ABSTRACT.
Toxocariasis is a rare and underdiagnosed disease, and data concerning epidemiological aspects of toxocariasis in Israel are lacking. We describe the epidemiology of toxocariasis in Israel. Epidemiological data of all serology tests between 2005 and 2019 were retrieved and compared with detailed demographics. Overall, 464 positive cases from a total of 10,896 tests conducted were identified, reflecting a mean positivity rate of 4.4% (yearly range, 2–22%). Over the years, a significant increase in yearly tests was noted, without a parallel change in the positivity rate. The greatest positivity was noted among males and children (< 18 years). No differences were noted when comparing urban/rural and Jewish/non-Jewish sub-groups. A significant correlation between toxocariasis incidence and dog ownership rate was observed in major cities (r[8] = 0.82, P < 0.005). Our study shows that, in Israel, toxocariasis is not restricted to specific populations or locations. The risk factor of dog contacts was reiterated by nationwide dog registration data. There is need for a seroprevalence survey to understand the disease situation more fully.
INTRODUCTION
Toxocariasis—also known as visceral larva migrans (VLM)/ocular larva migrans (OLM)—is a globally prevalent illness caused by an infestation of Toxocara canis or Toxocara cati larvae, which are ubiquitous gastrointestinal helminths in dogs and cats. Humans are paratenic hosts, usually infected by oral ingestion of embryonated Toxocara eggs from contaminated soil or food. 1 Canine and feline ownership is a somewhat controversial yet commonly cited risk factor. 2 Several studies challenge the traditional soil contamination hypothesis and suggest that direct contact with the fur of an infected dog may be an important zoonotic source of Toxocara eggs, yet soil contamination remains the leading hypothesis for human infection. 2 – 4 Studies worldwide have demonstrated high rates of soil contamination (10–30%) with Toxocara spp. eggs in backyards, sandpits, parks, playgrounds, lake beaches, and other public places. Toxocara canis eggs were found to be most common in public parks, whereas the majority of investigated sand boxes were contaminated with T. cati eggs, reflecting host defecation habits. 5, 6 A study of New York City playgrounds, quoted in a New York Times article, brought Toxocara to special attention and noted Toxocara in almost one third of sampled specimens. 7, 8
Children are affected more frequently clinically than adults, and both VLM and OLM are mainly seen in children younger than 10 years of age. 9 The global burden of human toxocariasis is poorly quantified. Serological surveys demonstrate that the infection is more frequent in tropical settings and in rural areas, with population-based seroprevalence ranging from 2.4% in Europe to 87% in the tropics. 10
The epidemiology of toxocariasis in Israel has been poorly studied. A couple of studies researched canine toxocariasis prevalence at a local level, 11, 12 two more describe case reports of VLM and OLM in humans, 13, 14 and two more were seroprevalence studies among institutionalized adults and children. 15, 16 Of note, all these studies were conducted before the year 2000. We are not aware of disease research on a national scale. We describe epidemiological characteristics and trends of all serologies for toxocariasis conducted in Israel between 2005 and 2019.
MATERIALS AND METHODS
Nationwide epidemiological data, including age, gender, residence, and serology results, were retrieved from the two laboratories in Israel that conduct Toxocara serology tests: the Ministry of Health’s Central Laboratory in Jerusalem and the Clinical Microbiology Laboratory, Soroka University Medical Center in Be’er Sheva. Until 2013, the Ministry of Health’s laboratory conducted serology tests using an ELISA kit (IVD Research, Carlsbad, CA). Between 2013 and June 2017, every positive or equivocal serum detected by ELISA (IVD Research, Carlsbad, CA, and, since 2015, SCIMEDX Corporation, Denville, NJ) was re-tested for confirmation of the result using a Western blot kit (LDBio Diagnostics, Lyon, France). Since July 2017, all tests are performed solely by Western blot (LDBio Diagnostics). The Soroka laboratory conducted ELISA tests (R-Biopharm, Darmstadt, Germany) throughout the study duration. Because of the differences between the laboratories and the inherent limitations of serological tests, borderline results were omitted. Tests were conducted only on account of clinical suspicion, and not as part of seroprevalence studies. Demographic data were obtained from Israeli Central Bureau of Statistics official open-source publications. Dog registration data were retrieved from official open-source publications of the Israeli Ministry of Agriculture and Rural Development.
Statistical analysis was performed using Microsoft Excel for Windows (version 2016; Microsoft Corporation, Redmond, WA) and Web-based platforms. P values less than 0.05 on a two-sided test were considered statistically significant. The relationship between categorical variables was evaluated using the χ 2 test. Correlation was calculated using Pearson’s correlation coefficient (r); distribution was verified using the Kolmogorov-Smirnov test for normality. The study was approved by the Sheba Medical Center and the Soroka Medical Center institutional review boards.
RESULTS
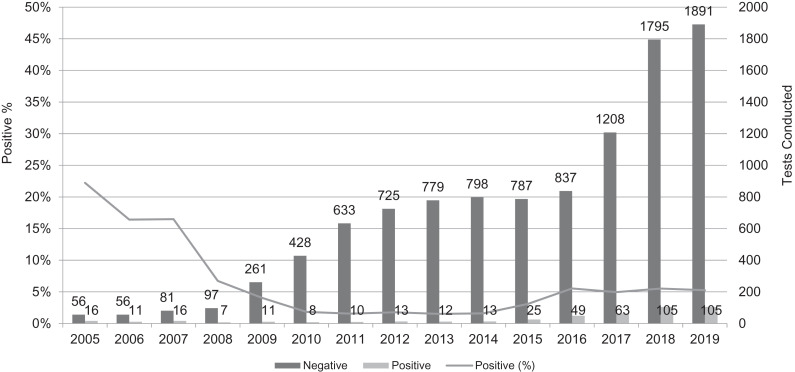
In total, 464 unique positive Toxocara serology tests were found. The total amount of Toxocara serologies conducted (10,896) demonstrated a substantial increase during the study period— from 72 in 2005 to 1996 in 2019. Of these, the proportion of positive tests remained fairly steady, at approximately 5% during the past decade. Figure 1 displays details and trends of tests conducted per year.
Figure 1.
Toxocara tests performed in Israel from 2005 to 2019. A total of 464 unique positive Toxocara serology tests were found. The number of Toxocara serologies conducted (N = 10,896) demonstrates a substantial increase during the study—from 72 in 2005 to 1996 in 2019. The proportion of positive tests remained fairly steady at approximately 5% during the past decade.
Demographic comparisons exhibited disparities of gender and age. The vast majority of tests were conducted among children (22.1%) and in the 18- to 40-year-old age group (62.1%), yet children had a significantly greater positivity rate (9.9% versus 2.7%, P < 0.00001). The gender ratio was heavily skewed, with females making up 76.5% of all tests (male-to female ratio, 1:3.4), yet the positivity rate was converse—8.2% for males and 3.2% for females—reflecting a male-to female ratio of 2.5:1.
In contrast, similarities in ethnicity and place of residence were observed. Of the positive patients, 350 of 440 (79.5%) were Jewish and 20.5% were non-Jewish. In the general population, 79% are Jewish and 21% are non-Jewish (P = 0.78). Again, among patients with toxocariasis, 402 of 440 (91.4%) reside in urban areas, similar to the general population (P = 0.99). Full demographic comparisons are listed in Table 1.
Table 1.
Basic demographics of patients with toxocariasis
| Demographic | Positive, n (%) | Negative, n (%) | Total, n | Positivity |
|---|---|---|---|---|
| Gender | ||||
| Male | 203 (44) | 2,264 (23) | 2,467 | 8.2% |
| Female | 255 (56) | 7,771 (77) | 8,026 | 3.2% |
| Total | 458 | 10,035 | 10,493 | 4.6% |
| Age, y | ||||
| Pediatric, < 18 | 208 (45) | 2,111 (21) | 2,309 | 9.9% |
| Adult, > 18 | 251 (55) | 7,923 (79) | 8,174 | 3.2% |
| 18–30 | 80 (17) | 3,118 (31) | 3,198 | 2.6% |
| 30–40 | 91 (20) | 3,229 (32) | 3,320 | 2.8% |
| 40–50 | 32 (7) | 886 (9) | 918 | 3.6% |
| 50–60 | 15 (3) | 318 (3) | 333 | 4.7% |
| 60+ | 33 (7) | 372 (4) | 405 | 8.9% |
| Mean | 23.5 | 29.0 | 28.8 | – |
| Total | 459 | 10,034 | 10,493 | 4.6% |
*Cases might not add up because of only partial demographic information collected in a subset of patients.
Geographic scattering of cases to subdistricts according to the official Israeli administrative division exhibits a rather homogenous dispersion of cases. When correlating toxocariasis incidence to the number of registered dogs in Israel’s 10 most populous cities, a significant correlation is demonstrated: r(8) = 0.82, P < 0.005 (range, –1 < r < +1). Figure 2 exhibits the correlation.
Figure 2.
Correlation between toxocariasis incidence and the number of registered dogs in Israel’s 10 most populous cities. A significant correlation is demonstrated (r[8] = 0.82, P < 0.005; range, –1 < r < 1), reiterating this well-known risk factor.
DISCUSSION
The increase in requests for Toxocara serology during the span of this study is a clear observation that warrants understanding. Because the percentage of positive samples remained quite consistent, we assume this is not related to laboratory reasons. There were no changes at the national level concerning the reportability or case definition of the disease. Therefore, we assume this increase may confer a rise in awareness among clinicians.
The common risk factors attributed to toxocariasis encompass environmental (e.g., contaminated soil, food, and water), socioecological (e.g., dog and cat ownership, occupation, low socioeconomic level, rural residences), and genetic (e.g., children, male) factors. 10, 17 Interestingly, demographic analysis in Israel is not in accordance with all these risk factors, revealing impressive comparability between the disease among rural versus urban and Jewish versus non-Jewish (Table 1). This might strengthen the growing evidence that contamination of soil with Toxocara eggs might be even greater in urban areas than in rural ones, or may suggest that pet dogs and not stray dogs account for the major source of infection. Conversely, significant differences were evident in the pediatric group versus the adult group. Toxocariasis is traditionally known as a pediatric disease, an actuality that was ratified by a recent systematic review and meta-analysis of seroprevalence worldwide, showing that young age is a risk factor for seropositivity. 19 Although the pediatric population did consist of a greater proportion of our patients, most of the cases were in fact encountered in adults. Thus, when a patient presents with signs and symptoms suggestive of toxocariasis, it is prudent to suspect the disease regardless of patient age. Efforts to educate clinicians about toxocariasis should be aimed at pediatricians and general practitioners alike.
The percentage of positive samples in our cohort is notably low (4.6%; range, 2–22%). When comparing this to similar studies (i.e., nationwide all-age serological studies among toxocariasis-suspected patients in developed countries), the low percentage is even more remarkable. Schneider and Auer 21 described 12.9% positivity in an Austrian cohort, Logar et al. 20 noted 24% in a Slovenian cohort, and Pinelli et al. 18 indicated 8.2% positivity in a Dutch cohort. The difference between our study and the others probably stems from a myriad of factors, including differences in dog prevalence, age differences, and laboratory artifacts (all studies conducted using ELISAs, yet only that of Schneider and Auer 21 added Western blot verification). Although true seroprevalence cannot be inferred from our study, it may imply low seroprevalence in Israel, because it is unlikely that the general population has greater seroprevalence than suspected patients. Of note, this approximation (4–5%) is not very different from the eastern Mediterranean regional estimation given by Rostami et al. 19 in their recent worldwide meta-analysis (8.2%; range, 5.1–12.0%), yet it still reflects very low seroprevalence from an international benchmark.
Proximity to dogs, because of the obvious biological factors, has long been known to be a risk factor for contracting toxocariasis. The correlation between toxocariasis and registered dogs in Israel’s 10 most populous cities corroborates this notion. Moreover, the disease was exceptionally rare in the Jerusalem subdistrict (which has large populations of Arabs and ultraorthodox Jewish people) and in selected ultraorthodox cities. Both of these populations are known to prefer limited contact with dogs. Rostami et al. 19 described religion-based dog aversions as a factor promoting low seroprevalence among Muslims in the Mediterranean region. Therefore, pet cats may be a more prominent vector in countries with major Muslim populations. 2 Concerning ultraorthodox Jewish people, the disdain has ancient roots as well, as the preeminent medieval Jewish scholar Maimonides stated: “Cursed be the one who raises dogs.” 22 To this day, dog ownership is scarce among this group. 23 Thus, religious traditions and prohibitions may confer protection from toxocariasis to a substantial part of Israel’s population.
An important caveat of Toxocara diagnosis, and consequently an important limitation of studies on toxocariasis, is that laboratory diagnosis is based on serology. Clinicians and researchers are limited in their ability to differentiate recent from previous infections and should be aware of possible cross-reactivity with antibodies to other pathogens. Performing Western blot analysis minimizes the possibility of cross-reactivity, which has been described especially, but not exclusively, with other nematodes such as Ascaris. 24 Therefore, since 2013, some positive samples in Israel have been validated or done directly with Western blot. From a practical standpoint, there is no gold standard test for the diagnosis of toxocariasis, and an antigen detection test is greatly needed. Apart from one attempt to diagnose Toxocara chorioretinitis using polymerase chain reaction of ocular fluids, 25 we are unaware of antigen-based or molecular tests in humans.
Our study has several limitations. First, because of the nature of the study, incidence and prevalence cannot be derived from it. The second includes the inherent limitations of serology tests as detailed herein. Third, the study data are from two different laboratories, with different and unharmonized detection methods. Fourth, a high proportion of tests, with a converse low proportion of seronegativity, were conducted on females of reproductive age. We assume this reflects physician confusion between Toxoplasmosis (probably ordered as screening during pregnancy) and Toxocariasis. In addition, we do not have data concerning the clinical suspicion leading to the serology request a priori. We assume that the large scale of the study compensates for these limitations in part.
In conclusion, our study sheds light on the nationwide epidemiological aspects of toxocariasis in Israel. Data imply underdiagnosis of the disease and epidemiological characteristics that differ from traditional assumptions—mainly that the disease is not restricted to any age, gender, ethnic group, or place of residence. However, published clinical data on toxocariasis are skewed heavily toward the pediatric population. The well-known risk factor of dog ownership was reiterated by nationwide dog registration data. There is a need for a seroprevalence survey to understand the disease situation more fully and to explore the clinical impact of the disease. Furthermore, toxocariasis is currently not a notifiable disease in Israel. Because of the important clinical ramifications of the disease, and its underdiagnosis, we recommend adding it to the list of national notifiable diseases.
References
- 1. Rubinsky-Elefant G Hirata CE Yamamoto JH Ferreira MU , 2010. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 104: 3–23. [DOI] [PubMed] [Google Scholar]
- 2. Overgaauw PAM van Knapen F , 2013. Veterinary and public health aspects of Toxocara spp. Vet Parasitol 193: 398–403. [DOI] [PubMed] [Google Scholar]
- 3. Roddie G Stafford P Holland C Wolfe A , 2008. Contamination of dog hair with eggs of Toxocara canis . Vet Parasitol 152: 85–93. [DOI] [PubMed] [Google Scholar]
- 4. Aydenizöz-Özkayhan M Yačci BB Erat S , 2008. The investigation of Toxocara canis eggs in coats of different dog breeds as a potential transmission route in human toxocariasis. Vet Parasitol 152: 94–100. [DOI] [PubMed] [Google Scholar]
- 5. Uga S Minami T Nagata K , 1996. Defecation habits of cats and dogs and contamination by Toxocara eggs in public park sandpits. Am J Trop Med Hyg 54: 122–126. [DOI] [PubMed] [Google Scholar]
- 6. Mizgajska-Wiktor H Jarosz W Fogt-Wyrwas R Drzewiecka A , 2017. Distribution and dynamics of soil contamination with Toxocara canis and Toxocara cati eggs in Poland and prevention measures proposed after 20 years of study. Vet Parasitol 234: 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Tyungu DL McCormick D Lau CL Chang M Murphy JR Hotez PJ Mejia R Pollack H , 2020. Toxocara species environmental contamination of public spaces in New York City. PLoS Negl Trop Dis 14: e0008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beil L , 2018. The Parasite on the Playground. New York Times. Available at; https://www.nytimes.com/2018/01/16/health/toxocara-children-new-york-playgrounds.html. Accessed January 25, 2022.
- 9. Despommier D , 2003. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 16: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma G Holland CV Wang T Hofmann A Fan CK Maizels RM Hotez PJ Gasser RB , 2018. Human toxocariasis. Lancet Infect Dis 18: e14–e24. [DOI] [PubMed] [Google Scholar]
- 11. Hoida G Greenberg Z Furth M Malsha Y Craig PS Schantz PM Sneir R El-On J , 1998. An epidemiological survey of Echinococcus granulosus and other helminths in animal populations in northern Israel. J Helminthol 72: 127–131. [DOI] [PubMed] [Google Scholar]
- 12. Gross EM Zeitan R Torok V , 1984. Toxocara canis infection in dogs in Beersheba, Israel. J Helminthol 58: 139–141. [DOI] [PubMed] [Google Scholar]
- 13. Ben-Ami M Katzuni E Hochman A Antonelli J Koren A , 1990. Toxocariasis in Emek Israel. Harefuah 119: 72–73. [PubMed] [Google Scholar]
- 14. Beiran I Cochavi O Miller B , 1998. “Silent” ocular toxocariasis. Eur J Ophthalmol 8: 195–196. [DOI] [PubMed] [Google Scholar]
- 15. Huminer D Symon K Groskope I Pietrushka D Kremer I Schantz PM Pitlik SD , 1992. Seroepidemiologic study of toxocariasis and strongyloidiasis in institutionalized mentally retarded adults. Am J Trop Med Hyg 46: 278–281. [DOI] [PubMed] [Google Scholar]
- 16. Pietrushka D Elian I Alaluf D Elian E , 1982. Intestinal parasites in inmates of a retarded children’s institution in central Israel. Isr J Med Sci 18: 313–314. [Google Scholar]
- 17. Berrett AN Erickson LD Gale SD Stone A Brown BL Hedges DW , 2017. Toxocara seroprevalence and associated risk factors in the United States. Am J Trop Med Hyg 97: 1846–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinelli E Herremans T Harms MG Hoek D Kortbeek LM , 2011. Toxocara and Ascaris seropositivity among patients suspected of visceral and ocular larva migrans in the Netherlands: trends from 1998 to 2009. Eur J Clin Microbiol Infect Dis 30: 873–879. [DOI] [PubMed] [Google Scholar]
- 19. Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y, Omrani VF, Hotez PJ, Gasser RB, 2019. Seroprevalence estimates for toxocariasis in people worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis 13: e0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Logar J Likar M Kraut A , 1993. Toxocara antibodies in patients with visceral or ocular disorder in Slovenia. Infection 21: 27–29. [DOI] [PubMed] [Google Scholar]
- 21. Schneider R Auer H , 2016. Incidence of Ascaris suum-specific antibodies in Austrian patients with suspected larva migrans visceralis (VLM) syndrome. Parasitol Res 115: 1213–1219. [DOI] [PubMed] [Google Scholar]
- 22.Maimonides M, 1470. Mishneh Torah, Sefer Nezikin, Nizqei Mamon, Chapter 5, Paragraph 9. [Google Scholar]
- 23. Bar-Tzuri R , 2011. Leisure Consumerism: The Pet Market and the Related Household Expenditure. Available at: https://employment.molsa.gov.il/Research/Documents/X11687.pdf. Accessed January 25, 2022.
- 24. Rudzińska M Kowalewska B Sikorska K , 2017. Clinical usefulness of Western blotting and ELISA avidity for the diagnosis of human toxocariasis. Parasite Immunol 39: 1–6. [DOI] [PubMed] [Google Scholar]
- 25. Tian JX O’Hagan S , 2015. Toxocara polymerase chain reaction on ocular fluids in bilateral granulomatous chorioretinitis. Int Med Case Rep J 8: 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]