Highlights
-
•
Heat stress did not alter the major carcass traits and meat quality variables.
-
•
Heat stress did not influence pH, drip loss, cooking loss, WHC, shear force and MFI.
-
•
MSTN, HSP27, CRYA and HSP90 genes could serve as biomarkers for meat quality in goats.
Keywords: Calpain system, Carcass, Climate resilience, Goat, Heat stress, HSPs, Meat quality
Abstract
A study was conducted to assess the impact of heat stress on various carcass traits, meat quality variables and gene expression patterns which governs meat quality in indigenous female Kodi Aadu breed. The study was conducted for 45 days in climate chamber with 12 animals randomly allocated into two groups of six animals each, KC (n = 6; Female; Control), KHS (n = 6; Female; heat stress). Majority of the major carcass traits and meat quality variables remained intact between KC and KHS groups. The myostatin (MSTN), calpain 1 (CAPN1) and Diacylglycerol Acyltransferase 1 (DGAT1) mRNA expression patterns were significantly (P < 0.01) lower in KHS group as compared to KC group. However, the calpain 2 (CAPN2), calpastatin (CAST) and Crytallin alpha (CRYA) mRNA expression patterns were significantly (P < 0.05) higher in KHS group. Thus, the study established that the major carcass traits and meat quality variables remained intact after heat stress exposure in female Kodi Aadu goats. Further, MSTN, HSP27, CRYA and HSP90 genes were identified as biomarkers for reflecting meat quality during heat stress exposure in female Kodi Aadu breed.
1. Introduction
Environmental variables, particularly high ambient temperature and humidity due to climate change not only causes physiological and metabolic changes in live animals but also impacts severely the meat production in terms of affecting the carcass yield, composition and meat quality attributes (Zhang et al., 2012, Abhijith et al., 2021). Heat stress causes dehydration in water deprived animals that makes the meat darker because of shrinkage of the myofibrils (Jacob et al., 2006). Heat stress also increases the lipid and protein oxidation resulting in decreased shelf life and safety of meat due to microbial growth (Wang et al., 2009). High ambient temperature significantly affects the quality of hot-boned psoas major and minor muscles of sheep and goats slaughtered during the hot season in Oman (Kadim et al., 2008). Heat stress increases the meat ultimate pH, myofibrillar fragmentation index (an effective tool in predicting meat tenderness early post-mortem) and decrease the drip losses (Kadim et al., 2008). These authors also observed that the darkness of meat increased in both Omani and Somali goats during hot season as compared to cold season. Impact of heat stress on carcass characteristics of Osmanabadi and Salem Black goats was studied by Archana et al. (2018) and they observed that the pH of the longissimus thoracis muscle was higher only in Osmanabadi goat and not in Salem Black goats suggesting the better adaptability of the later breed to hot and humid climatic conditions (Archana et al., 2018).
Heat shock protein (HSP) plays a significant role in farm animals during the heat stress condition which enhances cell survival, prevents cellular damage and protein degradation (Sejian et al., 2018). Significantly higher expression of HSP70 mRNA was observed in longissimus thoracis muscles of Osmanabadi and Salem Black goats exposed to heat stress (Archana et al., 2018). Higher expression of HSP27 was also observed in samples of less tender meat of cattle (Carvalho et al., 2014). This indicates that upregulation of HSPs in skeletal muscles could serve as potential biomarkers to reflect meat quality (Cassar-Malek and Picard, 2016). Apart from the classical molecular chaperones there are several other genes which determine the quality of the meat (Lonergan et al., 2010). Calpain system is the most well-known proteolytic system which affects meat tenderness (Huff-Lonergan & Lonergan, 1999). Increased activity of calpastatin causes a decrease in calpain activity by preventing the calpain proteolytic activation, membrane binding and the expression of catalytic activity that leads to decreased meat tenderness (Lonergan et al., 2010). Diacylglycerol acyltransferases 1 (DGAT1) is another gene involved in fatty acid metabolism and are considered as potential candidate genes for meat tenderness (Cases et al., 1998). However, not much information is available connecting the expression pattern of these genes with heat stress exposure in farm animals.
The potential to survive in diversified climatic condition, drought tolerance, ability to survive on limited pasture, ability to walk long distances in search of limited pasture, possessing unique feeding behaviour which imparts them the potential to consume any feed which is not suitable for consumption by any other livestock species makes goats to be the ideal species to survive and produce optimally in the changing climate scenario (Reshma Nair et al., 2021). However, breed variations were observed in all above traits in goats. Therefore, it is very vital to generate all baseline information pertaining to climate resilience in indigenous goat breeds. Such information would be very vital for identifying and disseminating the ideal goat breed to any specific agro-ecological zone for the marginal farmers to get benefit out of goat farming. This study is one such attempt to elucidate the production potential of female Kodi Aadu goats in terms of meat production characteristics and quality traits when these animals were subjected to heat stress. Special emphasis was given to understand the expression patterns of classical meat quality governing genes in the heat stressed Indigenous female Kodi Aadu goats.
2. Materials and methods
2.1. Location of the study
The experiment was conducted at the Centre for Climate Resilient Animal Adaptation Studies (CCRAAS), ICAR- National Institute of Animal Nutrition and Physiology experimental livestock farm (NIANP), Bengaluru, India which is located at the longitude 77° 38́E and the latitude of 12° 58́N and at altitude of 920 m above mean sea level. The experiment was conducted during May-June 2020 in state-of-the-art facility climate chamber. The control animals were kept in comfort condition with a temperature of 24 °C in thermo-neutral zone (TNZ) chamber and heat stress group was kept inside the heating/ cooling chamber with a simulated heat stress model from 10:00 h to 16:00 h (Supplementary Table 1). The parameters related to meat quality characteristics were conducted at Department of Livestock Products Technology, Veterinary College, Hebbal, Bengaluru. The study was conducted after obtaining due approval from the ethical committee for subjecting the animal to heat stress and for slaughtering the animals. The slaughter procedures were conducted as per the guidelines of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forest and Climate Change, Animal welfare Department, Government of India (CPCSEA/2/2017).
Table 1.
Effect of heat stress on major carcass traits and primal cuts in Kodi Aadu control and heat stressed groups.
| Traits |
Kodi Aadu |
Significance | |
|---|---|---|---|
| KC | KHS | ||
| Pre-slaughter weight (PSW) (kg) | 14.11 ± 0.66 | 14.21 ± 0.89 | NS |
| Hot carcass weight (HCW) (kg) | 6.65 ± 0.32 | 6.67 ± 0.48 | NS |
| Dressing Percentage (%) | 47.14 ± 0.67 | 46.84 ± 1.01 | NS |
| Loin eye area (LEA) (cm2) | 6.02 ± 0.05 | 5.73 ± 0.09 | NS |
| Fore Saddle (Kg) | 3.44 ± 0.18 | 3.42 ± 0.23 | NS |
| Fore Saddle (%) | 51.67 ± 0.33 | 51.35 ± 0.45 | NS |
| Neck (Kg) | 0.42 ± 0.02 | 0.41 ± 0.03 | NS |
| Shoulder (Kg) | 1.69 ± 0.09 | 1.73 ± 0.12 | NS |
| Rack (Kg) | 0.66 ± 0.05 | 0.64 ± 0.07 | NS |
| Breast (Kg) | 0.23 ± 0.02 | 0.25 ± 0.02 | NS |
| Shank (Kg) | 0.41 ± 0.01 | 0.41 ± 0.02 | NS |
| Hind Saddle | 3.08 ± 0.16 | 3.03 ± 0.23 | NS |
| Hind Saddle (%) | 46.26 ± 0.47 | 45.30 ± 0.35 | NS |
| Loin (Kg) | 0.53 ± 0.03 | 0.49 ± 0.05 | * |
| Flank (Kg) | 0.24 ± 0.02 | 0.23 ± 0.02 | NS |
| Leg (Kg) | 2.31 ± 0.12 | 2.30 ± 0.17 | NS |
*P < 0.05; NS- Non-Significant, KC- Kodi Aadu Control, KHS- Kodi Aadu Heat Stress
2.2. Animals used in the study
In the present study female Kodi Aadu breed goats was used as they are considered as one of the breeds that thrives on harsh environmental condition. It is an important meat goat breed in the Southern Tamil Nadu, India. Kodi Aadu goats are distributed in southern coastal regions of Tamil Nadu and are docile, hardy, meat purpose goats with fast growth rate and early maturity. They have a predominant white coat color with scattered black and brown patches. The average body weight of Kodi Aadu female ranges between 25 and 30 kg. Twelve female animals aged approximately one year were procured from Tirunelveli district of Tamil Nadu and were transported to the experimental facility at ICAR-NIANP, Bengaluru. The animals were kept for acclimatization for a period of 45 days in the experimental farm unit before the start of the experiment.
2.3. Experimental design
The study was conducted for a period of 45 days in controlled climate chamber between May-June 2020. Twelve female (one year old) Kodi Aadu goats were used in the present study. The animals were randomly allocated into two groups of six animals each, KC (n = 6; Control), KHS (n = 6; heat stress). The animals were stall fed with a diet consisting of 60 per cent roughage (Hybrid Napier hay) and 40 per cent concentrate (Maize 36 kg, wheat bran 37 kg, soybean meal 25 kg, mineral mixture 1.5 kg and common salt 0.5 kg per 100 kg). All animals were individually given access to ad-libitum feed and water. The control (C) goats were placed in the thermo neutral zone (TNZ), i.e., control chamber, while the heat stress (HS) goats were exposed to heat stress in heating chamber with a simulated heat stress model between 10.00AM to 4:00PM on all 45 days (Fig. 1a). Fig. 1b describes the animals in the heating climate chamber. All cardinal weather parameters were recorded twice daily for the entire duration of the study. The animals were slaughtered at the end of the study to assess the meat and carcass characteristics. The skeletal muscles were simultaneously collected for gene expression study.
Fig. 1.
(a) Climate chamber: Thermo-neutral Zone Chamber (left) and Heating/ Cooling Chamber (right) outside view; (b) Climate Chamber facility of ICAR-NIANP with the experimental animals inside the chambers.
2.4. Recording of weather variables & calculating THI
Both ambient temperature and relative humidity were recorded using thermo-hygrometer (Vaishno Instruments, Telangana, India). Additionally, climatic chambers are well equipped with weather sensors, which are connected to a server, from which we can collect the data in no time. The simulated temperature (Supplementary Table 1) model was used to generate heat stress in heating chamber. The model has been prepared as per the IMD data of Tamil Nadu region. The temperature humidity index during the current experiment was calculated with the following Mc Dowell (1972) formula:
THI = 0.72 (Tdb + Twb) + 40.6, where Tdb- Dry bulb temperature; Twb- Wet bulb temperature.
2.5. Slaughter procedure and carcass evaluation
All twelve animals were fasted for 12 h overnight with ad libitum access to water before the day of slaughter. The slaughter was conducted following all hygienic procedures in the slaughter house at Experimental Livestock Unit, ICAR-National Institute of Animal Nutrition and Physiology, Bengaluru. The animals were slaughtered by severing their jugular vein and carotid artery. After slaughter, the head was removed at the atlanto-occipital joint, fore and hint limbs were removed at the carpal and tarsal joints respectively. The animals were partially skinned lying on their back on the floor and then suspended to gambrel by the hind leg for further skinning. Sticking, legging, dressing and evisceration were performed as per the procedure described by Gerrard (1964). Details on the measurements of various carcass traits were described in detail in the supplementary materials.
2.5.1. Major carcass characteristics
The pre-slaughter weights (PSW) and hot carcass weights (HCW), loin eye area (LEA) were recorded using weighing machine (Essae-Teraoka Limited, Mumbai, India) in kg. Dressing percentage (DP) was calculated on the basis of hot carcass weight (HCW) and live weight (LW) using the following formula DP = HCW/LW * 100 Where DP- Dressing Percentage; HCW- Hot Carcass Weight; LW- Live Weight. The carcass was cut into different primal cuts viz., leg, loin, rack, neck and shoulder, flank, and breast and fore shank as per specifications of ISI (1995) and were individually weighed and recorded. The various linear carcass measurements were recorded on hot carcass using a flexible measuring tape and callipers. The edible offals (liver, blood, heart, liver, kidney,) and inedible offals (skin, head, feet, spleen, lung with trachea, Reproductive organ, Mammary gland) were separately weighed.
2.5.2. Physico-chemical characteristics
The pH of LTL muscle was estimated after 45 min and 24hrs of slaughter using a digital pH meter (Labman, LMPH-10, New Delhi, India). Immediately after death, one complete thigh and one complete thigh muscle was weighed and placed in a polyethylene bag. The samples were kept at + 4 °C during 24 h, then wiped and weighed. Drip loss was calculated and expressed as the percentage of the initial weight (Remignon et al., 1996). Cooking loss % was determined by weight loss after cooking of meat for 1 h in water bath at 80 °C (Babiker et al., 1990). The water holding capacity (WHC) was determined according to the protocol of Wardlaw et al. (1973). The shear force values were estimated using the protocol described by Wheeler et al. (1997) with certain modifications using Warner Bratzler Shear (G. R. Electric Manufacturing Company, Manhattan, USA). The mean shear force needed to shear through the core was assessed by taking the average of the six readings.
The LTL fiber diameter was measured as per the method outlined by Jeremiah and Martin (1977). Muscle fiber diameter was measured as the mean cross-sectional distance between exterior surfaces of Sarcolemma of 20 randomly selected muscle fibers and expressed in micrometer (µm). Sarcomere length of each muscle sample was measured as per the method outlined by Hostetler et al. (1972). Sarcomere lengths of 25 randomly selected fiber fragments were measured under Olympus BX53 phase contrast microscope with image analyser software under 100X and is expressed in micrometer (µm).
Hydroxyproline content of the meat sample was estimated based on the procedure of as described by Naveena et al. (2004). The collagen content was calculated by multiplying hydroxyproline content with 7.14 (Dransfield et al., 1983) and was expressed in mg/g tissue.
Collagen solubility was assessed using the method described by Mahendrakar et al. (1989). Myofibrillar fragmentation index was estimated by following the method given by Davis et al. (1980) with slight modifications as suggested by Hawkins et al. (1985). R- value was estimated as per the method outlined by (Honikel and Fischer, 1977). The R- value of the meat samples were estimated at 2 and 24 h after slaughter.
The LTL muscle samples were divided into approximately 2 × 2 × 2 cm sub samples which were cooked in a pressure cooker for 30 min. Then the samples were coded and serving sequence was randomized. A sensory panel of eight experienced members evaluated the different sensory attributes of the meat samples as per method described by Keeton (1983). The colour of the meat sample eight-point was measured using Hunter lab Mini scan XE plus Spectro- colorimeter (Model No. 45/O-L, Reston Virginia, USA) with geometry of diffuse/80 (sphere − 8 mm view) and an illuminant of D65/10 deg (Bindu et al., 2007). The myoglobin was extracted from meat sample using a procedure as detailed by Warris (1979) with slight modifications. The myoglobin (mg/g tissue) and Met-myoglobin (%) (Met-Mb) were calculated using the formula described by (Trout, 1989). The protein extractability, sarcoplasmic protein and total protein was determined according to the procedure given by Joo et al. (1999). Myofibrillar protein extractability was obtained by calculating the difference between total protein and sarcoplasmic protein extractability. Proximate compositions such as moisture, protein, fat and ash were estimated using the method by (Association of Official Analytical Chemists) AOAC, 2005.
2.6. Expression patterns of genes governing meat quality in LTL muscle
The total RNA was isolated from LTL muscles using GeneJET RNA Purification Kit (Thermo Scientific, Lithuania) and the procedure was done as per manufacturer’s protocol.
Total RNA isolated from different tissues was treated with DNase (TURBO DNA-free, Ambion, USA) in order to eliminate the genomic DNA contamination in total RNA. The total RNA was reverse transcribed into cDNA using Maxima first strand cDNA synthesis kit for RT-qPCR (Thermo Scientific, Lithuania).
Gene specific primers were designed using online NCBI primer design software (Primer 3, http://bioinfo.ut.ee/primer3/) and specificity was checked using Primer3 and BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) for targeted genes. The preferences were given to the primers binding to the exon-exon junction. Supplementary Table 2 describes the primer sequences for both the targeted genes and housekeeping genes.
The relative expression of targeted genes such as heat shock factor 1 (HSF1), heat shock protein 10 (HSP10), HSP27, HSP40, HSP60, HSP70, HSP90, HSP110, αB-crystallin (CRYAb), myostatin (MSTN), calpain 1 (CAPN1), CAPN2, CAST, and DAGT1 genes were studied using SYBR green chemistry (Maxima SYBR green qPCR master mix, Fermentas, USA) following methodology described by Archana et al. (2018). Both Hypoxanthine Phosphoribosyl transferase (HPRT) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were used as an internal control and the relative expression was analyzed using the formula, 2ΔΔCT method (Archana et al., 2018). The results were expressed in fold change as compared to untreated control (control = 1fold).
2.7. Statistical analysis
The data was analysed by one- way analysis of variance (ANOVA) SPSS (version 18.0) software. The changes in relative expression of all targeted genes in skeletal muscle in relation to HPRT and GAPDH as the house keeping genes were analyzed by t-test. Further, the correlation coefficient between the THI and all carcass traits and gene expression patterns were established by Pearson’s correlation coefficient test using SPSS (version 18.0) software. The R2 values were used to establish the correlation association between THI and various carcass traits. Results are shown as mean ± standard error (SE) and the significance level was set at P < 0.05.
3. Results
3.1. Temperature humidity index
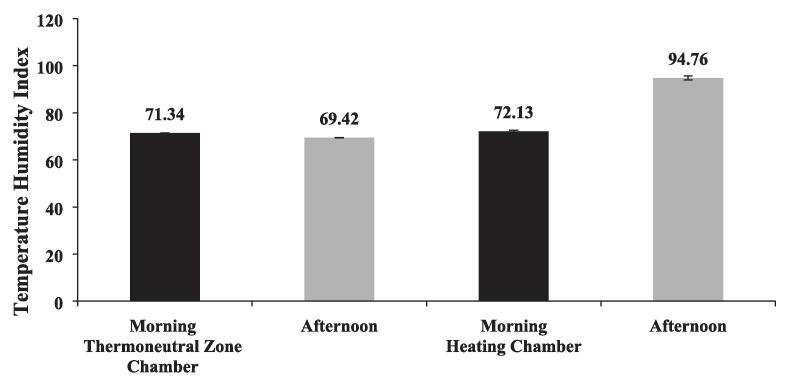
The THI values obtained between thermo-neutral and heating climate chambers both during morning and afternoon hours are depicted in Fig. 2. The THI calculated based on temperature and relative humidity also showed similar pattern to other weather variables between the chambers. The THI values obtained both during morning and afternoon in Thermo-neutral zone (TNZ) chamber and heating chambers were 71.34 ± 0.09, 69.42 ± 0.05, 72.13 ± 0.07 and 94.76 ± 0.45, respectively. The THI values were comparable during morning hours in both the climate chambers. However, during afternoon the THI values showed significant (P < 0.01) variation between the chambers with higher value recorded in the heating chamber.
Fig. 2.
Average temperature humidity index (THI) for the study period both in Thermo-neutral Chamber and Heating Chamber.
3.2. Major carcass traits
The variation in Mean ± SE of carcass traits viz., pre-slaughter weight, hot carcass weight, dressing percentage and loin eye area (LEA) are depicted in the Table 1. The correlation between THI and major carcass traits are described in supplementary Table 3. The pre-slaughter weight (PSW) in KC, KHS was 14.11 ± 0.66 and 14.21 ± 0.89 respectively. The hot carcass weight (HCW) in KC, KHS was 6.65 ± 0.32 and 6.67 ± 0.48 respectively. The dressing percentage (DP) in KC, KHS was 47.14 ± 0.67 and 46.84 ± 1.01, respectively. The loin eye area (LEA) in KC, KHS group was 6.02 ± 0.05 and 5.73 ± 0.09, respectively. The Mean ± SE of PSW, HCW, DP, LEA values showed no significant difference (P > 0.05) between control and heat stressed groups. However, the THI had a negative correlation (P < 0.05) only with LEA.
3.3. Primal cuts
The Mean ± SE weights of fore saddle, hind saddle, neck, shoulder, rack, loin, flank, shank, leg and breast are described in Table 1. Heat stress significantly (P < 0.05) influenced only loin variable among the primal cuts with significantly lower value in KHS group as compared to KC group. Further, the heat stress did not reveal any significant (P > 0.05) variation in other primal cuts between KC and KHS group animals. Further, THI also did not showed correlation with any of the primary cuts variables (Supplementary Table 4)
3.4. Linear carcass measurements
The Mean ± SE measurements of external carcass length, internal carcass length, buttock circumference, leg width, leg length, chest circumference, chest depth, chest width, and shoulder circumference are described in Table 2. The effects of heat stress were significant only on shoulder circumference (P < 0.05). The values of external carcass length, internal carcass length, leg width, chest circumference, chest depth, chest width and leg length were comparable (P > 0.05) between KC and KHS groups. Furthermore, no correlation was established between THI and other linear carcass variables (Supplementary Table 5).
Table 2.
Effect of heat stress on linear carcass measurements and non-carcass components and offals in Kodi Aadu control and heat stressed groups.
| Traits |
Kodi Aadu |
Significance | |
|---|---|---|---|
| KC | KHS | ||
| External Carcass Length (in) | 25.00 ± 0.45 | 26.00 ± 0.52 | NS |
| Internal Carcass Length (in) | 17.33 ± 0.42 | 18.17 ± 0.40 | NS |
| Buttock Circumference (in) | 15.17 ± 0.31 | 14.58 ± 0.46 | NS |
| Leg Width (in) | 3.58 ± 0.15 | 3.75 ± 0.25 | NS |
| Chest Circumference (in) | 22.17 ± 0.60 | 22.00 ± 0.52 | NS |
| Chest Depth (in) | 6.67 ± 0.17 | 6.83 ± 0.17 | NS |
| Chest Width (in) | 1.92 ± 0.08 | 2.17 ± 0.11 | NS |
| Shoulder Circumference (in) | 11.33 ± 0.40 | 11.67 ± 0.21 | * |
| Leg Length (in) | 13.83 ± 0.60 | 14.50 ± 0.56 | NS |
| Blood Wt. (kg) | 0.45 ± 0.03 | 0.45 ± 0.04 | NS |
| Head Wt. (kg) | 1.14 ± 0.04 | 1.14 ± 0.07 | NS |
| Skin Wt. (kg) | 1.11 ± 0.04 | 1.16 ± 0.07 | NS |
| Feet Wt. (kg) | 0.54 ± 0.03 | 0.54 ± 0.03 | NS |
| Heart (kg) | 0.09 ± 0.00 | 0.08 ± 0.01 | * |
| Liver (kg) | 0.26 ± 0.01 | 0.25 ± 0.01 | NS |
| Kidneys (kg) | 0.95 ± 0.01 | 0.10 ± 0.01 | NS |
| Spleen (kg) | 0.04 ± 0.00 | 0.04 ± 0.01 | NS |
| Lungs (kg) | 0.36 ± 0.02 | 0.36 ± 0.03 | NS |
| Brain (kg) | 0.09 ± 0.00 | 0.07 ± 0.01 | * |
| Reproductive Organ (kg) | 0.02 ± 0.00 | 0.06 ± 0.04 | * |
| Mammary Gland (kg) | 0.03 ± 0.01 | 0.02 ± 0.00 | NS |
*P < 0.05; NS- Non-Significant, KC- Kodi Aadu Control, KHS- Kodi Aadu Heat Stress.
3.5. Non-carcass components and offals
The Mean ± SE weights of blood, head, skin, feet, heart, liver, kidneys, spleen, lungs with trachea, reproductive organ, mammary gland in KC and KHS group are depicted in Table 2. The effects of heat stress were significant (P < 0.01) on some of the offals such as heart (P < 0.01), brain (P < 0.05) and reproductive organs (P < 0.05). Heart weight was observed to have significantly (P < 0.05) lower value in the KHS group (0.08 ± 0.01) as compared to the KC (0.09 ± 0.01) animals. Likewise, the brain weight also showed same trend with significantly (P < 0.05) lower value in KHS group (0.07 ± 0.01) as compared to KC group (0.09 ± 0.00) animals. However, reproductive organs showed contrasting result to this, with heat stressed group (0.06 ± 0.04) showing significantly higher (P < 0.05) value than the control group animals (0.02 ± 0.00). However, no significant difference (P > 0.05) was observed in blood, head, skin, feet, liver, kidneys, spleen, lungs and mammary gland between KC and KHS groups. Further, there was no any correlation established between THI and non-carcass components (Supplementary Table 6).
3.6. Physico-chemical characteristics
The variation in Mean ± SE of physico-chemical attributes viz., pH, drip loss, cooking loss, water holding capacity, shear force, muscle fiber diameter, sarcomere length, collagen content, myofibrillar fragmentation index, rigor value, collagen solubility are described in Table 3. Heat stress did not influence (P > 0.05) both pH45min, ultimate pH24hrs changes, drip loss, cooking loss, WHC, shear force, MFD, sarcomere length, collagen content, MFI and rigor value in LTL muscle. Collagen solubility percentage is the only physico-chemical variable influenced (P < 0.01) by heat stress across the groups. Further, THI and drip loss, WHC, shear force, MFD, sarcomere length, collagen content, collagen solubility, MFI and rigor value of LTL muscle did not show any correlation (Supplementary Table 7). However, a strong positive correlation (P < 0.05) was established between THI and pH24hrs of LTL muscle while a mild positive correlation (P < 0.05) was established between THI and cooking loss of LTL muscle (Supplementary Table 7).
Table 3.
Effect of heat stress on physico-chemical attributes, sensory attributes, meat colours, soluble proteins and proximate composition in Kodi Aadu control and heat stressed groups.
| Traits |
Kodi Aadu |
Significance | |
|---|---|---|---|
| KC | KHS | ||
| pH45min | 6.63 ± 0.07 | 6.76 ± 0.02 | NS |
| pH24hrs | 5.63 ± 0.03 | 6.03 ± 0.05 | NS |
| Drip loss % | 1.55 ± 0.21 | 1.85 ± 0.26 | NS |
| Cooking loss (%) | 22.33 ± 1.56 | 26.43 ± 0.83 | NS |
| Water holding capacity (%) | 16.17 ± 2.10 | 19.17 ± 2.24 | NS |
| Shear force (kg/cm2) | 7.63 ± 0.15 | 8.03 ± 0.14 | NS |
| Muscle Fiber Diameter (µm) | 40.52 ± 1.45 | 44.82 ± 2.40 | NS |
| Sarcomere Length (µm) | 1.73 ± 0.04 | 1.59 ± 0.09 | NS |
| Collagen Content (mg/g) | 4.08 ± 0.17 | 4.12 ± 0.12 | NS |
| Collagen Solubility (%) | 30.29 ± 1.48 | 27.35 ± 0.38 | ** |
| MFI | 531.83 ± 17.46 | 515.17 ± 15.62 | NS |
| Rigor value (Ratio) | 1.37 ± 0.01 | 1.36 ± 0.01 | NS |
| Appearance | 7.55 ± 0.13 | 7.35 ± 0.18 | NS |
| Flavour | 6.87 ± 0.08 | 6.85 ± 0.15 | NS |
| Juiciness | 6.68 ± 0.08 | 6.58 ± 0.14 | NS |
| Tenderness | 6.68 ± 0.05 | 6.27 ± 0.08 | NS |
| Over acceptability | 7.07 ± 0.08 | 6.83 ± 0.07 | NS |
| Lightness (L*) | 38.76 ± 1.95 | 30.51 ± 1.25 | NS |
| Redness (a*) | 11.68 ± 0.39 | 12.84 ± 0.24 | NS |
| Yellowness (b*) | 13.30 ± 0.37 | 15.16 ± 0.67 | ** |
| Myoglobin (mg/g) | 2.68 ± 0.10 | 2.71 ± 0.15 | NS |
| Met-Myoglobin (%) | 36.96 ± 2.94 | 45.76 ± 2.70 | NS |
| Sarcoplasmic Protein (mg/g) | 33.07 ± 3.42 | 29.22 ± 3.19 | NS |
| Total Protein (mg/g) | 125.09 ± 5.94 | 134.53 ± 3.41 | NS |
| Myofibrillar Protein (mg/g) | 100.45 ± 5.92 | 108.20 ± 3.30 | NS |
| Moisture (%) | 77.68 ± 0.30 | 76.94 ± 0.39 | NS |
| Protein (%) | 17.52 ± 0.27 | 17.51 ± 0.26 | NS |
| Fat (%) | 2.60 ± 0.22 | 2.61 ± 0.26 | NS |
| Ash (%) | 1.80 ± 0.05 | 1.84 ± 0.11 | NS |
**P < 0.01; NS- Non-Significant, KC- Kodii Aadu Control, KHS- Kodi Aadu Heat Stress
3.7. Sensory attributes
The Mean ± SE of organoleptic attributes of LTL muscle in KC and KHS groups were described in Table 3. Heat stress did not influence (P > 0.05) any of the organoleptic variables such as appearance, flavour, juiciness, tenderness and overall acceptability. Furthermore, THI showed a strong negative correlation (P < 0.01) with tenderness and a mild negative correlation (P < 0.05) with overall acceptability score of LTL muscle (Supplementary Table 8).
3.8. Meat color
The Mean ± SE of meat color attributes of LTL muscle in KC and KHS groups were described in Table 3. Analysis revealed that only yellowness (P < 0.01) of LTL muscle showed significant difference for heat stress treatment (Table 3). However, no significant difference could be observed in lightness, redness, myoglobin and met-myoglobin of LTL muscle (Table 3). Furthermore, THI showed a strong negative correlation (P < 0.01) with lightness and a mild positive correlation (P < 0.05) with redness and yellowness of LTL muscle (Supplementary Table 9).
3.9. Soluble proteins
The Mean ± SE of soluble proteins of LTL muscle in KC and KHS groups were described in Table 3. Analysis revealed heat stress did not influence (P > 0.05) any of the soluble proteins such as sarcopasmic protein, total protein and myofibrillar protein of LTL muscle (Table 3). Furthermore, THI also did not showed any correlation (P > 0.05) with any of the soluble proteins of LTL muscle (Supplementary Table 10).
3.10. Proximate composition
The Mean ± SE of proximate composition of LTL muscle in KC and KHS groups were described in Table 3. Heat stress did not influence (P > 0.05) any of the proximate composition such as moisture, fat, ash, and protein percentage of LTL muscle (Table 3). Furthermore, THI also did not showed any correlation (P > 0.05) with any of the proximate composition of LTL muscle (Supplementary Table 11).
3.11. Gene expression patterns
The Mean ± SE of different meat quality gene expressions in LTL muscle in KC and KHS groups were described in Table 4. The MSTN, CAPN1, DGAT1, HSP40, mRNA expression patterns were significantly (P < 0.01) lower in KHS group as compared to KC group. However, CAPN2, CAST, HSP27, CRYA, and HSP90 mRNA expression patterns were significantly (P < 0.01) higher in KHS group as compared to KC group. Further, HSF1, HSP10, HSP60, HSP70 and HSP110 mRNA expression patterns did not alter in KHS group as compared to KC group. Further, the THI had a mild positive correlation (P < 0.05) with MSTN, HSP40, HSP60 and a strong positive correlation (P < 0.01) with CAPN1, DGAT1 expression pattern. However, the THI had a mild negative correlation (P < 0.05) with CAPN2, CAST, CRYA, HSP90 and a strong negative correlation (P < 0.01) with HSP27 expression pattern expression pattern. Further no significant correlation was established between THI and HSF1, HSP10, HSP70, HSP110 expression patterns. The correlation between THI and different targeted genes were described in Supplementary Table 12.
Table 4.
Heat stress influence on the expression patterns of different target genes in relation to the house keeping genes.
| Gene | Group | Fold change | SE | p-value |
|---|---|---|---|---|
| MSTN | KC | 1 | 0.22 | 0.00 |
| KHS | 0.34 | 0.17 | ||
| CAPN1 | KC | 1 | 0.18 | 0.00 |
| KHS | 0.31 | 0.19 | ||
| CAPN2 | KC | 1 | 0.23 | 0.03 |
| KHS | 2.01 | 0.11 | ||
| CAST | KC | 1 | 0.19 | 0.02 |
| KHS | 2.09 | 0.18 | ||
| CRYA | KC | 1 | 0.39 | 0.04 |
| KHS | 4.58 | 0.50 | ||
| DAGT | KC | 1 | 0.18 | 0.01 |
| KHS | 0.30 | 0.05 | ||
| HSF1 | KC | 1 | 0.13 | 0.12 |
| KHS | 0.69 | 0.23 | ||
| HSP10 | KC | 1 | 0.03 | 0.78 |
| KHS | 0.98 | 0.10 | ||
| HSP27 | KC | 1 | 0.36 | 0.02 |
| KHS | 4.00 | 0.11 | ||
| HSP40 | KC | 1 | 0.18 | 0.02 |
| KHS | 0.47 | 0.22 | ||
| HSP60 | KC | 1 | 0.16 | 0.08 |
| KHS | 0.69 | 0.04 | ||
| HSP70 | KC | 1 | 0.23 | 0.36 |
| KHS | 0.82 | 0.18 | ||
| HSP90 | KC | 1 | 0.05 | 0.04 |
| KHS | 1.95 | 0.22 | ||
| HSP110 | KC | 1 | 0.26 | 0.09 |
| KHS | 0.59 | 0.10 |
MSTN-Myostatin, CAPN1- Calpain 1; CAPN2-Calpain 2; CAST-Capastatin; CRYA- Crytallin alpha; DAGT1- Diacylglycerol Acyltransferase 1; HSF1-Heat Shock Factor 1; HSP-Heat Shock Protein
4. Discussion
This study gains significance as the world is battling to feed the growing human population of 9.6 billion by 2050. Livestock and particularly the small ruminants are considered one of the ways to ensure protein availability for human consumption by 2050. Compared with sheep, goats are better adapted to tropical climate as it is evident from the higher distribution of goat population in tropical countries (Reshma Nair et al., 2021).
The THI was calculated as per McDowell (1972) and according to this thermal index any value over 78 are considered extremely stressful while any value above 72 is considered comfortable. The average THI obtained in this study for thermo-neutral and heating climate chambers were 69.42 and 94.76, respectively. Thus, the hypothesis of inducing heat stress to the KHS group animals was justified as these animals were exposed to the THI which falls in the extreme distress category. Similar observation of inducing heat stress based on McDowell (1972) index was also established by other researchers in goats (Aleena et al., 2018, Archana et al., 2018). These findings suggests that the heat stress group animals in this study was ensured of subjecting them to extremely severe heat stress while the control group animals were subjected to thermo-neutral condition.
Heat stress did not alter any of the major carcass traits variables such as pre-slaughter weight, hot carcass weight, dressing percentage and loin eye area (LEA). This shows the extreme climate resilience potential of Kodi Aadu breed as even the very high extremely severe heat stress could not induce any negative impact on the major carcass traits. Animals apart from adapting to the adverse environmental condition, when they maintain their productive responses are considered extremely climate resilient (Sejian et al., 2018). Thus, Kodi Aadu breed could be considered climate resilient breed. Hashem et al. (2013) also established similar non-significant influence of heat stress on both PSW and HCW in Black Bengal goats. However, Archana et al. (2018) observed negative influence of heat stress on PSW, HCW and LEA in both Osmanabadi and Salem Black goat breeds. These findings projects that even among indigenous breeds which are considered thermo-tolerant there are differences established in their productive response.
Like major carcass traits, most of the non-carcass components and offals also did not differ between the groups reflecting the coping ability of Kodi Aadu breed to heat stress challenges without compromising these productive variables. The heart, brain and reproductive organs differed for heat stress treatment. Additionally, there were no any correlation established between THI and non-carcass variables and offals indicating the extreme adaptive nature of Kodi Aadu breed. Likewise, Sen et al. (2004) also observed no influence of heat stress on non-carcass components in goats attributing this to the extreme adaptive nature of these animals. These findings again establish the fact that there are breed variations for the productive performance of thermo-tolerant goat breeds.
Except loin cut, rest all primal cut variables remained intact between the KC and KHS groups. This shows that Kodi Aadu breed maintained its productive response irrespective of being exposed to extremely severe heat stress. In a similar study Archana et al. (2018) established significant negative influence of heat stress only on rack cut in Osmanabadi breed and they attributed this to the adaptive nature of this breed being native to the location of the study. However, the same authors observed variations in fore saddle, leg and breast cuts in Salem Black goat breed. These findings across these studies again reflect the breed differences in response to heat stress in goats. Even almost all linear carcass measurements also remained intact between the KC and KHS groups except shoulder circumference. However, varied results were obtained for the effect of heat stress on linear carcass measurements in sheep and goat (Rana et al., 2014, Archana et al., 2018).
Again, except collagen solubility rest all physico-chemical attributes remained intact between KC and KHS group animals. The potential differences in collagen solubility might be attributed to the heat stress induced increase in lysyl oxidase (LOX) which induces cross-linking between collagen and elastin and thereby decreasing the solubility of collagen. The non-significant influence of heat stress on rest all physico-chemical attributes again reflects the supreme potential of Kodi Aadu breed to keep intact these vital meat quality variables even after exposure to chronic heat stress of very high magnitude. However, Archana et al. (2018) reported significant influence of heat stress on meat pH and shear force in Osmanabadi breed and cooking loss and shear force in Salem Black breed. The meat pH is usually altered in heat stressed animals as reported in goat (Kadim et al., 2008, Hashem et al., 2013) and cattle (Kadim et al., 2004). Like the ultimate pH, DL, CL, WHC also remained intact reflecting the extreme adaptive nature of Kodi Aadu breed to maintain these vital meat quality variables. However, contradictory reports of significant alteration in all these variables were reported in other indigenous goat breeds (Kadim et al., 2009, Archana et al., 2018, Van Wyk et al., 2020). Shear force is another vital meat quality variable which determines the meat tenderness and eating quality of meat (Chulayo & Muchenje, 2013). However, there are reports which suggested recording higher shear force value in heat stressed animals (Zhang et al., 2012, Archana et al., 2018). Contrary to these findings, Kadim et al. (2004) recorded lower shear force values in animals exposed to heat stress.
Heat stress did not influence any of the organoleptic attributes. This was evident from the no change in appearance, flavour, juiciness, tenderness and overall acceptability scores between KC and KHS groups. However, in a similar study conducted on Osmanabadi and Salem Black goat breeds Archana et al. (2018) reported significant reduction in appearance score in Osmanabadi breed while significantly reduced the scores for organoleptic attributes in Salem Black breed. Thus, the no effect of heat stress on any of the organoleptic attributes in this study clearly demonstrates the superiority of Kodi Aadu breed to cope with heat stress as compared to Osmanabadi and Salem Black breed.
Among the color variables, only yellowness showed significant variation for heat stress treatment between the groups. In general, the intensity of yellowness of muscle is influenced by the concentration of intramuscular fat. Meat yellowness has been shown to be closely associated with lipid oxidation (Lan et al., 2016, Wang et al., 2018). The effects of lipid oxidation on the formation of yellow pigments in meat are related to the non-enzymatic browning reactions between lipid oxidation products and the amine in the proteins or in the phospholipid head groups (Xia et al., 2009). In addition, as a result of oxidative stress, the accumulation of Schiff pigments from lipid oxidation products to protein complexes also contributes to the formation of yellowness (Rodríguez-Carpena et al., 2011). Thus, significantly higher b* value in the study in Kodi Aadu breed could be attributed to the increased oxidative stress in these heat stressed animals wherein more lipid and protein oxidation are expected. Both L* and a* remained intact in Kodi Aadu breed after heat stress exposure. Similar to our findings, Archana et al. (2018) also did not observed any changes in L* and a* after heat stress exposure in two indigenous Osmanabadi and Salem Black goat breeds (Archana et al., 2018). This shows the inherent potential of these indigenous breeds to maintain the vital color variables of meat even in extreme stressful condition. In contrast to L* and a*, increased b* was recorded in KHS group as compared to KC group. This was in contrast to the findings of Archana et al. (2018) who did not observe any changes in the b* after heat stress exposure in both Osmanabadi and Salem Black goat breed. This shows the breed variation for heat stress associated changes in b* among the indigenous breeds of goats. However, like L* and a* there was no significant difference in both myoglobin content and met-myoglobin percentage between KC and KHS group again reflecting the potential of Kodi Aadu breed to keep intact these vital color variables.
Again the heat stress did not alter any of the soluble proteins such as the sarcoplasmic, total and myofibrillar proteins remained intact between KC and KHS groups. However, Zhu et al. (2012) observed that meat with low WHC and low pH also exhibited substantially lower sarcoplasmic and total protein than normal meat. Presently not many reports are available lining heat stress with muscle soluble proteins and hence it was not possible to discuss these results further. It is the general observation that the body protein contents are reduced when the animals are subjected to heat stress of both acute and chronic magnitude (Zhang et al., 2012). Further the proximate composition variables also did not show any variations for the heat stress treatment in Kodi Aadu. The moisture, protein, ash, and fat percentages did not vary between KC and KHS groups. This was similar to the findings reported by Archana et al. (2018) in both Osmanabadi and Salem Black breeds wherein they reported no significant influence of heat stress on any of the proximate composition variables. These results indicate that inspite of subjecting the Kodi Aadu breed to high magnitude of heat stress still the nutritional composition of meat remained the same reflecting the excellent climate resilient potential of this breed.
The MSTN mRNA expression pattern was significantly lower in KHS group as compared to KC. The MSTN gene is the growth inhibitor and regulates the muscle development and differentiation (Gabriel et al., 2003). The reduced level of MSTN expression during heat stress exposure was similar to the finding established by Archana et al. (2018) in Osmanabadi breed. These authors opined that the reduced level of MSTN expression could be to avoid further deterioration of meat quality. This shows that heat stressed animals down regulate the expression pattern MSTN gene to maintain meat quality. This fact was supported by the no effect of heat stress on majority of meat quality variables such muscle pH, drip loss, WHC and shear force. By down regulating the MSTN expression Kodi Aadu goats exhibited their inherent ability to regulate muscle growth. However, Archana et al. (2018) also reported no effect of heat stress on MSTN expression pattern in Salem Black breed. This shows even among adapted goat breeds there is variation in the expression pattern of MSTN gene.
Heat stress significantly reduced the expression pattern of CAPN1 in LTL muscle. Calpain system is the most well-known proteolytic system which affects meat tenderness (Huff-Lonergan & Lonergan, 1999). Both CAPN1 and CAPN2 have the capability of degrading myofibrillar and cytoskeletal proteins to maintain tenderness in meat (Koohmaraie & Geesink, 2006). The significant low expression pattern of CAPN1 in the current study could indicate the compromised meat tenderness after heat stress exposure. However, the reduced CAPN1 did not reflected in actual meat tenderness in this study. This could be attributed to the higher expression of CAPN2 to nullify the adverse effect of CAPN1 on meat tenderness. The increased expression of CAPN2 could be the indicator of high meat tenderness but this could have primarily attributed to reverse the effect of CAPN1 resulting in no net effect on the actual meat tenderness in this study. These results show that Kodi Aadu breed has the capability to maintain tenderness to certain extent although exposed to very high magnitude of heat stress. Conversely, the high activity of their specific inhibitor, calpastatin, is related to the low degradation of myofibrillar proteins and low meat tenderness (Koohmaraie and Geesink, 2006, Lonergan et al., 2010). The expression pattern of CAST was significantly higher in KHS group as compared to KC group. This further indicates that the meat tenderness was getting compromised as a result of exposure to heart stress in Kodi Aadu breed. Two genes (CAPN2 and CAST) out of three targeted in calpain system got over expressed and although such expression pattern was correlated with low meat tenderness still the muscle tenderness was comparable between KC and KHS groups indicating coordinated activities of genes associated with calpain system to maintain tenderness in Kodi Aadu breed.
Heat stress significantly down regulated the expression pattern of DAGT1. The DAGT1 gene is involved in fatty acid metabolism are considered as potential candidate genes for meat tenderness (Cases et al., 1998). Therefore, the increased level of DAGT1 gene expression is directly proportional to muscle tenderness. However, in this study lower expression of DAGT1 was established after heat stress exposure in Kodi Aadu breed. But this lower expression may be of very less magnitude to induce low meat tenderness. This was evident from the no effect of heat stress on meat tenderness in this current study.
The HSF1 expression pattern was comparable between KC and KHS groups. The regulation of expression of HSF1 is highly complex with many external factors influencing it and no consistent reports are available especially on the level of heat stress that is required to trigger HSF1 expression (Madhusoodan et al., 2020). Exposure to heat stress triggers the activation of HSF1 to stimulate the production of HSPs (Das et al. 2016). Therefore, activation of HSF1 is very crucial to induce secretion of HSPs to regulate the cellular functions in heat stressed animals (Madhusoodan et al., 2020). Further, HSF1 was established to be an ideal marker for breeding dairy cattle for thermo-tolerance (Li et al., 2011). Therefore, the comparable level of HSF1 expression between KC and KHS indicates the better climate resilience capacity of Kodi Aadu breed. Similar to HSF1 expression, the expression pattern of HSP10, HSP60, HSP70 and HSP110 also was comparable between KC and KHS groups. This could be attributed to the non-significant level of HSF1 to trigger sufficient heat sock response in Kodi Aadu breed to induce changes in these classical molecular chaperones reflecting the extreme climate resilient potential of this breed.
There are reports which established HSP70 as marker for reflecting meat quality in livestock (Xing et al., 2019, Archana et al., 2018). HSP70 are molecular chaperones primarily involved in repairing damaged tissue and also helps in preventing the unfolding and misaggregation of proteins during stressful condition (Parkunan et al., 2015). Thus, quantifying heat stress associated HSP70 gene expression in skeletal muscle exposure gains significance from meat quality perspectives. The non-significant influence of heat stress on the expression pattern of HSP70 in LTL muscle clearly demonstrates the climate resilience of Kodi Aadu breed. In a similar study in Osmanabadi and Salem Black goat breeds, Archana et al. (2018) established heat stress induced higher expression pattern of HSP70 in LTL muscle and these authors correlated this to the higher adaptive capacity of these two breeds to heat stress. This shows that the non-significant influence of heat stress on the expression pattern of HSP70 in this study clearly demonstrates the better efficiency of Kodi Aadu breed to cope with heat stress as compared to Osmanabadi and Salem Black breeds. Thus, HSP70 mRNA expression in LTL muscle could serve as ideal biomarker for quantifying the heat stress associated deterioration in meat quality in indigenous goat breeds. However, the expression pattern of HSP40 was significantly lower in heat stressed animals. Both HSP70 and HSP40 similar in function and the main action of HSP40 were to reduce the oxidative stress in heat stressed animals (Mullins et al., 2016). Thus, the lower expression of HSP40 clearly demonstrates the sub threshold level of heat stress attained in the study reflecting the coping ability of Kodi Aadu goats when subjected even to extremely severe heat stress.
The HSP27 expression pattern was significantly higher in KHS group as compared to KC group. In particular, HSP27 can interrupt the apoptosis cascade and can stabilize and protect muscle proteins to calpains action (Lomiwes et al., 2014). Thus, the higher expression of HSP27 in the current study in Kodi Aadu breed reflects the superior ability of this breed to maintain meat quality. The HSP27 elicits its action in coordination with the action of other small HSP, such as αβ-crystallin (CRYA) (Lomiwes et al., 2014). The αβ-crystallin is also referred as HSPB5 or CRYA gene in farm animals. The higher expression of CRYA in KHS group supports this argument and thus the similar higher expression of both HSP27 and CRYA could be to act in coordinated way to maintain meat quality in Kodi Aadu breed. Further, the higher expression of HSP90 in KHS group as compared to KC group clearly demonstrates the thermo-tolerance of Kodi Aadu breed. The HSP90 was found to be associated with better thermo-tolerance in dairy cattle (Deb et al., 2014) and these authors observed that increased expression of HSP90 was to regulate effectively the body temperature and to prevent cellular damage in heat stressed indigenous Sahiwal cattle. Thus, the higher expression of HSP90 in the Kodi Aadu breed could be to maintain thermal balance and prevent cellular damage due to heat exposure. The significant alteration in the expression patterns of MSTN, CAPN1, CAPN2, HSP27, CRYA and HSP90 genes and the significant correlation of THI with these genes clearly indicates the significance of these genes for reflecting the productive potential of Kodi Aadu goat breed in tropical climate.
5. Conclusion
The study clearly demonstrated that the female Kodi Aadu goat breed possessed excellent climate resilient potential. This was evident from the non-influence of heat stress of very high magnitude on major carcass traits, non-carcass components and offals, primal cuts, linear carcass measurements, physic-chemical attributes, organoleptic attributes, meat color and LTL soluble proteins and meat quality associated gene expression patterns. Most of the diversified variables associated with carcass characteristics and meat quality remained intact in this breed after heat stress exposure, clearly demonstrates the very high potential of this breed to produce optimally after coping with the extremely stressful environment. Further, the study identified MSTN, HSP27, CRYA and HSP90 genes could serve as biomarkers for reflecting meat producing capability of goats in tropical climate. Hence, the female Kodi Aadu breed could be considered ideal meat producing indigenous goat breed to survive in hot tropical climate of Southern India. Further, these female Kodi Aadu goats could be recommended to the poor and marginal farmers of the specific agro-ecological zone of their origin to ensure their livelihood security. The study also warrants more such research efforts in males of this breed and other indigenous goat breeds to identify the best meat producing breed and such efforts could help the farming community to sustain their economy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Indian Council of Agricultural Research for funding this study and Karnataka Veterinary Animal and Fisheries Sciences University for extending the facilities for meat quality variables analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2021.100052.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abhijith A., Sejian V., Ruban W., Krishnan G., Bagath M., Pragna P., Manjunathareddy G.B., Bhatta R. Summer season induced heat stress associated changes on meat production and quality characteristics, myostatin and HSP70 gene expression patterns in indigenous goat. Small Ruminant Research. 2021;203:106490. doi: 10.1016/j.smallrumres.2021.106490. [DOI] [Google Scholar]
- Aleena J., Sejian V., Bagath M., Krishnan G., Beena V., Bhatta R. Resilience of three indigenous goat breeds to heat stress based on phenotypic traits and PBMC HSP70 expression. Int. J. Biometeorol. 2018;62(11):1995–2005. doi: 10.1007/s00484-018-1604-5. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) (2005). Official Method of Analysis (18th Ed.). Virginia, USA, pp.20-22.
- Archana P.R., Sejian V., Ruban W., Bagath M., Krishnan G., Aleena J.…Bhatta R. Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Science. 2018;141:66–80. doi: 10.1016/j.meatsci.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Babiker S.A., El Khider I.A., Shafie S.A. Chemical composition and quality attributes of goat meat and lamb. Meat Science. 1990;28(4):273–277. doi: 10.1016/0309-1740(90)90041-4. [DOI] [PubMed] [Google Scholar]
- Bindu J., Ravishankar C.N., Srinivasa Gopal T.K. Shelf-life evaluation of a ready-to-eat blackclam (Villorita cyprinoides) product in indigenous retortpouches. Journal of Food Engineering. 2007;78(3):995–1000. [Google Scholar]
- Carvalho M.E., Gasparin G., Poleti M.D., Rosa A.F., Balieiro J.C.C., Labate C.A.…Coutinho L.L. Heat shock and structural proteins associated with meat tenderness in Nellore beef cattle, a Bos indicus breed. Meat Science. 2014;96(3):1318–1324. doi: 10.1016/j.meatsci.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Cases S., Smith S.J., Zheng Y.-W., Myers H.M., Lear S.R., Sande E., Novak S., Collins C., Welch C.B., Lusis A.J., Erickson S.K., Farese R.V. Identification of a gene encoding an acyl CoA diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(22):13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassar-Malek I., Picard B. Expression Marker-Based Strategy to Improve Beef Quality. The Scientific World Journal. 2016;2016:1–11. doi: 10.1155/2016/2185323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulayo A.Y., Muchenje V. The effects of pre-slaughter stress and season on the activity of plasma creatine kinase and mutton quality from different sheep breeds slaughtered at a smallholder abattoir. Asian-Australasian Journal of Animal Sciences. 2013;26(12):1762–1772. doi: 10.5713/ajas.2013.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Sailo L., Verma N., Bharti P., Saikia J., Imtiwati, Kumar R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World. 2016;9(3):260–268. doi: 10.14202/vetworld.2016.260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G.W., Dutson T.R., Smith G.C., Carpenter Z.L. Fragmentation procedure for bovine longissimus muscle as an index of cooked steak tenderness. Journal of Food Science. 1980;45(4):880–884. [Google Scholar]
- Deb R., Sajjanar B., Singh U., Kumar S., Singh R., Sengar G., Sharma A. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus×Bos taurus) breed of cattle, A comparative study. Genes. 2014;536:435–440. doi: 10.1016/j.gene.2013.11.086. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Casey J.C., Boccard R., Touraille C., Buchter L., Hood D.E.…Tinbergen B.J. Comparison of chemical composition of meat determined at eight laboratories. Meat Science. 1983;8(2):79–92. doi: 10.1016/0309-1740(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Gabriel J.E., Alvares L.E., Gobet M.C., de Paz C.C.P., Packer I.U., Macari M., Coutinho L.L. Expression of MyoD, myogenin, myostatin and Hsp70 transcripts in chicken embryos submitted to mild cold or heat. Journal of Thermal Biology. 2003;28(4):261–269. [Google Scholar]
- Gerrard F. Leonard Hill; London: 1964. Meat Technology. [Google Scholar]
- Hashem M.A., Hossain M.M., Rana M.S., Hossain M.M., Islam M.S., Saha N.G. Effect of heat stress on blood parameter, carcass, and meat quality of Black Bengal goat. Bangladesh Journal of Animal Science. 2013;42(1):57–61. [Google Scholar]
- Hawkins R.R., Kemp J.D., Ely D.G., Fox J.D., Moody W.G., Vimini R.J. Carcass and meat characteristics of crossbred lambs born to ewes of different genetic types slaughtered at different weights. Livestock Production Science. 1985;12(3):241–250. [Google Scholar]
- Honikel K.O., Fischer C. A rapid method for detec-tion of PSE and DFD porcine muscles. Journal of Food Science. 1977;42:1633–1636. [Google Scholar]
- Hostetler R.L., Link B.A., Landmann W.A., Fitzhugh H.A. Effect of carcass suspension on sarcomere length and shear force of some major bovine muscles. Journal of Food Science. 1972;37(1):132–135. [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Quality Attributes of Muscle Foods. Springer US; Boston, MA: 1999. pp. 229–251. [DOI] [Google Scholar]
- Jacob R.H., Pethick D.W., Clark P., D’Souza D.N., Hopkins D.L., White J. Quantifying the hydration status of lambs in relation to carcass characteristics. Australian Journal of Experimental Agriculture. 2006;46(4):429–437. [Google Scholar]
- ISI. (1995). Indian standard, Meat and Meat products – Mutton and Goat meat (chevon) – Fresh, chilled and frozen, technical requirements (first revision). Bureau of Indian Standard Institution Ed. 2.1 (2004-05), New Delhi, India.
- Jeremiah L.E., Martin A.H. The influence of sex, within breed-of-sire groups, upon the histological properties of bovine longissimus dorsi muscle during post-mortem aging. Canadian Journal of Animal Science. 1977;57(1):7–14. [Google Scholar]
- Joo S.T., Kauffman R.G., Kim B.C., Park G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Science. 1999;52(3):291–297. doi: 10.1016/s0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Kadim I.T., Mahgoub O., Al-Ajmi D.S., Al-Maqbaly R.S., Al-Mugheiry S.M., Bartolome D.Y. The influence of season on quality characteristics of hot-boned beef m. longissimus thoracis. Meat Science. 2004;66(4):831–836. doi: 10.1016/j.meatsci.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kadim I.T., Mahgoub O., Al-Marzooqi W., Khalaf S., Al-Sinawi S.S.H., Al-Amri I.S. Effects of transportation during the hot season and low voltage electrical stimulation on histochemical and meat quality characteristics of sheep longissimus muscle. Livestock Science. 2009;126(1-3):154–161. [Google Scholar]
- Kadim I.T., Mahgoub O., Al-Marzooqi W., Al-Ajmi D.S., Al-Maqbali R.S., Al-Lawati S.M. The influence of seasonal temperatures on meat quality characteristics of hot-boned, m. psoas major and minor, from goats and sheep. Meat science. 2008;80(2):210–215. doi: 10.1016/j.meatsci.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Keeton J.T. Effects of fat and NaCl/phosphate levels on the chemical and sensory properties of porkpatties. Journal of Food Science. 1983;48(3):878–881. [Google Scholar]
- Koohmaraie M., Geesink G.H. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science. 2006;74(1):34–43. doi: 10.1016/j.meatsci.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Lan Y., Shang Y., Song Y., Dong Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Science. 2016;117:173–181. doi: 10.1016/j.meatsci.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Li Q.-L., Ju Z.-H., Huang J.-M., Li J.-B., Li R.-L., Hou M.-H.…Zhong J.-F. Two novel SNPs in HSF1 gene are associated with thermal tolerance traits in Chinese Holstein cattle. DNA Cell Biology. 2011;30(4):247–254. doi: 10.1089/dna.2010.1133. [DOI] [PubMed] [Google Scholar]
- Lomiwes D., Farouk M.M., Wiklund E., Young O.A. Small heat shock proteins and their role in meat tendernessA review. Meat Science. 2014;96(1):26–40. doi: 10.1016/j.meatsci.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Lonergan E.H., Zhang W., Lonergan S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010;86(1):184–195. doi: 10.1016/j.meatsci.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Madhusoodan A.P., Bagath M., Sejian V., Krishnan G., Rashamol V.P., Savitha S.T.…Bhatta R. Summer season induced changes in quantitative expression patterns of different heat shock response genes in Salem black goats. Tropical Animal Health and Production. 2020;52(5):2725–2730. doi: 10.1007/s11250-020-02242-5. [DOI] [PubMed] [Google Scholar]
- Mahendrakar N.S., Dani N.P., Ramesh B.S., Amla B.L. Studies on influence of age of sheep and post-mortem carcass conditioning treatments on muscular collagen content and its thermolability. Journal of Food Science and Technology. 1989;26(2):102–105. [Google Scholar]
- McDowell R.E. WH Freeman & Co; San Fransisco, USA: 1972. Improvement of livestock production in warm climate. [Google Scholar]
- Mullins C.R., Zerby H.N., Fitzpatrick L.A., Parker A.J. Bos indicus cattle possess greater basal concentrations of HSP27, alpha B-crystallin, and HSP70 in skeletal muscle in vivo compared with Bos taurus cattle. Journal of Animal Science. 2016;94:424–429. doi: 10.2527/jas.2015-9630. [DOI] [PubMed] [Google Scholar]
- Naveena B.M., Mendiratta S.K., Anjaneyulu A.S.R. Tenderization of buffalo meat using plantp roteases from Cucumistrigonus Roxb (Kachri) and Zingiber officinaleroscoe (Gingerrhizome) Meat Science. 2004;68(3):363–369. doi: 10.1016/j.meatsci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Parkunan T., Banerjee D., Mohanty N., Das P.K., Ghosh P., Mukherjee J., Paul A., Das A.K., Nanda P.K., Naskar S., Mohan N.H., Sarkar M., Das B.C. A comparative study on the expression profile of MCTs and HSPs in Ghungroo and Large White Yorkshire breeds of pigs during different seasons. Cell Stress Chaperones. 2015;20(3):441–449. doi: 10.1007/s12192-014-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana M.S., Hashem M.A., Akhter S., Habibullah M., Islam M.H., Biswas R.C. Effect of heat stress on carcass and meat quality of indigenous sheep of Bangladesh. Bangladesh Journal of Animal Science. 2014;43(2):147–153. [Google Scholar]
- Rémignon H., Desrosiers V., Marche G. Influence of increasing breast meat yield on muscle histology and meat quality in the chicken. Reproduction, Nutrition, Development. 1996;36(5):523–530. doi: 10.1051/rnd:19960508. [DOI] [PubMed] [Google Scholar]
- Reshma Nair M.R., Sejian V., Silpa M.V., Fonsêca V.F.C., de Melo Costa C.C., Devaraj C., Krishnan G., Bagath M., Nameer P.O., Bhatta R. Goat as the ideal climate resilient animal model in tropical environment: Revisiting advantages over other livestock species. International Journal of Biometeorology. 2021;65(12):2229–2240. doi: 10.1007/s00484-021-02179-w. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carpena J.G., Morcuende D.A., Estévez I.D., M.A.R.I.O. Avocado by-products as inhibitors of colour deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Science. 2011;89:166–173. doi: 10.1016/j.meatsci.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Sejian V., Bhatta R., Gaughan J.B., Dunshea F.R., Lacetera N. Adaptation of animals to heat stress. Animal. 2018;12(s2):s431–s444. doi: 10.1017/S1751731118001945. [DOI] [PubMed] [Google Scholar]
- Sen A.R., Santra A., Karim S.A. Carcass yield, composition and meat quality attributes of sheep and goat under semiarid conditions. Meat Science. 2004;66(4):757–763. doi: 10.1016/S0309-1740(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Trout G.R. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. Journal of Food Science. 1989;54(3):536–540. [Google Scholar]
- Van Wyk, G, L., Hoffman, L.C., Strydom, P. E., & Frylinck, L. (2020). Effect of breed types and castration on carcass characteristics of Boer and large frame indigenous veld goats of Southern Africa. Animal., 10, 188. [DOI] [PMC free article] [PubMed]
- Wang R.R., Pan X.J., Peng Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poultry Science. 2009;88(5):1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Wang Z., He Z., Gan X., Li H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Science. 2018;146:131–139. doi: 10.1016/j.meatsci.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Wardlaw F.B., Maccaskill L.H., Acton J.C. Effect of post-mortem muscle changes in poultry meat loaf properties. Journal of Food Science. 1973;38:421–424. [Google Scholar]
- Warris P.D. The extraction of haempigments from fresh meat. International Journal of Food Science and Technology. 1979;14(1):75–80. [Google Scholar]
- Wheeler T.L., Shackelford S.D., Johnson L.P., Miller M.F., Miller R.K., Koohmaraie M. A comparison of Warner-Bratzler shear force assessment within and among institutions. Journal of Animal Science. 1997;75(9):2423–2432. doi: 10.2527/1997.7592423x. [DOI] [PubMed] [Google Scholar]
- Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Science. 2009;83(2):239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Xing T., Gao F., Tume R.K., Zhou G., Xu X. Stress effects on meat quality, A mechanistic perspective. Comprehensive Reviews in Food Science and Food Safety. 2019;18(2):380–401. doi: 10.1111/1541-4337.12417. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poultry Science. 2012;91(11):2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhu X., Xu X., Min H., Zhou G. Occurrence and characterization of pale, soft, exudative-like broiler muscle commercially produced in China. Journal of Integrative Agriculture. 2012;11(8):1384–1390. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.