Highlights
-
•
Several fruits, plants, and roots are sources of betalains.
-
•
Betalains analysis can be performed by spectroscopic and chromatographic methods.
-
•
Betalains extraction techniques have advantages and disadvantages.
-
•
Betalains encapsulation increases its stability and possibility of incorporation in foods.
-
•
The incorporation of betalains in food packaging is a potential application.
Keywords: Alkaloid, Betalamic acid, Color, Encapsulation, Stability, Pigment, Wall material
Abstract
Betalains are water-soluble nitrogenous pigments with coloring properties and antioxidant activities, which is why they have been incorporated into several foods. However, their use is limited by their instability in response to different factors, such as, pH, oxygen, water activity, light, metals, among others. In this work, a review of up-to-date and relevant information is presented on the primary natural sources of betalains. Additionally, the advantages and disadvantages of the primary betalain extraction techniques are discussed and compared. The results of these studies were focused on the stability of betalains when incorporated into foods, either in pure or encapsulated form, and they are discussed through different technologies. Lastly, the most relevant information related to their stability and a projection of their promising future applications within the food industry is presented.
1. Introduction
Interest in incorporating natural additives, such as colorants and bioactive compounds into the food industry has increased in recent years, favoring the attention towards the nutritional value and sensory attributes of the products as well as improving food safety (Kanatt, 2020, Zin et al., 2020a). Given this concern, betalains constitute a group of compounds with great potential for the enrichment and supplementation of foods due to their pigmentation, antioxidant, antimicrobial properties, and other bioactivities associated with putative health benefits for humans (Prieto-Santiago et al., 2020, Zin et al., 2020b). However, the application of betalains as antioxidants and/or natural colorants in industrialized products is associated with challenges related to maintaining their chemical stability and, therefore, bioactivities and value as pigments (Castro-Enríquez et al., 2020). In this context, the present work provides a compilation of the most relevant and up to date findings that allow us to understand the chemical properties of betalains and the factors associated with their stability. We address the natural sources studied to date from which this group of pigments can be obtained, and we analyze the extraction and analysis methods applied to optimize and obtain these compounds more efficiently for future industrial applications. Lastly, the potential incorporation of betalains into foods to improve their acceptability and shelf life is discussed, to address the effect of the matrix in which each compound is incorporated. The effects of the storage conditions on the conservation of the betalain antioxidant and pigmentation capacity as well as potential projections and new application trends of these compounds in the food industry are noted.
2. Chemistry and natural sources of betalains
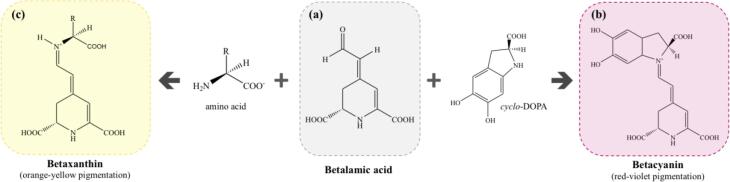
Betalains are nitrogenous pigments derived from betalamic acid (4-(2-oxoethylidene)-1,2,3,4-tetrahydropyridine-2,6-dicarboxylic acid) (Fig. 1a), constituting the basic structure of betalains (Slimen et al., 2017). Betalains have high hydrophilicity due to the hydroxyl groups (–OH) on their structures, and these groups lead to charge polarization and the formation of hydrogen bonds responsible for this property (Fathordoobady et al., 2016). From the basic structure of betalamic acid, condensations with different molecules are generated that originate from the two structural classifications of betalains. The first group is made up of structures in which betalamic acid is condensed by cyclo-DOPA (cyclo-L-3,4-dihydroxyphenylalanine) or its glucosyl derivatives, which are known as betacyanins (Fig. 1b). The second classification corresponds to betaxanthins (Fig. 1c), which originate from the condensation of betalamic acid with amino compounds (amino acids, amines, or derivatives) (Silva et al., 2020, Zin et al., 2020b).
Fig. 1.
(a) Betalamic acid, the basic structure of the betalains; (b) general structure of the betacyanins derived from the condensation of the betalamic acid with cyclo-DOPA; and (c) general structure of the betaxanthines derived from the condensation of the betalamic acid with amino acids or its derivatives.
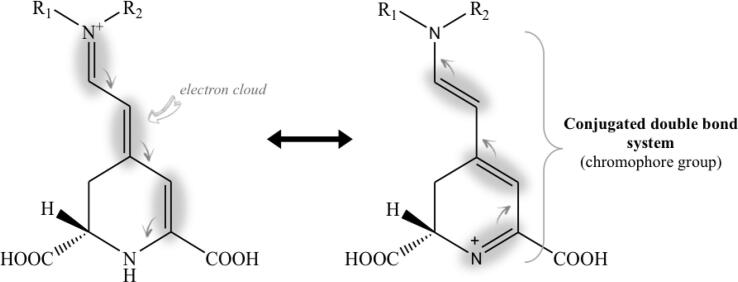
The type of substitution linked to the basic structure of betalain has a strong impact on its primary characteristic, its pigmentation, a property associated with the resonance between the electrons of the conjugated double bond system in the structure (Fig. 2). This structure acts as a chromophore with the absorption of visible light in the 457–485 nm region, producing the characteristic orange–yellow color of betaxanthins. For betacyanins, the substitution of the basic structure by an aromatic nucleus (such as cyclo-dopa) extends the electronic resonance system, producing a bathochromic shift of 50 to 70 nm, so its characteristic absorption is close to 532–550 nm, conferring a red-violet pigmentation (Choo, 2019, Rodriguez-Amaya, 2019).
Fig. 2.
Resonance structure of betalain. The gray shade represents the electron cloud within the conjugated double bond system, and the gray arrows indicate the conjugate displacement of electron clouds.
Betalains are secondary nitrogenous metabolites present in the seeds, fruits, flowers, leaves, stems, and roots of the Amaranthaceae, Cactaceae and Chenopodiaceae families to which they confer their characteristic red-yellow pigmentation, as well as multiple properties, including antioxidant, anti-cancer, antilipidemic, and antimicrobial activity (Gengatharan et al., 2016, Hu et al., 2020, Otálora et al., 2019). Therefore, a search was performed for plants that have been shown to contain this type of pigment. Table 1 provides a summary of the primary findings concerning the types and parts of these plants. The betalains content and the primary antioxidant properties exhibited by these pigments are given below. In the flowers/bracts of Amaranthus spp., betalain concentrations between 0.95 and 6.02 mg/100 g have been found (Li et al., 2015), and in Bougainvillea spp. 465 mg of betacyanins and 116 mg of betaxanthines/100 g show inhibition of the radicals ABTS and DPPH of 72.68 and 61.24%, respectively (Orozco-Villafuerte et al., 2019).
Table 1.
Summary of natural sources of betalains and their reported concentrations.
| Sample | Extraction method |
Betalains |
Betacyanins |
Betanines |
Amaranthins |
Betaxanthins |
Reference |
|---|---|---|---|---|---|---|---|
| (mg/100 g) | (mg/100 g) | (mg/100 g) | (mg/100 g) | (mg/100 g) | |||
| Flowers/Bracts | |||||||
| Amaranthus spp. | Solid-liquid extraction | 0.95–6.02 | – | – | 0.45–2.76 | – | Li et al., 2015 |
| Bougainvillea spectabilis | Solid-liquid extraction | – | 465 | – | – | 116 | Orozco-Villafuerte et al., 2019 |
| Fruits | |||||||
| Basella rubra L. | Maceration | 13.81 | – | – | – | – | Kumar et al., 2020 |
| Solid-liquid extraction | 143.76 | 124.18 | – | – | 19.16 | Kumar et al., 2015 | |
| Ultrasound-assisted extraction (UAE) | – | 142 | – | – | 535 | Maran & Priya, 2015 | |
| Prickly pear (Optunia ficus indica) | Solid-liquid extraction | 41.54 | – | – | – | – | Prakash-Maran, Manikandan, & Mekala, 2013 |
| Prickly pear (Opuntia spp.) | High Pressure Carbon Dioxide (HPCD) | 89 | – | – | – | – | Nunes et al., 2015 |
| High-Pulsed Electric Fields (HPEF) | 285 – 2,252 | 159 – 1,655 | – | – | 126 – 686 | Jiménez-Alvarado et al., 2015 | |
| Red dragon fruit (Hylocereus polyrhizus) | Solid-liquid extraction | – | 82.79 | – | – | – | Ramli et al., 2014 |
| Ultrasound-assisted extraction (UAE) | – | 71.34 | – | – | – | Ramli et al., 2014 | |
| Red pitaya (Stenocereus stellatus) | Ultrasound-assisted extraction (UAE) | 479.3 | – | – | – | – | Pérez-Loredo et al., 2017 |
| Xoconostle (Opuntia joconostle) | Solid-liquid extraction | – | 92 | – | – | – | Sanchez-Gonzalez et al., 2013 |
| Fruit peels | |||||||
| Opuntia engelmannii | Ultrasound-assisted extraction (UAE) | 20,160 | – | – | – | – | Melgar et al., 2019 |
| Microwave-assisted extraction (MAE) | 13,290 | – | – | – | – | Melgar et al., 2019 | |
| Red dragon fruit (Hylocereus polyrhizus) | Solid-liquid extraction | – | 18.67 | – | – | – | Ramli et al., 2014 |
| Ultrasound-assisted extraction (UAE) | – | 17.64 | – | – | – | Ramli et al., 2014 | |
| Leaves | |||||||
| Amaranthus spp. | Solid-liquid extraction | 16.90–20.93 | – | – | 7.75–9.67 | – | Li et al., 2015 |
| Red amaranth (Amaranthus cruentus) | Solid-liquid extraction | – | 159.09 | – | – | – | Das et al., 2019 |
| Roots | |||||||
| Grown red and golden beets (Beta vulgaris L.) | Ultrasound-assisted extraction (UAE) | – | – | 3.75 – 75.64 | – | – | Wang et al., 2020 |
| Red beetroot (Beta vulgaris L.) | Solid-liquid extraction | – | 390 | – | – | 214 | Silva et al., 2020 |
| Solid-liquid extraction | – | 156 | – | – | Neagu & Barbu, 2014 | ||
| Solid-liquid extraction | – | 30.9 | – | – | 16.3 | Swamy et al., 2014 | |
| Ultrasound-assisted extraction (UAE) | – | 445 | – | – | 242 | Silva et al., 2020 | |
| Seeds | |||||||
| Amaranthus spp. | Solid-liquid extraction | 0.07–0.96 | – | – | 0.04–0.44 | – | Li et al., 2015 |
| Whole plant | |||||||
| Alternanthera sessilis | Solid-liquid extraction | – | – | 7,310 | 7,270 | 7,450 | Yap et al., 2019 |
| Others | |||||||
| Amaranthus spp. stalks | Solid-liquid extraction | 0.56–1.54 | – | – | 0.28–0.70 | – | Li et al., 2015 |
| Amaranthus spp. sprouts | Solid-liquid extraction | 2.69 | – | – | 1.28 | – | Li et al., 2015 |
| Colored quinoa (Chenopodium quinoa Willd) hulls | Ultrasound-assisted extraction (UAE) | – | 96.47 | – | – | 201.01 | Laqui-Vilca et al., 2018 |
| Glasswort (Salicornia fruticosa) air parts | Solid-liquid extraction | 12,990 | – | – | – | – | Mohamed et al., 2018 |
Some fruits such as prickly pear (Opuntia spp.), red dragon fruit (Hylocereus polyrhizus), and xoconostle (Opuntia joconostle) have also been shown to be important sources of these pigments at concentrations ranging from 13.81 to 2.252 mg/100 g (Jiménez-Alvarado et al., 2015, Kumar et al., 2020, Pérez-Loredo et al., 2017, Ramli et al., 2014, Sanchez-Gonzalez et al., 2013). The roots of different varieties of the beet Beta vulgaris L. represent one of the most studied natural sources due to their high content of betalains, at between 30.9 and 445 mg of betacyanins/100 g and 16.3 to 242 mg of betaxanthins/100 g (Silva et al., 2020, Swamy et al., 2014). This natural source has shown a high ABTS scavenging activity of 229.83–300.76 mg of AAE/g in contents of 260–436.5 mg of betanin/100 g of fresh root (Wang et al., 2020). In addition, 0.19 mmol ET/g (by DPPH assay), 0.15 mmol ET/g (by FRAP), and 4.88 mmol ET/g (by ABTS) were found at concentrations of 4.6 mg of betacyanins and 2.6 mg of betaxanthins/g (Silva et al., 2020). The peels of red dragon fruits and prickly pears have also been reported as excellent sources of betalains, with up to 18.67 mg of betacyanins/100 g and 20,160 mg of betalains/100 g of peel, respectively. The peel of the red dragon fruits reflected an antioxidant activity (inhibition percentage, by ABTS assay) of 3.04% and a reducing power (by FRAP) of 200.83 mol Fe2 +/g but a low correlation with the betacyanin content (Ramli et al., 2014). In contrast to prickly pear peel, a synergistic effect was observed between the antioxidant activity of 2.4 mg/mL (IC50 value, by reducing power) and the betalain content (Melgar et al., 2019). These interesting results could be used to give value to fruit parts, generally considered a residue, that are used to obtain these pigments. Given these properties, other parts of the plant specimens can be used, including the leaves, stalks, sprouts, seeds, seed hulls, or the whole plant (Laqui-Vilca et al., 2018, Li et al., 2015, Mohamed et al., 2018, Yap et al., 2019). Li et al. (2015) observed a positive correlation between the betalains content of Amaranthus spp. and the antioxidant activity evaluated by FRAP method (0.63–62.21 mmol AAE/g) and ORAC (30.67–451.37 mmol TE/g), and concluded that the leaves of the species Amaranthus hypochondriacus had overwhelmingly higher antioxidant activities compared to other species and parts of the same plant, including its seeds, flowers, stems, and fruits. These findings are relevant when considering rapidly developing plant parts, such as aerial parts and leaves, as well as fruit residue fractions, such as the peel, when searching for alternative sources of betalains for use at an industrial level in an economical, sustainable, and renewable way.
3. Betalain extraction processes
In the interest of obtaining, studying, and evaluating the potential applicability of betalain pigments for the industry, multiple studies have been focused on the optimization of conventional extraction processes, using response surface methodology (RSM) (Kumar et al., 2017, Singh et al., 2017, Zin et al., 2020a, Zin et al., 2019) and multivariate analysis by principal components analysis (PCA) (Neagu and Barbu, 2014, Silva et al., 2020) to maximize yields and maintain their stability. The betalains extraction conditions from natural sources that have been optimized and/or suggested according to some results of the most recent studies are listed in Table 2.
Table 2.
Summary of the optimal or suggested conditions for the extraction of betalains by conventional solid–liquid extraction from natural sources.
| Sample | Compound(s) | RL/S | Solvent | Solvents ratio (%) | Temperature | Time | Other condition | Reference |
|---|---|---|---|---|---|---|---|---|
| Alternanthera sessilis (red) | Amaranthin, betaxanthin and betanin | 20 mL/g | ethyl acetate | 100 | 50 °C | 24 h | stirring speed of 200 rpm | Yap et al., 2019 |
| Bougainvillea glabra floral bracts | betalains (as optical density) | 17 mL/g | methanol:water | 25:75 | 22.5 °C | 6 h | Kumar et al., 2017 | |
| Glasswort (Salicornia fruticosa) air parts | betalains | 20 mL/g | ethanol:water (acidified with 0.5% citric acid) | 20:80 | 40 °C | 30 min | Mohamed et al., 2018 | |
| Hylocereus polyrhizus flesh | betacyanins | 10 mL/g | ethanol:water | 50:50 | room temp. | 20 min | stirring speed of 300 rpm | Fathordoobady et al., 2016 |
| betacyanins | 10 mL/g | ethanol:water | 70:30 | room temp. | 20 min | stirring speed of 300 rpm | Fathordoobady et al., 2016 | |
| Red amaranth (Amaranthus cruentus) | betacyanins | 40 mL/g | water (pH 5) | 100 | 50 °C | 60 min | Das et al., 2019 | |
| Red beetroot (Beta vulgaris L.) | betacyanins and betaxanthins | 75 mL/g | water | 100 | 30 °C | 30 min | stirring speed of 40 rpm | Silva et al., 2020 |
| betanin | 5 mL/g | water (acidified with 0.5% citric acid and 0.1% ascorbic acid) | 100 | 70 °C | Neagu & Barbu, 2014 | |||
| betalamic acid, betacyanin and betaxanthin | 33 mL/g | water | 100 | 60 °C | 84 min | Swamy et al., 2014 | ||
| Red beetroot (Beta vulgaris L.) peel | betacyanins and betaxanthins | 1.25 mL/g | ethanol:water | 15:85 | 20 °C | 60 min | stirring speed of 215 rpm | Zin et al., 2020 |
| Red beetroot (Beta vulgaris) powder | betalains | 100 mL/g | ethanol:water (pH 5) | 50:50 | 30 °C | Pandey et al., 2018 | ||
| Beetroots (Cylindra type) peel | betacyanins and betaxanthins | 10 mL/g | ethanol:water | 25:75 | 50 °C | 50 min | Zin et al., 2019 | |
| Red dragon fruit (Hylocereus polyrhizus) peel | betacyanin | 25 mL/g | water | 100 | 50 °C | 120 min | stirring speed of 200 rpm | Ramli et al., 2014 |
| Prickly pear (Optunia ficus indica) fruits | betalains | 42 mL/g | water (acidified with citric acid, pH 6.9) | 100 | 42 °C | 115 min | Prakash-Maran et al., 2013 | |
| Xoconostle (Opuntia joconostle) fruit | betacyanin | 20 mL/g | methanol:water (acidified with citric acid, pH 5) | 20:80 | 15 °C | 10 min | Sanchez-Gonzalez et al., 2013 |
RL/S mean: Ratio liquid (solvent)/solid.
Due to the hydrophilic nature of betalains, the methods developed for their extraction from different natural sources include the use of water, methanol- and ethanol–water mixtures in different ratios, and ethyl acetate. Water has a demonstrated efficiency at extracting betacyanins and betaxanthins from red amaranth (Amaranthus cruentus) (Das et al., 2019), red beet root (Beta vulgaris L.) (Neagu and Barbu, 2014, Silva et al., 2020, Swamy et al., 2014), red dragon fruit (Hylocereus polyrhizus) peel (Ramli et al., 2014), and prickly pear (Opuntia ficus indica) fruits (Prakash-Maran et al., 2013). Aqueous methanol solution has been found the most effective for extracting betalains from Bougainvillea glabra floral bracts (Kumar et al., 2017) and Xoconostle (Opuntia joconostle) fruit (Sanchez-Gonzalez et al., 2013). Ethanol solutions have provided a higher yield of betalains from glasswort (Salicornia fruticosa) (Mohamed et al., 2018), Hylocereus polyrhizus flesh (Fathordoobady et al., 2016), red beet root (Beta vulgaris L.) peel (Zin et al., 2020a) and powder (Pandey et al., 2018), and beet-roots (Cylindra type) peel (Zin et al., 2019). In addition, ethyl acetate is used to extract Alternanthera sessilis (Yap et al., 2019). It should be noted that during the selection of the appropriate solvent for extracting any phytochemical, the applicability of the final extraction product must be considered since, if it is a food additive, some options (such as methanol and ethyl acetate) are not suggested due to their potential toxicity. With this consideration in mind, solvents such as water, ethanol and a mixture of these solvents are recommended. The extraction of betacyanins with water has shown greater efficiency with respect to aqueous ethanol solutions due to the nucleophilic attack of ethanol on the aldimine bond (N = CH) of betalains originating from its degradation via decarboxylation (Das et al., 2019, Sanchez-Gonzalez et al., 2013). However, when samples with high pectin contents are used, such as red pitaya (Hylocereus polyrhizus) flesh and peel, a lower proportion of water in the aqueous ethanol extraction solvent is recommended to reduce mucilaginization due to the solubilization of water-soluble carbohydrates that hinder any subsequent filtration processes (Fathordoobady et al., 2016). In addition to choosing a suitable solvent, selecting the other extraction conditions has been shown to significantly improve betalains extraction efficiency, including the liquid/solid ratio, pH, temperature, and time (Kumar et al., 2017, Zin et al., 2020a). The liquid (solvent)/solid (sample) ratio (RL/S) recommended for conventional extraction varies over a range of 1.25–100 mL/g (Table 2) and is dependent on the type of sample. Having more sample available in the extraction mixture (solvent/sample) positively favors the betalains yield due to the more significant amount of extractable material in the medium (Prakash-Maran et al., 2013). However, a higher volume of solvent favors better hydration and the swelling of solid samples, reduces the viscosity of the medium and thus improves the extraction efficiency (Silva et al., 2020). Zin et al., (2020a) observed that the increase in RL/S improved the betacyanin and betaxanthin extraction yields from red beetroot (Beta vulgaris L.) when using an extraction temperature of 20 °C. Thus, the RL/S plays an important role when the extraction process is performed at low temperatures, in which a higher RL/S ratio must be considered to increase the diffusivity of the pigment in the medium and to decrease the time to reach the final equilibrium state, which improves the extraction efficiency (Mohamed et al., 2018, Neagu and Barbu, 2014, Zin et al., 2019).
3.1. Effect of pH on the extraction process
The pH also plays a very important role in the betalains extraction process since it can affect compound stability and thus decrease its extraction efficiency. Neagu and Barbu, (2014) observed that the pH has a positive impact on the extraction process when performed at a low temperature (20 °C) compared with a temperature of 70 °C, at which the pH does not have a significant influence. Das et al. (2019) observed an increase in the betalains extraction yield from red amaranth (Amaranthus cruentus) with acidification of the medium to pH 5. Similar results have been reported in red beet-root (Beta vulgaris L.) powder (Pandey et al., 2018) and Xoconostle (Opuntia joconostle) fruit (Sanchez-Gonzalez et al., 2013). Therefore, acidifying the extraction medium is recommended to improve the accumulation of betalains and prevent their degradation (Mohamed et al., 2018). Acidifying agents such as citric acid have been commonly used because they act as neutralizing agents for the electrophilic center of betalains, which improves its stability (Prakash-Maran et al., 2013).
3.2. Effect of heat and time on the extraction process
The extraction temperature and time significantly affect the extraction efficiency (Silva et al., 2020, Swamy et al., 2014). According to the results summarized in Table 2, an optimal conventional extraction temperature of 20–50 °C is recommended. An extraction at a low temperature of 10 °C is not enough for the complete extraction of betalains (Pandey et al., 2018). An increase in temperature to no higher than 55 °C, improves the extraction performance due to the softening of the plant tissue and the increased permeability of the cell membrane, favoring the more significant release of pigments whose solubility and diffusion coefficient are also increased (Maran and Priya, 2016, Zin et al., 2019). The time determines the period in which the extractable matter will be in contact with the extraction agent. A longer extraction time favors the process yield (Prakash-Maran et al., 2013). The extraction time has a significant effect when temperatures above 60 °C are used during the process, leading to a reduction in the betalains content due to the hydrolytic degradation associated with prolonged thermal exposure (Silva et al., 2020). However, at lower temperatures (30–50 °C), an extraction time of greater than 115 min also leads to the degradation of these pigments. The extraction time is also influenced by the RL/S, in which more sample and a longer extraction time are necessary to increase the yield levels (Kumar et al., 2017). This effect occurs because by increasing the mass of solute in the solvent, the time required to reach the equilibrium of mass and heat transfer is proportionally higher (Zin et al., 2019). Lastly, the extraction time is highly variable and depends on the type and nature of the sample. For example, (Fathordoobady et al., 2016) observed that the betacyanin extraction process in red dragon fruit peel (Hylocereus polyrhizus) required a longer extraction time compared to the fruit flesh. (Ramli et al., 2014) observed that if the same extraction time was used, the ethanol:water ratio of the solvent had to be increased in the peel compared to the flesh.
3.3. Other extraction methods
Other extraction methods have been studied in the search to improve the efficiency of conventional extraction when using enzymatic treatment with pectinases (Naderi et al., 2010), ultrasound-assisted extraction (Haq et al., 2020, Silva et al., 2020, Wang et al., 2020), β-cyclodextrin (CD)-enhanced ultrasound-assisted extraction (Tutunchi et al., 2019), microwave-assisted extraction (Ferreres et al., 2017, Melgar et al., 2019, Singh et al., 2017), and the application of pulsed electric fields (PEFs) (Jiménez-Alvarado et al., 2015, Nowacka et al., 2019) as well as the use of supercritical fluid extraction (SFE) as a safe alternative for the environment due to the lack or minimal use of solvents compared to conventional extraction (Fathordoobady et al., 2016, Nunes et al., 2015). Some of the alternative methods to conventional extraction as well as the primary process conditions are summarized in Table 3, again indicating that the nature of the sample will determine the conditions, time and intensity of the process as well as the type of solvent and the appropriate RL/S. However, the high cost involved in some of these extraction alternatives compared to traditional methods undoubtedly still needs to be considered and valued (Zin et al., 2020a).
Table 3.
Summary of some of the optimal or suggested conditions for the extraction of betalains by non-conventional extraction methods from natural sources.
| Extraction method | Sample | Compound(s) | RL/S | Solvent | Solvents ratio (%) | Temperature | Time | Other condition | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Enzimatic treatment | Hylocereus polyrhizus fruit | betanin, isobetanin, phylocactin, hylocerenin, isophyllocactin, and isohylocerenin | 1 mL/g | water (acidified with citric acid, pH 4) | 100 | 40 °C | 120 min | stirring speed of 250 rpm | Naderi et al., 2010 |
| Ultrasound-assisted | Colored quinoa (Chenopodium quinoa Willd) hulls | betacyanins | 100 mL/g | water | 100 | 9.2 s | power of 100 W, 30 kHz, 70% of amplitude, pulse of 0.6 | Laqui-Vilca et al., 2018 | |
| betaxanthins | 100 mL/g | water | 100 | 40 s | power of 100 W, 30 kHz, 90% of amplitude, pulse of 0.7 | Laqui-Vilca et al., 2018 | |||
| Grown red and golden beets (Beta vulgaris L.) | betacyanins and betaxanthins | 2 mL/g | methanol | 100 | 60 min | Wang et al., 2020 | |||
| Red beet (Beta vulgaris L.) | betacyanins and betaxanthins | 5 mL/g | ethanol:water (acidified with acetic acid, 0.5%) | 30:70–45:55 | 55 °C | 15 min | 37 kHz. After sonication, stirring at 320 rpm for 43 min at 40 °C | Haq et al., 2020 | |
| betacyanins and betaxanthins | 75 mL/g | water | 100 | 30 °C | 30 min | power of 83 W | Silva et al., 2020 | ||
| betacyanins | 25 mL/g | ethanol:water | 25:75 | 52 °C | 90 min | power of 165 W, 25 kHz | da Silva et al., 2018 | ||
| betaxanthins | 25 mL/g | ethanol:water | 25:75 | 37 °C | 90 min | power of 165 W, 25 kHz | da Silva et al., 2018 | ||
| betacyanins and betaxanthins | 15 mL/g | water (pH 2.5) | 50 °C | 10 min | Kushwaha et al., 2018 | ||||
| betacyanins and betaxanthins | 19 mL/g | water | 100 | 53 °C | 35 min | power of 89 W | Maran & Priya, 2016 | ||
| Red dragon fruit (Hylocereus polyrhizus) flesh | betacyanin | 25 mL/g | water | 100 | 25 °C | 30 min | 50 kHz | Ramli et al., 2014 | |
| Red pitaya (Stenocereus stellatus) | betacyanins and betaxanthins | ≈2 mL/g | water | 100 | 20 °C | 15 min | 40 kHz. After sonication, stirring at 3200 rpm | Pérez-Loredo et al., 2017 | |
| Opuntia engelmannii fruit peel | betacyanins | 200 mL/g | methanol:water (pH 7) | 17:83 | 33.9 °C | 1.2 min | 40 kHz, stirring speed of 200 rpm | Melgar et al., 2019 | |
| β-CD-enhanced ultrasound assisted | Red beets (Beta vulgaris L) | betanin | 10 mL/g | water:β-Ciclodextrin (β-CD) | 95:5 | 30 min | 28 kHz, 80 W. Prior to ultrasound treatment, the sample solution was homogenized for 180 min. | Tutunchi et al., 2019 | |
| Microwave assissted | Dragon fruit (Hylocereus polyzhirus) peel | betalains | 25 mL/g | water | 100 | 35°C | 8 min | microwave power of 100 W | Thirugnanasambandham & Sivakumar, 2015 |
| Red beetroot (Beta vulgaris L.) | betacyanins (betanin) | 250 mL/g | ethanol:water (acidified with ascorbic acid, 0.04 mol/L) | 50:50 | 1.17 min/1.7 min | microwave power of 400 W; duty cycle of 100% | Cardoso-Ugarte et al., 2014 | ||
| betaxanthins | 250 mL/g | ethanol:water (acidified with ascorbic acid, 0.04 mol/L) | 50:50 | 2.7 min/1.8 min | microwave power of 400 W; duty cycle of 100% | (Cardoso-Ugarte et al., 2014 | |||
| Red beetroot (Beta vulgaris L.) peel | betacyanins (betanin) | 5 mL/g | water (acidified with citric acid, pH 5.2) | 100 | 0.95 min | microwave power of 224.61 W | Singh et al., 2017 | ||
| betacyanins (betanin) | 5 mL/g | ethanol | 100 | 1.25 min | microwave power of 384.25 W | Singh et al., 2017 | |||
| betacyanins and betaxanthins | (4:1, 2:1, 2:1, and 1.5:1) | water | 100 | 12 min (4 times of 3 min) | microwave power of 450 W | Slavov et al., 2013 | |||
| Opuntia engelmannii fruit peel | betacyanins | 49 mL/g | methanol:water (pH 7) | 55:45 | 25°C | 8.8 min | microwave power of 400 W | Melgar et al., 2019 | |
| White-fleshed red pitaya (Hylocereus undatus) | betacyanins | 150 mL/g | water | 100 | 49.33°C | 5 min | microwave power of 600 W | Ferreres et al., 2017 | |
| Yellow pitaya (Hylocereus megalanthus) | betacyanins | 150 mL/g | water | 100 | 49.33°C | 5 min | microwave power of 600 W | Ferreres et al., 2017 | |
| Pulsed electric field | Red beet (Beta vulgaris L.) | betanin and vulgaxanthin | 100 mL/g | phosphate buffer, pH 6.5 | 100 | 20 μs pulses of electric field at 4.38 kV cm−1 of strength, Energy of 4.86 kJ/kg. | Nowacka et al., 2019 | ||
| betalains | 20 mL/g | water | 100 | 100 μs pulses with electric field strengthat 1 kV cm−1 of strength. | Loginova et al., 2011 | ||||
| High-Pulsed Electric Fields (HPEF) | Prickly Pear (Opuntia spp.) fruits | betacyanins and betaxanthins | 10 min | 8 kV cm−1, repetition rate of 5 Hz. | Jiménez-Alvarado et al., 2015 | ||||
| High Pressure Carbon Dioxide (HPCD) | Cactus pears (Opuntia spp.) fruit | betacyanins and betaxanthins | sample + water (acidified with citric acid, pH 5):CO2 pressurized | 20:80 | 40°C | 30 min | high pressure CO2 pre-treatment of dried sample pre-heated to 55°C, CO2 at 375 bar for 60 min | Nunes et al., 2015 | |
| Supercritical Fluid Extraction (SFE) | Hylocereus polyrhizus flesh and peel | betacyanins | co-solvent (ethanol:water 10:90):CO2 pressurized | 90:10 | 50°C | 90 min | pressure of 25 Mpa | Fathordoobady et al., 2016 |
RL/S mean: Ratio liquid (solvent)/solid.
4. Analysis of betalains
The color of betalains is due to their structural chromophore group (Fig. 2), which has allowed for UV–Vis spectroscopy to be the most widely used analytical technique for the quantitative identification of their two structural groups (betacyanins and betaxanthins) in natural sources. The measurement of the maximum absorption in the visible region at 480 nm is used for the quantification of betaxanthins and at 535–538 nm for betacyanins. Through a mathematical calculation that considers the dilution factor, the molecular weight of betalains (308 g/mol for betaxanthin, 550 g/mol for betanin, and 726.6 g/mol for amaranthin) and its extinction coefficient (ε) (48,000 L/mol·cm for betaxanthin, 60,000 L/mol·cm for betanin, and 56,600 L/mol·cm for amaranthin, in H2O), the total proximal content of betalain compounds can be determined (Haq et al., 2020, Kushwaha et al., 2018, Silva et al., 2020, Tutunchi et al., 2019, Yap et al., 2019, Zin et al., 2020a). However, the system of conjugated double bonds gives betalains the property of fluorescence absorption/emission, for a maximum excitation at 320–475 nm, corresponding to blue light, and emission at 506–660 nm correspondingly to green light (Slimen et al., 2017). Fourier transform infrared (FTIR) analysis has also been one of the tools used to evaluate the presence of betalains, although indirectly, since through this tool, it is only possible to identify the amine group of nitrogen (N–H) that would indicate the possible presence of these pigments. Some of the betalains signals studied by FTIR are located at 1651 cm−1, which is associated with the presence of the carbonyl group (C = O) in stretching mode associated with the amide bond; 1641 cm−1 is related to the N–H bend of the 1° amine group, the band at 1050 cm−1 represents the C–N stretching of the amine, and the measurement at 718 cm−1 confirms the presence of the amine group (N–H) (Kumar et al., 2017, Singh et al., 2017, Tutunchi et al., 2019).
Through tools such as liquid chromatography (LC), in the high-pressure liquid chromatography (HPLC) modality (Ferreres et al., 2017, García-Cruz et al., 2017, Wang et al., 2020), ultra-performance liquid chromatography (UPLC) (Melgar et al., 2019), or ultra-high-pressure liquid chromatography (UHPLC) (Cejudo-Bastante et al., 2014, Wang et al., 2020) in reverse-phase (RP), the separation of betalain mixtures has been very effective. Some of the solvents used as mobile phases to separate betalains by LC are acetonitrile–water (Fathordoobady et al., 2016, Ferreres et al., 2017, García-Cruz et al., 2017, Sawicki et al., 2016, Sawicki et al., 2017, Wang et al., 2020), methanol–water (Cejudo-Bastante et al., 2014, Slavov et al., 2013), and acetonitrile buffer of KH2PO4 (pH 2.74) (Sanchez-Gonzalez et al., 2013) in different proportions, which are applied by eluting in isocratic mode or modifying the gradient concentration of the phase. The acidification of the mobile phase with 0.012–1.0% formic acid (Fathordoobady et al., 2016, Ferreres et al., 2017, García-Cruz et al., 2017, Sawicki et al., 2016, Sawicki et al., 2017, Wang et al., 2020) or with trifluoroacetic acid (TFA) 0.05% (Naderi et al., 2010) is common in this analysis to maintain the stability of the betalains structure during the process. The coupling of LC to detectors, such as UV–Vis spectroscopy, diode array detection (DAD), and mass spectrometry (MS), has enabled the qualitative and quantitative analysis applied to the characterization of the betalain pigments profile from several natural sources, stability studies, and evaluations of the extraction efficiencies of specific betalains of particular interest. In detection by UV–Vis spectroscopy, monitoring at a wavelength of 480 or 540 nm is recommended (Melgar et al., 2019, Naderi et al., 2010, Slavov et al., 2013). For DAD, using a monitoring window at 477–484 and 535 nm is recommended (Fathordoobady et al., 2016, Ferreres et al., 2017, García-Cruz et al., 2017, Melgar et al., 2019, Wang et al., 2020). In detection by MS by time-of-flight (TOF) applying electrospray ionization (ESI) (Fathordoobady et al., 2016, Ferreres et al., 2017, García-Cruz et al., 2017, Melgar et al., 2019, Sawicki et al., 2016, Wang et al., 2020) or by tandem mass spectrometry (MS/MS) (Sawicki et al., 2017), operating the electrospray ionization source in positive mode is suggested.
5. Stability and encapsulation of betalains
Betalains are approved as colorants by the European Union and by the Food and Drug Administration and have been used in several food products (Khan, 2016), since they are also considered a valuable antioxidant resource, so their consumption could enhance protection against free radicals (Wybraniec, 2005). However, its use has been reduced due to its low stability, since its properties and coloring power are affected by several factors, which have been widely studied in various research studies (Table 4). In this regard, the temperature is one of the factors that has the greatest effect on the structure of betalains. An increase in temperature results in an increase in the degradation of betalains (Güneşer, 2016, Kayın et al., 2019, Laqui-Vilca et al., 2018, Prieto-Santiago et al., 2020), the structure of which is modified due to hydrolysis, isomerization, dehydrogenation, deglycosylation, and decarboxylation processes (Herbach et al., 2006). High temperatures cause the decarboxylation of betanin, generating neobetanin, which produces a color change due to the formation of an aglycone with less stability (Herbach et al., 2006, Reshmi et al., 2012). The thermal degradation of betalains has also been reported to produce mono-, di-, and tricarboxylic betacyanins (Wybraniec, 2005). The effect of the temperature on betalains degradation is influenced by the intensity of heating, the presence of oxygen, the concentration of pigments present, pressure, ultrasound, and other factors (dos Santos et al., 2018, Güneşer, 2016, Laqui-Vilca et al., 2018, Prieto-Santiago et al., 2020). Betalamic acid is susceptible to isomerization due to the temperature effect. The hydrolysis of betanin leads to the breakdown of the molecule, generating betalamic acid and cyclo-dopa-5-O-beta-glucoside, for an imminent decrease in coloration (Herbach et al., 2006). Oxygen is another critical factor in the degradation of betalains, especially since its effect has been related to joint degradation with other factors, such as the presence of light and temperature (Barba-Espin et al., 2018, von Elbe and Attoe, 1985). Betalains show stability at a pH of 4–6 and at a temperature of 4 °C; as a result, betalains are degraded at pH values outside this range and change color depending on the pH of the sample. Betalains below pH 3 present a violet color, and betalains at a pH greater than 7 exhibit a blue color (Wootton-Beard & Ryan, 2011). At pH >7, betanin is degraded by the hydrolysis of aldimine bonds, producing ferulic acid with an amine group (Khan, 2016). The presence of specific metals has also been reported to affect betalains degradation (Khan & Giridhar, 2014), so to reduce the effect of metal ions, chelating agents have been used, such as ascorbic acid or citric acid (Stintzing & Carle, 2008), which have been known to remove O2 from the solution and reduce the polarity at the N-1 position of betalains, which is susceptible to nucleophilic attack by water (Herbach et al., 2006). Betalains are easily degraded by light; this degradation is due to the absorption of UV light (Chhikara et al., 2019), and the degree of the effect depends on the light intensity, the presence or absence of oxygen, and the concentration and reactivity of betalains (Kayın et al., 2019). However, infrared light reportedly favors the accumulation of betacyanins in red beet roots (Shin et al., 2003). It has also been reported that water activity is a factor that must be controlled in products containing betalains, since high water activities potentiate betalains degradation, while low water activities improve their stability (Chhikara et al., 2019). There is also another series of processes, such as microwaving, boiling, roasting, vacuum, high pressure, ultrasound fermentation, pasteurization, and the use of additives, that have been shown to affect the stability of betalains in different products (Czyzowska et al., 2006, Laqui-Vilca et al., 2018, Moßhammer et al., 2007, Ravichandran et al., 2013, Sawicki et al., 2019). All these factors affect the structure of betalains in some way, which is reflected in a change in the color parameters (Güneşer, 2016, Moßhammer et al., 2007, Prieto-Santiago et al., 2020), so its control must be considered for use as a colorant and the development and incorporation into food products since the presence of all these factors limit their application in food, which is why several techniques have been used for their preservation. The encapsulation of betalains can help maintain their stability, increase their useful life, and improve their handling. Several factors have been reported that affect the retention of encapsulated betalains, such as the type and concentration of the wall material, the encapsulation technique, and the encapsulation conditions, among other factors. Among the wall materials, maltodextrin alone or in combination with other biopolymers is the polysaccharide that has been used most frequently for the encapsulation of betalains (Castro-Enríquez et al., 2020). However, maltodextrins have high solubility and hygroscopicity at high water activities (Prieto-Santiago et al., 2020), which is why materials such as gum arabic and whey protein concentrate, alginate, lecithin, and others have been used, which have been shown to delay or maintain the stability of the encapsulated betalains in addition to maintaining the integrity of the capsule at low water activities (Pitalua et al., 2010). Some studies have shown that the use of binary and ternary blends of polymers as wall materials produces higher retention and less degradation of betalains than using a single polymer, which is reflected in a higher encapsulation efficiency and greater retention of betalains during storage, and that the use of a second polymer generally increases the viscosity of the solution, leading to the formation of a thicker protective wall, which restricts the movement of betalains (Hogan et al., 2001).
Table 4.
Effect of processing factors on the stability of betalains obtained from different food sources.
| Process | Conditions | Products | Main Findings | Reference |
|---|---|---|---|---|
| Storage temperature and light | 25, 35 and 45 °C and light with/without aluminum foil | Red beet juice | Degradation of betalains, change in total phenols and color | Kayın et al., 2019 |
| Heating | 70–90 °C | Beet root | Degradation of betalains and color parameters. | Güneşer, 2016 |
| Heating | autoclave (120 °C) for 10, 20, 30, 40, 50, and 60 min | Beetroot juice, beetroot puree and whole peeled beetroots | Degradation of betalains and color parameters. | Prieto-Santiago et al., 2020 |
| Thermal stability and ultrasound treatment | 0–80 °C | Colored quinoa (Chenopodium quinoa Willd) hulls | Thermal stability was similar to that of betalains from beetroot | Laqui-Vilca et al., 2018 |
| High pressure processing (HPP) and high temperature short time (HTST) thermal treatment | HPP was applied at 000 bar for 10, 20 and 30 min and HTST treatment was applied at 75.7 °C for 80 s, 81.1 °C for 100 s and 85.7 °C for 120 s | Red beet stalks | HPP treatment did not show any improvement in the betalain stability. HTST was considered the most suitable to maintain betalain stability from red beet. |
dos Santos et al., 2018 |
| Presence of metals and ascorbic acid | Inorganic Se4+, Zn2+, and Cu2+ metal with/without ascorbic acid | Berry juice | Ascorbic acid protected the pigments from metal-induced bleaching | Khan & Giridhar, 2014 |
| Technological processes | Microwaving, boiling, roasting and vacuuming | Red beet | Vacuum and microwave produces increases in betalains, while boiling and roasting produces a decrease | Ravichandran et al., 2013 |
| Lactic acid fermentation | Three probiotic bacteria and three infant intestinal microbiota of Lactobacillus | Red beet juice | Lactic acid fermentation influenced color parameters | Czyzowska et al., 2006 |
| Food aditives And pH | Ascorbic, isoascorbic, and citric acid at pH 4 and 6 | Yellow-orange cactus pear | Pigment stability and color characteristics depended on type and concentration of the respective additive as well as on pH conditions. | Moßhammer et al., 2007 |
| Technological processes and in vitro digestion | Boiling, fermentation and microwave vacuuming treatment | Red beetroot products | Technological processes reduced the content of betalain by 42–70% in the obtained products. The contribution of betalains released from red beet products after in vitro digestion was detected within the range of 0.001–0.10%. | Sawicki et al., 2017 |
Notably, several betalains encapsulation techniques have been used, such as spray drying, lyophilization, coacervation, emulsion, ionic gelation, and sonication hydration. Spray drying turns out to be the most used method, primarily due to its low cost and the availability of equipment (Castro-Enríquez et al., 2020). The process has a high encapsulation efficiency, and the product obtained by this method is a highly manageable and versatile powder. However, for the high inlet temperatures used in the drying process, process yields may be less than 70%, and the high storage temperatures cause the degradation of the pigments encapsulated by this method (Chranioti et al., 2015; Soto-Castro et al., 2019). The primary problem with the powders obtained by spray drying is associated with the structural instability of the capsule or properties of the powder due to its high hygroscopicity that leads to a greater exposure of the betalains to environments with higher water activity and a greater exposure to oxygen, affecting their stability. Given this consideration, it is pertinent to deepen studies on new wall materials or mixtures thereof that offer these characteristics in the final powder. Another alternative encapsulation method, freeze-drying, has improved the stability of encapsulated betalains compared to spray drying (Ravichandran et al., 2014), but it is always recommended to consider the hygroscopicity of the system to guarantee its integrity and the stability of the pigment. Encapsulation in nanoliposomes using lecithin has protected the stability of betalains after ingestion in vitro (Amjadi et al., 2019), which implies that this type of system can favor the bioavailability of encapsulated betalains. The drawback of this liposomal system is that betalains have shown degradation problems during storage when they were incorporated in matrices such as gummy candies (Amjadi et al., 2018). However, the problem is possibly more associated with the composition and characteristics of the model matrix under study, such as its high hygroscopicity and water activity, which could participate in the oxidation of nanoliposomes. Ionic gelation is a technique that has effectively protected this group of pigments during storage (Otálora et al., 2019), while gelation using sodium alginate exhibited high encapsulation efficiency (Orozco-Villafuerte et al., 2019). However, more studies are still required to show the behavior and stability of this type of system under different storage conditions and in application matrices. Lastly, the encapsulation of betalains in emulsion systems has shown high degradation sensitivity influenced by temperature (Pagano et al., 2018), but despite these characteristics, the use of these emulsions as an intermediate product to obtain a powder by means of techniques such as spray drying or lyophilization to improve its stability or diversify its applicability should not be ruled out.
6. Applications of betalains as an additive in food
In the search for new sources of natural additives for use in food, numerous studies have evaluated the potential use of betalains as colorants, antioxidants, and antimicrobials. Attia et al. (2013) evaluated the effect of incorporating red beet extract as a colorant in jelly and ice sherbets for its sensory properties, observing that the general acceptability of the products is dependent on the concentration of added betalains and on properties comparable to those of a synthetic red colorant. Betalains have also been incorporated as colorants for ice cream, and they improve the acceptability of the product and have good color stability for 180 days under storage at −20 °C (Kumar et al., 2015, Roriz et al., 2018). Khan et al., (2015) incorporated betalains from berries (Rivina humilis) as a colorant for fruit spread and banana juice, and they observed that the stability of betalains in the fruit spread was not greater than 40% after six months storage at 5 °C. In the beverage, the proposed colorant was not viable due to the total loss of betalamic color during the pasteurization process. Güneşer (2016) observed that the betalains in beet roots could present moderate stability in response to thermal treatments (70–140 min, at 70–80 °C) when they are added as a colorant to cow milk. Gengatharan et al. (2016) evaluated the effect of pasteurization (30 min at 63 °C) on the stability of betalains from red pitahaya (Hylocereus polyrhizus) and red beet (Beta vulgaris; E-162) when added as a colorant to simulate a strawberry color in cow milk. The results showed that the stability and acceptability of the color were dependent on the betalains profile of the source of origin, indicating that the betalains in red pitahaya were more stable in response to the pasteurization process and 7 days of storage at 4 °C, and they showed a higher acceptability score color compared to E-162. Rodríguez-Sánchez et al. (2017) incorporated betaxanthins from yellow pitaya (S. pruinosus) fruit as a coloring for drinks and jelly gummies. They observed that the greatest betaxanthin stability was achieved when the product was stored at low temperatures and under dark conditions. In addition, they observed that these pigments were more stable in the gummies because of the food matrix (a protective effect was conferred by their interactions with proteins) and their low water activity. Kumar et al. (2020) observed that betalains from Basella rubra can be used as a colorant for banana spread with a stability of 95% after one year of storage at 5 °C, for an intermediate moisture food (making it a gel-like product) with a stability of 60% after two months, and for juices, bananas, and lemons, with a stability of 58% and 76%, respectively, after three months. In all cases, the proposed dye inhibited microbial growth and showed good sensory acceptability in the product. The results of these studies seem to indicate that the use of betalains as a colorant in food may be highly viable after considering three critical factors: 1) the betalains profile that constitutes the proposed natural colorant; 2) the composition of the food matrix (lower water activity, higher acidity, and presence of proteins favor color stability); and 3) the food storage conditions (products stored at −20–4 °C and protected from light are the best candidates).
The use of betalains as natural antioxidants has also been studied in several foods. Attia et al. (2013) studied the antioxidant effect of red beet roots in corn oil after seven days of storage at 60 °C, observing a decrease in the peroxide index with values similar to those obtained with BHT. Coria-Cayupán & Nazareno (2015) evaluated the protective effect of betalains from cactus pear fruits that were incorporated as natural pigments in dairy products (yogurt and cream), observing an inhibition of oxidative damage greater than 80% in yogurt and 50% in cream during the oxidation of the systems without the added pigments. da Silva et al. (2019) evaluated the lipid oxidation inhibition capacity of betanin when incorporated as an antioxidant in pork meat with results similar to those obtained by adding synthetic antioxidants such as BHA and BHT up to 6 days of storage to 4 °C. These results show that the use of betalains as antioxidant agents seems to meet the growing demand for increasingly natural foods by consumers; however, due to their pigmentation characteristics, the sensory impact on the food into which they are incorporated should not be disregarded.
7. New trends in applying betalains in the food industry
Faced with the demand to generate strategies that improve the shelf life of food, the monitoring of product quality in real time, the minimum use of synthetic preservatives, and the reduction of negative impacts on the environment, the development of new smart packaging based on biopolymers and natural extracts has increased in the food industry (Kanatt, 2020). The pH-sensitive property of betacyanins has been used in the development of smart films with potential applications in food packaging. Jamróz et al., (2019) observed that an extract rich in betalains from beet roots in furcellaran films changed from red to green when the films were exposed to ammonia. The developed film was applied as packaging to monitor the deterioration of fish fillets stored at 2 °C; however, the film's color change was not effective enough to inform trained panelists of the deterioration of the food. Under the same principle, Qin et al. (2020) incorporated an extract containing betalains from red pitaya in starch/polyvinyl alcohol films, yielding a film with antioxidant and antimicrobial properties that was successful as an intelligent packaging material to monitor the freshness of shrimp and had the potential to monitor the freshness of protein-rich animal foods. Similar results were observed by Hu et al. (2020) when incorporating amaranth betalains in a quaternary ammonium chitosan/fish gelatin film, yielding a functional film with improved antioxidant and antimicrobial properties against pathogens in food. The film also exhibited the ability to change color with pH sensitivity under alkaline conditions, which allowed its feasibility to be evaluated as a smart packaging material for monitoring the freshness of shrimp. Additionally, the effectiveness of the film's color change can be negatively affected by a higher content of betalains in the formulation. Lastly, Yao et al. (2020) developed antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan/polyvinyl alcohol with betalain extracts from cactus pears (Opuntia ficus-indica) and applied them as intelligent packaging materials that change color (from purple to orange) when shrimp lose their freshness. This finding indicates that some sources of betalains may have a place in the food industry not only as additive colorants, antioxidants, or antimicrobials but also because their participation is projected to become significant for the innovation and development of intelligent materials for the packaging of seafood industry products.
8. Conclusions
Each of the wall materials and encapsulation techniques used in the different investigations has advantages and disadvantages that must be considered during the development of microcapsules to obtain betalains with maximum stability and that affect their coloring properties to a lesser extent. Betalains have shown their potential as colorants, antioxidants, and antimicrobials in food matrices. Therefore, develop of intelligent and/or active materials for food packaging is very promising for the application and use of the properties of betalains in other fields of the food industry in the quest to extend shelf life, increase food safety, and reduce negative environmental impacts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
M. Jiménez-Fernández, Email: maribjimenez@uv.mx.
E. Lugo-Cervantes, Email: elugo@ciatej.mx.
References
- Amjadi S., Ghorbani M., Hamishehkar H., Roufegarinejad L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chemistry. 2018;256:156–162. doi: 10.1016/j.foodchem.2018.02.114. [DOI] [PubMed] [Google Scholar]
- Amjadi S., Mesgari Abbasi M., Shokouhi B., Ghorbani M., Hamishehkar H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. Journal of Functional Foods. 2019;59:119–128. doi: 10.1016/j.jff.2019.05.015. [DOI] [Google Scholar]
- Attia G.Y., Moussa M.E.M., Sheashea E.R. Characterization of red pigments extracted from red beet (Beta vulgaris L.) and its potential uses as antioxidant and natural food colorants. Egyptian. Journal of Agricultural Research. 2013;91(3):1095–1110. [Google Scholar]
- Barba-Espin G., Glied-Olsen S., Dzhanfezova T., Joernsgaard B., Lütken H., Müller R. Preharvest application of ethephon and postharvest UV-B radiation improve quality traits of beetroot (Beta vulgaris L. ssp. vulgaris) as source of colourant. BMC Plant Biology. 2018;18(1):1–12. doi: 10.1186/s12870-018-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Ugarte G.A., Sosa-Morales M.E., Ballard T., Liceaga A., San Martín-González M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris) LWT - Food Science and Technology. 2014;59(1):276–282. doi: 10.1016/j.lwt.2014.05.025. [DOI] [Google Scholar]
- Castro-Enríquez D.D., Montaño-Leyva B., Del Toro-Sánchez C.L., Juaréz-Onofre J.E., Carvajal-Millan E., Burruel-Ibarra S.E.…Rodríguez-Félix F. Stabilization of betalains by encapsulation—a review. Journal of Food Science and Technology. 2020;57(5):1587–1600. doi: 10.1007/s13197-019-04120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejudo-Bastante M.J., Chaalal M., Louaileche H., Parrado J., Heredia F.J. Betalain profile, phenolic content, and color characterization of different parts and varieties of opuntia ficus-indica. Journal of Agricultural and Food Chemistry. 2014;62(33):8491–8499. doi: 10.1021/jf502465g. [DOI] [PubMed] [Google Scholar]
- Chhikara N., Kushwaha K., Sharma P., Gat Y., Panghal A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chemistry. 2019;272:192–200. doi: 10.1016/j.foodchem.2018.08.022. [DOI] [PubMed] [Google Scholar]
- W.S. Choo Betalains: Application in Functional Foods 2019 10.1007/978-3-319-78030-6_38 1471 1498.
- Chranioti C., Nikoloudaki A., Tzia C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydrate Polymers. 2015;127:252–263. doi: 10.1016/j.carbpol.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Coria-Cayupán Y., Nazareno M.A. Cactus betalains can be used as antioxidant colorants protecting food constituents from oxidative damage. Acta Horticulturae. 2015;1067:319–325. doi: 10.17660/ActaHortic.2015.1067.44. [DOI] [Google Scholar]
- Czyzowska A., Klewicka E., Libudzisz Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. European Food Research and Technology. 2006;223(1):110–116. doi: 10.1007/s00217-005-0159-y. [DOI] [Google Scholar]
- da Silva D.V.T., dos Santos Baião D., de Oliveira Silva F., Alves G., Perrone D., Mere Del Aguila E.…V Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules. 2019;24(3):458. doi: 10.3390/molecules24030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva H.R.P., da Silva C., Bolanho B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.) Journal of Food Process Engineering. 2018;41(6):e12833. doi: 10.1111/jfpe.2018.41.issue-610.1111/jfpe.12833. [DOI] [Google Scholar]
- Das M., Saeid A., Hossain M.F., Jiang G.H., Eun J.B., Ahmed M. Influence of extraction parameters and stability of betacyanins extracted from red amaranth during storage. Journal of Food Science and Technology. 2019;56(2):643–653. doi: 10.1007/s13197-018-3519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos C.D., Ismail M., Cassini A.S., Marczak L.D.F., Tessaro I.C., Farid M. Effect of thermal and high pressure processing on stability of betalain extracted from red beet stalks. Journal of Food Science and Technology. 2018;55(2):568–577. doi: 10.1007/s13197-017-2966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathordoobady F., Mirhosseini H., Selamat J., Manap M.Y.A. Effect of solvent type and ratio on betacyanins and antioxidant activity of extracts from Hylocereus polyrhizus flesh and peel by supercritical fluid extraction and solvent extraction. Food Chemistry. 2016;202:70–80. doi: 10.1016/j.foodchem.2016.01.121. [DOI] [PubMed] [Google Scholar]
- Ferreres F., Grosso C., Gil-Izquierdo A., Valentão P., Mota A.T., Andrade P.B. Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chemistry. 2017;230:463–474. doi: 10.1016/j.foodchem.2017.03.061. [DOI] [PubMed] [Google Scholar]
- García-Cruz L., Dueñas M., Santos-Buelgas C., Valle-Guadarrama S., Salinas-Moreno Y. Betalains and phenolic compounds profiling and antioxidant capacity of pitaya (Stenocereus spp.) fruit from two species (S. Pruinosus and S. stellatus) Food Chemistry. 2017;234:111–118. doi: 10.1016/j.foodchem.2017.04.174. [DOI] [PubMed] [Google Scholar]
- Gengatharan A., Dykes G.A., Choo W.S. Stability of betacyanin from red pitahaya (Hylocereus polyrhizus) and its potential application as a natural colourant in milk. International Journal of Food Science and Technology. 2016;51(2):427–434. doi: 10.1111/ijfs.2016.51.issue-210.1111/ijfs.12999. [DOI] [Google Scholar]
- Güneşer O. Pigment and color stability of beetroot betalains in cow milk during thermal treatment. Food Chemistry. 2016;196:220–227. doi: 10.1016/j.foodchem.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Haq I.U., Butt M.S., Randhawa M.A., Shahid M. Extraction, characterization and optimization of betalains from red beetroot using response surface methodology. Pakistan Journal of Agricultural Sciences. 2020;57(2):535–543. doi: 10.21162/PAKJAS/20.8337. [DOI] [Google Scholar]
- Herbach K.M., Stintzing F.C., Carle R. Betalain stability and degradation - Structural and chromatic aspects. Journal of Food Science. 2006;71(4):R41–R50. doi: 10.1111/jfds.2006.71.issue-410.1111/j.1750-3841.2006.00022.x. [DOI] [Google Scholar]
- Hogan S.A., McNamee B.F., O’Riordan E.D., O’Sullivan M. Microencapsulating properties of sodium caseinate. Journal of Agricultural and Food Chemistry. 2001;49(4):1934–1938. doi: 10.1021/jf000276q. [DOI] [PubMed] [Google Scholar]
- Hu H., Yao X., Qin Y., Yong H., Liu J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. International Journal of Biological Macromolecules. 2020;159:675–684. doi: 10.1016/j.ijbiomac.2020.05.103. [DOI] [PubMed] [Google Scholar]
- Jamróz E., Kulawik P., Guzik P., Duda I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocolloids. 2019;97(April) doi: 10.1016/j.foodhyd.2019.105211. [DOI] [Google Scholar]
- Jiménez-Alvarado R., Aguirre-Álvarez G., Campos-Montiel R.G., Contreras-Esquivel J.C., Pinedo-Espinoza J.M., González-Aguayo E., Hernández-Fuentes A.D. Effect of High-Pulsed Electric Fields on the extraction yield and quality of juices obtained from the endocarp on nine prickly pear (Opuntia spp.) varieties. Jokull. 2015;65(3):414–435. [Google Scholar]
- Kanatt, S. R. (2020). Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packaging and Shelf Life, 24(December 2019), 100506. https://doi.org/10.1016/j.fpsl.2020.100506.
- Kayın N., Atalay D., Türken Akçay T., Erge H.S. Color stability and change in bioactive compounds of red beet juice concentrate stored at different temperatures. Journal of Food Science and Technology. 2019;56(11):5097–5106. doi: 10.1007/s13197-019-03982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I. Stabilization of betalains: A review. Food Chemistry. 2016;197:1280–1285. doi: 10.1016/j.foodchem.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Khan M.I., Giridhar P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L. berry juice. LWT - Food Science and Technology. 2014;58(2):649–657. doi: 10.1016/j.lwt.2014.03.027. [DOI] [Google Scholar]
- Khan M.I., Harsha P.S.C.S., Chauhan A.S., Vijayendra S.V.N., Asha M.R., Giridhar P. Betalains rich Rivina humilis L. berry extract as natural colorant in product (fruit spread and RTS beverage) development. Journal of Food Science and Technology. 2015;52(3):1808–1813. doi: 10.1007/s13197-013-1175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.N.A., Ritesh S.K., Sharmila G., Muthukumaran C. Extraction optimization and characterization of water soluble red purple pigment from floral bracts of Bougainvillea glabra. Arabian Journal of Chemistry. 2017;10:S2145–S2150. doi: 10.1016/j.arabjc.2013.07.047. [DOI] [Google Scholar]
- Kumar S.S., Manoj P., Shetty N.P., Prakash M., Giridhar P. Characterization of major betalain pigments -gomphrenin, betanin and isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. Journal of Food Science and Technology. 2015;52(8):4994–5002. doi: 10.1007/s13197-014-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.S., Arya M., Chauhan A.S., Giridhar P. Basella rubra fruit juice betalains as a colorant in food model systems and shelf-life studies to determine their realistic usability. Journal of Food Processing and Preservation. 2020;44(8):1–9. doi: 10.1111/jfpp.14595. [DOI] [Google Scholar]
- Kushwaha R., Kumar V., Vyas G., Kaur J. Optimization of different variable for eco-friendly extraction of betalains and phytochemicals from beetroot pomace. Waste and Biomass Valorization. 2018;9(9):1485–1494. doi: 10.1007/s12649-017-9953-6. [DOI] [Google Scholar]
- Laqui-Vilca C., Aguilar-Tuesta S., Mamani-Navarro W., Montaño-Bustamante J., Condezo-Hoyos L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Industrial Crops and Products. 2018;111:606–614. doi: 10.1016/j.indcrop.2017.11.034. [DOI] [Google Scholar]
- Li H., Deng Z., Liu R., Zhu H., Draves J., Marcone M.…Tsao R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. Journal of Food Composition and Analysis. 2015;37:75–81. doi: 10.1016/j.jfca.2014.09.003. [DOI] [Google Scholar]
- Loginova K.V., Lebovka N.I., Vorobiev E. Pulsed electric field assisted aqueous extraction of colorants from red beet. Journal of Food Engineering. 2011;106(2):127–133. doi: 10.1016/j.jfoodeng.2011.04.019. [DOI] [Google Scholar]
- Maran J.P., Priya B. Multivariate statistical analysis and optimization of ultrasound-assisted extraction of natural pigments from waste red beet stalks. Journal of Food Science and Technology. 2016;53(1):792–799. doi: 10.1007/s13197-015-1988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgar B., Dias M.I., Barros L., Ferreira I.C.F.R., Rodriguez-Lopez A.D., Garcia-Castello E.M. Ultrasound and microwave assisted extraction of Opuntia fruit peels biocompounds: Optimization and comparison using RSM-CCD. Molecules. 2019;24(19):3618. doi: 10.3390/molecules24193618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed E.E., Iwamoto S., Yamauchi R. Optimization of betalain extraction from Salicornia fruticosa and its encapsulation. Journal of Agroalimentary Processes and Technologies. 2018;24(1):1–8. [Google Scholar]
- Moßhammer M.R., Rohe M., Stintzing F.C., Carle R. Stability of yellow-orange cactus pear (Opuntia ficus-indica [L.] Mill. cv. ’Gialla’) betalains as affected by the juice matrix and selected food additives. European Food Research and Technology. 2007;225(1):21–32. doi: 10.1007/s00217-006-0378-x. [DOI] [Google Scholar]
- Naderi N., Stintzing F.C., Ghazali H.M., Manap Y.A., Jazayeri S.D. Betalain extraction from Hylocereus polyrhizus for natural food coloring purposes. Journal of the Professional Association for Cactus Development. 2010;12:143–154. [Google Scholar]
- Neagu C., Barbu V. Principal component analysis of the factors involved in the extraction of beetroot betalains. Agroalimentary Process Technology. 2014;20(4):311–318. http:// [Google Scholar]
- Nowacka M., Tappi S., Wiktor A., Rybak K., Miszczykowska A., Czyzewski J.…Tylewicz U. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods. 2019;8(7):244. doi: 10.3390/foods8070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, A. N., Saldanha Do Carmo, C., & Duarte, C. M. M. (2015). Production of a natural red pigment derived from Opuntia spp. using a novel high pressure CO2 assisted-process. RSC Advances, 5(101), 83106–83114. https://doi.org/10.1039/c5ra14998c.
- Orozco-Villafuerte J., Escobar-Rojas A., Buendía-González L., García-Morales C., Hernandez-Jaimes C., Alvarez-Ramirez J. Evaluation of the protection and release rate of bougainvillea (Bougainvillea spectabilis) extracts encapsulated in alginate beads. Journal of Dispersion Science and Technology. 2019;40(7):1065–1074. doi: 10.1080/01932691.2018.1496834. [DOI] [Google Scholar]
- Otálora M.C., de Jesús Barbosa H., Perilla J.E., Osorio C., Nazareno M.A. Encapsulated betalains (Opuntia ficus-indica) as natural colorants. Case study: Gummy candies. LWT. 2019;103:222–227. doi: 10.1016/j.lwt.2018.12.074. [DOI] [Google Scholar]
- Pagano A.P.E., Khalid N., Kobayashi I., Nakajima M., Neves M.A., Bastos E.L. Microencapsulation of betanin in monodisperse W/O/W emulsions. Food Research International. 2018;109:489–496. doi: 10.1016/j.foodres.2018.04.053. [DOI] [PubMed] [Google Scholar]
- Pandey G., Pandey V., Pandey P.R., Thomas G. Effect of extraction solvent temperature on betalain content, phenolic content, antioxidant activity and stability of beetroot (Beta vulgaris L.) powder under different storage conditions. Plant Archives. 2018;18(2):1623–1627. [Google Scholar]
- Pérez-Loredo M.G., De Jesús L.H., Barragán-Huerta B.E. Extraction of red pitaya (Stenocereus stellatus) bioactive compounds applying microwave, ultrasound and enzymatic pretreatments. Agrociencia. 2017;51(2):135–151. [Google Scholar]
- Pitalua E., Jimenez M., Vernon-Carter E.J., Beristain C.I. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food and Bioproducts Processing. 2010;88(2–3):253–258. doi: 10.1016/j.fbp.2010.01.002. [DOI] [Google Scholar]
- Prakash-Maran J., Manikandan S., Mekala V. Modeling and optimization of betalain extraction from Opuntia ficus-indica using Box-Behnken design with desirability function. Industrial Crops and Products. 2013;49:304–311. doi: 10.1016/j.indcrop.2013.05.012. [DOI] [Google Scholar]
- Prieto-Santiago V., Cavia M.M., Alonso-Torre S.R., Carrillo C. Relationship between color and betalain content in different thermally treated beetroot products. Journal of Food Science and Technology. 2020;57(9):3305–3313. doi: 10.1007/s13197-020-04363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Y., Liu, Y., Zhang, X., & Liu, J. (2020). Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocolloids, 100(October 2019), 105410. https://doi.org/10.1016/j.foodhyd.2019.105410.
- Ramli N.S., Ismail P., Rahmat A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus) The Scientific World Journal. 2014;2014:1–7. doi: 10.1155/2014/964731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K., Palaniraj R., Saw N.M.M.T., Gabr A.M.M., Ahmed A.R., Knorr D., Smetanska I. Effects of different encapsulation agents and drying process on stability of betalains extract. Journal of Food Science and Technology. 2014;51(9):2216–2221. doi: 10.1007/s13197-012-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K., Saw N.M.M.T., Mohdaly A.A.A., Gabr A.M.M., Kastell A., Riedel H.…Smetanska I. Impact of processing of red beet on betalain content and antioxidant activity. Food Research International. 2013;50(2):670–675. doi: 10.1016/j.foodres.2011.07.002. [DOI] [Google Scholar]
- Reshmi S.K., Aravindhan K.M., Suganya D. The effect of light, temperature, pH on stability of betacyanin pigments in Basella alba fruit. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(4):107–110. [Google Scholar]
- Rodriguez-Amaya D.B. Elsevier; 2019. Betalains. In Encyclopedia of Food Chemistry; pp. 35–39. [Google Scholar]
- Rodríguez-Sánchez J.A., Cruz y Victoria María.T., Barragán-Huerta B.E. Betaxanthins and antioxidant capacity in Stenocereus pruinosus: Stability and use in food. Food Research International. 2017;91:63–71. doi: 10.1016/j.foodres.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Roriz C.L., Barreira J.C.M., Morales P., Barros L., Ferreira I.C.F.R. Gomphrena globosa L. as a novel source of food-grade betacyanins: Incorporation in ice-cream and comparison with beet-root extracts and commercial betalains. LWT. 2018;92:101–107. doi: 10.1016/j.lwt.2018.02.009. [DOI] [Google Scholar]
- Sanchez-Gonzalez N., Jaime-Fonseca M.R., San Martin-Martinez E., Zepeda L.G. Extraction, stability, and separation of betalains from Opuntia joconostle cv. using response surface methodology. Journal of Agricultural and Food Chemistry. 2013;61(49):11995–12004. doi: 10.1021/jf401705h. [DOI] [PubMed] [Google Scholar]
- Sawicki T., Juśkiewicz J., Wiczkowski W. Using the SPE and Micro-HPLC-MS/MS method for the analysis of betalains in rat plasma after red beet administration. Molecules. 2017;22(12):2137. doi: 10.3390/molecules22122137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki T., Martinez-Villaluenga C., Frias J., Wiczkowski W., Peñas E., Bączek N., Zieliński H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. Journal of Functional Foods. 2019;55:229–237. doi: 10.1016/j.jff.2019.01.053. [DOI] [Google Scholar]
- Sawicki T., Surma M., Zieliński H., Wiczkowski W. Development of a new analytical method for the determination of red beetroot betalains using dispersive solid-phase extraction. Journal of Separation Science. 2016;39(15):2986–2994. doi: 10.1002/jssc.v39.1510.1002/jssc.201600196. [DOI] [PubMed] [Google Scholar]
- Shin K.S., Murthy H.N., Heo J.W., Paek K.Y. Induction of betalain pigmentation in hairy roots of red beet under different radiation sources. Biologia Plantarum. 2003;46(1):149–152. doi: 10.1023/A:1027313805930. [DOI] [Google Scholar]
- Silva, J. P. P., Bolanho, B. C., Stevanato, N., Massa, T. B., & da Silva, C. (2020). Ultrasound-assisted extraction of red beet pigments (Beta vulgaris L.): Influence of operational parameters and kinetic modeling. Journal of Food Processing and Preservation, June, e14762. https://doi.org/10.1111/jfpp.14762.
- Singh A., Ganesapillai M., Gnanasundaram N. Optimizaton of extraction of betalain pigments from Beta vulgaris peels by microwave pretreatment. IOP Conference Series: Materials Science and Engineering. 2017;263:032004. doi: 10.1088/1757-899X/263/3/032004. [DOI] [Google Scholar]
- Slavov A., Karagyozov V., Denev P., Kratchanova M., Kratchanov C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech Journal of Food Sciences. 2013;31(No. 2):139–147. [Google Scholar]
- Slimen I.B., Najar T., Abderrabba M. Chemical and antioxidant properties of betalains. Journal of Agricultural and Food Chemistry. 2017;65(4):675–689. doi: 10.1021/acs.jafc.6b04208. [DOI] [PubMed] [Google Scholar]
- Stintzing F.C., Carle R. In: Food colorants: Chemical and functional properties. Socaciu C., editor. CRC Press; Boca Raton: 2008. Betalains in food: Ocurrence, stability, and postharvest modificaciones; pp. 277–290. [Google Scholar]
- Swamy G.J., Sangamithra A., Chandrasekar V. Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box-Behnken design of experiments. Dyes and Pigments. 2014;111:64–74. doi: 10.1016/j.dyepig.2014.05.028. [DOI] [Google Scholar]
- Thirugnanasambandham K., Sivakumar V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. Journal of the Saudi Society of Agricultural Sciences. 2015;16(1):41–48. doi: 10.1016/j.jssas.2015.02.001. [DOI] [Google Scholar]
- Tutunchi P., Roufegarinejad L., Hamishehkar H., Alizadeh A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chemistry. 2019;297(March) doi: 10.1016/j.foodchem.2019.124994. [DOI] [PubMed] [Google Scholar]
- von Elbe J.H., Attoe E.L. Oxygen involvement in betanine degradation-Measurement of active oxygen species and oxidation reduction potentials. Food Chemistry. 1985;16(1):49–67. doi: 10.1016/0308-8146(85)90019-6. [DOI] [Google Scholar]
- Wang J., Jayaprakasha G.K., Patil B.S. UPLC-QTOF-MS fingerprinting combined with chemometrics to assess the solvent extraction efficiency, phytochemical variation, and antioxidant activities of Beta vulgaris L. Journal of Food and Drug Analysis. 2020;28(2):217–230. doi: 10.38212/2224-6614.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton-Beard P.C., Ryan L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. Proceedings of the Nutrition Society. 2011;70(OCE4):E135. doi: 10.1017/s0029665111001868. [DOI] [Google Scholar]
- Wybraniec S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. Journal of Agricultural and Food Chemistry. 2005;53(9):3483–3487. doi: 10.1021/jf048088d. [DOI] [PubMed] [Google Scholar]
- Yao X., Hu H., Qin Y., Liu J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocolloids. 2020;106(March) doi: 10.1016/j.foodhyd.2020.105896. [DOI] [Google Scholar]
- Yap C.H., Mat Junit S., Abdul Aziz A., Kong K.W. Multiple extraction conditions to produce phytochemical- and antioxidant-rich Alternanthera sessilis (red) extracts that attenuate lipid accumulation in steatotic HepG2 cells. Food Bioscience. 2019;32:100489. doi: 10.1016/j.fbio.2019.100489. [DOI] [Google Scholar]
- Zin M.M., Márki E., Bánvölgyi S. Conventional extraction of betalain compounds from beetroot peels with aqueous ethanol solvent. Acta Alimentaria. 2020;49(2):163–169. doi: 10.1556/066.2020.49.2.5. [DOI] [Google Scholar]
- Zin M.M., Anucha C.B., Bánvölgyi S. Recovery of phytochemicals via electromagnetic irradiation (microwave-assisted-extraction): Betalain and phenolic compounds in perspective. Foods. 2020;9(7):918. doi: 10.3390/foods9070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zin M.M., Borda F., Szilvia-Bánvölgyi E.M. 3rd International Conference on Biosystems and Food Engineering. 2019. Betalains, total polyphenols, and antioxidant contents in red beetroot peel (Cylindra type) [Google Scholar]