope protein, that suffer from high cross-reactivity. In addition, reports regarding long IgM persistence prevent its use in diagnosis of recent Zika infection. Following the Zika pandemic, a novel ELISA assay based on detection of IgM and IgG antibodies against Zika nonstructural 1 (NS1) protein was developed (NS1-IgM and NS1-IgG). Here, antibodies against NS1 were assessed in Israeli travelers diagnosed with Zika. NS1-IgM and NS1-IgG antibodies from 36 travelers diagnosed with ZIKV infection were detected as early as 5 days after symptom onset. However, while IgG levels were maintained for several months, IgM levels in all samples declined rapidly and by 31 days after symptom onset, no IgM positive samples were detected. Interval-censored survival analysis demonstrated 25%, 50%, and 75% decline in NS1-IgM levels in 29 days (95% CI: 22–34), 34 days (95% CI: 29–44), and 44 days (95% CI: 34–65), respectively. Our results suggest that IgM antibodies against ZIKV NS1 are short lived and can be used as a reliable marker for diagnosis of recent ZIKV infection.
INTRODUCTION
During 2015, Zika virus (ZIKV) has emerged in South America and in 2016, was declared as a public health emergency of international concern (PHEIC). 1 Since then, ZIKV has spread to over 80 countries and territories by both the endemic population as well as by travelers who substantially contributed to this rapid spread. 2, 3 The primary mode of transmission of this flavivirus is by mosquitoes, mostly via species from the Aedes genus; however, vertical transmission from mother to child, sexual transmission, and transmission through blood transfusion may also occur. 4 Most importantly, infection of the fetus, especially during the first trimester, can cause severe congenital malformation including microcephaly or other fetal brain defects such as problems with hearing and vision and possible developmental delays. 5
Molecular diagnosis of ZIKV RNA in bodily fluids by quantitative real-time-polymerase chain reaction (qRT-PCR) is possible; however, due to low and short viremia and viruria, 6, 7 a negative result does not rule out ZIKV infection. In addition, resources for molecular diagnosis are, in many cases and especially in ZIKV endemic countries, not available. Serological diagnosis is therefore, often essential for diagnosis of ZIKV infection. As in most viral infections, the presence of IgM antibodies is indicative of recent infection and therefore serves as the primary serological diagnosis for recent infection, while IgG antibodies are indicative of past infection. Serological diagnosis of ZIKV infection can be performed by detection of IgM and IgG antibodies against the envelope (E) protein; however, these assays suffer from cross-reaction with other flaviviruses. 8 In addition, recently, ZIKV IgM against the E protein (IgM-E) was shown to persist for months to years after infection, 9, 10 significantly complicating distinction of recent versus past infection and the assessment of the risk for ZIKV congenital syndrome among pregnant women. The nonstructural 1 (NS1) protein seems to be more specific with less cross-reaction with other flaviviruses. 11 Assessment of the role of IgM antibodies against Zika NS1 protein as a tool to distinguish between recent and past infection has never been published and in the case of a relatively recently known virus like ZIKV, the kinetics of antibodies (IgM and IgG) is still unclear.
In this study, we evaluated the detection time of ZIKV IgM and IgG against the NS1 protein in travelers with ZIKV infection.
METHODS
Patients.
Between December 2015 and October 2019, 36 Israeli travelers returning from ZIKV endemic countries 12 were diagnosed for ZIKV infection at the Israeli national center for zoonotic viruses. Twenty-nine of the cases were confirmed and seven were probable for ZIKV infection and thus were excluded from analysis. In most cases, more than one serum sample was available from each patient as a longitudinal follow up (Table 1).
Table 1.
Characteristics and serology results of confirmed ZIKV infected Israeli travelers diagnosed between 2015 and 2019
| Patient # | Country of possible exposure | Gender/age (year) | qRT-PCR result (first sample) | Neutralization result (titer) | Sample number | Time from onset/return (days) | IgM ELISA | IgG ELISA |
|---|---|---|---|---|---|---|---|---|
| 1 | Colombia | F/50 | Pos | Pos (640) | First | 5 | Pos | Pos |
| Second | 20 | Neg | Pos | |||||
| Third | 92 | Neg | Pos | |||||
| 2 | Colombia | F/32 | Pos | Pos (1280) | First | 10 | Pos | Pos |
| Second | 53 | Equ | Pos | |||||
| 3 | Dominican Republic | M/30 | Pos | Pos (1280) | First | 26 | Pos | Pos |
| Second | 46 | Neg | Pos | |||||
| 4 | Colombia | M/38 | Neg | Pos (1280) | First | 34 | Equ | Pos |
| 5 | Jamaica | M/23 | Pos | Pos (640) | First | 11 | Pos | Equ |
| Second | 25 | Pos | Pos | |||||
| Third | 49 | Neg | Pos | |||||
| 6 | United States (Miami) | F/30 | Pos | Pos (160) | First | 8 | Pos | Neg |
| Second | 160 | Neg | Pos | |||||
| 7 | Costa Rica | M/27 | Pos | Pos (1280) | First | 7 | Pos | Neg |
| Second | 18 | Equ | Equ | |||||
| Third | 37 | Neg | Pos | |||||
| 8 | Mexico | M/26 | Pos | Pos (640) | First | 27 | Equ | Pos |
| Second | 52 | Neg | Pos | |||||
| 9 | Mexico | F/30 | Pos | Pos (1280) | First | 12 | Pos | Equ |
| Second | 15 | Pos | Pos | |||||
| Third | 29 | Equ | Pos | |||||
| Fourth | 50 | Neg | Pos | |||||
| 10 | Mexico | M/37 | Pos | Pos (1280) | First | 5 | Equ | Neg |
| Second | 14 | Pos | Neg | |||||
| Third | 28 | Pos | Pos | |||||
| Fourth | 59 | Neg | Neg | |||||
| Fifth | 95 | Neg | Pos | |||||
| 11 | Costa Rica | M/26 | Pos | Pos (160) | First | 6 | Neg | Neg |
| Second | 12 | Pos | Neg | |||||
| 12 | Costa Rica | M/20 | Neg | Pos (1280) | First | 29 | Pos | Pos |
| 13 | Mexico/Cuba | F/21 | Pos | Pos (1280) | First | 6 | Neg | Neg |
| Second | 16 | Pos | Pos | |||||
| 14 | Colombia | F/22 | Neg | Pos (40) | First | 59 | Neg | Pos |
| Second | 91 | Neg | Pos | |||||
| 15 | Honduras | F/22 | Neg | Pos (160) | First | ∼60 | Neg | Pos |
| 16 | Central America | M/29 | Neg | Pos (320) | First | 105 | Neg | Pos |
| 17 | Panama | M/30 | Neg | Pos (40) | First | 30 | Pos | Pos |
| Second | 105 | Neg | Pos | |||||
| Third | 108 | Neg | Pos | |||||
| Fourth | 180 | Neg | Equ | |||||
| 18 | Cuba/Mexico | M/29 | Neg | Pos (320) | First | ∼75 | Neg | Pos |
| 19 | Thailand/Philippines | M/26 | Neg | Pos (160) | First | ∼30 | Neg | Pos |
| Second | ∼40 | Neg | Pos | |||||
| 20 | Porto-Rico | M/35 | Neg | Pos (20) | First | ∼70 | Neg | Pos |
| 21 | Vietnam | M/61 | Pos | Neg | First | 10 | Pos | Neg |
| Second | 58 | Neg | Neg | |||||
| Third | 80 | Neg | Neg | |||||
| 22 | Thailand | M/27 | Pos | Pos (1:40) | First | 10 | Pos | Neg |
| Second | 19 | Equ | Pos | |||||
| 23 | San Martin | M/32 | Neg | Pos (1:320) | First | 360 | Neg | Pos |
| 24 | M/24 | Neg | Pos (1:320) | First | 10 | Pos | Pos | |
| Second | ∼260 | Neg | Pos | |||||
| 25 | Thailand | F/53 | Pos | Pos (1:160) | First | 4 | Neg | Neg |
| Second | 11 | Equ | Pos | |||||
| Third | 22 | Neg | Pos | |||||
| 26 | Thailand | F/49 | Pos | Pos (1:320) | First | 3 | Neg | Neg |
| Second | 9 | Equ | Pos | |||||
| 27 | Thailand | F/22 | Pos | Pos (1:1280) | First | 9 | Pos | Pos |
| 28 | Thailand | F/43 | Pos | Pos (1:80) | First | 5 | Neg | Neg |
| Second | 34 | Neg | Pos | |||||
| 29 | Thailand | M/35 | Pos | Pos (1:20) | First | 5 | Equ | Neg |
| Second | 16 | Pos | Pos |
Laboratory ZIKV case definition.
ZIKV infection is considered confirmed if ZIKV nucleic acid is detected in, or isolated from, a clinical specimen; or if ZIKV specific IgM or IgG antibodies are detected in serum sample(s) and confirmed by neutralization test. ZIKV infection is considered probable in case ZIKV antibodies are detected in a serum sample without neutralization or RT-PCR.
ELISA.
Serological diagnosis by Euroimmun ZIKV ELISA (Anti- ZIKV IgM or IgG ELISA, # EI 2668-960G or M, Euroimmun, Lübeck, Germany) that determines IgM-NS1 and IgG-NS1 levels, was performed on all serum samples according to the manufacturer’s recommendation. Briefly, following 1:101 dilution in sample buffer, serum samples were added to ZIKV NS1 antigen coated plates and incubated at 37°C for 60 minutes. After several incubation and washing steps, the optical density (OD) was measured in a Tecan Sunrise system (Austria, GMBH). A signal-to-cut-off ratio was calculated, and values < 0.8 were regarded as negative, ≥ 0.8 to < 1.1 as borderline, and ≥ 1.1 as positive.
Zika qRT-PCR.
Quantitative real-time-PCR was performed as described by Lustig et al. 13 Briefly, total nucleic acids were extracted using the NucliSENS EasyMAG system (bioMérieux, Marcy l’Etoile, France) and Zika RNA was detected by qRT-PCR assay using specific primer probe set for the E protein as described by Lanciotti et al. 8
Zika neutralization assay.
Micro-neutralization assay was performed as described by Lustig et al. 13 Briefly, 100TCID50 Zika MR-677 was incubated with inactivated ELISA positive sera diluted 1:10–1:1280 in 96 well plates for 60 minutes in 37°C. Vero E-6 cells were added to each well and incubated for 5 days. Neutralizing dilution of each serum sample was determined by identifying the well with the highest serum dilution without observable cytopathic effect. A dilution equal to 1:10 or above was considered neutralizing.
Survival curve.
Estimation of the survival curve, that is, the proportion of individuals with IgM levels remaining above 0.8 plotted against time from symptom onset/return to Israel, was conducted in two stages. First, for those individuals who were serologically assessed more than once and had at least one level above 0.8 followed by at least one level below 0.8, a linear regression of the individual’s IgM log titer values against time was fit and the time to reach the 0.8 level was estimated. The time to reach 0.8 for individuals with serological levels only above 0.8 or only below 0.8 was taken as interval-censored either between 0 and the day of first serological measurement for those with all values below 0.8, or between the day of last serological measurement and infinity for those with all values above 0.8. Then a nonparametric maximum likelihood estimator (NPMLE) analysis for interval-censored data was performed using the R software, icfit, which provided an estimate of the survival curve with 95% bootstrap confidence limits for each point on the curve. 14
RESULTS
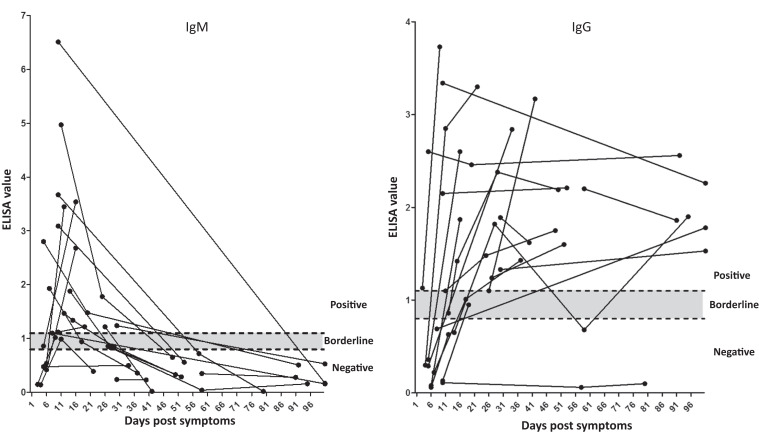
All 29 cases with confirmed ZIKV infection were obtained between 3 and 360 days post-symptom onset (PSO) or return from travel and had clinical data. Altogether 62 samples were tested. Table 1 summarizes the molecular and serological results of all 29 cases, including qRT-PCR, neutralization, and ELISA. To assess the kinetics of antibodies against NS1, IgM and IgG antibody titers were plotted against time PSO. Results (Figure 1 and Table 1) demonstrate that out of 19 samples obtained between days 5 and 15 PSO, 16 were positive or borderline for IgM and seven for IgG. By day 31 PSO, however, no IgM positive samples could be detected.
Figure 1.
Kinetics of NS1 -IgM and -IgG antibodies against Zika virus (ZIKV). ELISA values of IgM and IgG antibodies against the NS1 protein from 21 ZIKV positive patients with more than one sample are depicted against the time since onset of symptoms. Positive (≥ 1.1), negative (< 0.8), and borderline (between 0.8 and 1.1) areas are presented.
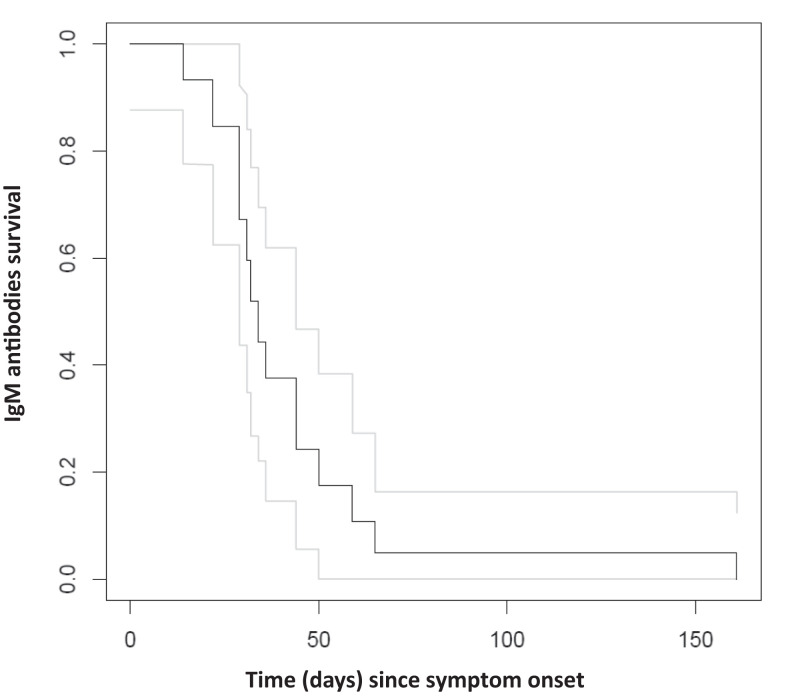
Our estimate of the duration of ZIKV IgM detection curve included samples from 28 travelers analyzed by ZIKV-NS1 ELISA. For 14 travelers, we could estimate a time period of detection, while for another 14, the time was interval-censored. The survival curve for persistence of IgM-NS1 (positive or borderline) detection estimated from these data is shown in Figure 2. Estimates of the time for 75%, 50%, and 25% IgM-NS1 persistence are 29 days (95% CI: 22–34), 34 days (95% CI: 29–44), and 44 days (95% CI: 34–65), respectively.
Figure 2.
Survival curve of IgM-NS1 antibodies. ELISA values of 42 samples from 28 travelers were used to evaluate the decay of ZIKV IgM-NS1 antibodies. The black and gray lines demonstrate the survival and 95%CI of IgM antibodies in correlation to the time since onset of symptoms.
DISCUSSION
The presence of IgM antibodies is usually indicative of recent viral infection. Flavivirus serological diagnosis is mainly based on detection of antibodies against the E protein. However, while enjoying high sensitivity, E-protein-based detection suffers from significant cross-reactivity. 8 In addition, long IgM persistence was shown for both Dengue and West Nile Virus (WNV): DENV E-IgM were shown to be detectable for 179 days 15 and WNV E-IgM can persist for 6 months to several years PSO. 16 Persistence of ZIKV IgM was not a significant issue until the recent ZIKV epidemic, where accurate diagnosis has become critical, especially among pregnant women. A recent study demonstrated that 76% of ZIKV IgM antibodies against the E protein are detectable 25 months PSO 9 in ZIKV-infected travelers from areas with none or limited Zika and dengue circulation. Another study evaluated the mean time to IgM seroreversion to be 237 days (95% CI: 128–459). 10 Importantly, serological diagnosis of ZIKV infection in the United States is based on detection of antibodies against the E protein. Due to the long IgM persistence, CDC guidelines do not recommend ZIKV serologic testing for both symptomatic and asymptomatic pregnant women and specifically mention that “Zika IgM antibodies can persist for months to years following infection. Therefore, detecting Zika IgM antibodies might not indicate a recent infection.” Despite the significant benefits and thorough characterization of E-based IgM detection, results from this study as well as the known high E cross-reactivity with other flaviviruses 17 suggest that NS1-based serology may be a more suitable tool to evaluate recent ZIKV infection and aid E-based serology in ZIKV diagnosis especially in travelers.
Our findings suggest that in travelers ZIKV IgM-NS1 levels decline rapidly and based on the survival analysis in most cases are no longer detected 6 weeks after infection. In this regard, the NS1-based ELISA can distinguish between recent and past infection. Notably, detection of NS1-IgG levels, in addition to IgM, significantly increases diagnostic sensitivity and allows detection of ZIKV-infected patients for years after infection. 11, 18, 19 Therefore, the combined detection of IgM and IgG antibodies against ZIKV NS1 may provide a good diagnostic estimate for recent and past ZIKV infection. Indeed, NS1-IgG was detected as early as 5–15 days PSO in 37% (7/19) of the samples; however, only together with IgM. Accordingly, the presence of NS1-IgM or NS1-IgM and NS1-IgG should indicate recent infection while NS1-IgG alone, past infection.
Based on samples from confirmed ZIKV-infected patients, studies found that a major disadvantage of IgM-NS1 based ELISA is low sensitivity 11, 18, 20 while the sensitivity of IgM-E based ELISA is higher. 11, 21 The results here suggest that low sensitivity in the ELISA may, at least partly, stem from the short duration of detectable IgM-NS1 antibodies. Indeed, when taking into account data from our previous study regarding samples from ZIKV-infected patients taken between 8 and 26 days PSO, 18 sensitivity of the IgM-NS1 based ELISA increased from 68% to 95%. Another study that evaluated samples from ZIKV-infected patients with several ELISA kits, demonstrated IgM- E and NS1 sensitivities of 100% and 37%, respectively; however, 83% of the samples were obtained before the first week or more than 30 days PSO, 11 which is not within the IgM detection time frame we observed in this study. Another method that measures IgM-NS1 levels by qualitative capture assay is indirect chemiluminescent immunoassay (CLIA), 22 which also offers higher sensitivity (98.7%) but should be evaluated for the duration that IgM can be detected. Therefore, sensitivities of the IgM-NS1 based ELISA should be reevaluated and assessed according to the time frames PSO to identify the optimal window of detection of this assay and take into account the survival of NS1-IgM antibodies. In addition, other NS1-based assays should be assessed for sensitivity and IgM persistence.
Limitation of the survival analysis include the use of convenience sampling and small cohort for evaluation of NS1-IgM persistence. Despite this, our survival analysis (Figure 2) clearly demonstrates that by 44 days, and if including also the 95% CI, by a maximum of 65 days after symptom onset only 25% of IgM antibodies may still be detected. Another limitation of our study is that it only included returning-travelers from nonendemic countries, and therefore IgM-NS1 persistence may not be similar in populations of endemic countries. A recent study that evaluated the kinetics of anti-ZIKV antibodies in 15 Zika-infected patients from a ZIKV endemic country using two ELISAs demonstrated median IgM persistence of 13 days for the Euroimmun assay and 53 days for an IgM-NS1 commercial kit by Dia. Pro. Serologic test, 23 suggesting short IgM persistence for both assays. Future studies should examine the persistence of NS1 and E-IgM in larger cohorts in travelers and in local population in areas with Zika and dengue co-circulation.
Similar to dengue, WNV, and Chikungunya, ZIKV will most probably continue to circulate and expand its geographical borders. However, unlike these other arboviruses, diagnosis of recent ZIKV infection will be essential for evaluating the risk for Zika congenital syndrome and the possibility of sexual transmission.
ACKNOWLEDGMENTS
Yaniv Lustig performed literature search, designed the study, analyzed the results, contributed to the analysis and wrote the article. Neta Zuckerman and Laurence Freedman analyzed the results, interpreted the data and contributed to writing the article. Ravit Koren, Shiri Karz-Likvornik and Mayan Yizchaki performed the data collection and some data interpretation. Ella Mendelson reviewed the study design and the article and contributed to the analysis and to writing the paper. Eli Schwartz performed literature search, designed the study, collected the data, contributed to the analysis and to writing the paper.
References
- 1. Wilder-Smith A Osman S , 2020. Public health emergencies of international concern: a historic overview. J Travel Med 27: taaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osman S, Preet R, 2020. Dengue, Chikungunya and Zika in GeoSentinel surveillance of international travellers: a literature review from 1995 to 2020. J Travel Med 27: taaa222. [DOI] [PubMed] [Google Scholar]
- 3. Angelo KM et al. 2020. Zika among international travellers presenting to GeoSentinel sites, 2012–2019: implications for clinical practice. J Travel Med 27: taaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masmejan S Musso D Vouga M Pomar L Dashraath P Stojanov M Panchaud A Baud D , 2020. Zika virus. Pathogens 9: 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vouga M Chiu YC Pomar L de Meyer SV Masmejan S Genton B Musso D Baud D Stojanov M , 2019. Dengue, Zika and Chikungunya during pregnancy: pre- and post-travel advice and clinical management. J Travel Med 26: taz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gourinat AC O’Connor O Calvez E Goarant C Dupont-Rouzeyrol M , 2015. Detection of Zika virus in urine. Emerg Infect Dis 21: 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joguet G et al. 2017. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 17: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 8. Lanciotti RS Kosoy OL Laven JJ Velez JO Lambert AJ Johnson AJ Stanfield SM Duffy MR , 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffin I et al. 2019. Zika virus IgM 25 months after symptom onset, Miami-Dade County, Florida, USA. Emerg Infect Dis 25: 2264–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone M et al. 2020. Zika virus RNA and IgM persistence in blood compartments and body fluids: a prospective observational study. Lancet Infect Dis 20: 1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safronetz D et al. 2017. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis 23: 1577–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leshem E Lustig Y Brosh-Nissimov T Paran Y Schwartz E , 2019. Incidence of laboratory-confirmed Zika in Israeli travelers to Thailand: 2016– 2019. J Travel Med 26: taz057. [DOI] [PubMed] [Google Scholar]
- 13. Lustig Y Mannasse B Koren R Katz-Likvornik S Hindiyeh M Mandelboim M Dovrat S Sofer D Mendelson E , 2016. Superiority of West Nile virus RNA detection in whole blood for diagnosis of acute infection. J Clin Microbiol 54: 2294–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fay MP Shaw PA , 2010. Exact and asymptotic weighted Logrank tests for interval censored data: the interval R package. J Stat Softw 36: i02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prince HE Matud JL , 2011. Estimation of dengue virus IgM persistence using regression analysis. Clin Vaccine Immunol 18: 2183–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray KO Garcia MN Yan C Gorchakov R , 2013. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg 89: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabe IB Staples JE Villanueva J Hummel KB Johnson JA Rose L Hills S Wasley A Fischer M Powers AM , 2016. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 65: 543–546. [DOI] [PubMed] [Google Scholar]
- 18. Lustig Y Zelena H Venturi G Van Esbroeck M Rothe C Perret C Koren R Katz-Likvornik S Mendelson E Schwartz E , 2017. Sensitivity and kinetics of an NS1-based Zika virus enzyme-linked immunosorbent assay in Zika virus-infected travelers from Israel, the Czech Republic, Italy, Belgium, Germany, and Chile. J Clin Microbiol 55: 1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michelson Y Lustig Y Avivi S Schwartz E Danielli A , 2019. Highly sensitive and specific Zika virus serological assays using a magnetic modulation biosensing system. J Infect Dis 219: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 20. De Ory F Sanchez-Seco MP Vazquez A Montero MD Sulleiro E Martinez MJ Matas L Merino FJ , 2018. Comparative evaluation of indirect immunofluorescence and NS-1-based ELISA to determine Zika virus-specific IgM. Viruses 10: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basile AJ Ao J Horiuchi K Semenova V Steward-Clark E Schiffer J , 2019. Performance of InBios ZIKV Detect 2.0 IgM capture ELISA in two reference laboratories compared to the original ZIKV Detect IgM capture ELISA. J Virol Methods 271: 113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van der Beken Y De Geyter D Van Esbroeck M , 2019. Performance evaluation of the Diasorin LIAISON(R) XL Zika capture IgM CLIA test. Diagn Microbiol Infect Dis 95: 144–148. [DOI] [PubMed] [Google Scholar]
- 23. Pasquier C Joguet G Mengelle C Chapuy-Regaud S Pavili L Prisant N Izopet J Bujan L Mansuy JM , 2018. Kinetics of anti-ZIKV antibodies after Zika infection using two commercial enzyme-linked immunoassays. Diagn Microbiol Infect Dis 90: 26–30. [DOI] [PubMed] [Google Scholar]