ABSTRACT.
Visceral leishmaniasis is treated with liposomal amphotericin B (L-AMB), which is associated with nephrotoxicity. Thus, we aimed to investigate nephrotoxicity through novel renal biomarkers in patients with visceral leishmaniasis during L-AMB use. Ours was a prospective study with 17 patients with visceral leishmaniasis treated with L-AMB during their hospital stay. Laboratory tests, renal parameters, urinary biomarkers (urinary kidney injury molecule 1, urinary monocyte chemoattractant protein 1 [uMCP-1], sodium–potassium–2 chloride cotransporter [NKCC2], sodium–hydrogen exchanger 3) [NHE3], aquaporin 2 [AQP2], tumor susceptibility gene 101 [TSH 101], and serum inflammatory biomarkers (MCP-1, interferon-γ, and IL-6) were evaluated in two periods: before and during L-AMB use. Glomerular filtration rate, creatinine, proteinuria, and albuminuria were similar before and during L-AMB use. IL-6 levels, AQT2, and NHE3 expression decreased, whereas uMCP-1 and urinary kidney injury molecule 1 levels increased during L-AMB treatment. In patients who developed acute kidney injury, uMCP-1 showed higher levels. L-AMB aggravated tubuloglomerular lesions, inflammation, and renal tubular disorders. Thus, patients treated with L-AMB need to be monitored for inflammatory and electrolyte disturbances to prevent acute kidney injury, longer length of hospital stay, higher public costs, and mortality.
INTRODUCTION
Visceral leishmaniasis is a parasitic disease caused by Leishmania sp. that affects 400,000 people worldwide and causes 40,000 deaths per year. 1 It is associated with nephropathy, which may be caused by glomerular ultrafiltration of excessive plasma proteins, the leishmaniasis infection itself, immune complex deposition, and renal inflammation. 2
Liposomal amphotericin B (L-AMB) is actually the first-line antileishmanial drug based on efficacy evaluated in different visceral leishmaniasis-endemic regions of the world. It has uncommon and mild toxicity, but it can cause nephrotoxicity and acute kidney injury (AKI), which could also be visceral leishmaniasis comorbidities. Patients with visceral leishmaniasis are monitored by ascertaining levels of serum creatinine, blood urea, and potassium, 3 which are traditional biomarkers with low sensitivity.
Novel kidney biomarkers have shown to be useful tools used to improve diagnosis and prognosis. In addition, urinary extracellular nanovesicles (exosomes) have been used as a non-invasive source of potential new biomarkers. 4 In this context, early diagnosis of AKI during the care of patients with visceral leishmaniasis may contribute to better management of L-AMB therapy and a decrease in AKI incidence, length of hospital stay, public costs, and mortality. 2 Thus, this work aims to investigate early kidney injury associated with L-AMB use in patients with visceral leishmaniasis, using urinary exosomes, and kidney and inflammatory biomarkers.
METHODS
Ours was a prospective study of patients with visceral leishmaniasis treated with L-AMB from April 2017 to January 2018 at São José Hospital, the hospital for Infectious Diseases located in the capital city of Fortaleza, state of Ceará, Brazil. The study included patients of both genders with a confirmed diagnosis of visceral leishmaniasis (positive K-39 antigen test and bone marrow aspirate) who were older than 18 years old and were treated with L-AMB only. Patients were excluded if they had previous kidney disease, used other nephrotoxic drugs, or had a hospital stay of less than 48 hours.
Blood and urine samples were collected at two time points: before treatment (hospital admission) and 3 to 7 days after the start of L-AMB therapy (during treatment). Hematological results were provided by the laboratory of São José Hospital. Biochemical measurements were performed using an automatic biochemistry analyzer (Cobas C111, Roche®, Basileia, CH). Serum sodium and potassium levels were measured using an ion selective electrode analyzer (9180 Electrolyte Analyzer, Roche®, Basileia, CH).
Proteinuria was measured using the colorimetric method (Labtest®), and albuminuria was measured using an immunoturbidimetry assay (Cobas C111). The glomerular filtration rate (GFR) was calculated by using the chronic kidney disease–Epidemiology Collaboration formula, 5 and Kidney Disease Improving Global Outcome criteria were used to establish whether the patients had AKI. 6
Novel urinary kidney biomarkers (urinary kidney injury molecule 1 [uKIM-1] and urinary monocyte chemoattractant protein 1 [uMCP-1]) and serum inflammatory biomarkers (monocyte chemoattractant protein 1 [MCP-1], interferon-γ, and IL-6) were quantified using ELISA (R&D Systems, Minneapolis, MN). All urinary clinical markers and novel biomarkers were adjusted by urinary creatinine levels and are expressed as milligrams per gram creatinine. 7
For the analysis of renal transporters, exosomes were isolated and Western blotting was performed, equalizing exosome content to urinary creatinine levels. 8 Bands corresponding to protein expression of aquaporin 2 (AQP2) (NB110-74682; Novus Biologicals, Chicago, IL), sodium–hydrogen exchanger 3 (NHE3) (NBP1-46581, Novus Biologicals), sodium–potassium–2 chloride cotransporter (NKCC2) (NBP1-57622, Novus Biologicals), and a constitutive exosome marker (tumor susceptibility gene 101 [TSG 101]) (NBP1-80659, Novus Biologicals) were scanned and quantified using the Image Laboratory system (Bio-Rad Laboratories, Redmond, WA). Protein expression was compared with that of healthy control subjects. The study protocol was approved by the Ethics Committee of São José Hospital (CAAE: 61488016.8.3001.5044).
Statistical analyses were carried out using SPSS version 23.0 for Macintosh (IBM, Armonk, NY). Continuous variables were expressed as the mean and standard deviation or as the median and interquartile range, according to the data distribution. The Shapiro-Wilk’s test was applied to assess normal or non-normal distribution. Categorical variables were expressed as absolute count and frequency, and are presented as a percentage of the group. To compare changes in patient parameters since hospital admission and during the use of L-AMB, Wilcoxon’s test or the paired t-test was used, according to the normality of the data. Comparisons of independent samples were carried out using Student’s t-test or the Mann-Whitney U test, as appropriate. Previous significant levels of kidney biomarkers were evaluated in correlation between traditional kidney injury markers measured before and during L-AMB use. Adjusted correlations were evaluated to investigate the inflammatory influence of visceral leishmaniasis on the urinary biomarker levels during L-AMB use. Spearman’s rho was used for the correlations. All analyses were two-tailed, with P < 0.05 as the level of significance.
RESULTS
During the study period, 41 patients had a confirmed diagnosis of visceral leishmaniasis. After applying the exclusion criteria, a total of 17 patients were included and they agreed to follow-up.
The patients were mainly men (71%) and the mean age was 43 ± 16 years. Five patients (29%) had some type of comorbidity, such as diabetes or hypertension, and seven patients (41%) developed AKI during their hospital stay. In addition, one of the 17 patients (6%) died. The patients had alterations in laboratory tests, including hematological disorders such as anemia, leukopenia, thrombocytopenia, hypoalbuminemia, and hypergammaglobulinemia, and liver disorders. During L-AMB use, there was a significant increase in the number of leukocytes only, in comparison with data at hospital admission. Traditional renal markers in patients with visceral leishmaniasis, such as GFR, creatinine, urea, proteinuria, albuminuria, fractional excretion of sodium, and fractional excretion of potassium showed no alteration between hospital admission and during the use of L-AMB. uMCP-1 and uKIM-1 levels increased during the use of L-AMB, whereas IL-6 and uMCP-1 decreased during the treatment. Interferon-γ levels did not show any significant differences when both moments were compared (Tables 1 and 2).
Table 1 .
General characteristics for patients with visceral leishmaniasis at admission and during liposomal amphotericin B use (N = 17)
| Parameter | Value |
|---|---|
| Age, y; mean ± SD | 42 ± 16 |
| Male gender, n (%) | 12 (71) |
| Systolic blood pressure, mmHg; mean ± SD | 122 ± 20 |
| Diastolic blood pressure, mmHg; mean ± SD | 72 ± 15 |
| Comorbidities, n (%) | 5 (29) |
| Hospital stay, d; mean (range) | 14 (6–33) |
| Acute kidney disease, n (%) | 7 (41) |
| Death, n (%) | 1 (6) |
Table 2.
Laboratory and renal parameters, and inflammatory biomarkers in patients with visceral leishmaniasis at admission and during liposomal amphotericin B use (N = 17)
| Parameters and biomarkers | At hospital admission | During liposomal amphotericin B use | P value |
|---|---|---|---|
| Laboratory parameters | |||
| Hemoglobin, g/dL; mean ± SD | 8.8 ± 2.5 | 8.5 ± 1.5 | 0.264 |
| White blood cell count, celles/mm3; median (IQR) | 2,260 (1,290–3,170) | 2,665 (2,210–3,050) | 0.008* |
| Platelet count, platelets 103/mm3; mean ± SD | 91.7 ± 73.5 | 92.7 ± 44.5 | 0.238 |
| Potassium, mEq/L; mean ± SD | 4 ± 0.5 | 3.9 ± 0.5 | 0.864 |
| Sodium, mEq/L; mean ± SD | 135.2 ± 5.6 | 137.8 ± 4.3 | 0.417 |
| Total protein, g/dL; mean ± SD | 7 ± 1.6 | 6.7 ± 0.8 | 0.850 |
| Albumin, g/dL; mean ± SD | 2.6 ± 0.9 | 2.5 ± 0.8 | 0.537 |
| Globulin, g/dL; mean ± SD | 4.5 ± 1.2 | 4.2 ± 1.1 | 0.564 |
| A/G ratio | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.606 |
| Aspartate aminotransferase, U/L; median (IQR) | 60 (35–138) | 66.5 (35.5–167) | 0.683 |
| Alanine aminotransferase, U/L; median (IQR) | 46 (32–64) | 46 (22.5–70.5) | 0.202 |
| Renal parameters | |||
| GFR, mL/min/1.73 m2; mean ± SD | 86.2 ± 31.8 | 89.8 ± 33.9 | 0.550 |
| Creatinine, mg/dL; mean ± SD | 1 ± 0.5 | 1 ± 0.4 | 0.645 |
| Urea, mg/dL; mean ± SD | 32.2 ± 12.6 | 31.8 ± 10.1 | 0.596 |
| Fractional excreation of sodium, median (IQR) | 1 (0.2–1.5) | 0.4 (0–2.4) | 0.594 |
| Fractional excreation of potassium, median (IQR) | 10.2 (8.3–18.7) | 14.1 (11–16.4) | 0.441 |
| Proteinuria, mg/g creatinine; median (IQR) | 555.7 (271.3–936.7) | 444.2 (228.2–700.2) | 0.422 |
| Albuminuria, mg/g creatinine; median (IQR) | 20.3 (13.5–30.5) | 26.6 (14.7–42.8) | 0.642 |
| uMCP-1, pg/mg creatinine; median (IQR) | 656 (413.8–929.6) | 1,871.9 (834.2–2,299.3) | 0.004* |
| uKIM-1, ng/mg creatinine; median (IQR) | 1.6 (1.2–2) | 2.5 (1.8–3.2) | 0.031* |
| uVEGF, pg/mg creatinine; median (IQR) | 59.8 (11.3–139.6) | 26.3 (12.5–92.6) | 0.564 |
| Inflammatory cytokines | |||
| Serum MCP-1, pg/mL; median (IQR) | 162.4 (31–419.3) | 144.6 (15–222) | 0.047* |
| IL-6, pg/mL; median (IQR) | 17.4 (8.7–49.1) | 0.2 (0–3.5) | 0.028* |
| Interferon-γ, pg/mL; median (IQR) | 15.6 (12.5–18.5) | 18.9 (15.4–39.8) | 0.125 |
A/G = albumin/globulin ratio; GFR = glomerular filtration rate; IQR = interquartile range; MCP-1 = monocyte chemoattractant protein 1; uKIM-1 = urinary kidney injury molecule 1; uMCP-1 = urinary monocyte chemoattractant protein 1; uVEGF = urinary vascular endothelial growth factor.
The P value was obtained using a paired t-test or McNemar’s test, as appropriate.
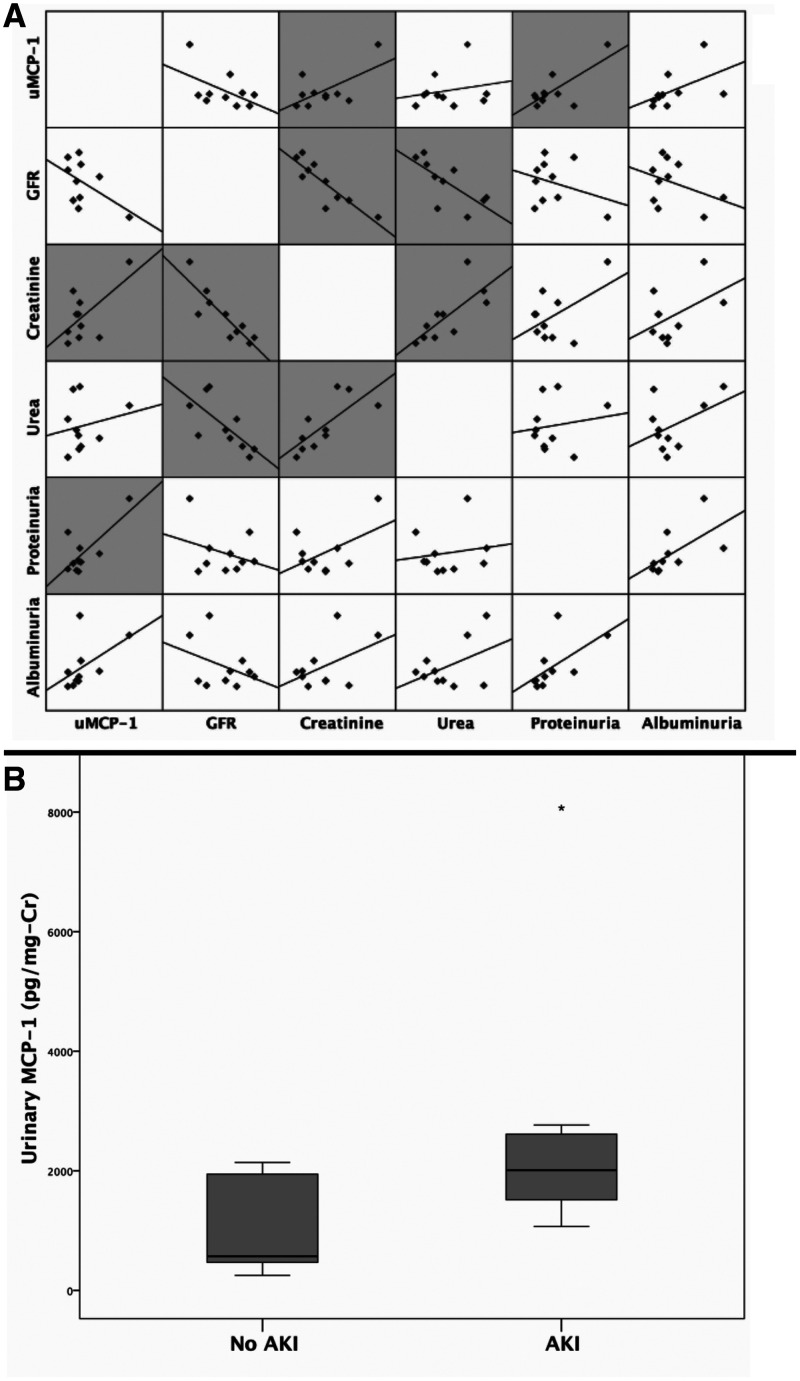
After observing the increase in uMCP-1 levels, we decided to investigate whether these levels during L-AMB use correlated with traditional renal parameters. The correlations analysis was adjusted by serum MCP-1 to verify the glomerular filtration bias of systemic MCP-1 over uMCP-1 levels. It was observed that urinary MCP-1 remained strongly correlated with proteinuria during the use of L-AMB (R = 0.849, P = 0.004) and also with creatinine (R = 0.750, P = 0.020). No significant correlations were observed among GFR, urea, and albuminuria (Figure 1A).
Figure 1.
Urinary monocyte chemoattractant protein 1 (MCP-1) evaluation in patients with visceral leishmaniasis at admission and during liposomal amphotericin B use. The scatterplot in the matrix represents correlations between urinary MCP-1 and other traditional renal biomarkers during liposomal amphotericin B use in patients with visceral leishmaniasis. (A) Significant correlations even after adjusting for serum MCP-1 are represented by gray shading in scatterplots. (B) Boxplot of urinary MCP-1 levels according to acute kidney infection development in patients hospitalized with visceral leishmaniasis during liposomal amphotericin B use. The Mann-Whitney U test was used (P = 0.040). Cr = creatinine; GFR = glomerular filtration rate.
Seven patients (41%) developed AKI during hospitalization, and these patients had greater uMCP-1 levels during the use of L-AMB than non-AKI patients (Figure 1B).
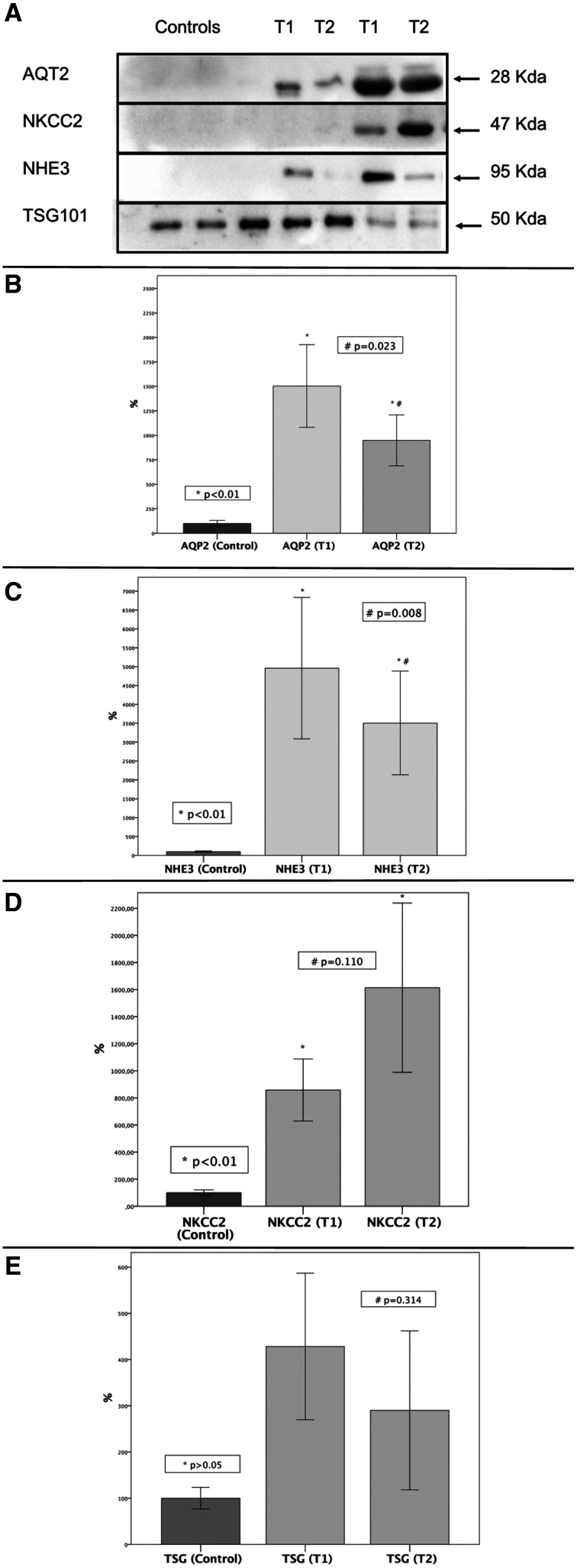
Nine patients were analyzed before and during the treatment for renal transporters in urinary exosomes by Western blot and compared with a control group consisting of healthy subjects. The other eight patients could not be analyzed because of methodological limitations (low urinary creatinine). Patient TSG 101 was evaluated and no significant differences were observed in TSG 101 expression at both time points and in healthy control subjects. An increase in urinary protein expression of AQP2, NHE3, and NKCC2 at both time points was observed compared with healthy control subjects. However, patients treated with L-AMB showed a significant decrease in AQP2 (P = 0.023) and NHE3 (P = 0.008) expression compared with the patients before treatment. A significant inverse correlation of uMCP-1 with NHE3 expression (R= –0.893, P = 0.007) was also observed (Figure 2).
Figure 2.
Renal transporters in the control group compared to patients with visceral leishmaniasis at hospital admission (T1) and during treatment (T2). * P < 0.05 for comparisons between healthy people and patients before and during liposomal amphotericin B use with the Wilcoxon test. # P < 0.05 for comparisons between patients before and during liposomal amphotericin B use with paired t-test. AQP2 = aquaporin 2; NKCC2 = sodium-potassium-2 chloride cotransporter; NHE3 = sodium-hydrogen exchanger 3; TSH 101 = tumor susceptibility gene 101.
DISCUSSION
L-AMB treatment in patients with visceral leishmaniasis is associated with nephrotoxicity, which is monitored through serum creatinine, blood urea, and potassium. 3 In our study, serum creatinine, blood urea, and potassium did not show any significant differences before and during treatment.
Proteinuria, another traditional clinical marker of renal damage, may be correlated with hypergammaglobulinemia, observed in patients with visceral leishmaniasis; this proteinuria may be related to glomerular damage. 9 In our study, we observed elevated levels of proteinuria and albuminuria before and during treatment with L-AMB without differences between them, suggesting renal damage, but nonspecific to the treatment.
Some studies have evaluated the correlation of novel biomarkers in AKI caused by visceral leishmaniasis before any treatment 2 and after treatment. 10 In our study, we evaluated novel biomarkers in AKI before and during treatment with L-AMB, and observed an increase in uMCP-1 and uKIM-1 in patients with visceral leishmaniasis undergoing L-AMB treatment.
MCP-1 is present in renal injury, being produced and released by different cells (such as mesangial, endothelial, and podocyte cells) by different inflammatory inducers. uMCP-1 has been associated primarily with tubulointerstitial and glomerular damage. 2 In our study, during L-AMB treatment, serum levels of MCP-1 decreased and uMCP-1 increased, suggesting that uMCP-1 was released from the kidney and the damage occurred in renal cells during treatment. Moreover, patients with visceral leishmaniasis who developed AKI had greater levels of uMCP-1 than those who did not. These results suggest the increase of uMCP-1 is related to renal tissue damage caused by the drug therapy.
Undifferentiated epithelial proximal tubular cells of the kidney express KIM-1 during ischemic insults, making this molecule a promising specific biomarker. 11 In our study, similar to uMCP-1, uKIM-1 levels also increased in patients during treatment with L-AMB.
Tubular dysfunction has been observed previously in patients with American cutaneous leishmaniasis, which was associated with dysregulation of major acid–base transporters in the proximal tubule (NHE3). 10 In our study, we observed an increase in the expression of AQP2, NKCC2, and NHE3 compared with the healthy control subjects before and during the treatment. Our results are similar to the findings obtained before the treatment, with the exception of AQP2, which suggests different mechanisms of tubular damage in distinct types of leishmaniasis (cutaneous or visceral). The comparison of the expression of transporters in patients before and during L-AMB use showed a decrease in AQP2 during treatment, which could be related to intravenous hydration at the hospital. NHE3 expression also showed a decrease during treatment, as well as a strong correlation between this decrease in its expression and high levels of uMCP-1.
NHE3 is an important transporter present in proximal tubular cells, and is responsible for tubular reabsorption of approximately 60% to 75% of sodium. It has been suggested that NHE3 excretion in urine is a marker of acute glomerular disease and acute tubular necrosis. 12 Apparently, an increase in transporter expression occurs to compensate the tubular cell dysfunction in visceral leishmaniasis. The damage increased with L-AMB use, as shown by the increase in uMCP-1, promoting a reduction in NHE3, which could be associated with worsening of the injury.
Increased levels of IL-6 have been strongly correlated with visceral leishmaniasis severity and death. The decrease in serum levels of IL-6 leads to an increase in macrophage activity, which helps in eliminating dead parasites. 13 In our study, the levels of serum IL-6 decreased in patients during treatment with L-AMB, which improved the condition of these patients during treatment.
The main limitation of our study was the low number of patients at the follow-up, which made a better evaluation of novel biomarkers during L-AMB treatment difficult. This limitation does not invalidate our findings; it shows the necessity for additional studies.
In conclusion, although L-AMB promoted an improvement in the parasitic disease, tubuloglomerular lesions and inflammation were aggravated, as evidenced by an increase in uMCP-1 (mostly in patients with AKI) and a decrease in NHE3 expression. Thus, we suggest using inflammatory and tubuloglomerular biomarkers, such as uMCP-1 and NHE3, to monitor AKI in patients with visceral leishmaniasis undergoing treatment with L-AMB.
ACKNOWLEDGMENTS
We are grateful to the patients and the team of physicians, nurses, pharmacists, residents, and medical students from São José Hospital in Fortaleza city.
References
- 1. Ready PD , 2014. Epidemiology of visceral leishmaniasis. Clin Epidemiol 6: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meneses GC De Francesco Daher E da Silva GB Jr. Bezerra GF da Rocha TP de Azevedo IEP Libório AB Martins AMC , 2018. Visceral leishmaniasis-associated nephropathy in hospitalised Brazilian patients: new insights based on kidney injury biomarkers. Trop Med Int Health 23: 1046–1057. [DOI] [PubMed] [Google Scholar]
- 3. van Griensven J Diro E , 2019. Visceral leishmaniasis: recent advances in diagnostics and treatment regimens. Infect Dis Clin North Am 33: 79–99. [DOI] [PubMed] [Google Scholar]
- 4. Salih M Zietse R Hoorn EJ , 2014. Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259. [DOI] [PubMed] [Google Scholar]
- 5. Levey AS et al. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellum JA Lameire N , KDIGO AKI Guideline Work Group, 2013. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waikar SS Sabbisetti VS Bonventre JV , 2010. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 78: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzales PA Pisitkun T Hoffert JD Tchapyjnikov D Star RA Kleta R Wang NS Knepper MA , 2009. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorriz JL Martinez-Castelao A , 2012. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando) 26: 3–13. [DOI] [PubMed] [Google Scholar]
- 10. Oliveira MJC et al., 2014. Preliminary study on tubuloglomerular dysfunction and evidence of renal inflammation in patients with visceral leishmaniasis. Am J Trop Med Hyg 91: 908--911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao X Tian L Xu W Zhang Z Wang C Qi C Ni Z Mou S , 2014. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Oliveira RA et al., 2011. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int 80: 1099--1106. [DOI] [PubMed] [Google Scholar]
- 13. Adiyanti SS Loho T , 2012. Acute kidney injury (AKI) biomarker. Acta Med Indones 44: 246–255. [PubMed] [Google Scholar]
- 14. Dos Santos PL et al. 2016. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Negl Trop Dis 10: e0004375. [DOI] [PMC free article] [PubMed] [Google Scholar]