ABSTRACT.
Repeated intravenous (IV) administration of radiation-attenuated sporozoite (RAS) vaccines induces Plasmodium-specific CD8+ liver-resident memory T (Trm) cells in mice and achieves sterile protection against challenge. Our heterologous “prime-and-trap” vaccine strategy was previously shown to simplify and improve upon RAS vaccination. Prime-and-trap vaccination combines epidermal priming by DNA-encoded circumsporozoite protein (CSP) antigen followed by a single IV dose of freshly dissected RAS (fresh-RAS) to direct and trap activated and expanding CD8+ T cells in the liver. Prime-and-trap vaccination protects mice against wild-type sporozoite (spz) challenge. Assessment of prime-and-trap vaccines in nonhuman primate (NHP) models and/or humans would be greatly enabled if fresh-RAS could be replaced by cryopreserved RAS (cryo-RAS). Here, we investigated if fresh-RAS could be replaced with cryo-RAS for prime-and-trap vaccination in BALB/cj mice. Despite a reduction in spz vaccine liver burden following cryo-RAS administration compared with fresh-RAS, cryo-RAS induced a similar level of Plasmodium yoelii (Py) CSP-specific CD8+ liver Trm cells and completely protected mice against Py spz challenge 112 days after vaccination. Additionally, when the glycolipid adjuvant 7DW8-5 was co-administered with cryo-RAS, 7DW8-5 permitted the dose of cryo-RAS to be reduced four-fold while still achieving high rates of sterile protection. In summary, cryo-RAS with and without 7DW8-5 were compatible with prime-and-trap malaria vaccination in a mouse model, which may accelerate the pathway for this vaccine strategy to move to NHPs and humans.
INTRODUCTION
Malaria is caused by Plasmodium parasites and is responsible for an estimated 229 million infections and 400,000 deaths each year. 1 Human Plasmodium infection begins when an individual is bitten by an infectious female Anopheles mosquito and sporozoites (spz) are transmitted into the skin. Sporozoites home to the liver and infect hepatocytes, replicating and differentiating for approximately 6–8 days before merozoites egress into the bloodstream to invade and begin replication within red blood cells. Blood stage infection is responsible for symptomatic malaria and further transmission. 2, 3 Candidate malaria vaccines target different stages of the parasite lifecycle with the most successful vaccines thus far targeting the pre-erythrocytic (PE) spz and liver stages (reviewed in reference 4). Plasmodium PE vaccines are particularly attractive as they would not only prevent blood stage infection and all clinical manifestations, but also stop further transmission of the parasite.
The only PE vaccines to ever induce greater than 90% sterile protection in humans are live-attenuated whole spz vaccines, Sanaria® PfSPZ Vaccine and PfSPZ-CVac. 5 – 11 Repeated intravenous (IV) administration of radiation attenuated spz (RAS) vaccines can achieve sterile protection in mice, non-human primates (NHPs), and humans. 5, 12 – 17 These RAS vaccines induce both humoral and cellular responses, which block spz invasion of hepatocytes 5, 15, 18 and kill infected cells, respectively. 19 The importance of cellular immune responses in the liver has been increasingly recognized as necessary for achieving sterile protection from RAS vaccines in mice. 20 – 24 The induction of CD8+ T cells, specifically liver-resident memory CD8+ T (Trm) cells, appears to be critical for long-term protection. 5, 13, 14, 25, 26 While the production of aseptic, purified, cryopreserved PfSPZ vaccines has seen significant advances in manufacturing, reducing the dosage or number of doses of PfSPZ would nonetheless significantly reduce the cost of goods.
Previously, we reported that a prime-and-trap vaccine strategy simplified and improved upon repeated RAS immunization of mice while maintaining the immunogenicity and protection of whole spz vaccines. 27 Prime-and-trap here comprises a two-step heterologous vaccine strategy that combines epidermal priming of DNA encoding the well-characterized circumsporozoite (CSP) spz antigen with a single IV dose of RAS to direct and trap the activated and expanding CD8+ T cells in the liver. This strategy induces robust malaria-specific CD8+ Trm cell responses in the liver and confers sterile protection in the Plasmodium yoelii (Py) rodent malaria model. 27 However, it is not possible to use freshly dissected RAS to immunize humans as any human vaccine must go extensive quality control release assay testing before administration.
Sanaria has pioneered the manufacture and use of aseptic, purified, cryopreserved PfSPZ products for use in humans including the radiation-attenuated PfSPZ Vaccine, 5, 8 – 10, 13, 28, 29 the chemo-attenuated Pf-CVac, 8, 15 the genetically attenuated PfSPZ-GA1 vaccine, 30 and infectious PfSPZ Challenge parasites used in controlled human malaria infections. 7, 31 Studies in mice have shown that it requires approximately seven times as many cryopreserved Py spz (cryo-RAS) as freshly dissected Py spz (fresh-RAS) to comparably infect mice 31 and three times as many cryo-RAS as fresh-RAS to comparably protect mice after immunization. 13 Sanaria’s PfSPZ vaccine achieved sterile protection in many malaria-naïve individuals 5, 26 and some malaria-exposed individuals. 7, 29 These findings suggested that cryo-RAS may be used as an alternative to fresh-RAS in prime-and-trap vaccination.
Here, we investigated if fresh-RAS can be replaced with Sanaria-produced irradiated, purified, cryopreserved Py spz (termed “cryo-RAS”) in our heterologous prime-and-trap vaccine. Mice primed with DNA encoding the PyCSP antigen administered via gene gun followed by trapping with fresh-RAS or cryo-RAS developed similar numbers of durable PyCSP-specific liver CD8+ Trm cells and were equivalently protected against Py spz challenge. We also show that the prime-and-cryo-RAS-trap vaccine can be further improved with the addition of a dose-sparing glycolipid adjuvant. These findings demonstrate that prime-and-trap is a versatile malaria vaccine that can induce protective liver-resident memory CD8+ T-cells using a single cryo-RAS dose, thereby making the strategy more feasible for translation into NHPs and humans.
MATERIALS AND METHODS
Mice.
BALB/cj mice (4–6 week old) were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in an Institutional Animal Care and Use Committee (IACUC)-approved animal facility at the University of Washington. All mice were female and used under an approved IACUC protocol (4317-01 to S. C. M.).
DNA vaccination by gene gun.
The PyCSP minigene DNA vaccine encoding the SYVPSAEQI epitope was constructed in the pUb.3 vector and tagged with an N-terminal ubiquitin tag as described. 27, 32, 33 For all vaccinations, Escherichia coli heat-labile toxin (LT)-encoding plasmid was used as an adjuvant in a 1:10 ratio with the PyCSP-minigene vector. 34 DNA was purified using an endotoxin-free purification kit (Qiagen, Hilden, Germany, #12362), loaded onto 0.8–1.5 μm diameter gold powder (Technic Inc., Cranston, RI, #12-507), and coated on tubing as cartridges as described previously. 33 Abdominal fur was trimmed and mice were vaccinated on the abdomen using a PowderJect-style gene gun by priming using two cartridges per day on Days 0 and 2 (0.5 μg DNA per cartridge). This method of priming with PyCSP-minigene/LT-encoding plasmid via gene gun is referred to as ggCSP.
Freshly-dissected spz vaccination and challenge.
Female Anopheles stephensi mosquitoes infected with wild-type (WT) Py (strain 17XNL) were reared at Seattle Children’s Research Institute (Seattle, WA). Fresh spz were obtained by salivary gland dissection 14–18 days post-infection followed by Accudenz gradient purification as described. 35 Fresh-RAS were generated by dissecting spz from mosquitoes, purifying them as above-mentioned, and then irradiating by X-ray exposure (10,000 rads; Rad Source, Buford, GA). As a control, heat-killed spz (HK-spz) were generated by incubating WT Py spz in a 55°C water bath for 30 minutes. All spz (WT, RAS, or HK) were administered IV in a volume of 100 μL of Schneider’s insect media (Gibco, Thermo Fisher Scientific, Waltham, MA). 1 × 103 freshly dissected WT Py spz were used for mouse challenge experiments, unless otherwise specified. Blood stage protection after spz challenge was assessed by Giemsa (Sigma-Aldrich, St. Louis, MO) stained thin blood smear microscopy on days 3–14 post-challenge. Mice were deemed protected if blood smears remained negative for parasites up to Day 14.
Cryopreserved spz vaccination and challenge.
Radiation-attenuated (100 Gy by C0-60), purified, vialed, cryopreserved 17XNL Py spz (cryo-RAS) were produced by Sanaria Inc. (Rockville, MD) as described. 13, 36 The vials were shipped to Seattle and stored in vapor phase liquid nitrogen per manufacturer recommendations. Cryo-RAS were thawed in a 37°C water bath for 30 seconds, diluted in 100 μL of Schneider’s insect media, and administered IV within 30 minutes. Sporozoites counts were confirmed on a hemocytometer within one hour of injection.
Glycolipid adjuvant preparation.
Good manufacturing practice (GMP) grade 7DW8-5 powder was dissolved in dimethyl sulfoxide (DMSO), aliquoted, and stored at −20°C. Single use aliquots were thawed at 56°C for 10 minutes and then sonicated in an ultrasonic water bath for five minutes to break micelle formation. The 7DW8-5 was mixed with diluted RAS immediately before administration to mice. All mice received 2 μg of 7DW8-5 adjuvant per injection.
Mouse plasma ELISA.
Interferon-γ (IFNγ) or interleukin-4 (IL-4) cytokine levels were determined by commercial ELISA kit according to manufacturer’s instructions (BioLegend, San Diego, CA, #430801 and #431104). Mouse blood was collected via submental bleed into tubes containing ethylenediaminetetraacetic acid (EDTA), and plasma was isolated and frozen. Plasma was diluted in the kit assay diluent, and absorbance was read on the CLARIOstar Plus plate reader (BMG Labtech, Ortenberg, Germany) according to kit instructions. Standard curves and cytokine concentrations were calculated in Microsoft Excel.
CD1d depletion.
Mice were injected intraperitoneally (IP) with 10 μg of anti-mouse CD1d (CD1.1) (BioXcell, Lebanon, NH, #BE0000) or matched isotype control (BioXcell, #BE0088) 24 hours before 7DW8-5 injection. Mouse blood was collected via submental bleed and plasma was isolated 12 and 24 hours postadjuvant administration. Plasma IFNγ cytokine levels were analyzed by ELISA as described earlier.
Liver burden reverse transcription polymerase chain reaction (RT-PCR).
Four hours post-RAS immunization, mice were euthanized and half of the liver was excised and pulverized by bead beating in 5 mL NucliSENS lysis buffer (bioMérieux, Durham, NC). Total RNA was extracted by processing 50 μL of the NucliSENS buffer-treated sample diluted 1:20 in NucliSENS lysis buffer on the EasyMag system (bioMérieux) as described for whole blood. 37 RNA was subjected to reverse transcription polymerase chain reaction (RT-PCR) using the SensiFAST™ Probe Lo-ROX Kit (Bioline, London, United Kingdom) using a predesigned HEX-labeled mouse GAPDH RT-PCR assay (IDT Inc, Coralville, IA) multiplexed with a Pan-Plasmodium 18S rRNA assay as described. 38 Conditions of 45°C for 10 minutes, 95°C for 2 minutes, and 45 cycles of 95°C for 5 seconds, 50°C for 35 seconds were run on a QuantStudio 5 real-time PCR machine (ThermoFisher, Waltham, MA). Data were normalized to mouse GAPDH, and copy numbers per reaction were determined using custom lot of quantified Armored RNA encoding full-length Plasmodium 18S rRNA (Asuragen, Austin, TX).
Liver lymphocyte isolation and flow cytometry.
Liver lymphocytes were isolated by mechanical dissociation and Percoll density gradient as previously described. 27, 39 Briefly, livers were perfused, mashed into a single cell suspension, and intrahepatic lymphocytes were isolated. Final liver lymphocyte pellets were resuspended in 150 μL 1X MACs buffer (phosphate-buffered saline [PBS], 1 mM EDTA, 0.5% fetal bovine serum [FBS]) and transferred to a U-bottom 96-well plate for blocking and staining for flow cytometry. All antibodies and flow cytometry analyses were as previously described 27 with the minor modification of the addition of a live/dead dye (Zombie NIR Fixable Viability Kit, BioLegend) to enable exclusion of dead cells from downstream analysis. All reagents used for flow cytometry are listed in Supplemental Table 1. Briefly, liver lymphocytes were treated with an Fc block and live/dead dye for 30 minutes (anti-CD16/32, clone 2.4G2; BD Biosciences), stained with antibody cocktail for 45 minutes, and fixed for 20 minutes (Cytofix/Cytoperm reagent; BD Biosciences, Franklin Lakes, NJ). 27 Flow cytometry was conducted on the LSRII instrument (BD Biosciences), and data were analyzed with FlowJo version 10.7.1 (BD Biosciences).
Statistics.
Comparisons of liver burden RT-PCR and ELISA groups were done using non-parametric Kruskal–Wallis one-way analysis of variance with Dunn’s multiple comparisons test. Comparisons of flow cytometry cell counts were done using the non-parametric two-tailed Mann–Whitney U test. Protection data was evaluated using Fisher’s exact test. All groups were compared against the ggCSP prime and 2 × 104 fresh-RAS trap positive control (unless otherwise specified). Error bars in figures are reported as standard deviation (SD) of the mean with individual mouse samples shown, if applicable (unless otherwise specified). All P values and 95% CI of the mean (if applicable) are listed in corresponding figure legends. Statistical significance was defined as P < 0.05. Prism GraphPad Prism 9.1.2 Software (San Diego, CA) was used for all calculations.
RESULTS
DNA prime and Py fresh- or cryo-RAS trap leads to substantial parasite liver burden.
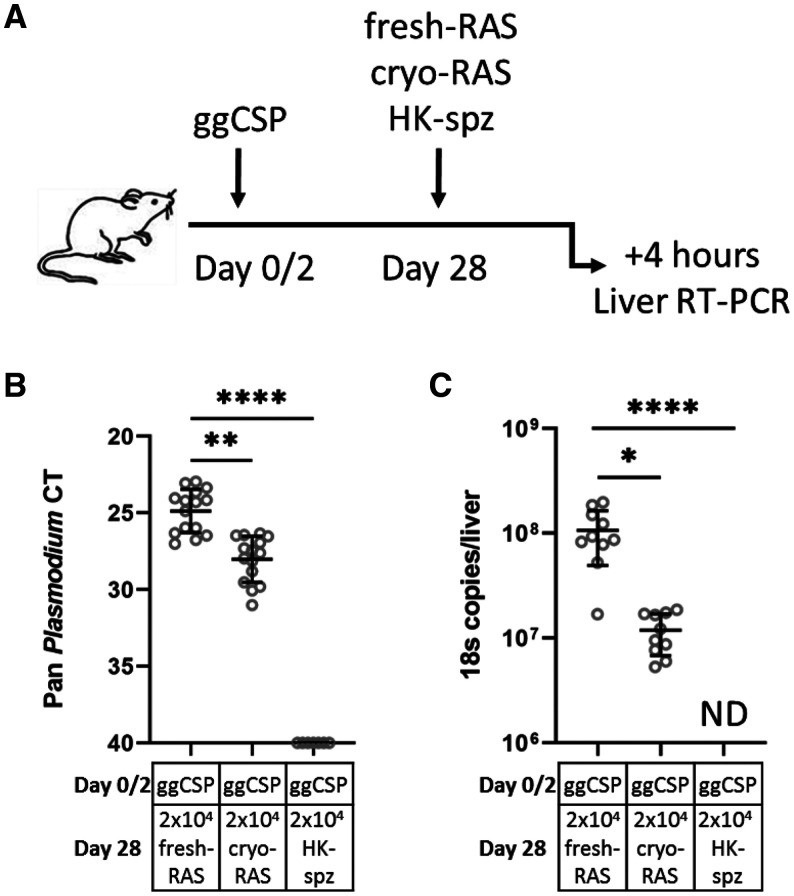
Based on earlier work, prime-and-trap vaccination using RAS for trapping is predicated on achieving a liver burden upon RAS trapping that is immunogenic for Trm cell formation. 27, 33 The working hypothesis is that if the vaccination phase RAS liver burden was significantly reduced, Trm cell formation may also be hindered, and protection could be lost. This was a possibility since the liver-homing ability of cryo-RAS was previously shown to be somewhat reduced compared with fresh-RAS. 40 To investigate the liver trapping potential of cryo-RAS, we DNA primed BALB/cj mice with ggCSP and then administered 2 × 104 Py fresh- or cryo-RAS trap 4 weeks later (Figure 1A). Non-viable HK-spz were used as a control. Four hours post-trapping, livers were harvested for RT-PCR to compare the liver parasite burden. This time point was selected to capture the number of parasites that initially invaded the liver, allowing time for the majority of circulating spz to home to the liver but not enough time to be targeted and/or killed there. 41 We found that the parasite burden in cryo-RAS immunized mice was significantly reduced compared with fresh-RAS, but was still very high relative to HK-spz (Figure 1). Cryo-RAS 18S rRNA copy numbers per mouse liver were approximately 9-fold lower than after fresh-RAS (Figure 1B and C). This was in striking contrast to the HK-spz liver burden, where 18S rRNA was not detectable in any mouse liver. Thus, we hypothesized that the known reduction in viability and motility of Py cryo-RAS compared with fresh-RAS led to a < 1 log reduction in parasite liver burden. We next sought to determine whether this difference would alter the suitability of cryo-RAS as a trapping candidate.
Figure 1.
DNA prime and Plasmodium yoelii (Py) fresh- or cryopreserved radiation attenuated sporozoite (cryo-RAS) trap leads to substantial parasite liver burden. (A) Experimental design of prime-and-trap liver burden studies. (B) Four hours after trapping with either fresh-RAS, cryo-RAS, or heat-killed sporozoite (HK-spz) mouse livers were excised and processed for real-time reverse transcription polymerase chain reaction (RT-PCR) to measure liver stage parasite burden with 18S pan Plasmodium primers. Data are shown as cycle thresholds (B) for all mice and for absolute 18S rRNA quantification for a subset of the mice where absolute calibrators were used (C). Error bars represent the SD of the mean of N = 15 mice across two experiments (N = 7 mice across two experiments for HK-spz). Data points correspond to individual mice. 95% CI of mean: fresh-RAS 24.1–25.7, cryo-RAS 27.2–28.9, HK-spz 45 cycles in (B) and fresh-RAS 6.5 × 107–1.5 × 108, cryo-RAS 8.2 × 106–1.5 × 107, HK-spz zero 18S copies in (C). Kruskal–Wallis with Dunn’s multiple comparisons test: fresh-RAS vs. all other groups. ND = not detected. * P < 0.05, ** P < 0.005, **** P < 0.0001.
Py fresh- or cryo-RAS trap induce comparable levels of PyCSP-specific liver CD8+ Trm cells.
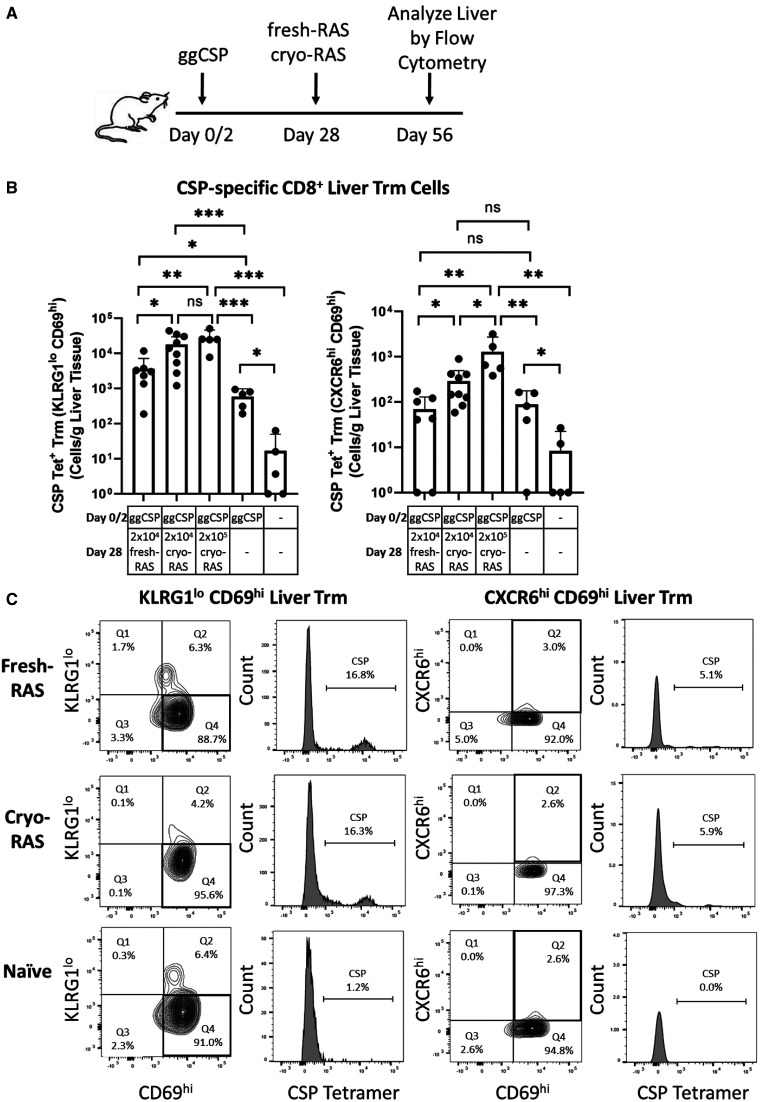
Despite the reduction of parasite burden following cryo-RAS trap, we hypothesized that the cryo-RAS present in the liver would be sufficient to induce protective PyCSP-specific liver CD8+ Trm cells. To investigate, mice were primed with ggCSP and administered 2 × 104 fresh- or cryo-RAS as abovementioned, then mouse livers were harvested 4 weeks post-trap for flow cytometry to compare PyCSP-specific CD8+ T cell frequencies. We previously showed that prime-and-trap induced high-frequency PyCSP-specific liver CD8+ Trm cells at this time point. 27 To define liver PyCSP tetramer-stained CD8+ Trm cells, we gated on either CD69+/KLRG1lo or CD69+/CXCR6hi expression as previously described. 14, 27, 42 We found that the levels of PyCSP-specific CD8+ Trm cells in livers from cryo- versus fresh-RAS trapped mice were significantly higher despite the differing parasite liver burdens observed by RT-PCR (Figure 2). The frequency of PyCSP-specific CD8+ Trm cells in the livers of cryo-RAS trapped mice were also higher than in either of the control groups, which received ggCSP prime only or were completely naïve (Figure 2). Furthermore, increasing the cryo-RAS trap dose 10-fold to 2 × 105 cryo-RAS only modestly increased the PyCSP-specific liver CD8+ T cells. Taken together, this data suggests that despite the reduced parasite liver burden observed following cryo-RAS trap, this immunization strategy induces high-frequency PyCSP-specific liver CD8+ Trm cells.
Figure 2.
Plasmodium yoelii (Py) fresh- or cryopreserved radiation attenuated sporozoite (cryo-RAS) trap induce comparable levels of circumsporozoite protein (CSP)-specific liver CD8+ Trm cells. (A) Experimental design of prime-and-trap studies. (B) Flow cytometry of CD69hi/KLRG1lo (Left) or CD69hi/CXCR6hi (Right) tetramer-stained, CSP-specific CD8+ liver Trm cells from (A) livers. (C) CSP-specific CD8+ liver Trm cell gating strategies, one representative animal per group is shown. Error bars represent 95% CI of the mean of N = 7–9 mice across two experiments (2 × 105 cryo-RAS, ggCSP only, and naïve groups N = 5 mice from one experiment). 95% CI of mean: fresh-RAS 194.2–7155, 2 × 104 cryo-RAS 6249–29752, 2 × 105 cryo-RAS 7586–46014, ggCSP 185.8–983.6, Naive 16.7–49.6 CSP-specific CD69hi/KLRG1lo Trm cells/g liver tissue and fresh-RAS 8.4–129.2, 2 × 104 cryo-RAS 87.7–497.0, 2 × 105 cryo-RAS 164.2–2730, ggCSP 0.1–177.2, Naive –7–22.5 CSP-specific CD69hi/CXCR6hi Trm cells/g liver tissue. Mann–Whitney two-tailed test. ns = P > 0.05. * P < 0.05, ** P < 0.008, *** P < 0.0001.
DNA priming followed by cryo-RAS trap protects mice against Py spz challenge 4–6 weeks post-vaccination.
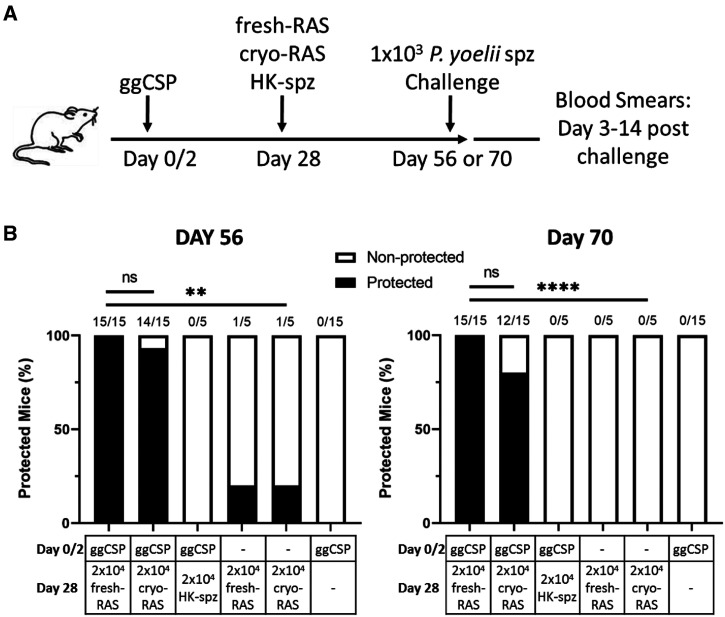
Next, we investigated whether DNA priming followed by cryo-RAS trap could protect mice against spz challenge at a memory time point. Previous studies by our group demonstrated that ggCSP prime and fresh-RAS trap completely protected mice when challenged at 4 weeks. 27 As CSP-specific liver Trm cell frequencies were similar in fresh- or cryo-RAS trapped mice, we hypothesized that cryo-RAS trap would similarly protect mice against spz challenge at a memory time point. Mice were ggCSP primed and trapped with 2 × 104 fresh- or cryo-RAS as before, then challenged with 1 × 103 fresh wild-type infectious Py spz 4–6 weeks later. Both fresh- and cryo-RAS achieved comparable protection at the 4-week challenge interval (Figure 3). Protection at 6 weeks was slightly reduced in the cryo-RAS trapped group compared with fresh-RAS, although this difference was non-significant (Figure 3). As expected, fresh- or cryo-RAS trap only or ggCSP prime only control group mice showed little to no protection (Figure 3). In addition, no protection was observed in mice that received ggCSP priming with HK-spz trap at either time point (Figure 3). Taken together, the data suggest that cryo-RAS can be substituted for fresh-RAS in prime-and-trap, can induce comparable levels of liver CD8+ Trm cells, and can similarly protect mice against spz challenge at a memory time point.
Figure 3.
DNA priming followed by cryopreserved radiation attenuated sporozoite (cryo-RAS) trap protects mice against Plasmodium yoelii sporozoite (Py spz) challenge 4–6 weeks post-vaccination. (A) Experimental design of prime-and-trap studies. (B) Results of protection studies after challenge with 1 × 103 wild-type purified Py spz administered on Day 56 (left) or Day 70 (right). N = 15 mice across two experiments (N = 5 mice from one experiment for HK-spz and RAS trap only [no ggCSP prime] groups). Fractions above bars indicate number of animals protected out of total group size. Protection was assessed with thin blood smears on Days 3–14 post-challenge. Fisher Exact Test: 2 × 104 fresh-RAS vs. all other groups. ns = P > 0.05. ** P < 0.005, **** P < 0.0001.
Py cryo-RAS trapping can be dose de-escalated by the co-administration of glycolipid adjuvant 7DW8-5.
In response to the trend for reduced vaccine efficacy following prime-and-trap with cryo-RAS versus fresh-RAS at the 6-week challenge time point, we investigated whether the addition of an adjuvant could both increase the vaccine efficacy and extend durability. Earlier studies identified the glycolipid adjuvant 7DW8-5 as a means to increase vaccine efficacy in mice and NHPs. 43 – 45 7DW8-5 is an α-galactosylceramide (α-GalCer) analog selected for its potent adjuvant activity and reduced toxicity compared with other earlier α-GalCer analogs. 43 Mechanistically, 7DW8-5 is presented on CD1d molecules to activate invariant natural killer T (iNKT) cells. When activated, iNKT cells produce Th1 and Th2 cytokines, including IFNγ and IL-4. 46 7DW8-5 is an attractive adjuvant for malaria as it co-localizes in the draining lymph nodes with RAS and increases activation of CD8+ T cells. 43 – 45 Here, we tested the effect of 7DW8-5 on the required trapping dose and protection durability of prime and cryo-RAS trap vaccination.
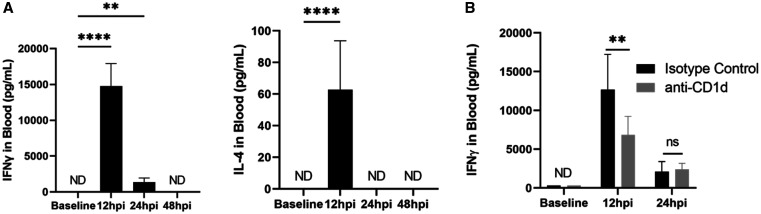
First, we characterized the cytokine responses induced following IV administration of 7DW8-5. α-GalCer analogs containing phenyl groups were previously shown to induce Th1 skewed cytokine responses. 47 Here, IV administration of 7DW8-5 induced a potent transient spike of IFNγ and to a lesser extent IL-4 in mouse blood (Figure 4). Consistent with the literature, 44, 45 IFNγ expression peaked approximately 12 hours post-injection and returned to baseline levels by 48 hours (Figure 4A). To demonstrate the specificity of the adjuvant response, we transiently blocked mouse CD1d prior to 7DW8-5 injection using an anti-mouse CD1d antibody and monitored IFNγ expression. IFNγ was significantly blocked by anti-CD1d, but not a matched isotype control (Figure 4B). This data demonstrates that 7DW8-5 can be IV administered to mice and induces a specific and transient spike of IFNγ in the blood that is cleared within 48 hours.
Figure 4.
Intravenous (IV) administration of glycolipid adjuvant 7DW8-5 induces a systemic and potent interferon (IFNγ) and interleukin 4 (IL-4) spike. (A) Quantification of IFNγ (Left) and IL-4 (Right) in mouse blood plasma induced by 7DW8-5 injection over time measured by ELISA. Kruskal–Wallis with Dunn’s multiple comparisons test, baseline vs. all other groups. 95% CI of mean for 12 hpi: IFNγ 8,792–16,077, IL-4 40.5–84.9 pg/mL. (B) Results of anti-CD1d blocking studies after administration of 7DW8-5. Multiple unpaired t tests. 95% CI of mean for 12 hpi: Isotype control 9434–15941, anti-CD1d 4458–9238 pg/mL. Error bars represent SD of mean of N = 10 mice across two experiments. ND = not detected; ns = P > 0.05. ** P < 0.005, **** P < 0.0001.
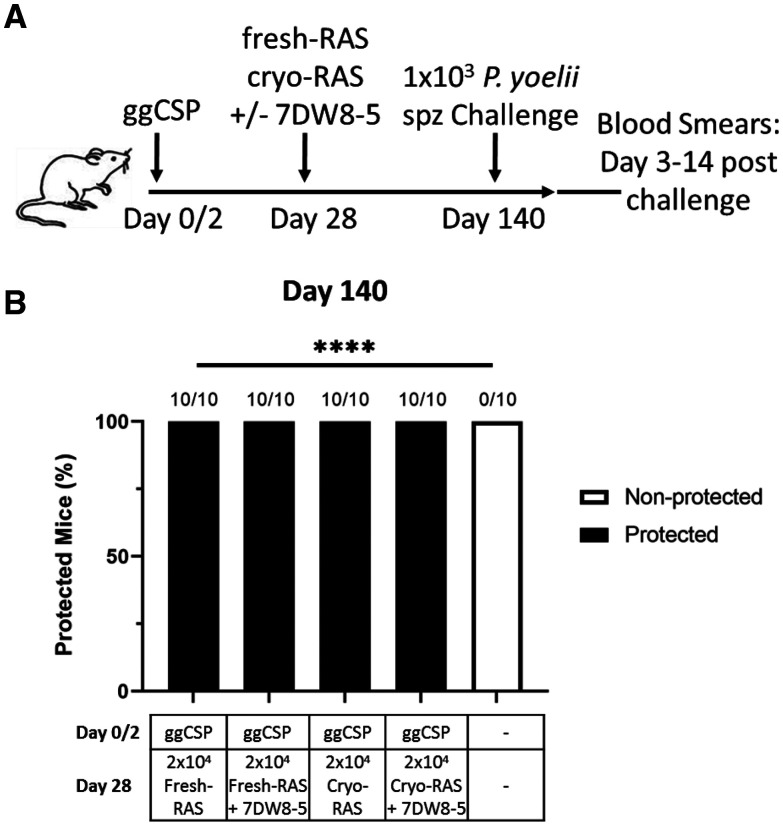
Expression of IFNγ is correlated with protection in NHPs and human clinical trails. 9 Based on our preliminary mouse studies, we hypothesized that the IFNγ induced by 7DW8-5 administration would improve cryo-RAS efficacy and durability. Preliminary experiments in the laboratory investigated the durability of prime-and-trap with fresh-RAS trapping and found that sterile protection against spz challenge at 4 months could be achieved with a high dose of fresh-RAS (5 × 104) or with the 2 × 104 fresh-RAS combined with a ggCSP boost (Supplemental Figure 2). Thus, we hypothesized that prime-and-trap with 2 × 104 fresh- or cryo-RAS would protect a substantial portion of mice at 4 months and that any reduced protection could be rescued by addition of 7DW8-5 at the trapping step. To investigate this possibility, mice were ggCSP primed and then trapped with either 2 × 104 fresh- or cryo-RAS with or without 7DW8-5. Four months post-trapping, mice were challenged with 1 × 103 freshly dissected WT Py spz and protection was assessed. Somewhat surprisingly, given the data in Figure 3 suggesting reduced protection with cryo-RAS trap at 6 weeks, we found that prime-and-trap with fresh- or cryo-RAS achieve complete protection against spz challenge at 4 months with or without the addition of 7DW8-5 (Figure 5). The reasons for slight differences in protection in the 4–6 week challenge (86–100% protection) versus 4-month challenge (100% protection) are unclear, but could include interoperator variations in IV techniques and/or differences in batches of spz. Nonetheless, in summary, the data indicates that PyCSP DNA prime and cryo-RAS trap vaccination achieves long-lasting sterile protection in mice.
Figure 5.
DNA priming followed by Plasmodium yoelii (Py) fresh- or cryopreserved radiation attenuated sporozoite (cryo-RAS) trap +/−7DW8-5 induces durable protection in mice. (A) Experimental design of prime-and-trap studies. (B) Results of protection studies after challenge with 1 × 103 wild-type purified Py sporozoite (spz) administered 4 months (Day 140) after trapping. N = 10 mice across two experiments. Fractions above bars indicate number of animals protected out of total group size. Protection was assessed with thin blood smears on Days 3–14 post-challenge. Fisher Exact Test: fresh-RAS vs. all other groups. ns = P > 0.05. **** P < 0.0001.
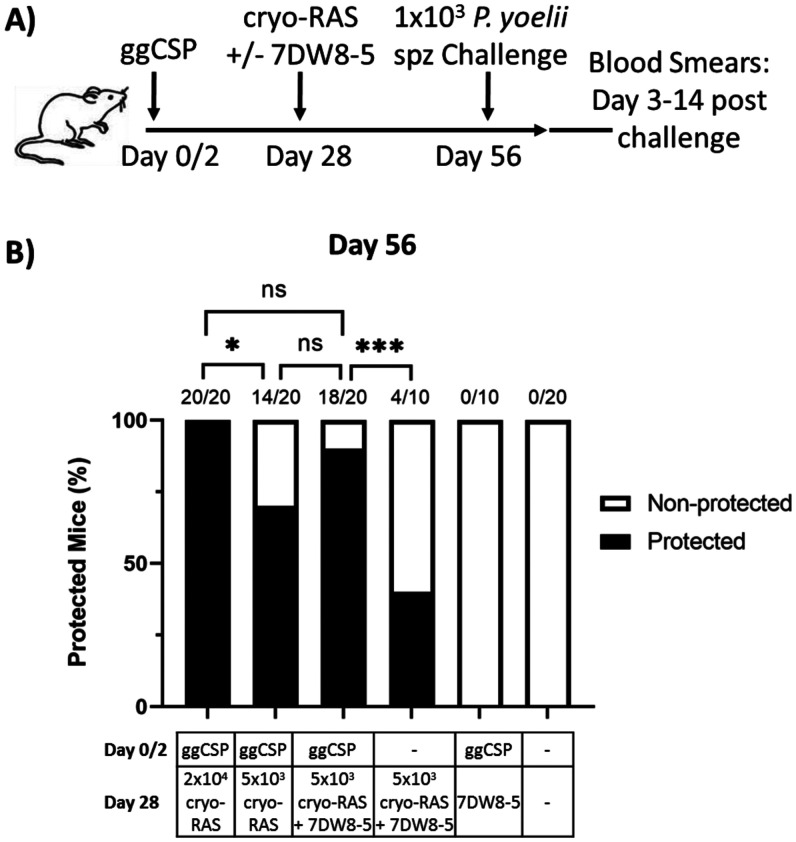
Next, we determined whether we could decrease the cryo-RAS dose required for sterile protection. Preliminary experiments investigating prime-and-trap with fresh-RAS dose de-escalation in our laboratory suggested that 2 × 104 fresh-RAS were required for reliable sterile protection (Supplemental Figure 3). Thus, we investigated whether the cryo-RAS trapping dose could be reduced by co-administration with the glycolipid adjuvant 7DW8-5. Mice were ggCSP primed followed by 2 × 104 or 5 × 103 cryo-RAS with or without the addition of 7DW8-5. The full dose of 2 × 104 cryo-RAS was required for complete protection in the absence of 7DW8-5, but the dose of cryo-RAS could be reduced four-fold by co-administration with 7DW8-5 while still maintaining ≥ 90% sterile protection (Figure 6). As expected, control mice receiving ggCSP prime followed by 7DW8-5 trap without any cryo-RAS, and those immunized with only cryo-RAS+7DW8-5 (no ggCSP prime) were not protected against spz challenge. In summary, we demonstrate that prime-and-trap vaccination with cryo-RAS is feasible and durable, and that the cyro-RAS trapping dose can be reduced by the co-administration of 7DW8-5.
Figure 6.
Plasmodium yoelii (Py) cryopreserved radiation attenuated sporozoite (cryo-RAS) trap dose can be reduced by the co-administration of 7DW8-5. (A) Experimental design of prime-and-trap studies. (B) Results of protection studies after challenge with 1 × 103 wild-type purified Py sporozoite (spz) administered 4 weeks (Day 56) after trapping. N = 10–20 mice across two to four experiments. Fractions above bars indicate the number of animals protected out of total group size. Protection was assessed with thin blood smears on Days 3–14 post-challenge. Fisher Exact Test: 2 × 104 cryo-RAS vs. all other groups and 5 × 103 ggCSP+(cryo-RAS+7DW8-5) vs. all other groups. ns = P > 0.05. * P < 0.05, *** P < 0.0005.
DISCUSSION
In this study, we optimized the two-step malaria prime-and-trap vaccine strategy for translation to NHPs and humans. Our original prime-and-trap vaccine combined epidermal priming with DNA encoding the PyCSP antigen followed by a single IV dose of freshly dissected liver-homing RAS and a concurrent ggCSP DNA boost. 27 Here, we investigated the potential to substitute fresh-RAS trap with cryo-RAS, which can be moved to humans based on progress with PfSPZ Vaccine, 5, 9 PfSPZ—CVac, 15 and PfSPZ Challenge. 31 We demonstrated that prime-and-trap can be improved with the use of cryo-RAS in the place of fresh-RAS, and that a lower trapping dose can be used if cryo-RAS are co-administered with the glycolipid adjuvant 7DW8-5. This trapping approach is therefore quite versatile in that liver-homing cryo-RAS can be administered either with or without immunostimulatory 7DW8-5. We further demonstrated that ggCSP boosting during trapping, which was used in the original vaccine strategy, 27 is dispensable for achieving protection by prime-and-trap.
Previously, we showed that the fresh-RAS trap positions protective CD8+ Trm cells in the liver. 27 Here, we demonstrated that despite the reduced liver burden observed following cryo-RAS trap compared with fresh-RAS, both formulations (fresh or cryopreserved) are sufficient for inducing durable sterile protection in mice. The reduction in parasite liver burden is likely because of reduced cryo-RAS infectivity, as previous studies in mice have shown a seven-fold decrease in cryo-RAS liver infectivity compared with fresh-RAS. 31, 48 However, despite reduced infectivity, cryo-RAS trapping induced higher levels of protective PyCSP-specific liver CD8+ Trm cells compared with fresh-RAS at 4 weeks and similarly achieved sterile protection at 4 months in mice. Differences in spz purification methods (fresh-RAS purified by Accudenz gradient and cryo-RAS purified at Sanaria) could be responsible for the discrepancy observed between liver burden and liver CD8+ Trm formation. Since it is known that mosquito debris can reduce spz immunogenicity, 38 it is tempting to speculate that ultra-purified aseptic cryo-RAS are more immunogenic than fresh-RAS and can thus induce more CD8+ Trm cells in the liver. In contrast, HK-spz did not show any measureable liver burden in ggCSP DNA-primed mice, and their use as a trap did not provide any protection against spz challenge. The lack of protection induced from non-viable HK-spz trapping offers further evidence that liver invasion by RAS is likely key for protective CD8+ Trm formation. A prior study evaluated HK-spz immunization in C57Bl/6 mice previously adoptively transferred with parasite-specific PbT-1 cells. Unadjuvanted HK-spz conferred no sterile protection in such mice, and the addition of α-GalCer protected only one of eight mice, 49 suggesting that viable spz are critical for protection. RAS have limited intrahepatic development but do actively invade hepatocytes. Based on our findings, RAS trapping induces liver CD8+ cells and achieves sterile protection but HK-spz do not. Thus, our results suggest that hepatocyte invasion is important for liver immunity. Recent studies have also demonstrated that liver CD8+ Trm cells are long lasting and can have a half-life of ∼425 days and still maintain protective efficacy 200 days post-vaccination. 50 Consistent with this data, we observed no loss of protective efficacy with our prime-and-trap vaccine using fresh- or cryo-RAS trap 4 months (112 days) post-trap. This data suggests that the durability of CSP-specific liver CD8+ Trm cells induced from fresh- or cryo-RAS trap are similarly long lasting.
Many studies have revealed the beneficial adjuvanting effects of glycolipids in mice. 45, 50, 51 The α-GalCer analog 7DW8-5 has emerged as a promising candidate adjuvant for malaria vaccines, demonstrating efficacy in both mice and NHPs. 43 – 45 Mechanistically, 7DW8-5 is presented on CD1d molecules to iNKT cells (reviewed in reference 52), which are found normally patrolling liver sinusoids in mice. 53 Although iNKT cells are conserved between mice and humans, 54 many differences exist in iNKT cell frequencies, functions, and behaviors. 55 Despite over 25 years of research, many cancer and infectious disease clinical trials with α-GalCer have shown suboptimal results. The majority of α-GalCer studies occur in preclinical mouse models with no standard protocol for clinical translation of experimental strategies (reviewed in reference 56). Thus, 7DW8-5 may face similar translational challenges. However, preclinical studies in mice have shown that 7DW8-5 binds CD1d with a higher affinity and has a 100-fold higher dose-sparing effect than α-GalCer. 57 This data and promising studies in humanized mice 58 and NHPs 44 suggest that 7DW8-5 is beneficial as a malaria vaccine adjuvant in larger animal models and potentially even humans.
This optimized prime-and-trap strategy was designed to improve translation of the existing strategy, but still has several shortcomings. First, this vaccine strategy contains two unique components each with separate regulatory, GMP, storage, and administration requirements. To our knowledge, there are no licensed human vaccines utilizing truly heterologous prime/boost doses, even though heterologous prime/boost strategies are widely understood to maximize immunogenicity in pre-clinical models 59 – 61 and human clinical trials. 62, 63 Second, although widely used in veterinary medicine, DNA vaccines have not yet been licensed for any disease in humans. 64, 65 Numerous clinical trials have been completed and are ongoing demonstrating efficacy and safety of DNA (and mRNA) vaccines for various pathogens including P. falciparum, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Zika virus, and Ebola virus. 66 – 69 Clinical trials involving gene gun-delivered DNA have shown good tolerability and immunogenicity for other pathogens, 70, 71 in agreement with pre-clinical studies in small and large animal models. 72 – 75 Specifically, gene gun–delivered vaccines have been shown to increase immunogenicity, 27, 76 be highly reproducible, 77 painless, needle-free, and dose-sparing in mice and NHPs. 78, 79 Lastly, the use of cryo-RAS for prime-and-trap is an improvement over the original fresh-RAS-dependent strategy, but faces some implementation hurdles. Cryo-RAS, like any eukaryotic cells, require liquid nitrogen vapor phase storage and currently require IV administration. Ongoing clinical trials involving PfSPZ vaccines have demonstrated that large-scale implementation in endemic regions is achievable 8, 29 and is in fact easier than distribution of products requiring 4°C storage because liquid nitrogen does not require electricity in the cold chain. Coupled with these PfSPZ manufacturing, storage, and administration successes, the fact that prime-and-trap requires only a single dose of spz further simplifies their use here, which may make it easier to translate these findings to the clinic. To further aid this translation to clinical candidates, additional studies in our laboratory are also exploring alternative priming strategies as well as non-IV routes of cryo-RAS trapping. Beyond DNA vaccination, mRNA vaccines for malaria are also under consideration here given the recent successes with safe and efficacious mRNA vaccines to combat SARS-CoV-2. 80
In summary, prime-and-trap with cryo-RAS is a vaccine strategy with considerable clinical potential. For priming, nucleic acid vaccines are now being widely used to fight SARS-CoV-2, and there are many other DNA and mRNA vaccines in clinical development. For trapping, GMP-grade, aseptic, purified cryopreserved PfSPZ have been used in clinical trials in thousands of subjects in seven countries in Africa, 7, 29 five countries in Europe, 81 and at multiple sites in the United States 5, 13 and are known to be safe and efficacious (reviewed in reference 6). The one-time use of cryo-RAS in prime-and-trap vaccination here greatly simplifies the immunization schedule, which will aid manufacturing and likely improve adherence as well. 82 Lastly, the adjuvant 7DW8-5 was dose-sparing for cryo-RAS, which could also simplify manufacturing. Overall, the data suggest that prime-and-trap with cryo-RAS and the glycolipid adjuvant 7DW8-5 should be further investigated in preclinical vaccine studies in NHPs and in human clinical trials.
Supplemental Material
ACKNOWLEDGMENTS
We thank Tess Seltzer, Alexis Kaushansky, Will Betz, and Stefan H. I. Kappe (Seattle Children’s Research Institute) for assistance and support of Plasmodium yoelii–infected mosquito production and the NIH Tetramer Core Facility (contract number 75N93020D00005) for providing PyCSP monomers. We thank the veterinary staff of the UW Department of Comparative Medicine. We also thank Sanaria for the cryopreserved P. yoelii sporozoites.
Note: Supplemental table and figures appear at www.ajtmh.org.
References
- 1. World Health Organization , 2020. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Geneva, Switzerland: WHO. [Google Scholar]
- 2. Hodgson SH et al. 2015. Increased sample volume and use of quantitative reverse-transcription PCR can improve prediction of liver-to-blood inoculum size in controlled human malaria infection studies. Malar J 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fairley NH , 1947. Sidelights on malaria in man obtained by subinoculation experiments. Trans R Soc Trop Med Hyg 40: 621–676. [DOI] [PubMed] [Google Scholar]
- 4. Draper SJ Sack BK King CR Nielsen CM Rayner JC Higgins MK Long CA Seder RA , 2018. Malaria vaccines: recent advances and new horizons. Cell Host Microbe 24: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seder RA et al. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 6. Richie TL et al. 2015. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33: 7452–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jongo SA et al. 2018. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg 99: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jongo SA et al. 2021. Immunogenicity and protective efficacy of radiation-attenuated and chemo-attenuated PfSPZ vaccines in Equatoguinean adults. Am J Trop Med Hyg 104: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishizuka AS et al. 2016. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22: 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Epstein JE et al. 2017. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2: e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyke KE et al. 2017. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA 114: 2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nussenzweig RS Vanderberg J Most H Orton C , 1967. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei . Nature 216: 160–162. [DOI] [PubMed] [Google Scholar]
- 13. Epstein JE et al. 2011. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334: 475–480. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Ruiz D et al. 2016. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage. Infect Immun 45: 889–902. [DOI] [PubMed] [Google Scholar]
- 15. Mordmuller B et al. 2017. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mwakingwe-Omari A et al. 2021. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 595: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss WR Jiang CG , 2012. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLOS ONE 7: e31247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keitany GJ et al. 2014. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect Immun 82: 5143–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doolan DL Hoffman SL , 2000. The complexity of protective immunity against liver-stage malaria. J Immunol 165: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 20. Holz LE Fernandez-Ruiz D Heath WR , 2016. Protective immunity to liver-stage malaria. Clin Transl Immunology 5: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schofield L Villaquiran J Ferreira A Schellekens H Nussenzweig R Nussenzweig V , 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330: 664–666. [DOI] [PubMed] [Google Scholar]
- 22. Weiss WR Sedegah M Beaudoin RL Miller LH Good MF , 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA 85: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seguin MC Klotz FW Schneider I Weir JP Goodbary M Slayter M Raney JJ Aniagolu JU Green SJ , 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med 180: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt NW Butler NS Harty JT , 2011. Plasmodium-host interactions directly influence the threshold of memory CD8 T cells required for protective immunity. J Immunol 186: 5873–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghilas S Valencia-Hernandez A-M Enders MH Heath WR Fernandez-Ruiz D , 2020. Resident memory T cells and their role within the liver. Int J Mol Sci 21: 8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishizuka AS et al. 2016. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22: 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen TM Stone BC Chuenchob V Murphy SC , 2018. Prime-and-trap malaria vaccination to generate protective CD8+ liver-resident memory T cells. J Immunol 201: 1984–1993. [DOI] [PubMed] [Google Scholar]
- 28. Oneko M et al. 2021. Safety, immunogenicity and efficacy of PfSPZ vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med 27: 1636–1645. [DOI] [PubMed] [Google Scholar]
- 29. Sissoko MS et al. 2017. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 17: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roestenberg M et al. 2020. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med 12: eaaz5629. [DOI] [PubMed] [Google Scholar]
- 31. Roestenberg M et al. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 88: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imai J Otani M Sakai T Hatta S , 2016. Purification of the subcellular compartment in which exogenous antigens undergo endoplasmic reticulum-associated degradation from dendritic cells. Heliyon 2: e00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stone BC Kas A Billman ZP Fuller DH Fuller JT Shendure J Murphy SC , 2016. Complex minigene library vaccination for discovery of pre-erythrocytic Plasmodium T cell antigens. PLOS ONE 11: e0153449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arrington J et al. 2002. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J Virol 76: 4536–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kennedy M et al. 2012. A rapid and scalable density gradient purification method for Plasmodium sporozoites. Malar J 11: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chattopadhyay R Conteh S Li M James ER Epstein JE Hoffman SL , 2009. The effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine. Vaccine 27: 3675–3680. [DOI] [PubMed] [Google Scholar]
- 37. Murphy SC et al. 2012. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg 86: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Billman ZP Seilie AM Murphy SC , 2016. Purification of Plasmodium sporozoites enhances parasite-specific CD8+ T cell responses. Infect Immun 84: 2233–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blom KG Qazi MR Matos JB Nelson BD DePierre JW Abedi-Valugerdi M , 2009. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol 155: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prinz H Sattler JM Roth A Ripp J Adams JH Frischknecht F , 2018. Immunization efficacy of cryopreserved genetically attenuated Plasmodium berghei sporozoites. Parasitol Res 117: 2487–2497. [DOI] [PubMed] [Google Scholar]
- 41. Murphy SC et al. 2018. Plasmodium 18S rRNA of intravenously administered sporozoites does not persist in peripheral blood. Malar J 17: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mackay LK et al. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 43. Li X Fujio M Imamura M Wu D Vasan S Wong CH Ho DD Tsuji M , 2010. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA 107: 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Padte NN et al. 2013. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLOS ONE 8: e78407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X et al. 2015. Colocalization of a CD1d-binding glycolipid with a radiation-attenuated sporozoite vaccine in lymph node-resident dendritic cells for a robust adjuvant effect. J Immunol 195: 2710–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bricard G et al. 2010. α-Galactosylceramide analogs with weak agonist activity for human iNKT cells define new candidate anti-inflammatory agents. PLOS ONE 5: e14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hung J-T Huang J-R Yu AL , 2017. Tailored design of NKT-stimulatory glycolipids for polarization of immune responses. J Biomed Sci 24: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruben A et al. 2013. 157 Cryopreservation of Plasmodium falciparum sporozoites and Sanaria® PfSPZ vaccine. Cryobiology 67: 442. [Google Scholar]
- 49. Ghilas S et al. 2021. Development of Plasmodium-specific liver-resident memory CD8(+) T cells after heat-killed sporozoite immunization in mice. Eur J Immunol 51: 1153–1165. [DOI] [PubMed] [Google Scholar]
- 50. Holz LE et al. 2020. Glycolipid-peptide vaccination induces liver-resident memory CD8+ T cells that protect against rodent malaria. Sci Immunol 5: eaaz8035. [DOI] [PubMed] [Google Scholar]
- 51. Li X Huang J Kawamura A Funakoshi R Porcelli SA Tsuji M , 2017. Co-localization of a CD1d-binding glycolipid with an adenovirus-based malaria vaccine for a potent adjuvant effect. Vaccine 35: 3171–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Godfrey DI Uldrich AP McCluskey J Rossjohn J Moody DB , 2015. The burgeoning family of unconventional T cells. Nat Immunol 16: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 53. Geissmann F Cameron TO Sidobre S Manlongat N Kronenberg M Briskin MJ Dustin ML Littman DR , 2005. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 3: e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brossay L Chioda M Burdin N Koezuka Y Casorati G Dellabona P Kronenberg M , 1998. CD1d-mediated recognition of an α -galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med 188: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X Polacino P Garcia-Navarro R Hu S-L Tsuji M , 2012. Peripheral blood invariant natural killer T cells of pig-tailed macaques. PLoOS ONE 7: e48166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y Springfield R Chen S Li X Feng X Moshirian R Yang R Yuan W , 2019. Alpha-GalCer and iNKT cell-based cancer immunotherapy: realizing the therapeutic potentials. Front Immunol 10: 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coelho-Dos-Reis JG Li X Tsuji M , 2018. Development of a novel mechanism-based glycolipid adjuvant for vaccination. F1000 Res 7: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li X Huang J Kaneko I Zhang M Iwanaga S Yuda M Tsuji M , 2017. A potent adjuvant effect of a CD1d-binding NKT cell ligand in human immune system mice. Expert Rev Vaccines 16: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sedegah M et al. 2002. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8(+)-T-cell populations. Infect Immun 70: 3493–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gilbert SC Schneider J Hannan CM Hu JT Plebanski M Sinden R Hill AV , 2002. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 61. Schneider J et al. 2001. A prime-boost immunisation regimen using DNA followed by recombinant modified vaccinia virus Ankara induces strong cellular immune responses against the Plasmodium falciparum TRAP antigen in chimpanzees. Vaccine 19: 4595–4602. [DOI] [PubMed] [Google Scholar]
- 62. McConkey SJ et al. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med 9: 729–735. [DOI] [PubMed] [Google Scholar]
- 63. Ogwang C et al. 2015. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med 7: 286re5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jazayeri SD Poh CL , 2019. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet Res 50: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hobernik D Bros M , 2018. DNA vaccines-how far from clinical use? Int J Mol Sci 19: 3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tebas P et al. 2021. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, phase 1 clinical trial. EClinicalMedicine 31: 100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tebas P et al. 2017. Safety and immunogenicity of an anti–Zika virus DNA vaccine — preliminary report. N Engl J Med 385: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tebas P et al. 2019. Intradermal SynCon® Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis 220: 400–410. [DOI] [PubMed] [Google Scholar]
- 69. Epstein JE et al. 2004. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/AS02A. Vaccine 22: 1592–1603. [DOI] [PubMed] [Google Scholar]
- 70. Roy MJ et al. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19: 764–778. [DOI] [PubMed] [Google Scholar]
- 71. Swain WE et al. 2000. Tolerability and immune responses in humans to a PowderJect DNA vaccine for hepatitis B. Dev Biol (Basel) 104: 115–119. [PubMed] [Google Scholar]
- 72. Fuller DH Loudon P Schmaljohn C , 2006. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods 40: 86–97. [DOI] [PubMed] [Google Scholar]
- 73. Dincer Z Jones S Haworth R , 2006. Preclinical safety assessment of a DNA vaccine using particle-mediated epidermal delivery in domestic pig, minipig and mouse. Exp Toxicol Pathol 57: 351–357. [DOI] [PubMed] [Google Scholar]
- 74. Fry LM Bastos RG Stone BC Williams LB Knowles DP Murphy SC , 2019. Gene gun DNA immunization of cattle induces humoral and CD4 T-cell-mediated immune responses against the Theileria parva polymorphic immunodominant molecule. Vaccine 37: 1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bergmann-Leitner ES Leitner WW , 2013. Gene Gun Immunization to Combat Malaria. Methods Mol Biol 940: 269–284. [DOI] [PubMed] [Google Scholar]
- 76. Belperron AA Feltquate D Fox BA Horii T Bzik DJ , 1999. Immune responses induced by gene gun or intramuscular injection of DNA vaccines that express immunogenic regions of the serine repeat antigen from Plasmodium falciparum. Infect Immun 67: 5163–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoshida A Nagata T Uchijima M Higashi T Koide Y , 2000. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 18: 1725–1729. [DOI] [PubMed] [Google Scholar]
- 78. Bergmann-Leitner ES, Leitner WW, 2015. Vaccination Using Gene-Gun Technology. New York, NY: Springer, 289–302. [DOI] [PubMed] [Google Scholar]
- 79. Walsh DS et al. 2006. Heterologous prime-boost immunization in rhesus macaques by two, optimally spaced particle-mediated epidermal deliveries of Plasmodium falciparum circumsporozoite protein-encoding DNA, followed by intramuscular RTS,S/AS02A. Vaccine 24: 4167–4178. [DOI] [PubMed] [Google Scholar]
- 80. Chaudhary N Weissman D Whitehead KA , 2021. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov 20: 817–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bastiaens GJH et al. 2016. Safety, immunogenicity, and protective efficacy of intradermal immunization with aseptic, purified, cryopreserved Plasmodium falciparum sporozoites in volunteers under chloroquine prophylaxis: a randomized controlled trial. Am J Trop Med Hyg 94: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hadjipanayis A , 2019. Compliance with vaccination schedules. Hum Vaccin Immunother 15: 1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.