ABSTRACT.
COVID-19, a respiratory viral infection, has affected 388 million individuals worldwide as of the February 4, 2022. In this review, we have outlined the important liver manifestations of COVID-19 and discussed the possible underlying pathophysiological mechanisms and their diagnosis and management. Factors that may contribute to hepatic involvement in COVID-19 include direct viral cytopathic effects, exaggerated immune responses/systemic inflammatory response syndrome, hypoxia-induced changes, vascular changes due to coagulopathy, endothelitis, cardiac congestion from right heart failure, and drug-induced liver injury. The majority of COVID-19-associated liver symptoms are mild and self-limiting. Thus management is generally supportive. Liver function tests and abdominal imaging are the primary investigations done in relation to liver involvement in COVID-19 patients. However, imaging findings are nonspecific. Severe acute respiratory syndrome coronavirus 2 RNA has been found in liver biopsies. However, there is limited place for liver biopsy in the clinical context, as it does not influence management. Although, the management is supportive in the majority of patients without previous liver disease, special emphasis is needed in those with nonalcoholic fatty liver disease, cirrhosis, hepatocellular carcinoma, hepatitis B and C infections, and alcoholic liver disease, and in liver transplant recipients.
INTRODUCTION
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and as of November 28, 2021, there have been more than 260 million cases worldwide and over 5 million deaths. 1 Several vaccines have already been developed with a view to controlling this pandemic. There are two genera of human coronaviruses: alpha (human coronavirus [HCoV]-229E and HCoV-NL63) and beta (HCoV-HKU1, HCoV-OC43, severe acute respiratory syndrome coronavirus 1 [SARS-CoV-1] and Middle East respiratory syndrome coronavirus [MERS-CoV]). The coronaviruses HCoV-OC43, HCoV-HKU1, HCoV-229E, and HCoV-NL63 cause mild disease, whereas the SARS-CoV-1, MERS-CoV, and SARS-CoV-2 may potentially cause severe disease. 2, 3 Outbreaks of SARS-CoV-1 and MERS-CoV infections occurred in 2002 and 2012, respectively. 4 Severe acute respiratory syndrome coronavirus 2 has 70% and 40% genetic sequence similarity with SARS-CoV-1 and MERS-CoV. 5 Although fever and respiratory symptoms predominate in coronavirus infections, a range of liver manifestations is seen in SARS-CoV-1, MERS, and SARS-CoV-2 patients. 6, 7
Table 1 shows a summary of the liver findings in SAR-CoV-1, MERS, and SARS-CoV-2. 6, 8 – 17 Hepatic impairment was seen in up to 60% of patients with SARS-CoV-1. The main laboratory findings of SARS-CoV-1 were moderate to a marked elevation of alanine transaminase (ALT), decreased serum albumin, and increased serum bilirubin levels. 11, 16 Pathological findings included prominent mitosis, acidophilic bodies, and mild to moderate lobular inflammation. Severe acute respiratory syndrome coronavirus 1 induced liver injury was supported by the presence of viral RNA in liver tissue. 16 Autopsies of SARS-CoV-1 patients found large numbers of viral particles in hepatocytes and hepatic vascular endothelial cells. 18 Some patients with severe MERS-CoV had raised liver aminotransferase (ALT and aspartate transaminase [AST]) levels and hyperbilirubinemia. 12, 13 A low albumin level on the day of diagnosis was a predictor of disease severity. 12 As with SARS-CoV-1, mild portal tract and lobular lymphocytic infiltration, moderate steatosis, and scattered calcification were observed in MERS-CoV infections. 19 The incidence of liver injury in severe COVID-19 cases (74.4%) was higher than that of patients with mild disease (43%). The incidence of liver injury in COVID-19-associated deaths was 58%. 8 In this review, we have outlined the important liver manifestations of COVID-19 and discussed the possible pathophysiological mechanisms and their diagnosis and management.
Table 1.
Liver involvement of SARS-CoV-1, MERS, and SARS-CoV-2
| SARS-CoV-1 | MERS-CoV | SARS-CoV-2 | |
|---|---|---|---|
| Incidence of liver injury | 60% 6 | 60% 6 | 14.8–53% 8 |
| Expression of entry receptor on cholangiocytes | NA | NA | ACE2 receptor expression is higher than on hepatocytes 9 |
| Expression of entry receptor on hepatocytes | ACE2 receptor expression is abundant 9 | DPP-4 receptor expression is high in liver 10 | ACE2 receptor expression is low 9 |
| Expression of entry receptor in Kupffer cells, liver endothelial cells, and other inflammatory cells | NA | NA | ACE2 receptor is expressed 9 |
| Liver enzyme level | Mild to moderate elevation of ALT and AST—53% 11 | Elevation of ALT and/or AST 12, 13 | Elevation of ALT 23.3% and AST 23.4% 14 |
| Albumin level | Decreased serum albumin 1 | Decreased levels of albumin 12, 13, 15 | Decreased levels of albumin 61.3% 14 |
| Bilirubin level | Increased serum bilirubin 6 | Increased serum bilirubin 12, 13 | Increased serum bilirubin 27.9% 4 |
| Serum GGT level | NA | NA | Increased in severe cases 27.9% 14 |
| Pathological manifestations of liver injury | Antemortem Mild lobular activities with occasional acidophilic bodies and prominent Kupffer cell Mildly inflamed portal tracts with lymphocytic infiltration Nonspecific inflammation in the liver in biopsy Hydropic degeneration Steatosis Focal necrosis 6 Postmortem histopathological findings- Necrosis Nodular cirrhosis Minor inflammatory changes Hydropic and fatty degeneration Interstitial cell proliferation Mild fatty acid degeneration Mild congestion 6 Significant increase in mitotic cells with eosinophilic bodies and balloon-like hepatocytes 15 | Postmortem histopathological findings Mild chronic lymphocytic portal and lobular inflammation Reactive parenchyma with mild cellular hydropic degeneration Rare multinucleated hepatocytes and mild disarray of the hepatic plates Mild sinusoidal lymphocytosis and small necroinflammatory foci in the hepatic lobules Congestion, hemorrhage, and focal perivenular loss of hepatocytes Macrovesicular perivenular steatotic change, sinusoidal congestion, hemorrhage, and focal perivenular loss of hepatocytes Scattered calcifications Nonspecific hepatitis 6 | Postmortem histopathological findings Microvescicular steatosis Mild lobular and portal activity 16 Hepatomegaly Hepatocyte degeneration Lobular focal necrosis Neutrophil infiltration (lymphocytes and monocytes in portal area) Congestion of hepatic sinuses with microthrombosis Mild sinusoidal dilatation Mild lobular lymphocytic infiltration Patchy hepatic necrosis in the periportal and centrilobular areas Over activation of T cells 17 |
ACE2 = angiotensin-converting enzyme 2; ALT = alanine aminotransferase; AST = aspartate aminotransferase; DPP-4 = dipeptidyl peptidase 4; MERS-CoV = Middle East respiratory syndrome coronavirus; NA = not available/applicable; SARS-CoV-1 = severe acute respiratory syndrome coronavirus 1; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
LITERATURE SEARCH
We searched PubMed, Google Scholar, and Google from January 2020 to November 28, 2021, for articles written in English that describe the liver effects of COVID-19, using the search terms “coronaviruses and liver,” “COVID-19 and liver,” “COVID-19, and liver symptoms,” “COVID-19 and hepatic,” “COVID-19 and liver function tests,” “COVID-19 and liver inflammation,” “SARS-Cov-2 and liver,” and “transplantation during COVID-19.” Reference lists of the articles were scanned to identify any additional studies. The article title and abstract were read for the initial selection and then the full-text article was read. Reference lists of the full-text articles were scanned to identify any additional studies. All types of research articles, including original research articles, reviews, case series, short communications, and case reports were considered. Of the 103 articles identified, 59 were analyzed further (Figure 1).
Figure 1.
PRISMA flow chart. This figure appears in color at www.ajtmh.org.
Liver-related outcomes associated with COVID-19.
The current literature has several studies on liver-related outcomes in COVID-19. However, the definition of liver injury tends to vary among the different studies. Furthermore, specifying liver-related outcomes in COVID-19 patients is made difficult because of the studies describing different etiologies, different disease severities, small numbers of study participants from a single geographical location, and the lack of correlation of liver test results with preexisting liver conditions. Preexisting chronic liver disease (CLD) may predispose a person to adverse outcomes following COVID-19 because of immune dysregulation. 20 Marjot’s international registry study found mortality to be high in cirrhosis patients (32%) compared with those without cirrhosis (8%). 20 Furthermore, a strong correlation between the stage of liver disease and the rate of intensive care unit (ICU) admissions, renal replacement therapy, and death was found. 20 The cause of death in patients with CLD/cirrhosis was respiratory related in the majority (71%) and 19% were liver related. 20 On admission, although respiratory symptoms were similar among the CLD and non-CLD individuals, gastrointestinal (GI) side effects were comparatively higher in CLD patients. 20 Furthermore, baseline liver disease stage and alcohol-related liver disease were risk factors for death from COVD-19. 20 In a multicenter cohort study by Lavarone et al., the mortality of those with cirrhosis was significantly higher than those without cirrhosis (34% versus 18%, respectively). 21 Further, the mortality associated with cirrhosis was higher than among those with cirrhosis and bacterial infection. 21 Another study by Marjot et al. 22 in autoimmune hepatitis patients found that autoimmune hepatitis (AIH) and immunosuppression were not significantly associated with death despite the use of medications that suppressed the immune system. This may be because of the low sample number (N = 77) of AIH patients.
PATHOPHYSIOLOGY OF LIVER INVOLVEMENT IN COVID-19
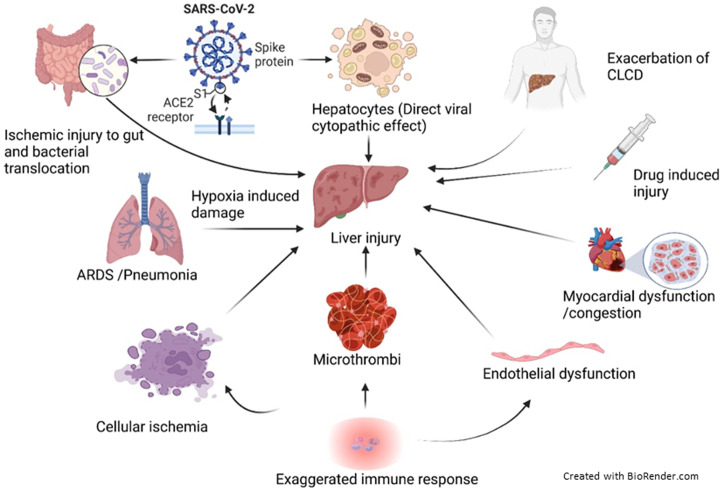
Factors that may contribute to liver involvement in COVID-19 include direct viral cytopathic effects, exaggerated immune responses/systemic inflammatory response syndrome (SIRS), hypoxia-induced changes, vascular changes due to coagulopathy, 23 endothelitis, cardiac congestion from right heart failure, and drug-induced liver injury. 8, 24 These factors may also exacerbate any underlying liver disease. The pathophysiological processes involved in liver impairment in COVID-19 are summarized in Figure 2.
Figure 2.
Pathophysiological processes that may lead to liver injury in COVID-19. Following SARS-CoV-2 infection, liver injury may result due to direct cytopathic effects (due to viral entry through ACE2 receptors on hepatocytes and cholangiocytes) or hypoxia-induced damage (resulting from ARDS or pneumonia-associated hypoxia) or bacterial translocation and inflammation (direct viral injury and ischemic injury of the gut or disruption of gut–mucosal barrier) or systemic hypotension and cellular ischemia, abnormal coagulation/microthrombi, endothelial dysfunction (resulting from exaggerated immune responses/systemic inflammation) or cardiac congestion from right heart failure (due to myocardial dysfunction), or drug-induced liver injury or exacerbation of chronic liver disease. SARS-CoV-2 = severe acute respiratory syndrome corona virus 2; ACE2 = angiotensin-converting enzyme 2; ARDS = acute respiratory distress syndrome; CLCD = chronic liver cell disease. This figure appears in color at www.ajtmh.org.
Angiotensin-converting enzyme 2 receptors.
Angiotensin-converting enzyme 2 (ACE2) receptors provide a gateway for viral entry, and its tissue distribution determines the pattern of viral tropism. There is high expression of ACE2 on cholangiocytes (epithelial cells of the bile duct) and low expression on hepatocytes, Kupffer cells (liver macrophages), and endothelial cells. 9 Levels of expression on bile ducts are similar to type II alveolar cells. 25 Cholangiocytes undergo syncytia formation following SARS-CoV-2 infection and similar observations have been noted when the virus infects adult human cholangiocyte organoids. The virus is able to replicate within the bile duct epithelium. Levels of ACE2 expression may be affected by many factors. Preexisting liver disease, hypoxia, drug-induced liver injury, and inflammation increase the levels of expression 24 and may, in turn, enhance viral-induced cytotoxicity. In vitro studies found pretreatment of ACE2 receptors with trypsin increases the binding affinity of SARS-CoV-2 spike protein. Liver epithelial cells express trypsin, and this may facilitate viral entry despite low ACE2 expression levels. Furthermore, the spike protein of SARS-CoV-2 has a furin-like proteolytic site. As furin is predominantly expressed in the liver, it may support viral entry. Cell line studies have found viral entry to depend on the PIKfyve-TCP2 endocytotic pathway that is expressed in the liver and gall bladder, at comparable levels to the lung.
Direct viral cytotoxicity.
The renin-angiotensin system (RAS) plays a major role in liver inflammation, tissue remodeling, and fibrosis. Angiotensin-converting enzyme 2 is a key negative regulator of the RAS and limits fibrosis through the degradation of Angiotensin II and the formation of Angiotensin (1–7). Upon binding of the SARS-CoV-2 virus, ACE2 is endocytosed and levels are reduced on the cell surface. Murine studies found reduced ACE2 levels to worsen liver fibrosis in chronic liver injury models. 26 Direct viral cytotoxicity gives rise to steatohepatitis by interfering with lipogenesis and in turn, may worsen chronic liver diseases such as nonalcoholic fatty liver disease (NAFLD) and alcoholic hepatitis. 24
Immune-mediated effects.
An exaggerated inflammatory response in COVID-19 leads to lymphocyte activation, neutrophilia, and an increase in C-reactive protein (CRP) and inflammatory cytokines. Levels of serum interleukin (IL)-2, IL-6, IL-7, IL-10, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-inducible-protein-10, monocyte chemotactic protein-1, and macrophage-inflammatory-protein-1 alpha are significantly higher in severe COVID-19. 9, 27 A CRP ≥ 20 mg/L and a lymphocyte count < 1.1 × 109/L are independent risk factors for liver injury. Lymphopenia is noted in 63–70.3% of COVID-19 patients. Postmortem liver histology shows microvesicular steatosis and T cell accumulation, pointing to the presence of immune-mediated damage. 28 The systemic inflammatory response secondary to the infection causes systemic hypotension, cellular ischemia, abnormal coagulation, microthrombi, and endothelial dysfunction and may further exacerbate the liver damage caused by direct viral cytopathic effects. Thus liver damage should be suspected and treated promptly in a clinically deteriorating patient with systemic manifestations of COVID-19.
Hypoxia-related effects.
Liver hypoxia (because of microvascular thrombosis and gas exchange defects secondary to lung injury) may cause hepatic damage. Ischemic injury to the gut with resulting intestinal endotoxemia, and activation of the sympathetic nervous and adrenocortical systems may further contribute to liver damage. 9, 29 Furthermore, COVID-19-induced myocardial dysfunction can potentially give rise to right heart failure, adding to the existing damage, and worsening ischemic liver injury. Elevated transaminases in the context of respiratory failure, shock, and heart failure in severe COVID-19 may be indicators of this pathophysiological mechanism. 30
Drug-related cytotoxicity.
As most COVID-19 patients have fever, antipyretics containing acetaminophen are frequently used. Higher doses of this medication are known to cause liver damage. Many antiviral drugs are administered (alone or in combination) and some of them may have adverse effects on the liver (Table 2). 31 – 48 It should be noted that some of the medications are no longer in use for COVID-19 in current clinical practice. Lopinavir/ritonavir increases the odds of liver injury by fourfold. Thus close monitoring is needed in such patients especially when abnormal liver function tests (LFTs) have been observed at admission. 49
Table 2 .
Potential liver side effects of currently and previously used medications in COVID-19
| Class | Drug | Dosage | Administration | Liver side effects | References |
|---|---|---|---|---|---|
| Antivirals | Remdesivir (In phase 3 clinical trials) | Loading dose 200 mg over 30–120 minutes on day 1 followed by 100 mg once daily for remaining 4/9 days Not needing invasive mechanical ventilation/ECMO: for 5 days Needs mechanical ventilation or ECMO for 10 days | Intravenous | 1–10%—liver enzyme derangement, hyperbilirubinemia | 31, 32, 33, 34 |
| Paxlovid ((PF-07321332 150 mg and ritonavir 100 mg) | 300 mg PF-07321332 (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) all taken together orally every 12 hours for 5 days | Oral | May cause liver damage because of ritonavir. No dosage adjustment is needed for patients with either mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. | 47 | |
| Molnupiravir | 800 mg (administered as four 200 mg capsules) taken orally every 12 hours with or without food for 5 days | Oral | N/A | 48 | |
| Lopinavir/ ritonavir (LPV/r) (Kaletra) | 400/100 mg twice daily or 800/200 mg once daily for 14 days. | Oral (administer with or without food) | 1–10%—hepatic disorders, cholangitis, hyperbilirubinemia | 35 | |
| Ribavirin (In phase 2 clinical trials) | 400 mg twice daily for 14 days (in clinical trials)—dosing not defined | Oral (administer with food) | 0.1–1%—Hepatic disorders Less than 0.1%—Cholangitis, hepatic failure | 36 | |
| Darunavir | 1 pill of DRV/c (a single-tablet regimen containing 800 mg of darunavir and 150 mg of cobicistat) per day for 5 days | Oral | Moderate to severe elevations in serum aminotransferase levels (> 5 × ULN) in 3–10% of patients overall | 37 | |
| Favipiravir | 1,800 mg twice daily on day 1 followed by 800 mg twice daily on days 2 to a maximum of 14 days | Oral | Liver enzyme derangement (2%) | 38 | |
| Immunomodulatory drugs | Tocilizumab | 4–8 mg/kg (maximum 800 mg) over 1 hour; or 400 mg once Consider an additional dose 8–12 hours later if continued clinical deterioration (maximum of 2 doses) | Intravenous | Frequency not known—Hepatic disorders | 31 |
| Interferon α/β | INF-β-1b 0.25 mg alternated for 3 days (in clinical trial)—dosing not established | Subcutaneous injection | 0.1–1%—Hepatic disorders, autoimmune hepatitis | 31 | |
| Baricitinib (completed clinical trial) | 4 mg once daily | Oral | Frequency not known—Abnormal liver enzymes | 39, 40 | |
| Baricitinib + antiviral therapy administration for 2 weeks | |||||
| Imatinib | 400 mg daily for 14 days | Oral | Common elevations in serum aminotransferase levels mild elevations in serum bilirubin can occur. These abnormalities are usually mild, asymptomatic, and resolve despite continuing therapy. Linked to rare instances of clinically apparent acute liver injury with jaundice. | 41, 42 | |
| Antiparasitic | Chloroquine | 500 mg twice/day for 10 days. | Oral (administer with food) | Less than 0.1%—Hepatitis | ( 33 |
| Hydroxychloroquine | Loading dose of 400 mg twice daily for 1 day, followed by 200 mg twice daily for 4 days. | Oral (administer with food) | Frequency not known—Acute hepatic failure | 31, 43 | |
| Steroids | Dexamethasone | 6mg daily for 7–10 days | Oral | Frequency not known—Acute hepatic failure | 44, 45 |
| Antibiotic | Azithromycin | NA | NA | Low rate of acute, transient, and asymptomatic elevation in serum aminotransferases which occurs in 1–2% of patients treated for short periods, and a somewhat higher proportion of patients given azithromycin long term. Rarely cause clinically apparent liver injury. | 46 |
ALT = alanine transaminase; ECMO = extracorporeal membrane oxygenation; INF-β = interferon-beta; LPV/r = lopinavir/ritonavir; NA = not applicable.
Gut microbiota.
Recent studies on gut microbiota have suggested an alteration in intestinal microbiota composition (i.e., dysbiosis) contributes to different immune-mediated inflammatory diseases. 50 Similarly, in COVID-19, gut microbiota dysbiosis might play an important role in determining the clinical outcome of patients with underlying comorbid conditions such as diabetes, hypertension, and obesity. 51 For instance, gut microbiota diversity is generally decreased in older individuals and COVID-19 is also more severe and fatal in this group of individuals raising a potential role of the gut microbiota in overall pathogenesis and outcomes. 52 Furthermore, it has been suggested that COVID-19 patients are depleted of gut bacteria with known immunomodulatory potential. 53 Additionally, inflammation induced by gut dysbiosis represents an important factor in cardiometabolic and diabetic pathogenesis and may contribute to increasing the severity of COVID-19 in the most vulnerable patients. 54 As diet plays a critical role in modulating the gut microbiota, there has been increased interest in evaluating the health benefits and disease-preventing properties of diet and dietary habits and their association with favorable patient outcomes. 55, 56 The GI blood supply drains to the liver by the portal venous system. Thus disruption of the gut microbiota, with breach of the gut–mucosal barrier may lead to sepsis-induced hepatic dysfunction.
Mitochondrial damage.
Preliminary observations suggest that SARS‐CoV‐2 affects mitochondrial activity. 57 Furthermore, Wang et al. identified mitochondrial crista abnormalities in liver specimens from COVID‐19 patients. Interestingly, impaired mitochondrial activity has also been implicated in the pathogenesis of NAFLD/non-alcoholic steatohepatitis. 58 Thus, SARS‐CoV‐2 infection might worsen the metabolic state and aggravate preexisting NAFLD by these mechanisms.
HEPATIC MANIFESTATIONS IN COVID-19
The COVID-19-associated liver injury is defined as liver damage occurring due to the virus or its treatment in those with or without preexisting liver damage. 59 Several biochemical definitions for liver injury have been proposed. These include, ALT or AST exceeding three times the upper limit of normal, and alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), or total bilirubin exceeding two times the upper limit of normal. The overall incidence of liver damage due to COVID-19 varies from 14.8% to 53%, 24 and is more frequent in severe than in mild disease. The degree of liver injury is generally mild 60 and predominantly hepatocellular rather than cholestatic. 61 Those with GI symptoms were more prone to developing liver involvement. 62 Li and Xiao 63 classified liver involvement in COVID-19 into two types—specific and nonspecific. The specific type caused three or higher and two or higher fold elevations in ALT/AST and total bilirubin levels, respectively. The nonspecific type caused mild and transient LFT abnormalities, was due to general inflammation, and usually does not need any special treatment. 60 Hepatic injury is commonly associated with decreased lymphocyte counts, raised neutrophil counts, and male gender. 64 This reflects the role of innate immunity/inflammation in COVID-19-associated hepatic injury. More studies are needed to support the relationship between male gender and hepatic injury. The highest ALT, AST, APT, and GGT levels are significantly associated with high body temperatures during the illness. 65 This suggests that changes in the body temperature may contribute to the pathophysiology of COVID-19-associated liver disease. The presence of hepatic injury has been associated with the development of acute respiratory distress syndrome (ARDS). Larger cohort studies should help to better understand this process. Acute liver injury is associated with high mortality. This fulminate hepatic failure may result from direct viral replication or increased inflammation. A summary of the liver-related investigations findings and the treatments used are shown in Table 3. 28, 49, 66 – 80
Table 3.
Clinical characteristics, liver manifestations, and treatments of patients with COVID-19
| First author, year, and country | Article type | Total no of patients (had GI symptoms) | No of males n (%) | Average age (Years) | No of patients had livers abnormalities before or after COVID-19 (%) | Increase of aminotransferase levels U/L, mean/median (n) | Increase of bilirubin mg/dL, n (%) | Decrease of albumin g/L | Alkaline phosphatase U/L, mean ± SD (n) | Gamma-glutamyl transferase U/L (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | ALT | AST | |||||||||
| Beigmohammadi, 2020 66 (Iran) | RA | 7 | 5 (71%) | 67.85 | 1 (peptic ulcer disease) | NA | NA | NA | NA | NA | NA | NA |
| Cardoso, 2020 67 (Portugal) | RL | 20 | 18 (90%) | 67 | 18 | 0 | (≤ 55 IU/L) On admission— 31 U/L Peaked on ICU day 8–82 | (≤ 34 IU/L) On admission— 51 U/L Peaked on ICU day 5—69 U/L | (≤ 1.2 mg/dL) On admission— 0.65 mg/dL Peaked on ICU day 3–1.16 mg/dL | (Normal 40.0– 55.0 g/L) 31.6 g/L 97 (98%) | NA | NA |
| Cai, 2020 49 (China) | RA | 417 | 198 (47.5%) | 47- M | 21 (5.04%) (NAFLD, alcoholic liver disease, and chronic hepatitis B) | 396 (95%) | On admission 27 U/L—AbLT 47 U/L—LI During hospitalization 69 U/L—nonsevere 79 U/L—severe | On admission 34 U/L—AbLT 47.2 U/L—LI During hospitalization 34 U/L—nonsevere 58 U/L—severe | On admission 16.8 μmol/L—AbLT 17.2 μmol/L—LI During hospitalization 19 μmol/L—nonsevere 22 μmol/L—severe | NA | NA | On admission 36.45 U/L—AbLT 134.91 U/L—LI During hospitalization 40 U/L—nonsevere 92 U/L—severe |
| Chen N, 2020 68 (China) | RA | 99 | 67 (68%) | 55.5 | 0 | NA | (U/L; normal range 9.0–50.0) 39 U/L 28 (28%) | (U/L; normal range 15.0–40.0 34 U/L 35 (35%) | (μmol/L; normal range 0.0–21.0) 15.1 μmol/L (7.3%) 18 (18%) | (g/L; normal range 40.0–55.0) 31.6 g/L (4.0%) 97 (98%) | NA | NA |
| Guan W-J, 2020 69 (China) | RA | 1,099 | 601 (55%) | NA | 23 (2.3%) Hep B | NA | > 40 U/L 168/757 (22.2%) | > 40 U/L 158/741 (21.3%) | > 17.1 μmol/L 76/722 (10.5%) | NA | NA | NA |
| Effenberger, 2020 70 (Austria) | RA | 32 | NA | 73.5—with liver damage 69.9—no liver damage | 3 (9.4%) hepatitis C and successful antiviral therapy or signs of MAFLD | NA | No liver damage— 31.3 U/L Liver damage— 76.3 U/L | No liver damage— 19.5 U/L Liver damage— 67.1 U/L | NA | NA | NA | NA |
| Ji D, 2020 28 (China) | RA | 140 | 82 (58.6) | 41.9 | 54 (38.6%) had NAFLD 7 (5.0%) had positive HBsAg | 22 (15.7%) had CLD (3— cirrhosis 6—CHB 13—NAFLD) | Non—CLD n (%) 59 (50.0) CLD—15 (68.2) | Non—CLD n (%) 19 (16.1) CLD—6 (27.3) | Non—CLD n (%)—7 (5.9) CLD—2 (9.1) | NA | Non—CLD n (%)— 4 (3.4) CLD—0 (0) | NA |
| Jin X, 2020 71 (China) | RA | 651 (74%) | 37 (50%) | 46.14 | 8 (10.8%) Chronic liver disease | NA | (U/L; normal range 9–50) With GI symptoms— 25.0 Without GI symptoms—21.5 | (U/L; normal range 15–40 With GI symptoms—29.35 Without GI symptoms—24.4 | (umol/L; normal range 0–26) With GI symptoms— 10.0 Without GI symptoms—9.6 | (g/L; normal range 40–55) with GI symptoms— 40.13 g/L Without GI symptoms— 41.50 g/L | NA | NA |
| Lin, 2020 72 (China) | RA | 95 (58%) | 45 (47%) | 49.5 | 0 | NA | (U/L; normal range 7–40 in female, 9–50 in male) Initial—0 During hospitalization— 22 (23.2%) | (U/L; normal rage 13–35 in female, 15–40 in male) Initial—1 (1.1) During hospitalization— 4 (4.2) | (μmol/L; normal range 3.0–24.0) During hospitalization— 22 (23.2) | NA | NA | NA |
| Luo, 2020 73 (China) | RA | 1,141 (183%) | 102 (55.7%) | 53.8 | NA | NA | (Normal range 9–50 U/L) 66.4% | (Normal range 15–40 U/L) 65.8% | NA | NA | NA | NA |
| Mo, 2020 74 (China) | RA | 155 | 86 (55.5%) | 54 | NA | NA | 23 U/L | 32 U/L | NA | 38 g/L (34–41) | NA | NA |
| Pan, 2020 75 (China) | RA | 204 (103) | 107 (52.5%) | 52.9 | 7 (3.4%) digestive disease | NA | Without digestive symptoms— 29.53 mmol/L With digestive symptoms— 42.24 mmol/L | Without digestive symptoms—27.48 With digestive symptoms— 35.12 | NA | Without digestive symptoms— 35.84 g/L With digestive symptoms— 36.16 g/L | NA | NA |
| Singh and Khan, 2020 76 (USA) | RA | 2780 | 1,070 (38.5%) | 5 LD group— 55.2 Non-LD—51.6 | NA | NA | With liver disease— 100 U/L (130) Without liver disease— 80 UL (70) | With liver disease— 221 U/L (130) Without liver disease— 133 U/L (770) | With liver disease— 1.2 mg/dL (120) Without liver disease— 0.8 mg/dL (770) | With liver disease— 2.6 (120) Without liver disease— 2.5 (770) | With liver disease— 153 U/L (120) Without liver disease— 93 U/L (770) | With liver disease— 278 U/L (10) Without liver disease— 99 U/L (30) |
| Xu X-W, 2020 77 (China) | RA | 62 | 36 (58%) | 41 | 7 (11%) | NA | 22 U/L | 10 (16%) 26 U/L | NA | NA | NA | NA |
| Xu Y, 2020 78 (China) | BC | 10 | 6 (60%) | 12 | NA | NA | One patient had above normal (9–50 U/L) | Two patients had above normal (5–60 U/L) | NA | All within normal range (40–55 g/L) | NA | NA |
| Zhang H, 2020 79 (China) | RA | 505 (164) | 228 (45.1%) | 51.2 | NA | NA | (Normal range 7–40 U/L) 42.1 U/L | (Normal range 13–35 UL) 48.1 U/L | NA | NA | NA | (Normal range 7–45 U/L) 45.8 U/L |
| Zhou, 2020 80 (China) | RA | 191 | 119 (62%) | 56 | NA | NA | > 40 U/L 59/189 (31%) | NA | NA | 32·3 g/L | NA | NA |
AbLT = abnormal liver tests; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CLD = chronic liver disease; CRRT = continuous renal replacement therapy; ECMO = extracorporeal membrane oxygenation; HBsAg = hepatitis B surface antigen; ICU = intensive care unit; LI = liver injury; MAFLD = metabolic associated fatty liver disease; NA = not available; NAFLD = nonalcoholic fatty liver disease; NSAIDs = nonsteroidal anti-inflammatory drugs; PaO2/FiO2 = ratio of arterial oxygen partial pressure to fractional inspired oxygen.
Elevation in aminotransferase levels.
The commonest liver test abnormalities reported are mild to moderate elevations in ALT and AST levels (seen in 14–53% of cases). 81, 82 Significant elevations of ALT and AST are commoner in those with other digestive symptoms. 83 Raised liver aminotransferases are associated with significantly longer hospital stays. 84, 85 A multicenter retrospective cohort study of 5,771 adult COVID-19 patients found AST to increase initially followed by ALT. Aspartate transaminase abnormalities were associated with the highest risk of mortality. 64 Although ALT is more specific to the liver, higher AST levels may be associated with injury to other organs or because of mitochondrial injury and should thus be interpreted with caution.
Elevations in bilirubin levels.
Elevated bilirubin levels are observed in 20–40% of patients and 10% had very high levels. 63, 69 Bilirubin levels are significantly higher in those with severe disease and are associated with a poorer prognosis. 86 Most of the studies do not indicate whether the hypebilirubinemia is of the direct or indirect type. A study from Spain found a biphasic pattern of hyperbilirubinemia, initially hepatocellular and later cholestatic in type. 87 This suggests that the elevation of bilirubin may be because of both direct hepatic injury and cholestasis. In addition to the increase in serum total bilirubin levels, raised conjugated bilirubin levels and conjugated to unconjugated bilirubin ratios were observed in COVID-19 patients. The high bilirubin levels may also be related to hemolysis. 88 Further studies would delineate the predominant pathogenesis of elevated bilirubin levels in COVID-19.
Reduced synthetic function.
Up to 4% of patients with severe COVID-19 had reduced albumin levels. 69 Studies have found lower albumin levels to be associated with a poorer prognosis (severe pneumonia, longer hospital stays, and higher mortality). 8, 87, 89 This may be due to a direct effect of the virus on the liver or due to systemic inflammation in severe COVID-19. The low albumin levels may be due to switching off of albumin production by the liver, increased catabolism or loss of protein through the GI tract during COVID-19. Thus, the low albumin level mentioned in the studies should not be considered a direct marker of reduced liver function. As histological data do not suggest severe hepatic injury, it would be unlikely that low albumin is mainly contributed by hepatic dysfunction. Prothrombin time (PT) has been suggested as a predictive factor for clinical outcomes in COVID-19 patients. The survival rate is significantly lower in patients with prolonged PT. 90 Baranovskii et al. found significantly prolonged admission PT in ICU-transferred patients compared with stable COVID-19 patients. 91 Such findings may be due to systemic inflammation–related coagulopathy rather than reduced hepatic function.
Raised gamma-GT levels.
Elevations of serum GGT levels point to the presence of cholangiocyte injury 81, 92 and are observed in a sizeable proportion of those with severe COVID-19. Elevation of GGT in association with a rise in ALP would suggest cholestasis. The need for ICU care and reduced survival was observed in COVID-19 patients with a cholestatic pattern of hepatic injury. 24, 93
Pathological changes on liver histology.
The described pathological changes in liver histology are mainly ascertained from postmortem studies. Most of the studies do not indicate whether the patients had preexisting liver disease or the severity of the liver derangement, precluding useful interpretation of the pathological findings. The liver histology changes noted in COVID-19 include moderate microvascular and macrovascular steatosis and mild lobular portal inflammation. 17, 83 In autopsy studies, centrilobular steatosis was seen, 94 with significant increases in mitotic cells, eosinophils, and balloon-like liver cells. 16 Lagana et al. found lobular necroinflammation (50%), portal inflammatory infiltrates (50%), cholestasis (38%), lobular apoptosis (25%), and macrovesicular steatosis (75%). 95 However, again the presence of preexisting liver disease or severity of the liver disease was not considered. A study by Wang et al, where the preexisting liver disease was excluded, found viral structures within hepatocytes by electron microscopy and raised the possibility of a direct cytopathic effect of the virus. 79
MANAGEMENT OF LIVER INVOLVEMENT IN COVID-19
The majority of patients with COVID-19 have no or mild liver function abnormalities during the illness. 96, 97 In mild COVID-19, hepatic damage may be transient and generally returns to normal without any special measures. 63, 96 Thus, management is generally supportive with monitoring of LFTs. A summary of the liver management and recommendations are given in Table 4. 98 – 110
Table 4.
Management of liver disease in COVID-19
| Investigations | Pharmacological management | Nonpharmacological management | Recommendations | ||
|---|---|---|---|---|---|
| Laboratory and other biochemistry | Imaging and biopsy | ||||
| No preexisting liver disease | LFTs on admission (baseline) and at least twice weekly during hospital stay 100 Screen for HBV if systemic immunosuppression and tocilizumab has been given for > 7 days 73 | Indicated only in suspicion of vascular or biliary disease 96, 97 Limited/no place for liver biopsy 102 | In moderate to severe liver injury lopinavir-ritonavir, tocilizumab are contraindicated 102 | N/A | Baseline LFTs should be performed on admission to identify preexisting liver disease Transient elevation in LFTs may be seen and needs monitoring at least twice weekly in patients receiving hepatotoxic medication |
| Preexisting liver disease (NAFLD, cirrhosis, HCC, chronic Hepatitis B, alcoholic liver disease) | LFTs on admission (baseline) and at least every other day during hospital stay 103 | Indicated only in suspicion of vascular or biliary disease 101 Limited/no place for liver biopsy 102 | Concomitant administration of tenofovir derivatives with lopinavir-ritonavir—> increases tenofovir concentrations 104 Caution in use of Paxlovid (combination of Ritonavir + Nirmatrelvir) in preexisting liver disease, liver enzyme abnormality or liver inflammation 110 Paxlovid may induce hepatic enzymes and breakdown of nirmatrelvir or ritonavir 110 Paracetamol > 2 g per day to be avoided 104 NSAIDs used with caution 104 Corticosteroids used with caution in hepatitis B as they may increase the risk of hepatitis in chronic HBV 104 Continue treatment of HBV even during treatment of COVID-19, discontinuation of antiviral treatment of hepatitis B discouraged 105 Anti-HBV drugs may be considered when patients are on immunosuppressive treatment with careful monitoring 105 | Strict measures to minimize exposure to COVID-19 especially in HCC due to very high risk of hospital-acquired COVID-19. 98 COVID-19 vaccines as early as possible 109 Treat HCC without delay. 98 Pneumococcal and influenza vaccines irrespective of the age. 99 | Apart from HCV without decompensated cirrhosis, all other preexiting liver diseases should be managed as before. Use of immunosuppression in chronic liver disease requires caution and close monitoring. HCC should be treated without delay taking all precautions. |
| Liver transplant | Test donor and recipient for COVID-19 preoperatively | No specific recommendations | Remdesivir—risk of hepatotoxicity Increased levels with liver enzyme inducers 108 Tocilizumab—minor interaction with cyclosporine, tacrolimus, and sirolimus May reduce concentrations of calcineurin inhibitors. Use with chloroquine and hydroxychloroquine may produce additive toxicity. Myelosuppressive effect may potentiate hematological toxicity of ribavirin and interferon-beta. 102 | Liver transplantation should not be postponed during pandemic 106 COVID-19 vaccines as early as possible 109 | Although challenging, LT should not be postponed due to COVID-19 Early COVID-19 vaccination with third booster dose 1–2 months after second dose Perform baseline LFT before starting remdesivir therapy and monitor during therapy. Discontinue infusions if ALT and AST > 10 times ULN. |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; LFT = liver function test; LT = liver transplant; NAFLD = nonalcoholic fatty liver disease; NSAIDs = nonsteroidal anti-inflammatory drugs; ULN = upper limit of normal.
Diagnostic aspects.
Liver function tests and abdominal imaging are the primary investigations done in relation to liver involvement in COVID-19 patients. Liver biochemistry including the liver enzymes (ALT and AST), serum bilirubin, albumin, and PT should be monitored for diagnosing liver damage. 61 However, the reasons for derangement of these blood tests are multifactorial and systemic inflammatory response because of COVID-19 may play a greater role than liver injury. Nevertheless, the liver tests should be performed during admission to establish a baseline and also to identify patients with suspected or known underlying liver disease. Further biochemical investigations may be needed in patients with known liver disease for example, known hepatitis B, C, and so on. The optimal interval for undertaking LFTs is uncertain. It has been suggested that LFTs be monitored at least twice weekly in COVID-19 patients receiving potential liver-toxic medications, whereas those with abnormal LFT results or with preexisting liver disease should be monitored more frequently. 100 Increased serum AST and lactate dehydrogenase (LDH) with normal ALT levels should raise the suspicion of alternative diagnoses such as skeletal muscle or myocardial injury. Abnormal LFTs are frequently noted at admission before antiviral treatment of COVID-19 is commenced. Abnormal LFTs at the onset of a COVID-19 infection may indicate underlying chronic liver disease (CLD) 61 and the treating physicians should take this into account. 30 The imaging modalities include abdominal ultrasonography and computed tomography scans. The imaging findings are nonspecific and are usually indicated when there is suspicion of portal venous thrombosis or biliary obstruction.
Lei et al. found liver hypo-echogenicity (homogeneous or heterogeneous) and peri-cholecystic fat stranding to be common positive findings on abdominal computed tomography. However, such findings are only detected in a subset of patients with liver derangement and hence such investigations should be used very selectively. 101 Postmortem liver biopsy often shows moderate microvascular steatosis and mild lobular and portal activity, but these are not specific for COVID-19. Therefore, there is limited or no place in liver biopsy in a clinical context. Although clues about the underlying pathological processes may be obtained, the influence of such findings on clinical management would be limited. 102
The American Association for the Study of Liver Diseases (AASLD) recommends the consideration of causes unrelated (e.g., hepatitis B virus [HBV] or hepatitis C virus [HCV]) to COVID-19 and other causes (e.g., myositis, ischemia, and cytokine release syndrome) for any liver test abnormalities. 102 In areas where viral hepatitis is prevalent, serological investigations for viral hepatitis may be considered depending on the clinical circumstances. In regions where it is less prevalent, monitoring of hepatic functions would suffice and further investigation for causes, may be restricted to cases where the hepatic functions do not normalize within a reasonable time frame (such as 2–3 months of its first detection). 111 The choice of evaluation for patients with persistent liver function abnormalities should include investigation for chronic parenchymal liver diseases and other infective causes and is based on the clinical presentation.
Specific management in patients without previous liver diseases.
Liver functions should be monitored in COVID-19 patients at admission and during hospitalization. 103 The presence of abnormal liver tests is not a contraindication for investigational or off-label treatment of COVID-19. 102 However, such patients should be closely monitored while receiving any antivirals and off-label agents with potential liver toxicity. Known liver-toxic medications such as lopinavir-ritonavir and tocilizumab should be withheld if there is moderate to severe liver injury. If systemic immunosuppression such as corticosteroids and tocilizumab is administered for more than 7 days, screening for the HBV is recommended especially in regions where this infection is prevalent. 111 Drug interactions need to be considered when prescribing for COVID-19 patients with chronic liver disease. Paracetamol (doses > 2 g per day to be avoided in patients with chronic liver disease) and nonsteroidal anti-inflammatory drugs NSAIDs should be used with caution. The use of corticosteroids in COVID-19 may increase the risk of hepatitis in chronic HBV patients. Thus, one should be cautious with its use and if used done with close monitoring. Certain combinations of drugs are best avoided in patients with preexisting liver disease. For example, concomitant administration of tenofovir derivatives with lopinavir–ritonavir is relatively contraindicated as the concentration of tenofovir may be increased due to a drug interaction. In such cases, suitable alternatives should be used. 104
COVID-19 IN PATIENTS WITH DIAGNOSED LIVER DISEASES
Those with preexisting liver disease, the elderly, or individuals who consume high amounts of alcohol or are obese should be monitored closely. The AASLD recommends the consideration of causes unrelated to COVID-19 (e.g., HBV or HCV) and other causes (e.g., myositis, ischemia, and cytokine release syndrome) for any observed liver test abnormalities. Patients receiving immunosuppressive medications (liver transplant recipients or those with autoimmune hepatitis) should be managed as they were prior to the pandemic. Those with chronic HBV should continue their treatment and in HCV patients without decompensated cirrhosis treatment may be delayed. Hepatocellular carcinoma should be treated without delay. 102
Some preexisting liver diseases are risk factors for poorer prognosis in COVID-19. Preexisting liver disease increases ACE2 expression on hepatocytes (observed in murine and human studies) and may thus increase the hepatic tropism of SARS-CoV-2. 24 Grasselli et al. found 3% of 1,591 COVID-19 patients admitted to ICUs had a history of chronic liver disease. 105
Nonalcoholic fatty liver disease
Patients with NAFLD have a higher risk of COVID-19 progression (6.6% versus 44.7%) and a higher likelihood of abnormal LFTs (70% versus 11.1%). 68 It is a risk factor for hospitalization in COVID-19, and was suggested as a more significant factor than age, gender, obesity, or other comorbidities. 112 Although the underlying mechanism is yet to be clarified, a possible reason could be impaired innate immune responses to the virus. Hepatic macrophages/Kupffer cells may be skewed from an inflammatory-promoting to inflammatory-suppressing type. 68 Nonalcoholic fatty liver disease is associated with an increased risk of severe COVID-19, even after adjusting for obesity. 113 Zheng et al. found a 6-fold higher risk of severe COVID-19 in patients with NAFLD. The severity of COVID-19 was much higher in obese than nonobese NAFLD patients. 114 According to Prins et al., the liver contains the highest number of macrophages, and NAFLD patients often present with elevated cytokine levels. Nonalcoholic fatty liver disease progression could also be hastened by COVID‐19. 115
Cirrhosis.
These patients are more susceptible to SARS-CoV-2 infections, owing to their immunocompromised status. Increased disease severity and complications lead to higher mortality. A multicenter study of 50 cirrhosis patients with COVID-19 found a 30-day mortality rate of 34%. 116 Higher severity of the underlying liver disease was associated with an increased risk of mortality in COVID-19. In a study of 152 COVID-19 patients with chronic liver disease (including 103 with cirrhosis), the mortality rate was 40%. The mortality rates in patients with Child-Pugh (CP) class A cirrhosis, CP class B cirrhosis, and CP class C cirrhosis were 24%, 43%, and 63% respectively. CP class B or C cirrhosis were independent predictors of mortality. 117
Hepatocellular carcinoma.
Patients with hepatocellular carcinoma (HCC) often need to visit a hospital for their treatment (chemotherapy and or immunotherapy) and thus need to be managed and monitored carefully. They have a higher risk of getting hospital-associated COVID-19, especially those who underwent surgery or received systemic treatment in the prior month. Post-epatectomy liver failure (PHLF) is a life-threatening situation following hepatectomy. Increased inflammation during COVID-19 may predispose the patient to PHLF. Secretion of IL-6 after hepatectomy and during PHLF, may further increase inflammation during COVID-19. 118 In patients with HCC, COVID-19 may exacerbate existing chronic liver disease and complicate cancer management. Cancer patients have a higher risk of infection and worse outcomes, especially those who have recently undergone cancer treatment. Hepatocellular carcinoma was underrepresented in COVID-19 series. Mitigation measures should be implemented to minimize the exposure of such patients to the virus. A decision on the treatment of HCC should be balanced with the availability of medical resources and the level of risk of getting COVID-19. 119
Hepatitis B infections.
Globally, there are over 250 million people living with HBV infection. 120
Thus, it is important to study the clinical characteristics of COVID-19 patients with preexisting HBV infection. These patients tend to have a more severe form of COVID-19. 69, 121 However, according to the COVID-HBV-Chinese Portal Hypertension Diagnosis and Monitoring Study Group study, patients with preexisting HBV infections had a lower incidence of ICU admission or death. 8 Similar findings were noted with SARS-CoV-1 and HBV coinfection. 122 In a systematic review done by Hossein Mirzaie et al., the mortality was 6% in COVID-19-HBV, coinfected persons. The low ICU admission and death rates in preexisting HBV patients maybe because of host immune responses that result from indirect interactions between HBV and SARS-CoV-2 virus. 8 Furthermore, early COVID-19 vaccination in HBV-infected populations, additional precautionary measures, and early identification and treatment of COVID-19 infection may have contributed to better outcomes. During treatment, discontinuation of antiviral treatment of hepatitis B is discouraged, so as to prevent its reactivation. Anti-HBV drugs may be considered when patients are on immunosuppressive treatment and the patients should be monitored carefully. 123
Alcoholic liver disease.
Both alcohol-associated liver disease (ALD) and alcohol use disorders (AUD) have been affected during the COVID-19 pandemic. Economic and social stresses resulting from the pandemic increased alcohol consumption in some individuals and delays in care have led to increased mortality from alcohol-associated hepatitis. 117
Liver transplant recipients.
A meta-analysis comparing 1522 COVID-19-infected liver transplant (LT) patients and around 240,000 COVID-19-infected non-LT patients showed similar mortality rates. 106 The LT patients had a cumulative mortality rate of 17.4%. 106 The graft dysfunction rate was 2.3% (1.3–4.1%). However, 23% developed severe infections. 106 A review by Kullar et al. showed that 80% of LT patients with COVID-19 required hospital admission and 17% required intensive care. 124 Around 21% required mechanical ventilation and the overall mortality was 17%. 124 Therefore, these patients would require close monitoring during the active stage of infection with observation for graft rejection. Vaccination as a preventive strategy is recommended. Postexposure prophylaxis should be considered in selected high-risk individuals. Due to lack of consensus, management strategies varied widely, including variations in immunosuppressive therapy and different investigational therapies to manage COVID-19 in transplant patients. 125
Surgical aspects.
Surgical services, especially routine surgeries for both benign and malignant conditions, have been affected worldwide during the COVID-19 pandemic. 126 – 128 The effect of surgery and anesthesia has negative implications on COVID-19 patients. Furthermore, healthcare workers were also affected because of increased high-risk exposures and the lack of clinical exposure/training due to the postponement of routine surgeries. 129 – 131 Surgery should not be delayed for HCC patients. However, adequate precautions should be taken to minimize complications, which include prior vaccination and proper timing if previously infected by SARS-CoV-2 and thromboprophylaxis. 126, 130, 132 Patients are especially vulnerable to pulmonary complications and venous thromboembolism and these should be prevented. 133, 134 Routine preoperative screening for SARS-CoV-2 is mandatory and COVID-19-free pathways have been shown to be beneficial. 135, 136 However, preoperative isolation is controversial. 137 During periods of societal restriction, the resilience of elective surgery systems requires strengthening to prevent postponement of cancer surgeries. 138, 139
Liver transplantation has been affected due to COVID-19 infection worldwide. 140 Individuals with a liver transplant need preoperative, surgical intervention, and postoperative care, which is challenging during this pandemic. The healthcare facilities are overwhelmed with the management of COVID-19 and the need for resources such as ICU beds and ventilators. 136 In addition, to limited facilities, the exclusion of donors with COVID-19 is a major problem encountered in the transplantation programs. Furthermore, immunosuppressive therapy in transplanted individuals and drug interactions may make them more vulnerable for COVID-19 infection and hence optimal protective measures should be maintained. The European Association for the Study of the Liver (EASL) and AASLD have suggested that LT should not be postponed during the pandemic. Preliminary data has shown that despite immunosuppression in LT patients, no increased risk was found with post-LT patients. 107 This may suggest that immunosuppression in LT patients was not associated with an increased risk of COVID-19 infection. However, further studies are needed prior to this becoming routine clinical practice.
In Italy, though liver transplantation was carried out during COVID-19, a 25% reduction in procured organs was observed during the first 4 weeks of the outbreak. 107 Both living and deceased donor LTs were performed and increased mortality was seen in patients who needed to remain on the waiting list. 141 Saracco et al. found no significant difference in numbers of patients undergoing LT from deceased donors in 2020 compared with 2019. The rate of early graft dysfunction was 24% and 33% in 2020 and 2019. In 2020, the Median Model for End-stage Liver Disease (MELD) score was higher (17 versus 13) and there were no deaths in those on the waiting list. 107 Thus careful testing of symptomatic patients and careful testing all transplant donors for SARS-CoV-2 RNA and team-work helps overcome the constraints in LT during the COVID-19 pandemic. Furthermore, a patient’s liver transplantation candidacy should not be affected by PCR test results alone, as the COVID-19 PCR may remain positive in absence of active COVID-19. 108
Drug interactions.
Drug–drug interaction is a problem associated with LT during the COVID-19 era. Patients who underwent LT are usually poly-medicated mainly with immunosuppressive drugs. Since a number of drugs used in COVID-19 have only been recently authorized, monitoring for potential drug interactions is important. 141 Remdesivir has been approved by US Food and Drug Administration (US FDA) for the treatment of hospitalized patients with COVID-19. It is potentially hepatotoxic and should be used with caution. Increased liver transaminase levels are a common adverse effect, and discontinuation of remdesivir infusions should be considered if elevations in ALT or AST above 10 times the upper limit of normal are noticed. Baseline LFTs should be done before initiation of therapy and these should be monitored closely during therapy. 109, 142 The concentration of remdesivir may be affected by enzyme inducers such as clarithromycin, rifampin, phenytoin, and phenobarbital. 143 Favipiravir increases the concentration of pioglitazone, rosiglitazone, paracetamol, oseltamivir, and hormonal replacement therapy, but does not have significant interactions with immunosuppressive medications or steroids. 143, 144 Paxlovid, an oral antiviral agent contains the protease inhibitor nirmatrelvir and a low dose of ritonavir. Ritonavir may cause liver injury and thus caution needs to be exercised when Paxlovid is considered for patients with liver enzyme abnormalities, hepatic inflammation, or preexisting liver diseases. 145 Tociluzumab has minor interactions with ciclosporin, tacrolimus, and sirolimus. It may also reduce the concentrations of calcineurin inhibitors and drug level monitoring should be performed. Its use with chloroquine and hydroxychloroquine may produce additive toxicity. Tocilizumab has a myelosuppressive effect and it may thus potentiate hematological toxicity of ribavirin and interferon-beta if used together. In the setting of LT, interferon-beta has no interactions with immunosuppressive drugs or steroids. However, as it induces myelosuppression, it should not be combined with tocilizumab. Also, potential interaction with chloroquine and hydroxychloroquine may increase its toxicity. 141
COVID-19 VACCINES IN LIVER DISORDERS
Patients with CLD (predominantly cirrhosis), hepatobiliary malignancies, candidates for liver transplantation, and immunosuppressed individuals 110 after liver transplantation appear to be at increased risk of COVID-19 infection and increased mortality. This risk might occur through cirrhosis-associated immune dysfunction, acute hepatic decompensation, and a systemic inflammatory response. 146 Therefore, COVID-19 vaccines should be administered as early as possible to patients with CLD. In general, vaccines are less effective in CLD and post-LT patients. The impaired immune response in such patients may result in an incomplete immediate and long-term immune protection following vaccination. 147 The original vaccine trials included only small numbers of patients with mild to moderate liver disease and excluded those on immunosuppressive medications. 148 Chronic liver disease patients represented less than 0.5% of those enrolled in phase II clinical trials. From these clinical trial results, it was difficult to speculate which COVID-19 vaccine type would be most effective in those with CLD. Liver transplant individuals usually have reduced rates of seroconversion and lower antibody titers in response to vaccination and this may be similar with the COVID-19 vaccines. 146 In a recent US study, 63% of post-LT patients seroconverted after the second dose, whereas 100% of cirrhotic patients did so. Furthermore, 28% of LT patients did not develop humoral or T cell responses, pointing to the need for routine serological testing with third vaccine dose administration in such patients. 149 Strauss et al. found that LT patients who received two doses of an mRNA vaccine to have a greater antibody response than other solid organ transplants (SOT). This may be due to milder induction immunosuppression given to LT patients when compared with other SOT patients such as heart and lung transplant patients. 150 Early vaccination and avoiding the use of antimetabolite medications (if this were at all possible) should be considered for obtaining better postvaccine immune outcomes in these patients. A third dose of the COVID-19 vaccine needs to be considered for LT recipients, at around 1 or 2 months after their second dose. In patients with CLD, vaccination may not result in a robust immune response due to immunosuppression. 151 Hence, monoclonal antibody therapy may be beneficial in these patients. Vaccine-related adverse effects in LT recipients are similar to other individuals. 152
LIMITATIONS
A limitation of this review is that the majority of studies are observational and have small numbers of subjects making it difficult to provide more definitive conclusions. It is possible that subtle liver findings were not documented (and thus underestimated) during the early part of the pandemic. Well-conducted studies from different regions of the world would help expand the evidence base and provide better answers to the many questions at hand. Ours is a broad overview of the main reported hepatic manifestations in COVID-19 and their management. A more comprehensive and detailed profile of specific aspects should emerge as more data are published from different countries.
CONCLUSION
In conclusion, liver involvement is observed in COVID-19 patients and may influence disease prognosis and outcomes. The factors that may contribute to liver involvement in COVID-19 include direct viral cytopathic effects, exaggerated immune responses, hypoxia-induced changes, vascular changes due to coagulopathy, endothelitis, cardiac congestion from right heart failure, and drug-induced liver injury. Further clinical and laboratory studies should help ascertain more details on the potential mechanisms of SARS-CoV-2 infections and the liver. The COVID-19 vaccines should be administered as early as possible to patients with CLD and a third dose of the vaccine needs to be considered for LT recipients.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene has waived the Open Access fee for this article due to the ongoing COVID-19 pandemic and has assisted with publication expenses.
REFERENCES
- 1. World Health Organization, 2020. Country Reports. Available at: https://www.who.int/countries/. Accessed February 27, 2021.
- 2. Zhu Z Lian X Su X Wu W Marraro GA Zeng Y , 2020. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res 21: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu DX Liang JQ Fung TS , 2021. Human coronavirus-229E,-OC43,-NL63, and-HKU1 (Coronaviridae). Encycl Virol 2: 428–440. [Google Scholar]
- 4. Abdelghany T Ganash M Bakri MM Qanash H Al-Rajhi AM Elhussieny NI , 2021. SARS-CoV-2, the other face to SARS-CoV and MERS-CoV: future predictions. Biomed J 44: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu H Chan JF-W Wang Y Yuen TT-T Chai Y Shuai H Yang D Hu B Huang X Zhang X , 2021. SARS-CoV-2 induces a more robust innate immune response and replicates less efficiently than SARS-CoV in the human intestines: an ex vivo study with implications on pathogenesis of COVID-19. Cell Mol Gastroenterol Hepatol 11: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kukla M Skonieczna-Żydecka K Kotfis K Maciejewska D Łoniewski I Lara L Pazgan-Simon M Stachowska E Kaczmarczyk M Koulaouzidis A , 2020. COVID-19, MERS and SARS with concomitant liver injury—systematic review of the existing literature. J Clin Med 9: 1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jayasekara D Seneviratne SL Jayasekara A De Zoysa I , 2020. Atypical presentations of COVID-19. Adv Infect Dis 10: 136. [Google Scholar]
- 8. Jothimani D Venugopal R Abedin MF Kaliamoorthy I Rela M , 2020. COVID-19 and the liver. J Hepatol 73: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J Fan J-G , 2020. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itou M Kawaguchi T Taniguchi E Sata M , 2013. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol 19: 2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu K-L Lu S-N Changchien C-S Chiu K-W Kuo C-H Chuah S-K Liu J-W Lin M-C Eng H-L Chen S-S , 2004. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg 71: 125–128. [PubMed] [Google Scholar]
- 12. Saad M Omrani AS Baig K Bahloul A Elzein F Matin MA Selim MA Al Mutairi M Al Nakhli D Al Aidaroos AY , 2014. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 29: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Hameed F Wahla AS Siddiqui S Ghabashi A Al-Shomrani M Al-Thaqafi A Tashkandi Y , 2016. Characteristics and outcomes of Middle East respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J Intensive Care Med 31: 344–348. [DOI] [PubMed] [Google Scholar]
- 14. Kumar-M P Mishra S Jha DK Shukla J Choudhury A Mohindra R Mandavdhare HS Dutta U Sharma V , 2020. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int 14: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong K-H Choi J-P Hong S-H Lee J Kwon J-S Kim S-M Park SY Rhee J-Y Kim B-N Choi HJ , 2018. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax 73: 286–289. [DOI] [PubMed] [Google Scholar]
- 16. Chau TN Lee KC Yao H Tsang TY Chow TC Yeung YC Choi KW Tso YK Lau T Lai ST , 2004. SARS‐associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 39: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Portincasa P Krawczyk M Machill A Lammert F Di Ciaula A , 2020. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med 77: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, 2004. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng DL Al Hosani F Keating MK Gerber SI Jones TL Metcalfe MG Tong S Tao Y Alami NN Haynes LM , 2016. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 186: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marjot T Moon AM Cook JA Abd-Elsalam S Aloman C Armstrong MJ Pose E Brenner EJ Cargill T Catana M-A , 2021. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol 74: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iavarone M D’Ambrosio R Soria A Triolo M Pugliese N Del Poggio P Perricone G Massironi S Spinetti A Buscarini E , 2020. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol 73: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marjot T et al. 2021. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol 74: 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahman A Niloofa R Jayarajah U De Mel S Abeysuriya V Seneviratne SL , 2021. Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg 104: 1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nardo AD Schneeweiss‐Gleixner M Bakail M Dixon ED Lax SF Trauner M , 2021. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int 41: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J Song S Cao HC Li LJ , 2020. Liver diseases in COVID-19: etiology, treatment and prognosis. World J Gastroenterol 26: 2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osterreicher CH et al. 2009. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abeysuriya V. et al. , 2021. Combination of cycle threshold time, absolute lymphocyte count and neutrophil: lymphocyte ratio is predictive of hypoxia in patients with SARS-CoV-2 infection. Trans R Soc Trop Med Hyg. 10.1093/trstmh/trab182. [DOI] [PMC free article] [PubMed]
- 28. Ji D Qin E Xu J Zhang D Cheng G Wang Y Lau G , 2020. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol 73: 451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rahman A Niloofa R De Zoysa IM Cooray AD Kariyawasam J Seneviratne SL , 2020. Neurological manifestations in COVID-19: a narrative review. SAGE Open Med 8: 2050312120957925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertolini A et al. 2020. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology 72: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barlow A Landolf KM Barlow B Yeung SYA Heavner JJ Claassen CW Heavner MS , 2020. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. J Hum Pharmacol Drug Ther 40: 416–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M Cao R Zhang L Yang X Liu J Xu M Shi Z Hu Z Zhong W Xiao G , 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ClinicalTrials.gov [Internet] , 2020. Severe 2019-nCoV Remdesivir RCT. Bethesda MNLoMUF-IN, Identifier NCT04257656. February 24, 2020 [cited July 29, 2020]. Available at: https://clinicaltrials.gov/ct2/show/NCT04257656.
- 34. ClinicalTrials.gov [Internet] , 2020. Mild/Moderate 2019-nCoV. Bethesda MNLoMUF-IN, Identifier NCT04252664. February 24, 2020 [cited July 29, 2020]. Available at: https://clinicaltrials.gov/ct2/show/NCT04252664.
- 35. Deng L Li C Zeng Q Liu X Li X Zhang H Hong Z Xia J , 2020. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect 81: e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov [Internet] , 2020. Lopinavir/Ritonavir, Ribavirin and IFN-Beta Combination for nCoV Treatment. Cg, Bethesda MNLoMUF-IN IN. February 28, 2020. [Cited July 29, 2020]; [about 4 screens].
- 37. ClinicalTrials.gov, [Internet] , 2012. Darunavir. LCaRIoD-ILI, Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. [Updated September 1, 2017].
- 38. Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, Patil S, Barkate H, 2020. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis 102: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richardson P Griffin I Tucker C Smith D Oechsle O Phelan A Stebbing J , 2020. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. ClinicalTrials.gov [Internet] , 2020. Baricitinib Therapy in COVID-19. Cg, Bethesda MNLoMUM-IN IN. Identifire NCT04358614. July 22, 2020 [Cited July 29, 2020].
- 41. Emadi A Chua JV Talwani R Bentzen SM Baddley J , 2020. Safety and efficacy of imatinib for hospitalized adults with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 21: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] , 2012. Imatinib. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. [Updated Apr 24, 2018]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK547959/. Accessed July 29, 2020. [PubMed]
- 43. ClinicalTrials.gov [Internet] , 2012. Hydroxychloroquine. LCaRIoD-ILI, Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. [Updated March 25, 2018].
- 44. ClinicalTrials.gov [Internet] , 2020. Efficacy of Dexamethasone Treatment for Patients with ARDS Caused by COVID-19 (DEXA-COVID19). Bethesda, MD: MNLoMUMIN IN. September 2, 2020 [Cited July 29, 2020].
- 45. Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences University of Oxford, 2020. Available at: https://www.phc.ox.ac.uk/research/oxford-centre-for-evidence-based-medicine. Accessed February 19, 2022.
- 46. ClinicalTrials.gov [Internet] , 2020. Azithromycin in Hospitalized COVID-19 Patients (AIC). Cg, Bethesda MNLoMUJIN0. Identifier: NCT04359316. [Cited December 6, 2020].
- 47. Anonymous , 2021. Conditions for use, conditions for distribution and patients targeted and conditions for safety monitoring addressed to member states for unauthorized product PAXLOVID (PF-07321332 150 mg and ritonavir 100 mg). Available at: https://www.ema.europa.eu/en/documents/referral/paxlovid-pf-07321332-ritonavir-covid-19-article-53-procedure-conditions-use-conditions-distribution_en.pdf. Accessed December 24, 2021.
- 48. Anonymous, 2021. Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available at: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/#:~:text=At%20the%20interim%20analysis%2C%20molnupiravir,%2F377)%3B%20p%3D0.0012. Accessed December 20, 2020.
- 49. Cai Q et al. 2020. COVID-19: abnormal liver function tests. J Hepatol 73: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forbes JD Van Domselaar G Bernstein CN , 2016. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol 7: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chattopadhyay I Shankar EM , 2021. SARS-CoV-2-indigenous microbiota nexus: does gut microbiota contribute to inflammation and disease severity in COVID-19? Front Cell Infect Microbiol 11: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dhar D Mohanty A , 2020. Gut microbiota and COVID-19-possible link and implications. Virus Res 285: 198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yeoh YK et al. 2021. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terruzzi I Senesi P , 2020. Does intestinal dysbiosis contribute to an aberrant inflammatory response to SARS-CoV-2 in frail patients? Nutrition 79: 110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Q Liang Q Balakrishnan B Belobrajdic DP Feng Q-J Zhang W , 2020. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients 12: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rishi P Thakur K Vij S Rishi L Singh A Kaur IP Patel SK Lee J-K Kalia VC , 2020. Diet, gut microbiota and COVID-19. Indian J Microbiol 60: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller B, Silverstein A, Flores M, Xiang W, Cao K, Kumagai H, Mehta HH, Yen K, Kim S-J, Cohen P, 2020. SARS-CoV-2 induces a unique mitochondrial transcriptome signature. 10.21203/rs.3.rs-36568/v1. [DOI] [PMC free article] [PubMed]
- 58. Koliaki C et al. 2015. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746. [DOI] [PubMed] [Google Scholar]
- 59. Sun J, Aghemo A, Forner A, Valenti L, 2020. COVID-19 and liver disease. Liver Int 40: 1278--1281. [DOI] [PubMed]
- 60. Ghoda A Ghoda M , 2020. Liver injury in COVID-19 infection: a systematic review. Cureus 12: e9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang R-X Zheng R-D Fan J-G , 2020. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol 26: 4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kariyawasam JC, Jayarajah U, Riza R, Abeysuriya V, Seneviratne SL, 2021. Gastrointestinal manifestations in COVID-19. Trans R Soc Trop Med Hyg trab042. [DOI] [PMC free article] [PubMed]
- 63. Li Y Xiao SY , 2020. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications. J Med Virol 92: 1491–1494. [DOI] [PubMed] [Google Scholar]
- 64. Lei F et al. 2020. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 72: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abe K et al. 2020. Clinical features and liver injury in patients with COVID-19 in the Japanese population. Intern Med 59: 2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beigmohammadi MT Jahanbin B Safaei M Amoozadeh L Khoshavi M Mehrtash V Jafarzadeh B Abdollahi A , 2021. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol 29: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cardoso FS Pereira R Germano N , 2020. Liver injury in critically ill patients with COVID-19: a case series. Crit Care 24: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen N et al. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guan WJ et al. 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Effenberger M et al. 2020. Liver stiffness by transient elastography accompanies illness severity in COVID-19. BMJ Open Gastroenterol 7: e000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jin X et al. 2020. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69: 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lin L et al. 2020. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 69: 997–1001. [DOI] [PubMed] [Google Scholar]
- 73. Luo S Zhang X Xu H , 2020. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol 18: 1636–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mo P. et al. , 2020. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis ciaa270.
- 75. Pan L et al. 2020. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 115: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Singh S Khan A , 2020. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 159: 768–771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu XW et al. 2020. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368: m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xu Y et al. 2020. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 26: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Y et al. 2020. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 73: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou F et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang C Shi L Wang FS , 2020. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 5: 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun J Aghemo A Forner A Valenti L , 2020. COVID-19 and liver disease. Liver Int 40: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 83. Xu Z et al. 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fan Z Chen L Li J Cheng X Yang J Tian C Zhang Y Huang S Liu Z Cheng J , 2020. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 18: 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie H Zhao J Lian N Lin S Xie Q Zhuo H , 2020. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int 40: 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Paliogiannis P Zinellu A , 2020. Bilirubin levels in patients with mild and severe COVID-19: a pooled analysis. Liver Int 40: 1787–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bernal-Monterde V et al. 2020. SARS-CoV-2 infection induces a dual response in liver function tests: association with mortality during hospitalization. Biomedicines 8: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu Z et al. 2020. Bilirubin levels as potential indicators of disease severity in coronavirus disease patients: a retrospective cohort study. Front Med 7: 598870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de la Rica R Borges M Aranda M Del Castillo A Socias A Payeras A Rialp G Socias L Masmiquel L Gonzalez-Freire M , 2020. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain: a retrospective cohort study. Microorganisms 8: 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang L He WB Yu XM Hu DL Jiang H , 2020. Prolonged prothrombin time at admission predicts poor clinical outcome in COVID-19 patients. World J Clin Cases 8: 4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baranovskii DS Klabukov ID Krasilnikova OA Nikogosov DA Polekhina NV Baranovskaia DR Laberko LA , 2021. Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia. Curr Med Res Opin 37: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shao T et al. 2020. γ-Glutamyltransferase elevations are frequent in patients with COVID-19: a clinical epidemiologic study. Hepatol Commun 4: 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roedl K et al. 2021. Severe liver dysfunction complicating course of COVID-19 in the critically ill: multifactorial cause or direct viral effect? Ann Intensive Care 11: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barton LM Duval EJ Stroberg E Ghosh S Mukhopadhyay S , 2020. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 153: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lagana SM et al. 2020. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol 33: 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Borges do Nascimento IJ et al. 2020. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PLOS ONE 15: e0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Borges do Nascimento IJ Cacic N Abdulazeem HM von Groote TC Jayarajah U Weerasekara I Esfahani MA Civile VT Marusic A Jeroncic A , 2020. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med 9: 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Inchingolo R Acquafredda F Tedeschi M Laera L Surico G Surgo A Fiorentino A Spiliopoulos S de’Angelis N Memeo R , 2021. Worldwide management of hepatocellular carcinoma during the COVID-19 pandemic. World J Gastroenterol 27: 3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Im H Ser J Sim U Cho H , 2021. Promising expectations for pneumococcal vaccination during COVID-19. Vaccines (Basel) 9: 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong GL et al. 2020. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol 5: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lei P Zhang L Han P Zheng C Tong Q Shang H Yang F Hu Y Li X Song Y , 2020. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int 14: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chang JPE et al. 2020. Chapter of Gastroenterologists professional guidance for management of patients with liver disease in Singapore during the COVID-19 pandemic. Singapore Med J 61: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo S Zhang X Xu H , 2020. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol 18: 1636–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Da BL Im GY Schiano TD , 2020. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology 72: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 105. Grasselli G et al. 2020. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kulkarni AV Tevethia HV Premkumar M Arab JP Candia R Kumar K Kumar P Sharma M Rao PN Reddy DN , 2021. Impact of COVID-19 on liver transplant recipients-a systematic review and meta-analysis. EClinicalMedicine 38: 101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saracco M Martini S Tandoi F Dell’Olio D Ottobrelli A Scarmozzino A Amoroso A Fonio P Balagna R Romagnoli R , 2020. Carrying on with liver transplantation during the COVID-19 emergency: report from piedmont region. Clin Res Hepatol Gastroenterol 45: 101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nickerson AM, Sobotka LA, Kelly SG, 2021. PRO: liver transplantation in the times of COVID-19: “to transplant or not to transplant.” Clin Liver Dis (Hoboken) 18: 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Seneviratne SL, Niloofa R, De Zoysa I, de Mel S, Abeysuriya V, 2020. Remdesivir and COVID-19. Int J Adv Res 8: 565--567. [Google Scholar]
- 110. Seneviratne SL, Yasawardene P, Hettiarachchi D, Amarasena DK, Wijerathne W, Samaraweera B, Mathew DKD, Abeysuriya V, 2021. The Delta variant of SARS-CoV-2: the current global scourge. Sri Lankan Fam Phys 36: 17--25. [Google Scholar]
- 111. Wong GL-H et al. 2020. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol 5: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S, 2020. Nonalcoholic fatty liver disease (NAFLD) and risk of hospitalization for COVID-19. medRxiv. 10.1101/2020.09.01.20185850. [DOI] [Google Scholar]
- 113. Sachdeva S Khandait H Kopel J Aloysius MM Desai R Goyal H , 2020. NAFLD and COVID-19: a pooled analysis. SN Compr Clin Med 2: 2726–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zheng KI et al. 2020. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism 108: 154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Prins GH Olinga P , 2020. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int 40: 2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Iavarone M et al. 2020. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol 73: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moon AM et al. 2020. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol 73: 705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Triki H Jeddou H Boudjema K , 2020. Surgical resection for liver cancer during the COVID-19 outbreak. Updates Surg 72: 305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chan SL Kudo M , 2020. Impacts of COVID-19 on liver cancers: during and after the pandemic. Liver Cancer 9: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cooke GS et al. 2019. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology and Hepatology Commission. Lancet Gastroenterol Hepatol 4: 135–184. [DOI] [PubMed] [Google Scholar]
- 121. Sarin SK , 2020. “Fast, faster, and fastest: science on the run during COVID-19 drama”-“do not forget the liver.” Hepatol Int 14: 454–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chan HL Kwan AC To KF Lai ST Chan PK Leung WK Lee N Wu A Sung JJ , 2005. Clinical significance of hepatic derangement in severe acute respiratory syndrome. World J Gastroenterol 11: 2148–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yang RX Zheng RD Fan JG , 2020. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol 26: 4753–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kullar R Patel AP Saab S , 2021. COVID-19 in liver transplant recipients. J Clin Transl Hepatol 9: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. NIH, 2021. Special considerations in solid organ transplant, hematopoietic stem cell transplant, and cellular immunotherapy candidates, donors, and recipients. Available at: https://www.covid19treatmentguidelines.nih.gov/special-populations/transplant/. Accessed November 20, 2021.
- 126. Collaborative C , 2020. Delaying surgery for patients with a previous SARS‐CoV‐2 infection. Br J Surg 107: e601–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wijerathne PK Nanayakkara K Basnayake O Gunapala N Jayarajah U , 2020. Impact of COVID-19 on postgraduate surgical training: the trainees’ perspective. Sri Lanka Journal of Surgery 38: 53–55. [Google Scholar]
- 128. Jayasinghe R Jayarajah U Seneviratne S , 2020. Consensus on peri-operative surgical practice during the COVID-19 pandemic: an appraisal of the literature. Sri Lanka J Surg 38: 57–61. [Google Scholar]
- 129. Jayasinghe R Welikala N Gunaratne R Jayarajah U Dassanayake V Seneviratne S , 2021. Anaesthetic implications during the COVID-19 pandemic: an appraisal of the literature. Sri Lanka J Surg 39: 48–52. [Google Scholar]
- 130. COVIDSurg Collaborative, GlobalSurg Collaborative , 2021. Timing of surgery following SARS‐CoV‐2 infection: an international prospective cohort study. Anaesthesia 76: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Basnayake O Jayarajah U Gunawardena K Samarasekera DN , 2021. Impact of COVID-19 on postgraduate education and mental wellbeing of surgical trainees: a systematic review. Sri Lanka Journal of Surgery 39: 43–48. [Google Scholar]
- 132. COVIDSurg Collaborative C, GlobalSurg Collaborative , 2021. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br J Surg 108: 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. COVIDSurg Collaborative, GlobalSurg Collaborative , 2021. SARS‐CoV‐2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia 77: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. COVIDSurg Collaborative , 2021. Death following pulmonary complications of surgery before and during the SARS-CoV-2 pandemic. Br J Surg 108: 1448–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. COVIDSurg Collaborative , 2021. Preoperative nasopharyngeal swab testing and postoperative pulmonary complications in patients undergoing elective surgery during the SARS-CoV-2 pandemic. Br J Surg 108: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Glasbey JC et al. 2020. Elective cancer surgery in COVID-19–free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol 20: 01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. COVIDSurg Collaborative, GlobalSurg Collaborative , 2021. Effects of pre‐operative isolation on postoperative pulmonary complications after elective surgery: an international prospective cohort study. Anaesthesia 76: 1454–1464. [DOI] [PubMed] [Google Scholar]
- 138. Collaborative ISR, Group TARSW , 2021. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol 22: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. BJS Commission Team , 2021. BJS commission on surgery and perioperative care post-COVID-19. Br J Surg 108: 1162–1180. [DOI] [PubMed] [Google Scholar]
- 140. Fix OK et al. 2020. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology 72: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. El Kassas M et al. 2020. Liver transplantation in the era of COVID-19. Arab J Gastroenterol 21: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Aleem A Mahadevaiah G Shariff N Kothadia JP , 2021. Hepatic Manifestations of COVID-19 and Effect of Remdesivir on Liver Function in Patients with COVID-19 Illness. Proceedings (Bayl Univ Med Cent) 34: 473--477. [DOI] [PMC free article] [PubMed]
- 143. El Kassas M et al. 2020. Liver transplantation in the era of COVID-19. Arab J Gastroenterol 21: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Seneviratne SL Abeysuriya V De Mel S De Zoysa I Niloofa R , 2020. Favipiravir in COVID-19. Int J Progr Sci Technol 19: 143–145. [Google Scholar]
- 145. Mahase E, 2021. COVID-19: Pfizer’s Paxlovid Is 89% Effective in Patients at Risk of Serious Illness, Company Report. BMJ 375: n2713. [DOI] [PubMed]
- 146. Marjot T, Webb GJ, 2021. COVID-19 and Liver Disease: Mechanistic and Clinical Perspectives. 1–17. [DOI] [PMC free article] [PubMed]
- 147. Seneviratne SL Jayarajah U Abeysuriya V Rahman A Wanigasuriya K , 2020. COVID-19 vaccine landscape. J Ceylon Coll Phys 51: 120–131. [Google Scholar]
- 148. Cornberg M Buti M Eberhardt CS Grossi PA Shouval D , 2021. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol 74: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ruether DF. et al. , 2021. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol 20: 162–172. [DOI] [PMC free article] [PubMed]
- 150. Strauss AT. et al. , 2021. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl 27: 1852–1856. [DOI] [PMC free article] [PubMed]
- 151. Rabascall CX Lou BX Navetta-Modrov B Hahn SS , 2021. Effective use of monoclonal antibodies for treatment of persistent COVID-19 infection in a patient on rituximab. BMJ Case Reports CP 14: e243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mann R Sekhon S Sekhon S , 2021. Drug-induced liver injury after COVID-19 vaccine. Cureus 13: e16491. [DOI] [PMC free article] [PubMed] [Google Scholar]