Abstract
The anthrax toxin protective antigen (PA), the membrane binding and pore-forming component of the anthrax toxin, was studied using 19F NMR. We site-specifically labeled PA with p-fluorophenylalanine (pF-Phe) at Phe427, a critically important residue that comprises the ϕ-clamp that is required for translocation of edema factor (EF) and lethal factor (LF) into the host cell cytosol. We utilized 19F NMR to follow low-pH-induced structural changes in the prepore, alone and bound to the N-terminal PA binding domain of LF, LFN. Our studies indicate that pF-Phe427 is dynamic in the prepore state and then becomes more dynamic in the transition to the pore. An increase in dynamic behavior at the ϕ-clamp may provide the necessary room for movement needed in translocating EF and LF into the cell cytosol.
The anthrax toxin protective antigen (PA) is secreted by Bacillus anthracis and binds to host immune cells.2 Upon binding, proteolytic cleavage4 of PA leads to the formation of either a heptameric5 or octameric prepore,6 providing a surface that allows binding of two enzymatic components of the toxin: edema factor (EF) and/or lethal factor (LF). Following binding, the toxin is then endocytosed into the cell7 and at low pH forms a membrane-spanning pore that allows entry of EF and LF into the cell cytosol, where they target functions that severely disrupt the immune response.8 Several toxins utilize a pore mechanism to allow entry of toxin components into the cell, including the Clostridioides difficile toxin9 and Clostridium perfringens iota toxin.10 The mechanism by which these toxins transport their cargo into cells remains unclear in part due to the very narrow channel comprised of phenylalanines, termed the ϕ-clamp,11 that the polypeptides must traverse to gain entry into the cell cytosol. Early studies showed that Phe427 in PA played a key role in translocation of EF and LF into cells. Mutations at this site have been shown to prevent translocation of LFN-DTA, a fusion of the N-terminal PA binding domain of LF and the catalytic domain of diphtheria toxin, into CHO-KI cells.12,13 X-ray structural studies of the heptameric prepore by Lacy and co-workers showed that residues near Phe427 (Phe427 was not observed in the crystal structure) resided within the lumen of the prepore.14 Finally, seminal studies by Krantz and Melnyk showed that the low-pH-induced transition to the pore state caused a constriction of the Phe427 residues to form a narrow iris, the ϕ-clamp.11 Mutations at this site also revealed that translocation was dependent on the integrity of the ϕ-clamp and that while LFN could bind and engage the ϕ-clamp and prevent ion conductance, certain mutations (Phe427 to Ala, Gly, Ileu, and Asp for instance) would not translocate LFN across the bilayer membrane.
The recent cryo-electron microscopy (cryo-EM) structure of the pore state at high resolution (2.9 Å) confirmed that the ϕ-clamp forms a narrow 6 Å iris, where the Phe427 rings lie in a nearly face-to-face arrangement (Figure 1), and suggested that only completely unfolded polypeptides lacking secondary structure can pass through.1 However, even in a completely unfolded polypeptide chain, the Cα to side chain end atoms of arginine, lysine, or tryptophan are on the order of 6 Å, suggesting that to translocate EF or LF in an unfolded state, the ϕ-clamp must be able to expand and contract to allow these side chains to pass through. We modeled the first 10 amino acids from the N-terminal end of LF (AGGHG-DVGMH), using the single poly-L-proline II helix from the collagen α2(I) chain as a template,15 and placed this through the ϕ-clamp iris. The modeled structure shows obvious steric clashes among the His, Met, and Asp residues, highlighting the need for expansion to allow translocation (Figure 1).
Figure 1.
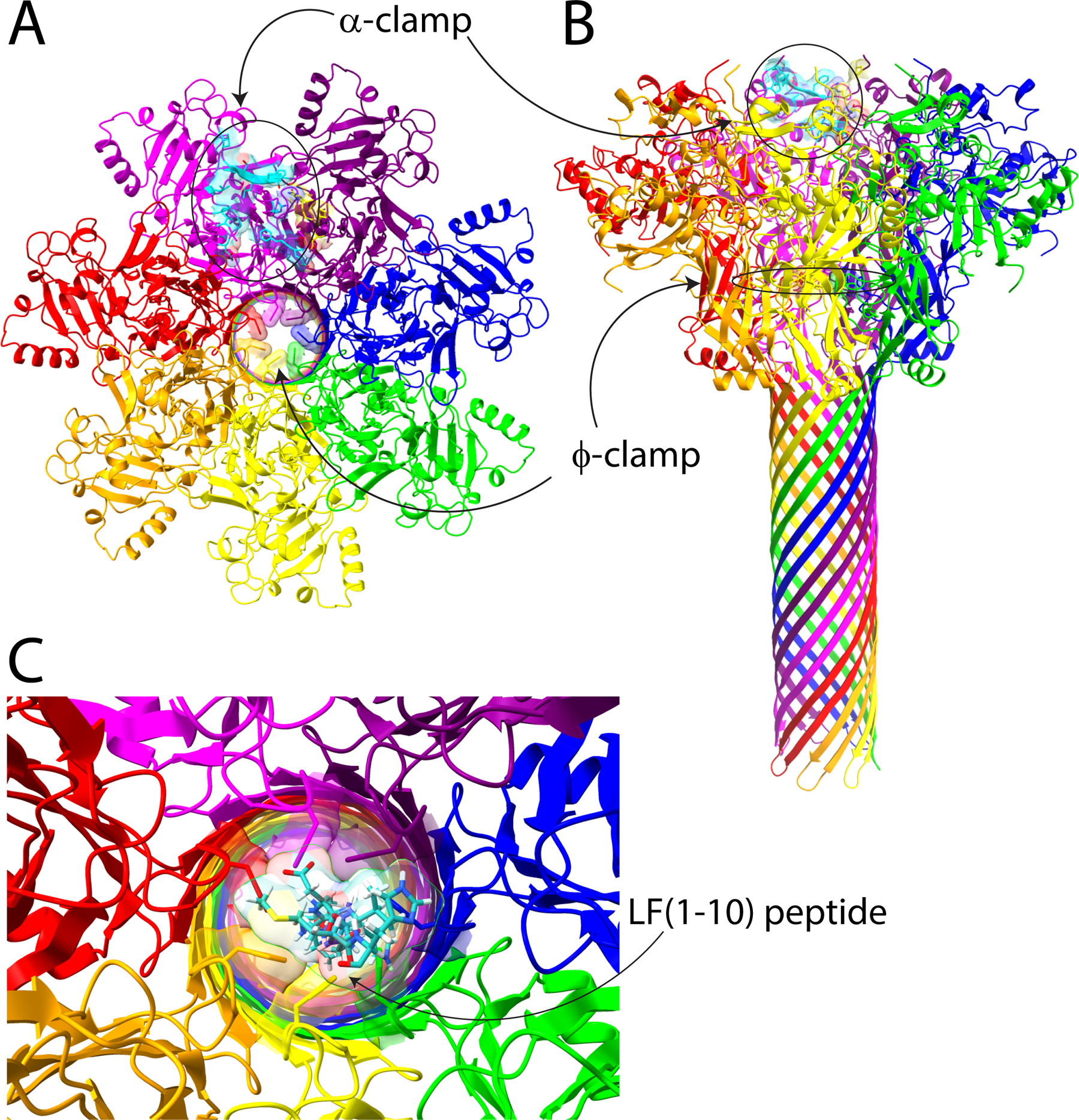
Anthrax toxin pore and regions encompassing the α-clamp and the ϕ-clamp. Panels A and B show top-down and side views, respectively, of the cryo-EM structure of the PA pore (Protein Data Bank entry 3J9C).1 We have highlighted the α-clamp region in cyan and yellow on the surface of the pore, and the ϕ-clamp ring of Phe427 residues is also highlighted. In panel C, we modeled LF (residues 1–10) (cyan) into the center of the ϕ-clamp iris. One can see that there are clear steric clashes between the Phe427 residues and the side chains of LF, including Met, His, and Asp. This figure was generated using ChimeraX.3
Although the 6 Å diameter seen by cryo-EM suggests that the passage is too narrow to allow unfolded polypeptides to pass through, early ion conductance studies of the anthrax pore in planar lipid bilayers using tetraalkylammonium ions of differing sizes placed an upper diameter of the pore at 12 Å.16,17 Similarly, studies using different sized polyethylene glycols (PEGs) suggested an upper diameter of 20 Å,18 which even if these are overestimates suggests that despite the narrow ϕ-clamp structure observed by cryo-EM, larger diameters can be achieved. We now present direct evidence, using 19F NMR, that the ϕ-clamp is in fact highly dynamic.
To do this, we utilized the Fürter system19 that allowed us to site-specifically incorporate p-fluorophenylalanine (pF-Phe) into the 83 kDa form of PA (pF-PhePA83) at Phe427. The purified pF-PhePA83 was ~73% labeled as determined by LC-MS/MS (Supplementary Data S1). Partial digestion with trypsin allows cleavage of the first 157 amino acids of PA within domain 1, yielding a 63 kDa fragment that oligomerizes into a heptameric prepore (pF-PhePA63)7.5
Figure 2A shows the 19F NMR spectrum of 3 μM prepore (pF-Phe427-PA63)7 at pH 8.5, collected at 600 MHz using a fluorine cryoprobe, and shows that there are two overlapping resonances in the prepore state (−36.97p and −37.21p). This was quite striking, as the MW of the heptameric prepore is 63 × 7 = 441 kDa, and we attribute the ability to see resonances in this state to the increased dynamic behavior of pF-Phe427. As mentioned, in the crystal structure of the heptameric prepore state, electron density is missing for Phe427, suggestive of increased dynamic motion.14 The middle figure is after addition of the detergent Fos-14 (1:1000, pF-PhePA63:Fos-14) and shows that addition of Fos-14 resolves a smaller upfield peak (~37.34p), suggesting that the pF-Phe427 in the prepore structure occupies at least two different conformational states. It also indicates that the prepore remains intact in this detergent; Fos-14 was deemed the best detergent in a myriad of detergents tested that could largely prevent aggregation of the pore at low pH while maintaining structural integrity.20 Finally, the top figure presents the spectrum after the addition of sufficient 1 M phosphoric acid to induce a transition to pH 5.5, a pH that allows pore formation to occur.21 The spectrum shows that the −37.34p resonance is now sharper (Lorentzian line fitting gives an approximate line width change from 316 to 190 Hz) with a higher intensity, suggesting that there is a major population shift to the upfield resonance.
Figure 2.
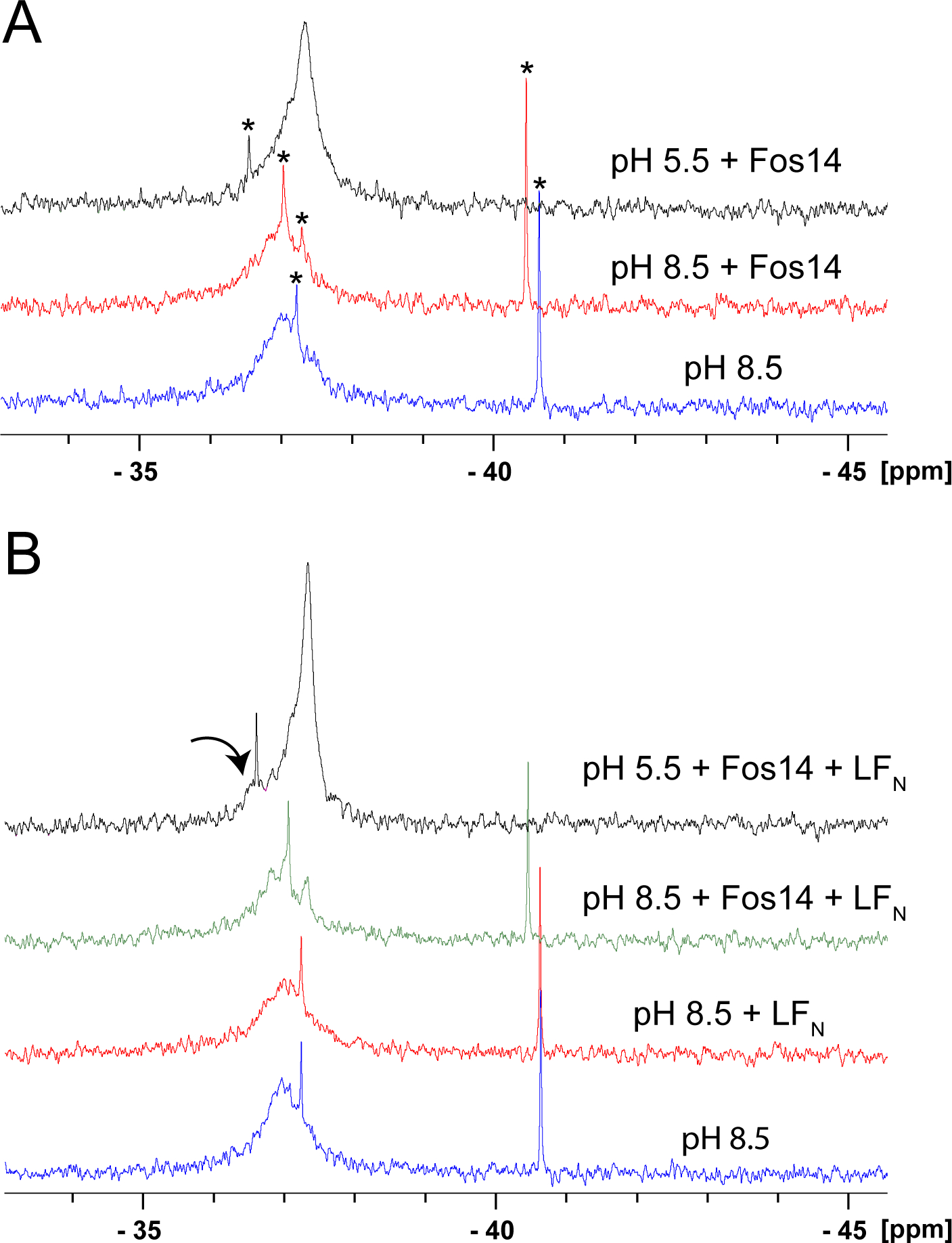
(A) 19F NMR spectra of the heptameric (pFPhe427PA63)7 prepore and pore states. The bottom (blue) represents 3.3 μM heptameric prepore in 20 mM Tris (pH 8.5), 0.4 M NaCl, and 1 mM CaCl2 buffer, with the addition of 50 μL of D2O and 12.5 μL of 2 mM 5-fluorotryptophan as an internal reference (−46.03 ppm). The middle spectrum (red) is after the addition of 20 μL of 1 M Fos-14 detergent in water, and the top spectrum (black) after the addition of 10 μL of 1 M phosphoric acid. (B) 19F NMR spectra of the heptameric (pFPhe427PA63)7 prepore and effects of the addition of LFN. Spectra and sample conditions are the same as in panel A, except the concentration of PA was higher [7 μM heptameric prepore (blue)], with the addition of LFN (red) to give a final ratio of LFN to heptameric prepore of 3:1. The green spectrum is after the addition of Fos-14, and the black spectrum after the addition of phosphoric acid. Note the new resonance in the spectrum at pH 5.5 (arrow). Asterisks denote small molecule impurities in the samples. Spectra in panels A and B represent 40000 transients collected at 20 °C on a Bruker 600 MHz instrument equipped with a 19F cryoprobe and were processed with 10 Hz of line broadening.
LF and EF bind to the prepore, and the N-terminal α-helical regions of LF and EF have been shown in recent cryo-EM studies to engage the α-clamp (Figure 1), which positions each LF or EF for translocation through the pore.22,23 Engagement of the α-clamp has also been shown to favor an allosteric change at the ϕ-clamp, which in single-channel conductance measurements could exist in the pore state in either a more conductive open state (unclamped empty) or less conductive open state (clamped empty).24 Engagement favors a transition to the less conductive clamped state, and in planar lipid bilayer experiments, LFN (the N-terminal PA binding domain of LF) is able to engage the ϕ-clamp and block channel conductance.25 To determine if the pF-Phe resonance can sense an environmental change when LFN is bound, we repeated the experiments described above in the same manner but in the presence of LFN. In Figure 2B, after the addition of LFN in a 1:3 (pF-Phe427-PA63)7:LFN ratio, the resonance appears to broaden, and after the addition of Fos-14, at least three resonances become apparent. The sharp resonance (asterisk) in the spectra is likely a small molecule contaminant from the purification process. After the addition of phosphoric acid, the resonance we attribute to the pore state is now sharper, the line width decreasing to ~90 Hz, but is asymmetric, suggesting that other states are likely present in smaller populations. We see another resonance that becomes more apparent at −36.6 ppm (arrow). Because this resonance is not present in the prepore in the absence of LFN, we take this to indicate that LFN is likely causing an environmental change at the ϕ-clamp.
The narrowed line width and greater intensity observed in our spectrum of the pore state (either with or without LFN) are surprising. As mentioned above, the cryo-EM structure of the pore state showed that the Phe427 side chains form a narrow iris that is only 6 Å wide, and each of the Phe427 side chains is in a face–face orientation.1 In studies of crystals of pF-Phe, the aromatic rings are oriented in a face-to-face arrangement and showed a relatively high barrier to rotation about the Cβ–aromatic bond (~20 kcal/mol), in contrast to crystals of Phe (~5 kcal/mol).26 The higher barrier was attributed to the closer packing of the pF-Phe rings; that if rotation does occur, other planar rings within van der Waals contact distance must also rotate in a cooperative manner. Given the proximity of the Phe rings in the pore state, rotation around the Cβ–aromatic bond should also be sterically hindered from rotation and assume a rotational correlation time (τi) that matches that of the entire pore structure.
In elegant studies of fluorotyrosine-labeled alkaline phosphatase, the theoretical basis for relaxation processes that give rise to fluorine line widths was described.27,28 It was shown that relaxation via chemical shift anisotropy (CSA) contributes substantially to the observed line widths, increases as the rotational correlation time (τc) of the molecule increases, increases as the field strength increases, and increases as the degree of motion around the Cβ–aromatic bond decreases (τi approaches isotropic motion).28 Assuming a spherical shape for the prepore, a minimum estimate of τc is ~185 ns and is expected to increase in the transition to the more elongated pore.1 Thus, our expectation was that the transition to the pore state would lead to extreme broadening of the pF-Phe427 resonance into the baseline. However, the line width of the pore resonance is narrower than the prepore and narrows even further when LFN (which is expected to increase τc even further) is bound.
Due to sample limitations, we could not account for relaxation mechanisms (T1 and T2) or CSA contributions to the observed line widths. However, our data suggest that the narrower resonance observed in the pore state is due to increased dynamics at the pF-Phe427 site. In early planar lipid bilayer experiments, current fluctuations between open and closed conductance states of the pore could be observed from single channels that occur on a subsecond time scale.29 Further single-channel conductance studies also indicated a fast (~20 ms) flickering between open and closed conductive states.30 The fast flickering between open and closed states of the PA pore has also been observed by others,31,32 and closures are not due to contaminating molecules present in solution.33 The narrowed line width of the pore state may represent this fast-flickering motion between the open and closed states. How then can we explain the even narrower line shape of the pore resonance in the presence of LFN?
One explanation is that the presence of LFN may prevent solvent exposure at the ϕ-clamp, narrowing the line width by decreasing the level of solvent dipolar relaxation. However, as mentioned above in the recent cryo-EM structure of the pore with bound EF and/or LF, N-terminal portions of LF and EF bind to the α-clamp.22 Using peptides representing the N-terminal end of LF (residues 1–50), binding of peptides to the α-clamp induces an allosteric change at the ϕ-clamp.24 In these studies, peptide binding shifts the equilibrium between two separate open subconductance states, favoring a more closed, clamped state.24 If these states were present in our studies, binding of LFN would shift the equilibrium to the more closed clamped state, limiting the fast flickering to a more narrow range of open conformational states. This could give rise to the narrower line width that we observe. In addition, single-channel recordings of the PA pore in the presence of helix-favoring peptides revealed at least two intermediate conductance states, aside from the open and closed states, again indicating that the ϕ-clamp is a dynamic structure and not static.34 To allow for translocation of EF and LF through the narrow ϕ-clamp, there must be some dynamic flexibility in this structure, and our 19F NMR data would support a more dynamic ϕ-clamp that is likely able to expand and contract to sterically accommodate all 20 amino acids and combinations of sequences translocating through the channel.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Prof. Chien Ho (Carnegie Mellon University, Pittsburgh, PA), Prof. Jake Schaefer (Washington University in St. Louis, St. Louis, MO), and Prof. David Peyton (Portland State University, Portland, OR) for their comments and discussions on fluorine line widths. The authors are also grateful to Dr. Hang Pham, who is now a practicing physician in Eugene, OR, for initial mutagenesis and 19F NMR studies of the pF-PhePA83 protein.
Funding
This study was supported through a Kansas INBRE award, which is supported through the IDeA Program of the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Grant P20 GM103418. This study also made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH Grant P41GM103399 (NIGMS) (formerly P41RR002301). Equipment was purchased with funds from the University of Wisconsin—Madison, the NIH (P41GM103399, S10RR02781, S10RR08438, S10RR023438, S10RR025062, and S10RR029220), the National Science Foundation (DMB-8415048, OIA-9977486, and BIR-9214394), and the U.S. Department of Agriculture.
ABBREVIATIONS
- NMR
nuclear magnetic resonance
- PA
protective antigen
- EF
edema factor
- LF
lethal factor
- T1
longitudinal relaxation time
- T2
transverse relaxation time
- CSA
chemical shift anisotropy
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.0c00833.
LC-MS/MS analysis of pF-Phe-labeled PA83, expression and purification of pF-Phe PA and LFN, and 19F NMR experimental conditions (PDF)
Accession Codes
UniprotKB P13423 (PAG_BACAN).
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.0c00833
Contributor Information
Srinivas Gonti, Department of Chemistry and Biochemistry, Wichita State University, Wichita, Kansas 67260, United States.
William M. Westler, National Magnetic Resonance Facility at Madison and Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706-1544, United States
Masaru Miyagi, Department of Pharmacology, Case Western Reserve University, Cleveland, Ohio 44106, United States.
James G. Bann, Department of Chemistry and Biochemistry, Wichita State University, Wichita, Kansas 67260, United States.
REFERENCES
- (1).Jiang J, Pentelute BL, Collier RJ, and Zhou ZH (2015) Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature 521 (7553), 545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C, and Pulendran B (2003) Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424 (6946), 329–34. [DOI] [PubMed] [Google Scholar]
- (3).Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, and Ferrin TE (2021) UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30 (1), 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, and Thomas G (1992) Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem 267 (23), 16396–402. [PubMed] [Google Scholar]
- (5).Christensen KA, Krantz BA, Melnyk RA, and Collier RJ (2005) Interaction of the 20 kDa and 63 kDa fragments of anthrax protective antigen: kinetics and thermodynamics. Biochemistry 44 (3), 1047–53. [DOI] [PubMed] [Google Scholar]
- (6).Kintzer AF, Thoren KL, Sterling HJ, Dong KC, Feld GK, Tang II, Zhang TT, Williams ER, Berger JM, and Krantz BA (2009) The protective antigen component of anthrax toxin forms functional octameric complexes. J. Mol. Biol 392 (3), 614–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rainey GJ, Wigelsworth DJ, Ryan PL, Scobie HM, Collier RJ, and Young JA (2005) Receptor-specific requirements for anthrax toxin delivery into cells. Proc. Natl. Acad. Sci. U. S. A 102 (37), 13278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hanna PC, Acosta D, and Collier RJ (1993) On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. U. S. A 90 (21), 10198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Anderson DM, Sheedlo MJ, Jensen JL, and Lacy DB (2020) Structural insights into the transition of Clostridioides difficile binary toxin from prepore to pore. Nat. Microbiol 5 (1), 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Knapp O, Maier E, Waltenberger E, Mazuet C, Benz R, and Popoff MR (2015) Residues involved in the pore-forming activity of the Clostridium perfringens iota toxin. Cell. Microbiol 17 (2), 288–302. [DOI] [PubMed] [Google Scholar]
- (11).Krantz BA, Melnyk RA, Zhang S, Juris SJ, Lacy DB, Wu Z, Finkelstein A, and Collier RJ (2005) A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 309 (5735), 777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sellman BR, Nassi S, and Collier RJ (2001) Point mutations in anthrax protective antigen that block translocation. J. Biol. Chem 276 (11), 8371–6. [DOI] [PubMed] [Google Scholar]
- (13).Sellman BR, Mourez M, and Collier RJ (2001) Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292 (5517), 695–7. [DOI] [PubMed] [Google Scholar]
- (14).Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, and Collier RJ (2004) Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. U. S. A 101 (36), 13147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Boudko SP, and Bachinger HP (2016) Structural insight for chain selection and stagger control in collagen. Sci. Rep 6, 37831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Blaustein RO, and Finkelstein A (1990) Diffusion limitation in the block by symmetric tetraalkylammonium ions of anthrax toxin channels in planar phospholipid bilayer membranes. J. Gen. Physiol 96 (5), 943–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Blaustein RO, and Finkelstein A (1990) Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Effects on macroscopic conductance. J. Gen. Physiol 96 (5), 905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nablo BJ, Halverson KM, Robertson JW, Nguyen TL, Panchal RG, Gussio R, Bavari S, Krasilnikov OV, and Kasianowicz JJ (2008) Sizing the Bacillus anthracis PA63 channel with nonelectrolyte poly(ethylene glycols). Biophys. J 95 (3), 1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Furter R (1998) Expansion of the genetic code: site-directed p-fluoro-phenylalanine incorporation in Escherichia coli. Protein Sci. 7 (2), 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Vernier G, Wang J, Jennings LD, Sun J, Fischer A, Song L, and Collier RJ (2009) Solubilization and characterization of the anthrax toxin pore in detergent micelles. Protein Sci. 18 (9), 1882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Miller CJ, Elliott JL, and Collier RJ (1999) Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38 (32), 10432–41. [DOI] [PubMed] [Google Scholar]
- (22).Zhou K, Liu S, Hardenbrook NJ, Cui Y, Krantz BA, and Zhou ZH (2020) Atomic Structures of Anthrax Prechannel Bound with Full-Length Lethal and Edema Factors. Structure 28 (8), 879–887.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hardenbrook NJ, Liu S, Zhou K, Ghosal K, Hong Zhou Z, and Krantz BA (2020) Atomic structures of anthrax toxin protective antigen channels bound to partially unfolded lethal and edema factors. Nat. Commun 11 (1), 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Das D, and Krantz BA (2016) Peptide- and proton-driven allosteric clamps catalyze anthrax toxin translocation across membranes. Proc. Natl. Acad. Sci. U. S. A 113 (34), 9611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhang S, Finkelstein A, and Collier RJ (2004) Evidence that translocation of anthrax toxin’s lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc. Natl. Acad. Sci. U. S. A 101 (48), 16756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hiyama Y, Silverton JV, Torchia DA, Gerig JT, and Hammond SJ (1986) Molecular structure and dynamics of crystalline p-fluoro-D,L-phenylalanine. A combined x-ray/NMR investigation. J. Am. Chem. Soc 108 (10), 2715–2723. [Google Scholar]
- (27).Hull WE, and Sykes BD (1974) Fluorotyrosine alkaline phosphatase. 19F nuclear magnetic resonance relaxation times and molecular motion of the individual fluorotyrosines. Biochemistry 13 (17), 3431–7. [DOI] [PubMed] [Google Scholar]
- (28).Hull WE, and Sykes BD (1975) Fluorotyrosine alkaline phosphatase: internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J. Mol. Biol 98 (1), 121–53. [DOI] [PubMed] [Google Scholar]
- (29).Blaustein RO, Koehler TM, Collier RJ, and Finkelstein A (1989) Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. U. S. A 86 (7), 2209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Blaustein RO, Lea EJ, and Finkelstein A (1990) Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. J. Gen. Physiol 96 (5), 921–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Orlik F, Schiffler B, and Benz R (2005) Anthrax toxin protective antigen: inhibition of channel function by chloroquine and related compounds and study of binding kinetics using the current noise analysis. Biophys. J 88 (3), 1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nestorovich EM, Karginov VA, Berezhkovskii AM, and Bezrukov SM (2010) Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys. J 99 (1), 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Yamini G, and Nestorovich EM (2017) Relevance of the alternate conductance states of anthrax toxin channel. Proc. Natl. Acad. Sci. U. S. A 114 (13), E2545–E2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ghosal K, Colby JM, Das D, Joy ST, Arora PS, and Krantz BA (2017) Dynamic Phenylalanine Clamp Interactions Define Single-Channel Polypeptide Translocation through the Anthrax Toxin Protective Antigen Channel. J. Mol. Biol 429 (6), 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


