Highlights
-
•
Integrated valorization of grape stalks generated lignin, fermentable sugars and nanocellulose.
-
•
For the first time, xylose was recover from grape stalk.
-
•
First cellulose nanocrystals recovered from grape stalk.
-
•
First proof of concept of use of a generated stream from an integrated valorization process.
-
•
Grape stalks fermentable sugars are consumed to produce bioethanol.
-
•
Biorefinery is a sustainable way for the processing of grape stalk.
Keywords: Grape stalk, Biosugars, Fermentation, Lignin, Cellulose nanocrystals
Abstract
White and red grape stalks biomass were fractioned to maximize its economic value by the production of fermentable sugars, as other value streams. High yields of extractives and lignin were first obtained, originating a biomass rich in cellulose and hemicellulose, which was subject to acid and enzymatic hydrolysis for production of fermentable sugars. Higher concentrations of sugars were obtained by enzymatic than by dilute acid hydrolysis. These biosugars were used for fermentation processes with Pichia stipitis and Saccharomyces cerevisiae. The presence of higher quantities of xylose favoured P. stipitis to produce higher ethanol yields than S. cerevisiae which is glucose lover. Cellulose nanocrystals were produced from the resulting biomass without monosaccharides. For the first time an integrated valorization of grape stalks followed by an application of one of the valorized streams is presented.
1. Introduction
Wine is an alcoholic beverage from fermentable carbon sources performed by yeasts mainly (but not exclusively) by strains of Saccharomyces cerevisiae (Jackson, 2008). It is the oldest and most economically important of all biotechnologies, that has been enjoyed from ancient times to modern times by many people for more than 7.5 thousand years (Vidigal & Rangel, 2021). Even today wine industry constitutes an important part of the economy in several regions of the world. According to OIV (The International Organization of Vine and Wine), the world production of wine was of ca. 292 Mhl in 2018, especially by countries such as Italy (54.8 Mhl), France (49.2 Mhl) and Spain (44.9 Mhl), which are the main wine producers in Europe and worldwide (OIV, 2020). Wine can be produced with honey, grains, rice, sugarcane and fruits usually grape. Grapes are one of the main agroeconomic crops in the world, with >77.8 million tons produced every year and ca. 57 % is used in the wine-making process (OIV, 2019, Zhu et al., 2020). The process of wine making process transforms sugar of the grapes into ethanol and involves the generation of wastes that can be divided into two main categories, solid and liquid wastes. Solid wastes of grape are the stalk, pomace, and lees. Grape stalk (5-7%) is obtained after the destemming process, while grape pomace (20-25%) after the pressing process and consists of processed skins (10-12%) and seeds (3-6%). On the other hand, lees (2-6%) are produced throughout the fermentation and sedimentation steps and contain dead yeast cells. Winery wastewater is a liquid waste (~0.5-14 L per liter of wine) result of the washing equipment and bottles and purges from the cooling process of the wine making process (Chowdhary et al., 2021, Oliveira and Duarte, 2016, Zacharof, 2017). Grape stalks are the skeletons of grape bunch and are composed mainly by lignocelluloses. Lignocelluloses contains polysaccharides such as cellulose and hemicelluloses and fiber as lignin, with other minor compounds and ash. Grape stalk waste can be up to 5-7% of the raw material used in processing and are not part of the vine-making process, but their production is directly linked to the winemaking process, so they are considered an important winery waste (Ahmad et al., 2020). This by-product is a potential waste for bioactive compounds and has a significant value for extraction of essential compounds, less thought out compared to pomace and seed. The common use of stalks is for animal feed and it is often dumped in landfills (Ahmad et al., 2020). Grape stalks are not intrinsically hazardous; however, they have high content of organic matter and the fact that production is concentrated in a period of the year poses potential pollution problems. According to recent studies, discharging of grape stalks to soil originates inhibition of the germination properties of soil, because of the biological oxygen demand, carbon, and phenolic compounds (Lafka et al., 2007). For this reason, environmental and economically sustainable grape stalk management should be a priority for the industry worldwide. Therefore, valorization of grape stalk has become an important field of research for minimizing environmental impact and generation of value added compounds. Reducing sugars are an example of compounds that can be obtained by processing complex carbohydrates (cellulose and hemicellulose). Some processes such as acid and enzymatic hydrolysis of polysaccharides have been studied and optimized in recent years (Egüés et al., 2013, Spigno et al., 2008, 2013). However, applying this process exclusively to produce ethanol with stalk sugars is unattractive in terms of costs and benefits. Therefore, other valued streams must be obtained to turn feasible to use these wastes in an integrated manner, which increases profits and attracts companies to discover these materials.
In this context, this work was focused on the valorization of two types of grape stalk (red and white) as biomass for biorefineries to produce second generation ethanol and value-added compounds (cellulose, hemicellulose, lignin, cellulose nanocrystals), through biotechnological interventions and green processing approaches, to improve both environmental and the economic sustainability.
2. Materials and methods
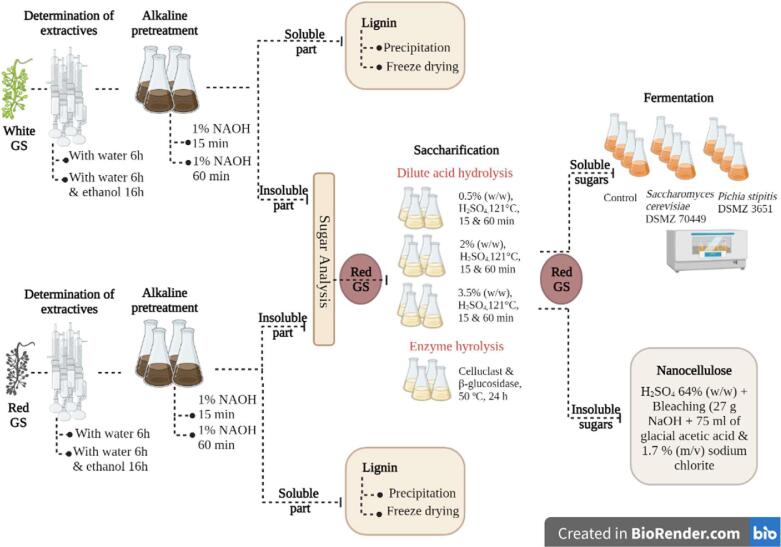
Red grape stalk (Vinhão variety) was provided by Quinta do Mascate (Braga, Portugal) and white grape stalk (Loureiro variety) from Sogrape company (Barcelos, Portugal). After their arrival they were kept at −80 °C in sealed plastic bags. Both varieties material were dried in an oven at 60 °C for 24 h and then milled with a small kitchen grinder. The milled material was called GS red or white. The process is illustrated in Fig. 1.
Fig. 1.
Schematic illustration of process used in this study. Created with BioRender.com.
2.1. Extractives fractioning
Extractives were separated from the two GS materials (red and white) owing to their solubility in water or neutral organic solvents following the protocols described in National Renewable Energy Laboratory (NREL/TP-510–42619) (Sluiter, Ruiz, et al., 2008). Two processes were tested to decrease the time of extraction and save production costs. The first process with deionized water was performed for 6 h, while the second one was the recommended by the protocol, which consisted of a successive solvent extraction with water and ethyl alcohol (Carlo Erba, Vel De Reul, France) by 6 and 16 h, respectively. Both were performed in a Soxhlet extractor equipment.
Extractives content solubilized by each solvent were determined using the difference between the mass values of the original material and solid residue after drying at 100 °C for 24 h. After extraction, the liquid phase (extract) was kept at −20 °C. The final solid material without extractives was called GSWE (grape stalk water extracted) and GSWEE (grape stalk water and ethanol extracted) red or white.
Results were reported as a percentage of the oven dry weight (ODW), according to equation (1):
| (1) |
where the weight flask plus extractives corresponds to the mass of the sample after treatment in grams (g), the weight flask to the mass of the empty flask in grams (g) and weight initial sample to the mass of the initial samples in grams (g).
2.2. Alkaline pretreatment of grape stalk (delignification)
Red and white grape stalks without extracts were pretreated following optimized parameters by other researchers (Egüés et al., 2013, Pujol et al., 2013). These were soaked with 1% NaOH (Eka, Bohus, Sweden) solution at the ratio of 1: 10 (solid: liquid) for 15 and 60 min in autoclave (100 °C). After this process, samples were centrifuged and cleaned with deionized water until the black color disappearance and achievement of neutral pH values. All supernatants were collected and join in a final solution called black liquor, which was stored at refrigerated conditions until further use. The resulting delignified solid material was further subject to drying at 100 °C during 24 h and was called delignified gape stalk (DGS). The delignification % was calculated according equation (2):
| (2) |
where the weight flask plus pretreated sample corresponds to the mass of the sample after alkaline treatment in grams (g), the weight flask to the mass of the empty flask in grams (g) and weight initial sample to the mass of the initial samples in grams (g).
2.3. Extraction of lignin derivatives from black liquors
The black liquors previously obtained were subject to the concentration of the soluble lignin using two methods. The first method tested was liquor freeze drying (Zhang et al., 2016), while the second method was a controlled precipitation of the lignin of the liquor by acidification with sulfuric acid (95%) (Honeywell, Seelze, Germany) until pH 2. After the pH reduction, solutions were kept for 24 h to allow the precipitation of lignin. The next steps were to centrifuge the samples at 8000 rpm for 20 min and wash them twice with distilled water to remove possible impurities such as sugar or inorganic particles. Finally, the samples were dried in an oven at 60 °C for 48 h (Domínguez-Robles et al., 2017). The yields and purity of the rich lignin fractions were calculated according to equations (3), (4):
| (3) |
where the weight lignin after dry means mass of dry lignin after treatment (g) and the weight lignin on black liquor is the mass of lignin on black liquor (g).
| (4) |
where the mass of the pure lignin are the grams of total pure lignin obtained after treatment and the mass of total lignin are the grams of total lignin in sample.
2.4. Dilute acid hydrolysis process
The effect of the type of acid, concentration and reaction times were tested in the saccharification process. DGS-red were subject to weak hydrolysis using H2SO4 (Honeywell, Seelze, Germany) and acetic acid (Sigma, Steinhelm, Germany) at low concentrations (0.5%, 2%, 3.5% w/w based on the dry matter), in a ratio solid to liquid of 1:10 and processed at 121 °C for two periods of time (15 and 60 min) in an autoclave. At the end of the treatment, samples were centrifuged at 5000 rpm during 10 min. The supernatant with the higher yield of sugars was used for fermentation and the pellet was dried and stored for further use for production of cellulose nanocrystals, which was called hydrolyzed grape stalk (HGS).
2.5. Enzymatic hydrolysis
Enzymatic hydrolysis was performed using the enzymatic Celluclast mixture preparation (Novozymes, Bagsvaerd, Denmark) and β-glucosidase (Sigma, Saint Louis, USA). The enzymatic activity units were estimated considering that red DGS had 43.79% of cellulose. Samples were suspended in citrate buffer (pH 5.0, 0.05 M) (Gama et al., 2015) with 20 FPU (filter paper units) of Celluclast and 40 IU (international unit) of β-glucosidase per gram cellulose (Ping et al., 2011). The hydrolysis was carried out at 50 °C for 24 h and with further centrifugation at 5000 rpm for 10 min (Verardi et al., 2012). A second hydrolysis was carried for 48 h to obtain a higher yield of sugars. Sugar rich supernatants were used for fermentation procedures as described below, while the pellet was used for production of cellulose nanocrystals and called HGS. For both hydrolysis processes (acid and enzymatic), the saccharification % were calculated according to equation (5):
| (5) |
where reducing sugars is the concentration of reducing sugars after saccharification in mg/ml and the cellulose content in pretreated substrate is concentration of sugar (glucose) content before saccharification in mg/ml.
2.6. Extraction of cellulose nanocrystals
HGS-red fractions were subject to two processes. The first was a strong acid hydrolysis with H2SO4 64% (w/w) at a ratio 1:20 (HGS:acid) performed at 50 °C, for 30 min, under vigorous and constant stirring (Flauzino Neto et al., 2013). The resulting suspension was diluted 10-fold with cold water to stop the hydrolysis and centrifuged twice for 10 min at 7000 rpm to remove excess acid. A second process was applied to bleach the enzyme resulting HGS-red and remove the color of the resulting fraction rich in cellulose nanocrystals. Bleaching was performed with an acetate buffer with 27 g NaOH + 75 ml of glacial acetic acid in 1 L of water and 1.7% (m/v) sodium chlorite. The process was performed by immersion of samples in both solutions at 80 °C and applying subsequently the process of hydrolysis, as explained before (Flauzino Neto et al., 2013). In both cases, the resulting precipitates were dialyzed with deionized water to remove non-reactive sulfate groups, salts and soluble sugars until a neutral pH value was obtained (5–7 days). The resulting dialyzed suspension was then sonicated at 70% intensity for 5 min in a VCX 130 ultrasonicator (Sonics & Materials, Newtown, USA), with the sample tubes immersed in an ice bath to prevent heating. Colloidal suspensions were stored in a refrigerator at 4 °C with a few drops of chloroform added to prevent any bacterial growth until freeze-drying. Freeze drying was carried out using a Vacuum Freeze Dryer (Model FT33, Armfield, UK), under a vacuum pressure of 100 millitorr, at −46 °C in the freezing chamber and 15 °C in the sample chamber. The cellulose nanocrystals were labeled CNC acid or CNC enzymatically. The cellulose nanocrystals were labeled CNC acid or CNC enzymatically. Cellulose nanocrystals % was calculated according to equation (6):
| (6) |
where the weight flask plus treated sample corresponds to the mass of the sample after freeze-drying process in grams (g), the weight flask to the mass of the empty flask in grams (g) and weight initial sample to the mass of the initial samples in grams (g).
2.7. Analytical methods
2.7.1. Sugars and lignin quantification methods
Monosaccharides (glucose, xylose, arabinose) that can be used to estimate cellulose (glucose) and hemicelluloses (xylose, arabinose) were quantified in the GS, GSWE, GSWEE and DGS fractions, following the protocols described in NREL/TP-510–42618 (Sluiter et al., 2011). Briefly, samples were hydrolyzed using 3 ml of 72% H2SO4 (Honeywell, Seelze, Germany) per 300 mg in a water bath set at 30 °C for 1 h, added with 28 ml of deionized water and autoclaved for 1 h 121 °C. After this time, the samples were vacuum filtered through a crucible and washed with boiling distilled water. The Klason lignin was determined by mass residue after drying at 100 °C. In addition, soluble lignin was determined on the combined filtrates by measuring the absorbance at 206 nm using a UV–vis spectrophotometer (Shimadzu-UV mini-1240) (Pujol et al., 2013). The values of Klason and soluble lignin were added to obtain the total lignin content. Glucose, xylose and arabinose were measured by an HPLC system that consisted of a Beckman Coulter (California, USA) unit equipped with Karat32 software coupled to detectors: Beckman Diode Array (Wavelenght 220 nm) and a refractive index detector (RI Detector K-2301, Knauer, Germany). Ion exchange Aminex HPX-87H Column (300 × 7.8 mm) (Bio-Rad) was maintained at 55 °C (CH-150 Column Oven; Eldex, California, USA) to analyze sugars and organic acids.
The mobile phase used was 13 mM sulphuric acid at a flow rate of 0.5 ml/min. Running time was 30 min, and the injection volume was 50 μL. Each sample was injected in duplicate. A standard curve was done with different concentrations for all sugars (0.05-2 mg/ml) and for fermentation inhibitors such as acetic acid, 5-hydroxymethylfurfural (HMF) and furfural (0.3125-5 g/L). Sugars and lignin concentrations were calculated according to equation (7),8,9 provided by the method NREL/TP-510–42618 (Sluiter et al., 2011) and inhibitors were calculated according to equation (10):
| (7) |
where C is the concentration in mg/ml of sugars as determined by HPLC, V is the volume of filtrate 86.73 ml, weight initial sample is the mass of the initial samples (mg).
| (8) |
where weight crucible plus insoluble lignin is the insoluble residue of lignin (g), weight crucible is the weight of empty crucible (g), weight initial sample is the mass of the initial samples in grams (g).
| (9) |
where UV abs is the average UV–Vis absorbance for sample at appropriate wavelength, volume filtrate is the volume of filtrate (86.73 ml), dilution is the dilution factor (if it is performed), Ɛ is the absorptivity of biomass at specific wavelength, weight initial sample is the mass of the initial samples in milligram, pathlength is the pathlength of UV–Vis cell in cm.
| (10) |
where y is the peak area, m is the slope of regression line, c is the intercept of the regression line with the y-axis. Dilution factors (D) and multipliers (M) may be used to calculate the final analyte concentration, if required.
2.7.2. Ash content determination
Ash content was determined according to NREL/TP-510–42622 (Sluiter, Hames, et al., 2008). Porcelain markers were placed in the muffle furnace at 575 °C for 4 h. Crucibles were removed from the furnace directly into a desiccator to cool down. The crucibles and two g of samples were weighed (to the nearest 0.1 mg) and placed in the muffle furnace at 575 °C for 24 h. The weight of the samples was recorded after cooling. Dried samples were used, and each sample was analyzed in duplicate. Ash was calculated according to following equation (11):
| (11) |
where weight crucible plus ash is the sample and crucible weight after treatment/g, weight crucible is the weight of empty crucible/g, weight initial sample is the mass of the initial samples in grams (g).
2.7.3. Fourier-transformed infrared spectroscopy (FTIR) analysis of lignin from grape stalks
Fourier transform infrared spectroscopy (FTIR) was successfully used to identify the precipitated lignin of GS, GSWE and GSWEE. Spectra were obtained using KBr pellets and recorded on an IRPrestige-21 infrared spectrophotometer (Shimadzu, Japan). Precipitated dried lignin of GS, GSWEE and GSWEE were ground and mixed with KBr (sample / KBr ratio = 1/100) to prepare the discs. The experiments were performed using a wavelength range of 500-4000 cm−1, resolution of 4 cm−1 and a total of 32 scans for each sample.
2.8. Fermentation trials using the biosugars for production of bioethanol
2.8.1. Microorganisms
Saccharomyces cerevisiae DSMZ 70449 and Pichia stipitis DSMZ 3651 yeast strains were used. Yeasts were obtained as a freeze-dried powder and the activation was done accordingly the indications of the supplier DSMZ in Potato Dextrose Broth (PDB) (Conda, Madrid, Spain), along three days at 25 °C and then passed to slants. Stock cultures of these strains were prepared in 30% glycerol water and stored at −80 °C for further use.
2.8.2. Preparation of the fermentation media
The supernatants obtained from both hydrolysis processes were tested as liquid media for fermentation processes. These were supplemented with nutrients, such as 3 g/l (NH4)2SO4 (Sigma, St. Louis, USA), 3 g/l K2HPO4 (Sigma, Steinheim, Germany), 1 g/l MgSO4, 5 g/l yeast extract, 3.5 g/l peptone. To obtain sterile media for fermentations, they were first autoclaved at 121 °C for 15 min in Erlenmeyer’s flasks sealed with cotton caps covered with aluminum paper. Control media was prepared with 10 g/l glucose, 10 g/l xylose, 3 g/l (NH4)2SO4 (Sigma, St. Louis, USA), 3 g/l K2HPO4 (Sigma, Steinheim, Germany), 1 g/l MgSO4, 5 g/l yeast extract, 3.5 g/l peptone.
2.8.3. Preparation of the yeast inoculums, fermentation process and sampling along incubation time
Overnight yeast cultures were grown in PDB at 25–30 °C. These were inoculated in the previously prepared fermentation media, to standardize an initial cell concentration of 1x105 CFU/ml. The inoculated Erlenmeyer’s were incubated at 25-30 °C for 7 days. Three ml of samples were taken at times 0, 1, 3 and 7 days, 1 ml for evaluation of the yeast growth and 2 ml for pH analyses and glucose, xylose and ethanol quantification by HPLC, according to the methods described previously in section 2.7.1. The yeast growth was evaluated by performing decimal dilutions in peptone water 0.1% (w/v) and plating in potato dextrose agar by spread pour plating technique. Plates were incubated during 24 h at 25-30 °C and the log CFU/ml were estimated. Ethanol yield, fermentation efficiency and ethanol productivity were calculated according to following equations:
| (12) |
where △P/△S stands for product (g/L of ethanol) produced per amount of substrate (g/L of glucose) consumed.
| (13) |
where the amount of ethanol produced is the amount of product (g/L of ethanol) produced, amount of sugar consumed is the amount of substrate (g/L of sugars) consumed and 0.511 is the conversion factor of glucose or xylose to ethanol.
2.9. Statistics
Each trial was run in triplicate and values are reported as mean ± SD. IBM SPSS® 19.0 (SPSS, Chicago, IL, USA) software for Windows was used to perform statistical analysis of variance (ANOVA) followed by Tukey’s post hoc test (for means discrimination) to evaluate the significance of dilute acid hydrolysis and T-test was used to perform statistical analysis to evaluate the significance of ex-red and ex-white. Variance homogeneity was confirmed according to Levene’s test. All significance tests were conducted at P ≤ 0.05.
3. Results and discussion
3.1. Composition of the red and white grape stalks
Two methods were tested for the removal of extractives from red and white grape stalks biomass, nevertheless no differences between both methods were detected (p > 0.05). In terms of varieties, the white presented a higher quantity of extractives. Extractives are water-soluble material, such as non-structural sugar, terpenes, etc. and ethanol soluble material including chlorophyll, waxes, etc. These values are not in agreement with other research works (Pujol et al., 2013), which obtained ca. 21% red grape stalk water extractives and ca. 28% of ethanol extractives. Also, the water extractives of red grape stalk are ca. 36.5% (Spigno et al., 2013).
The information available for the composition of grape stalks is scarce. The different methods generate different values of the polysaccharides and lignin in some cases. There is significant difference between cellulose quantities of GS-red and white varieties (p < 0.05). Cellulose quantities are higher in red than white variety, which is accordingly to the found in literature (Spigno et al., 2013). In addition, the extractives are higher in GS-white, which indicates that some cellulose maybe is lost during the process of extraction with water and ethanol. For hemicellulose and arabinose quantities, no differences were found between both varieties and extractives separation methods. Total lignin (Klason or insoluble lignin and soluble lignin) content was lower in GS-red than in GS-white and are according to the found in literature between 40 and 47.3% (Ping et al., 2011, Spigno et al., 2008). Nevertheless, other authors showed lower values around 17% (Prozil et al., 2012). These significant differences can be attributed first to the protocols used to quantify, to the interpretation of what is soluble or Klason lignin and finally to variations in the source variety and type of grape stalk. The ash content of GS agrees with the reported for grape stalks ca. 3.9% (Ping et al., 2011). As can be seen, there is a decrease on ash content along the processing and red stalks present the higher content. This can be due to the significant amounts of potassium and other inorganic elements that are present (Pujol et al., 2013). Grape stalk has very low ash content which is a potential benefit to increase the efficiency of the hydrolysis (Bin & Hongzhang, 2010). The carbohydrate composition shows that glucose is the most abundant neutral monosaccharide and xylose is the second most abundant monomer followed by arabinose. Red GS has more sugar available than white GS (p < 0.05).
Concerning the effect of the method used to separate the extractives, the water extraction during 6 h showed to be an alternative method to the advised by the standard NREL/TP-510-42619 (Sluiter, Ruiz, et al., 2008), since it does not influence the content on cellulose and hemicellulose, which will be the source of sugars for fermentation. The ethanol used in the advised method certainly extracts more dyes, waxes but in this specific case does not bring any extra function than using only water (Table 1).
Table 1.
Composition of the GS extractives (ODW, mean ± SD) in samples subject to the two types of extractives processes tested, using water (GSWE) or using the advised method by the standard protocol water followed by ethanol (GSWEE). Composition of samples without extractives in terms of their polysaccharides, lignin and ash contents.
| Red grape stalk |
White grape stalk |
|||
|---|---|---|---|---|
| Compounds | GSWE | GSWEE | GSWE | GSWEE |
| Extractives (%, w/w) | 56.29 ± 3.46 | 58.86 ± 4.43 | 75.08 ± 2.11 | 76.28 ± 3.89 |
| After extractives removal | ||||
| Cellulose (glucose) | 19.43 ± 0.32 | 10.50 ± 0.63 | 5.19 ± 0.51 | 8.55 ± 0.20 |
| Hemicellulose | 8.36 ± 0.73 | 8.01 ± 0.68 | 7.76 ± 0.57 | 8.22 ± 0.34 |
| Xylose | 7.55 ± 0.52 | 7.20 ± 0.55 | 7.41 ± 0.52 | 7.54 ± 0.26 |
| Arabinose | 0.81 ± 0.21 | 0.81 ± 0.13 | 0.65 ± 0.05 | 0.68 ± 0.08 |
| Total lignin | 39.16 ± 2.95 | 48.47 ± 1.88 | 54.62 ± 5.35 | 60.22 ± 0.98 |
| Klason lignin | 37.81 ± 2.91 | 45.39 ± 1.81 | 53.70 ± 5.23 | 56.12 ± 0.98 |
| Soluble lignin | 1.35 ± 0.04 | 3.08 ± 0.07 | 0.92 ± 0.12 | 4.10 ± 0.005 |
| Ash (%, w/w) | 4.90 ± 0.03 | 2.27 ± 0.09 | 3.12 ± 0.08 | 1.54 ± 0.28 |
* All data are yields of components (g) per 100 g of oven-dried grape stalk, GSWE: Grape stalk water extracted, GSWEE: Grape stalk water and ethanol extracted.
3.2. Effect of the alkaline pretreatment and different times of reaction in the delignification process
The red and white GSWE were pretreated with 1% w/v NaOH solution at the ratio of 1: 10 (solid: liquid) for 15 and 60 min in autoclave. The delignification % (or % of solubility) of GSWE red and white samples were respectively 47.81%, 42.04%, when using 15 min, and 50.61% and 45.58%, when using 60 min of processing time. No significant differences were obtained between processing times and grape types (p > 0.05), but the major proportion was obtained in GSWE-red with 60 min. The samples with higher % of delignification were subject to sugars analyses to observe what remained after delignification. The higher quantity of sugars was detected in delignified samples from red variety (Table 2). For this reason, DGS-red was chosen for the enzymatic and dilute acid hydrolysis process and production of bio-sugars with potential for fermentation purposes.
Table 2.
Data concerning delignification and lignin fractions obtained. Delignification % of the GSWE-red and GSWE-white samples and sugar analysis of GSWE treated with NaOH 1% (w/v) tested with two reaction times 15 and 60 min. Yields (%) and purity of lignin obtained from grape stalks without extractives using water (GSWE) or water plus ethanol (GSWEE) procedures.
| Delignification process | ||||
| Time of process | % Delignification | |||
| GSWE-red | GSWE-white | |||
| 15 min | 47.81 ± 2.61 | 42.04 ± 2.02 | ||
| 60 min | 50.61 ± 1.62 | 45.58 ± 0.86 | ||
| Compounds (monosaccharides) | % Sugars in DGS samples | |||
| with 60 min | ||||
| Glucose | 43.79 ± 5.92 | 26.33 ± 6.58 | ||
| Xylose | 16.38 ± 2.50 | 9.54 ± 1.42 | ||
| Arabinose | 0.38 ± 0.07 | N.D. | ||
| Total | 60.55 | 35.87 | ||
| Dried lignin fractions | ||||
| Material | Yield (%, w/w) | Purity (%, w/w) | ||
| Freeze dry | Precipitation | Freeze dry | Precipitation | |
| GSWE-red | 9.23 | 1.4 | 40.84 | 70.57 |
| GSWEE-red | 1.48 | 1.15 | 30.08 | 67.91 |
| GSWE-white | 9.47 | 1.98 | 56.02 | 73.73 |
| GSWEE-white | 1.42 | 4.27 | 39.5 | 67.01 |
* N.D.: Not detected; Sugars data are yields of components (g) per 100 g of oven-dried grape stalk. GSWE: Grape stalk water extracted, GSWEE: Grape stalk water and ethanol extracted.
3.3. Recovered lignin from grape stalks
3.3.1. Red and white varieties differences in lignin contents and impact of extractives removal processes
The delignification was performed under the eco-friendly and cost-effective alkaline method with NaOH solution, in which the ether bonds break due to the NaOH and then lignin degrades gradually in the form of alkali lignin (Ricardo Soccol et al., 2011). Black color comes from lignin compounds colored by alkali and dissolved to liquor. Afterwards, two extraction methods for lignin were tested i.e., precipitation or freeze drying. Results showed that freeze-dried extracts from white GS generated higher lignin yields (9.47%) comparing with precipitation process that had (1.98%) (Table 2), but the precipitation method generates purer lignin. In addition, a higher yield and purity of lignin was obtained from water extracted materials in contrast with water and ethanol derived ones, which indicates that lignin is lost during the process with water and ethanol.
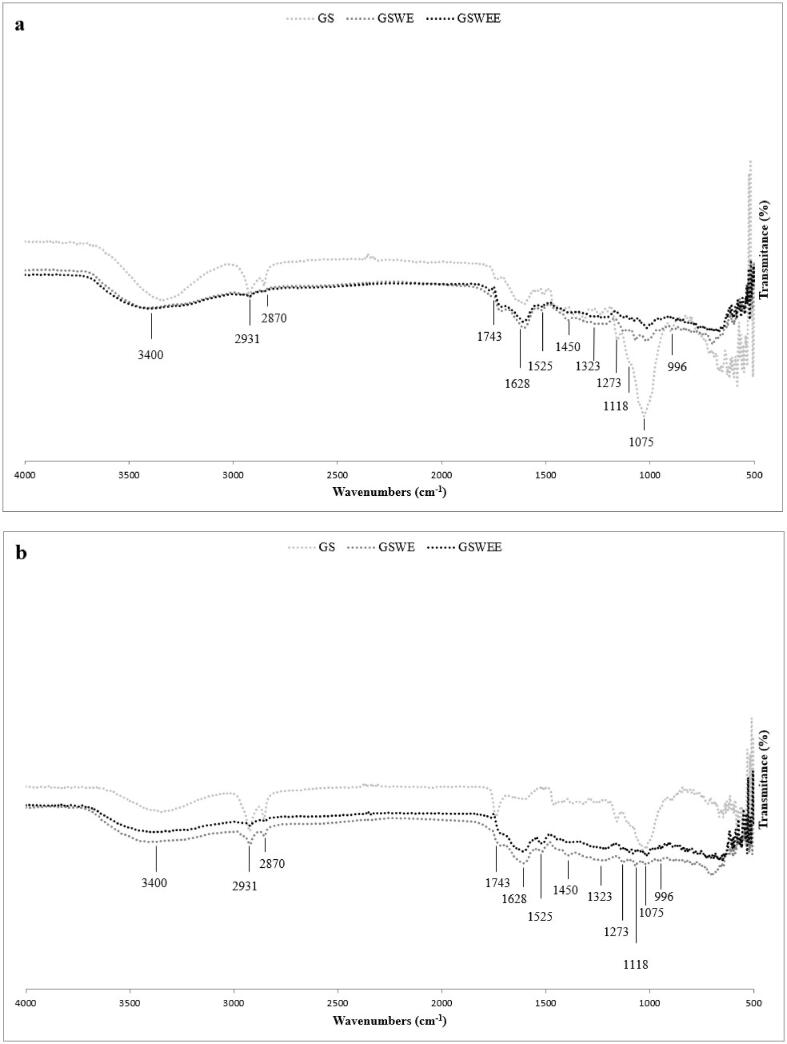
The lignin obtained with precipitation was characterized with FTIR spectroscopic analysis. FTIR spectroscopy is a versatile, rapid, and reliable technique for lignin characterization, and using this technique, the phydroxyphenyl, guaiacyl, and syringyl units, methoxyl groups, carbonyl groups, and the ratio of phenolic hydroxyl to aliphatic hydroxyl groups can be identified.
The spectra in Fig. 2 show multiple adsorption peaks, suggesting the material's complexity. Hidroxilo (OH) vibration modes are indicated by the broad peak at around 3400 cm−1. The two sharp peaks at 2931 cm−1 and 2870 cm−1 are due to the asymmetric and symmetric vibrations of C–H in olephinic chains, respectively, while the peak at 1743 cm−1 is attributed to the carbonyl C = O in ester groups. The existence of lignin is indicated by the presence of typical lignin bands at 1323 cm−1, 1273 cm−1, and 1525 cm−1, being the first two bands attributed to skeletal vibrations of syringil and guayacil aromatic rings with CO stretching, respectively, and the last one to aromatic skeletal vibrations (Pujol et al., 2013). The band at 1450 cm−1 associated with the deformation vibration of C–H in the aromatic ring of lignin fragments is less dense. The existence of polyphenols is indicated by the characteristic band at 2931 cm−1, and polysaccharide peaks can be found at 1075 cm−1, 1118 cm−1, and 996 cm−1 (Kacuráková, 2000, Pujol et al., 2013). Initially, comparison between the different FTIR spectra of GSWE and GSWEE shows that the above identified peaks are present in all fractions. Slight differences in the density of the strip can be observed in the white grape stalk compared to the red grape stalk. For the initial grape stalk, the most significant differences are observed in bands attributed to polysaccharides (1075 cm−1, 1118 cm−1 and 996 cm−1) and lignin (1525 cm−1 and 1450 cm−1), which show lower peak densities than extracted grape stalk.
Fig. 2.
FTIR analysis of initial grape stalks (GS) material, and lignin’s from grape stalks without extractives using water (GSWE) and water plus ethanol (GSWEE) of a) red and b) white grape stalks using precipitation process.
3.3.2. Biosugars production from the delignified materials
3.3.2.1. Dilute acid hydrolysis biosugars production
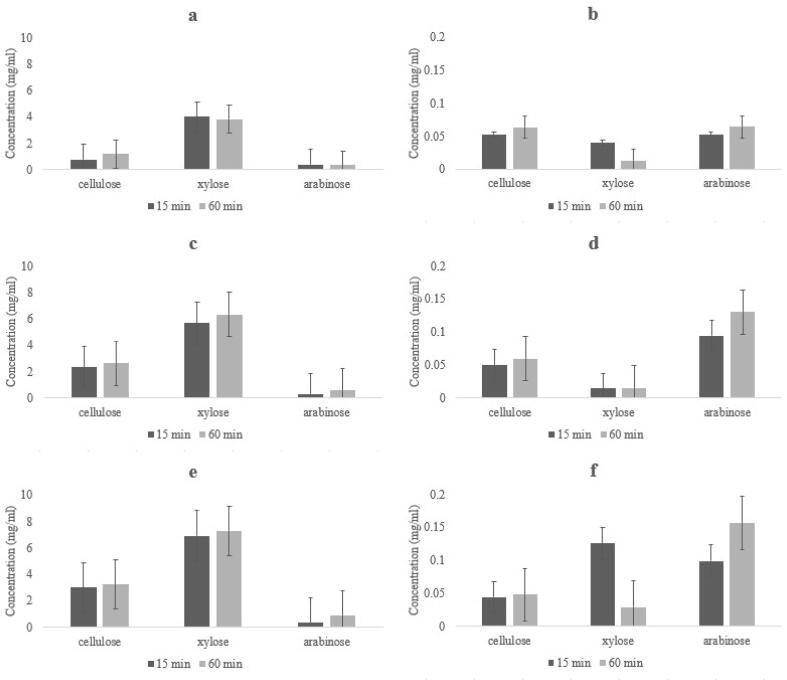
Sulphuric and acetic acids were tested at three concentrations 0.5, 2 and 3.5%, at 15 and 60 min of reaction time (Fig. 3). Different sugar concentrations were obtained for the two types of acids (p < 0.05), but no differences between the used concentrations (p > 0.05). Maximum glucose, xylose and arabinose concentrations were obtained from hydrolysis with sulphuric acid at the higher concentration of 3.5% and is according with the saccharification rates (Table 3). Since cellulose is the major contributor of glucose and is present at higher concentrations, the % of saccharification is always lower than the others, which are present in lower quantities such as xylose and arabinose which are almost all released. With these concentrations it was possible to obtain ca. 7 g/L of xylose and ca. 3 g/L of glucose. The time of hydrolysis was also important, and it was possible to observe that with 60 min, the higher content of sugars was obtained with values of xylose with 7.29 g/L, glucose with 3.22 g/L of and arabinose with 0.91 g/L. The results were compared with some literature works reporting sugars concentrations of grape stalk obtained for sulphuric acid. The results are not in agreement with reported by Egüés et al., 2013 (glucose and xylose ca. 12–9 g/L respectively). These differences can be due to the hydrolysis time in autoclave which in the case of Egüés et al. (2013) was 90 min (Egüés et al., 2013).
Fig. 3.
Monosaccharides composition and concentrations (means ± SD) in red DGS samples after dilute acid hydrolysis using two times of processing and 0.5 % (a and b), 2% (c and d) and 3.5% (e and f) of sulphuric acid and acetic acid, respectively.
Table 3.
Saccharification rates (%) of cellulose and hemicellulose in glucose, xylose and arabinose and production of inhibitors after acid hydrolysis procedures, as well for enzymatic hydrolysis (means ± SD). Remaining cellulose and hemicellulose in samples after hydrolysis processes and conversion rate (%) of cellulose and hemicellulose in glucose and xylose.
| Acid hydrolysis | |||||
|---|---|---|---|---|---|
| Sugars/Compounds | Acid concentration (%) | Saccharification rates (%) |
|||
| Sulphuric acid |
Acetic acid |
||||
| 15 min | 60 min | 15 min | 60 min | ||
| Glucose | 0.5 | 1.6 ± 0.03 | 2.4 ± 0.04 | 0.1 ± 0.01 | 0.12 ± 0.08 |
| 2 | 4.8 ± 0.01 | 5.4 ± 0.02 | 0.1 ± 0.05 | 0.12 ± 0.05 | |
| 3.5 | 6.1 ± 0.03 | 6.6 ± 0.09 | 0.08 ± 0.03 | 0.1 ± 0.09 | |
| Xylose | 0.5 | 22.1 ± 0.6 | 21.0 ± 0.06 | 0.22 ± 0.01 | 0.05 ± 0.01 |
| 2 | 31.2 ± 0.02 | 34.7 ± 0.03 | 0.05 ± 0.07 | 0.11 ± 0.05 | |
| 3.5 | 38.0 ± 0.09 | 40.1 ± 0.01 | 0.71 ± 0.03 | 0.16 ± 0.07 | |
| Arabinose | 0.5 | 12.5 ± 0.05 | 32.2 ± 0.04 | 4.47 ± 0.01 | 5.4 ± 0.03 |
| 2 | 26.8 ± 0.01 | 52.7 ± 0.07 | 8.04 ± 0.04 | 14.3 ± 0.05 | |
| 3.5 | 27.7 ± 0.06 | 81.3 ± 0.01 | 8.04 ± 0.03 | 11.6 ± 0.08 | |
| HMF | 0.5 | 0.083 ± 0.97 | 0.077 ± 1.46 | 0.086 ± 1.23 | 0.088 ± 0.29 |
| 2 | 0.084 ± 0.11 | 0.078 ± 1.94 | 0.087 ± 0.17 | 0.090 ± 0.55 | |
| 3.5 | 0.087 ± 1.77 | 0.097 ± 1.95 | 0.089 ± 0.56 | 0.094 ± 0.75 | |
| Acetic acid | 0.5 | 1.1 ± 97.75 | 1.2 ± 138.50 | 0.3 ± 193.08 | 0.4 ± 177.69 |
| 2 | 1.3 ± 43.76 | 1.6 ± 4.61 | 1.5 ± 216.08 | 1.8 ± 176.04 | |
| 3.5 | 1.5 ± 7.46 | 2.5 ± 196.45 | 2.7 ± 166.78 | 2.9 ± 153.96 | |
| Enzymatic hydrolysis | ||
| Procedure | Remaining concentration of Cellulose (g/L) | Remaining concentration of Hemicellulose (g/L) |
| GSWE-red/ First | 6.06 ± 0.06 | 8.08 ± 0.06 |
| GSWE-red/ Second | 2.15 ± 0.39 | 0.004 ± 0.15 |
| Process | Glucose (%) | Xylose (%) |
| First saccharification | 25.97 | 82.73 |
| Second saccharification | 12.9 | 3.27 |
| Total (some of two processes) | 38.87 | 86.00 |
Concluding the use of 3.5% of sulfuric acid and 60 min of hydrolysis time were the best conditions to obtain a high saccharification and so these conditions were chosen to produce the media broths for fermentation used in the next section.
Acid hydrolysis is a common process to obtain sugar monomers that can be used e.g., in fermentation. Here, acid hydrolysis will involve breaking down the polysaccharide structure. In this study, sulphuric and acetic acids were chosen as suitable for weak/dilute acid hydrolysis. The use of these acids is related with the fact that sulphuric acid is the most used catalysts for hydrolysis of lignocellulosic biomass (Lenihan et al., 2010) and acetic acid is already present in lignocellulosic biomass in the form of acetyl groups on the hemicellulose. Moreover, acetic acid can work as a co-solvent (Huber et al., 2006, Sun et al., 2009).
On the other hand, the cost of highly concentrated acid treatment on biomass and the need for recovery limit the process of sugars released by concentrated acid hydrolysis. Another disadvantage is the effect of high acid concentration and processing time, which can lead to the formation of HMF and furfural due to the degradation of complex polysaccharides (Taherzadeh et al., 2000). In this dilute hydrolysis, inhibitor compounds can be formed by hydrolysis of hemicellulose into xylose and further dehydration into acetic acid, furfural and HMF (Klinke et al., 2004, Lu et al., 2009). The concentrations of inhibitors produced are also presented in Table 3. Furfural was not detected and as it was expected, the inhibitors increased with increasing sulphuric and acetic acid concentration (van Spronsen et al., 2011). According to this, the results showed that the inhibitors (acetic acid and 5-HMF) concentrations were not significantly different (P > 0.05) when using 15 and 60 min as processing times. The concentration of acetic acid and HMF was increased with the 60 min of reaction time and 3.5% acid concentration. These compounds inhibit the growth of yeast cells and subsequent fermentation in a dose-dependent manner which is 4 g/L, 2 g/L, 1.86 g/L, respectively (Delgenes et al., 1996, Favaro et al., 2013). And the values obtained are at lower concentrations than these ones, so the media rich in these sugars can be used as fermentation media.
3.3.2.2. Enzymatic hydrolysis
In this experiment, polysaccharides of DGS were hydrolyzed to monosaccharides with commercial enzymes i.e., celluclast and β-glucosidase. Celluclast and β-glucosidase were chosen to achieve high cellulose conversion and prevent cellulose accumulation. Cellulase breaks the branched cellulose chains, while the cellobiose formed is broken down with the action of ß-glucosidases producing glucose. The combined action of both enzymes significantly reduces the time required for hydrolysis (García-Cubero et al., 2010). The main carbohydrates after enzymatic hydrolysis were glucose and xylose.
Higher concentrations of sugars were obtained by enzymatic hydrolysis than by dilute acid hydrolysis, with values of glucose with 6.06 g/L and xylose with 8.08 g/L. Also, saccharification % was calculated and the higher values were obtained for xylose than glucose, 82.73% and 25.97%, respectively (Table 3). The results show that xylose conversion was higher than glucose conversion in the DGS-red. Xylose recovery with these enzymes has not been mentioned before for grape stalk. Nevertheless, cellulose-to-glucose conversion agrees with the reported for grape stalk by Ping et al. (2011), which was ca. 25% (Ping et al., 2011). Hemicellulose conversion to five basic monosaccharides (xylose, mannose, glucose, arabinose and galactose) is much higher than cellulose conversion, this can be because of the chain length in hemicellulose is much shorter (Andersen, 2007, Horn et al., 2012). According to literature, increasing of reaction time can be recover more glucose (Han et al., 2012, Li et al., 2012). Considering this, a second enzymatic hydrolysis process was carried out with more time. Anyway, the amount of sugar seems too low to economically exploit the stalks for industrial fermentation, but this deserves further research to boost that concentration.
3.4. Fermentation procedures using the biosugars
3.4.1. Fermentation profiles
Growth curves and sugar consumption of the yeast species in control, acid dilute, and enzymatic sugars media were evaluated. Through all series of fermentations, P. stipitis and S. cerevisiae were also evaluated on their ability to produce ethanol and consequent consumption of sugars. In control media, both yeasts behaved differently, especially in what concerns the consumption of xylose, which was higher for P. stipitis. Also, this strain produced slightly more ethanol than S. cerevisiae, but with similar profiles of glucose. In the enzymatic sugars media, the fermentations were like the observed in control media, in what concerns glucose consumption and production of ethanol for both strains, but in acid dilute sugars media, strains had a slower metabolic activity, maybe because of the presence of lower quantities of sugars, with low ethanol quantities production.
The same happened with P. stipitis, which showed to be well adapted to enzymatic sugars media than dilute acid media and agrees with the reported elsewhere (Gonçalves et al., 2016, Groves, 2009). To produce ethanol, the fermentation efficiency was 42.41% for P. stipitis compared to the S. cerevisiae 26.71% in the enzymatic sugars media. The ethanol productivity and yields in the enzymatic media was doubled, when compared to acid dilute media. The results show that fermentations of grape stalk can be optimized based on the enzymatic media with P. stipitis to obtain significant ethanol yields, since this strain is able to use xylose and glucose at the same time for production of ethanol.
The total sugars concentration was determined as the sum of monosaccharide (glucose and xylose) found in the dilute acid and enzymatic sugars media. A maximum total sugars concentration ca. 14 g/L was achieved with enzymatic medias. This followed with ca. 9 g/L with dilute acid medias. The ethanol yields of both media were lower than the corresponding theoretical yields for glucose fermentations (0.51 g/g) (Table 4). Nevertheless, the enzymatic media results were always higher than in acid media. This can be the effect of having low sugar concentration or fermentation time (Agbogbo et al., 2007, Groves, 2009). However, considering the different media and fermentation results, the P. stipitis enzyme media was determined to be the best media as substrate (14.42 g/L) owing to the results on the ethanol fermentation yield (0.22 g/L).
Table 4.
Ethanol yields obtained by P. stipitis and S. cerevisiae using as culture media added with sugars obtained from dilute acid and enzymatic hydrolysis as fermentation media.
| Microorganisms | Species and type of media | Initial total concentration of sugars (g/L)* | Final total concentration of sugars (g/L) | Ethanol yield (g/L) | Fermentation efficiency (%) | Ethanol productivity (g/L/h) |
|---|---|---|---|---|---|---|
| P. stipitis | Control | 20.17 | 0.81 | 0.24 | 46.08 | 0.65 |
| Enzyme | 14.42 | 3.15 | 0.22 | 42.41 | 0.39 | |
| Dilute acid | 9.10 | 1.63 | 0.13 | 26.20 | 0.14 | |
| S. cerevisiae | Control | 20.22 | 6.74 | 0.21 | 40.94 | 0.40 |
| Enzyme | 14.26 | 3.71 | 0.14 | 26.71 | 0.21 | |
| Dilute acid | 9.65 | 1.38 | 0.005 | 8.99 | 0.05 |
*All sugars included (glucose, xylose and arabinose).
3.5. Cellulose nanocrystals
Hydrolyzed grape stalks fractions were processed without bleach process to produce CNC. The yields of CNC obtained were 5.79% and 1.20% from enzymatic and acid processes, respectively. At the end of second enzyme hydrolysis, samples were bleached to remove color, because samples were not able to be analyzed by DLS. After the bleaching process, samples were without color, but they lost their whiteness using the sulfuric acid solution. After dialysis, the use of ultrasonication allowed the defibrillation of cellulose fiber with the hydrodynamic forces of the ultrasound. Samples were analysed by DLS to determine the CNC particle sizes in suspension and charge. The sizes obtained were high due to the fact that nanocrystals lengths were measured using a DLS scatter, which is dynamic and detect agglomeration events that may occur in solution. However, small CNCs were obtained with ca. 294 nm. The polydispersity index (PI) measures the difference in particle size distribution. A high PI identifies the existence of particle families of various sizes, which may indicate aggregation (Hanaor et al., 2012). In general, all samples showed PI values much higher than 0.3, indicating the polydisperse distribution of CNCs. On the other hand, the zeta potential (ZP) can give an indication of whether repulsion will occur between adjacent, similarly charged particles in the dispersion. At the point when ZP is high (regardless of whether positive or negative qualities) it implies stability between particles, while when the potential is low, the particles will in general coagulate/flocculate as the attraction exceeds the repulsion in the dispersion. In CNCs extracted from grape stalks, ZP values were about 30 mV, indicating moderate stability. Since sulfuric acid destroys amorphous regions in cellulose fibers, leaving only very normal crystal regions remaining, the values were negative, resulting in negatively charged sulfonated nanoparticles.
Several researchers investigated shapes and size distributions of cellulose nanocrystals obtained in hydrolysis of different type of fiber using acid or enzymes (Abraham et al., 2011, Filson et al., 2009, Mandal and Chakrabarty, 2011, Oksman et al., 2011, Tsukamoto et al., 2013). However, this study is reporting for the first time, that biomass from grape stalk is a source of cellulose nanocrystals, which potentially may add value to grape stalk.
4. Conclusions
Similar quantities of extractives and in high quantities were obtained in white variety GS. These extractives could be a good source of phenolic compounds known for their applications as antioxidants and antimicrobial. These properties could be investigated in the future. The alkaline pretreatment of grape stalks performed for 60 min showed to be the best method for delignification. Pure lignin was recovered in high quantities from water extrated materials and using the precipitation process. Nowadays, lignin is a very popular compound known for its antioxidant, material properties for packaging, as other applications. Delignified red GS material was selected to follow the integrated process, owing to the higher sugar quantities. For dilute acid process, the best conditions were using 3.5% sulphuric acid during 60 min, to the recovery of structural reducing sugars from red GS, because of the sugars yields and lower production of inhibitors. Comparing acid and enzymatic, the last one was the best process for recovery of fermentable monosaccharides. Ethanol was successfully produced using via S. cerevisiae and P. stipitis fermentation. Pichia stipitis produced higher ethanol yields than the S. cerevisiae in enzymatic sugars derived media. The resulting biomass from hydrolysis already without sugars was successfully purified to cellulose nanocrystals. It is very important to find and present new raw materials for the use of renewable energy producing sectors, to guarantee a sustainable future. This study suggests that the integrated valorization of grape stalks allows to give a second life to, while also contributing to the reduction of production costs and residual quantity. For the first time, an integrated evaluation of grape stalks has been carried out and the use of the resulting biosugars (cellulose, hemicellulose), has proven the concept of producing different compounds (lignin, cellulose nanocrystals) in biorefinery. Thus, it contributes to the development of new trade opportunities for the respective processing industries and follows the current worldwide trend for recycling of agro-industrial waste.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding for the preparation of this work was provided by: MultiBiorefinery project (POCI-01-0145-FEDER-016403).
References
- Abraham E., Deepa B., Pothan L.A., Jacob M., Thomas S., Cvelbar U., Anandjiwala R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydrate Polymers. 2011;86(4):1468–1475. doi: 10.1016/j.carbpol.2011.06.034. [DOI] [Google Scholar]
- Agbogbo F.K., Coward-Kelly G., Torry-Smith M., Wenger K., Jeffries T.W. The effect of initial cell concentration on xylose fermentation by Pichia stipitis. Applied Biochemistry and Biotechnology. 2007;137–140(1–12):653–662. doi: 10.1007/s12010-007-9086-7. [DOI] [PubMed] [Google Scholar]
- Ahmad B., Yadav V., Yadav A., Rahman M.U., Yuan W.Z., Li Z., Wang X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Science of The Total Environment. 2020;719:137315. doi: 10.1016/j.scitotenv.2020.137315. [DOI] [PubMed] [Google Scholar]
- Andersen, N. (2007). General rights Enzymatic Hydrolysis of Cellulose Experimental and Modeling Studies.
- Bin Y., Hongzhang C. Effect of the ash on enzymatic hydrolysis of steam-exploded rice straw. Bioresource Technology. 2010;101(23):9114–9119. doi: 10.1016/j.biortech.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Chowdhary P., Gupta A., Gnansounou E., Pandey A., Chaturvedi P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environmental Pollution. 2021;278:116796. doi: 10.1016/j.envpol.2021.116796. [DOI] [PubMed] [Google Scholar]
- Delgenes J.P., Moletta R., Navarro J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme and Microbial Technology. 1996;19(3):220–225. doi: 10.1016/0141-0229(95)00237-5. [DOI] [Google Scholar]
- Domínguez-Robles J., Sánchez R., Espinosa E., Savy D., Mazzei P., Piccolo A., Rodríguez A. Isolation and Characterization of Gramineae and Fabaceae Soda Lignins. International Journal of Molecular Sciences. 2017;18(2):327. doi: 10.3390/ijms18020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egüés I., Serrano L., Amendola D., de Faveri D.M., Spigno G., Labidi J. Fermentable sugars recovery from grape stalks for bioethanol production. Renewable Energy. 2013;60:553–558. doi: 10.1016/j.renene.2013.06.006. [DOI] [Google Scholar]
- Favaro L., Basaglia M., Trento A., van Rensburg E., García-Aparicio M., van Zyl W.H., Casella S. Exploring grape marc as trove for new thermotolerant and inhibitor-tolerant Saccharomyces cerevisiae strains for second-generation bioethanol production. Biotechnology for Biofuels. 2013;6(1):168. doi: 10.1186/1754-6834-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filson P.B., Dawson-Andoh B.E., Schwegler-Berry D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chemistry. 2009;11(11):1808. doi: 10.1039/b915746h. [DOI] [Google Scholar]
- Flauzino Neto W.P., Silvério H.A., Dantas N.O., Pasquini D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue – Soy hulls. Industrial Crops and Products. 2013;42:480–488. doi: 10.1016/j.indcrop.2012.06.041. [DOI] [Google Scholar]
- Gama R., van Dyk J.S., Pletschke B.I. Optimisation of enzymatic hydrolysis of apple pomace for production of biofuel and biorefinery chemicals using commercial enzymes. 3. Biotech. 2015;5(6):1075–1087. doi: 10.1007/s13205-015-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cubero M.T., Marcos M., Bolado S., Coca M., González-Benito G. Optimization of operating conditions in enzymatic hydrolysis of pretreated lignocellulosic materials. Chemical Engineering Transactions. 2010;21:1285–1290. doi: 10.3303/CET1021215. [DOI] [Google Scholar]
- Gonçalves F.A., Ruiz H.A., Silvino dos Santos E., Teixeira J.A., de Macedo G.R. Bioethanol production by Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis from delignified coconut fibre mature and lignin extraction according to biorefinery concept. Renewable Energy. 2016;94:353–365. doi: 10.1016/j.renene.2016.03.045. [DOI] [Google Scholar]
- Groves, S. L. (2009). Optimization of ethanol production by yeasts from lignocellulosic feedstocks. doi: 10.37099/mtu.dc.etds/195.
- Han L., Feng J., Zhang S., Ma Z., Wang Y., Zhang X. Alkali pretreated of wheat straw and its enzymatic hydrolysis. Brazilian Journal of Microbiology. 2012;43(1):53–61. doi: 10.1590/S1517-83822012000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaor D., Michelazzi M., Leonelli C., Sorrell C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. Journal of the European Ceramic Society. 2012;32(1):235–244. doi: 10.1016/j.jeurceramsoc.2011.08.015. [DOI] [Google Scholar]
- Horn S., Vaaje-Kolstad G., Westereng B., Eijsink V.G. Novel enzymes for the degradation of cellulose. Biotechnology for Biofuels. 2012;5(1):45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G.W., Iborra S., Corma A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chemical Reviews. 2006;106(9):4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- Jackson, R. (2008). Wine Science - 3rd Edition. https://www.elsevier.com/books/wine-science/jackson/978-0-12-373646-8.
- Kacuráková M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydrate Polymers. 2000;43(2):195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- Klinke H.B., Thomsen A.B., Ahring B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Applied Microbiology and Biotechnology. 2004;66(1):10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- Lafka T.-I., Sinanoglou V., Lazos E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chemistry. 2007;104(3):1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- Lenihan P., Orozco A., O’Neill E., Ahmad M.N.M., Rooney D.W., Walker G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chemical Engineering Journal. 2010;156(2):395–403. doi: 10.1016/j.cej.2009.10.061. [DOI] [Google Scholar]
- Li Y., Qin C., Lei Y. The Study of Enzyme Hydrolysis Saccharification Process of Stems and Leaves of Banana. Energy Procedia. 2012;16:223–228. doi: 10.1016/j.egypro.2012.01.037. [DOI] [Google Scholar]
- Lu X., Yamauchi K., Phaiboonsilpa N., Saka S. Two-step hydrolysis of Japanese beech as treated by semi-flow hot-compressed water. Journal of Wood Science. 2009;55(5):367–375. doi: 10.1007/s10086-009-1040-6. [DOI] [Google Scholar]
- Mandal A., Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydrate Polymers. 2011;86(3):1291–1299. doi: 10.1016/j.carbpol.2011.06.030. [DOI] [Google Scholar]
- OIV. (2019). The International Organisation of Vine and Wine, 2019. Statistical Report on World Vitiviniculture. https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf.
- OIV. (2020). International Organisation of Vine and Wine; 2020 WINE PRODUCTION OIV First Estimates.
- Oksman K., Etang J.A., Mathew A.P., Jonoobi M. Cellulose nanowhiskers separated from a bio-residue from wood bioethanol production. Biomass and Bioenergy. 2011;35(1):146–152. doi: 10.1016/j.biombioe.2010.08.021. [DOI] [Google Scholar]
- Oliveira M., Duarte E. Integrated approach to winery waste: Waste generation and data consolidation. Frontiers of Environmental Science & Engineering. 2016;10(1):168–176. doi: 10.1007/s11783-014-0693-6. [DOI] [Google Scholar]
- Ping L., Brosse N., Sannigrahi P., Ragauskas A. Evaluation of grape stalks as a bioresource. Industrial Crops and Products. 2011;33(1):200–204. doi: 10.1016/j.indcrop.2010.10.009. [DOI] [Google Scholar]
- Prozil S.O., Evtuguin D.v., Lopes L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Industrial Crops and Products. 2012;35(1):178–184. doi: 10.1016/j.indcrop.2011.06.035. [DOI] [Google Scholar]
- Pujol D., Liu C., Fiol N., Olivella M.À., Gominho J., Villaescusa I., Pereira H. Chemical characterization of different granulometric fractions of grape stalks waste. Industrial Crops and Products. 2013;50:494–500. doi: 10.1016/j.indcrop.2013.07.051. [DOI] [Google Scholar]
- Ricardo Soccol, C., Faraco, V., Karp, S., Vandenberghe, L. P. S., Thomaz-Soccol, V., Woiciechowski, A., & Pandey, A. (2011). Lignocellulosic Bioethanol. In Biofuels (pp. 101–122). Elsevier. doi: 10.1016/B978-0-12-385099-7.00005-X.
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008). Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42622; Issue Date: 7/17/2005. www.nrel.gov.
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2011). Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; Issue Date: April 2008; Revision Date: July 2011 (Version 07-08-2011). http://www.nrel.gov/biomass/analytical_procedures.html.
- Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008). Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42619; Issue Date 7/17/2005. http://www.nrel.gov/biomass/analytical_procedures.html.
- Spigno G., Maggi L., Amendola D., Dragoni M., de Faveri D.M. Influence of cultivar on the lignocellulosic fractionation of grape stalks. Industrial Crops and Products. 2013;46:283–289. doi: 10.1016/j.indcrop.2013.01.034. [DOI] [Google Scholar]
- Spigno G., Pizzorno T., de Faveri D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresource Technology. 2008;99(10):4329–4337. doi: 10.1016/j.biortech.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Sun N., Rahman M., Qin Y., Maxim M.L., Rodríguez H., Rogers R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chemistry. 2009;11(5):646. doi: 10.1039/b822702k. [DOI] [Google Scholar]
- Taherzadeh M.J., Gustafsson L., Niklasson C., Lidén G. Inhibition effects of furfural on aerobic batch cultivation of Saccharomyces cerevisiae growing on ethanol and/or acetic acid. Journal of Bioscience and Bioengineering. 2000;90(4):374–380. doi: 10.1016/S1389-1723(01)80004-9. [DOI] [PubMed] [Google Scholar]
- Tsukamoto J., Durán N., Tasic L. Nanocellulose and Bioethanol Production from Orange Waste using Isolated Microorganisms. Journal of the Brazilian Chemical Society. 2013 doi: 10.5935/0103-5053.20130195. [DOI] [Google Scholar]
- van Spronsen J., Cardoso M.A.T., Witkamp G.-J., de Jong W., Kroon M.C. Separation and recovery of the constituents from lignocellulosic biomass by using ionic liquids and acetic acid as co-solvents for mild hydrolysis. Chemical Engineering and Processing: Process Intensification. 2011;50(2):196–199. doi: 10.1016/j.cep.2010.12.010. [DOI] [Google Scholar]
- Verardi, A., De, I., Ricca, E., & Calabr, V. (2012). Hydrolysis of Lignocellulosic Biomass: Current Status of Processes and Technologies and Future Perspectives. In Bioethanol. InTech. doi: 10.5772/23987.
- Vidigal S.S.M.P., Rangel A.O.S.S. Exploiting flow-based separation techniques for sample handling in wine analysis. Food Analytical Methods. 2021;2021:1–14. doi: 10.1007/S12161-021-02138-6. [DOI] [Google Scholar]
- Zacharof M.-P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste and Biomass Valorization. 2017;8(4):1011–1025. doi: 10.1007/s12649-016-9674-2. [DOI] [Google Scholar]
- Zhang F., Lin J., Zhao G. Preparation and characterization of modified soda lignin with polyethylene glycol. Materials. 2016;9(10):822. doi: 10.3390/ma9100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Hao M., Zhang N. Influence of contents of chemical compositions on the mechanical property of sisal fibers and sisal fibers reinforced PLA composites. Journal of Natural Fibers. 2020;17(1):101–112. doi: 10.1080/15440478.2018.1469452. [DOI] [Google Scholar]