Abstract
We examined the relationship between penicillin susceptibility, peritoneal virulence in Swiss mice, and capsular type in a selection of 122 clinical Streptococcus pneumoniae isolates belonging to 24 serotypes. Regardless of the serotype, all 32 virulent strains were susceptible to penicillin, and all 41 strains with diminished susceptibility or resistance to penicillin were avirulent. The remaining 49 strains were both susceptible to penicillin and avirulent, irrespective of the serotype. On the basis of their capsular type and pathogenic behavior, strains fell into one of four groups. In the group consisting of serotypes 1, 3, and 4 (n = 16), strains were predominantly virulent (81.3%), and all were penicillin susceptible. In the serotype 6 group (n = 32), the frequency of virulence was significantly lower (34.4 versus 81.3%, P = 0.002), and strains were predominantly penicillin susceptible (71.9%). In the group composed of serotypes 9, 14, 19, and 23 (n = 50), all strains were avirulent, and 56% had decreased susceptibility (n = 12) or resistance to (n = 16) penicillin. The fourth group was heterogenous, as it pooled 24 strains of 15 different serotypes; in this group the frequency of virulence was 33.3%, and strains were predominantly penicillin susceptible (83.3%). These data point to a complex relationship between penicillin susceptibility and virulence in mice but do not entirely separate these characteristics from the role of the capsular type. The possibility that the mechanisms conferring penicillin resistance are related to those leading to a loss of virulence is supported by these findings.
Streptococcus pneumoniae remains a leading cause of human morbidity and mortality worldwide (14, 19, 23). Antimicrobial agents have been used successfully to treat infections by this pathogen for over six decades, but genes expressing resistance to these agents have emerged and disseminated, interfering seriously with antimicrobial therapy. In the last two decades, and more rapidly in the last 7 years, the global incidence of strains displaying resistance to penicillin and other β-lactams and multiresistance has increased alarmingly (1, 2, 11, 15, 16, 21).
Epidemiological, clinical, and preliminary laboratory investigations of pneumococcal infection have suggested a relationship between penicillin susceptibility and capsular type (6, 10, 13, 17, 20, 27) and between serotype and virulence (6, 9). However, penicillin resistance is less common in blood isolates than in isolates recovered from other sites (16), and pneumonia caused in adults by penicillin-nonsusceptible pneumococci has been linked to milder clinical manifestations (12). Experimental pneumococcal pneumonia cannot be induced in immunocompetent mice by inoculation with wild resistant strains, independent of serotype (4). These data, and others suggesting that invasive strains are largely penicillin susceptible while less invasive strains are largely penicillin nonsusceptible (7, 20, 21, 22, 26), point to a correlation between penicillin susceptibility and virulence.
By studying a collection of 122 well-characterized clinical isolates of S. pneumoniae belonging to 24 serotypes, we investigated the relationship between penicillin susceptibility, virulence in mice, and capsular type.
MATERIALS AND METHODS
S. pneumoniae.
The 122 S. pneumoniae study strains were isolated in France between 1991 and 1993 from patients 15 to 90 years old with infections thought to be caused by pneumococci. Most strains (105 of 122) came from the Microbiological Laboratory of Bichat-Claude Bernard Hospital, a 1,200-bed tertiary university hospital in Paris, and the rest were kindly provided by P. Geslin, Centre de Référence du Pneumocoque, Créteil, France. Isolates cultured from blood (n = 45), cerebrospinal fluid (n = 8), and the lower respiratory tract (n = 33) were clinically defined as invasive; the others, originated from the upper respiratory tract (n = 14) and middle ear (n = 19), were defined as noninvasive; and the last three, originated from lymph node, vagina, and knee infections, were also defined as noninvasive. None of the strains in this selected collection was considered a colonizing strain.
Serotype.
All strains were tested by duplicate slide agglutination assays with capsular type-specific antisera (P. Geslin).
Susceptibility testing.
MICs were measured by the microdilution method in Mueller-Hinton broth supplemented with 5% sterile horse serum (24) (Diagnostics Pasteur, Marnes-la-Coquette, France). The MIC was defined as the lowest antibiotic concentration that inhibited all visible growth after 18 h of incubation at 37°C. Penicillin susceptibility was defined according to current breakpoints (25), i.e., susceptibility, MIC ≤ 0.06 μg/ml; decreased susceptibility, 0.12 < MIC ≤ 1 μg/ml; and resistance, MIC ≥ 2 μg/ml.
Experimental virulence.
Female Swiss mice weighing 20 to 22 g (Iffa-Credo Laboratories, l'Arbresle, France) were used in virulence assays as previously described (5). In brief, pneumococcal strains were grown at 37°C in brain-heart infusion broth (bioMérieux, Marcy l'Etoile, France) supplemented with 5% sterile horse serum. Mice were infected intraperitoneally with serial 10-fold dilutions of log-phase cultures in a volume of 0.5 ml per mouse. Groups of five mice received 103 to 108 CFU/mouse. Mortality was assessed daily, and the death rates 7 days postinfection were used to calculate the 100% lethal dose (LD100). As previously defined (5), a strain was considered virulent when an intraperitoneal inoculum of ≤105 CFU killed 100% of mice within 1 week. A strain was considered avirulent when the intraperitoneal LD100 was >105 CFU. With the avirulent strains, lethal pneumonia was only consistently induced by ≥107 CFU intratracheally in leukopenic mice.
Statistical analysis.
Group percentages were compared by using Fisher's exact Chi2 test. Regression analysis was performed between log LD100 and log MIC. P values of 0.05 or less were considered significant.
RESULTS AND DISCUSSION
The 122 clinical strains were divided into four groups on the basis of previous studies (6, 9) on pneumococcal strain behavior in experimental virulence models. The four groups comprised serotypes 1, 3, and 4, mainly virulent; serotype 6, both virulent and avirulent; serotypes 9, 14, 19, and 23, all avirulent; and serotypes 7 to 38, mainly avirulent. In the four groups, the respective proportions of clinically invasive strains were 93.8, 62.5, 66.0, and 75.0%. Strains of invasive origin were more frequently virulent than noninvasive strains (29.1 versus 13.9%), but this difference was not statistically significant (P = 0.106). However, the frequency of virulence among the 53 strains originating from blood or cerebrospinal fluid was significantly higher than among all other strains (35.8 versus 15.9%, P = 0.0187).
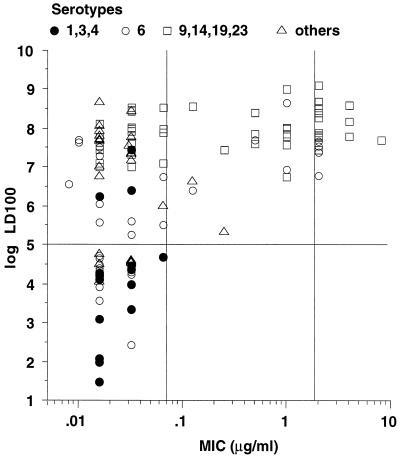
Among the 122 clinical isolates of S. pneumoniae, regardless of the serotype, penicillin-susceptible strains (MIC ≤ 0.06) were either virulent (LD100 ≤ 105) or avirulent (LD100 > 105), but all nonsusceptible strains were avirulent (Fig. 1). By analyzing the overall serotypes, a positive significant correlation (P < 0.0001) was found between log MIC and log LD100. When the relationship was analyzed individually for the most frequent serotypes, i.e., 9 (n = 13), 14 (n = 8), 19 (n = 10), and 23 (n = 19), no significant relation was found, although there was a trend with serotype 14 (P = 0.054). Conversely, with serotype 6, a negative correlation between penicillin resistance and virulence was shown to be significant (P = 0.0017).
FIG. 1.
Relationship between virulence in mice and susceptibility to penicillin for 122 clinical S. pneumoniae strains of various serotypes.
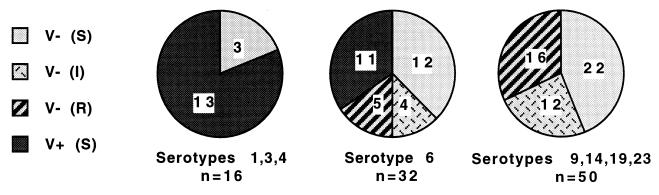
As shown in Fig. 2, 81.3% of strains belonging to serotypes 1, 3, and 4 (all of which were susceptible to penicillin) were virulent. However, these virulent isolates clustered in the serotypes that contained no resistant strains, meaning that no role of resistance could be inferred in this particular group. In serotype 6 (71.9% susceptible), the proportion of virulent strains was significantly lower (34.4 versus 81.3%, P = 0.002). Serotype 6 was particular, as the virulent strains (34.4%) were all penicillin susceptible, whereas the avirulent strains (65.6%) were either susceptible (37.5%) or resistant (28.1%). On the other hand, serotypes 9, 14, 19, and 23 were all avirulent, whether they were penicillin susceptible (44%), intermediate (24%), or resistant (32%), indicating that the primary determinant of decreased virulence might be the serotype rather than acquired penicillin resistance.
FIG. 2.
Relationship between virulence in mice and susceptibility to penicillin within three main groups of S. pneumoniae serotypes. Symbols: V−, avirulent; V+, virulent; S, I, R, penicillin susceptible, intermediate, and resistant, respectively.
The potential reason for the clustering of less virulent strains among serotypes 9, 14, 19, and 23 is unclear. For instance, less-pathogenic serotypes might be more prone to long-term colonization and thus more prone to multiple antibiotic exposure. In our study, the virulent phenotype was 100% predictive of penicillin susceptibility, and the penicillin-resistant phenotype was never associated with virulence. Björkman et al. (8) studied the impact of acquired resistance to streptomycin or rifampin of Salmonella enterica serovar Typhimurium in mice and found that most resistant mutants were less virulent than their wild counterparts. Our experimental results support the clinical study by Einarsson et al. (12), which suggested that adult pneumonia caused by penicillin-nonsusceptible pneumococci was associated with milder clinical manifestations than that caused by penicillin-susceptible pneumococci, indicating either that resistance “carries a cost” or that the serotypes of penicillin-nonsusceptible pneumococci are less virulent. Moreover, Austrian (3) and Knecht et al. (18) reported a correlation between the pathogenicity of certain serotypes in humans and their virulence in rodents. Type 3 bacteremic pneumococcal pneumonia treated with penicillin has a case fatality rate of 50%, and 1 CFU may cause lethal infection in mice or rats. By contrast, type 37 pneumococci are rarely pathogenic in humans, and 107 organisms are required to cause lethal infection in rodents. Type 3 (capsule composed of glucose and glucuronic acid) is among the most invasive type and is associated with a poor prognosis, whereas type 37 (homopolymeric glucose capsule) is rarely associated with infection.
In conclusion, the relationship between susceptibility, serotype, and virulence is complex. The serotype appears to be the primary determinant of virulence and resistance, but none of the strains studied here exhibited both decreased susceptibility and virulence. In our preliminary experiments (Azoulay-Dupuis et al., 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C35, 1997), the genetic transformation of a virulent-susceptible serotype 6 strain with genomic DNA from a penicillin-resistant isolate led to resistance and decreased virulence at the same time. Further investigations are needed to establish the precise relationship between susceptibility, serotype, and virulence in S. pneumoniae and to establish the underlying genetic mechanisms involved in each phenomenon.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C. Emergence of resistance to antimicrobial agents in gram-positive bacteria-pneumococci. Drugs. 1996;51(Suppl. 1):1–5. doi: 10.2165/00003495-199600511-00003. [DOI] [PubMed] [Google Scholar]
- 3.Austrian R. The enduring pneumococcus: unfinished business and opportunities for the future. Microb Drug Resist. 1997;3:111–115. doi: 10.1089/mdr.1997.3.111. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay-Dupuis E, Vallée E, Veber B, Bédos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob Agents Chemother. 1992;36:2698–2703. doi: 10.1128/aac.36.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay-Dupuis E, Moine P, Bédos J P, Rieux V, Vallée E. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob Agents Chemother. 1996;40:941–946. doi: 10.1128/aac.40.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bédos J P, Rollin O, Bouanchaud D H, Pocidalo J J. Relationship between virulence and resistance to antimicrobials in pneumococci. Contribution of experimental data obtained in an animal model. Pathol Biol. 1991;39:984–990. [PubMed] [Google Scholar]
- 7.Bédos J P, Chevret S, Chastang C, Geslin P, Régnier B the French Cooperative Pneumococcus Study Group. Epidemiologic features of and risk factors for infection by Streptococcus pneumoniae strains with diminished susceptibility to penicillin: findings of a French survey. Clin Infect Dis. 1996;22:63–72. doi: 10.1093/clinids/22.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Björkman J, Hugues D, Andersson D I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:3949–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler J C, Hofman J, Cetron M S, Elliot J A, Facklam R R, Breiman R F the Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's pneumococcal sentinel surveillance system. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 11.Doern G V, Brueggemann A B, Blocker M, Dunne M, Holley H P, Kehl K S, Duval J, Kugler K, Putnam S, Rauch A, Pfaller M A. Clonal relationships among high-level penicillin-resistant Streptococcus pneumoniae in the United States. Clin Infect Dis. 1998;27:757–761. doi: 10.1086/514937. [DOI] [PubMed] [Google Scholar]
- 12.Einarsson S, Kristjansson M, Kristinsson K G, Kjartansson G, Jonsson S. Pneumonia caused by penicillin-nonsusceptible and penicillin-susceptible pneumococci in adults: a case-control study. Scand J Infect Dis. 1998;30:253–256. doi: 10.1080/00365549850160882. [DOI] [PubMed] [Google Scholar]
- 13.Fenoll A, Jado I, Vicioso D, Pérez A, Casal J. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996) J Clin Microbiol. 1998;36:3447–3454. doi: 10.1128/jcm.36.12.3447-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillat J. Epidemiology of systemic Streptococcus pneumoniae infections. Presse Med. 1998;27(Suppl. 1):9–16. [PubMed] [Google Scholar]
- 15.Geslin P, Frémaux A, Sissia G, Spicq C. Streptococcus pneumoniae: serotypes, invasive and antibiotic resistant strains. Current situation in France. Presse Med. 1998;27(Suppl. 1):21–27. [PubMed] [Google Scholar]
- 16.Ho P L, Que T L, Ngai-Chong Tsang D, Ng T K, Chow K H, Seto W H. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–1313. doi: 10.1128/aac.43.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knecht J C, Schiffman G, Austrian R. Some biological properties of pneumococcus type 37 and the chemistry of its capsular polysaccharide. J Exp Med. 1970;132:475–487. doi: 10.1084/jem.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman D, Schlaeffer F, Boldur H, Lieberman D, Horowitz S, Leinonen M, Horowitz O, Manor E, Porath A. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax. 1996;51:179–184. doi: 10.1136/thx.51.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linares J, Pallares R, Alonso T, Perez J L, Ayats J, Gudiol F, Viladrich P F, Martin R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1990) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 21.Marton A, Gulyas M, Munos R, Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. J Infect Dis. 1991;163:542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin V A, Riley T V, Roberts C L. Penicillin resistance in laboratory isolates of Streptococcus pneumoniae, in Western Australia, 1990–1994. Eur J Epidemiol. 1998;14:611–615. doi: 10.1023/a:1007446304166. [DOI] [PubMed] [Google Scholar]
- 23.Moine P, Vercken J B, Chevret S, Chastang C, Gajdos P the French Study Group for Community-Acquired Pneumonia in the Intensive Care Unit. Severe community-acquired pneumonia. Etiology, epidemiology, and prognosis factors. Chest. 1994;105:1487–1495. doi: 10.1378/chest.105.5.1487. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1987. [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, approved standard, seventh edition. Villanova, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 26.Pastor P, Medley F, Murphy T V. Invasive pneumococcal disease in Dallas County, Texas: results from population-based surveillance in 1995. Clin Infect Dis. 1998;26:590–595. doi: 10.1086/514589. [DOI] [PubMed] [Google Scholar]
- 27.Verhaegen J, Glupczynski Y, Verbist L, Blogie M, Verbiest N, Vandeven J, Yourassowsky E. Capsular types and antibiotic susceptibility of pneumococci isolated from patients in Belgium with serious infections, 1980–1993. Clin Infect Dis. 1995;20:1339–1345. doi: 10.1093/clinids/20.5.1339. [DOI] [PubMed] [Google Scholar]