ABSTRACT.
Monocyte dysfunction in helminth infection is one of the mechanisms proposed to explain the diminished parasite antigen-specific T cell responses seen with patent filarial infection. In fact, monocytes from filariae-infected individuals demonstrate internalized filarial antigens and, as a consequence, express inhibitory surface molecules and have diminished cytokine production. To investigate the mechanisms underlying monocyte dysfunction in filarial infections, purified human monocytes were exposed to live microfilariae (mf) of Brugia malayi, and the mRNA and protein expression of important inhibitory and/or autophagy-related molecules were assessed. Our data indicate that mf-induced autophagy in human monocytes shown by the formation of autophagic vesicles, by the upregulation in the mRNA expression of autophagy-related genes BCN1, LC3B, ATG5, ATG7 (P < 0.05), and by increase in the levels of LC3B protein. Furthermore, this mf-induced autophagy increased the levels of monocyte CD206 expression. In addition, mf significantly induced the frequency of interferon (IFN)-γ+ human monocytes and at the same time induced the mRNA expression of indoleamine 2,3-dioxygenase (IDO) through an IFN-γ-dependent mechanism; significantly enhanced tryptophan degradation (an indicator of IDO activity; P < 0.005). Interestingly, this autophagy induction by mf in monocytes was IFN-γ-dependent but IDO-independent as was reversed by anti-IFN-γ but not by an IDO inhibitor. Our data collectively suggest that mf of Brugia malayi regulate the function of monocytes by induction of IDO and IFN-γ, induce autophagy through an IFN-γ-dependent mechanism, and increase M2 phenotype through induction of autophagy; all acting in concert to drive monocyte dysfunction.
INTRODUCTION
Lymphatic filariasis is a mosquito-borne disease caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori and a major cause of morbidity in tropical and subtropical regions of the world. 1 Microfilariae (mf) released in the lymphatics of the infected individual encounter a variety of antigen presenting cells including monocytes and dendritic cells, 2 and monocyte dysfunction is one of the causes of impaired antigen-specific T cell responses seen among patient lymphatic filariasis. 2 In addition, monocytes of microfilaremic individuals have been shown to exhibit diminished expression of genes involved in antigen presentation and processing, and produce fewer proinflammatory cytokines in response to activation. 3 Monocytes from patients with asymptomatic filarial infection exhibit hallmarks of alternative activation. 4 Previously, we reported that Brugia malayi mf use mechanisms of metabolic modulations in dendritic cells (DC). In fact, mf downregulate mTOR signaling pathway resulting in increased autophagy in human DC, therefore, regulate the host immune response. 5 Autophagy, a physiological process of organelle degradation, is characterized by the formation of double membrane vesicles called autophagosomes 6 that eliminates intracellular pathogens by direct killing or silencing their replication cycle by modulating transcriptional and translational machinery of the host cell. 7 In addition, it also favors the intracellular survival of certain microorganisms through exerting the anti-inflammatory effect by downregulation of the inflammasome. 8
Interferon-γ is a potent autophagy inducer, and can facilitate the ability of macrophages to kill intracellular parasites by overcoming the phagosome maturation block. 9, 10 Interferon-γ also plays an important role in protection against helminth infection. 11 In fact, M2 cytokines (also induced by helminth parasites) can downregulate Th1 responses, result in decreased levels of IFN-γ and inhibition of autophagy resulting in human macrophages. 12 Furthermore, this cytokine can induce indoleamine 2,3 dioxygenase (IDO) a rate limiting enzyme involved in the catabolism of tryptophan (Trp) through kynurenine (Kyn) pathway that inhibits T cell activation and induce the proliferation of immunosuppressive Tregs. 13
Apart from the involvement of autophagy in host–pathogen interactions, autophagy is also important in monocyte–macrophage differentiation. 14 Based on the responses to different stimuli, monocytes/macrophages can be categorized into classically activated M1 (proinflammatory activated by LPS or IFN-γ) or alternatively activated M2 (anti-inflammatory activated by interleukin 4 [IL-4] or IL-13). In monocyte/macrophage differentiation and polarization to M1/M2 phenotype, induction of autophagy is considered to be essential. It has been reported that intracellular bacteria inhibit M1 polarization through LC3-dependent autophagy resulting in dysregulation of host immune response. 8 In fact, loss of autophagy in macrophages exhibit increased proinflammatory M1 and decreased anti-inflammatory M2 polarization in mice with high-fat diet. 15
The effect of Brugia malayi mf on human monocyte polarization remains to be elucidated. In this study, we sought to determine whether mf induce autophagy in human monocytes and whether this parasite-induced autophagy plays a role in monocyte polarization. Our data indicate that mf of Brugia malayi induce autophagy by the formation of autophagic vesicles and genes including Beclin-1, ATG5, ATG7, and protein levels of LC3 in human monocytes. Although mf induce IFN-γ production in human monocytes and increase the mRNA levels and the frequencies of IDO positive cells, the mf-induced autophagy was shown to solely be IFN-γ dependent. Moreover, our data suggest that mf-induced autophagy is involved in monocyte upregulation of CD206; an M2 marker.
MATERIALS AND METHODS
Ethics statement.
Human monocytes were isolated from leukopacks of healthy adult volunteers from North America by counterflow centrifugal elutriation under a protocol approved by the Institutional Review Board (IRB) of the Department of Transfusion Medicine, Clinical Center, National Institutes of Health (NIH; IRB 99-CC-0168). The healthy adult volunteers were given informed written consent.
mf preparations.
Live Brugia malayi mf (provided under contract with the University of Georgia, Athens, GA) were collected by peritoneal lavage of infected jirds and separated from peritoneal cells by Ficoll diatrizoate density centrifugation. The mf were then washed repeatedly in RPMI medium with antibiotics and cultured overnight at 37°C in 5% CO2 before use.
In vitro exposure of monocytes to live mf.
Human monocytes were cultured at 50 × 106 per 6-well plate in serum-free media RPMI 1640 medium supplemented with 20 mM glutamine (Lonza, Allendale, NJ) P/S for 2 hours, after which the media was removed and the adherent cells were harvested. Monocytes were then cultured alone or exposed torecombinant human IL-4 (rhIL-4; 50 ng/mL; PeproTech, Rocky Hill, NJ), or a combination of LPS (1 µg/mL; InvivoGen, San Diego, CA) and IFN-γ (20 ng/mL), or live mf (50,000 per million cells to reflect physiologically relevant concentrations) for 24 hours. This number of mf used in culture is roughly equivalent to the number in individuals with 1,000 mf per milliliter of blood (∼0.2 million monocytes in 1 mL of blood). For anti-IFN-γ and IDO inhibitor assays, human monocytes were cultured alone or exposed to live mf with anti-IFN-γ antibody (5µg/mL; Ebiosciences, San Jose, CA) or the IDO inhibitor 1 methyl Trp (1mM/mL; Sigma-Aldrich, St. Louis, MO) or Bafilomycin 1 (BAf1) (50 nM/mL; Sigma-Aldrich, Cat no. B1793). After 24 hours, the cells were harvested with Versene/EDTA (Biofluids Division, Rockville, MD), washed twice with phosphate-buffered saline (PBS; without Ca2+/Mg2+), counted by trypan blue exclusion, and used for functional studies.
RNA preparation and real-time reverse transcription polymerase chain reaction.
Total RNA was prepared from 8 to 15 independent monocyte donors using an RNAEasy Minikit (Qiagen, Germantown, MD). RNA (1 µg) from the cells was used to generate cDNA and then assessed by standard TaqMan assays (Applied Biosystems Inc., Beverly, MA) using an ABI VIIA7 system (Applied Biosystems, Inc.). Briefly, random hexamers were used to prime RNA samples for reverse transcription using MultiScribe reverse transcriptase (Applied Biosystems Inc.), after which PCR products IDO, BECN1, ATG5, ATG7, as well as an endogenous 18S rRNA control, were assessed using TaqMan predeveloped assay reagents. The threshold cycle (CT), defined as the PCR cycle at which a statistically significant increase in reaction concentration is first detected, was calculated for the genes of interest and the 18S control and used to determine relative transcript levels. Relative transcript levels were determined by the formula 1/ΔCT, where ΔCT is the difference between the CT of the target gene and that of the corresponding endogenous 18S reference.
Electron microscopy.
Monocytes and mixtures of monocytes and mf were fixed by resuspension in Karnovsky’s fixative (Electron Microscopy Sciences, Hatfield, PA), and held overnight on ice before further processing at room temperature with microwave irradiation as follows. Samples were irradiated at 167 W for three cycles of 2 minutes on, 2 minutes off, and 2 minutes on (2-2-2), recovered by centrifugation for 5 minutes at 800 ×g, and washed twice by gentle resuspension in 0.1M sodium phosphate pH 7.2 for 3 minutes each time. The cells were postfixed with 1% osmium tetroxide and 0.8% potassium ferrocyanide in 0.1M sodium phosphate pH 7.2 with three cycles of 2-2-2, washed twice for 3 minutes each in water, and contrasted with 1% uranyl acetate in water for three cycles of 2-2-2. Following two more 3 minutes washes in water, the samples were dehydrated with 3 minutes changes in 70, 100, 100, and 100% ethanol at 250 W, and infiltrated with Durcupan epoxy resin (Electron Microscopy Sciences) at 250 W, using exchanges in 50% resin-ethanol for 20 minutes, 75% resin-ethanol for 40 minutes, and 3 changes of 100% resin for 60 minutes each. Resin blocks were hardened overnight at 65°C, trimmed, and sectioned using a 35-degree diamond knife (Diatome USA, Hatfield, PA). Silver sections were collected on 200 mesh Cu grids, poststained with Sato’s lead citrate, and examined at 80 kV with a model H7800 transmission electron microscope (Hitachi High Technologies, Dallas, TX). Images were captured with an XR-81 CCD camera (Advanced Microscopy Techniques, Inc., Woburn, MA).
Determination of IDO enzymatic activity.
To monitor enzyme activity, monocytes were harvested and were washed and resuspended in Hank's Balanced Salt Solution (HBSS). Supernatants were harvested and assayed for the degradation of Trp or presence of Kyn, the first stable catabolite downstream of IDO. Kyn was detected by either high-pressure liquid chromatography (HPLC) or using a modified spectrophotometric assay. High-pressure liquid chromatography was performed according to Yong and Lau with minor modifications. 16 Kynurenine was measured according to a published procedure. 17 Supernatant of MDM cultures (360 µL) was incubated with 180 µL of 30% trichloroacetic acid (TCA) for 30 minutes at 50°C. After centrifugation at 3000 ×g for 10 minutes, the supernatant was collected, mixed with an equal volume of freshly prepared Ehrlich Reagent (2% p-dimethylaminobenzaldehyde in glacial acetic acid), and incubated for 12–30 minutes at ambient temperature. The absorbance was measured at 492 nm and compared with a calibration curve obtained with L-Kyn (Santa Cruz, Heidelberg, Germany).
Flow cytometry staining.
Staining of cells with antibody was carried out according to standard protocols. Human monocytes were cultured alone or with mf as mentioned previously. During last 4 hours, based on the experiments Brefeldin A (3 ug/mL) (BD Biosciences, San Jose, CA, Cat. No 555029) were added to measure the intracellular cytokine secretion (eBioscience Foxp3/Transcription Factor Staining Buffer Set, Cat No. 00-5523-00). After 24 hours, cells were harvested, washed with PBS, and incubated with 10 µL human IgG (10 mg/mL; Sigma-Aldrich) for 10 minutes at 4°C to inhibit nonspecific binding through FcγRs and then incubated with marker-specific mAb conjugated with CD14-APC Cy7 (eBioscience, Cat No. 47-0149-42), IDO-PerCPefluor 710 (eBioscience Cat No. 46-9477-42), CD206 (Mannose receptor)-Percp efluor 710 (eBioscience, Cat No. 46-2069-42), anti-human IFN-γ -FiTC (eBioscience, Cat No. 11-7311-41)at saturating concentrations for 30 minutes at 4°C and washed twice with FACS medium. Monocyte cell populations (CD14+) were then identified and gated to measure the expression of cell surface markers.
Flow cytometric analysis.
For flow cytometric analysis, cells were analyzed by acquisition of 100,000 events per tube using a BD LSRII flow cytometer (BD Biosciences). Compensation was performed in every experiment using BD CompBeads (BD Biosciences) for single-color controls and unstained cells as negative controls. Data were analyzed using FlowJo Software (Tree Star, Ashland, OR). Nonviable cells were excluded from our analysis on the basis of forward and side scatter. Percentage of cell surface marker expression and mean fluorescence intensity (MFI) was measured for all markers.
Stimulation of monocytes for immunoblotting and autophagy.
To measure autophagy, 1 × 106 monocytes were left unexposed or were exposed to live mf at concentrations with or without anti-IFN-γ antibody or isotype control or 1 methyl Trp in 2% heat-inactivated human AB serum (Gemini Bioproducts, Sacramento, CA) supplemented with 100 IU/mL penicillin and 100 g/mL streptomycin (Biofluids, Inc.) overnight. Monocytes were harvested after 24 hours with Versene-EDTA (Biofluids Inc.), washed twice with PBS (without Ca2+/Mg2+), counted by trypan blue exclusion.
Immunoblot analysis.
Cell lysates were prepared and boiled for 5 minutes in Laemmli sample buffer (Bio-Rad Laboratories, Inc., Hercules, CA); 20 µL of protein was then loaded into a 1.5-mm 4–20% Tris gel and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). After blocking using 5% nonfat milk for 1 hour, the membranes were incubated overnight at 4°C with rabbit anti-LC3A/B (Cell Signaling Technology, Inc., Beverly, MA). After washing, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cell Signaling Technology) at 1:2,000 at room temperature for 2 hours. For the β-actin control, membranes were stripped in stripping buffer (Thermo Scientific, Grand Island, NJ) for 10 minutes and reprobed with anti-β-actin (Cell Signaling Technology) antibody. Proteins were detected by a chemiluminescence detection system (Cell Signaling Technology). β-Actin was used as an internal control because of low background detection and a molecular weight distinct from those of the proteins of interest. LC3II/actin ratios were calculated by quantification of intensity of LC3II and actin bands and graphed. ImageJ was used to quantify the intensity of bands in immunoblots. P values were calculated based on protein expression in exposed monocytes compared with unexposed monocytes with and without treatment.
Statistical analysis.
Unless noted otherwise, geometric means were used as a measure for central tendency. Omnibus2 normality test was performed to confirm the data is not normally distributed and then the nonparametric Wilcoxon signed-rank test was used for paired group comparisons. All analyses were performed using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
mf-induced autophagy in human monocytes.
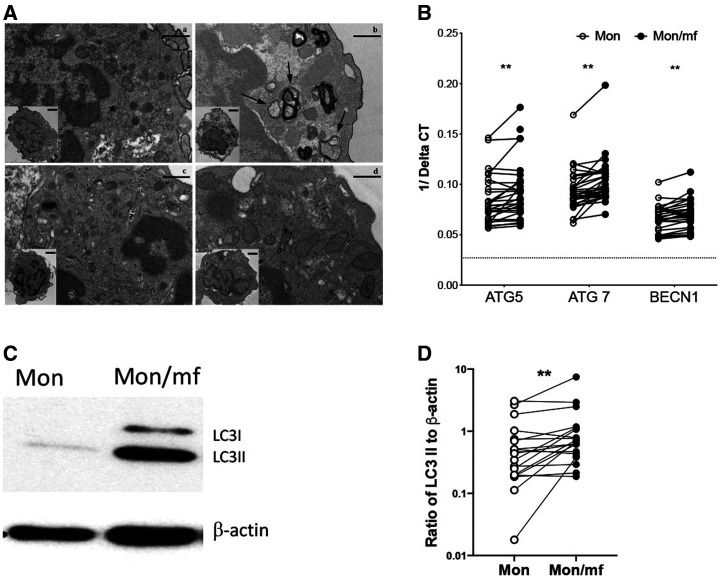
Several studies have addressed the role of autophagy in host–pathogen interactions. 7 In fact, autophagy is designed to effectively remove intracellular and microbial pathogens that can damage the cellular microenvironment. 18, 19 Previously, we have shown that mf regulate the host immune response by downmodulating the mTOR signaling pathway in monocyte-derived DC leading to increased autophagy. 5 In this study, we have investigated the effect of mf in regulation of autophagy in human monocytes. To this end, exposure to mf resulted in the formation of autophagic vesicles (Figure 1Ab). Similar vesicles were not observed in sections from cells using the autophagy inhibitor BAf1 (Figure 1Ad). Also, mf significantly upregulated the mRNA expression of genes associated with autophagy (ATG5, ATG7, and BCN1 [Figure 1B] and facilitated the conversion of LC3I to LC3II allowing for LC3II accumulation in these cells (Figure 1C and D). Although, this increase was statistically significant but not all donors (N = 19–25) showed the trend (Figure 1B and D).
Figure 1.
Microfilariae (mf)-induced autophagy in human monocytes. (A) Human monocytes were either a) unexposed, or exposed to b) live mf, c) live mf and anti-IFN-γ ab, or d) live mf and BAf1 for 24 hours. Cells were harvested, prepared for electron microscopy, and examined. Extranuclear regions in which autophagosomes occurred or would be expected are shown. Inserts depict lower magnification images of the entire cell section. Arrows demark autophagosomes. Human monocytes were either unexposed (Mon) or exposed to mf (Mon/mf) for 24 hours. Cells were harvested and (B) the mRNA expression of autophagy associated genes were assessed using real-time polymerase chain reaction (RT-PCR). Line graphs are shown as 1/average delta CT (N = 25). (C) One representative image of LC3I and LC3II expression using Western blot analysis are shown. (D) Line graphs represent the ratio of intensity of protein to β-actin (N = 19). Each circle represents an independent donor, where open circles represent Mon and closed circles represent Mon/mf. * P < 0.05, ** P < 0.005.
mf-induced autophagy results in the induction of CD206 expression in human monocytes.
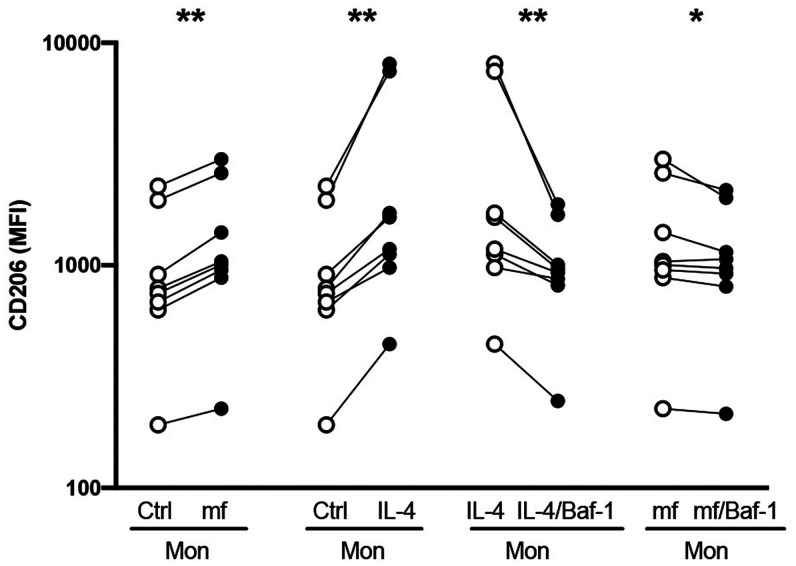
Autophagy has been shown to have an anti-inflammatory effect through the downmodulation of the inflammasome thereby downregulating proinflammatory cytokine production. 15 In fact, pathogen dysregulation and polarization of monocyte/macrophage through an LC3-dependent autophagy has been observed. 8 To assess how this mf-induced autophagy affects monocyte/macrophage polarization, human monocytes were exposed to live mf either alone or in the presence of Baf1, a known inhibitor of autophagy. Following the exposure of monocytes to mf, the cell surface expression of CD206, an M2 marker was significantly upregulated (Figure 2; N = 8). Interestingly, all monocyte donors that had an increased CD206 expression following the exposure to mf, also exhibited an increase in autophagy-related genes. However, upon treatment with Baf1, mf-induced CD206 in human monocytes was decreased significantly (P < 0.05). Furthermore, Baf1 inhibited the IL-4-induced CD206 expression in these cells (Figure 2). Our data collectively suggest that mf-induced autophagy leads to upregulation of CD206 in monocytes.
Figure 2.
Microfilariae (mf)-induced autophagy results in the induction of CD206+ monocytes. Human monocytes were exposed to mf, or recombinant human interleukin 4 (IL-4), with or without an autophagy inhibitor (BAF1) for 48 hours, cells were harvested and assessed for the expression of CD206 by flow cytometry. Line graphs represent mean fluorescence intensity (MFI) and each circle represents an independent donor. * P < 0.05, ** P < 0.005 (N = 8).
mf-induced intracellular IFN-γ and IDO expression and function in human monocytes
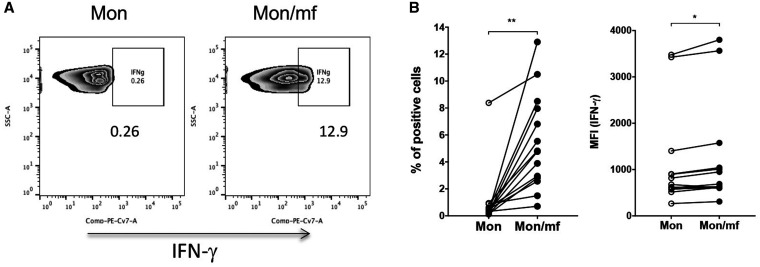
The interplay between cytokines such as IFN-γ and autophagy has been shown in many settings. 20, 21 In fact, IFN-γ promotes Trp depletion by induction of IDO and induce autophagic flux in human kidney epithelial cells. 22 Therefore, first we asked whether mf induce either IFN-γ or IDO in human monocytes. When elutriated monocytes from healthy volunteers were exposed to live mf for 24 hours, the frequency of intracellular IFN-γ positive (P < 0.005) monocytes were increased following the mf exposure as was its expression (MFI [P < 0.05]) (Figure 3A and B).
Figure 3.
Microfilariae (mf) significantly induce the frequency and mean fluorescence intensity (MFI) of interferon (IFN)-γ producing monocytes. Human monocytes were either unexposed (Mon) or exposed to live mf (Mon/mf) for 24 hours. Cells were harvested and the intracellular expression of IFN-γ was measured using flow cytometry (cells are gated on CD14+ cells). (A) One representative set of flow pseudo blot demonstrating the frequency of IFN-γ+ cells. Line graphs are expressed as (B) percentage or (C) mean fluorescence intensity (MFI) of IFN-γ+ cells. Each circle represents an independent donor, where open circles represent Mon and closed circles represent Mon/mf. * P < 0.05, ** P < 0.005 (N = 14).
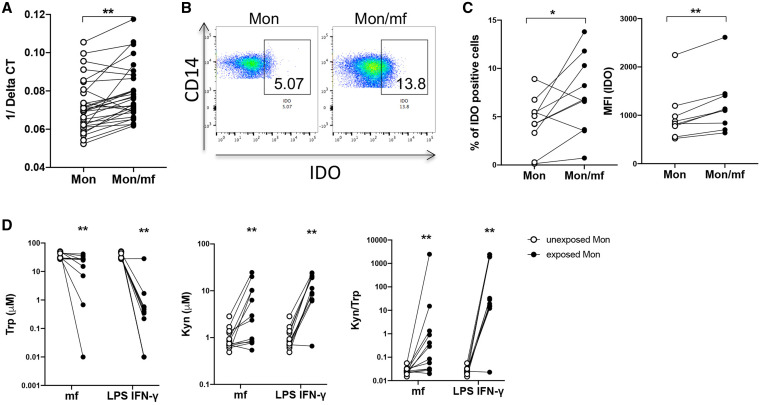
It has been shown that intracellular parasites are capable of inducing immunosuppression in human DC through upregulation of IDO, 23 and IDO is regulated by IFN-γ. 24, 25 Because mf was shown to induce IFN-γ (Figure 3), next we assessed the expression of IDO in mf-exposed monocytes. Our data indicate that the exposure of human monocytes to mf significantly induced the IDO mRNA expression (Figure 4A), and the frequency of IDO positive cells (Figure 4B and C). Moreover, the mf-exposed monocytes showed significantly higher expression of IDO as measured by MFI (Figure 4C). To assess the enzymatic activity of IDO, we measured the levels of Trp and Kyn (Figure 4D), following the exposure to mf, or to the combination of LPS and IFN-γ (LPS/IFN-γ). As seen, mf significantly increased the levels of Kyn and decreased the levels of Trp resulting in a decrease in the ratio of Tryp to Kyn in these cells (Figure 4D).
Figure 4.
Microfilariae (mf) significantly induce monocyte indoleamine 2,3-dioxygenase (IDO) expression and function. Human monocytes were either unexposed (Mon) or exposed to live mf (Mon/mf) for 24 hours. Cells were harvested and the (A) mRNA or (B) and (C) intracellular expression of IDO was measured using real-time polymerase chain reaction (PCR) or flow cytometry respectively. (A) Line graphs are shown as 1/Delta CT (N = 27). (B) One representative set (N = 9) of flow pseudoblot demonstrating frequency of IDO+ cells. (C) Line graphs are expressed as percentage or mean fluorescence intensity (MFI) of IDO+ cells. D) IDO activity was measure using HPLC to assess the depletion of Trp (right), induction of Kyn (middle) and the ratio of Trp/Kyn (left) in Mon, Mon/mf and monocytes exposed to LPS and interferon (IFN)-γ (Mon/LPS.IFN-γ) (N = 11). Each circle represents an independent donor, where open circles represent Mon and closed circles represent Mon/mf or Mon/LPS.IFN-γ. * P < 0.05, ** P < 0.005. This figure appears in color at www.ajtmh.org.
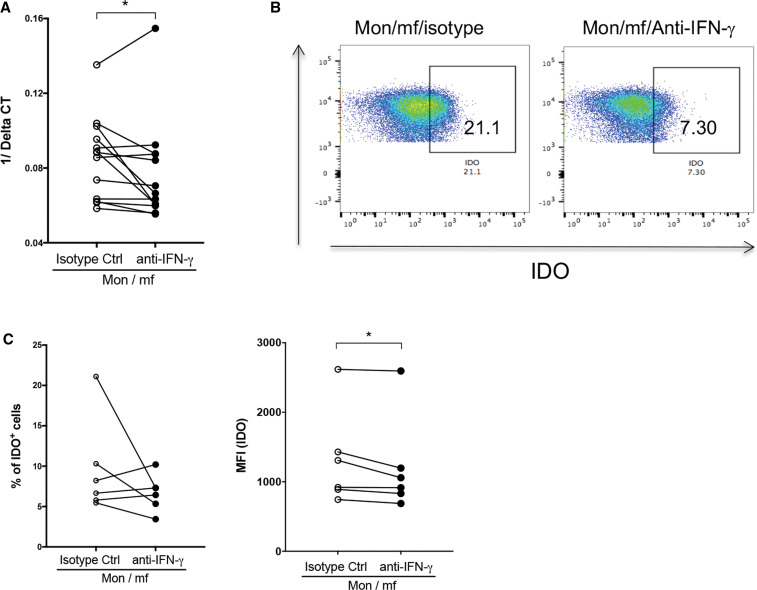
As IDO is induced and regulated through both IFN-γ-dependent 24, 26, 27 or IFN-γ-independent pathways, 28, 29 we assessed mf-induced IDO expression in the presence or absence of neutralizing anti-IFN-γ (Figure 5). Neutralizing anti-IFN-γ (compared with isotype control) significantly downmodulated the IDO mRNA (Figure 5A) and the intracellular IDO expression (MFI) in mf-exposed monocytes (Figure 5B and C), suggesting that the mf-induced IDO expression is IFN-γ dependent.
Figure 5.
Microfilariae (mf) induction of indoleamine 2,3-dioxygenase (IDO) in monocytes is interferon (IFN)-γ-dependent. Mf-exposed human monocytes (Mon/mf) were treated with either neutralizing anti- interferon (IFN)-γ antibody, or isotype control for 24 hours. Cells were harvested and the (A) mRNA or (B) and (C) intracellular expression of IDO was measured using real-time polymerase chain reaction (PCR) or flow cytometry respectively. (A) Line graphs are shown as 1/Delta CT (N = 11) with and without neutralizing anti-IFN-γ antibody. (B) One representative set of flow demonstrating frequency of IDO+ cells. (C) Line graphs are expressed as percentage or mean fluorescence intensity (MFI) of IDO+ cells (N = 6). Each circle represents an independent donor, where open circles represent Mon/mf/isotype control and closed circles represent Mon/mf/anti-IFN-γ. * P < 0.05, ** P < 0.005. This figure appears in color at www.ajtmh.org.
mf-induced autophagy is IFN-γ but not IDO dependent.
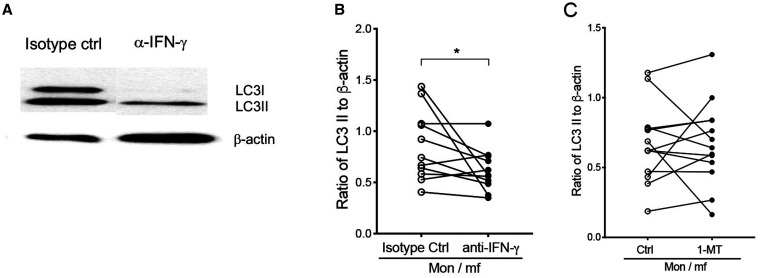
Next, we asked whether mf-induced autophagy in human monocytes is IFN-γ and/or IDO dependent. To this end, human monocytes were exposed to mf in the presence of neutralizing anti-IFN-γ antibody or IDO inhibitor (1 methyl Trp). Following 24 hours exposure, the cells were harvested and the accumulation of LC3II was measured by immunoblot analysis. As seen in Figure 6, anti-IFN-γ antibody significantly reduced the mf-induced LC3II accumulation in human monocytes when compared with the isotype control (Figure 6A and B), whereas the treatment with the IDO inhibitor did not show any significant decrease in LC3-II levels (Figure 6C). In addition, neutralizing anti-IFN-γ reversed the morphology (autophagic vesicles; Figure 1Ac) of mf-exposed monocytes as compared with the mf-unexposed monocytes. These results suggest that mf-induced autophagy is IFN-γ dependent but not dependent on IDO.
Figure 6.
Microfilariae (mf)-induced autophagy in human monocytes is interferon (IFN)-γ but not IDO-dependent. Human monocytes were either exposed to mf in the presence of either isotype control, anti-IFN-γ antibody, or indoleamine 2,3-dioxygenase (IDO) inhibitor for 24 hours, cells were harvested and assessed for the expression of LC3-I and LC3-II using western blot analysis. (A) One representative image of LC3-I and LC3-II expression using Western blot analysis is shown for isotype control and anti-IFN-γ antibody. (B) Line graphs represent the ratio of intensity of protein to β-actin with and without anti-IFN-γ antibody (N = 11). (C) Line graphs represent the ratio of intensity of protein to β-actin with and without IDO inhibitor 1 MT(N = 11). Bars: 500 nm, 1 µm in inserts. Each circle represents an independent donor, where open circles represent Mon/mf/isotype control (B) or Mon/mf (C) and closed circles represent Mon/mf in the presence of either anti-IFN-γ (B) or IDO inhibitor (C). * P < 0.05, ** P < 0.005 (N = 11).
DISCUSSION
Helminth parasites regulate host antigen presenting cells through different cellular and molecular mechanisms largely unknown. Patent lymphatic filariasis patients have impaired monocytes and CD4+ T cells function reflected by their inability to produce inflammatory cytokines in response to activating stimuli. 30 Previously, we have shown that live mf of Brugia malayi impair the viability and alter the function and phenotype of monocytes and monocyte-derived DC. 5, 31 Furthermore, secreted products from live mf attributed to altered viability and function of DC but to a lesser extent. 32 The present study elucidates some of the mechanisms underlying this altered monocyte function.
Autophagy is a cellular process in eukaryotic cells for degrading cellular proteins and organelles and thereby maintaining homeostasis. 33 Autophagy is an important mechanism used by the host immune cells to eliminate intracellular microbes and is a regulator of innate and adaptive immunity and inflammation. 34 It has been shown that preventing the induction of autophagy in monocytes hinders differentiation and cytokine production. 14 The induction of autophagy by intracellular pathogens such Helicobacter pylori, Leishmania amazonensis, and Toxoplasma gondii has been reported previously. 7, 35 Earlier, we have shown that the extracellular helminth parasite Brugia malayi mf induces autophagy in monocyte-derived DC through inhibition of mTOR pathway. 5 Furthermore, extracellular vesicles from mf inhibited mTOR pathway in monocytic cell line. 36 In addition, it has been shown that ES-62, a phosphorylcholine (PC)-containing immunomodulatory glycoprotein secreted by the filarial nematode Acanthocheilonema viteae blocks inflammatory responses of dendritic cells by inducing the autophagy machinery leading to degradation of signaling molecules involved in cytokine production. 37 In this study, we demonstrated that mf of Brugia malayi are able to induce autophagy in human monocytes by two strategies: 1) increasing the gene expression of the autophagy-related genes ATG5, ATG7 and Beclin-1, suggesting that human monocytes undergo the elongation and closure of autophagosome when exposed to live mf; and 2) by the conversion of LC3II allowing for autophagosome maturation in these cells. ATGs such as ATG5 and ATG7 are required for the induction of autophagy in human DCs and the subsequent promotion of antigen presentation. 38 In fact, blocking ATG5 and ATG7 can lead to the escape of intracellular pathogens from the autophagic machinery. 39 Furthermore, LC3II has been used as an effective marker for investigating the activation of the autophagic pathway, as LC3II is integrated into the membranes of newly formed autophagosomes, and its level correlates positively with numbers of cellular autophagosomes. 40 These data suggest that mf induce the lipidated form of LC3 that is known to be recruited to autophagosomes and associated with the autophagosome membrane.
It has been reported in literature that autophagy extensively regulates the response of macrophages to microenvironmental stimuli and modulates the production, function, maintenance, and polarization of macrophages. 41 It is well known that autophagy exhibits an anti-inflammatory effect through the downregulation of inflammasome. 42 The CCL2 and IL-6 have a potent effect in inducing autophagy in macrophages and also in polarizing monocytes toward an M2 phenotype. 43 In this study, we show that mf-induced autophagy may regulate human monocytes through M2 polarization. Upon treatment with Baf1, mf-induced CD206 expression was decreased, suggesting the inhibition of autophagy downregulates the mf-induced M2 polarization in these cells. It has been shown that the nonspecific lysosomal inhibitors such as chloroquine and Baf1 impair M2 polarization in a tumor microenvironment. 44, 45 In the Atg-deficient mouse, effective autophagy is required to promote an anti-inflammatory environment through LC3-associated phagocytosis and appropriate cytokine release. 46 Although several reports mentioned the increased expression of CD206 upon parasitic helminth infection, its exact role remains unclear. In our previous study, 5-day exposure of monocytes to mf did not induce CD206. In this study, 48-hour exposure of these cells to mf induces the expression of CD206. This discrepancy could be because of the timing of the assay. Further studies on helminth-induced autophagy and increased mannose receptor will address the interaction between the helminth and CD206.
Interferon-γ is one of the major cytokines that activate macrophages and enhances their ability to kill microorganisms. 47 Several studies have shown that monocytes possess the ability to produce IFN-γ 47, 48 and nitric oxide (NO), both involved in the elimination of filarial parasites. 49 – 51 Although the role of IFN-γ in controlling intracellular parasite infection has been extensively studied, 52 the exact mechanism of IFN-γ’s role in filarial parasite elimination is unclear. In this study, we have showed that live mf of Brugia malayi exposure induces IFN-γ in human monocytes. Furthermore, IFN-γ induces IDO, an inflammatory cytokine-inducible enzyme involved in immune modulation. 53 This enzyme catalyzes the degradation of L-tryptophan to immunoregulatory catabolite N-formylkynurenine and can be measured by the ratio of Trp to Kyn (Trp/Kyn). 54, 55 In fact, IDO-producing APCs can inhibit the T cell proliferation and also can suppress the specific T cell immune responses against parasitic infection. 56 On the other hand, activation of IDO controls the replication of intracellular parasite Trypanosoma cruzi infection and improves the survival of lethally infected mice. 57
In this study, mf induces the protein levels and gene expression of IDO in human monocytes, suggesting that mf of Brugia malayi is able to immune suppress host by inducing IDO expression/and activity in human monocytes. Indoleamine 2,3-dioxygenase is induced and regulated through both IFN-γ-dependent 24, 26, 27 or IFN-γ-independent pathways. 28, 29 In murine models of schistosomiasis, IFN-dependent IDO production from macrophages is involved in limiting the inflammation and thereby protects the infected host against excess damage. 58 In this study, the induction of IDO gene expression and protein levels are IFN-γ dependent as evidenced by neutralizing anti-IFN-γ downmodulated the IDO mRNA and the intracellular IDO expression in mf-exposed monocytes.
Several studies reported the role of IFN-γ in elimination of intracellular pathogens by inducing autophagy in host immune cells. 39, 59, 60 Activation of autophagy by IFN-γ in human epithelial cells relies on Trp depletion and the activation of the GCN2–eIF2α pathway has been shown. 22 In fact, depletion of Trp is tolerogenic, as it inhibits Th cell proliferation and promotes regulatory T cell amplification, and Kyns, the Trp metabolites, promote T cell death. 53 Interferon-γ-induced IDO and thereby Trp depletion promotes autophagy expands the spectrum of immunoregulatory properties of IDO. 22 In our study, mf-induced IFN-γ and IDO exhibits the immunomodulatory potential of helminth on human monocytes. Further to elucidate whether mf-induced autophagy through IFN-γ or IDO in human monocytes, we used neutralizing antibody IFN-γ and IDO inhibitor. Our results suggest that live mf of Brugia malayi induced autophagy in human monocytes through IFN-γ-dependent mechanism.
In conclusion, helminth parasites alter the innate immune responses through different mechanisms. First, mf-induced autophagy in human monocytes through an IFN-γ dependent mechanism. This induction of autophagy resulted in the upregulation of an M2 marker CD206. Whether the induction of CD206 is IFN-γ-dependent and/or M2 polarization by this parasite is autophagy-dependent is not fully understood and requires further investigation. Second, this parasite regulates monocyte response through induction of IDO (IFN-γ-dependent; but autophagy-independent); a known enzyme involved in peripheral tolerance and immunosuppression.
ACKNOWLEDGMENTS
We thank Dr. Andrew Miller and his group at Cancer Immunology, Inflammation and Tolerance Program, Georgia Cancer Center, Augusta University, Augusta, GA, for their assistance in measuring IDO enzymatic activities. We thank Dr. Sasisekhar Bennuru for his assistance with the figures.
References
- 1. Babu S Nutman TB , 2014. Immunology of lymphatic filariasis. Parasite Immunol 36: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Semnani RT , 2013. The interaction between filarial parasites and human monocyte/macrophage populations. Adv Exp Med Biol 785: 49–56. [DOI] [PubMed] [Google Scholar]
- 3. Semnani RT et al. 2006. Filaria-induced monocyte dysfunction and its reversal following treatment. Infect Immun 74: 4409–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babu S Kumaraswami V Nutman TB , 2009. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis 199: 1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narasimhan PB Bennuru S Meng Z Cotton RN Elliott KR Ganesan S McDonald-Fleming R Veenstra TD Nutman TB Tolouei Semnani R , 2016. Microfilariae of Brugia malayi inhibit the mTOR pathway and induce autophagy in human dendritic cells. Infect Immun 84: 2463–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehrpour M Esclatine A Beau I Codogno P , 2010. Overview of macroautophagy regulation in mammalian cells. Cell Res 20: 748–762. [DOI] [PubMed] [Google Scholar]
- 7. Levine B Mizushima N Virgin HW , 2011. Autophagy in immunity and inflammation. Nature 469: 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y et al. 2017. Brucella dysregulates monocytes and inhibits macrophage polarization through LC3-dependent autophagy. Front Immunol 8: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar P , 2017. IFNγ-producing CD4 T lymphocytes: the double-edged swords in tuberculosis. Clin Transl Med 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rovetta AI et al. 2014. IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis. Autophagy 10: 2109–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babu S Ganley LM Klei TR Shultz LD Rajan TV , 2000. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi . Infect Immun 68: 3034–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghadimi D de Vrese M Heller KJ Schrezenmeir J , 2010. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol 10: 694–706. [DOI] [PubMed] [Google Scholar]
- 13. Acovic A Gazdic M Jovicic N Harrell CR Fellabaum C Arsenijevic N Volarevic V , 2018. Role of indoleamine 2,3-dioxygenase in pathology of the gastrointestinal tract. Therap Adv Gastroenterol 11: 1756284818815334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y Morgan MJ Chen K Choksi S Liu ZG , 2012. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 119: 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu K Zhao E Ilyas G Lalazar G Lin Y Haseeb M Tanaka KE Czaja MJ , 2015. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yong S Lau S , 1979. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr A 175: 343–346. [DOI] [PubMed] [Google Scholar]
- 17. Braun D Longman RS Albert ML , 2005. A two-step induction of indoleamine 2,3-dioxygenase (IDO) activity during dendritic-cell maturation. Blood 106: 2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McEwan DG , 2017. Host–pathogen interactions and subversion of autophagy. Essays Biochem 61: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su CW Cao Y Zhang M Kaplan J Su L Fu Y Walker WA Xavier R Cherayil BJ Shi HN , 2012. Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages. J Immunol 189: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuzawa T Kim BH Shenoy AR Kamitani S Miyake M Macmicking JD , 2012. IFN-γ elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol 189: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang Y-P et al. 2010. Autophagy facilitates IFN-γ-induced Jak2-STAT1 activation and cellular Inflammation. J Biol Chem 285: 28715–28722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fougeray S Mami I Bertho G Beaune P Thervet E Pallet N , 2012. Tryptophan depletion and the kinase GCN2 mediate IFN-γ-induced autophagy. J Immunol 189: 2954–2964. [DOI] [PubMed] [Google Scholar]
- 23. Donovan MJ Tripathi V Favila MA Geraci NS Lange MC Ballhorn W McDowell MA , 2012. Indoleamine 2,3-dioxygenase (IDO) induced by Leishmania infection of human dendritic cells. Parasite Immunol 34: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jürgens B Hainz U Fuchs D Felzmann T Heitger A , 2009. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood 114: 3235–3243. [DOI] [PubMed] [Google Scholar]
- 25. Wolf B Posnick D Fisher JL Lewis LD Ernstoff MS , 2009. Indoleamine-2,3-dioxygenase enzyme expression and activity in polarized dendritic cells. Cytotherapy 11: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlin JM Borden EC Sondel PM Byrne GI , 1989. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol 45: 29–34. [DOI] [PubMed] [Google Scholar]
- 27. MacKenzie CR González RG Kniep E Roch S Däubener W , 1999. Cytokine mediated regulation of interferon-gamma-induced IDO activation. Adv Exp Med Biol 467: 533–539. [DOI] [PubMed] [Google Scholar]
- 28. Saito K Suyama K Nishida K Sei Y Basile AS , 1996. Early increases in TNF-α, IL-6 and IL-1β levels following transient cerebral ischemia in gerbil brain. Neurosci Lett 206: 149–152. [DOI] [PubMed] [Google Scholar]
- 29. Fujigaki S Saito K Sekikawa K Tone S Takikawa O Fujii H Wada H Noma A Seishima M , 2001. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol 31: 2313–2318. [DOI] [PubMed] [Google Scholar]
- 30. Sasisekhar B Aparna M Augustin DJ Kaliraj P Kar SK Nutman TB Narayanan RB , 2005. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect Immun 73: 3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB, 2011. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun 79: 3957–3965. [DOI] [PMC free article] [PubMed]
- 32. Semnani RT Liu AY Sabzevari H Kubofcik J Zhou J Gilden JK Nutman TB , 2003. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol 171: 1950–1960. [DOI] [PubMed] [Google Scholar]
- 33. Chun Y Kim J , 2018. Autophagy: an essential degradation program for cellular homeostasis and life. Cells 7: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deretic V Levine B , 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5: 527–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cyrino LT, Araújo AP, Joazeiro PP, Vicente CP, Giorgio S, 2012. In vivo and in vitro Leishmania amazonensis infection induces autophagy in macrophages. Tissue Cell 44: 401–408. [DOI] [PubMed] [Google Scholar]
- 36.Ricciardi et al., 2021. Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway. PLoS Negl Trop Dis 15: e0008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eason RJ Bell KS Marshall FA Rodgers DT Pineda MA Steiger CN Al-Riyami L Harnett W Harnett MM , 2016. The helminth product, ES-62 modulates dendritic cell responses by inducing the selective autophagolysosomal degradation of TLR-transducers, as exemplified by PKCδ. Sci Rep 6: 37276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saitoh T, Akira S, 2010. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol 189: 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siqueira M da S Ribeiro R de M Travassos LH , 2018. Autophagy and its interaction with intracellular bacterial pathogens. Front Immunol 9: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shibutani ST Saitoh T Nowag H Münz C Yoshimori T , 2015. Autophagy and autophagy-related proteins in the immune system. Nat Immunol 16: 1014–1024. [DOI] [PubMed] [Google Scholar]
- 41. Chen P Cescon M Bonaldo P , 2014. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy 10: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong Z Sanchez-Lopez E Karin M , 2016. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roca H Varsos ZS Sud S Craig MJ Ying C Pienta KJ , 2009. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284: 34342–34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang CP Su YC Hu CW Lei HY , 2013. TLR2-dependent selective autophagy regulates NF-κB lysosomal degradation in hepatoma-derived M2 macrophage differentiation. Cell Death Differ 20: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang M Liu J Shao J Qin Y Ji Q Zhang X Du J , 2014. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol Cancer 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee DE Bareja A Bartlett DB White JP , 2019. Autophagy as a therapeutic target to enhance aged muscle regeneration. Cells 8: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamaguchi R Kawata J Yamamoto T Ishimaru Y Sakamoto A Ono T Narahara S Sugiuchi H Hirose E Yamaguchi Y , 2015. Mechanism of interferon-gamma production by monocytes stimulated with myeloperoxidase and neutrophil extracellular traps. Blood Cells Mol Dis 55: 127–133. [DOI] [PubMed] [Google Scholar]
- 48. Kraaij MD Vereyken EJF Leenen PJM van den Bosch TPP Rezaee F Betjes MGH Baan CC Rowshani AT , 2014. Human monocytes produce interferon-gamma upon stimulation with LPS. Cytokine 67: 7–12. [DOI] [PubMed] [Google Scholar]
- 49. Rajan TV Porte P Yates JA Keefer L Shultz LD , 1996. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi . Infect Immun 64: 3351–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verma SK Joseph SK Verma R Kushwaha V Parmar N Yadav PK Thota JR Kar S Murthy PK , 2015. Protection against filarial infection by 45–49 kDa molecules of Brugia malayi via IFN-γ-mediated iNOS induction. Vaccine 33: 527–534. [DOI] [PubMed] [Google Scholar]
- 51. Taylor MJ Cross HF Mohammed AA Trees AJ Bianco AE , 1996. Susceptibility of Brugia malayi and Onchocerca lienalis microfilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFN gamma-activated macrophages. Parasitology 112: 315–322. [DOI] [PubMed] [Google Scholar]
- 52. Rodrigues AA et al. 2012. IFN-γ plays a unique role in protection against low virulent Trypanosoma cruzi strain. PLoS Negl Trop Dis 6: e1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mbongue JC Nicholas DA Torrez TW Kim NS Firek AF Langridge WHR , 2015. The role of indoleamine 2,3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel) 3: 703–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xiao C Chen Y Liang X Xie Z Zhang M Li R Li Z Fu X Yu X Shi W , 2014. A modified HPLC method improves the simultaneous determination of plasma kynurenine and tryptophan concentrations in patients following maintenance hemodialysis. Exp Ther Med 7: 907–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mellor AL Lemos H Huang L , 2017. Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol 8: 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andersen MH , 2012. The specific targeting of immune regulation: T-cell responses against Indoleamine 2,3-dioxygenase. Cancer Immunol Immunother 61: 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Knubel CP Martínez FF Fretes RE Díaz Lujan C Theumer MG Cervi L Motrán CC , 2010. Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J 24: 2689–2701. [DOI] [PubMed] [Google Scholar]
- 58. Rani R Jordan MB Divanovic S Herbert DR , 2012. IFN-γ–driven IDO production from macrophages protects IL-4Rα–deficient mice against lethality during Schistosoma mansoni infection. Am J Pathol 180: 2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jo EK Yuk JM Shin DM Sasakawa C , 2013. Roles of autophagy in elimination of intracellular bacterial pathogens. Front Immunol 4: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalvakolanu DV, Gade P, 2012. IFNG and autophagy. A critical role for the ER-stress mediator ATF6 in controlling bacterial infections. Autophagy 8: 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]