Figure 1.
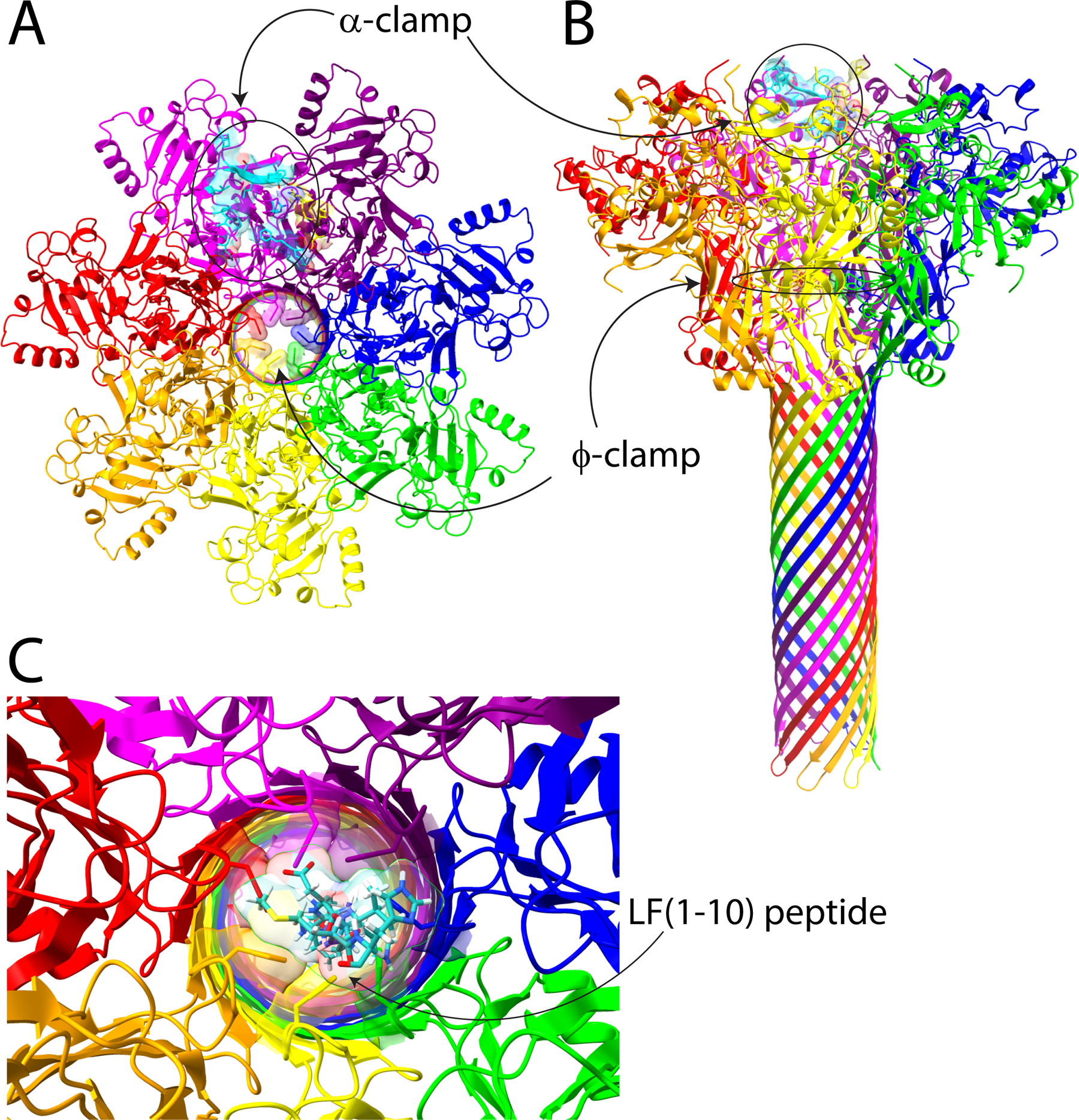
Anthrax toxin pore and regions encompassing the α-clamp and the ϕ-clamp. Panels A and B show top-down and side views, respectively, of the cryo-EM structure of the PA pore (Protein Data Bank entry 3J9C).1 We have highlighted the α-clamp region in cyan and yellow on the surface of the pore, and the ϕ-clamp ring of Phe427 residues is also highlighted. In panel C, we modeled LF (residues 1–10) (cyan) into the center of the ϕ-clamp iris. One can see that there are clear steric clashes between the Phe427 residues and the side chains of LF, including Met, His, and Asp. This figure was generated using ChimeraX.3
