Highlights
-
•
Crude polyphenols from bignay fruit differing in maturity had varied bioactivities.
-
•
Kalabaw cultivar had higher in vitro lipid-lowering activities than Common cultivar.
-
•
Cholesterol-binding ability of bignay crude polyphenols was similar to the control.
-
•
Thermal processing decreased lipase inhibitory activity of bignay crude polyphenols.
-
•
Bignay fruits have huge potential as functional food in relation to dyslipidemia.
Keywords: Bignay, Bile acid, Cholesterol micellar solubility, Lipid-lowering, Pancreatic lipase, Polyphenols
Abstract
Bignay [Antidesma bunius (L). Spreng] fruit contains an array of polyphenols and information on how these bioactive compounds vary with cultivar type, maturity stage, and process treatment are unclear. Also, the effects of these variations on the lipid-lowering potential of this Philippine indigenous berry have not been reported. This study aimed to evaluate the lipid-lowering properties of the fruits of two bignay cultivars as affected by maturity stage and thermal processing. In vitro lipid-lowering assays revealed that both bignay cultivars had appreciable pancreatic lipase inhibitory activity, bile acid binding capacity, and cholesterol micellar solubility inhibition, which were comparable to those of the known lipid-lowering agents used as positive controls in this study. Freeze-dried samples of the freshly harvested fruits of both bignay cultivars [i.e., Common Cultivar (CC) and Kalabaw cultivar (KC)] had the highest bile acid binding activity (41.9–45.5% for CC and 43.4–54.0% for KC) for all the three maturity stages implying the beneficial effects of fresh bignay fruits related to lipid metabolism. Steam-blanched fruits had the highest pancreatic lipase inhibition activity (17.8–37.4% for CC and 29.2–39.0% for KC), regardless of maturity stage, while water-blanched samples exhibited the highest cholesterol micellar solubility inhibition (39.6–42.2% for CC and 40.2–47.6% for KC). Thermal processing tended to lower the lipid-lowering properties of the bignay fruits relative to their freeze-dried fresh fruits. Results of this study showed the potential of Philippine bignay fruit as a functional food that may be helpful in the management of dyslipidemia.
1. Introduction
Bignay [Antidesma bunius (L). Spreng] is a fruit-bearing tree commonly found in the Philippines. It belongs to the Euphorbiaceous family and has ovoid-shaped fruit clustered in a bunch of 30–40 tiny fruits. The fruits are typically eaten raw (Butkhup and Samappito, 2008), processed as wines (Belina-Aldemita, Sabularse, Dizon, Hurtada, & Torio, 2013), tea, jam, and jellies (Lizardo, Mabesa, Dizon, & Aquino, 2015). Furthermore, the fruit extract has been observed to have antibacterial properties (Lizardo, Mabesa, Dizon, & Aquino, 2015), α-glucosidase inhibitory activity (Lawag, Aguinaldo, Naheed, & Mosihuzzaman, 2012), anti-diabetic properties (El-Tantawy, Soliman, El-Naggar, & Shafei, 2015), and antioxidant properties (Belina-Aldemita, Sabularse, Dizon, Hurtada, & Torio, 2013). Due to these health-related properties of bignay, it can be considered as a functional food. Functional food refers to food items and products that are marketed with an increased quantity of biologically active food components providing health benefits apart from nutritive value (Crowe, 2013).
Bignay’s importance has grown as a significant source of polyphenols (Butkhup and Samappito, 2008, Santiago, Garcia, Dizon, & Merca, 2007). Polyphenols, having at least one aromatic ring with one or more hydroxyl groups, are secondary metabolites widely distributed in all plants synthesized during normal plant development as well as in response to stress conditions such as ultraviolet radiation, infections, bruising, etc. (Beckman, 2000, Malenčićet al., 2013). However, variety of factors, such as environmental condition, genotype, cultivar, harvest time, storage condition, and processing could affect the levels of polyphenols in foods and plants. Moreover, species is still considered to be the primary factor resulting in different quantities in different products.
In order to preserve the safety and quality attributes of fruits and vegetables, blanching is one of the simplest and oldest thermal processing treatments employed. It denatures enzymes including lipoxygenase, polyphenol oxidase, polygalacturonase, peroxidases, and chlorophyllase which are associated with quality and nutrient losses (Patras, Brunton, O’Donnell, & Tiwari, 2010, Xiao et al., 2017). Blanching can be done via water-blanching or steam-blanching.
The polyphenol-rich extracts from certain temperate berries have been shown to effectively inhibit pancreatic lipase (McDougall, Kulkarni, & Stewart, 2009), bile acid absorption (Kahlon & Smith, 2007), cholesterol micellar solubility (Liu et al., 2018) in vitro and thereby influence dietary lipids digestion and absorption that consequently aids in managing weight gain and obesity which contribute to the development of non-communicable lifestyle-related diseases such as diabetes and cardiovascular diseases. Currently, there is limited information on the in vitro lipid-lowering properties of bignay either as raw or in processed form. This study focused on yielding scientific evidence in support of the utilization of bignay fruit as a functional food, especially in addressing obesity through the evaluation of its in vitro lipid-lowering properties. Specifically, the in vitro bile acid binding capacity, pancreatic lipase inhibition, and cholesterol micellar solubility inhibition of two cultivars of Philippine bignay fruit were studied as affected by maturity stage (i.e., unripe, half-ripe, and fully ripe) and thermal processing treatments such as steam- and water-blanching.
2. Materials and methods
2.1. Plant materials, reagents, and sample preparation
The fruits of two bignay cultivars were obtained from the Institute of Plant Breeding, College of Agriculture and Food Science, University of the Philippines Los Baños (UPLB), College, Laguna, Philippines in May–August 2019. The fruits' flesh of two bignay cultivars namely the Common Cultivar (CC) and Kalabaw Cultivar (KC) were used in this study. The maturity stages at which bignay fruits were harvested included the unripe (green), half-ripe (red), and fully ripe (black) stages. Cholestyramine resin, p-nitrophenylbutyrate, cholesterol, sodium taurocholate, oleic acid, lipase (from porcine pancreas), methanol, Triton X-100, and other salts/reagents used in buffer and solution preparations were all purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All reagents used in this study were analytical and/or HPLC grade and distilled water was used all throughout, unless otherwise specified.
The fruits were washed thoroughly under running tap water to remove any dirt on the surface, air-dried, and separated into three portions. Equal portions (in grams) of the air-dried fruit samples were water-blanched or steam-blanched to employ thermal processing treatments at 90 ± 5 °C for 2 min and at 105 ± 5 °C for 5 min, respectively. The blanched fruits were cooled to room temperature (25–28 °C) and were manually pressed to separate the flesh from the seeds which were eventually discarded. The fruits' flesh samples were then homogenized manually using mortar and pestle until a semi-fine paste was formed. All treatments were freeze-dried using a freeze dryer fabricated for the Department of Science and Technology (DOST) (Gecar Machine Solutions, Inc. Quezon, Philippines) for at least 48 h including an equal portion (in grams) of the unprocessed freshly harvested fruits considered as control samples. The freeze-dried samples were ground accordingly using a DV Tech Grinder Model 525 (Madrid, Spain) and sieved using a commercially available fine-mesh (60 mm) sieve and then stored in polyethylene zip locks at 4 °C in a desiccator until further use.
2.2. Extraction of crude polyphenols from bignay fruits’ flesh
The method described by De Souza et al. (2014), with slight modifications, was used for the extraction of crude polyphenols from bignay fruits. Freeze-dried sample was placed into a beaker containing 50:50 (v/v) methanol:water with 1% glacial acetic acid and mixed using a Junior Orbit Shaker Model 3520 (Lab-Line Instruments Inc., IL, USA) set at 200 rpm for 1 h at room temperature (25–28 °C). The solution was then poured into a centrifuge tube and was centrifuged at 12,000 rpm for 30 min at 10 °C. It was decanted into an amber glass bottle and stored in the freezer at −10 °C until further use. These polyphenol crude extracts from freeze-dried fruits’ flesh of the two bignay cultivars harvested at different maturity stages and subjected to thermal process treatments prior to lyophilization were used for the lipid-lowering activity assays and parallel metabolomics profiling studies of which results will be published elsewhere.
2.3. Bile acid binding assay
The bile acid binding was carried out following the method of Kongo-Dia-Moukala, Zhang, & Irakoze (2011). Bile acid solution (2 mM) was prepared using sodium taurocholate dissolved in 50 mM phosphate buffer (pH 6.5). A 400-μL test sample was added to each microcentrifuge tube containing 400 μL bile acid solution while cholestyramine was used as a positive control. The solutions were incubated at 37 °C for 30 min, centrifuged at room temperature (25–28 °C) for 20 min with a speed of 6000 rpm using a Labnet Prism™ Model C1801-230 V-EU centrifuge (Labnet International Inc., NJ, USA), and supernatant was collected in a new set of microcentrifuge tubes. The supernatant was filtered using a 25-mm nylon-welded syringe filter (0.22 μm) prior to transferring into sample vials for separation and analysis via Acquity Ultra-Performance Liquid Chromatography H Class with RP BEH C18 column (130 Å, 1.7 μm, 2.1 mm × 50 mm) (Waters Corp., Prague, Czech Republic) maintained at 35 °C and detected through a photodiode-array detector (Waters Corp., Prague, Czech Republic). The injected sample volume was 3 μL for each sample and the bile acid (taurocholate) was eluted with methanol:0.4% KH2PO4 (65:35) at a flow rate of 0.1 mL/min for 6 min. The absorbance of the eluate was monitored continuously at 210 nm and quantified using a standard calibration curve generated from the peak area responses of the standard solutions. Two injections with three replicates each sample were conducted for this assay. From the standard calibration curve, unbound bile acid concentration was calculated.
2.4. Pancreatic lipase inhibition assay
The pancreatic lipase inhibition assay was carried out using the method described by Chedda et al. (2016). Phosphate buffer (pH 7.2) was prepared with 150 mM of sodium chloride and 0.5% (v/v) of Triton X-100. Porcine pancreatic lipase activity was measured using p-nitrophenylbutyrate (PNPB) as substrate. Lipase inhibition activity was determined on a 96-well plate where each well was added with a mixture of 25 μL of the test solution, 50 μL lipase enzyme solution, 100 μL phosphate buffer, and 25 μL PNPB solution, then incubated at 37 °C for 30 min. The amount of the released p-nitrophenol from the reaction mixture was measured at 400 nm using a Multiskan™ GO microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Orlistat (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) dissolved in dimethylsulphoxide was used as a positive control. Two determinations per test solution with three replicates each were conducted for this assay.
2.5. Cholesterol micellar solubility inhibition assay and cholesterol binding capacity
The inhibition of cholesterol micellar solubility was determined following the method of Zlatkis, Zak, & Boyle (1953). A cholesterol micellar solution was prepared by sonicating 10 mM sodium taurocholate, 0.4 mM cholesterol, 1 mM oleic acid in 132 mM NaCl, and 15 mM sodium phosphate buffer (pH 7.4). Test sample (450 μL) was mixed with 400 μL cholesterol micellar solution and incubated at 37 °C for 24 h followed by centrifugation at room temperature (25–28 °C) at a speed of 12,000 rpm for 30 min. The supernatant was collected for cholesterol concentration determination. Cholesterol micellar solubility activity was determined on a 96-well plate with each well containing a mixture of 80 μL of the incubated solution, 100 μL glacial acetic acid, and 120 μL Zak’s coloring reagent, then incubated for 30 min at room temperature. The cholesterol binding capacity was determined at 560 nm using the same microplate reader and quantified using a standard calibration curve. Two determinations with three replicates for each test solution were conducted for this assay.
2.6. Statistical analysis
All data were subjected to two-factor Analysis of Variance (ANOVA) at p ≤ 0.05 considering thermal processing treatments and maturity stages as main factors. Post-hoc mean comparisons were analyzed using Tukey’s Honestly Significant Difference (HSD) test at 95% level of confidence using IBM® Statistical Package for the Social Sciences (SPSS)® Version 20 Statistical Software.
3. Results
3.1. Varying concentrations of crude polyphenols extracted from bignay Kalabaw fruits subjected to different thermal processing treatments and their lipid-lowering properties in vitro
Pancreatic lipase inhibition and cholesterol micellar solubility inhibition were generally significantly higher in all crude polyphenols extracted from ground freeze-dried fully ripe fresh fruits (control) of bignay Kalabaw cultivar than in steam- or water-blanched bignay fruits, ranging from 46.7% to 40.6% (Table 1). Highest lipase inhibitory activity was noted for the 100 µg/mL test solution obtained from the control followed by the 80 µg/mL test solution. Inhibition of pancreatic lipase activity decreased from as low as 0.1% to as high as 14.4% when the fruits were subjected to thermal processing treatments with the highest degree of activity lowering observed for water-blanched samples. The lipase inhibitory activities of the crude polyphenols extracted from thermally processed bignay fruits did not go beyond 40% and the mean pancreatic lipase inhibitory activity of the water-blanched samples (37.1%) tended to be lower than that of the steam-blanched samples (38.3%). Highest inhibition was noted for 40 µg/mL test solution for the steam-blanched fruits while 60–80 µg/mL test solutions for the water-blanched fruits showed comparable and highest activity. Nonetheless, the test solutions having the highest concentration of crude polyphenols (i.e., 100 µg/mL) were used in succeding in vitro lipid-lowering experiments based on the results of the fresh (control) fully ripe bignay fruits since lipase inhibitory activity variations due to polyphenols concentration did not reach statistical significance within each process treatment (data not shown).
Table 1.
Percent inhibition of pancreatic lipase activity and cholesterol micellar solubility as affected by varying concentrations of crude polyphenols (20–100 μg/mL) extracted from bignay Kalabaw fruits subjected to steam-blanching and water-blanching.
| Percent Inhibition per Process Treatment | Concentration of Crude Polyphenols Test Solutions (μg/mL) |
||||
|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |
| Pancreatic lipase inhibition (%) | |||||
| Control (Fresh) | 43.5 ± 6.50a | 40.6 ± 4.16a | 42.1 ± 5.36a | 46.4 ± 1.84a | 46.7 ± 3.84a |
| Steam-blanched | 37.6 ± 10.40b | 40.5 ± 2.80b | 39.5 ± 6.66c | 36.8 ± 2.20b | 37.5 ± 5.08b |
| Water-blanched | 34.4 ± 3.84c | 32.3 ± 2.75c | 40.0 ± 2.60b | 40.0 ± 3.20c | 38.8 ± 2.63c |
| Cholesterol micellar solubility inhibition (%) | |||||
| Control (Fresh) | 45.3 ± 1.25a | 44.9 ± 1.51b | 44.5 ± 0.96a | 43.8 ± 2.29ab | 44.6 ± 0.74ab |
| Steam-blanched | 44.9 ± 2.03ab | 48.6 ± 1.20a | 42.0 ± 1.78b | 42.3 ± 7.11b | 37.4 ± 2.23b |
| Water-blanched | 40.2 ± 2.53b | 40.9 ± 2.47ab | 43.4 ± 2.09ab | 46.0 ± 0.86a | 46.4 ± 0.93a |
Means in the same column (per inhibition type) followed by different letters are significantly different via Tukey’s Honestly Significant Difference (HSD) test at p ≤ 0.05.
Cholesterol micellar solubility inhibition showed similar trend as in the lipase activity inhibition earlier mentioned but generally showed higher degree of inhibitory activity than the latter. Interestingly, unlike in the lipase inhibition assay, at 100 µg/mL crude polyphenols (i.e. highest test solution concentration), the cholesterol micellar solubility inhibition for the water-blanched bignay fruits yielded the highest activity followed by the fresh (control) samples, then by the steam-blanched samples. On the other hand, 40 µg/mL crude polyphenols from steam-blanched fruits showed the highest cholesterol micellar solubility inhibition among all the samples tested. The observed variations due to concentration of crude polyphenols were narrower than that observed for the lipase inhibitory activity and the differences did not reach statistical significance (Table 1). In addition, the mean activity was comparable for the steam-blanched and water-blanched bignay fruits (~43.4%) while that of the fresh (control) bignay fruits was significantly higher than these two in terms of mean cholesterol micellar solubility inhibitory activity.
3.2. Bile acid binding capacity
The bile acid (taurocholate) binding capacity of the crude polyphenols extracted from bignay Common cultivar (CC) fruits subjected to either steam-blanching or water-blanching varied widely but all were significantly lower than that of cholestyramine (positive control, including the fresh control) samples (Fig. 1A). In contrast, the taurocholate binding capacity of the crude polyphenols from thermally processed Kalabaw cultivar (KC) were comparable and were significantly lower than the results for cholestyramine (positive control) and the fresh (control) samples (Fig. 1B). With CC, the bile acid binding capacity increased as the fruit matured to fully ripe stage and it was noted that the half-ripe CC fruits yield the lowest binding capacity for both thermal process treatments as well as for the control (mean = 41.8%). Overall, KC crude polyphenols tended to have higher bile acid binding capacity as compared to CC even when fruits were thermally processed and considering all the three maturity stages in this study (Fig. 1).
Fig. 1.
Bile acid (taurocholate) binding of crude polyphenols extracted from the fruits of two bignay cultivars (A - Common cultivar; B - Kalabaw cultivar) harvested at different maturity stages and subjected to steam- and water-blanching. Control means freeze-dried fresh samples and Cholestyramine (a hypocholesterolemic drug) was used as positive control. Values followed by different superscript letters are significantly different via Tukey’s Honestly Significant Difference (HSD) test at p ≤ 0.05.
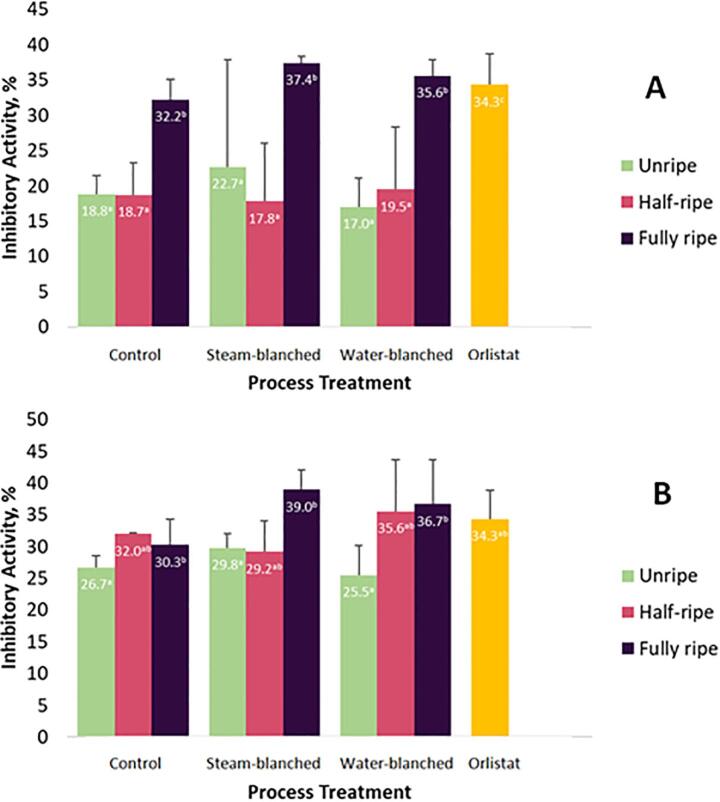
3.3. Pancreatic lipase inhibition assay
The percent inhibition of pancreatic lipase due to crude polyphenols extracts from fresh and thermally processed CC and KC showed consistently high activity for the fully ripe fruits except the fresh (control) samples of KC (Fig. 2). Interestingly, extracts from the fully ripe fruits of both cultivars yielded significantly higher lipase inhibitory activity than the positive control Orlistat with around 1.3–4.7% difference in activity. Inhibitory activity of the extracts from steam-blanched and water-blanched CC fruits were generally low as compared to that of Orlistat and those obtained for KC samples, similar to the observations in the bile acid binding capacity discussed above. This implies varietal differences in in vitro lipid-lowering properties as far as these two Philippine indigenous bignay berries are concerned. It was also observed that there was an increase of pancreatic lipase inhibition for CC fruits as maturity progressed towards fully ripe stage, but this trend was not perfectly observed in the case of KC fruits. Furthermore, unripe and half-ripe fruits yielded statistically similar and low lipase inhibitory activities, regardless of thermal processing treatments employed as well as in the case of fresh (control) samples. This suggests that fully ripe bignay fruits exert appreciable lipase inhibitory action, either as raw fresh fruits, steam-blanched or water-blanched fruits (Fig. 2). Highest lipase inhibition was noted for crude polyphenols extracted from steam-blanched fully ripe KC fruits while lowest was recorded for the crude polypehonols extracted from water-blanched unripe fruits of CC. Generally, the difference in percent lipase inhibition was around 15–20% for CC fruits, while 5–14% for KC samples considering fruit maturity stage, regardless of the thermal process employed.
Fig. 2.
Pancreatic lipase inhibition of crude polyphenols extracted from the fruits of two bignay cultivars (A - Common cultivar; B – Kalabaw cultivar) harvested at different maturity stages and subjected to steam- and water-blanching. Control means freeze-dried fresh samples and Orlistat (a lipase-inhibiting drug) was used as positive control. Values followed by different superscript letters are significantly different via Tukey’s Honestly Significant Difference (HSD) test at p ≤ 0.05.
3.4. Cholesterol binding capacity
The cholesterol binding capacity of crude polyphenols extracted from CC fruits tended to be lower than that of the positive control cholestyramine (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) while KC fruits had comparable or higher capacity, regardless of maturity stage and thermal processing treatments employed, except for the water-blanched fully ripe KC fruits (Fig. 3). This corroborates the suggestion that with the two bignay cultivars used in this study, varietal difference in terms of in vitro lipid-lowering properties is apparent as mentioned in the discussion above. For CC fruits, only the water-blanched samples had a significant increase in cholesterol binding capacity as maturity progressed to fully ripe stage while the half-ripe fruits showed highest binding capacity in both fresh (control) and steam-blanched fruits (Fig. 3A). Cholestyramine also had significantly higher cholesterol binding capacity. For KC fruits, it was noteworthy that the crude polyphenols extracted from the water-blanched fully ripe fruit samples had significantly lower binding capacity (~40%) than cholestyramine (~46%), however, all other treatments, including the fresh (control) samples showed over 40% cholesterol binding capacity, some even higher than that of cholestyramine, as in the case of steam- and water-blanched half-ripe KC fruits. This interestingly suggests that unripe and half-ripe KC fruits, may they be thermally processed or in the raw fresh form, may exert comparable cholesterol binding action at this level with the commercially available hypocholesterolemic cholestyramine and comparison with other over-the-counter blood cholesterol-lowering drugs warrants further studies. Furthermore, steam- and water-blanched KC fruits tended to have increased cholesterol binding activity from unripe to half-ripe and similar trend was observed for the fresh (control) and steam-blanched CC fruits (Fig. 3). An opposite trend was noted for the unripe and half-ripe fruits of water-blanched CC and fresh (control) KC. The marked lowering of cholesterol binding capacity of water-blanched fully ripe KC fruit crude polyphenols, on the other hand, needs validation. Results of the in vitro cholesterol binding activity assay showed the superiority of crude polyphenols extracted from KC fruits in the unripe and half-ripe stages over cholestyramine and CC fruits, though consistent statistical significance was not achieved. Nonetheless, further studies on other food processing treatments involving this particular bignay cultivar may support the results of the present work on the cholesterol-lowering potential of KC fruits, regardless of maturity stage.
Fig. 3.
Cholesterol binding capacity of crude polyphenols extracted from the fruits of two bignay cultivars (A - Common cultivar; B - Kalabaw cultivar) harvested at different maturity stages and subjected to steam- and water-blanching. Control means freeze-dried fresh samples and Cholestyramine (a hypocholesterolemic drug) was used as positive control. Values followed by different superscript letters are significantly different via Tukey’s Honestly Significant Difference (HSD) test at p ≤ 0.05.
4. Discussion
Berries, like bignay, contain various dietary polyphenols that may vary in terms of bioavailability (Koli et al., 2010). The mechanism of action of polyphenols in relation to different digestive processes and on lipid metabolism is not yet clear. However, it has been hypothesized that possible metabolic interactions and physiological processes such as binding and increasing excretion of bile acids as well as hampered enzymatic lipolysis may possibly result in the reduction of plasma cholesterol and triglyceride levels in vivo. Cholestyramine, a bile acid sequestrant, binds bile acid via ionic interaction that disrupts the solubilization of cholesterol in the gut and its enterohepatic circulation, thus, impeding reabsorption. This consequently reduces the bile acid level in the pool. In order to maintain a steady level of bile acid, de novo synthesis of bile acid from cholesterol will take place. In combination, these scenarios could lead to decreased plasma cholesterol (Insull, 2006). Ogawa et al. (2016) reported that epigallocatechin gallate (EGCG), a tea polyphenol, strongly interacts with the steroid skeleton of sodium taurocholate, a common bile salt, through hydrogen bonding with the gallate moiety thereby disrupting bile acid emulsifying activity and preventing the formation of lipid and cholesterol micelles. The complex formation between the hydroxyl groups in bignay fruit polyphenols with the steroid backbone of sodium taurocholate might similarly explain the observed inhibitory activity of the samples in this study. The taurocholate binding capacity of the bignay crude polyphenols reported here ranging from 41.5% to 54.0%, were generally higher as compared to gallic acid, a phenolic acid from grape seed, reported to have ~43% bile acid binding capacity at 2 mg/mL concentration (Ngamukote et al., 2011). In addition, the bile acid binding activity of spinach at different harvest time was observed to be significantly higher at 20 and 40 days as compared to 30, 50, and 60 days and the levels of bound sodium cholate were significantly higher at 30 and 50 days (Barkat, Singh, Jayaprakasha, & Patil, 2018). The differences in the levels of polyphenols in spinach might be attributed to the growing conditions and stresses experienced by the food crop, both biotic and abiotic (Howard, Pandjaitan, Morelock, & Gil, 2002). Similar trend was observed in the present study; wherein as maturity of bignay fruits progressed from unripe to fully ripe and regardless of the thermal processing treatments employed for both cultivars, the bile acid binding capacity significantly decreased from unripe to half-ripe and then increased from half-ripe to fully ripe.
Pancreatic lipase is the primary lipolytic enzyme that hydrolyzes dietary fats or triglycerides. Lipase inhibitors, such as Orlistat, are substances that reduce the activity of lipases in order to decrease the gastrointestinal absorption of dietary fats and subsequently eliminate them via fecal route in vivo (Heck, Yanovski, & Calis, 2000). Martinez-Gonzalez et al. (2017) proposed that polyphenols tend to form a complex with pancreatic lipase in a static-quenching mechanism. Furthermore, it was shown in a previous molecular docking experiment that polyphenols bind at the active site of pancreatic lipase rendering competitive inhibition against the substrate and that the hydrogen bonds and π-stacking interactions were mainly involved and may explain the mechanism in play in the case of crude polyphenols extracted from the bignay fruits reported here (Martinez-Gonzalez et al., 2017). The present study showed that bignay polyphenols inhibited pancreatic lipase by 18.7%−39.0%. Chedda et al. (2016) reported that Indian spices exhibited pancreatic lipase inhibition, but not in a concentration-dependent manner. The highest inhibitory activity for 30 μg/mL Fenugreek, 1 μg/mL Bishop’s weed, and 1 μg/mL Black cumin were reported as 66%, 61%, and 80.5%, respectively. These Indian spices contained relatively high amounts of quercetin, a flavonoid and a potent pancreatic lipase inhibitor (Sergent, Vanderstraeten, Winand, Beguin, & Schneider, 2012, Youet al., 2012). The pancreatic lipase inhibition observed in this study might also be attributed to the quercetin content found in bignay cultivars which have been previously reported to have varying amounts depending on the cultivar (Butkhup and Samappito, 2008).
Dietary lipids need to be solubilized as micelles in the small intestine prior to absorption (Smet, Mensink, & Plat, 2012). The monoacylglycerides and free fatty acids liberated from the action of pancreatic lipase retain their association with bile forming amphipathic micelles that act as carriers of non-polar molecules to the brush border of the small intestine for absorption via diffusion (Iqbal & Hussain, 2009). Crude polyphenols in two Philippine indigenous bignay cultivars were shown here to appreciably bind sodium taurocholate by 41.5–54.0%. Bile salts such as sodium taurocholate are required for micelle formation and disruption of bile acid function resulting to lower plasma cholesterol (Coreta-Gomes, Vaz, Wasielewski, Geraldes, & Moreno, 2016). Consequently, polyphenols from bignay fruits significantly reduced the solubility of cholesterol micelles in vitro by 33.2–47.6%, higher than individual phenolics in grape seed extracts, such as gallic acid, catechin, epicatechin at 0.2 mg/mL concentration (27.2, 11.9, and 19.5%, respectively) (Ngamukote et al., 2011). Tea polyphenols, on the other hand, were found to form a complex with phosphatidylcholine (PC), a predominant bile phospholipid, which decreases the micellar solubility of cholesterol (Kobayashi et al., 2014). It has also been suggested that EGCG decreases the micellar solubility of cholesterol through specific interactions with PC. Similarly, the formation of complex interactions with the bile acid and the interference of bignay polyphenols with the structure of micellar cholesterol might have caused the micelles to be larger and insoluble resulting in the cholesterol micellar solubility inhibition observed here. Similar studies have shown that polyphenols from berries and plant extracts, specifically mulberry and sweetleaf, were able to inhibit the cholesterol micellar solubility by more than 50% (Adisakwattana, Intrawangso, Hemrid, Chanathong, & Mäkynen, 2012) as noted in the present study and were similar to the positive control. This suggests that bignay crude polyphenols, specifically those extracted from KC fruits, have fairly high cholesterol micellar solubility reduction capability as that of cholestyramine.
Polyphenol contents may vary especially during ripening. At earlier stages of ripening, polyphenols were at highest concentration (Eid, Al-Awadi, Vauzour, Oruna-Concha, & Spencer, 2013). During berry ripening, an upsurge of anthocyanin concentration was observed while non-anthocyanin polyphenols decreased due to competition for substrates during anthocyanin synthesis (Liang et al., 2011). Thermal processing may also cause complex physical and chemical reactions among phenolic compounds including degradation of polyphenols and release of bound phenolics (Xu & Chang, 2008). These main factors greatly influence polyphenol contents that consequently affect the organoleptic quality as well as the health benefits of berries (Liang et al., 2011). Variations in the in vitro lipid-lowering properties of Philippine bignay fruits of two cultivars harvested at different maturity stages and subjected to steam- and water-blanching were described in this study. Anthocyanins, which constitute the main group of phenolic compounds in berries have been observed to increase with maturation in many fruits (Delgado, Martín, Del Álamo, & González, 2004, Vulić et al., 2011). In contrast, as reported by Ivanova et al. (2011), other polyphenols have been shown to decrease due to oxidation and enzymatic browning during ripening.
Aside from fruit maturity, the magnitude and duration of thermal processing can strongly influence polyphenols content, stability, and bioactivity, specifically anthocyanins (Patras, Brunton, O’Donnell, & Tiwari, 2010). Brownmiller, Howard, & Prior (2008) have shown that blanching of blueberry purees at 95 °C for three minutes, in combination with pasteurization, resulted in 43% reduction of total anthocyanins as compared to original levels found in the fresh blueberry fruits. Furthermore, anthocyanin losses ranging from 10% to 80% was reported in jams manufactured by boiling raw foods for 10–15 minutes (García-Viguera & Zafrilla, 2000). In contrast, other previous reports showed that blanching or heating to about 70–100 °C could have a positive effect on the retention of anthocyanins and other polyphenols at a certain amount of time, typically in less than 10 minutes (Xiao et al., 2017). Processing time and temperature combinations may not be the only factors affecting anthocyanin stability but also the berries’ intrinsic factors such as pH, anthocyanin content, enzymes, proteins and metallic ions and other extrinsic factors as well (i.e., storage temperature, light, and oxygen) (Rein, 2005). These factors may influence the bioactivities of polyphenols and anthocyanins in berries and may explain the variations in the in vitro lipid-lowering properties of Philippine indigenous berries observed in this study.
5. Conclusion
Crude polyphenols extracted from the fruit flesh of two Philippine bignay cultivars showed promising lipid-lowering properties in vitro. The crude polyphenols extracted from the fruits of Kalabaw cultivar generally showed higher in vitro lipid-lowering capacity over the Common cultivar crude polyphenols, considering sodium taurocholate binding, pancreatic lipase inhibition, and to cholesterol micellar solubility inhibition, and cholesterol binding. Lipid-lowering potentials of crude polyphenols from two Philippine indigenous berries reported here were comparable to those obtained from popular lipid-lowering agents available in the market. Bignay fruits have huge potential as functional food in relation to dyslipidemia, warrant further in vivo validation studies, and may be further developed into an effective food product/ingredient with notable lipid-lowering properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We gratefully acknowledge the Science Education Institute (SEI) of the Department of Science and Technology (DOST) through its Accelerated Science and Technology Human Resource Development Program-National Science Consortium (ASTHRDP-NSC) for BRA Crieta's graduate scholarship grant. The authors also thankfully acknowledge the DOST-National Research Council of the Philippines (DOST-NRCP) and the DOST-Philippine Council for Health Research and Development (DOST-PCHRD) for additional funding. We also thank Lloyd Earl L. Flandez, Abbie Glenn M. Estribillo, and Joshua B. Benedicto of the Institute of Food Science and Technology, College of Agriculture and Food Science (IFST-CAFS), University of the Philippines Los Baños (UPLB) for their technical assistance in the conduct of this study. We thank Dr. Pablito M. Magdalita of the Institute of Plant Breeding, CAFS, UPLB for the kind gift of bignay fruits used in this study.
Funding sources
This study was part of the research project funded by the Department of Science and Technology-Philippine Council for Health Research and Development (DOST-PCHRD).
Author contributions
BRAC, APPT, and KATCI conceptualized and designed the study. BRAC, PJVG, and JCV performed the experiments. KATCI and APPT supervised the conduct of experiments. KATCI as program leader, spearheaded all in vitro characterization studies and related activities under the Philippine Indigenous Berries Program (BERRYPINOY) of IFST-CAFS, UPLB funded by DOST-PCHRD. JCV and MAOT previously optimized and validated the in vitro assays prior to use in this study. BRAC, APPT, and KATCI interpreted the results and wrote the paper. All authors approved the final version of the manuscript prior to submission.
References
- Adisakwattana S., Intrawangso J., Hemrid A., Chanathong B., Mäkynen K. Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids. Food Technology and Biotechnology. 2012;50(1):11–16. [Google Scholar]
- Barkat N., Singh J., Jayaprakasha G.K., Patil B.S. Effect of harvest time on the levels of phytochemicals, free radical-scavenging activity, α-amylase inhibition and bile acid-binding capacity of spinach (Spinacia oleracea) Journal of the Science of Food and Agriculture. 2018;98(9):3468–3477. doi: 10.1002/jsfa.8862. [DOI] [PubMed] [Google Scholar]
- Beckman C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiological and Molecular Plant Pathology. 2000;57(3):101–110. [Google Scholar]
- Belina-Aldemita M.D., Sabularse V.C., Dizon E.I., Hurtada W.A., Torio M.A.O. Antioxidant properties of bignay [Antidesma bunius (L.) Spreng.] wine at different stages of processing. Philippine Science Letters. 2013;6:249–256. [Google Scholar]
- Butkhup L., Samappito S. An analysis on flavonoids contents in Mao Luang fruits of fifteen cultivars (Antidesma bunius), grown in northeast Thailand. Pakistan Journal of Biological Sciences. 2008;11(7):996–1002. doi: 10.3923/pjbs.2008.996.1002. [DOI] [PubMed] [Google Scholar]
- Brownmiller C., Howard L.R., Prior R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. Journal of Food Science. 2008;73(5):H72–H79. doi: 10.1111/j.1750-3841.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- Chedda U., Kaikini A., Bagle S., Seervi M., Sathaye S. In vitro pancreatic lipase inhibitionpotential of commonly used Indian spices. IOSR Journal of Pharmacy. 2016;6(10):10–13. [Google Scholar]
- Coreta-Gomes F.M., Vaz W.L., Wasielewski E., Geraldes C.F., Moreno M.J. Quantification of cholesterol solubilized in dietary micelles: Dependence on human bile salt variability and the presence of dietary food ingredients. Langmuir. 2016;32(18):4564–4574. doi: 10.1021/acs.langmuir.6b00723. [DOI] [PubMed] [Google Scholar]
- Crowe K.M. Designing functional foods with bioactive polyphenols: Highlighting lessons learned from original plant matrices. Journal of Human Nutrition and Food Science. 2013;1(3):1018–1019. [Google Scholar]
- De Souza V.R., Pereira P.A.P., da Silva T.L.T., de Oliveira Lima L.C., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chemistry. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- Delgado R., Martín P., Del Álamo M., González M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. Journal of the Science of Food and Agriculture. 2004;84(7):623–630. [Google Scholar]
- Eid N.M., Al-Awadi B., Vauzour D., Oruna-Concha M.J., Spencer J.P. Effect of cultivar type and ripening on the polyphenol content of date palm fruit. Journal of Agricultural and Food Chemistry. 2013;61(10):2453–2460. doi: 10.1021/jf303951e. [DOI] [PubMed] [Google Scholar]
- El-Tantawy W.H., Soliman N.D., El-Naggar D., Shafei A. Investigation of antidiabetic action of Antidesmabunius extract in type 1 diabetes. Archives of Physiology and Biochemistry. 2015;121(3):116–122. doi: 10.3109/13813455.2015.1038278. [DOI] [PubMed] [Google Scholar]
- García-Viguera C., Zafrilla P. In: Chemistry and Physiology of Selected Food Colorants. Ames J.M., Hofmann T, editors. Vol. 775. American Chemical Society; USA: 2000. Changes in anthocyanins during food processing: influence on color; pp. 56–65. (ACS Symposium Series). [DOI] [Google Scholar]
- Heck A.M., Yanovski J.A., Calis K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2000;20(3):270–279. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L.R., Pandjaitan N., Morelock T., Gil M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. Journal of Agricultural and Food Chemistry. 2002;50(21):5891–5896. doi: 10.1021/jf020507o. [DOI] [PubMed] [Google Scholar]
- Insull W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. Southern Medical Journal. 2006;99(3):257–274. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Hussain M.M. Intestinal lipid absorption. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(6):E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova V., Stefova M., Vojnoski B., Dörnyei Á., Márk L., Dimovska V., Kilár F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Research International. 2011;44(9):2851–2860. [Google Scholar]
- Kahlon T.S., Smith G.E. In vitro binding of bile acids by blueberries (Vaccinium spp.), plums (Prunus spp.), prunes (Prunus spp.), strawberries (Fragaria X ananassa), cherries (Malpighia punicifolia), cranberries (Vaccinium macrocarpon) and apples (Malus sylvestris) Food Chemistry. 2007;100(3):1182–1187. [Google Scholar]
- Kobayashi M., Nishizawa M., Inoue N., Hosoya T., Yoshida M., Ukawa Y., Ikeda I. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. Journal of Agricultural and Food Chemistry. 2014;62(13):2881–2890. doi: 10.1021/jf405591g. [DOI] [PubMed] [Google Scholar]
- Koli R., Erlund I., Jula A., Marniemi J., Mattila P., Alfthan G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. Journal of Agricultural and Food Chemistry. 2010;58(7):3927–3932. doi: 10.1021/jf9024823. [DOI] [PubMed] [Google Scholar]
- Kongo-Dia-Moukala J.U., Zhang H., Irakoze P.C. In vitro binding capacity of bile acids by defatted corn protein hydrolysate. International Journal of Molecular Sciences. 2011;12(2):1066–1080. doi: 10.3390/ijms12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawag I.L., Aguinaldo A.M., Naheed S., Mosihuzzaman M. α-Glucosidase inhibitory activity of selected Philippine plants. Journal of Ethnopharmacology. 2012;144(1):217–219. doi: 10.1016/j.jep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Liang Z., Sang M., Fan P., Wu B., Wang L., Duan W., Li S. Changes of polyphenols, sugars, and organic acid in 5 Vitis genotypes during berry ripening. Journal of Food Science. 2011;76(9):C1231–C1238. doi: 10.1111/j.1750-3841.2011.02408.x. [DOI] [PubMed] [Google Scholar]
- Liu S., You L., Zhao Y., Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Research International. 2018;107:73–83. doi: 10.1016/j.foodres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Lizardo R.C.M., Mabesa L.B., Dizon E.I., Aquino N.A. Functional and antimicrobial properties of bignay [Antidesma bunius (L.) Spreng.] extract and its potential as natural preservative in a baked product. International Food Research Journal. 2015;22(1):88. [Google Scholar]
- Malenčić D., Cvejić J., Tepavčević V., Bursać M., Kiprovski B., Rajković M. Changes in L-phenylalanine ammonia-lyase activity and isoflavone phytoalexins accumulation in soybean seedlings infected with Sclerotinia sclerotiorum. Open Life Sciences. 2013;8(9):921–929. [Google Scholar]
- Martinez-Gonzalez A.I., Alvarez-Parrilla E., Díaz-Sánchez Á.G., de la Rosa, Núñez-Gastélum J.A., Gonzalez-Aguilar G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technology and Biotechnology. 2017;55(4):519–530. doi: 10.17113/ftb.55.04.17.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G.J., Kulkarni N.N., Stewart D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chemistry. 2009;115(1):193–199. [Google Scholar]
- Ngamukote S., Mäkynen K., Thilawech T., Adisakwattana S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011;16(6):5054–5061. doi: 10.3390/molecules16065054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Hirose S., Nagaoka S., Yanase E. Interaction between tea polyphenols and bile acid inhibits micellar cholesterol solubility. Journal of Agricultural and Food Chemistry. 2016;64(1):204–209. doi: 10.1021/acs.jafc.5b05088. [DOI] [PubMed] [Google Scholar]
- Patras A., Brunton N.P., O’Donnell C., Tiwari B.K. Effect of thermal processing on anthocyanin stability in foods: Mechanisms and kinetics of degradation. Trends in Food Science and Technology. 2010;21(1):3–11. [Google Scholar]
- Rein M. University of Helsinki; Finland: 2005. Copigmentation reactions and color stability of berry anthocyanins. PhD Dissertation. [Google Scholar]
- Santiago D.M.O., Garcia V.V.G., Dizon E.I., Merca F.E. Antioxidant activities, flavonol and flavanol content of selected Southeast Asian indigenous fruits. Philippine Agricultural Scientist. 2007;90(2):123–130. [Google Scholar]
- Sergent T., Vanderstraeten J., Winand J., Beguin P., Schneider Y.J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chemistry. 2012;135(1):68–73. [Google Scholar]
- Smet E.D., Mensink R.P., Plat J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: suggested mechanisms from past to present. Molecular Nutrition and Food Research. 2012;56(7):1058–1072. doi: 10.1002/mnfr.201100722. [DOI] [PubMed] [Google Scholar]
- Vulić J.J., Tumbas V.T., Savatović S.M., Đilas S.M., Ćetković G.S., Čanadanović-Brunet J.M. Polyphenolic content and antioxidant activity of the four berry fruits pomace extracts. Acta PeriodicaTechnologica. 2011;42:271–279. [Google Scholar]
- Xiao H.W., Pan Z., Deng L.Z., El-Mashad H.M., Yang X.H., Mujumdar A.S., Zhang Q. Recent developments and trends in thermal blanching - A comprehensive review. Information Processing in Agriculture. 2017;4(2):101–127. [Google Scholar]
- Xu B., Chang S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. Journal of Agricultural and Food Chemistry. 2008;56(16):7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- You Q., Chen F., Wang X., Jiang Y., Lin S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT-Food Science and Technology. 2012;46(1):164–168. [Google Scholar]
- Zlatkis A., Zak B., Boyle A.J. A new method for the direct determination of serum cholesterol. Journal of Laboratory and Clinical Medicine. 1953;41(3):486–492. [PubMed] [Google Scholar]